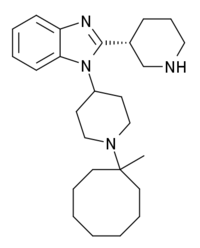
MCOPPB
Appearance
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H40N4 |
| Molar mass | 408.621 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
MCOPPB is a drug which acts as a potent and selective agonist for the nociceptin receptor, with a pKi of 10.07 and much weaker activity at other opioid receptors. It has only moderate affinity for the mu opioid receptor, weak affinity for the kappa opioid receptor and negligible binding at the delta opioid receptor. In animal studies, MCOPPB produces potent anxiolytic effects, with no inhibition of memory or motor function, and only slight sedative side effects which do not appear until much higher doses than the effective anxiolytic dose range.[1][2]
References
- ^ Hirao A, Imai A, Sugie Y, Yamada Y, Hayashi S, Toide K. Pharmacological characterization of the newly synthesized nociceptin/orphanin FQ-receptor agonist 1-[1-(1-methylcyclooctyl)-4-piperidinyl]-2-[(3R)-3-piperidinyl]-1H-benzimidazole as an anxiolytic agent. Journal of Pharmacological Sciences. 2008 Mar;106(3):361-8. PMID 18319566
- ^ Hayashi S, Hirao A, Imai A, Nakamura H, Murata Y, Ohashi K, Nakata E. Novel non-peptide nociceptin/orphanin FQ receptor agonist, 1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3R)-3-piperidinyl]-1H-benzimidazole: design, synthesis, and structure-activity relationship of oral receptor occupancy in the brain for orally potent antianxiety drug. Journal of Medicinal Chemistry. 2009 Feb 12;52(3):610-25. PMID 19125610
