Moderna
 | |
| Formerly | ModeRNA Therapeutics (2010–2018) |
|---|---|
| Company type | Public |
| |
| Industry | Biotechnology |
| Founded | September 2010 |
| Founders |
|
| Headquarters | 200 Technology Square Cambridge, Massachusetts, U.S.[3] |
Key people |
|
| Products | Moderna COVID-19 vaccine[6] |
| Revenue | |
| Total assets | |
| Total equity | |
Number of employees | 830 (2019)[7] |
| Website | modernatx |
Moderna (/məˈdɜːrnə/ mə-DUR-nə)[8] is an American pharmaceutical and biotechnology company based in Cambridge, Massachusetts. It focuses on drug discovery, drug development, and vaccine technologies based exclusively on messenger RNA (mRNA).[9][10]
Moderna's technology platform inserts synthetic nucleoside-modified mRNA (modRNA) into human cells. This mRNA then reprograms the cells to prompt immune responses. It is a novel technique, previously abandoned due to the side effects of inserting mRNA into cells.[11][12][13] As of November 2020[update], the Moderna COVID-19 vaccine candidate, mRNA-1273, had shown preliminary evidence of 94% efficacy in preventing COVID-19 disease in a Phase III trial, with only minor flu-like side effects. This led to its submission for emergency use authorization (EUA) in Europe, the United States, and Canada.[14][15] On December 18, 2020, mRNA-1273 was issued an EUA in the United States.[16] It was authorized for use in Canada on December 23, 2020,[17][18] as well as in the European Union on January 6, 2021.[19] Subsequently the mRNA-1273 was authorized in the United Kingdom on 8 January 2021.[20]
History
2010–2016

In 2010, ModeRNA Therapeutics was formed to commercialize the research of stem cell biologist Derrick Rossi. Rossi had developed a method of modifying mRNA by first transfecting it into human cells, then dedifferentiating it into stem cells which could then be further redifferentiated into desired target cell types.[21][22] Rossi approached fellow Harvard University faculty member Tim Springer, who solicited co-investment from Kenneth Chien, Bob Langer, and venture capital firm Flagship Ventures.[22][23]
In 2011, the CEO of Flagship Ventures (now Flagship Pioneering), Noubar Afeyan, brought in European pharma sales and operations executive Stéphane Bancel as CEO.[22][11] Afeyan personally owned 19.5% of Moderna and was the largest single shareholder, while his fund, Flagship Pioneering, owned 18%.[24]
In March 2013, Moderna and AstraZeneca signed a five-year exclusive option agreement to discover, develop, and commercialize mRNA for treatments in the therapeutic areas of cardiovascular, metabolic, and renal diseases, and selected targets for cancer.[11][25][26] The agreement included a $240 million upfront payment to Moderna, a payment that was "one of the largest ever initial payments in a pharmaceutical industry licensing deal that does not involve a drug already being tested in clinical trials",[25] and an 8% share in Moderna.[24] As of May 2020[update], only one candidate has passed Phase I trials, a treatment for myocardial ischemia, labelled AZD8601.[a][28]
In January 2014, Moderna and Alexion Pharmaceuticals entered a $125 million deal for orphan diseases in need of therapies. Alexion paid Moderna $100 million for 10 product options to develop rare-disease treatments, including for Crigler-Najjar syndrome, using Moderna's mRNA therapeutics platform.[29] By 2016, Bancel told an audience of JPMorgan Chase investors that the work with Alexion would shortly enter human trials. However, by 2017, the program with Alexion had been scrapped as the animal trials showed that Moderna's treatment would never be safe enough for use in humans.[11][13]
In February 2016, an op-ed in Nature criticized Moderna for not publishing any peer-reviewed papers on its technology, unlike most other emerging and established biotech companies, and compared its approach to that of the controversially failed Theranos.[30] In September 2018, Thrillist published article titled, "Why This Secretive Tech Start-Up Could Be The Next Theranos",[31] criticizing its reputation for secrecy and the absence of scientific validation or independent peer-review of its research, though having the highest valuation of any U.S. private biotech company at more than $5 billion.[11][12] A former Moderna scientist told Stat: "It's a case of the emperor's new clothes. They're running an investment firm, and then hopefully it also develops a drug that's successful".[11]
2018–2020
In 2018, the company rebranded as "Moderna Inc." with the ticker symbol MRNA, and further increased its portfolio of vaccine development.[10] In December 2018, Moderna became the largest biotech initial public offering in history, raising $621 million (27 million shares at $23 per share) on NASDAQ, and implying an overall valuation of $7.5 billion for the entire company.[32][33] The year-end 2019 SEC filings showed that Moderna had accumulated losses of $1.5 billion since inception, with a loss of $514 million in 2019 alone, and had raised $3.2 billion in equity since 2010.[10][24] As of December 2020[update], Moderna was valued at $60 billion.[34]
In March 2020, in a White House meeting between the Trump administration and pharmaceutical executives, Bancel told the president that Moderna could have a COVID-19 vaccine ready in a few months.[10] The next day, the FDA approved clinical trials for the Moderna vaccine candidate, with Moderna later receiving investment of $483 million from Operation Warp Speed.[10] Moderna board member, Moncef Slaoui, was appointed head scientist for the Operation Warp Speed project.[10]
Overview
Moderna develops mRNAs that are delivered in lipid nanoparticle, using mRNA with pseudouridine nucleosides. Candidates are designed to have improved folding and translation efficiency via insertional mutagenesis.[35]
The Moderna COVID-19 vaccine candidate, mRNA-1273, was shown in a Phase I trial to be immunogenic in a small number of volunteers aged 18–55 years.[36] As of December 2020[update], no mRNA vaccine had completed Phase III clinical trials or had been licensed for prophylactic use against COVID-19, although the Moderna and Pfizer COVID-19 vaccines had been approved for marketing under emergency use authorizations from the FDA and regulatory agencies in other countries.[37][38]
Leadership
Since 2011, Moderna has been led by CEO Stéphane Bancel, a French businessman with a pharmaceutical sales and operations background.[11][10] Bancel has been described as having a secretive approach to Moderna, and as being a tough operator.[11][10] Though never having worked with RNA before, Stat noted that Bancel "is listed as a co-inventor on more than 100 of Moderna's early patent applications, unusual for a CEO who is not a PhD scientist".[11] After Noubar Afeyan and Robert Langer, Bancel is the largest individual shareholder in the company.[39]
Stephen Hoge, M.D., is president and a former McKinsey & Company management consultant who joined in 2012; he is the fourth-largest individual shareholder in the company.[39][5]
Board
Noubar Afeyan, CEO of Flagship Pioneering, has been the chairman of Moderna's board of directors since 2011.[40] Afeyan who has a Ph.D. in biochemical engineering[41] holds his interest in Moderna through various Flagship Pioneering vehicles. At the 2018 IPO, documents filed stated that Afeyan owned 19.5% of the company, while Flagship owned 18%, thus giving Afeyan control over 37.5% of the company.[24]
In May 2020, board member Dr. Moncef Slaoui resigned from the company to become Chief Scientist for the Trump administration's "Operation Warp Speed", a group designed to accelerate the development of a vaccine for the coronavirus. Slaoui continued to hold more than $10 million in stock options in the company in his new role while the federal government invested $483 million in the company to assist in coronavirus vaccine trials. Senator Elizabeth Warren called the holding a conflict of interest and stated that Slaoui should have divested his options.[42]
COVID-19 vaccine
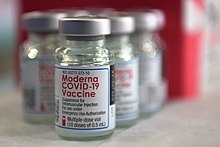
The Moderna COVID‑19 vaccine, sold under the brand name Spikevax, is a COVID-19 vaccine developed by the American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). Depending on the jurisdiction, it is authorized for use in humans aged six months,[43] twelve years, or eighteen years and older. It provides protection against COVID-19, which is caused by infection by the SARS-CoV-2 virus.[44][45][46][47]
It is designed to be administered in two or three 0.5-mL doses given by intramuscular injection, primarily into the deltoid muscle, at an interval of at least 28 days apart.[48][49][50][51] The World Health Organization advises an eight-week interval between doses to optimize efficacy. Additional booster doses are approved in some regions to maintain immunity. Clinical trials and real-world data have demonstrated the vaccine's high efficacy, with significant effectiveness observed two weeks post-administration of the second dose, offering 94% protection against Covid and robust defense against severe cases. The vaccine's efficacy spans various demographics, including age, sex, and those with high-risk medical conditions.
It is an mRNA vaccine composed of nucleoside-modified mRNA (modRNA) encoding a spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles.[52] In August and September 2022, bivalent versions of the vaccine (Moderna COVID-19 Vaccine, Bivalent) containing elasomeran/elasomeran 0-omicron (Spikevax Bivalent Zero/Omicron)[53] were authorized for use as booster doses in individuals aged 18 or older in the United Kingdom,[54][55] Switzerland,[56] Australia,[57] Canada,[58][59] the European Union,[45] and the United States.[60][61][62][63][64][65] The second component of the version of the bivalent vaccine used in the United States[66] is based on the Omicron BA.4/BA.5 variant,[60] while the second component of the bivalent vaccine version used in other countries is based on the Omicron BA.1 variant.[45][53][55][54][59] The vaccine's effectiveness against variants has been extensively studied, indicating varying degrees of protection. For instance, during the prevalence of the Delta variant, effectiveness against infection slightly decreased over time. The vaccine's longevity and continuous protection are under study, with ongoing research focusing on its duration of effectiveness, which remains partially undetermined as of the latest updates.
The safety profile of the vaccine is favorable, with common side effects including injection site pain, fatigue, and headaches. Severe reactions like anaphylaxis are exceedingly rare. Concerns regarding myocarditis, have been identified but are typically mild and manageable. The vaccine's formulation utilizes mRNA technology, encapsulated within lipid nanoparticles to ensure cellular uptake and immune system response.See also
Notes
References
- ^ "What we know about Moderna's COVID-19 vaccine candidate — and what we don't". PBS News Hour. November 16, 2020. Archived from the original on December 4, 2020.
Noubar Afeyan is a co-founder and chairman of Moderna...
- ^ "Moderna chairman: We don't need deep-freeze conditions for vaccine". CNN. November 16, 2020. Archived from the original on December 4, 2020.
- ^ "Key Facts". Moderna. Retrieved May 19, 2020.
- ^ Alspach, Kyle (May 22, 2013). "Moderna CEO Bancel joins Flagship Ventures as senior partner". Boston Business Journal (Blog). American City Business Journals. Archived from the original on January 31, 2014. Retrieved March 12, 2020.
- ^ a b c "Executive Committee & Leadership". Archived from the original on November 16, 2020. Retrieved August 13, 2020.
- ^ "Christmas comes early as first Moderna vaccines arrive in Canada". Global News. December 24, 2020. Retrieved December 25, 2020.
- ^ a b c d e f "Moderna Reports Third Quarter 2020 Financial Results". Moderna. October 29, 2020. Retrieved December 19, 2020.
- ^ Moderna (October 23, 2019). mRNA-3704 and Methylmalonic Acidemia (Video). YouTube. Retrieved January 19, 2021.
- ^ Catherine Shaffer (December 6, 2013). "Moderna Makes Entrance with $40M Round for mRNA Work". BioWorld. Archived from the original on November 16, 2020. Retrieved December 11, 2013.
- ^ a b c d e f g h Elizabeth Cohen (November 30, 2020). "Moderna applies for FDA authorization for its Covid-19 vaccine". CNN. Retrieved December 4, 2020.
- ^ a b c d e f g h i Garade, Damien (September 13, 2016). "Ego, ambition, and turmoil: Inside one of biotech's most secretive startups". Stat. Archived from the original on November 16, 2020. Retrieved May 18, 2020.
- ^ a b Crow, David (September 6, 2017). "Secretive Moderna yet to convince on $5bn valuation". Financial Times. Archived from the original on February 3, 2019. Retrieved May 18, 2020.
- ^ a b Garde, Damien (January 10, 2017). "Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine". Stat. Archived from the original on November 16, 2020. Retrieved May 19, 2020.
- ^ Ludwig Burger (December 1, 2020). "COVID-19 vaccine sprint as Pfizer-BioNTech, Moderna seek emergency EU approval". Reuters. Retrieved December 4, 2020.
- ^ Hannah Kuchler (November 30, 2020). "Canada could be among the first to clear Moderna's COVID-19 vaccine for use". The Financial Post. Retrieved December 4, 2020.
- ^ "Statement from NIH and BARDA on the FDA Emergency Use Authorization of the Moderna COVID-19 Vaccine". US National Institutes of Health. December 18, 2020. Retrieved December 19, 2020.
- ^ "Regulatory Decision Summary - Moderna COVID-19 Vaccine". Health Canada. December 23, 2020. Retrieved December 23, 2020.
- ^ "Moderna COVID-19 Vaccine (mRNA-1273 SARS-CoV-2)". COVID-19 vaccines and treatments portal. December 23, 2020. Retrieved December 23, 2020.
- ^ Strauss, Marine (January 6, 2021). "UPDATE 1-European Commission gives final approval to Moderna vaccine". Reuters. Retrieved January 10, 2021.
- ^ releases, 8:30am to 5pm For real-time updates including the latest press; Statements, News; https://www.twitter.com/mhragovuk, see our Twitter channel at. "Moderna vaccine becomes third COVID-19 vaccine approved by UK regulator". GOV.UK. Retrieved January 10, 2021.
{{cite web}}:|last2=has generic name (help); External link in|last3= - ^ Erin Kutz (October 4, 2010). "ModeRNA, Stealth Startup Backed By Flagship, Unveils New Way to Make Stem Cells". Xconomy. Archived from the original on February 8, 2017. Retrieved February 7, 2017.
- ^ a b c Gregory Huang (December 6, 2012). "Moderna, $40M in Tow, Hopes to Reinvent Biotech with "Make Your Own Drug"". Xconomy. Archived from the original on November 22, 2018. Retrieved June 21, 2013.
- ^ Catherine Elton (March 2013). "The NEXT Next Big Thing". Boston Magazine. Archived from the original on November 16, 2020. Retrieved December 11, 2013.
- ^ a b c d Terry, Mark (November 29, 2018). "Can Moderna Live up to the Hype? mRNA Company Increases IPO Goal to $600 Million". BioSpace. Archived from the original on November 16, 2020. Retrieved May 19, 2020.
The filings with the U.S. Securities and Exchange Commission (SEC) not only updated the proposed IPO but disclosed the size of the company's largest investors' stakes. Noubar B. Afeyan, company chairman, holds 19.5 percent, or 58,882,696 shares. Flagship Pioneering holds 18 percent of Moderna shares
- ^ a b Andrew Pollack (March 21, 2013). "AstraZeneca Makes a Bet on an Untested Technique". The New York Times. Archived from the original on November 16, 2020. Retrieved December 11, 2013.
- ^ Robert Weisman (March 21, 2013). "Moderna in line for $240m licensing deal". The Boston Globe. Archived from the original on November 16, 2020. Retrieved December 11, 2013.
- ^ "Moderna's gamble: what's behind biotech's biggest-ever IPO?". Pharmaceutical Technology. February 21, 2019. Archived from the original on November 16, 2020. Retrieved May 20, 2020.
- ^ "Our Pipeline". Modena. Archived from the original on November 16, 2020. Retrieved May 19, 2020.
- ^ Chris Reidy (January 13, 2014). "Alexion, Moderna announce agreement to develop messenger RNA therapeutics". The Boston Globe. Archived from the original on November 16, 2020. Retrieved March 18, 2020.
- ^ "Research not fit to print". Nature. 34 (115). February 5, 2016. Archived from the original on November 16, 2020. Retrieved May 19, 2020.
- ^ McGauley, Joe (September 23, 2018). "Why This Secretive Tech Start-Up Could Be The Next Theranos". Thrillist. Retrieved May 18, 2020.
- ^ Mukherjee, Sy (December 8, 2018). "Moderna Had the Biggest Biotech IPO Ever. Here's What That Says About the Industry's Future". Fortune. Archived from the original on November 16, 2020. Retrieved May 18, 2020.
- ^ Ramsey, Lydia (December 7, 2018). "Moderna just priced the biggest IPO in biotech history, valuing the startup at $7.5 billion". Business Insider. Archived from the original on November 16, 2020. Retrieved May 18, 2020.
- ^ "Moderna stock price and market value". Yahoo Finance. December 4, 2020. Retrieved December 4, 2020.
- ^ Servick, Kelly (February 1, 2017). "This mysterious $2 billion biotech is revealing the secrets behind its new drugs and vaccines". Science. doi:10.1126/science.aal0686. Archived from the original on November 16, 2020. Retrieved May 17, 2018.
- ^ Jackson, Lisa A.; Anderson, Evan J.; Rouphael, Nadine G.; Roberts, Paul C.; Makhene, Mamodikoe; Coler, Rhea N.; McCullough, Michele P.; Chappell, James D.; Denison, Mark R.; Stevens, Laura J.; Pruijssers, Andrea J. (July 14, 2020). "An mRNA Vaccine against SARS-CoV-2 — Preliminary Report". New England Journal of Medicine. 0 (20): 1920–1931. doi:10.1056/NEJMoa2022483. ISSN 0028-4793. PMC 7377258. PMID 32663912.
At the 100-microgram dose, the one Moderna is advancing into larger trials, all 15 patients experienced side effects, including fatigue, chills, headache, muscle pain, and pain at the site of injection. All side effects were considered mild or moderate. A higher, 250-microgram dose led to more serious reactions and has been set aside.
- ^ Singh, Jerome Amir; Upshur, Ross E G (2020). "The granting of emergency use designation to COVID-19 candidate vaccines: implications for COVID-19 vaccine trials". The Lancet Infectious Diseases. doi:10.1016/s1473-3099(20)30923-3. ISSN 1473-3099.
- ^ Safura Abdool Karim (December 18, 2020). "Emergency use authorization of Covid-19 vaccines could hinder global access to them". STAT. Retrieved December 27, 2020.
- ^ a b Wink, Ben (May 18, 2020). "Here are the 5 multimillionaire scientists and executives getting the richest off Moderna's spike to record highs". Business Insider. Archived from the original on November 16, 2020. Retrieved May 19, 2020.
- ^ "Moderna's Board of Directors". Moderna. Archived from the original on November 16, 2020. Retrieved May 19, 2020.
- ^ "One Of Biotech's Biggest Investors Says The Industry Must Be 'Unreasonable'". www.wbur.org. Archived from the original on November 16, 2020. Retrieved July 15, 2020.
- ^ Corcoran, Kieran (May 16, 2020). "The ex-pharma exec leading Trump's COVID-19 vaccine program has $10 million in stock options for a company getting federal funding". Business Insider. Archived from the original on November 16, 2020. Retrieved May 18, 2020.
- ^ Mandavilli A (June 19, 2022). "CDC recommends COVID-19 vaccines for children under 5". The New York Times. Archived from the original on June 21, 2022. Retrieved June 21, 2022.
- ^ Cite error: The named reference
Moderna COVID-19 vaccine Moderna COVID-19 Vaccine FDA labelwas invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
Moderna COVID-19 vaccine Spikevax EPARwas invoked but never defined (see the help page). - ^ "Moderna Spikevax COVID-19 vaccines". Health Canada. September 12, 2023. Archived from the original on September 15, 2023. Retrieved September 15, 2023.
- ^ Cite error: The named reference
Moderna COVID-19 vaccine TGA provisional approval 2was invoked but never defined (see the help page). - ^ Cite error: The named reference
Moderna COVID-19 vaccine CDC standing orderwas invoked but never defined (see the help page). - ^ "Moderna COVID-19 Vaccine". Dosing & Administration. Infectious Diseases Society of America. January 4, 2021. Archived from the original on December 20, 2020. Retrieved January 5, 2021.
- ^ "FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals". U.S. Food and Drug Administration (FDA) (Press release). August 12, 2021. Archived from the original on December 7, 2021. Retrieved August 13, 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "COVID-19 Vaccines for Moderately to Severely Immunocompromised People". U.S. Centers for Disease Control and Prevention (CDC). August 13, 2021. Archived from the original on December 10, 2021. Retrieved August 13, 2021.
- ^ Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T (February 2021). "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine". The New England Journal of Medicine. 384 (5): 403–416. doi:10.1056/NEJMoa2035389. PMC 7787219. PMID 33378609.
- ^ a b "Moderna Announces Clinical Update on Bivalent COVID-19 Booster Platform". Moderna (Press release). April 19, 2022. Archived from the original on May 6, 2022. Retrieved May 7, 2022.
- ^ a b "First bivalent COVID-19 booster vaccine approved by UK medicines regulator" (Press release). Medicines and Healthcare products Regulatory Agency (MHRA). August 15, 2022. Archived from the original on August 16, 2022. Retrieved August 16, 2022.
- ^ a b "Regulatory approval of Spikevax bivalent Original/Omicron booster vaccine". Medicines and Healthcare products Regulatory Agency (MHRA). August 15, 2022. Archived from the original on August 16, 2022. Retrieved August 16, 2022.
- ^ "Swissmedic approves first bivalent COVID-19 booster vaccine in Switzerland". Swissmedic (Press release). August 29, 2022. Archived from the original on August 29, 2022. Retrieved September 1, 2022.
- ^ "TGA provisionally approves Moderna bivalent COVID-19 vaccine for use as a booster dose in adults" (Press release). Therapeutic Goods Administration. August 30, 2022. Archived from the original on September 1, 2022. Retrieved September 1, 2022.
- ^ "Regulatory Decision Summary - Spikevax Bivalent". Health Canada. September 1, 2022. Archived from the original on September 1, 2022. Retrieved September 1, 2022.
- ^ a b Boisvert N (September 1, 2022). "Health Canada approves updated Moderna vaccine for Omicron variant". CBC News. Archived from the original on September 1, 2022. Retrieved September 1, 2022.
- ^ a b "Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose" (Press release). U.S. Food and Drug Administration (FDA). August 31, 2022. Archived from the original on September 1, 2022. Retrieved September 1, 2022.
- ^ "CDC Recommends the First Updated COVID-19 Booster" (Press release). U.S. Centers for Disease Control and Prevention (CDC). September 1, 2022. Archived from the original on September 2, 2022. Retrieved September 1, 2022.
- ^ "COVID-19 Vaccine Tracker: Moderna: mRNA-1273". McGill University. Archived from the original on February 1, 2022.
- ^ "Moderna COVID-19 Vaccine". U.S. Food and Drug Administration (FDA). July 7, 2021. Archived from the original on January 23, 2021. Retrieved August 5, 2021.
- ^ Stevis-Gridneff M (January 6, 2021). "The E.U. drug regulator approves the Moderna vaccine". The New York Times. Archived from the original on August 6, 2021. Retrieved August 5, 2021.
- ^ "Moderna Reports Second Quarter Fiscal Year 2021 Financial Results and Provides Business Updates". Moderna, Inc. (Press release). August 5, 2021. Archived from the original on August 6, 2021. Retrieved August 5, 2021.
- ^ "Moderna Completes Application to U.S. Food and Drug Administration For Emergency Use Authorization of Omicron-targeting Bivalent COVID-19 Booster Vaccine, mRNA-1273.222". Moderna, Inc. August 23, 2022. Archived from the original on September 25, 2022. Retrieved September 25, 2022.
External links
- Official website

- Business data for Moderna, Inc.:
- Companies in the Nasdaq-100
- Companies listed on the Nasdaq
- 2010 establishments in Massachusetts
- 2018 initial public offerings
- American companies established in 2010
- Biotechnology companies of the United States
- Biotechnology companies established in 2010
- Companies based in Cambridge, Massachusetts
- COVID-19
- Health care companies based in Massachusetts
- Medical research
- Medical responses to the COVID-19 pandemic
- RNA vaccines
- Pharmaceutical companies of the United States
- Vaccine producers
