Piperine: Difference between revisions
| Line 66: | Line 66: | ||
==Research== |
==Research== |
||
A component of [[pungency]] by piperine results from activation of the heat- and acidity-sensing [[TRPV]] [[ion channel]]s, [[TRPV1]] and [[TRPA1]], on [[nociceptors]], the pain-sensing [[neuron|nerve cells]].<ref name="pmid15685214">{{cite journal | last1 = McNamara | first1= F. N. |last2=Randall |first2=A. |last3=Gunthorpe |first3=M. J. | title = Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) | journal = British Journal of Pharmacology | volume = 144 | issue = 6 | pages = 781–790 | date = March 2005 | pmid = 15685214 | pmc = 1576058 | doi = 10.1038/sj.bjp.0706040 }}</ref> Piperine is under [[basic research|preliminary research]] for its potential to affect [[bioavailability]] of other compounds in food and [[dietary supplement]]s, such as a possible effect on the [[bioavailability]] of [[curcumin]].<ref>{{cite journal | last1= Shoba|first1= G. |last2=Joy |first2=D. |last3=Joseph |first3=T. |last4=Majeed |first4=M. |last5=Rajendran |first5=R. |last6=Srinivas |first6=P. S. | title = Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers | journal = Planta Medica | volume = 64 | issue = 4 | pages = 353–356 | date = May 1998 | doi = 10.1055/s-2006-957450 | pmid = 9619120 }}</ref> It has been reported that piperine has an antibacterial activity against gastric cancer pathogen Helicobacter pylori. |
A component of [[pungency]] by piperine results from activation of the heat- and acidity-sensing [[TRPV]] [[ion channel]]s, [[TRPV1]] and [[TRPA1]], on [[nociceptors]], the pain-sensing [[neuron|nerve cells]].<ref name="pmid15685214">{{cite journal | last1 = McNamara | first1= F. N. |last2=Randall |first2=A. |last3=Gunthorpe |first3=M. J. | title = Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) | journal = British Journal of Pharmacology | volume = 144 | issue = 6 | pages = 781–790 | date = March 2005 | pmid = 15685214 | pmc = 1576058 | doi = 10.1038/sj.bjp.0706040 }}</ref> Piperine is under [[basic research|preliminary research]] for its potential to affect [[bioavailability]] of other compounds in food and [[dietary supplement]]s, such as a possible effect on the [[bioavailability]] of [[curcumin]].<ref>{{cite journal | last1= Shoba|first1= G. |last2=Joy |first2=D. |last3=Joseph |first3=T. |last4=Majeed |first4=M. |last5=Rajendran |first5=R. |last6=Srinivas |first6=P. S. | title = Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers | journal = Planta Medica | volume = 64 | issue = 4 | pages = 353–356 | date = May 1998 | doi = 10.1055/s-2006-957450 | pmid = 9619120 }}</ref> It has been reported that piperine has an antibacterial activity against gastric cancer pathogen Helicobacter pylori. |
||
== See also == |
== See also == |
||
Revision as of 15:35, 27 October 2017
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
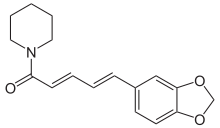
(2E,4E)-5-(2H-1,3-Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one | |
| Other names
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one
Piperoylpiperidine Bioperine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.135 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H19NO3 | |
| Molar mass | 285.343 g·mol−1 |
| Density | 1.193 g/cm3 |
| Melting point | 130 °C (266 °F; 403 K) |
| Boiling point | Decomposes |
| 40 mg/l | |
| Solubility in alcohol | 1 g/15 ml |
| Solubility in ether | 1 g/36 ml |
| Solubility in chloroform | 1 g/1.7 ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Piperine | |
|---|---|
| Heat | |
| Scoville scale | 100,000 SHU |
Piperine, along with its isomer chavicine, is the alkaloid[1] responsible for the pungency of black pepper and long pepper. It has been used in some forms of traditional medicine.
Preparation
Piperine is extracted from black pepper using dichloromethane.[2] Aqueous hydrotropes can be used in the extraction to result in high yield and selectivity.[3] The amount of piperine varies from 1–2% in long pepper, to 5–10% in commercial white and black peppers.[4] Further, it may be prepared by treating the solvent-free residue from an alcoholic extract of black pepper, with a solution of potassium hydroxide to remove resin (said to contain chavicine, an isomer of piperine) and solution of the washed, insoluble residue in warm alcohol, from which the alkaloid crystallises on cooling.[5]
Reactions
Piperine yields salts only with strong acids. The platinichloride B4·H2PtCl6 forms orange-red needles ("B" denotes one mole of the alkaloid base in this and the following formulae). Iodine in potassium iodide added to an alcoholic solution of the base in the presence of a little hydrochloric acid gives a characteristic periodide, B2·HI·I2, crystallising in steel-blue needles, melting point 145 °C.
History
Piperine was discovered in 1819 by Hans Christian Ørsted, who isolated it from the fruits of Piper nigrum, the source plant of both the black and white pepper grains.[6] Flückiger and Hanbury found piperine in Piper longum and Piper officinarum (Miq.) C. DC. (=Piper retrofractum Vahl), two species called "long pepper".[7] West African pepper also contains piperine.[8]
Anderson[9][10][11] first hydrolysed piperine by alkalis into a base and an acid, which were later named[12] piperidine and piperic acid respectively. The alkaloid was first synthesised[13] by the action of piperoyl chloride on piperidine.
Research
A component of pungency by piperine results from activation of the heat- and acidity-sensing TRPV ion channels, TRPV1 and TRPA1, on nociceptors, the pain-sensing nerve cells.[14] Piperine is under preliminary research for its potential to affect bioavailability of other compounds in food and dietary supplements, such as a possible effect on the bioavailability of curcumin.[15] It has been reported that piperine has an antibacterial activity against gastric cancer pathogen Helicobacter pylori.
See also
- Piperidine, a cyclic six-membered amine that results from hydrolysis of piperine
- Capsaicin, the active piquant chemical in chili peppers
- Allyl isothiocyanate, the active piquant chemical in mustard, radishes, horseradish, and wasabi
- Allicin, the active piquant flavor chemical in raw garlic and onions (see those articles for discussion of other chemicals in them relating to pungency, and eye irritation)
- Ilepcimide
- Piperlongumine
References
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, p. 7442, ISBN 091191028X
- ^ Epstein, William W.; Netz, David F.; Seidel, Jimmy L. (1993). "Isolation of piperine from black pepper". J. Chem. Ed. 70 (7): 598. doi:10.1021/ed070p598.
- ^ US 6365601, Gaikar, "Process for extraction of piperine from Piper species", issued 2002-04-02
- ^ "Pepper". Tis-gdv.de. Retrieved 2 September 2017.
- ^ Ikan, Raphael (1991). Natural Products: A Laboratory Guide (2nd ed.). San Diego, CA: Academic Press. pp. 223–224. ISBN 0123705517.
- ^ Ørsted, Hans Christian (1820). "Über das Piperin, ein neues Pflanzenalkaloid" [On piperine, a new plant alkaloid]. Schweiggers Journal für Chemie und Physik (in German). 29 (1): 80–82.
- ^ Friedrich A. Fluckiger; Daniel Hanbury (1879). Pharmacographia : a History of the Principal Drugs of Vegetable Origin, Met with in Great Britain and British India. London: Macmillan. p. 584. ASIN B00432KEP2.
- ^ Stenhouse (1855). Pharm. J. 14: 363.
{{cite journal}}: Missing or empty|title=(help)[full citation needed] - ^ Annalen. 75: 82. 1850.
{{cite journal}}: Missing or empty|title=(help)[full citation needed] - ^ Annalen. 84: 345.
{{cite journal}}: Missing or empty|title=(help)[full citation needed] - ^ Wertheim; Rochleder (1845). Annalen. 54: 255.
{{cite journal}}: Missing or empty|title=(help)[full citation needed] - ^ Babo; Keller (1857). J. Pr. Chem. 72: 53.
{{cite journal}}: Missing or empty|title=(help)[full citation needed] - ^ Rugheimer (1882). Ber. 15: 1390.
{{cite journal}}: Missing or empty|title=(help)[full citation needed] - ^ McNamara, F. N.; Randall, A.; Gunthorpe, M. J. (March 2005). "Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1)". British Journal of Pharmacology. 144 (6): 781–790. doi:10.1038/sj.bjp.0706040. PMC 1576058. PMID 15685214.
- ^ Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. S. (May 1998). "Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers". Planta Medica. 64 (4): 353–356. doi:10.1055/s-2006-957450. PMID 9619120.
