Hydrocodone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | dihydrocodeinone |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601006 |
| Dependence liability | Moderate |
| Routes of administration | oral, intranasal, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High (80% +) |
| Metabolism | Hepatic |
| Elimination half-life | 3.8–6 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.304 |
| Chemical and physical data | |
| Formula | C18H21NO3 |
| Molar mass | 299.368 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Hydrocodone or dihydrocodeinone is a synthetic opioid derived from either of two naturally occurring opiates: codeine[1] and thebaine[2]. It is an orally active narcotic analgesic and antitussive. It is available in tablet, capsule, and syrup form.
Hydrocodone is often compounded with other, generally less effective non-opioid compounds such as paracetamol (also known as acetaminophen) or ibuprofen, both often added to discourage recreational use [citation needed] (as paracetamol can cause potentially fatal liver toxicity at high doses), and to provide a possible synergy of analgesic effects between hydrocodone and the non-opioid compounds present. The particular niche in which hydrocodone is most commonly used is as an intermediate centrally acting analgesic. Abrupt discontinuation of hydrocodone (Vicodin, Vicodin ES, and Norco) may result in withdrawal symptoms.
History
Hydrocodone was first synthesized in Germany in 1920 by Carl Mannich and Helene Löwenheim.[3] It was approved by the Food and Drug Administration on 23 March 1943 for sale in the United States and approved by Health Canada for sale in Canada under the brand name Hycodan.[4][5]
Hydrocodone and compounds containing it are marketed, in varying forms, under a number of trademarks, including Anexsia, Biocodone, Damason-P, Dicodid, Duodin, Hycet, Hycodan (or, generically, Hydromet), Hycomine, Hydrococet, Hydrokon, Hydrovo, Kolikodol, Lorcet, Lortab, Mercodinone, Norco, Norgan, Novahistex, Orthoxycol, Panacet, Symtan, Synkonin, Vicodin, Xodol and Zydone. Hycodan was the original trade name.
The trade name Dicodid was chosen because hydrocodone is the codeine analogue of hydromorphone (Dilaudid) and the naming scheme extended to related drugs like Dihydrin (dihydrocodeine) and Dinarkon (oxycodone). The trade name Vicodin refers to hydrocodone being six times stronger than codeine by mouth, as in the Roman numeral VI.[citation needed]
Medical uses
Hydrocodone is used to treat moderate to severe pain and as an antitussive to treat cough.[6]
Pharmacology
As a narcotic, hydrocodone relieves pain by binding to opioid receptors in the brain and spinal cord. It can be taken with or without food as desired. When taken with alcohol, it can intensify drowsiness. It may interact with monoamine oxidase inhibitors, as well as other drugs that cause drowsiness.
It is in FDA pregnancy category C. Animal reproduction studies have shown an adverse effect on the fetus, and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. In addition, a newborn of a mother taking the medication may exhibit breathing problems or withdrawal symptoms.
Studies have shown hydrocodone is stronger than codeine but only one-tenth as potent as morphine at binding to receptors and reported to be only 59% as potent as morphine in analgesic properties. However, in tests conducted on rhesus monkeys, the analgesic potency of hydrocodone was actually found to be higher than that of morphine.[7] Per os hydrocodone has a MEDD factor of .4, meaning that 1 mg of hydrocodone is equivalent to .4 mg of intravenous morphine. However, because of morphine's low oral bioavailability, there is a 1:1 correspondence between orally administered morphine and orally administered hydrocodone.[8]
Hydrocodone can be habit-forming, which leads to physical and psychological dependence. The potential for addiction varies from individual to individual depending on unique biological differences. Sales and production of this drug have increased significantly in recent years, as have diversion and illicit use.
In the U.S., formulations containing more than 15 mg per dosage unit are considered Schedule II drugs, as would any formulation consisting of just hydrocodone alone. Those containing less than or equal to 15 mg per dosage unit in combination with paracetamol or another non-controlled drug are called hydrocodone compounds and are considered Schedule III drugs. Hydrocodone is typically found in combination with other drugs such as paracetamol, aspirin, ibuprofen and homatropine methylbromide. The purpose of the non-controlled drugs in combination is often twofold: 1) To provide increased analgesia via drug synergy. 2) To limit the intake of hydrocodone by causing unpleasant and often unsafe side effects at higher-than-prescribed doses.
In the UK, it is listed as a Class A drug under the Misuse of Drugs Act 1971. Hydrocodone is not commercially available in pure form in the United States due to a separate regulation, and is always sold with an NSAID, paracetamol, antihistamine, expectorant, or homatropine. Pure hydrocodone is a more strictly controlled Schedule II drug[9] and sold by compounding pharmacies. The cough preparation Codiclear DH is the purest commercial US hydrocodone item, containing guaifenesin and small amounts of ethanol as active ingredients. In Germany and elsewhere, hydrocodone is available as single-active-ingredient tablets as Dicodid (by analogy to the original manufacturer's other products Dilaudid and Dinarkon and others) available in 5- and 10-mg strengths.
As with many other opioids, it is quite possible to reduce the amount of hydrocodone needed to stop a certain level of pain by having the patient take the hydrocodone along with one of the medications with analgesic-sparing properties, also known as potentiators. The most common, one of the most effective with hydrocodone, and safest is hydroxyzine (often marketed under the brand name Vistaril). Orphenadrine, nefopam, carisoprodol, and antihistamines also potentiate most opioids. Especially in the case of carisoprodol, it is imperative that the titration and addition of the potentiator be done under strict supervision of a physician.
Hydrocodone also interacts relatively well with most adjuvant and atypical analgesics used for severe and neuropathic pain such as first-generation anti-depressants, anticholinergics, anticonvulsants, centrally acting stimulants, NMDA antagonists, etc. Hydrocodone can usually be successfully used with duloxetine (Cymbalta) for neuropathic pain, especially that from diabetic neuropathy, provided that the patient has normal relative and absolute levels of Cytochrome P450-related liver enzymes.
Compounds

When sold commercially in the United States, hydrocodone is always combined with another medication. Those combined with paracetamol (acetaminophen) are known by various trademark names, such as Vicodin and Lortab. Hydrocodone also can be combined with aspirin (e.g., Lortab ASA) and ibuprofen (e.g., Vicoprofen).
Combining an opioid such as hydrocodone with another analgesic can increase the effectiveness of the drug without increasing opioid-related side effects (e.g., nausea, constipation, sedation). Another argument for combining hydrocodone with paracetamol (acetaminophen) is that it limits the potential for misuse. As with other opioid analgesics, with a few exceptions, there is no ceiling dose for hydrocodone in users tolerant to its effects; however the hepatotoxicity of the paracetamol it is often combined with begins to manifest itself with doses of around 4,000 mg/day.[10]
Pharmacokinetics
Hydrocodone is biotransformed by the liver into several metabolites, and has a serum half-life that averages 3.8 hours.[7] The hepatic cytochrome P450 enzyme CYP2D6 converts it into hydromorphone, a more potent opioid.
Pharmacogenomics
Analgesic effect by hydrocodone is highly dependent on metabolism to O-demethylated morphine, hydromorphone, by the cytochrome 450 CYP2D6.[11] In the population, there are a group of patients that are less responsive to hydrocodone opioid (~10% of the Caucasian population).[12] Genotyping this group showed that they have inherited polymorphisms in their CYP2D6 allele.[13]
These patients are regarded to be poor or intermediate metabolizers of opioid medications and would need higher concentrations of opioids to experience the same therapeutic benefits as a person without the polymorphism. Therefore, these patients are more likely to encounter adverse drug reactions.[14] On the other hand, ultra-rapid metabolizers (up to 7% of Caucasians and up to 30% of Asian and African populations) may have increased toxicity due to rapid conversion.[15]
Contraindications and interactions
Mixing hydrocodone with alcohol, cocaine, amphetamines, methylphenidate, benzodiazapines, barbiturates, and a number of other medications can have severe adverse reactions including but not limited to heart failure, heart attack, respiratory distress, pulmonary failure, liver or kidney failure, jaundice, amnesia, seizures, blackouts, and coma.[citation needed] Also, hydrocodone can cause false indications on blood and urinalysis testing for morphine, codeine, hydromorphone and cocaine depending on usage and dosing. Generally this effect is only present when doses are taken for long periods of time, and the effect ceases after cessation of use. It isn't a false positive if the test is designed to detect opiates.
- Alcohol
There are serious health risks posed by concurrently consuming alcohol with hydrocodone compounds.
The most common medication compounded with hydrocodone is paracetamol (acetaminophen), which is metabolized solely by the liver. Therefore the risk of fatal overdose due to hepatotoxicity can occur with significantly lower levels of paracetamol when mixed with ethanol. Also the mixture can potentially cause serious damage to the liver, kidneys, and stomach wall. Paracetamol may increase the potential for coma, respiratory problems, and can damage the CNS.[16]
Adverse effects
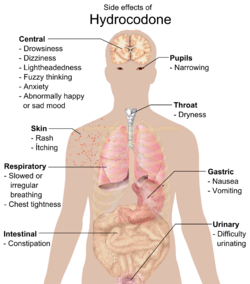
Common side effects include dizziness, itching, lightheadedness, nausea, sweating, drowsiness, constipation, vomiting, and euphoria. Vomiting in some patients is so severe that hospitalization is required, although this can be due to alcohol consumption before taking the medication.[citation needed] Some less common side effects are allergic reaction, blood disorders, changes in mood, racing heartbeat, mental fogginess, anxiety, lethargy, difficulty urinating, spasm of the ureter, irregular or depressed respiration, and rash.
Hydrocodone in particular, and the -codone family of opioids in general, have been shown to have a liability to cause long term hearing loss over periods of use, though these actual findings are quite rare.[18][19]

Symptoms of hydrocodone overdose include respiratory depression; extreme somnolence; blue, clammy, or cold skin; narrowed or widened pupils; bradycardia; coma; seizures; cardiac arrest; and death.[20]
Daily consumption of hydrocodone should not exceed 40 milligrams in patients not tolerant to opiates.[citation needed] The 2006 Physicians Desk Reference states that Norco 10, containing 10 milligrams of hydrocodone and 325 mg of paracetamol can be taken at a dosage of up to twelve tablets per day (120 mg of hydrocodone). This restriction is only limited by the fact that twelve tablets, each containing 325 mg of paracetamol, puts the patient right below the 24-hour FDA maximum of 4,000 mg of paracetamol.[21] Some specially compounded products are routinely given to chronic pain patients in doses of up to 180 mg of hydrocodone per day.[citation needed]
Some of the effects of hydrocodone come from the fact that a fraction of it is changed to hydromorphone in the liver, as is the case with all codeine-based analgesics (codeine into morphine, dihydrocodeine into dihydromorphine, nicocodeine into nicomorphine etc.). The percentage can vary based on both other medications taken and inherited metabolic quirks involving the Cytochrome P450 metabolic pathways—some cannot process it at all, whereas a smaller percentage can get even more strength from it than usual. These factors can also cause hydrocodone and related drugs to have a threshold effect, cause significant lengthening or shortening of the duration of effects in the absence of tolerance, and increase or decrease the de facto conversion ratio between hydrocodone and other drugs like morphine, hydromorphone, and synthetics like levorphanol and methadone.
Testosterone
Hydrocodone, along with most other opioids, may also severely decrease testosterone levels in men and may cause menstrual irregularities in women. Short-term use of opioids will usually result in a decrease in testosterone with a subsequent rebound post-cessation. However, chronic use is much more dangerous. In a study on cancer survivors using opioids for chronic pain relief, 90% of the subjects had hypogonadal levels of testosterone.[citation needed]
This may occur due to both a negative feedback at both the hypothalamus-pituitary and at the gonadal (testicular) level. This is known as "central hypogonadism". Patients using opioid therapy should be screened for such endocrinological problems periodically through blood tests and inquiry of symptoms, which include loss of libido, erectile dysfunction, anxiety, fatigue, loss of muscle mass, and infertility. Treatment should first consist of opioid rotation. If that does not work, then testosterone replacement should commence.[citation needed]
Recreational use
Hydrocodone presents much of the same side-effects as other opioids including euphoria, sedation and somnolence. Hydrocodone is one of the most common recreational prescription drugs in America, along with oxycodone. Recreational hydrocodone use is particularly prevalent among teenagers and young adults because of the drug's widespread availability.[citation needed]
Many users of hydrocodone report a sense of satisfaction, especially at higher doses. A number of users also report a warm or pleasant numbing sensation throughout the body, one of the most well known effects of narcotics.[22] Withdrawal effects may include, but are not limited to; severe pain, pins and needles sensation throughout body, sweating, extreme anxiety and restlessness, sneezing, watery eyes, fever, depression, and extreme drug cravings, among others. The presence of paracetamol in hydrocodone-containing products allegedly deters many users, at least in theory, from taking excessive amounts. However, some users will bypass this danger by using cold water extraction to extract and dispose of a portion of the paracetamol, taking advantage of the water-soluble element of the drug. It is common for users to have liver problems from consuming excessive amounts of paracetamol over a long period of time; taking 10,000 to 15,000 milligrams (10 to 15 grams) of paracetamol in a period of 24 hours typically results in paracetamol overdose and severe hepatotoxicity; doses in the range of 15,000–20,000 milligrams a day have been reported as fatal.[23] It is this factor that leads many recreational users to use only single-entity opioids such as oxycodone. One of the major problems today with the illicit use of hydrocodone, especially in younger populations, is that users may not be aware that hydrocodone pills contain paracetamol.[10]
Detection in body fluids
Hydrocodone may be quantitated in blood, plasma or urine to monitor for misuse, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Many commercial opiate screening tests cross-react appreciably with hydrocodone and its metabolites, but chromatographic techniques can easily distinguish hydrocodone from other opiates. Blood or plasma hydrocodone concentrations are typically in the 5-30 µg/L range in persons taking the drug therapeutically, 100-200 µg/L in abusers and 0.1-2.0 mg/L in cases of acute fatal overdosage.[24][25]
Regulation
- Belgium
In Belgium, hydrocodone is no longer available for medical use.
- Luxembourg
In Luxembourg, hydrocodone is available by prescription under name Biocodone. Prescriptions are more commonly given for use as a cough suppressant (antitussive) rather than for pain relief (analgesic).
- Germany
In Germany, hydrocodone is available as single-active-ingredient tablets as Dicodid (by analogy to the original manufacturer's other products Dilaudid and Dinarkon and others) available in 5- and 10-mg strengths. Hydrocodone is listed under the Betäubungsmittelgesetz as a Suchtgift in the same category as morphine.
- Austria
Hydrocodone is regulated in the same fashion as in Germany under the Austrian Suchtmittelgesetz; since 2002 it has been available in the form of German products and those produced elsewhere in the European Union under Article 76 of the Schengen Treaty—prior to this, no Austrian companies produced hydrocodone products, with dihydrocodeine and nicomorphine being more commonly used for the same levels of pain and the former for coughing.
- The Netherlands
In the Netherlands, hydrocodone is not available for medical use and is classified as a List 1 drug under the Opium Law.
- Sweden
Hydrocodone is no longer available for medical use. The last remaining formula banned in 1967.
- United Kingdom
In the UK, hydrocodone is not available for medical use and is listed as a Class A drug under the Misuse of Drugs Act 1971. Various formulations of dihydrocodeine, a weaker opioid, are frequently used as an alternative for the aforementioned indications of hydrocodone use.
- United States
Under the Controlled Substances Act (CSA) hydrocodone is listed as both a Schedule II and Schedule III substance depending on the formulation.
- Schedule II lists hydrocodone in pure form and any formulations of combination products containing more than 15 mg hydrocodone per dosage unit.
- Schedule III lists hydrocodone in formulations of combination products containing up to 15 mg hydrocodone per dosage unit.
Hydrocodone was until recently the active antitussive in more than 200 formulations of cough syrups and tablets sold in the United States. In late 2006, the FDA began forcing the recall of many of these formulations due to reports of deaths in infants and children under the age of six. The legal status of drug formulations originally sold between 1938 and 1962—before FDA approval was required—was ambiguous. As a result of FDA enforcement action, 88% of the hydrocodone-containing medications have been removed from the market.[26]
At the present time, doctors, pharmacists, and codeine-sensitive or allergic patients or sensitive to the amounts of histamine released by its metabolites must choose among rapidly dwindling supplies of the Hycodan-Codiclear-Hydromet type syrups, Tussionex—an extended-release suspension similar to the European products Codipertussin (codeine hydrochloride), Paracodin suspension (dihydrocodeine hydroiodide), Tusscodin (nicocodeine hydrochloride) and others—and a handful of weak dihydrocodeine syrups. The low sales volume and Schedule II status of Dilaudid cough syrup predictably leads to under-utilisation of the drug. There are several conflicting views concerning the US availability of cough preparations containing ethylmorphine (also called dionine or codethyline)—Feco Syrup and its equivalents were first marketed circa 1895 and still in common use in the 1940s and 1950s, and the main ingredient is treated like codeine under the Controlled Substances Act of 1970.
C-III and higher prescriptions are generally valid for 6 months (including any refills). In the U.S., hydrocodone is always found in combination with other drugs such as paracetamol, aspirin, ibuprofen and homatropine methylbromide due to compounding regulations. These combinations are considered C-III substances. The purpose of the non-controlled drugs in combination is often twofold: 1) To provide increased analgesia via drug synergy. 2) To limit the intake of hydrocodone by causing unpleasant and often unsafe side effects at higher-than-prescribed doses (See Below). As stated above, hydrocodone is not available in pure form in the United States due to a separate regulation, and is always sold with an NSAID, paracetamol, antihistamine, expectorant, or homatropine. The cough preparation Codiclear DH is the purest US hydrocodone item, containing guaifenesin and small amounts of ethanol as active ingredients.
As of July 2010, the FDA is considering banning some hydrocodone and oxycodone fixed-combination proprietary prescription drugs—based on the paracetamol content and the widespread occurrence of liver problems. FDA action on this suggestion would ostensibly also affect codeine, dihydrocodeine, and propoxyphene products such as the Tylenol With Codeine and Panlor series of drugs and Darvocet. An extended-release hydrocodone-only product is apparently close to final approval for marketing in the United States, and single-ingredient tablets of oxycodone and codeine are currently marketed. Mixtures of these drugs with other drugs such as Vicoprofen (hydrocodone & ibuprofen), Combunox (ibuprofen and oxycodone), Synalgos DC (aspirin and dihydrocodeine), and the Empirin With Codeine series are also currently available. Presently it is the most prescribed drug in the USA. In 2010 131.2 million prescriptions of Hydrocodone (combined with acetaminophen) were made.[27]
See also
References
- ^ US Patent Application 2011/0071016 A1
- ^ WO Patent Application 2006/91885 A2
- ^ Mannich, C.; Löwenheim, H. (1920). "Ueber zwei neue Reduktionsprodukte des Kodeins". Archiv der Pharmazie. 258 (2–4): 295–316. doi:10.1002/ardp.19202580218.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Drugs@FDA—Approval History: Hycodan". FDA. Retrieved 7 January 2006.
- ^ "FDA Docket No. 2007N-0353, Drug Products Containing Hydrocodone; Enforcement Action Dates". FDA. Retrieved 7 January 2006. See section I. B., DESI Review of Hydrocodone Products
- ^ "Hydrocodone bitartrate". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ a b Davis, Mellar P. (2005). "Hydrocodone". Opioids for cancer pain. Oxford UK: Oxford University Press. pp. 59–68. ISBN 0-19-852943-0.
- ^ "Instructions for Mean Equivalent Daily Dose (MEDD)" (PDF). Retrieved 22 August 2010.
- ^ "Controlled Substances Act". Wikipedia. Retrieved 8 February 2012.
- ^ a b "Acetaminophen (Tylenol)—side effects, drug class, medical uses, and drug interactions by". Medicinenet.com. 21 September 2007. Retrieved 22 August 2010.
- ^ "Hydrocodone". Drugbank. Retrieved 14 June 2011.
- ^ "Inhibition of Cytochrome P450 2D6 Metabolism of Hydrocodone to Hydromorphone Does Not Importantly Affect Abuse Liability—JPET". Jpet.aspetjournals.org. 1 April 1997. Retrieved 22 August 2010.
- ^ Paul J Jannetto, Nancy C Bratanow (23 October 2009). "Utilization of Pharmacogenomics and Therapeutic Drug Monitoring for Opioid Pain Management". Medscape. Retrieved 14 June 2011.
- ^ Paul J Jannetto, Nancy C Bratanow (23 October 2009). "Utilization of Pharmacogenomics and Therapeutic Drug Monitoring for Opioid Pain Management". Medscape. Retrieved 14 June 2011.
- ^ "hydrocodone/acetaminophen". Medscape. Retrieved 14 June 2011.
- ^ Draganov, P.; Durrence, H.; Cox, C.; Reuben, A. (2000). "Alcohol-acetaminophen syndrome" (archived page). Postgraduate Medicine. 107 (1): 189–95. PMID 10649673.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b MedlinePlus (The American Society of Health-System Pharmacists)—Drug Information: Hydrocodone. Last Revised—1 October 2008. Retrieved on 21 February 2009.
- ^ Friedman RA, House JW, Luxford WM, Gherini S, Mills D (2000). "Profound hearing loss associated with hydrocodone/acetaminophen abuse". Am J Otol. 21 (2): 188–91. doi:10.1016/S0196-0709(00)80007-1. PMID 10733182.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ho T, Vrabec JT, Burton AW (2007). "Hydrocodone use and sensorineural hearing loss". Pain Physician. 10 (3): 467–72. PMID 17525781.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b "MedlinePlus drug information: Hydrocodone. Last revised: Last Revised—10/01/2008. Retrieved on 15 Mars, 2009". Nlm.nih.gov. 28 July 2010. Retrieved 22 August 2010.
- ^ "Slide 1" (PDF). Retrieved 22 August 2010.
- ^ "Hydrocodone". http://www.justice.gov. United States Government. 1 July 2007. Retrieved 13 January 2010.
{{cite web}}:|first=missing|last=(help); Check|archiveurl=value (help); External link in|work= - ^ Rx List
- ^ Spiller HA. Postmortem oxycodone and hydrocodone blood concentrations. J. Forensic Sci. 48: 429-431, 2003.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp. 812-814.
- ^ "Medical News: FDA Pulls Plug on 200-Plus Unapproved Cough Syrups With Hydrocodone—in Product Alert, Prescriptions from". MedPage Today. Retrieved 22 August 2010.
- ^ http://www.emedicinehealth.com/script/main/art.asp?articlekey=143443
External links
- Erowid Hydrocodone (Vicodin) Vault
- U.S. National Library of Medicine: Drug Information Portal – Hydrocodone
