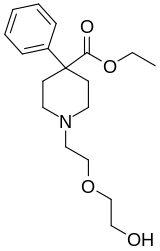
Etoxeridine
Appearance
 | |
| Clinical data | |
|---|---|
| Other names | Etoxeridine, Carbetidine, Atenos |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.750 |
| Chemical and physical data | |
| Formula | C18H27NO4 |
| Molar mass | 321.417 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Etoxeridine (Carbetidine, Atenos) is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine).
Etoxeridine was developed in the 1950s[1] and investigated for use in surgical anesthesia, however it was never commercialized and is not currently used in medicine.[2][3][4] As with other opioids which were not in clinical use during the drafting of the Controlled Substances Act, it is categorized as a Schedule I narcotic.
References
- ^ BE 558883
- ^ Merlevede E, Levis S (1958). "Pharmacological study of carbetidine, a new synthetic analgesic". Archives Internationales de Pharmacodynamie et de Thérapie (in French). 115 (1–2): 213–232.
- ^ Sironi PG (1959). "Brief note on a new synthetic analgesic: carbetidine hydrochloride". Minerva Anestesiologica (in Italian). 25 (6): 251–254.
- ^ Crawford JS, Foldes FF (August 1959). "Studies on the respiratory and circulatory effects of carbetidine HCI used for supplementation of thiopentone sodium-nitrous oxide-oxygen anaesthesia". British Journal of Anaesthesia. 31 (8): 348–51. doi:10.1093/bja/31.8.348. PMID 13812715.
