Abiogenesis: Difference between revisions
Continuing comprehensive clean-up: ref.# 114–117. Questioning book edited by Ahuja: reliable source? |
Continuing comprehensive clean-up: ref.# 118–120, 248. |
||
| Line 125: | Line 125: | ||
In 1961, it was shown that the nucleic acid purine base [[adenine]] can be formed by heating aqueous [[ammonium cyanide]] solutions.<ref>{{cite journal |last=Oró |first=Joan |authorlink=Joan |date=16 September 1961 |title=Mechanism of Synthesis of Adenine from Hydrogen Cyanide under Possible Primitive Earth Conditions |journal=Nature |location=London |publisher=Nature Publishing Group |volume=191 |issue=4794 |pages=1193–1194 |bibcode=1961Natur.191.1193O |doi=10.1038/1911193a0 |issn=0028-0836 |pmid=13731264}}</ref> Other pathways for synthesizing bases from inorganic materials were also reported.<ref name="Basile1984">{{cite journal |last1=Basile |first1=Brenda |last2=Lazcano |first2=Antonio |authorlink2=Antonio Lazcano |last3=Oró |first3=Joan |year=1984 |title=Prebiotic syntheses of purines and pyrimidines |journal=[[Advances in Space Research]] |location=Amsterdam, the Netherlands |publisher=Elsevier |volume=4 |issue=12 |pages=125–131 |doi=10.1016/0273-1177(84)90554-4 |issn=0273-1177 |pmid=11537766}}</ref> [[Leslie Orgel]] and colleagues have shown that freezing temperatures are advantageous for the synthesis of purines, due to the concentrating effect for key precursors such as [[hydrogen cyanide]].<ref>{{cite journal |last=Orgel |first=Leslie E. |authorlink=Leslie Orgel |date=August 2004 |title=Prebiotic Adenine Revisited: Eutectics and Photochemistry |journal=Origins of Life and Evolution of Biospheres |publisher=Kluwer Academic Publishers |volume=34 |issue=4 |pages=361–369 |bibcode=2004OLEB...34..361O |doi=10.1023/B:ORIG.0000029882.52156.c2 |issn=0169-6149 |pmid=15279171}}</ref> Research by [[Stanley Miller]] and colleagues suggested that while adenine and guanine require freezing conditions for synthesis, cytosine and uracil may require boiling temperatures.<ref>{{cite journal |last1=Robertson |first1=Michael P. |last2=Miller |first2=Stanley L. |date=29 June 1995 |title=An efficient prebiotic synthesis of cytosine and uracil |journal=Nature |location=London |publisher=Nature Publishing Group |volume=375 |issue=6534 |pages=772–774 |bibcode=1995Natur.375..772R |doi=10.1038/375772a0 |issn=0028-0836 |pmid=7596408}}</ref> Research by the Miller group notes the formation of seven different amino acids and 11 types of nucleobases in ice when ammonia and [[cyanide]] were left in a freezer from 1972 to 1997.<ref>{{cite journal |last=Fox |first=Douglas |date=February 2008 |url=http://discovermagazine.com/2008/feb/did-life-evolve-in-ice |title=Did Life Evolve in Ice? |journal=[[Discover (magazine)|Discover]] |location=Waukesha, WI |publisher=[[Kalmbach Publishing]] |issn=0274-7529 |accessdate=2008-07-03}}</ref><ref>{{cite journal |last1=Levy |first1=Matthew |last2=Miller |first2=Stanley L. |last3=Brinton |first3=Karen |last4=Bada |first4=Jeffrey L. |authorlink4=Jeffrey L. Bada |date=June 2000 |title=Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions |journal=[[Icarus (journal)|Icarus]] ||location=Amsterdam, the Netherlands |publisher=Elsevier |volume=145 |issue=2 |pages=609–13 |bibcode=2000Icar..145..609L |doi=10.1006/icar.2000.6365 |issn=0019-1035 |pmid=11543508}}</ref> Other work demonstrated the formation of s-[[triazine]]s (alternative [[nucleobase]]s), [[pyrimidine]]s (including cytosine and uracil), and adenine from urea solutions subjected to freeze-thaw cycles under a reductive atmosphere (with spark discharges as an energy source).<ref>{{cite journal |last1=Menor-Salván |first1=César |last2=Ruiz-Bermejo |first2=Marta |last3=Guzmán |first3=Marcelo I. |last4=Osuna-Esteban |first4=Susana |last5=Veintemillas-Verdaguer |first5=Sabino |date=20 April 2009 |title=Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases |journal=[[Chemistry: A European Journal]] |location=Weinheim, Germany |publisher=[[Wiley-VCH]] on behalf of [[ChemPubSoc Europe]] |volume=15 |issue=17 |pages=4411–4418 |doi=10.1002/chem.200802656 |issn=0947-6539 |pmid=19288488}}</ref> The explanation given for the unusual speed of these reactions at such a low temperature is [[Eutectic point|eutectic freezing]]. As an ice crystal forms, it stays pure: only molecules of water join the growing crystal, while impurities like salt or cyanide are excluded. These impurities become crowded in microscopic pockets of liquid within the ice, and this crowding causes the molecules to collide more often. Mechanistic exploration using quantum chemical methods provide a more detailed understanding of some of the chemical processes involved in chemical evolution, and a partial answer to the fundamental question of molecular biogenesis.<ref>{{cite journal |last1=Roy |first1=Debjani |last2=Najafian |first2=Katayoun |last3=von Ragué Schleyer |first3=Paul |authorlink3=Paul von Ragué Schleyer |date=30 October 2007 |title=Chemical evolution: The mechanism of the formation of adenine under prebiotic conditions |journal=Proc. Natl. Acad. Sci. U.S.A. |location=Washington, D.C. |publisher=National Academy of Sciences |volume=104 |issue=44 |pages=17272–17277 |doi=10.1073/pnas.0708434104 |issn=0027-8424 |pmc=2077245 |pmid=17951429}}</ref> |
In 1961, it was shown that the nucleic acid purine base [[adenine]] can be formed by heating aqueous [[ammonium cyanide]] solutions.<ref>{{cite journal |last=Oró |first=Joan |authorlink=Joan |date=16 September 1961 |title=Mechanism of Synthesis of Adenine from Hydrogen Cyanide under Possible Primitive Earth Conditions |journal=Nature |location=London |publisher=Nature Publishing Group |volume=191 |issue=4794 |pages=1193–1194 |bibcode=1961Natur.191.1193O |doi=10.1038/1911193a0 |issn=0028-0836 |pmid=13731264}}</ref> Other pathways for synthesizing bases from inorganic materials were also reported.<ref name="Basile1984">{{cite journal |last1=Basile |first1=Brenda |last2=Lazcano |first2=Antonio |authorlink2=Antonio Lazcano |last3=Oró |first3=Joan |year=1984 |title=Prebiotic syntheses of purines and pyrimidines |journal=[[Advances in Space Research]] |location=Amsterdam, the Netherlands |publisher=Elsevier |volume=4 |issue=12 |pages=125–131 |doi=10.1016/0273-1177(84)90554-4 |issn=0273-1177 |pmid=11537766}}</ref> [[Leslie Orgel]] and colleagues have shown that freezing temperatures are advantageous for the synthesis of purines, due to the concentrating effect for key precursors such as [[hydrogen cyanide]].<ref>{{cite journal |last=Orgel |first=Leslie E. |authorlink=Leslie Orgel |date=August 2004 |title=Prebiotic Adenine Revisited: Eutectics and Photochemistry |journal=Origins of Life and Evolution of Biospheres |publisher=Kluwer Academic Publishers |volume=34 |issue=4 |pages=361–369 |bibcode=2004OLEB...34..361O |doi=10.1023/B:ORIG.0000029882.52156.c2 |issn=0169-6149 |pmid=15279171}}</ref> Research by [[Stanley Miller]] and colleagues suggested that while adenine and guanine require freezing conditions for synthesis, cytosine and uracil may require boiling temperatures.<ref>{{cite journal |last1=Robertson |first1=Michael P. |last2=Miller |first2=Stanley L. |date=29 June 1995 |title=An efficient prebiotic synthesis of cytosine and uracil |journal=Nature |location=London |publisher=Nature Publishing Group |volume=375 |issue=6534 |pages=772–774 |bibcode=1995Natur.375..772R |doi=10.1038/375772a0 |issn=0028-0836 |pmid=7596408}}</ref> Research by the Miller group notes the formation of seven different amino acids and 11 types of nucleobases in ice when ammonia and [[cyanide]] were left in a freezer from 1972 to 1997.<ref>{{cite journal |last=Fox |first=Douglas |date=February 2008 |url=http://discovermagazine.com/2008/feb/did-life-evolve-in-ice |title=Did Life Evolve in Ice? |journal=[[Discover (magazine)|Discover]] |location=Waukesha, WI |publisher=[[Kalmbach Publishing]] |issn=0274-7529 |accessdate=2008-07-03}}</ref><ref>{{cite journal |last1=Levy |first1=Matthew |last2=Miller |first2=Stanley L. |last3=Brinton |first3=Karen |last4=Bada |first4=Jeffrey L. |authorlink4=Jeffrey L. Bada |date=June 2000 |title=Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions |journal=[[Icarus (journal)|Icarus]] ||location=Amsterdam, the Netherlands |publisher=Elsevier |volume=145 |issue=2 |pages=609–13 |bibcode=2000Icar..145..609L |doi=10.1006/icar.2000.6365 |issn=0019-1035 |pmid=11543508}}</ref> Other work demonstrated the formation of s-[[triazine]]s (alternative [[nucleobase]]s), [[pyrimidine]]s (including cytosine and uracil), and adenine from urea solutions subjected to freeze-thaw cycles under a reductive atmosphere (with spark discharges as an energy source).<ref>{{cite journal |last1=Menor-Salván |first1=César |last2=Ruiz-Bermejo |first2=Marta |last3=Guzmán |first3=Marcelo I. |last4=Osuna-Esteban |first4=Susana |last5=Veintemillas-Verdaguer |first5=Sabino |date=20 April 2009 |title=Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases |journal=[[Chemistry: A European Journal]] |location=Weinheim, Germany |publisher=[[Wiley-VCH]] on behalf of [[ChemPubSoc Europe]] |volume=15 |issue=17 |pages=4411–4418 |doi=10.1002/chem.200802656 |issn=0947-6539 |pmid=19288488}}</ref> The explanation given for the unusual speed of these reactions at such a low temperature is [[Eutectic point|eutectic freezing]]. As an ice crystal forms, it stays pure: only molecules of water join the growing crystal, while impurities like salt or cyanide are excluded. These impurities become crowded in microscopic pockets of liquid within the ice, and this crowding causes the molecules to collide more often. Mechanistic exploration using quantum chemical methods provide a more detailed understanding of some of the chemical processes involved in chemical evolution, and a partial answer to the fundamental question of molecular biogenesis.<ref>{{cite journal |last1=Roy |first1=Debjani |last2=Najafian |first2=Katayoun |last3=von Ragué Schleyer |first3=Paul |authorlink3=Paul von Ragué Schleyer |date=30 October 2007 |title=Chemical evolution: The mechanism of the formation of adenine under prebiotic conditions |journal=Proc. Natl. Acad. Sci. U.S.A. |location=Washington, D.C. |publisher=National Academy of Sciences |volume=104 |issue=44 |pages=17272–17277 |doi=10.1073/pnas.0708434104 |issn=0027-8424 |pmc=2077245 |pmid=17951429}}</ref> |
||
At the time of the [[Miller–Urey experiment]], scientific consensus was that the [[early Earth]] had a [[reducing atmosphere]] with compounds relatively rich in [[hydrogen]] and poor in [[oxygen]] (e.g., {{chem|[[carbon|C]]|[[hydrogen|H]]|4}} and {{chem|[[nitrogen|N]]|[[hydrogen|H]]|3}} as opposed to {{chem|[[carbon|C]]|[[oxygen|O]]|2}} and {{chem|[[nitrogen|N]]|[[oxygen|O]]|2}}). However, current scientific consensus describes the primitive atmosphere as either weakly reducing or neutral<ref name="Cleaves 2008">{{cite journal |last1=Cleaves |first1=H. James |last2=Chalmers |first2=John H. |last3=Lazcano |first3=Antonio |last4=Miller |first4=Stanley L. |last5=Bada |first5=Jeffrey L. |display-authors=3 |date=April 2008 |title=A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres |journal=Origins of Life and Evolution of Biospheres |location=Dordrecht, the Netherlands |publisher=[[Springer Science+Business Media|Springer]] |volume=38 |issue=2 |pages=105–115 |bibcode=2008OLEB...38..105C |doi=10.1007/s11084-007-9120-3 |issn=0169-6149 |pmid=18204914}}</ref><ref name="Chyba 2005">{{cite journal |last=Chyba |first=Christopher F. |date=13 May 2005 |title=Rethinking Earth's Early Atmosphere |journal=Science |location=Washington, D.C. |publisher=American Association for the Advancement of Science |volume=308 |issue=5724 |pages=962–963 |doi=10.1126/science.1113157 |issn=0036-8075 |pmid=15890865}}</ref> (see also [[Oxygen catastrophe]]). Such an atmosphere would diminish both the amount and variety of amino acids that could be produced, although studies that include iron and carbonate minerals (thought present in early oceans) in the experimental conditions have again produced a diverse array of amino acids.<ref name="Cleaves 2008"/> Other scientific research has focused on two other potential reducing environments: outer space and deep-sea thermal vents<ref>{{harvnb|Barton|Briggs|Eisen|Goldstein|2007|pp=93–95}}</ref><ref>{{harvnb|Bada|Lazcano|2009|pp=56–57}}</ref><ref name="Bada 2003">{{cite journal |last1=Bada |first1=Jeffrey L. |last2=Lazcano |first2=Antonio |date=2 May 2003 |url=http://astrobiology.berkeley.edu/PDFs_articles/Bada_Science2003.pdf |format=PDF |title=Prebiotic Soup--Revisiting the Miller Experiment |journal=Science |location=Washington, D.C. |publisher=American Association for the Advancement of Science |volume=300 |issue=5620 |pages=745–746 |doi=10.1126/science.1085145 |issn=0036-8075 |pmid=12730584}}</ref> |
At the time of the [[Miller–Urey experiment]], scientific consensus was that the [[early Earth]] had a [[reducing atmosphere]] with compounds relatively rich in [[hydrogen]] and poor in [[oxygen]] (e.g., {{chem|[[carbon|C]]|[[hydrogen|H]]|4}} and {{chem|[[nitrogen|N]]|[[hydrogen|H]]|3}} as opposed to {{chem|[[carbon|C]]|[[oxygen|O]]|2}} and {{chem|[[nitrogen|N]]|[[oxygen|O]]|2}}). However, current scientific consensus describes the primitive atmosphere as either weakly reducing or neutral<ref name="Cleaves 2008">{{cite journal |last1=Cleaves |first1=H. James |last2=Chalmers |first2=John H. |last3=Lazcano |first3=Antonio |last4=Miller |first4=Stanley L. |last5=Bada |first5=Jeffrey L. |display-authors=3 |date=April 2008 |title=A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres |journal=Origins of Life and Evolution of Biospheres |location=Dordrecht, the Netherlands |publisher=[[Springer Science+Business Media|Springer]] |volume=38 |issue=2 |pages=105–115 |bibcode=2008OLEB...38..105C |doi=10.1007/s11084-007-9120-3 |issn=0169-6149 |pmid=18204914}}</ref><ref name="Chyba 2005">{{cite journal |last=Chyba |first=Christopher F. |date=13 May 2005 |title=Rethinking Earth's Early Atmosphere |journal=Science |location=Washington, D.C. |publisher=American Association for the Advancement of Science |volume=308 |issue=5724 |pages=962–963 |doi=10.1126/science.1113157 |issn=0036-8075 |pmid=15890865}}</ref> (see also [[Oxygen catastrophe]]). Such an atmosphere would diminish both the amount and variety of amino acids that could be produced, although studies that include iron and carbonate minerals (thought present in early oceans) in the experimental conditions have again produced a diverse array of amino acids.<ref name="Cleaves 2008"/> Other scientific research has focused on two other potential reducing environments: outer space and deep-sea thermal vents<ref>{{harvnb|Barton|Briggs|Eisen|Goldstein|2007|pp=93–95}}</ref><ref>{{harvnb|Bada|Lazcano|2009|pp=56–57}}</ref><ref name="Bada 2003">{{cite journal |last1=Bada |first1=Jeffrey L. |last2=Lazcano |first2=Antonio |date=2 May 2003 |url=http://astrobiology.berkeley.edu/PDFs_articles/Bada_Science2003.pdf |format=PDF |title=Prebiotic Soup--Revisiting the Miller Experiment |journal=Science |location=Washington, D.C. |publisher=American Association for the Advancement of Science |volume=300 |issue=5620 |pages=745–746 |doi=10.1126/science.1085145 |issn=0036-8075 |pmid=12730584 |accessdate=2015-06-13}}</ref> |
||
The spontaneous formation of complex [[polymer]]s from abiotically generated monomers under the conditions posited by the "soup" theory is not at all a straightforward process. Besides the necessary basic organic monomers, compounds that would have prohibited the formation of polymers were formed in high concentration during the Miller–Urey and Oró experiments.<ref>{{cite journal |last=Oró |first=Joan |last2=Kimball |first2=Aubrey P. |date=February 1962 |title=Synthesis of purines under possible primitive earth conditions: II. Purine intermediates from hydrogen cyanide |journal=[[Archives of Biochemistry and Biophysics]] |location=Amsterdam, the Netherlands |publisher=Elsevier |volume=96 |issue=2 |pages=293–313 |doi=10.1016/0003-9861(62)90412-5 |issn=0003-9861 |pmid=14482339}}</ref> The Miller–Urey experiment, for example, produces many substances that would react with the amino acids or terminate their coupling into [[peptide|peptide chains]].<ref>{{cite book |editor-last=Ahuja |editor-first=Mukesh |year=2006 |chapter=Origin of Life |chapterurl=https://books.google.com/books?id=VJF12TlT58kC&pg=PA11#v=onepage&q&f=false |title=Life Science |volume=1 |location=Delhi |publisher=Isha Books |page=11 |oclc=297208106 |ref=harv}}{{Unreliable source?|reason=What material is Ahuja editing? Further, see use of Ahuja material in the Iron-sulfur world section in this WP article, among others. See also: Wikipedia talk:Noticeboard for India-related topics/Archive 42#Problem with ISHA books as references|date=June 2015}}</ref> |
The spontaneous formation of complex [[polymer]]s from abiotically generated monomers under the conditions posited by the "soup" theory is not at all a straightforward process. Besides the necessary basic organic monomers, compounds that would have prohibited the formation of polymers were formed in high concentration during the Miller–Urey and Oró experiments.<ref>{{cite journal |last=Oró |first=Joan |last2=Kimball |first2=Aubrey P. |date=February 1962 |title=Synthesis of purines under possible primitive earth conditions: II. Purine intermediates from hydrogen cyanide |journal=[[Archives of Biochemistry and Biophysics]] |location=Amsterdam, the Netherlands |publisher=Elsevier |volume=96 |issue=2 |pages=293–313 |doi=10.1016/0003-9861(62)90412-5 |issn=0003-9861 |pmid=14482339}}</ref> The Miller–Urey experiment, for example, produces many substances that would react with the amino acids or terminate their coupling into [[peptide|peptide chains]].<ref>{{cite book |editor-last=Ahuja |editor-first=Mukesh |year=2006 |chapter=Origin of Life |chapterurl=https://books.google.com/books?id=VJF12TlT58kC&pg=PA11#v=onepage&q&f=false |title=Life Science |volume=1 |location=Delhi |publisher=Isha Books |page=11 |oclc=297208106 |ref=harv}}{{Unreliable source?|reason=What material is Ahuja editing? Further, see use of Ahuja material in the Iron-sulfur world section in this WP article, among others. See also: Wikipedia talk:Noticeboard for India-related topics/Archive 42#Problem with ISHA books as references|date=June 2015}}</ref> |
||
| Line 132: | Line 132: | ||
[[Autocatalysis|Autocatalysts]] are substances that catalyze the production of themselves, and therefore are simple molecular replicators. The simplest self-replicating chemical systems are autocatalytic, and typically contain three components: two precursors that join together to form a product molecule, and the product molecule itself. The product molecule catalyzes the reaction by providing a complementary template that binds to the precursors, thus bringing them together. Such systems have been demonstrated both in biological macromolecules and in small organic molecules.<ref name="Paul2004">{{cite journal |last1=Paul |first1=Natasha |last2=Joyce |first2=Gerald F. |date=December 2004 |title=Minimal self-replicating systems |journal=[[Current Opinion|Current Opinion in Chemical Biology]] |location=Amsterdam, the Netherlands |publisher=Elsevier |volume=8 |issue=6 |pages=634–639 |doi=10.1016/j.cbpa.2004.09.005 |issn=1367-5931 |pmid=15556408}}</ref><ref name="Bissette2013">{{cite journal |last1=Bissette |first1=Andrew J. |last2=Fletcher |first2=Stephen P. |date=2 December 2013 |title=Mechanisms of Autocatalysis |journal=[[Angewandte Chemie|Angewandte Chemie International Edition]] |location=Weinheim, Germany |publisher=Wiley-VCH on behalf of the [[Gesellschaft Deutscher Chemiker|German Chemical Society]] |volume=52 |issue=49 |pages=12800–12826 |doi=10.1002/anie.201303822 |issn=1433-7851 |pmid=24127341}}</ref> Systems that do not proceed by template mechanisms, such as the self-reproduction of [[micelle]]s and [[Vesicle (biology and chemistry)|vesicle]]s, have also been observed.<ref name="Bissette2013"/> |
[[Autocatalysis|Autocatalysts]] are substances that catalyze the production of themselves, and therefore are simple molecular replicators. The simplest self-replicating chemical systems are autocatalytic, and typically contain three components: two precursors that join together to form a product molecule, and the product molecule itself. The product molecule catalyzes the reaction by providing a complementary template that binds to the precursors, thus bringing them together. Such systems have been demonstrated both in biological macromolecules and in small organic molecules.<ref name="Paul2004">{{cite journal |last1=Paul |first1=Natasha |last2=Joyce |first2=Gerald F. |date=December 2004 |title=Minimal self-replicating systems |journal=[[Current Opinion|Current Opinion in Chemical Biology]] |location=Amsterdam, the Netherlands |publisher=Elsevier |volume=8 |issue=6 |pages=634–639 |doi=10.1016/j.cbpa.2004.09.005 |issn=1367-5931 |pmid=15556408}}</ref><ref name="Bissette2013">{{cite journal |last1=Bissette |first1=Andrew J. |last2=Fletcher |first2=Stephen P. |date=2 December 2013 |title=Mechanisms of Autocatalysis |journal=[[Angewandte Chemie|Angewandte Chemie International Edition]] |location=Weinheim, Germany |publisher=Wiley-VCH on behalf of the [[Gesellschaft Deutscher Chemiker|German Chemical Society]] |volume=52 |issue=49 |pages=12800–12826 |doi=10.1002/anie.201303822 |issn=1433-7851 |pmid=24127341}}</ref> Systems that do not proceed by template mechanisms, such as the self-reproduction of [[micelle]]s and [[Vesicle (biology and chemistry)|vesicle]]s, have also been observed.<ref name="Bissette2013"/> |
||
In 1993, [[Stuart Kauffman]] proposed that life initially arose as autocatalytic chemical networks.<ref>{{ |
In 1993, [[Stuart Kauffman]] proposed that life initially arose as autocatalytic chemical networks.<ref>{{harvnb|Kauffman|1993|loc=chpt. 7}}</ref> [[United Kingdom|British]] [[ethologist]] [[Richard Dawkins]] wrote about [[autocatalysis]] as a potential explanation for the origin of life in his 2004 book ''[[The Ancestor's Tale]]''.<ref>{{harvnb|Dawkins|2004}}</ref> In his book, Dawkins cites experiments performed by [[Julius Rebek]] and his colleagues at the [[Scripps Research Institute]] in [[California]] in which they combined [[amino adenosine]] and [[pentafluorophenyl esters]] with the autocatalyst amino adenosine triacid ester (AATE). One system from the experiment contained variants of AATE, which catalysed the synthesis of themselves. This experiment demonstrated the possibility that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of natural selection.{{Citation needed|date=January 2012}} |
||
In the early 1970s, Manfred Eigen and [[Peter Schuster]] examined the transient stages between the molecular chaos and a self-replicating [[Hypercycle (chemistry)|hypercycle]] in a prebiotic soup.<ref>{{ |
In the early 1970s, Manfred Eigen and [[Peter Schuster]] examined the transient stages between the molecular chaos and a self-replicating [[Hypercycle (chemistry)|hypercycle]] in a prebiotic soup.<ref>{{harvnb|Eigen|Schuster|1979}}</ref> In a hypercycle, the [[information|information storing system]] (possibly RNA) produces an [[enzyme]], which catalyzes the formation of another information system, in sequence until the product of the last aids in the formation of the first information system. Mathematically treated, hypercycles could create [[Quasispecies model|quasispecies]], which through natural selection entered into a form of Darwinian evolution. A boost to hypercycle theory was the discovery of [[ribozyme]]s capable of catalyzing their own chemical reactions. The hypercycle theory requires the existence of complex biochemicals, such as nucleotides, which do not form under the conditions proposed by the Miller–Urey experiment. |
||
[[Geoffrey W. Hoffmann]], a student of Eigen, contributed to the concept of life involving both replication and metabolism emerging from catalytic noise. His contributions included showing that an early sloppy translation machinery can be stable against an error catastrophe of the type that had been envisaged as problematical by Leslie Orgel ("Orgel's paradox")<ref>{{cite journal |last=Hoffmann |first=G. W. |title=On the Origin of the Genetic Code and the Stability of the Translation Apparatus |journal=J. Mol. Biol. |volume=86 |pages=349–362 |year=1974 | doi=10.1016/0022-2836(74)90024-2 |pmid=4414916 |issue=2 }}</ref><ref>{{cite journal | last=Orgel | first=L. | title=The Maintenance of the Accuracy of Protein Synthesis and its Relevance to Ageing | journal=Proceedings of the National Academy of Sciences of the United States of America | volume=49 | pages=517–521 | year=1963 | doi=10.1073/pnas.49.4.517 | pmid=13940312 | pmc=299893 | issue=4 | bibcode=1963PNAS...49..517O }}</ref> and calculations regarding the occurrence of a set of required catalytic activities together with the exclusion of catalytic activities that would be disruptive.<ref>{{cite journal | last=Hoffmann | first=G. W. | title=The Stochastic Theory of the Genetic Code | year=1975 | journal=Annual Review of Physical Chemistry | volume=26 | pages=123–144 | editor-first=H. | editor-last=Eyring | doi=10.1146/annurev.pc.26.100175.001011 | bibcode=1975ARPC...26..123H }}</ref> |
[[Geoffrey W. Hoffmann]], a student of Eigen, contributed to the concept of life involving both replication and metabolism emerging from catalytic noise. His contributions included showing that an early sloppy translation machinery can be stable against an error catastrophe of the type that had been envisaged as problematical by Leslie Orgel ("Orgel's paradox")<ref>{{cite journal |last=Hoffmann |first=G. W. |title=On the Origin of the Genetic Code and the Stability of the Translation Apparatus |journal=J. Mol. Biol. |volume=86 |pages=349–362 |year=1974 | doi=10.1016/0022-2836(74)90024-2 |pmid=4414916 |issue=2 }}</ref><ref>{{cite journal | last=Orgel | first=L. | title=The Maintenance of the Accuracy of Protein Synthesis and its Relevance to Ageing | journal=Proceedings of the National Academy of Sciences of the United States of America | volume=49 | pages=517–521 | year=1963 | doi=10.1073/pnas.49.4.517 | pmid=13940312 | pmc=299893 | issue=4 | bibcode=1963PNAS...49..517O }}</ref> and calculations regarding the occurrence of a set of required catalytic activities together with the exclusion of catalytic activities that would be disruptive.<ref>{{cite journal | last=Hoffmann | first=G. W. | title=The Stochastic Theory of the Genetic Code | year=1975 | journal=Annual Review of Physical Chemistry | volume=26 | pages=123–144 | editor-first=H. | editor-last=Eyring | doi=10.1146/annurev.pc.26.100175.001011 | bibcode=1975ARPC...26..123H }}</ref> |
||
| Line 288: | Line 288: | ||
{{main|Gard model}} |
{{main|Gard model}} |
||
The [[Gard model|lipid world]] theory postulates that the first self-replicating object was lipid-like.<ref>{{cite web | url=http://ool.weizmann.ac.il/ | title=Origin of Life at the Weizmann Institute | publisher=Ool.weizmann.ac.il |date=6 January 2008 }}</ref><ref>{{cite journal | last=Segré | first=D. | last2=Ben-Eli | first2=D. | last3=Deamer | first3=D | last4=Lancet | first4=D. | title=The Lipid World | date=February–April 2001 | journal=Origins of Life and Evolution of Biospheres | volume=31 | issue=1–2 | pages=119–45 | pmid=11296516 | url=http://ool.weizmann.ac.il/Segre_Lipid_World.pdf | format=PDF | doi=10.1023/A:1006746807104 | accessdate=2008-09-11 }}</ref> It is known that [[phospholipids]] form [[lipid bilayers]] in water while under agitation – the same structure as in cell membranes. These molecules were not present on early Earth, but other [[amphiphilic]] long chain molecules also form membranes. Furthermore, these bodies may expand (by insertion of additional lipids), and under excessive expansion may undergo spontaneous splitting which preserves the same size and composition of lipids in the two [[Offspring|progenies]]. The main idea in this theory is that the molecular composition of the lipid bodies is the preliminary way for information storage, and evolution led to the appearance of polymer entities such as RNA or DNA that may store information favorably. Studies on vesicles from potentially [[Prebiotic (chemistry)|prebiotic]] amphiphiles have so far been limited to systems containing one or two types of amphiphiles. This in contrast to the output of simulated prebiotic chemical reactions, which typically produce very heterogeneous mixtures of compounds.<ref>{{cite journal | last=Chen | first=I. A. | last2=Walde | first2=P | title=From Self-Assembled Vesicles to Protocells | journal=Cold Spring Harbor Perspectives in Biology | date=2 June 2010 | volume=2 | issue=7 | pages=a002170–a002170 | doi=10.1101/cshperspect.a002170 | url=http://cshperspectives.cshlp.org | pmid=20519344 | pmc=2890201}}</ref> |
The [[Gard model|lipid world]] theory postulates that the first self-replicating object was lipid-like.<ref>{{cite web | url=http://ool.weizmann.ac.il/ | title=Origin of Life at the Weizmann Institute | publisher=Ool.weizmann.ac.il |date=6 January 2008 }}</ref><ref>{{cite journal | last=Segré | first=D. | last2=Ben-Eli | first2=D. | last3=Deamer | first3=D | last4=Lancet | first4=D. | title=The Lipid World | date=February–April 2001 | journal=Origins of Life and Evolution of Biospheres | volume=31 | issue=1–2 | pages=119–45 | pmid=11296516 | url=http://ool.weizmann.ac.il/Segre_Lipid_World.pdf | format=PDF | doi=10.1023/A:1006746807104 | accessdate=2008-09-11 }}</ref> It is known that [[phospholipids]] form [[lipid bilayers]] in water while under agitation – the same structure as in cell membranes. These molecules were not present on early Earth, but other [[amphiphilic]] long chain molecules also form membranes. Furthermore, these bodies may expand (by insertion of additional lipids), and under excessive expansion may undergo spontaneous splitting which preserves the same size and composition of lipids in the two [[Offspring|progenies]]. The main idea in this theory is that the molecular composition of the lipid bodies is the preliminary way for information storage, and evolution led to the appearance of polymer entities such as RNA or DNA that may store information favorably. Studies on vesicles from potentially [[Prebiotic (chemistry)|prebiotic]] amphiphiles have so far been limited to systems containing one or two types of amphiphiles. This in contrast to the output of simulated prebiotic chemical reactions, which typically produce very heterogeneous mixtures of compounds.<ref>{{cite journal | last=Chen | first=I. A. | last2=Walde | first2=P | title=From Self-Assembled Vesicles to Protocells | journal=Cold Spring Harbor Perspectives in Biology | date=2 June 2010 | volume=2 | issue=7 | pages=a002170–a002170 | doi=10.1101/cshperspect.a002170 | url=http://cshperspectives.cshlp.org | pmid=20519344 | pmc=2890201}}</ref> |
||
Within the hypothesis of a lipid bilayer membrane composed of a mixture of various distinct amphiphilic compounds there is the opportunity of a huge number of theoretically possible combinations in the arrangements of these amphiphiles in the membrane. Among all these potential combinations, a specific local arrangement of the membrane would have favored the constitution of an [[hypercycle (chemistry)|hypercycle]],<ref>{{cite journal | first1=Manfred | |
Within the hypothesis of a lipid bilayer membrane composed of a mixture of various distinct amphiphilic compounds there is the opportunity of a huge number of theoretically possible combinations in the arrangements of these amphiphiles in the membrane. Among all these potential combinations, a specific local arrangement of the membrane would have favored the constitution of an [[hypercycle (chemistry)|hypercycle]],<ref>{{cite journal |last1=Eigen |first1=Manfred |authorlink1=Manfred Eigen |last2=Schuster |first2=Peter |authorlink2=Peter Schuster |date=November 1977 |title=The Hypercycle. A Principle of Natural Self-Organization. Part A: Emergence of the Hypercycle |url=http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1977Naturwissenschaften64.pdf |format=PDF |journal=Naturwissenschaften |location=Berlin |publisher=Springer-Verlag |volume=64 |issue=11 |pp=541–565 |issn=0028-1042 |pmid=593400 |accessdate=2015-06-13}} |
||
*{{cite journal |last1=Eigen |first1=Manfred |last2=Schuster |first2=Peter |year=1978 |title=The Hypercycle. A Principle of Natural Self-Organization. Part B: The Abstract Hypercycle |url=http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1978Naturwissenschaften65a.pdf |format=PDF |journal=Naturwissenschaften |location=Berlin |publisher=Springer-Verlag |volume=65 |pages=7–41 |issn=0028-1042 |accessdate=2015-06-13}} |
|||
*{{cite journal |last1=Eigen |first1=Manfred |last2=Schuster |first2=Peter |date=July 1978 |title=The Hypercycle. A Principle of Natural Self-Organization. Part C: The Realistic Hypercycle |url=http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1978Naturwissenschaften65b.pdf |format=PDF |journal=Naturwissenschaften |location=Berlin |publisher=Springer-Verlag |volume=65 |issue=7 |pages=341–369 |issn=0028-1042 |accessdate=2015-06-13}}</ref><ref>{{cite journal | last1=Markovitch | first1=O. | last2=Lancet | first2=D. | year=2012 | title=Excess Mutual Catalysis Is Required for Effective Evolvability | journal=Artificial Life | volume=18 | issue=3 | pages=243–266 | doi=10.1162/artl_a_00064 | url=http://www.mitpressjournals.org/doi/abs/10.1162/artl_a_00064 | pmid=22662913 }}</ref> actually a positive [[feedback]] composed of two mutual [[catalysts]] represented by a membrane site and a specific compound trapped in the vesicle. Such site/compound pairs are transmissible to the daughter vesicles leading to the emergence of distinct [[Lineage (evolution)|lineages]] of vesicles which would have allowed Darwinian [[natural selection]].<ref>{{cite journal | last=Tessera | first=Marc | title=Origin of Evolution versus Origin of Life: A Shift of Paradigm | journal=International Journal of Molecular Sciences | date=1 June 2011 | volume=12 | issue=6 | pages=3445–3458 | doi=10.3390/ijms12063445 | pmid=21747687 | pmc=3131571 }}</ref> |
|||
===Polyphosphates=== |
===Polyphosphates=== |
||
| Line 362: | Line 364: | ||
* {{cite book |last=Davies |first=Geoffrey F. |year=2007 |chapter=Chapter 2.3 Dynamics of the Hadean and Archaean Mantle |editor1-last=van Kranendonk |editor1-first=Martin J. |editor2-last=Smithies |editor2-first=R. Hugh |editor3-last=Bennett |editor3-first=Vickie C. |title=Earth's Oldest Rocks |series=Developments in Precambrian Geology |volume=15 |location=Amsterdam, the Netherlands; Boston |publisher=[[Elsevier]] |doi=10.1016/S0166-2635(07)15023-4 |isbn=978-0-444-52810-0 |lccn=2009525003 |ref=harv}} |
* {{cite book |last=Davies |first=Geoffrey F. |year=2007 |chapter=Chapter 2.3 Dynamics of the Hadean and Archaean Mantle |editor1-last=van Kranendonk |editor1-first=Martin J. |editor2-last=Smithies |editor2-first=R. Hugh |editor3-last=Bennett |editor3-first=Vickie C. |title=Earth's Oldest Rocks |series=Developments in Precambrian Geology |volume=15 |location=Amsterdam, the Netherlands; Boston |publisher=[[Elsevier]] |doi=10.1016/S0166-2635(07)15023-4 |isbn=978-0-444-52810-0 |lccn=2009525003 |ref=harv}} |
||
* {{cite book |last=Davies |first=Paul |authorlink=Paul Davies |year=1999 |title=The Fifth Miracle: The Search for the Origin of Life |location=London |publisher=[[Penguin Books]] |isbn=0-14-028226-2 |ref=harv}} |
* {{cite book |last=Davies |first=Paul |authorlink=Paul Davies |year=1999 |title=The Fifth Miracle: The Search for the Origin of Life |location=London |publisher=[[Penguin Books]] |isbn=0-14-028226-2 |ref=harv}} |
||
* {{cite book |last=Dawkins |first=Richard |authorlink=Richard Dawkins |year=2004 |title=[[The Ancestor's Tale|The Ancestor's Tale: A Pilgrimage to the Dawn of Evolution]] |location=Boston, MA |publisher=[[Houghton Mifflin Harcourt|Houghton Mifflin]] |isbn=0-618-00583-8 |lccn=2004059864 |oclc=56617123 |ref=harv}} |
|||
* {{cite book |last=Dobell |first=Clifford |authorlink=Clifford Dobell |title=Antony van Leeuwenhoek and His 'Little Animals' |year=1960 |origyear=Originally published 1932; New York: [[Harcourt (publisher)|Harcourt, Brace & Company]] |location=New York |publisher=[[Dover Publications]] |lccn=60002548 |ref=harv}} |
* {{cite book |last=Dobell |first=Clifford |authorlink=Clifford Dobell |title=Antony van Leeuwenhoek and His 'Little Animals' |year=1960 |origyear=Originally published 1932; New York: [[Harcourt (publisher)|Harcourt, Brace & Company]] |location=New York |publisher=[[Dover Publications]] |lccn=60002548 |ref=harv}} |
||
* {{cite book |last=Dyson |first=Freeman |authorlink=Freeman Dyson |year=1999 |title=Origins of Life |edition=Revised |location=Cambridge, UK; New York |publisher=[[Cambridge University Press]] |isbn=0-521-62668-4 |lccn=99021079 |ref=harv}} |
* {{cite book |last=Dyson |first=Freeman |authorlink=Freeman Dyson |year=1999 |title=Origins of Life |edition=Revised |location=Cambridge, UK; New York |publisher=[[Cambridge University Press]] |isbn=0-521-62668-4 |lccn=99021079 |ref=harv}} |
||
* {{cite book |last1=Eigen |first1=M. |authorlink1=Manfred Eigen |last2=Schuster |first2=P. |authorlink2=Peter Schuster |year=1979 |title=The Hypercycle: A Principle of Natural Self-Organization |location=Berlin; New York |publisher=[[Springer Science+Business Media|Springer-Verlag]] |isbn=0-387-09293-5 |lccn=79001315 |oclc=4665354 |ref=harv}} |
|||
* {{cite book |last=Fesenkov |first=V. G. |authorlink=Vasily Fesenkov |year=1959 |chapter=Some Considerations about the Primaeval State of the Earth |editor1-last=Oparin |editor1-first=A. I. |editor1-link=Alexander Oparin |editor2-last=Braunshtein |editor2-first=A. E. |editor3-last=Pasynskii |editor3-first=A. G. |editor4-last=Pavlovskaya |editor4-first=T. E. |display-editors=1 |title=The Origin of Life on the Earth |url=https://archive.org/details/proceedings00inte |series=I.U.B. Symposium Series |volume=1 |others=Edited for the [[International Union of Biochemistry and Molecular Biology|International Union of Biochemistry]] by Frank Clark and [[Richard Laurence Millington Synge|R. L. M. Synge]] |edition=English-French-German |location=London; New York |publisher=[[Pergamon Press]] |isbn=978-1-4832-2240-0 |lccn=59012060 |accessdate=2015-06-03 |ref=harv}} International Symposium on the Origin of Life on the Earth (held at Moscow, 19–24 August 1957) |
* {{cite book |last=Fesenkov |first=V. G. |authorlink=Vasily Fesenkov |year=1959 |chapter=Some Considerations about the Primaeval State of the Earth |editor1-last=Oparin |editor1-first=A. I. |editor1-link=Alexander Oparin |editor2-last=Braunshtein |editor2-first=A. E. |editor3-last=Pasynskii |editor3-first=A. G. |editor4-last=Pavlovskaya |editor4-first=T. E. |display-editors=1 |title=The Origin of Life on the Earth |url=https://archive.org/details/proceedings00inte |series=I.U.B. Symposium Series |volume=1 |others=Edited for the [[International Union of Biochemistry and Molecular Biology|International Union of Biochemistry]] by Frank Clark and [[Richard Laurence Millington Synge|R. L. M. Synge]] |edition=English-French-German |location=London; New York |publisher=[[Pergamon Press]] |isbn=978-1-4832-2240-0 |lccn=59012060 |accessdate=2015-06-03 |ref=harv}} International Symposium on the Origin of Life on the Earth (held at Moscow, 19–24 August 1957) |
||
* {{cite book |last=Huxley| first=Thomas Henry |authorlink=Thomas Henry Huxley |year=1968 |origyear=Originally published 1897 |chapter=VIII Biogenesis and Abiogenesis [1870] |chapterurl=http://aleph0.clarku.edu/huxley/CE8/B-Ab.html |title=Discourses, Biological and Geological |series=Collected Essays |volume=VIII |edition=Reprint |location=New York |publisher=[[Greenwood Publishing Group|Greenwood Press]] |lccn=70029958 |accessdate=2014-05-19 |ref=harv}} |
* {{cite book |last=Huxley| first=Thomas Henry |authorlink=Thomas Henry Huxley |year=1968 |origyear=Originally published 1897 |chapter=VIII Biogenesis and Abiogenesis [1870] |chapterurl=http://aleph0.clarku.edu/huxley/CE8/B-Ab.html |title=Discourses, Biological and Geological |series=Collected Essays |volume=VIII |edition=Reprint |location=New York |publisher=[[Greenwood Publishing Group|Greenwood Press]] |lccn=70029958 |accessdate=2014-05-19 |ref=harv}} |
||
* {{cite book |last=Kauffman |first=Stuart |authorlink=Stuart Kauffman |year= |
* {{cite book |last=Kauffman |first=Stuart |authorlink=Stuart Kauffman |year=1993 |title=The Origins of Order: Self-Organization and Selection in Evolution |location=New York |publisher=[[Oxford University Press]] |isbn=978-0-19-507951-7 |lccn=91011148 |oclc=23253930 |ref=harv}} |
||
* {{cite book |last=Kauffman |first=Stuart |year=1995 |title=At Home in the Universe: The Search for Laws of Self-Organization and Complexity |location=New York |publisher=Oxford University Press |isbn=0-19-509599-5 |lccn=94025268 |ref=harv}} |
|||
* {{cite conference |url=http://www.panspermia.org/oseti.htm |title=Panspermia Asks New Questions |first=Brig |last=Klyce |date=22 January 2001 |conference=The Search for Extraterrestrial Intelligence (SETI) in the Optical Spectrum III |conference-url=http://www.coseti.org/4273-sch.htm |editor1-last=Kingsley |editor1-first=Stuart A. |editor1-link=Stuart Kingsley |editor2-last=Bhathal |editor2-first=Ragbir |editor2-link=Ragbir Bhathal |volume=4273 |publisher=[[SPIE]] |location=Bellingham, WA |isbn=0-8194-3951-7 |lccn=2001279159 |doi=10.1117/12.435366 |accessdate=2015-06-09 |ref=harv}} Proceedings of the SPIE held at San Jose, CA, 22–24 January 2001 |
* {{cite conference |url=http://www.panspermia.org/oseti.htm |title=Panspermia Asks New Questions |first=Brig |last=Klyce |date=22 January 2001 |conference=The Search for Extraterrestrial Intelligence (SETI) in the Optical Spectrum III |conference-url=http://www.coseti.org/4273-sch.htm |editor1-last=Kingsley |editor1-first=Stuart A. |editor1-link=Stuart Kingsley |editor2-last=Bhathal |editor2-first=Ragbir |editor2-link=Ragbir Bhathal |volume=4273 |publisher=[[SPIE]] |location=Bellingham, WA |isbn=0-8194-3951-7 |lccn=2001279159 |doi=10.1117/12.435366 |accessdate=2015-06-09 |ref=harv}} Proceedings of the SPIE held at San Jose, CA, 22–24 January 2001 |
||
* {{cite book |last=Lennox |first=James G. |authorlink=James G. Lennox |year=2001 |title=Aristotle's Philosophy of Biology: Studies in the Origins of Life Science |series=Cambridge Studies in Philosophy and Biology |location=Cambridge, UK; New York |publisher=Cambridge University Press |isbn=0-521-65976-0 |lccn=00026070 |ref=harv}} |
* {{cite book |last=Lennox |first=James G. |authorlink=James G. Lennox |year=2001 |title=Aristotle's Philosophy of Biology: Studies in the Origins of Life Science |series=Cambridge Studies in Philosophy and Biology |location=Cambridge, UK; New York |publisher=Cambridge University Press |isbn=0-521-65976-0 |lccn=00026070 |ref=harv}} |
||
Revision as of 09:05, 13 June 2015

Abiogenesis (/ˌeɪbaɪ.[invalid input: 'ɵ']ˈdʒɛn[invalid input: 'ɨ']sɪs/ AY-by-oh-JEN-ə-siss[1]), or biopoiesis,[2] is the natural process of life arising from non-living matter, such as simple organic compounds.[3][4][5][6] It is thought to have occurred between 3.8 and 4 billion years ago, and is studied through a combination of laboratory experiments and extrapolation from the genetic information of modern organisms in order to make reasonable conjectures about what pre-life chemical reactions may have given rise to a living system.[7]
The study of abiogenesis involves three main types of considerations: the geophysical, the chemical, and the biological,[8] with more recent approaches attempting a synthesis of all three. Many approaches investigate how self-replicating molecules, or their components, came into existence. It is generally accepted that current life on Earth descended from an RNA world,[9] although RNA-based life may not have been the first life to have existed.[10][11] The Miller–Urey experiment and similar experiments demonstrated that most amino acids, basic chemicals of life, can be synthesized from inorganic compounds in conditions intended to be similar to early Earth. Several mechanisms have been investigated, including lightning and radiation. Other approaches ("metabolism first" hypotheses) focus on understanding how catalysis in chemical systems in the early Earth might have provided the precursor molecules necessary for self-replication.[12] Complex organic molecules have been found in the solar system and in interstellar space, and these molecules may have provided starting material for the development of life on Earth.[13][14][15][16]
According to the panspermia hypothesis, microscopic life—distributed by meteoroids, asteroids and other small Solar System bodies—may exist throughout the universe.[17] It is speculated that the biochemistry of life may have begun shortly after the Big Bang, 13.8 billion years ago, during a habitable epoch when the age of the Universe was only 10–17 million years.[18][19]
Nonetheless, Earth is the only place in the universe known to harbor life.[20][21] The age of the Earth is about 4.54 billion years.[22][23][24] The earliest undisputed evidence of life on Earth dates at least from 3.5 billion years ago,[25][26][27] during the Eoarchean Era after a geological crust started to solidify following the earlier molten Hadean Eon. There are microbial mat fossils found in 3.48 billion-year-old sandstone discovered in Western Australia.[28][29][30] Other early physical evidence of a biogenic substance is graphite in 3.7 billion-year-old metasedimentary rocks discovered in Western Greenland.[31] More than 99 percent of all species, amounting to over five billion species,[32] that ever lived on Earth are estimated to be extinct.[33][34] Estimates on the number of Earth's current species range from 10 million to 14 million,[35] of which about 1.2 million have been documented and over 86 percent have not yet been described.[36]
Early geophysical conditions
Based on recent computer model studies, the complex organic molecules necessary for life may have formed in the protoplanetary disk of dust grains surrounding the Sun before the formation of the Earth.[37] According to the computer studies, this same process may also occur around other stars that acquire planets.[37] (Also see Extraterrestrial organic molecules).
The Hadean Earth is thought to have had a secondary atmosphere, formed through degassing of the rocks that accumulated from planetesimal impactors. At first, it was thought that the Earth's atmosphere consisted of hydrides—methane, ammonia and water vapour—and that life began under such reducing conditions, which are conducive to the formation of organic molecules. During its formation, the Earth lost a significant part of its initial mass, with a nucleus of the heavier rocky elements of the protoplanetary disk remaining.[38] However, based on today's volcanic evidence, it is now thought that the early atmosphere would have probably contained 60% hydrogen, 20% oxygen (mostly in the form of water vapour), 10% carbon dioxide, 5 to 7% hydrogen sulfide, and smaller amounts of nitrogen, carbon monoxide, free hydrogen, methane and inert gases. As Earth lacked the gravity to hold any molecular hydrogen, this component of the atmosphere would have been rapidly lost during the Hadean period, along with the bulk of the original inert gases. Solution of carbon dioxide in water is thought to have made the seas slightly acidic, with a pH of about 5.5.[39] The atmosphere at the time has been characterized as a "gigantic, productive outdoor chemical laboratory."[40] It is similar to the mixture of gases released by volcanoes, which still support some abiotic chemistry today.[40]
Oceans may have appeared first in the Hadean eon, as soon as two hundred million years (200 Ma) after the Earth was formed, in a hot 100 °C (212 °F) reducing environment, and the pH of about 5.8 rose rapidly towards neutral.[41] This has been supported by the dating of 4.404 Ga-old zircon crystals from metamorphosed quartzite of Mount Narryer in Western Australia, which are evidence that oceans and continental crust existed within 150 Ma of Earth's formation.[42] Despite the likely increased vulcanism and existence of many smaller tectonic "platelets", it has been suggested that between 4.4 and 4.3 Ga, the Earth was a water world, with little if any continental crust, an extremely turbulent atmosphere and a hydrosphere subject to high UV, from a T Tauri sun, cosmic radiation and continued bolide impact.[43]
The Hadean environment would have been highly hazardous to modern life. Frequent collisions with large objects, up to 500 kilometres (310 mi) in diameter, would have been sufficient to sterilise the planet and vaporise the ocean within a few months of impact, with hot steam mixed with rock vapour becoming high altitude clouds that would completely cover the planet. After a few months, the height of these clouds would have begun to decrease but the cloud base would still have been elevated for about the next thousand years. After that, it would have begun to rain at low altitude. For another two thousand years, rains would slowly have drawn down the height of the clouds, returning the oceans to their original depth only 3,000 years after the impact event.[44]
The earliest biological evidence for life on Earth
The earliest life on Earth existed before 3.5 billion years ago,[25][26][27] during the Eoarchean Era when sufficient crust had solidified following the molten Hadean Eon. Physical evidence has been found in biogenic graphite in 3.7 billion-year-old metasedimentary rocks from Western Greenland[31] and microbial mat fossils found in 3.48 billion-year-old sandstone from Western Australia.[28][30] Gustaf Arrhenius of the Scripps Institution of Oceanography has found evidence of early life in rocks from Akilia Island, near Isua, Greenland, dating to 3.7 billion years ago: using a mass spectrometer, he has identified biogenic carbon isotopes.[45] At Strelley Pool, in the Pilbarra Region of Western Australia, compelling evidence of early life has been found in pyrite-bearing sandstone in a fossilized beach, that showed rounded tubular cells that oxidised sulfur by photosynthesis in the absence of oxygen.[46]
In the earlier period between 3.8 and 4.1 Ga, changes in the orbits of the gas giant planets may have caused a late heavy bombardment by asteroids[47] that pockmarked the Moon and the other inner planets (Mercury, Mars, and presumably Earth and Venus). This would likely have repeatedly sterilized the planet, had life appeared before that time.[40] Geologically, the Hadean Earth would have been far more active than at any other time in its history. Studies of meteorites suggests that radioactive isotopes such as aluminium-26 with a half-life of 7.17×105 years, and potassium-40 with a half-life of 1.250×109 years, isotopes mainly produced in supernovae, were much more common.[48] Coupled with internal heating as a result of gravitational sorting between the core and the mantle, there would have been a great deal of mantle convection, with the probable result of many more smaller and much more active tectonic plates than in modern times.
The time periods between such devastating environmental events give time windows for the possible origin of life in various early environments. A study by Maher and Stevenson shows that if the deep marine hydrothermal setting provides a suitable site for the origin of life, abiogenesis could have happened as early as 4.0 to 4.2 Ga, whereas if it occurred at the surface of the Earth, abiogenesis could only have occurred between 3.7 and 4.0 Ga.[49]
Further evidence of the early appearance of life comes from the Isua supercrustal belt in Western Greenland and from similar formations in the nearby Akilia Island. Isotopic fingerprints typical of life, preserved in the sediments, have been used to suggest that life existed on the planet already by 3.85 billion years ago.[50]
Conceptual history
Spontaneous generation
Belief in the present ongoing spontaneous generation of certain forms of life from non-living matter goes back to Aristotle and ancient Greek philosophy and continued to have support in Western scholarship until the 19th century.[51] This belief was paired with a belief in heterogenesis, i.e., that one form of life derived from a different form (e.g. bees from flowers).[52] Classical notions of spontaneous generation, which can be considered under the modern term abiogenesis, held that certain complex, living organisms are generated by decaying organic substances. According to Aristotle, it was a readily observable truth that aphids arise from the dew that falls on plants, flies from putrid matter, mice from dirty hay, crocodiles from rotting logs at the bottom of bodies of water, and so on.[53] In the 17th century, people began to question such assumptions. In 1646, Sir Thomas Browne published his Pseudodoxia Epidemica (subtitled Enquiries into Very many Received Tenets, and Commonly Presumed Truths), which was an attack on false beliefs and "vulgar errors." His contemporary, Alexander Ross erroneously refuted him, stating: "To question this (i.e., spontaneous generation) is to question reason, sense and experience. If he doubts of this let him go to Egypt, and there he will find the fields swarming with mice, begot of the mud of Nylus, to the great calamity of the inhabitants."[54]
In 1665, Robert Hooke published the first drawings of a microorganism. Hooke was followed in 1676 by Anton van Leeuwenhoek, who drew and described microorganisms that are now thought to have been protozoa and bacteria.[55] Many felt the existence of microorganisms was evidence in support of spontaneous generation, since microorganisms seemed too simplistic for sexual reproduction, and asexual reproduction through cell division had not yet been observed. Van Leeuwenhoek took issue with the ideas common at the time that fleas and lice could spontaneously result from putrefaction, and that frogs could likewise arise from slime. Using a broad range of experiments ranging from sealed and open meat incubation and the close study of insect reproduction, by the 1680s he became convinced that spontaneous generation was incorrect.[56]
The first experimental evidence against spontaneous generation came in 1668 when Francesco Redi showed that no maggots appeared in meat when flies were prevented from laying eggs. It was gradually shown that, at least in the case of all the higher and readily visible organisms, the previous sentiment regarding spontaneous generation was false. The alternative seemed to be biogenesis: that every living thing came from a pre-existing living thing (omne vivum ex ovo, Latin for "every living thing from an egg").
In 1768, Lazzaro Spallanzani demonstrated that microbes were present in the air, and could be killed by boiling. In 1861, Louis Pasteur performed a series of experiments that demonstrated that organisms such as bacteria and fungi do not spontaneously appear in sterile, nutrient-rich media, but only invade them from outside.
The belief that spontaneous self-ordering of spontaneous generation is impossible led to an alternative. By the middle of the 19th century, the theory of biogenesis had accumulated so much evidential support, due to the work of Louis Pasteur and others, that the alternative theory of spontaneous generation had been effectively disproven. John Desmond Bernal suggests that earlier theories such as spontaneous generation were based upon an explanation that life was continuously created as a result of chance events.[57]
The origin of the terms biogenesis and abiogenesis
The term biogenesis is usually credited to either Henry Bastian or to Thomas Henry Huxley.[58] Bastian used the term (around 1869) in an unpublished exchange with John Tyndall to mean life-origination or commencement. In 1870, Huxley, as new president of the British Association for the Advancement of Science, delivered an address entitled Biogenesis and Abiogenesis.[59] In it he introduced the term biogenesis (with an opposite meaning to Bastian) and also introduced the term abiogenesis:
- And thus the hypothesis that living matter always arises by the agency of pre-existing living matter, took definite shape; and had, henceforward, a right to be considered and a claim to be refuted, in each particular case, before the production of living matter in any other way could be admitted by careful reasoners. It will be necessary for me to refer to this hypothesis so frequently, that, to save circumlocution, I shall call it the hypothesis of Biogenesis; and I shall term the contrary doctrine–that living matter may be produced by not living matter–the hypothesis of Abiogenesis.[60]
Subsequently, in the preface to Bastian's 1871 book, The Modes of Origin of Lowest Organisms,[61] the author refers to the possible confusion with Huxley's usage and he explicitly renounced his own meaning:
- A word of explanation seems necessary with regard to the introduction of the new term archebiosis. I had originally, in unpublished writings, adopted the word biogenesis to express the same meaning—viz, life-origination or commencement.
- But in the mean time the word biogenesis has been made use of, quite independently, by a distinguished biologist [Huxley], who wished to make it bear a totally different meaning. He also introduced the term abiogenesis. I have been informed, however, on the best authority, that neither of these words can—with any regard to the language from which they are derived—be supposed to bear the meanings which have of late been publicly assigned to them. Wishing to avoid all needless confusion, I therefore renounced the use of the word biogenesis, and being, for the reason just given, unable to adopt the other term, I was compelled to introduce a new word, in order to designate the process by which living matter is supposed to come into being, independently of pre-existing living matter.[62]
Pasteur and Darwin

Pasteur himself remarked, after a definitive finding in 1864, "Never will the doctrine of spontaneous generation recover from the mortal blow struck by this simple experiment."[63][64] One alternative was that life's origins on Earth had come from somewhere else in the Universe. Periodically resurrected (see Panspermia, above) Bernal said that this approach "is equivalent in the last resort to asserting the operation of metaphysical, spiritual entities... it turns on the argument of creation by design by a creator or demiurge".[65] Such a theory, Bernal said was unscientific and a number of scientists defined life as a result of an inner life force, which in the late 19th century was championed by Henri Bergson.
The concept of evolution proposed by Charles Darwin put an end to these metaphysical theologies. In a letter to Joseph Dalton Hooker on 1 February 1871,[66] Charles Darwin addressed the question, suggesting that the original spark of life may have begun in a "warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c., present, that a proteine compound was chemically formed ready to undergo still more complex changes." He went on to explain that "at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed."[67] In other words, the presence of life itself makes the search for the spontaneous origin of life dependent on the artificial production of organic compounds in the sterile conditions of the laboratory.
"Primordial soup" hypothesis
No new notable research or theory on the subject appeared until 1924, when Alexander Oparin reasoned that atmospheric oxygen prevents the synthesis of certain organic compounds that are necessary building blocks for the evolution of life. In his book The Origin of Life,[68][69] Oparin proposed that the "spontaneous generation of life" that had been attacked by Louis Pasteur did in fact occur once, but was now impossible because the conditions found on the early Earth had changed, and preexisting organisms would immediately consume any spontaneously generated organism. Oparin argued that a "primeval soup" of organic molecules could be created in an oxygenless atmosphere through the action of sunlight. These would combine in ever more complex ways until they formed coacervate droplets. These droplets would "grow" by fusion with other droplets, and "reproduce" through fission into daughter droplets, and so have a primitive metabolism in which factors that promote "cell integrity" survive, and those that do not become extinct. Many modern theories of the origin of life still take Oparin's ideas as a starting point.
Robert Shapiro has summarized the "primordial soup" theory of Oparin and Haldane in its "mature form" as follows:[70]
- The early Earth had a chemically reducing atmosphere.
- This atmosphere, exposed to energy in various forms, produced simple organic compounds ("monomers").
- These compounds accumulated in a "soup" that may have concentrated at various locations (shorelines, oceanic vents etc.).
- By further transformation, more complex organic polymers – and ultimately life – developed in the soup.
Around the same time, J. B. S. Haldane suggested that the Earth's prebiotic oceans—different from their modern counterparts—would have formed a "hot dilute soup" in which organic compounds could have formed. J.D. Bernal, a pioneer in x-ray crystallography, called this idea biopoiesis or biopoesis, the process of living matter evolving from self-replicating but nonliving molecules,[71][72] and proposed that biopoiesis passes through a number of intermediate stages.
One of the most important pieces of experimental support for the "soup" theory came in 1952. Stanley Miller and Harold Urey, performed an experiment that demonstrated how organic molecules could have spontaneously formed from inorganic precursors, under conditions like those posited by the Oparin-Haldane Hypothesis. The now-famous "Miller–Urey experiment" used a highly reduced mixture of gases—methane, ammonia and hydrogen—to form basic organic monomers, such as amino acids.[73] This provided direct experimental support for the second point of the "soup" theory, and it is around the remaining two points of the theory that much of the debate now centers. In the Miller–Urey experiment, a mixture of water, hydrogen, methane, and ammonia was cycled through an apparatus that delivered electrical sparks to the mixture. After one week, it was found that about 10% to 15% of the carbon in the system was now in the form of a racemic mixture of organic compounds, including amino acids, which are the building blocks of proteins.
Bernal shows that based upon this and subsequent work there is no difficulty in principle in forming most of the molecules we recognise as the basic molecules of life from their inorganic precursors. The underlying hypothesis held by Oparin, Haldane, Bernal, Miller and Urey, for instance, was that multiple conditions on the primeval Earth favored chemical reactions that synthesized the same set of complex organic compounds from such simple precursors. A 2011 reanalysis of the saved vials containing the original extracts that resulted from the Miller and Urey experiments, using current and more advanced analytical equipment and technology, has uncovered more biochemicals than originally discovered in the 1950s. One of the more important findings was 23 amino acids, far more than the five originally found.[74] However Bernal said that "it is not enough to explain the formation of such molecules, what is necessary" he says "..is a physical-chemical explanation of the origins of these molecules that suggests the presence of suitable sources and sinks for free energy".[75]
Proteinoid microspheres
In trying to uncover the intermediate stages of abiogenesis mentioned by Bernal, Sidney W. Fox in the 1950s and 1960s, studied the spontaneous formation of peptide structures under conditions that might plausibly have existed early in Earth's history. He demonstrated that amino acids could spontaneously form small chains called peptides. In one of his experiments, he allowed amino acids to dry out as if puddled in a warm, dry spot in prebiotic conditions. He found that, as they dried, the amino acids formed long, often cross-linked, thread-like, submicroscopic polypeptide molecules now named "proteinoid microspheres".[76]
In another experiment using a similar method to set suitable conditions for life to form, Fox collected volcanic material from a cinder cone in Hawaii. He discovered that the temperature was over 100 °C (212 °F) just 4 inches (100 mm) beneath the surface of the cinder cone, and suggested that this might have been the environment in which life was created—molecules could have formed and then been washed through the loose volcanic ash and into the sea. He placed lumps of lava over amino acids derived from methane, ammonia and water, sterilized all materials, and baked the lava over the amino acids for a few hours in a glass oven. A brown, sticky substance formed over the surface and when the lava was drenched in sterilized water a thick, brown liquid leached out. It turned out that the amino acids had combined to form proteinoids, and the proteinoids had combined to form small globules that Fox called "microspheres". His proteinoids were not cells, although they formed clumps and chains reminiscent of cyanobacteria, but they contained no functional nucleic acids or any encoded information. Based upon such experiments, Colin S. Pittendrigh stated in December 1967 that "laboratories will be creating a living cell within ten years," a remark that reflected the typical contemporary levels of innocence of the complexity of cell structures.[77]
Current models
There is still no "standard model" of the origin of life. Most currently accepted models draw at least some elements from the framework laid out by Alexander Oparin (in 1924) and J.B.S. Haldane (in 1925), who postulated the molecular or chemical evolution theory of life.[78] According to them, the first molecules constituting the earliest cells "were synthesized under natural conditions by a slow process of molecular evolution, and these molecules then organized into the first molecular system with properties with biological order."[78] Oparin and Haldane suggested that the atmosphere of the early Earth may have been chemically reducing in nature, composed primarily of methane (CH4), ammonia (NH3), water (H2O), hydrogen sulfide (H2S), carbon dioxide (CO2) or carbon monoxide (CO), and phosphate (PO43−), with molecular oxygen (O2) and ozone (O3) either rare or absent. The current scientific model, however, is an atmosphere that contained 60% hydrogen, 20% oxygen (mostly in the form of water vapor), 10% carbon dioxide, 5 to 7% hydrogen sulfide, and smaller amounts of nitrogen, carbon monoxide, free hydrogen, methane and inert gases.[79][80] In the atmosphere proposed by Oparin and Haldane, electrical activity can catalyze the creation of certain basic small molecules (monomers) of life, such as amino acids. This was demonstrated in the Miller–Urey experiment by Stanley L. Miller and Harold C. Urey reported in 1953.
John Desmond Bernal coined the term biopoiesis in 1949 to refer to the origin of life.[81] In 1967, he suggested that it occurred in three "stages": 1) the origin of biological monomers; 2) the origin of biological polymers; and 3) the evolution from molecules to cells. He suggested that evolution commenced between stage 1 and 2. The first stage is now fairly well understood, and the discovery of alkaline vents and the similarity with the "proton pump" found as the basis of biological life has begun to provide evidence about the second stage. Bernal considered the third, the discovery of methods by which biological reactions were incorporated behind cell walls, to be the most difficult. Modern work on the self organising capacities by which cell membranes self-assemble, and the work on micropores in various substrates as a half-way house towards the development of independent free-living cells, is ongoing research designed to answer this problem.[82][83][84]
The chemical processes that took place on the early Earth are called chemical evolution. Both Manfred Eigen and Sol Spiegelman demonstrated that evolution, including replication, variation, and natural selection, can occur in populations of molecules as well as in organisms.[40] Spiegelman took advantage of natural selection to synthesize Spiegelman's Monster, which had a genome with just 218 bases. Eigen built on Spiegelman's work and produced a similar system with just 48 or 54 nucleotides.[85]
Chemical evolution was followed by the initiation of biological evolution, which led to the first cells.[40] No one has yet synthesized a "protocell" using basic components with the necessary properties of life (the so-called "bottom-up-approach"). Without such a proof-of-principle, explanations have tended to focus on chemosynthesis.[86] However, some researchers are working in this field, notably Steen Rasmussen and Jack Szostak. Others have argued that a "top-down approach" is more feasible. One such approach, successfully attempted by Craig Venter and others at The Institute for Genomic Research, involves engineering existing prokaryotic cells with progressively fewer genes, attempting to discern at which point the most minimal requirements for life were reached.[87][88]
Chemical origin of organic molecules
The elements, except for hydrogen, ultimately derive from stellar nucleosynthesis. Complex molecules, including organic molecules, form naturally both in space and on planets.[13] There are two possible sources of organic molecules on the early Earth:
- Terrestrial origins – organic synthesis driven by impact shocks or by other energy sources (such as ultraviolet light, redox coupling, or electrical discharges) (e.g. Miller's experiments)
- Extraterrestrial origins – formation of organic molecules in interstellar dust clouds and rained down on planets.[89][90] (See pseudo-panspermia)
Estimates of these sources suggest that the heavy bombardment before 3.5 Ga within the early atmosphere made available quantities of organics comparable to those produced by other energy sources.[91][92]

It has been estimated that the Late Heavy Bombardment may also have effectively sterilised the Earth's surface to a depth of tens of metres. If life evolved deeper than this, it would have also been shielded from the early high levels of ultraviolet radiation from the T Tauri stage of the sun's evolution. Simulations of geothermically heated oceanic crust yield far more organics than those found in the Miller-Urey experiments (see below). In the deep hydrothermal vents, Everett Shock has found "there is an enormous thermodynamic drive to form organic compounds, as seawater and hydrothermal fluids, which are far from equilibrium, mix and move towards a more stable state".[93] Shock has found that the available energy is maximised at around 100 – 150 degrees Celsius, precisely the temperatures at which the hyperthermophilic bacteria and archaea have been found, at the base of the tree of life closest to the Last Universal Common Ancestor.[94]
Chemical synthesis
While features of self-organization and self-replication are often considered the hallmark of living systems, there are many instances of abiotic molecules exhibiting such characteristics under proper conditions. Palasek showed that self-assembly of RNA molecules can occur spontaneously due to physical factors in hydrothermal vents.[95] Virus self-assembly within host cells has implications for the study of the origin of life,[96] as it lends further credence to the hypothesis that life could have started as self-assembling organic molecules.[97][98]
Multiple sources of energy were available for chemical reactions on the early Earth. For example, heat (such as from geothermal processes) is a standard energy source for chemistry. Other examples include sunlight and electrical discharges (lightning), among others.[40] Unfavorable reactions can also be driven by highly favorable ones, as in the case of iron-sulfur chemistry. For example, this was probably important for carbon fixation (the conversion of carbon from its inorganic form to an organic one).[note 1] Carbon fixation via iron-sulfur chemistry is highly favorable, and occurs at neutral pH and 100 °C (212 °F). Iron-sulfur surfaces, which are abundant near hydrothermal vents, are also capable of producing small amounts of amino acids and other biological metabolites.[40]
Formamide produces all four ribonucleotides and other biological molecules when warmed in the presence of various terrestrial minerals. Formamide is ubiquitous in the universe, produced by the reaction of water and HCN (hydrogen cyanide). It has several advantages as a prebiotic precursor, including the ability to easily become concentrated through the evaporation of water.[99][100] Although HCN is poisonous, it only affects aerobic organisms (eukaryotes and aerobic bacteria). It can play roles in other chemical processes as well, such as the synthesis of the amino acid glycine.[40]
In 1961, it was shown that the nucleic acid purine base adenine can be formed by heating aqueous ammonium cyanide solutions.[101] Other pathways for synthesizing bases from inorganic materials were also reported.[102] Leslie Orgel and colleagues have shown that freezing temperatures are advantageous for the synthesis of purines, due to the concentrating effect for key precursors such as hydrogen cyanide.[103] Research by Stanley Miller and colleagues suggested that while adenine and guanine require freezing conditions for synthesis, cytosine and uracil may require boiling temperatures.[104] Research by the Miller group notes the formation of seven different amino acids and 11 types of nucleobases in ice when ammonia and cyanide were left in a freezer from 1972 to 1997.[105][106] Other work demonstrated the formation of s-triazines (alternative nucleobases), pyrimidines (including cytosine and uracil), and adenine from urea solutions subjected to freeze-thaw cycles under a reductive atmosphere (with spark discharges as an energy source).[107] The explanation given for the unusual speed of these reactions at such a low temperature is eutectic freezing. As an ice crystal forms, it stays pure: only molecules of water join the growing crystal, while impurities like salt or cyanide are excluded. These impurities become crowded in microscopic pockets of liquid within the ice, and this crowding causes the molecules to collide more often. Mechanistic exploration using quantum chemical methods provide a more detailed understanding of some of the chemical processes involved in chemical evolution, and a partial answer to the fundamental question of molecular biogenesis.[108]
At the time of the Miller–Urey experiment, scientific consensus was that the early Earth had a reducing atmosphere with compounds relatively rich in hydrogen and poor in oxygen (e.g., CH
4 and NH
3 as opposed to CO
2 and NO
2). However, current scientific consensus describes the primitive atmosphere as either weakly reducing or neutral[109][110] (see also Oxygen catastrophe). Such an atmosphere would diminish both the amount and variety of amino acids that could be produced, although studies that include iron and carbonate minerals (thought present in early oceans) in the experimental conditions have again produced a diverse array of amino acids.[109] Other scientific research has focused on two other potential reducing environments: outer space and deep-sea thermal vents[111][112][113]
The spontaneous formation of complex polymers from abiotically generated monomers under the conditions posited by the "soup" theory is not at all a straightforward process. Besides the necessary basic organic monomers, compounds that would have prohibited the formation of polymers were formed in high concentration during the Miller–Urey and Oró experiments.[114] The Miller–Urey experiment, for example, produces many substances that would react with the amino acids or terminate their coupling into peptide chains.[115]
Autocatalysis
Autocatalysts are substances that catalyze the production of themselves, and therefore are simple molecular replicators. The simplest self-replicating chemical systems are autocatalytic, and typically contain three components: two precursors that join together to form a product molecule, and the product molecule itself. The product molecule catalyzes the reaction by providing a complementary template that binds to the precursors, thus bringing them together. Such systems have been demonstrated both in biological macromolecules and in small organic molecules.[116][117] Systems that do not proceed by template mechanisms, such as the self-reproduction of micelles and vesicles, have also been observed.[117]
In 1993, Stuart Kauffman proposed that life initially arose as autocatalytic chemical networks.[118] British ethologist Richard Dawkins wrote about autocatalysis as a potential explanation for the origin of life in his 2004 book The Ancestor's Tale.[119] In his book, Dawkins cites experiments performed by Julius Rebek and his colleagues at the Scripps Research Institute in California in which they combined amino adenosine and pentafluorophenyl esters with the autocatalyst amino adenosine triacid ester (AATE). One system from the experiment contained variants of AATE, which catalysed the synthesis of themselves. This experiment demonstrated the possibility that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of natural selection.[citation needed]
In the early 1970s, Manfred Eigen and Peter Schuster examined the transient stages between the molecular chaos and a self-replicating hypercycle in a prebiotic soup.[120] In a hypercycle, the information storing system (possibly RNA) produces an enzyme, which catalyzes the formation of another information system, in sequence until the product of the last aids in the formation of the first information system. Mathematically treated, hypercycles could create quasispecies, which through natural selection entered into a form of Darwinian evolution. A boost to hypercycle theory was the discovery of ribozymes capable of catalyzing their own chemical reactions. The hypercycle theory requires the existence of complex biochemicals, such as nucleotides, which do not form under the conditions proposed by the Miller–Urey experiment.
Geoffrey W. Hoffmann, a student of Eigen, contributed to the concept of life involving both replication and metabolism emerging from catalytic noise. His contributions included showing that an early sloppy translation machinery can be stable against an error catastrophe of the type that had been envisaged as problematical by Leslie Orgel ("Orgel's paradox")[121][122] and calculations regarding the occurrence of a set of required catalytic activities together with the exclusion of catalytic activities that would be disruptive.[123]
Homochirality
Homochirality refers to the geometric property of some materials that are composed of chiral units. Chiral refers to nonsuperimposable 3D forms that are mirror images of one another, as are left and right hands. Living organisms use molecules that have the same chirality ("handedness"): with almost no exceptions,[124] amino acids are left-handed while nucleotides and sugars are right-handed. Chiral molecules can be synthesized, but in the absence of a chiral source or a chiral catalyst, they are formed in a 50/50 mixture of both enantiomers. This is called a racemic mixture. Known mechanisms for the production of non-racemic mixtures from racemic starting materials include: asymmetric physical laws, such as the electroweak interaction; asymmetric environments, such as those caused by circularly polarized light, quartz crystals, or the Earth's rotation; and statistical fluctuations during racemic synthesis.[125]
Once established, chirality would be selected for.[126] A small enantiomeric excess can be amplified into a large one by asymmetric autocatalysis, such as in the Soai reaction.[127] In asymmetric autocatalysis, the catalyst is a chiral molecule, which means that a chiral molecule is catalysing its own production. An initial enantiomeric excess, such as can be produced by polarized light, then allows the more abundant enantiomer to outcompete the other.[128]
Clark has suggested that homochirality may have started in outer space, as the studies of the amino acids on the Murchison meteorite showed that L-alanine is more than twice as frequent as its D form, and L-glutamic acid was more than three times prevalent than its D counterpart. Various chiral crystal surfaces can also act as sites for possible concentration and assembly of chiral monomer units into macromolecules.[129] Compounds found on meteorites suggest that the chirality of life derives from abiogenic synthesis, since amino acids from meteorites show a left-handed bias, whereas sugars show a predominantly right-handed bias, the same as found in living organisms.[130]
Self-enclosement, reproduction, duplication and the RNA world
Protocells
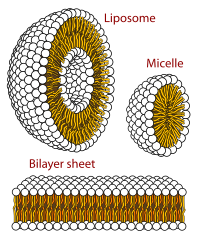
A protocell is a self-organized, endogenously ordered, spherical collection of lipids proposed as a stepping-stone to the origin of life.[131] A central question in evolution is how simple protocells first arose and differed in reproductive contribution to the following generation driving the evolution of life. Although a functional protocell has not yet been achieved in a laboratory setting, the goal appears well within reach.[132][133][134]
Self-assembled vesicles are essential components of primitive cells.[131] The second law of thermodynamics requires that the universe move in a direction in which disorder (or entropy) increases, yet life is distinguished by its great degree of organization. Therefore, a boundary is needed to separate life processes from non-living matter.[135] Researchers Irene A. Chen and Jack W. Szostak (Nobel Prize in Physiology or Medicine 2009) amongst others, demonstrated that simple physicochemical properties of elementary protocells can give rise to essential cellular behaviors, including primitive forms of differential reproduction competition and energy storage. Such cooperative interactions between the membrane and encapsulated contents could greatly simplify the transition from replicating molecules to true cells.[133] Furthermore, competition for membrane molecules would favor stabilized membranes, suggesting a selective advantage for the evolution of cross-linked fatty acids and even the phospholipids of today.[133] This micro-encapsulation allowed for metabolism within the membrane, exchange of small molecules and prevention of passage of large substances across it.[136] The main advantages of encapsulation include increased solubility of the cargo and storing energy in the form of a chemical gradient.
A 2012 study led by Armen Mulkidjanian of Germany's University of Osnabrück, suggests that inland pools of condensed and cooled geothermal vapour have the ideal characteristics for the origin of life.[137] Scientists confirmed in 2002 that by adding a montmorillonite clay to a solution of fatty acid micelles (lipid spheres), the clay sped up the rate of vesicles formation 100-fold.[134] So this one mineral can get precursors (nucleotides) to spontaneously assemble into RNA and membrane precursors to assemble into membrane.
Another protocell model is the Jeewanu. First synthesized in 1963 from simple minerals and basic organics while exposed to sunlight, it is still reported to have some metabolic capabilities, the presence of semipermeable membrane, amino acids, phospholipids, carbohydrates and RNA-like molecules.[138][139] However, the nature and properties of the Jeewanu remains to be clarified.
RNA world
The RNA world hypothesis describes an early Earth with self-replicating and catalytic RNA but no DNA or proteins.[141] It is generally accepted that current life on Earth descends from an RNA world,[9][142][143] although RNA-based life may not have been the first life to exist.[10][11] This conclusion is drawn from many independent lines of evidence, such as the observations that RNA is central to the translation process and that small RNAs can catalyze all of the chemical groups and information transfers required for life.[11][144] The structure of the ribosome has been called the "smoking gun," as it showed that the ribosome is a ribozyme, with a central core of RNA and no amino acid side chains within 18 angstroms of the active site where peptide bond formation is catalyzed.[10] The concept of the RNA world was first proposed in 1962 by Alexander Rich,[145] and the term was coined by Walter Gilbert in 1986.[11][146]
Possible precursors for the evolution of protein synthesis include a mechanism to synthesize short peptide cofactors or from a mechanism for the duplication of RNA. It is likely that the ancestral ribosome was composed entirely of RNA, although some roles have since been taken over by proteins. Major remaining questions on this topic include identifying the selective force for the evolution of the ribosome and determining how the genetic code arose.[147]
Eugene Koonin said, "Despite considerable experimental and theoretical effort, no compelling scenarios currently exist for the origin of replication and translation, the key processes that together comprise the core of biological systems and the apparent pre-requisite of biological evolution. The RNA World concept might offer the best chance for the resolution of this conundrum but so far cannot adequately account for the emergence of an efficient RNA replicase or the translation system. The MWO (Ed.: "many worlds in one"[148]) version of the cosmological model of eternal inflation could suggest a way out of this conundrum because, in an infinite multiverse with a finite number of distinct macroscopic histories (each repeated an infinite number of times), emergence of even highly complex systems by chance is not just possible but inevitable."[148]
RNA synthesis and replication
The RNA world has spurred scientists to try to determine if RNA molecules could have spontaneously formed that were capable of catalyzing their own replication.[149][150][151] Evidences suggest chemical conditions (including the presence of boron, molybdenum and oxygen) for initially producing RNA molecules may have been better on the planet Mars than those on the planet Earth.[149][150] If so, life-suitable molecules, originating on Mars, may have later migrated to Earth via meteor ejections.[149][150]
A number of hypotheses of modes of formation have been put forward. As of 1994, there were difficulties in the abiotic synthesis of the nucleotides cytosine and uracil.[152] Subsequent research has shown possible routes of synthesis; for example, formamide produces all four ribonucleotides and other biological molecules when warmed in the presence of various terrestrial minerals.[99][100] Early cell membranes could have formed spontaneously from proteinoids, which are protein-like molecules produced when amino acid solutions are heated while in the correct concentration in aqueous solution. These are seen to form micro-spheres which are observed to behave similarly to membrane-enclosed compartments. Other possibilities include systems of chemical reactions that take place within clay substrates or on the surface of pyrite rocks.
Factors supportive of an important role for RNA in early life include its ability to act both to store information and to catalyze chemical reactions (as a ribozyme); its many important roles as an intermediate in the expression and maintenance of the genetic information (in the form of DNA) in modern organisms; and the ease of chemical synthesis of at least the components of the molecule under the conditions that approximated the early Earth. Relatively short RNA molecules have been artificially produced in labs, which are capable of replication.[153] Such replicase RNA, which functions as both code and catalyst provides its own template upon which copying can occur. Jack Szostak has shown that certain catalytic RNAs can, indeed, join smaller RNA sequences together, creating the potential, in the right conditions for self-replication. If these conditions were present, Darwinian selection would favour the proliferation of such autocatalytic sets, to which further functionalities could be added.[154] Lincoln and Joyce have identified such autocatalytic systems of RNA capable of self-sustained replication.[155] The systems, which include two ribozymes that catalyze each other's synthesis, replicated with doubling time of about one hour, and were subject to natural selection.[156] In evolutionary competition experiments, this led to the emergence of new systems which replicated more efficiently.[10] This was the first demonstration of evolutionary adaptation occurring in a molecular genetic system.[156]
Depending on the specific definition for life being used, life can be considered to have emerged when RNA chains began to express the basic conditions necessary for natural selection to operate as conceived by Darwin: heritability, variation of type, and differential reproductive output. Fitness of an RNA replicator (its per capita rate of increase) would likely be a function of adaptive capacities that were intrinsic (in the sense that they were determined by the nucleotide sequence) and the availability of resources.[157][158] The three primary adaptive capacities may have been (1) the capacity to replicate with moderate fidelity (giving rise to both heritability and variation of type), (2) the capacity to avoid decay, and (3) the capacity to acquire and process resources.[157][158] These capacities would have been determined initially by the folded configurations of the RNA replicators that, in turn, would be encoded in their individual nucleotide sequences. Relative reproductive success among different replicators would have depended on the relative values of these adaptive capacities.
Pre-RNA world
It is possible that a different type of nucleic acid, such as PNA, TNA or GNA, was the first to emerge as a self-reproducing molecule, only later replaced by RNA.[159][160] Larralde et al., say that "the generally accepted prebiotic synthesis of ribose, the formose reaction, yields numerous sugars without any selectivity."[161] and they conclude that their "results suggest that the backbone of the first genetic material could not have contained ribose or other sugars because of their instability." The ester linkage of ribose and phosphoric acid in RNA is known to be prone to hydrolysis.[162]
Pyrimidine ribonucleosides and their respective nucleotides have been prebiotically synthesised by a sequence of reactions which by-pass the free sugars, and are assembled in a stepwise fashion by using nitrogenous or oxygenous chemistries. John Sutherland has demonstrated high yielding routes to cytidine and uridine ribonucleotides built from small 2 and 3 carbon fragments such as glycolaldehyde, glyceraldehyde or glyceraldehyde-3-phosphate, cyanamide and cyanoacetylene. One of the steps in this sequence allows the isolation of enantiopure ribose aminooxazoline if the enantiomeric excess of glyceraldehyde is 60% or greater.[163] This can be viewed as a prebiotic purification step, where the said compound spontaneously crystallised out from a mixture of the other pentose aminooxazolines. Ribose aminooxazoline can then react with cyanoacetylene in a mild and highly efficient manner to give the alpha cytidine ribonucleotide. Photoanomerization with UV light allows for inversion about the 1' anomeric centre to give the correct beta stereochemistry.[164] In 2009 they showed that the same simple building blocks allow access, via phosphate controlled nucleobase elaboration, to 2',3'-cyclic pyrimidine nucleotides directly, which are known to be able to polymerise into RNA. This paper also highlights the possibility for the photo-sanitization of the pyrimidine-2',3'-cyclic phosphates.[165]
Origin of biological metabolism
Laboratory research suggests that metabolism-like reactions could have occurred naturally in early oceans, before the first organisms evolved.[12][166] The findings suggests that metabolism predates the origin of life and evolved through the chemical conditions that prevailed in the worlds earliest oceans. Reconstructions in laboratories show that some of these reactions can produce RNA, and some others resemble two essential reaction cascades of metabolism: glycolysis and the pentose phosphate pathway, that provide essential precursors for nucleic acids, amino acids and lipids.[166] Following are some observed discoveries and related hypotheses.
Iron-sulfur world
Proposed in the 1980s by Günter Wächtershäuser, encouraged and supported by Karl R. Popper,[167][168][169] in his iron–sulfur world theory, this hypothesis postulates the evolution of (bio)chemical pathways as fundamentals of the evolution of life. Moreover, it presentes a consistent system of tracing today's biochemistry back to ancestral reactions that provide alternative pathways to the synthesis of organic building blocks from simple gaseous compounds.
In contrast to the classical Miller experiments, which depend on external sources of energy (such as simulated lightning or ultraviolet irradiation), "Wächtershäuser systems" come with a built-in source of energy, sulfides of iron and other minerals (e.g. pyrite). The energy released from redox reactions of these metal sulfides is not only available for the synthesis of organic molecules, but also for the formation of oligomers and polymers. It is therefore hypothesized that such systems may be able to evolve into autocatalytic sets of self-replicating, metabolically active entities that would predate the life forms known today.[12][166] The experiment produced a relatively small yield of dipeptides (0.4% to 12.4%) and a smaller yield of tripeptides (0.10%) although under the same conditions, dipeptides were quickly broken down.[170]
Several models reject the idea of the self-replication of a "naked-gene" and postulate the emergence of a primitive metabolism which could provide an environment for the later emergence of RNA replication. The centrality of the Krebs cycle to energy production in aerobic organisms, and in drawing in carbon dioxide and hydrogen ions in biosynthesis of complex organic chemicals, including amino acids and nucleotides, suggests that it was one of the first parts of the metabolism to evolve.[171] Somewhat in agreement with these notions, geochemist Michael Russell has proposed that "the purpose of life is to hydrogenate carbon dioxide" (as part of a "metabolism-first", rather than a "genetics-first", scenario).[172][173] Physicist Jeremy England of MIT has proposed that thermodynamically, life was bound to eventually arrive, as based on established physics, he mathematically indicates "...that when a group of atoms is driven by an external source of energy (like the sun or chemical fuel) and surrounded by a heat bath (like the ocean or atmosphere), it will often gradually restructure itself in order to dissipate increasingly more energy. This could mean that under certain conditions, matter inexorably acquires the key physical attribute associated with life.".[174][175]
One of the earliest incarnations of this idea was put forward in 1924 with Alexander Oparin's notion of primitive self-replicating vesicles which predated the discovery of the structure of DNA. Variants in the 1980s and 1990s include Günter Wächtershäuser's iron-sulfur world theory and models introduced by Christian de Duve based on the chemistry of thioesters. More abstract and theoretical arguments for the plausibility of the emergence of metabolism without the presence of genes include a mathematical model introduced by Freeman Dyson in the early 1980s and Stuart Kauffman's notion of collectively autocatalytic sets, discussed later in that decade.
Leslie Orgel summarized his analysis of the proposal by stating, "There is at present no reason to expect that multistep cycles such as the reductive citric acid cycle will self-organize on the surface of FeS/FeS2 or some other mineral."[176] It is possible that another type of metabolic pathway was used at the beginning of life. For example, instead of the reductive citric acid cycle, the "open" acetyl-CoA pathway (another one of the five recognised ways of carbon dioxide fixation in nature today) would be compatible with the idea of self-organisation on a metal sulfide surface. The key enzyme of this pathway, carbon monoxide dehydrogenase/acetyl-CoA synthase harbours mixed nickel-iron-sulfur clusters in its reaction centers and catalyses the formation of acetyl-CoA (which may be regarded as a modern form of acetyl-thiol) in a single step.
Zn-World hypothesis
The Zn-World (zinc world) theory of Armen Mulkidjanian[177] is an extension of Wächtershäuser's pyrite hypothesis. Wächtershäuser based his theory of the initial chemical processes leading to informational molecules (i.e. RNA, peptides) on a regular mesh of electric charges at the surface of pyrite that may have made the primeval polymerization thermodynamically more favourable by attracting reactants and arranging them appropriately relative to each other.[178] The Zn-World theory specifies and differentiates further.[177][179] Hydrothermal fluids rich in H2S interacting with cold primordial ocean (or "Darwin pond") water leads to the precipitation of metal sulfide particles. Oceanic vent systems and other hydrothermal systems have a zonal structure reflected in ancient volcanogenic massive sulfide deposits (VMS) of hydrothermal origin. They reach many kilometers in diameter and date back to the Archean eon. Most abundant are pyrite (FeS2), chalcopyrite (CuFeS2), and sphalerite (ZnS), with additions of galena (PbS) and alabandite (MnS). ZnS and MnS have a unique ability to store radiation energy, e.g. provided by UV light. Since during the relevant time window of the origins of replicating molecules the primordial atmospheric pressure was high enough (>100 bar) to precipitate near the Earth's surface and UV irradiation was 10 to 100 times more intense than now, the unique photosynthetic properties mediated by ZnS provided just the right energy conditions to energize the synthesis of informational and metabolic molecules and the selection of photostable nucleobases.
The Zn-World theory has been further filled out with experimental and theoretical evidence for the ionic constitution of the interior of the first proto-cells before Archea, Eubacteria and Proto-Eukarya evolved. Archibald Maccallum noted the resemblance of organismal fluids such as blood, lymph to seawater;[180] however, the inorganic composition of all cells differ from that of modern sea water, which led Mulkidjanian and colleagues to reconstruct the "hatcheries" of the first cells combining geochemical analysis with phylogenomic scrutiny of the inorganic ion requirements of universal components of modern cells. The authors conclude that ubiquitous, and by inference primordial, proteins and functional systems show affinity to and functional requirement for K+, Zn2+, Mn2+, and phosphate. Geochemical reconstruction shows that the ionic composition conducive to the origin of cells could not have existed in what we today call marine settings but is compatible with emissions of vapor-dominated zones of what we today call inland geothermal systems. Under the anoxic, CO2-dominated primordial atmosphere, the chemistry of water condensates and exhalations near geothermal fields would resemble the internal milieu of modern cells. Therefore, the precellular stages of evolution may have taken place in shallow "Darwin-ponds" lined with porous silicate minerals mixed with metal sulfides and enriched in K+, Zn2+, and phosphorus compounds.[181][182]
Deep sea vent hypothesis

The deep sea vent, or alkaline hydrothermal vent, theory for the origin of life on Earth posits that life may have begun at submarine hydrothermal vents,[183] where hydrogen-rich fluids emerge from below the sea floor, as a result of serpentinization of ultra-mafic olivine with sea water and a pH interface with carbon dioxide-rich ocean water. Sustained chemical energy in such systems is derived from redox reactions, in which electron donors, such as molecular hydrogen, react with electron acceptors, such as carbon dioxide (see iron-sulfur world theory). These are highly exothermic reactions.[note 2]
Michael Russell demonstrated that alkaline vents created an abiogenic proton-motive force chemiosmotic gradient,[184] in which conditions are ideal for an abiogenic hatchery for life. Their microscopic compartments "provide a natural means of concentrating organic molecules", composed of iron-sulfur minerals such as mackinawite, endowed these mineral cells with the catalytic properties envisaged by Günter Wächtershäuser.[171] This movement of ions across the membrane depends on a combination of two factors:
- Diffusion force caused by concentration gradient – all particles including ions tend to diffuse from higher concentration to lower.
- Electrostatic force caused by electrical potential gradient – cations like protons H+ tend to diffuse down the electrical potential, anions in the opposite direction.
These two gradients taken together can be expressed as an electrochemical gradient, providing energy for abiogenic synthesis. The proton-motive force (PMF) can be described as the measure of the potential energy stored as a combination of proton and voltage gradients across a membrane (differences in proton concentration and electrical potential).

Nobel laureate Szostak suggested that geothermal activity provides greater opportunities for the origination of life in open lakes where there is a buildup of minerals. In 2010, based on spectral analysis of sea and hot mineral water as well as cactus juice, Ignat Ignatov and Oleg Mosin demonstrated that life may have predominantly originated in hot mineral water. The hot mineral water that contains bicarbonate and calcium ions has the most optimal range.[185] This is similar case as the origin of life in hydrothermal vents, but with bicarbonate and calcium ions in hot water. This water has a pH of 9–11 and is possible to have the reactions in sea water. According to Nobel winner Melvin Calvin, certain reactions of condensation-dehydration of amino acids and nucleotides in individual blocks of peptides and nucleic acids can take place in the primary hydrosphere with pH 9-11 at a later evolutionary stage.[186] Some of these compounds like hydrocyanic acid (HCN) have been proven in the experiments of Miller. This is the environment in which the stromatolites have been created. David Ward described the formation of stromatolites in hot mineral water at the Yellowstone National Park. Stromatolites have lived in hot mineral water and in proximity to areas with volcanic activity.[187] Processes have evolved in the sea near geysers of hot mineral water. In 2011 Tadashi Sugawara created a protocell in hot water.[188]
Experimental research and computing modeling indicate that the surfaces of mineral particles inside hydrothermal vents have similar catalytic properties to enzymes under ambient conditions and are able to create simple organic molecules, such as methanol (CH3OH) and formic, acetic and pyruvic acid out of the dissolved CO2 in the water.[189][190]
Thermosynthesis
Today's bioenergetic process of fermentation is carried out by either the aforementioned citric acid cycle or the Acetyl-CoA pathway, both of which have been connected to the primordial iron-sulfur world. In a different approach, the thermosynthesis hypothesis considers the bioenergetic process of chemiosmosis, which plays an essential role in cellular respiration and photosynthesis, more basal than fermentation: the ATP synthase enzyme, which sustains chemiosmosis, is proposed as the currently extant enzyme most closely related to the first metabolic process.[191][192]
First, life needed an energy source to bring about the condensation reaction that yielded the peptide bonds of proteins and the phosphodiester bonds of RNA. In a generalization and thermal variation of the binding change mechanism of today's ATP synthase, the "first protein" would have bound substrates (peptides, phosphate, nucleosides, RNA 'monomers') and condensed them to a reaction product that remained bound until after a temperature change it was released by thermal unfolding.
The energy source under the thermosynthesis hypothesis was thermal cycling, the result of suspension of protocells in a convection current, as is plausible in a volcanic hot spring; the convection accounts for the self-organization and dissipative structure required in any origin of life model. The still ubiquitous role of thermal cycling in germination and cell division is considered a relic of primordial thermosynthesis.
By phosphorylating cell membrane lipids, this "first protein" gave a selective advantage to the lipid protocell that contained the protein. This protein also synthesized a library of many proteins, of which only a minute fraction had thermosynthesis capabilities. As proposed by Dyson,[193] it propagated functionally: it made daughters with similar capabilities, but it did not copy itself. Functioning daughters consisted of different amino acid sequences.
Whereas the iron-sulfur world identifies a circular pathway as the most simple—and therefore assumes the existence of enzymes—the thermosynthesis hypothesis does not even invoke a pathway, and does not assume the existence of regular enzymes: ATP synthase's binding change mechanism resembles a physical adsorption process that yields free energy,[194] rather than a regular enzyme's mechanism, which decreases the free energy. The RNA world also implies the existence of several enzymes. It has been claimed that the emergence of cyclic systems of protein catalysts is implausible.[195]
Other models of abiogenesis
Clay hypothesis
Montmorillonite, an abundant clay, is a catalyst for the polymerization of RNA and for the formation of membranes from lipids.[196] A model for the origin of life using clay was forwarded by A. Graham Cairns-Smith in 1985 and explored as a plausible illustration by several scientists.[197] The clay hypothesis postulates that complex organic molecules arose gradually on a pre-existing, non-organic replication surfaces of silicate crystals in solution.
James Ferris's studies have also confirmed that clay minerals of montmorillonite catalyze the formation of RNA in aqueous solution, by joining activated nucleotides to join together to form longer chains.[198]
In 2007, Kahr and colleagues reported their experiments that tested the idea that crystals can act as a source of transferable information, using crystals of potassium hydrogen phthalate. "Mother" crystals with imperfections were cleaved and used as seeds to grow "daughter" crystals from solution. They then examined the distribution of imperfections in the new crystals and found that the imperfections in the mother crystals were reproduced in the daughters, but the daughter crystals also had many additional imperfections. For gene-like behavior to be observed, the quantity of inheritance of these imperfections should have exceeded that of the mutations in the successive generations, but it did not. Thus Kahr concluded that the crystals "were not faithful enough to store and transfer information from one generation to the next".[199][200]
Gold's "deep-hot biosphere" model
In the 1970s, Thomas Gold proposed the theory that life first developed not on the surface of the Earth, but several kilometers below the surface. It is claimed that discovery of microbial life below the surface of another body in our solar system would lend significant credence to this theory. Thomas Gold also asserted that a trickle of food from a deep, unreachable, source is needed for survival because life arising in a puddle of organic material is likely to consume all of its food and become extinct. Gold's theory is that the flow of such food is due to out-gassing of primordial methane from the Earth's mantle; more conventional explanations of the food supply of deep microbes (away from sedimentary carbon compounds) is that the organisms subsist on hydrogen released by an interaction between water and (reduced) iron compounds in rocks.
Panspermia
Exogenesis is related to, but not the same as, the notion of panspermia. Neither hypothesis actually answers the question of how life first originated, but merely shifts it to another planet or a comet. However, the advantage of an extraterrestrial origin of primitive life is that life is not required to have evolved on each planet it occurs on, but rather in a single location, and then spread about the galaxy to other star systems via cometary and/or meteorite impact. Evidence to support the hypothesis is scant, but it finds support in studies of Martian meteorites found in Antarctica and in studies of extremophile microbes' survival in outer space.[201][202][203][204][205][206][207]
Extraterrestrial organic molecules

An organic compound is any member of a large class of gaseous, liquid, or solid chemicals whose molecules contain carbon. Carbon is the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen.[208] Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets.[209] Organic compounds are relatively common in space, formed by "factories of complex molecular synthesis" which occur in molecular clouds and circumstellar envelopes, and chemically evolve after reactions are initiated mostly by ionizing radiation.[13][210][211][212] Based on computer model studies, the complex organic molecules necessary for life may have formed on dust grains in the protoplanetary disk surrounding the Sun before the formation of the Earth.[37] According to the computer studies, this same process may also occur around other stars that acquire planets.[37]
Observations suggest that the majority of organic compounds introduced on Earth by interstellar dust particles are considered principal agents in the formation of complex molecules, thanks to their peculiar surface-catalytic activities.[213][214] Studies reported in 2008, based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, suggested that the RNA component uracil and related molecules, including xanthine, were formed extraterrestrially.[215][216] On 8 August 2011, a report based on NASA studies of meteorites found on Earth was published suggesting DNA components (adenine, guanine and related organic molecules) were made in outer space.[213][217][218][219] Scientists also found that the cosmic dust permeating the universe contains complex organics ("amorphous organic solids with a mixed aromatic-aliphatic structure") that could be created naturally, and rapidly, by stars.[220][221][222] A scientist who suggested that these compounds may have been related to the development of life on Earth said that "If this is the case, life on Earth may have had an easier time getting started as these organics can serve as basic ingredients for life."[220]

Glycolaldehyde, the first example of an interstellar sugar molecule, was detected in the star-forming region near the center of our galaxy. It was discovered in 2000 by Jes Jørgensen and Jan M. Hollis.[223] Then, on 29 August 2012, the same team reported the detection of glycolaldehyde in a distant star system. The molecule was found around the protostellar binary IRAS 16293-2422 400 light years from Earth.[224][225][226] Glycolaldehyde is needed to form ribonucleic acid (RNA), which is similar in function to DNA. These findings suggest that complex organic molecules may form in stellar systems prior to the formation of planets, eventually arriving on young planets early in their formation.[227] Because sugars are associated with both metabolism and the genetic code, two of the most basic aspects of life, it is thought the discovery of extraterrestrial sugar increases the likelihood that life may exist elsewhere in our galaxy.[223]
NASA announced in 2009 that scientists had identified another fundamental chemical building block of life in a comet for the first time, glycine, an amino acid, which was detected in material ejected from Comet Wild-2 in 2004 and grabbed by NASA's Stardust probe. Glycine has been detected in meteorites before. Carl Pilcher, who leads NASA's Astrobiology Institute commented that "The discovery of glycine in a comet supports the idea that the fundamental building blocks of life are prevalent in space, and strengthens the argument that life in the universe may be common rather than rare."[228] Comets are encrusted with outer layers of dark material, thought to be a tar-like substance composed of complex organic material formed from simple carbon compounds after reactions initiated mostly by ionizing radiation. It is possible that a rain of material from comets could have brought significant quantities of such complex organic molecules to Earth.[229][230][231] Amino acids which were formed extraterrestrially may also have arrived on Earth via comets.[40] It is estimated that during the Late Heavy Bombardment, meteorites may have delivered up to five million tons of biogenic elements to Earth per year.[40]

Polycyclic aromatic hydrocarbons (PAH) are the most common and abundant of the known polyatomic molecules in the visible universe, and are considered a likely constituent of the primordial sea.[232][233][234] PAHs, along with fullerenes (or "buckyballs"), have been recently detected in nebulae.[235][236]
On 3 April 2013, NASA reported that complex organic chemicals could arise on Titan, a moon of Saturn, based on studies simulating the atmosphere of Titan.[237]
On 24 January 2014, NASA reported that current studies on the planet Mars by the Curiosity and Opportunity rovers will now search for evidence of ancient life, including a biosphere based on autotrophic, chemotrophic and/or chemolithoautotrophic microorganisms, as well as ancient water, including fluvio-lacustrine environments (plains related to ancient rivers or lakes) that may have been habitable.[238][239][240][241] The search for evidence of habitability, taphonomy (related to fossils), and organic carbon on the planet Mars is now a primary NASA objective.[238] Nitrogen, which was not attributed to biological organisms, was found on Mars, indicating a possible abiotic nitrogen cycle.[242][243]
In March 2015, NASA scientists reported that, for the first time, complex DNA and RNA organic compounds of life, including uracil, cytosine and thymine, have been formed in the laboratory under outer space conditions, using starting chemicals, such as pyrimidine, found in meteorites. Pyrimidine, like polycyclic aromatic hydrocarbons (PAHs), the most carbon-rich chemical found in the Universe, may have been formed in red giants or in interstellar dust and gas clouds, according to the scientists.[244]
Lipid world
The lipid world theory postulates that the first self-replicating object was lipid-like.[245][246] It is known that phospholipids form lipid bilayers in water while under agitation – the same structure as in cell membranes. These molecules were not present on early Earth, but other amphiphilic long chain molecules also form membranes. Furthermore, these bodies may expand (by insertion of additional lipids), and under excessive expansion may undergo spontaneous splitting which preserves the same size and composition of lipids in the two progenies. The main idea in this theory is that the molecular composition of the lipid bodies is the preliminary way for information storage, and evolution led to the appearance of polymer entities such as RNA or DNA that may store information favorably. Studies on vesicles from potentially prebiotic amphiphiles have so far been limited to systems containing one or two types of amphiphiles. This in contrast to the output of simulated prebiotic chemical reactions, which typically produce very heterogeneous mixtures of compounds.[247] Within the hypothesis of a lipid bilayer membrane composed of a mixture of various distinct amphiphilic compounds there is the opportunity of a huge number of theoretically possible combinations in the arrangements of these amphiphiles in the membrane. Among all these potential combinations, a specific local arrangement of the membrane would have favored the constitution of an hypercycle,[248][249] actually a positive feedback composed of two mutual catalysts represented by a membrane site and a specific compound trapped in the vesicle. Such site/compound pairs are transmissible to the daughter vesicles leading to the emergence of distinct lineages of vesicles which would have allowed Darwinian natural selection.[250]
Polyphosphates
A problem in most scenarios of abiogenesis is that the thermodynamic equilibrium of amino acid versus peptides is in the direction of separate amino acids. What has been missing is some force that drives polymerization. The resolution of this problem may well be in the properties of polyphosphates.[251][252] Polyphosphates are formed by polymerization of ordinary monophosphate ions PO4−3. Several mechanisms for such polymerization have been suggested. Polyphosphates cause polymerization of amino acids into peptides. They are also logical precursors in the synthesis of such key biochemical compounds as ATP. A key issue seems to be that calcium reacts with soluble phosphate to form insoluble calcium phosphate (apatite), so some plausible mechanism must be found to keep calcium ions from causing precipitation of phosphate. There has been much work on this topic over the years, but an interesting new idea is that meteorites may have introduced reactive phosphorus species on the early Earth.[253]
PAH world hypothesis
Polycyclic aromatic hydrocarbons (PAHs) are known to be abundant in the universe,[232][233][234] including in the interstellar medium, in comets, and in meteorites, and are some of the most complex molecules so far found in space.[209]
Other sources of complex molecules have been postulated, including extraterrestrial stellar or interstellar origin. For example, from spectral analyses, organic molecules are known to be present in comets and meteorites. In 2004, a team detected traces of PAHs in a nebula.[254] In 2010, another team also detected PAHs, along with fullerenes (or "buckyballs"), in nebulae.[255] The use of PAHs has also been proposed as a precursor to the RNA world in the PAH world hypothesis.[citation needed] The Spitzer Space Telescope has detected a star, HH 46-IR, which is forming by a process similar to that by which the sun formed. In the disk of material surrounding the star, there is a very large range of molecules, including cyanide compounds, hydrocarbons, and carbon monoxide. In September 2012, NASA scientists reported that PAHs, subjected to interstellar medium (ISM) conditions, are transformed, through hydrogenation, oxygenation and hydroxylation, to more complex organics – "a step along the path toward amino acids and nucleotides, the raw materials of proteins and DNA, respectively".[256][257] Further, as a result of these transformations, the PAHs lose their spectroscopic signature which could be one of the reasons "for the lack of PAH detection in interstellar ice grains, particularly the outer regions of cold, dense clouds or the upper molecular layers of protoplanetary disks."[256][257]
NASA maintains a database for tracking PAHs in the universe.[209][258] More than 20% of the carbon in the universe may be associated with PAHs,[209][259] possible starting materials for the formation of life. PAHs seem to have been formed shortly after the Big Bang, are widespread throughout the universe,[232][233][234] and are associated with new stars and exoplanets.[209]
Radioactive beach hypothesis
Zachary Adam claims that tidal processes that occurred during a time when the moon was much closer may have concentrated grains of uranium and other radioactive elements at the high-water mark on primordial beaches, where they may have been responsible for generating life's building blocks.[260] According to computer models reported in Astrobiology,[261] a deposit of such radioactive materials could show the same self-sustaining nuclear reaction as that found in the Oklo uranium ore seam in Gabon. Such radioactive beach sand might have provided sufficient energy to generate organic molecules, such as amino acids and sugars from acetonitrile in water. Radioactive monazite material also has released soluble phosphate into the regions between sand-grains, making it biologically "accessible". Thus amino acids, sugars, and soluble phosphates might have been produced simultaneously, according to Adam. Radioactive actinides, left behind in some concentration by the reaction, might have formed part of organo-metallic complexes. These complexes could have been important early catalysts to living processes.
John Parnell has suggested that such a process could provide part of the "crucible of life" in the early stages of any early wet rocky planet, so long as the planet is large enough to have generated a system of plate tectonics which brings radioactive minerals to the surface. As the early Earth is thought to have had many smaller plates, it might have provided a suitable environment for such processes.[262]
Thermodynamic dissipation
Karo Michaelian from the National Autonomous University of Mexico (UNAM) points out that any model for the origin of life must take into account the fact that life is an irreversible thermodynamic process and, like all irreversible processes, its origin and persistence as a "self-organized" system is due to its dissipation an imposed generalized chemical potential, i.e. the production of entropy. That is, entropy production is not incidental to the process of life, but rather the fundamental reason for its existence. Present day life augments the entropy production of Earth in its solar environment by dissipating ultraviolet and visible photons into heat through organic pigments in water. This heat then catalyzes a host of secondary dissipative processes such as the water cycle, ocean and wind currents, hurricanes, etc.[263][264] Michaelian argues that if the thermodynamic function of life today is to produce entropy through photon dissipation, then this probably was its function at its very beginnings.[265][266] It turns out that both RNA and DNA when in water solution are very strong absorbers and extremely rapid dissipaters of ultraviolet light within the 230–290 nm wavelength region, which is a part of the sun's spectrum that could have penetrated the prebiotic atmosphere.[267] The amount of ultraviolet (UV-C) light reaching the Earth's surface within this spectral range in the Archean could have been on the order of 4 W/m2,[268] or some 31 orders of magnitude greater than it is today at 260 nm where RNA and DNA absorb most strongly.[269] In fact, not only RNA and DNA, but many fundamental molecules of life (those common to all three domains of life, archea, bacteria, and eucaryote) are also pigments that absorb in the UV-C, and many of these also have a chemical affinity to RNA and DNA.[270] Nucleic acids may thus have acted as acceptor molecules to the UV-C photon excited antenna pigment donor molecules by providing an ultrafast channel for dissipation. Michaelian has shown that there would have existed a non-linear, non-equilibrium thermodynamic imperative to the abiogenic UV-C photochemical synthesis [165] and proliferation of these pigments over the entire Earth surface if they augmented the solar photon dissipation rate.[271]
A simple mechanism to explain enzyme-less replication of RNA and DNA can be given within the same dissipative thermodynamic framework by assuming that life arose when the temperature of the primitive seas had cooled to somewhat below the denaturing temperature of RNA or DNA. The ratio of 18O/16O found in cherts of the Barberton greenstone belt of South Africa indicates that the Earth’s surface temperature was around 80 °C at 3.8 Ga,[272][273] falling to 70±15 °C about 3.5 to 3.2 Ga,[274] suggestively close to RNA or DNA denaturing (uncoiling and separation) temperatures. During the night, the surface water temperature would drop below the denaturing temperature and single strand RNA/DNA could act as extension template for the formation of double strand RNA/DNA. During the daylight hours, RNA and DNA would absorb UV-C light and convert this directly into heat at the ocean surface, thereby raising the local temperature enough to allow for denaturing of RNA and DNA. Direct experimental evidence for the denaturing of DNA through UV-C light dissipation has now been obtained.[275][276]
The copying process would have been repeated with each diurnal cycle.[265][266] Such an ultraviolet light and temperature assisted mechanism of replication (UVTAR) bears similarity to polymerase chain reaction (PCR), a routine laboratory procedure employed to multiply DNA segments. Since denaturation would be most probable in the late afternoon when the Archean sea surface temperature would be highest, and since late afternoon submarine sunlight is somewhat circularly polarized, the homochirality of the organic molecules of life can also be explained within the proposed thermodynamic framework.[277][278]
The fact that the aromatic amino acids have been shown to have chemical affinity to their codons, or anti-codons, and that they also absorb strongly in the UV-C, suggests that they might have originally acted as antenna pigments to increase dissipation and to provide more local heat for UVTAR replication of RNA and DNA as the sea surface temperature cooled. The accumulation of information, e.g. coding for the aromatic amino acids, in RNA or DNA would thus be related to reproductive success under this mechanism. Michaelian suggests that the traditional origin of life research, that expects to describe the emergence of life without overwhelming reference to entropy production through dissipation, is erroneous and that imposed environmental potentials, such as the solar photon flux, and the dissipation of this flux, must be considered to understand the emergence, proliferation, and evolution of life.
Multiple genesis
Different forms of life with variable origin processes may have appeared quasi-simultaneously in the early history of Earth.[279] The other forms may be extinct, leaving distinctive fossils through their different biochemistry (e.g., using arsenic instead of phosphorus), survive as extremophiles, or simply be unnoticed through their being analogous to organisms of the current life tree. Hartman,[280] for example, proposed that:
The first organisms were self-replicating iron-rich clays which fixed carbon dioxide into oxalic and other dicarboxylic acids. This system of replicating clays and their metabolic phenotype then evolved into the sulfide rich region of the hotspring acquiring the ability to fix nitrogen. Finally phosphate was incorporated into the evolving system which allowed the synthesis of nucleotides and phospholipids. If biosynthesis recapitulates biopoiesis, then the synthesis of amino acids preceded the synthesis of the purine and pyrimidine bases. Furthermore the polymerization of the amino acid thioesters into polypeptides preceded the directed polymerization of amino acid esters by polynucleotides.
Lynn Margulis's endosymbiotic theory suggests that multiple forms of archea entered into symbiotic relationship to form the eukaryotic cell. The horizontal transfer of genetic material between archea promotes such symbiotic relationships, and thus many separate organisms may have contributed to building what has been recognised as the Last Universal Common Ancestor (LUCA) of modern organisms.
See also
Notes
- ^ The reactions are:
- FeS + H2S → FeS2 + 2H+ + 2e−
- FeS + H2S → FeS2 + HCOOH
- ^ The reactions are:
Reaction 1: Fayalite + water → magnetite + aqueous silica + hydrogen- 3Fe2SiO4 + 2H2O → 2Fe3O4 + 3SiO2 + 2H2
- 3Mg2SiO4 + SiO2 + 4H2O → 2Mg3Si2O5(OH)4
- 2Mg2SiO4 + 3H2O → Mg3Si2O5(OH)4 + Mg(OH)2
Analogy of reaction 3 with belite hydration in ordinary Portland cement: Belite + water → C-S-H phase + portlandite- 2 Ca2SiO4 + 4 H2O → 3 CaO · 2 SiO2 · 3 H2O + Ca(OH)2
References
- ^ Pronunciation: "/ˌeɪbʌɪə(ʊ)ˈdʒɛnɪsɪs/". Pearsall, Judy; Hanks, Patrick, eds. (1998). "abiogenesis". The New Oxford Dictionary of English (1st ed.). Oxford, UK: Oxford University Press. p. 3. ISBN 0-19-861263-X.
- ^ Bernal 1960, p. 30
- ^ Oparin 1953, p. vi
- ^ Warmflash, David; Warmflash, Benjamin (November 2005). "Did Life Come from Another World?". Scientific American. 293 (5). Stuttgart: Georg von Holtzbrinck Publishing Group: 64–71. doi:10.1038/scientificamerican1105-64. ISSN 0036-8733.
- ^ Yarus 2010, p. 47
- ^ Peretó, Juli (2005). "Controversies on the origin of life" (PDF). International Microbiology. 8 (1). Barcelona: Spanish Society for Microbiology: 23–31. ISSN 1139-6709. PMID 15906258. Retrieved 1 June 2015.
- ^ Voet & Voet 2004, p. 29
- ^ Dyson 1999
- ^ a b
- Copley, Shelley D.; Smith, Eric; Morowitz, Harold J. (December 2007). "The origin of the RNA world: Co-evolution of genes and metabolism" (PDF). Bioorganic Chemistry. 35 (6). Amsterdam, the Netherlands: Elsevier: 430–443. doi:10.1016/j.bioorg.2007.08.001. ISSN 0045-2068. PMID 17897696. Retrieved 8 June 2015.
The proposal that life on Earth arose from an RNA World is widely accepted.
- Orgel, Leslie E. (April 2003). "Some consequences of the RNA world hypothesis". Origins of Life and Evolution of the Biosphere. 33 (2). Kluwer Academic Publishers: 211–218. doi:10.1023/A:1024616317965. ISSN 0169-6149. PMID 12967268.
It now seems very likely that our familiar DNA/RNA/protein world was preceded by an RNA world...
- Robertson & Joyce 2012: "There is now strong evidence indicating that an RNA World did indeed exist before DNA- and protein-based life."
- Neveu, Kim & Benner 2013: "[The RNA world's existence] has broad support within the community today."
- Copley, Shelley D.; Smith, Eric; Morowitz, Harold J. (December 2007). "The origin of the RNA world: Co-evolution of genes and metabolism" (PDF). Bioorganic Chemistry. 35 (6). Amsterdam, the Netherlands: Elsevier: 430–443. doi:10.1016/j.bioorg.2007.08.001. ISSN 0045-2068. PMID 17897696. Retrieved 8 June 2015.
- ^ a b c d Robertson, Michael P.; Joyce, Gerald F. (May 2012). "The origins of the RNA world". Cold Spring Harbor Perspectives in Biology. 4 (5). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press: a003608. doi:10.1101/cshperspect.a003608. ISSN 1943-0264. PMC 3331698. PMID 20739415.
{{cite journal}}: Invalid|ref=harv(help) - ^ a b c d Cech, Thomas R. (July 2012). "The RNA Worlds in Context". Cold Spring Harbor Perspectives in Biology. 4 (7). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press: a006742. doi:10.1101/cshperspect.a006742. ISSN 1943-0264. PMC 3385955. PMID 21441585.
- ^ a b c Keller, Markus A.; Turchyn, Alexandra V.; Ralser, Markus (25 March 2014). "Non‐enzymatic glycolysis and pentose phosphate pathway‐like reactions in a plausible Archean ocean". Molecular Systems Biology. 10 (725). Heidelberg, Germany: EMBO Press on behalf of the European Molecular Biology Organization. doi:10.1002/msb.20145228. ISSN 1744-4292. PMC 4023395. PMID 24771084.
- ^ a b c Ehrenfreund, Pascale; Cami, Jan (December 2010). "Cosmic carbon chemistry: from the interstellar medium to the early Earth". Cold Spring Harbor Perspectives in Biology. 2 (12). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press: a002097. doi:10.1101/cshperspect.a002097. ISSN 1943-0264. PMC 2982172. PMID 20554702.
- ^ Perkins, Sid (8 April 2015). "Organic molecules found circling nearby star". Science (News). Washington, D.C.: American Association for the Advancement of Science. ISSN 1095-9203. Retrieved 2 June 2015.
- ^ King, Anthony (14 April 2015). "Chemicals formed on meteorites may have started life on Earth". Chemistry World (News). London: Royal Society of Chemistry. ISSN 1473-7604. Retrieved 17 April 2015.
- ^ Saladino, Raffaele; Carota, Eleonora; Botta, Giorgia; et al. (13 April 2015). "Meteorite-catalyzed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation". Proc. Natl. Acad. Sci. U.S.A. 112 (21). Washington, D.C.: National Academy of Sciences: E2746–E2755. doi:10.1073/pnas.1422225112. ISSN 1091-6490. PMID 25870268.
- ^ Rampelotto, Pabulo Henrique (26 April 2010). Panspermia: A Promising Field Of Research (PDF). Astrobiology Science Conference 2010. Houston, TX: Lunar and Planetary Institute. p. 5224. Bibcode:2010LPICo1538.5224R. Retrieved 3 December 2014. Conference held at League City, TX
- ^ Loeb, Abraham (October 2014). "The habitable epoch of the early Universe". International Journal of Astrobiology. 13 (4). Cambridge, UK: Cambridge University Press: 337–339. doi:10.1017/S1473550414000196. ISSN 1473-5504.
- Loeb, Abraham (3 June 2014). "The Habitable Epoch of the Early Universe". arXiv:1312.0613v3 [astro-ph.CO].
- ^ Dreifus, Claudia (2 December 2014). "Much-Discussed Views That Go Way Back". The New York Times. New York: The New York Times Company. p. D2. ISSN 0362-4331. Retrieved 3 December 2014.
- ^ Graham, Robert W. (February 1990). "Extraterrestrial Life in the Universe" (PDF) (NASA Technical Memorandum 102363). Lewis Research Center, Cleveland, Ohio: NASA. Retrieved 2 June 2015.
- ^ Altermann 2009, p. xvii
- ^ "Age of the Earth". United States Geological Survey. 9 July 2007. Retrieved 10 January 2006.
- ^ Dalrymple 2001, p. 205–221
- ^ Manhesa, Gérard; Allègre, Claude J.; Dupréa, Bernard; Hamelin, Bruno (May 1980). "Lead isotope study of basic-ultrabasic layered complexes: Speculations about the age of the earth and primitive mantle characteristics". Earth and Planetary Science Letters. 47 (3). Amsterdam, the Netherlands: Elsevier: 370–382. Bibcode:1980E&PSL..47..370M. doi:10.1016/0012-821X(80)90024-2. ISSN 0012-821X.
- ^ a b Schopf, J. William; Kudryavtsev, Anatoliy B.; Czaja, Andrew D.; Tripathi, Abhishek B. (5 October 2007). "Evidence of Archean life: Stromatolites and microfossils". Precambrian Research. 158 (3–4). Amsterdam, the Netherlands: Elsevier: 141–155. doi:10.1016/j.precamres.2007.04.009. ISSN 0301-9268.
- ^ a b Schopf, J. William (29 June 2006). "Fossil evidence of Archaean life". Philosophical Transactions of the Royal Society B. 361 (1470). London: Royal Society: 869–885. doi:10.1098/rstb.2006.1834. ISSN 0962-8436. PMC 1578735. PMID 16754604.
- ^ a b Raven & Johnson 2002, p. 68
- ^ a b Borenstein, Seth (13 November 2013). "Oldest fossil found: Meet your microbial mom". Excite. Yonkers, NY: Mindspark Interactive Network. Associated Press. Retrieved 2 June 2015.
- ^ Pearlman, Jonathan (13 November 2013). "'Oldest signs of life on Earth found'". The Daily Telegraph. London: Telegraph Media Group. Retrieved 15 December 2014.
- ^ a b Noffke, Nora; Christian, Daniel; Wacey, David; Hazen, Robert M. (16 November 2013). "Microbially Induced Sedimentary Structures Recording an Ancient Ecosystem in the ca. 3.48 Billion-Year-Old Dresser Formation, Pilbara, Western Australia". Astrobiology. 13 (12). New Rochelle, NY: Mary Ann Liebert, Inc.: 1103–1124. Bibcode:2013AsBio..13.1103N. doi:10.1089/ast.2013.1030. ISSN 1531-1074. PMC 3870916. PMID 24205812.
- ^ a b Ohtomo, Yoko; Kakegawa, Takeshi; Ishida, Akizumi; et al. (January 2014). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience. 7 (1). London: Nature Publishing Group: 25–28. doi:10.1038/ngeo2025. ISSN 1752-0894.
- ^ McKinney 1997, p. 110
- ^ Stearns & Stearns 1999, p. x
- ^ Novacek, Michael J. (8 November 2014). "Prehistory's Brilliant Future". The New York Times. New York: The New York Times Company. ISSN 0362-4331. Retrieved 25 December 2014.
- ^ Miller & Spoolman 2012, p. 62
- ^ Mora, Camilo; Tittensor, Derek P.; Adl, Sina; et al. (23 August 2011). "How Many Species Are There on Earth and in the Ocean?". PLOS Biology. 9 (8). San Francisco, CA: PLOS: e1001127. doi:10.1371/journal.pbio.1001127. ISSN 1545-7885. PMC 3160336. PMID 21886479.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Moskowitz, Clara (29 March 2012). "Life's Building Blocks May Have Formed in Dust Around Young Sun". Space.com. Salt Lake City, UT: Purch. Retrieved 30 March 2012.
- ^ Fesenkov 1959, p. 9
- ^ Russell 2010
- ^ a b c d e f g h i j Follmann, Hartmut; Brownson, Carol (November 2009). "Darwin's warm little pond revisited: from molecules to the origin of life". Naturwissenschaften. 96 (11). Berlin: Springer-Verlag: 1265–1292. doi:10.1007/s00114-009-0602-1. ISSN 0028-1042. PMID 19760276.
- ^ Morse, John W.; MacKenzie, Fred T. (1998). "Hadean Ocean Carbonate Geochemistry". Aquatic Geochemistry. 4 (3–4). Kluwer Academic Publishers: 301–319. doi:10.1023/A:1009632230875. ISSN 1380-6165.
- ^ Wilde, Simon A.; Valley, John W.; Peck, William H.; Graham, Colin M. (11 January 2001). "Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago" (PDF). Nature. 409 (6817). London: Nature Publishing Group: 175–178. doi:10.1038/35051550. ISSN 0028-0836. PMID 11196637. Retrieved 3 June 2015.
- ^ Rosing, Minik T.; Bird, Dennis K.; Sleep, Norman H.; et al. (22 March 2006). "The rise of continents—An essay on the geologic consequences of photosynthesis" (PDF). Palaeogeography, Palaeoclimatology, Palaeoecology. 232 (2–4). Amsterdam, the Netherlands: Elsevier: 99–113. doi:10.1016/j.palaeo.2006.01.007. ISSN 0031-0182. Retrieved 8 June 2015.
- ^ Sleep, Norman H.; Zahnle, Kevin J.; Kasting, James F.; et al. (9 November 1989). "Annihilation of ecosystems by large asteroid impacts on early Earth". Nature. 342 (6246). London: Nature Publishing Group: 139–142. Bibcode:1989Natur.342..139S. doi:10.1038/342139a0. ISSN 0028-0836. PMID 11536616.
- ^ Davies 1999
- ^ O'Donoghue, James (21 August 2011). "Oldest reliable fossils show early life was a beach". New Scientist. London: Reed Business Information. ISSN 0262-4079. Retrieved 13 October 2014.
- Wacey, David; Kilburn, Matt R.; Saunders, Martin; et al. (October 2011). "Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia". Nature Geoscience. 4 (10). London: Nature Publishing Group: 698–702. doi:10.1038/ngeo1238. ISSN 1752-0894.
- ^ Gomes, Rodney; Levison, Hal F.; Tsiganis, Kleomenis; Morbidelli, Alessandro (26 May 2005). "Origin of the cataclysmic Late Heavy Bombardment period of the terrestrial planets". Nature. 435 (7041). London: Nature Publishing Group: 466–469. Bibcode:2005Natur.435..466G. doi:10.1038/nature03676. ISSN 0028-0836. PMID 15917802.
- ^ Davies 2007, pp. 61–73 harvnb error: multiple targets (2×): CITEREFDavies2007 (help)
- ^ Maher, Kevin A.; Stevenson, David J. (18 February 1988). "Impact frustration of the origin of life". Nature. 331 (6157). London: Nature Publishing Group: 612–614. Bibcode:1988Natur.331..612M. doi:10.1038/331612a0. ISSN 0028-0836. PMID 11536595.
- ^ Mojzsis, Stephen J.; Arrhenius, Gustaf; McKeegan, Kevin D.; et al. (7 November 1996). "Evidence for life on Earth before 3,800 million years ago". Nature. 384 (6604). London: Nature Publishing Group: 55–59. Bibcode:1996Natur.384...55Mdoi=10.1038/384055a0. doi:10.1038/384055a0. ISSN 0028-0836. PMID 8900275.
{{cite journal}}: Check|bibcode=length (help) - ^ Sheldon 2005
- ^ Vartanian 1973, pp. 307–312
- ^ Lennox 2001, pp. 229–258
- ^ Balme, D. M. (1962). "Development of Biology in Aristotle and Theophrastus: Theory of Spontaneous Generation". Phronesis. 7 (1–2). Leiden, the Netherlands: Brill Publishers: 91–104. doi:10.1163/156852862X00052. ISSN 0031-8868.
- ^ Dobell 1960
- ^ Bondeson 1999
- ^ Bernal 1967
- ^ "Biogenesis". Hmolpedia. Ancaster, Ontario, Canada: WikiFoundry, Inc. Retrieved 19 May 2014.
- ^ Huxley 1968
- ^ Huxley 1968
- ^ Bastian 1871
- ^ Bastian 1871, p. xi
- ^ Oparin 1953, p. 196
- ^ Tyndall 1905, IV, XII (1876), XIII (1878)
- ^ Bernal 1967, p. 139
- ^ Priscu, John C. "Origin and Evolution of Life on a Frozen Earth". Arlington County, VA: National Science Foundation. Retrieved 1 March 2014.
- ^ Darwin 1887, p. 18: "It is often said that all the conditions for the first production of a living organism are now present, which could ever have been present. But if (and oh! what a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c., present, that a proteine compound was chemically formed ready to undergo still more complex changes, at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed." — Charles Darwin, 1 February, 1871
- ^ Bernal 1967, The Origin of Life (A. I. Oparin, 1924), pp. 199–234
- ^ Oparin 1953
- ^ Shapiro 1987, p. 110
- ^ Bernal 1967
- ^ Bryson 2004, pp. 300–302
- ^ Miller, Stanley L. (15 May 1953). "A Production of Amino Acids Under Possible Primitive Earth Conditions". Science. 117 (3046). Washington, D.C.: American Association for the Advancement of Science: 528–529. Bibcode:1953Sci...117..528M. doi:10.1126/science.117.3046.528. ISSN 0036-8075. PMID 13056598.
- ^ Parker, Eric T.; Cleaves, Henderson J.; Dworkin, Jason P.; et al. (5 April 2011). "Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment" (PDF). Proc. Natl. Acad. Sci. U.S.A. 108 (14). Washington, D.C.: National Academy of Sciences: 5526–5531. Bibcode:2011PNAS..108.5526P. doi:10.1073/pnas.1019191108. PMC 3078417. PMID 21422282. Retrieved 8 June 2015.
- ^ Bernal 1967, p. 143
- ^ Walsh, J. Bruce (1995). "Part 4: Experimental studies of the origins of life". Origins of life (Lecture notes). Tucson, AZ: University Of Arizona. Archived from the original on 13 January 2008. Retrieved 8 June 2015.
- ^ Woodward 1969, p. 287
- ^ a b Bahadur, Krishna (1973). "Photochemical Formation of Self–sustaining Coacervates" (PDF). Proceedings of the Indian National Science Academy. 39B (4). New Delhi: Indian National Science Academy: 455–467. ISSN 0370-0046.
- Bahadur, Krishna (1975). "Photochemical Formation of Self-Sustaining Coacervates". Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 130 (3). Jena, Germany: Gustav Fischer Verlag: 211–218. doi:10.1016/S0044-4057(75)80076-1. OCLC 641018092. PMID 1242552.
- ^ Kasting, James F. (12 February 1993). "Earth's early atmosphere". Science. 259 (5097). Washington, D.C.: American Association for the Advancement of Science: 920–926. doi:10.1126/science.11536547. ISSN 0036-8075. PMID 11536547.
- ^ Trail, Dustin; Watson, E. Bruce; Tailby, Nicholas D. (1 December 2011). "The oxidation state of Hadean magmas and implications for early Earth's atmosphere". Nature. 480 (7375). London: Nature Publishing Group: 79–82. doi:10.1038/nature10655. ISSN 0028-0836. PMID 22129728.
- ^ Bernal 1951
- ^ Bernal, John Desmond (September 1949). "The Physical Basis of Life". Proceedings of the Physical Society. Section A. 62 (9). Bristol, UK: Physical Society: 537–558. Bibcode:1949PPSA...62..537B. doi:10.1088/0370-1298/62/9/301. ISSN 0370-1298.
- ^ Russell 2010
- ^ Kauffman 1995
- ^ Oehlenschläger, Frank; Eigen, Manfred (December 1997). "30 Years Later – a New Approach to Sol Spiegelman's and Leslie Orgel's in vitro EVOLUTIONARY STUDIES Dedicated to Leslie Orgel on the occasion of his 70th birthday". Origins of Life and Evolution of Biospheres. 27 (5–6). Kluwer Academic Publishers: 437–457. doi:10.1023/A:1006501326129. ISSN 0169-6149. PMID 9394469.
- ^ McCollom, Thomas; Mayhew, Lisa; Scott, Jim (7 October 2014). "NASA awards CU-Boulder-led team $7 million to study origins, evolution of life in universe" (Press release). Boulder, CO: University of Colorado Boulder. Retrieved 8 June 2015.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.1190719 , please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.1190719instead. - ^ Swaby, Rachel (20 May 2010). "Scientists Create First Self-Replicating Synthetic Life". Wired. New York: Condé Nast. Retrieved 8 June 2015.
- ^ Gawlowicz, Susan (6 November 2011). "Carbon-based organic 'carriers' in interstellar dust clouds? Newly discovered diffuse interstellar bands". Science Daily. Rockville, MD: ScienceDaily, LLC. Retrieved 8 June 2015.
- Geballe, Thomas R.; Najarro, Francisco; Figer, Donald F.; et al. (10 November 2011). "Infrared diffuse interstellar bands in the Galactic Centre region". Nature. 479 (7372). London: Nature Publishing Group: 200–202. doi:10.1038/nature10527. ISSN 0028-0836. PMID 22048316.
- ^ Klyce 2001
- ^ Chyba, Christopher; Sagan, Carl (9 January 1992). "Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life". Nature. 355 (6356). London: Nature Publishing Group: 125–132. Bibcode:1992Natur.355..125C. doi:10.1038/355125a0. ISSN 0028-0836. PMID 11538392.
- ^ Furukawa, Yoshihiro; Sekine, Toshimori; Oba, Masahiro; et al. (January 2009). "Biomolecule formation by oceanic impacts on early Earth". Nature Geoscience. 2 (1). London: Nature Publishing Group: 62–66. Bibcode:2009NatGe...2...62F. doi:10.1038/NGEO383. ISSN 1752-0894.
- ^ Davies 1999, p. 155
- ^ Bock & Goode 1996
- ^ Palasek, Stan (23 May 2013). "Primordial RNA Replication and Applications in PCR Technology". arXiv:1305.5581v1 [q-bio.BM].
- ^ Koonin, Eugene V.; Senkevich, Tatiana G.; Dolja, Valerian V. (19 September 2006). "The ancient Virus World and evolution of cells". Biology Direct. 1. London: BioMed Central: 29. doi:10.1186/1745-6150-1-29. ISSN 1745-6150. PMC 1594570. PMID 16984643.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Vlassov, Alexander V.; Kazakov, Sergei A.; Johnston, Brian H.; et al. (August 2005). "The RNA World on Ice: A New Scenario for the Emergence of RNA Information". Journal of Molecular Evolution. 61 (2). Berlin: Springer-Verlag: 264–273. doi:10.1007/s00239-004-0362-7. ISSN 0022-2844. PMID 16044244.
- ^ Nussinov, Mark D.; Otroshchenko, Vladimir A.; Santoli, Salvatore (1997). "The emergence of the non-cellular phase of life on the fine-grained clayish particles of the early Earth's regolith". BioSystems. 42 (2–3). Amsterdam, the Netherlands: Elsevier: 111–118. doi:10.1016/S0303-2647(96)01699-1. ISSN 0303-2647. PMID 9184757.
- ^ a b Saladino, Raffaele; Crestini, Claudia; Pino, Samanta; et al. (March 2012). "Formamide and the origin of life". Physics of Life Reviews. 9 (1). Amsterdam, the Netherlands: Elsevier: 84–104. doi:10.1016/j.plrev.2011.12.002. ISSN 1571-0645. PMID 22196896.
- ^ a b Saladino, Raffaele; Botta, Giorgia; Pino, Samanta; et al. (July 2012). "From the one-carbon amide formamide to RNA all the steps are prebiotically possible". Biochimie. 94 (7). Amsterdam, the Netherlands: Elsevier: 1451–1456. doi:10.1016/j.biochi.2012.02.018. ISSN 0300-9084. PMID 22738728.
- ^ Oró, Joan (16 September 1961). "Mechanism of Synthesis of Adenine from Hydrogen Cyanide under Possible Primitive Earth Conditions". Nature. 191 (4794). London: Nature Publishing Group: 1193–1194. Bibcode:1961Natur.191.1193O. doi:10.1038/1911193a0. ISSN 0028-0836. PMID 13731264.
- ^ Basile, Brenda; Lazcano, Antonio; Oró, Joan (1984). "Prebiotic syntheses of purines and pyrimidines". Advances in Space Research. 4 (12). Amsterdam, the Netherlands: Elsevier: 125–131. doi:10.1016/0273-1177(84)90554-4. ISSN 0273-1177. PMID 11537766.
- ^ Orgel, Leslie E. (August 2004). "Prebiotic Adenine Revisited: Eutectics and Photochemistry". Origins of Life and Evolution of Biospheres. 34 (4). Kluwer Academic Publishers: 361–369. Bibcode:2004OLEB...34..361O. doi:10.1023/B:ORIG.0000029882.52156.c2. ISSN 0169-6149. PMID 15279171.
- ^ Robertson, Michael P.; Miller, Stanley L. (29 June 1995). "An efficient prebiotic synthesis of cytosine and uracil". Nature. 375 (6534). London: Nature Publishing Group: 772–774. Bibcode:1995Natur.375..772R. doi:10.1038/375772a0. ISSN 0028-0836. PMID 7596408.
- ^ Fox, Douglas (February 2008). "Did Life Evolve in Ice?". Discover. Waukesha, WI: Kalmbach Publishing. ISSN 0274-7529. Retrieved 3 July 2008.
- ^ Levy, Matthew; Miller, Stanley L.; Brinton, Karen; Bada, Jeffrey L. (June 2000). "Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions". Icarus. 145 (2). Amsterdam, the Netherlands: Elsevier: 609–13. Bibcode:2000Icar..145..609L. doi:10.1006/icar.2000.6365. ISSN 0019-1035. PMID 11543508.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ Menor-Salván, César; Ruiz-Bermejo, Marta; Guzmán, Marcelo I.; Osuna-Esteban, Susana; Veintemillas-Verdaguer, Sabino (20 April 2009). "Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases". Chemistry: A European Journal. 15 (17). Weinheim, Germany: Wiley-VCH on behalf of ChemPubSoc Europe: 4411–4418. doi:10.1002/chem.200802656. ISSN 0947-6539. PMID 19288488.
- ^ Roy, Debjani; Najafian, Katayoun; von Ragué Schleyer, Paul (30 October 2007). "Chemical evolution: The mechanism of the formation of adenine under prebiotic conditions". Proc. Natl. Acad. Sci. U.S.A. 104 (44). Washington, D.C.: National Academy of Sciences: 17272–17277. doi:10.1073/pnas.0708434104. ISSN 0027-8424. PMC 2077245. PMID 17951429.
- ^ a b Cleaves, H. James; Chalmers, John H.; Lazcano, Antonio; et al. (April 2008). "A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres". Origins of Life and Evolution of Biospheres. 38 (2). Dordrecht, the Netherlands: Springer: 105–115. Bibcode:2008OLEB...38..105C. doi:10.1007/s11084-007-9120-3. ISSN 0169-6149. PMID 18204914.
- ^ Chyba, Christopher F. (13 May 2005). "Rethinking Earth's Early Atmosphere". Science. 308 (5724). Washington, D.C.: American Association for the Advancement of Science: 962–963. doi:10.1126/science.1113157. ISSN 0036-8075. PMID 15890865.
- ^ Barton et al. 2007, pp. 93–95
- ^ Bada & Lazcano 2009, pp. 56–57
- ^ Bada, Jeffrey L.; Lazcano, Antonio (2 May 2003). "Prebiotic Soup--Revisiting the Miller Experiment" (PDF). Science. 300 (5620). Washington, D.C.: American Association for the Advancement of Science: 745–746. doi:10.1126/science.1085145. ISSN 0036-8075. PMID 12730584. Retrieved 13 June 2015.
- ^ Oró, Joan; Kimball, Aubrey P. (February 1962). "Synthesis of purines under possible primitive earth conditions: II. Purine intermediates from hydrogen cyanide". Archives of Biochemistry and Biophysics. 96 (2). Amsterdam, the Netherlands: Elsevier: 293–313. doi:10.1016/0003-9861(62)90412-5. ISSN 0003-9861. PMID 14482339.
- ^ Ahuja, Mukesh, ed. (2006). "Origin of Life". Life Science. Vol. 1. Delhi: Isha Books. p. 11. OCLC 297208106.
{{cite book}}: External link in|chapterurl=|ref=harv(help); Unknown parameter|chapterurl=ignored (|chapter-url=suggested) (help)[unreliable source?] - ^ Paul, Natasha; Joyce, Gerald F. (December 2004). "Minimal self-replicating systems". Current Opinion in Chemical Biology. 8 (6). Amsterdam, the Netherlands: Elsevier: 634–639. doi:10.1016/j.cbpa.2004.09.005. ISSN 1367-5931. PMID 15556408.
- ^ a b Bissette, Andrew J.; Fletcher, Stephen P. (2 December 2013). "Mechanisms of Autocatalysis". Angewandte Chemie International Edition. 52 (49). Weinheim, Germany: Wiley-VCH on behalf of the German Chemical Society: 12800–12826. doi:10.1002/anie.201303822. ISSN 1433-7851. PMID 24127341.
- ^ Kauffman 1993, chpt. 7
- ^ Dawkins 2004
- ^ Eigen & Schuster 1979
- ^ Hoffmann, G. W. (1974). "On the Origin of the Genetic Code and the Stability of the Translation Apparatus". J. Mol. Biol. 86 (2): 349–362. doi:10.1016/0022-2836(74)90024-2. PMID 4414916.
- ^ Orgel, L. (1963). "The Maintenance of the Accuracy of Protein Synthesis and its Relevance to Ageing". Proceedings of the National Academy of Sciences of the United States of America. 49 (4): 517–521. Bibcode:1963PNAS...49..517O. doi:10.1073/pnas.49.4.517. PMC 299893. PMID 13940312.
- ^ Hoffmann, G. W. (1975). Eyring, H. (ed.). "The Stochastic Theory of the Genetic Code". Annual Review of Physical Chemistry. 26: 123–144. Bibcode:1975ARPC...26..123H. doi:10.1146/annurev.pc.26.100175.001011.
- ^ Chaichian, Masud; Rojas, HugoPerez; Tureanu, Anca (2014). "12". Basic Concepts in Physics - Undergraduate Lecture Notes in Physics. Heidelberg: Springer. pp. 353–364. doi:10.1007/978-3-642-19598-3_12. ISBN 978-3-642-19597-6. Retrieved 26 May 2015.
- ^ Plasson, R; Kondepudi, DK; Bersini, H; Commeyras, A; Asakura, K (2007). "Emergence of homochirality in far-from-equilibrium systems: mechanisms and role in prebiotic chemistry". Chirality. 19 (8): 589–600. doi:10.1002/chir.20440. PMID 17559107.
- ^ Clark, S. (1999). "Polarised starlight and the handedness of Life". American Scientist. 97 (4): 336–43. Bibcode:1999AmSci..87..336C. doi:10.1511/1999.4.336.
- ^ Shibata, Takanori; Morioka, Hiroshi; Hayase, Tadakatsu; Choji, Kaori; Soai, Kenso (1996). "Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol". J. Am. Chem. Soc. 118 (2): 471–472. doi:10.1021/ja953066g.
- ^ Soai, K; Sato, I; Shibata, T (2001). "Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds". Chem Rec. 1 (4): 321–32. doi:10.1002/tcr.1017. PMID 11893072.
- ^ Hazen, Robert M. (2005). Genesis: the scientific quest for life's origin. Washington, D.C: Joseph Henry Press. ISBN 0-309-09432-1.
- ^ Mullen, L (5 September 2005). "Building Life from Star-Stuff". Astrobiology Magazine.
- ^ a b Chen, Irene A.; Walde, Peter (July 2010). "From Self-Assembled Vesicles to Protocells" (PDF). Cold Spring Harb Perspect Biol. 2 (7): a002170. doi:10.1101/cshperspect.a002170. PMC 2890201. PMID 20519344.
- ^ National Science Foundation (2013). "Exploring Life's Origins – Protocells". Retrieved 18 March 2014.
- ^ a b c Chen, Irene A. (8 December 2006). "The Emergence of Cells During the Origin of Life". Science. 314 (5805): 1558–1559. doi:10.1126/science.1137541.
- ^ a b Zimmer, Carl (26 June 2004). "What Came Before DNA?". Discover Magazine: 1–5.
- ^ Shapiro, Robert (12 February 2007). "A Simpler Origin for Life". Scientific American.
- ^ Chang, Thomas Ming Swi (2007). Artificial cells : biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation, cell/stem cell therapy. Hackensack, N.J.: World Scientific. ISBN 981-270-576-7.
- ^ Switek, Brian (13 February 2012). "Debate bubbles over the origin of life". Nature – News.
- ^ Grote, M (September 2011). "Jeewanu, or the 'particles of life'" (PDF). Journal of Biosciences. 36 (4): 563–570. doi:10.1007/s12038-011-9087-0. Archived from the original (PDF) on 23 March 2014.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Gupta, V. K.; Rai, R. K. (2013). "Histochemical localisation of RNA-like material in photochemically formed self-sustaining, abiogenic supramolecular assemblies 'Jeewanu'". Int. Res. J. of Science & Engineering. 1 (1): 1–4. ISSN 2322-0015.
- ^ Wimberly, BT; Brodersen, DE; Clemons, WM Jr; Morgan-Warren, RJ; Carter, AP; Vonrhein, C; Hartsch, T; Ramakrishnan, V (September 2000). "Structure of the 30S ribosomal subunit". Nature. 407 (6802): 327–39. doi:10.1038/35030006. PMID 11014182.
- ^ Zimmer, Carl (25 September 2014). "A Tiny Emissary From the Ancient Past". New York Times. Retrieved 26 September 2014.
- ^ Wade, Nicholas (4 May 2015). "Making Sense of the Chemistry That Led to Life on Earth". New York Times. Retrieved 10 May 2015.
- ^ Pater, Bhavesh H.; Percivalle, Claudia; Ritson, Dougal J.; Duffy, Colm D.; Sutherland, John D. (16 March 2015). "Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism". Nature Chemistry. 7: 301–307. doi:10.1038/nchem.2202. Retrieved 10 May 2015.
- ^ Yarus, M (2011). "Getting past the RNA world: the initial Darwinian ancestor". Cold Spring Harb Perspect Biol. 3 (4): a003590. doi:10.1101/cshperspect.a003590. PMC 3062219. PMID 20719875.
- ^ Neveu, Marc; Kim, Hyo-Joong; Benner, Steven A. (22 April 2013). "The 'Strong' RNA World Hypothesis: Fifty Years Old". Astrobiology. 13 (4). New Rochelle, NY: Mary Ann Liebert, Inc.: 391–403. doi:10.1089/ast.2012.0868. ISSN 1531-1074. PMID 23551238.
{{cite journal}}: Invalid|ref=harv(help) - ^ Gilbert, Walter (20 February 1986). "Origin of life: The RNA world". Nature. 319 (6055): 618–618. Bibcode:1986Natur.319..618G. doi:10.1038/319618a0.
- ^ Noller, HF (2012). "Evolution of protein synthesis from an RNA world". Cold Spring Harb Perspect Biol. 4 (4): a003681. doi:10.1101/cshperspect.a003681. PMC 3312679. PMID 20610545.
- ^ a b Koonin, Eugene V. (31 May 2007). "The cosmological model of eternal inflation and the transition from chance to biological evolution in the history of life". Biol. Direct. p. 15. doi:10.1186/1745-6150-2-15. PMC 1892545.
{{cite web}}: CS1 maint: unflagged free DOI (link) - ^ a b c Zimmer, Carl (12 September 2013). "A Far-Flung Possibility for the Origin of Life". New York Times.
- ^ a b c Webb, Richard (29 August 2013). "Primordial broth of life was a dry Martian cup-a-soup". New Scientist.
- ^ Ma, W; Yu, C; Zhang, W; Hu, J (November 2007). "Nucleotide synthetase ribozymes may have emerged first in the RNA world". RNA. 13 (11): 2012–9. doi:10.1261/rna.658507. PMC 2040096. PMID 17878321.
- ^ Orgel, L. (1994). "The origin of life on Earth". Scientific American. 271 (4): 81. doi:10.1038/scientificamerican1094-76. PMID 7524147.
- ^ Johnston, W. K.; et al. (2001). "RNA-Catalyzed RNA Polymerization: Accurate and General RNA-Templated Primer Extension". Science. 292 (5520): 1319–25. Bibcode:2001Sci...292.1319J. doi:10.1126/science.1060786. PMID 11358999.
- ^ Szostak, Jack W. (4 June 2008). "The Origins of Function in Biological Nucleic Acids, Proteins, and Membranes". HHMI.
- ^ Lincoln, Tracey A.; Joyce, Gerald F. (8 January 2009). "Self-Sustained Replication of an RNA Enzyme". Science. 323 (5918): 1229–32. Bibcode:2009Sci...323.1229L. doi:10.1126/science.1167856. PMC 2652413. PMID 19131595.
- ^ a b Joyce, GF (2009). "Evolution in an RNA world". Cold Spring Harb Symp Quant Biol. 74: 17–23. doi:10.1101/sqb.2009.74.004. PMC 2891321. PMID 19667013.
- ^ a b Bernstein, H; Byerly, HC; Hopf, FA; Michod, RA; Vemulapalli, GK (1983). "The Darwinian Dynamic". Quarterly Review of Biology. 58: 185–207. doi:10.1086/413216.
- ^ a b Michod, RE (1999). "Darwinian Dynamics: Evolutionary Transitions in Fitness and Individuality". Princeton, New Jersey: Princeton University Press. ISBN 978-0-691-05011-9.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Orgel, Leslie (2000). "A Simpler Nucleic Acid". Science. 290 (5495): 1306–7. doi:10.1126/science.290.5495.1306. PMID 11185405.
- ^ Nelson, K. E.; Levy, M.; Miller, S. L. (2000). "Peptide nucleic acids rather than RNA may have been the first genetic molecule". PNAS. 97 (8): 3868–71. Bibcode:2000PNAS...97.3868N. doi:10.1073/pnas.97.8.3868. PMC 18108. PMID 10760258.
- ^ Larralde, R.; Robertson, M. P.; Miller, S. L. (1995). "Rates of Decomposition of Ribose and Other Sugars: Implications for Chemical Evolution". PNAS. 92 (18): 8158–60. Bibcode:1995PNAS...92.8158L. doi:10.1073/pnas.92.18.8158. PMC 41115. PMID 7667262.
- ^ Lindahl, Tomas (1993). "Instability and decay of the primary structure of DNA". Nature. 362 (6422): 709–15. Bibcode:1993Natur.362..709L. doi:10.1038/362709a0. PMID 8469282.
- ^ Anastasi, C; Crowe, MA; Powner, MW; Sutherland, JD (2006). "Direct Assembly of Nucleoside Precursors from Two- and Three-Carbon Units". Angewandte Chemie International Edition. 45 (37): 6176–9. doi:10.1002/anie.200601267. PMID 16917794.
- ^ Powner, MW; Sutherland, JD (2008). "Potentially Prebiotic Synthesis of Pyrimidine β-D-Ribonucleotides by Photoanomerization/Hydrolysis of α-D-Cytidine-2-Phosphate". ChemBioChem. 9 (15): 2386–7. doi:10.1002/cbic.200800391. PMID 18798212.
- ^ a b Powner, MW; Gerland, B; Sutherland, JD (May 2009). "Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions". Nature. 459 (7244): 239–42. Bibcode:2009Natur.459..239P. doi:10.1038/nature08013. PMID 19444213.
- ^ a b c "Metabolism May Have Started in Early Oceans Before the Origin of Life". Wellcome Trust. Astrobiology Web. 25 April 2014. Retrieved 26 April 2014.
- ^ "Evolutionary Epistemology and Sir Karl Popper's Latest Intellectual Interest: A First-Hand Report". Tkpw.net. Retrieved 13 August 2012.
- ^ "Amateur Shakes Up Ideas on Recipe for Life". The New York Times. 22 April 1997.
- ^ Popper, K. (1990). "Pyrite and the origin of life". Nature. 344 (6265): 387. doi:10.1038/344387a0.
letters to the editor
- ^ Huber, C.; Wächtershäuser, G. (1998). "Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: implications for the origin of life". Science. 281 (5377): 670–2. Bibcode:1998Sci...281..670H. doi:10.1126/science.281.5377.670. PMID 9685253.
- ^ a b Lane, Nick (2010). Life Ascending: the 10 great inventions of evolution.
- ^ Musser, George (23 September 2011). "How Life Arose on Earth, and How a Singularity Might Bring It Down". Scientific American.
- ^ Carroll, Sean (10 March 2010). "Free Energy and the Meaning of Life".
- ^ "A new physics theory of life". Quanta Magazine. Simonds Foundation.
- ^ England, J (April 2013). "Statistical Physics of Self Replication" (PDF). J. Chem. Phys. 139: 121923. doi:10.1063/1.4818538.
- ^ Orgel, LE (November 2000). "Self-organizing biochemical cycles". Proceedings of the National Academy of Sciences of the United States of America. 97 (23): 12503–7. Bibcode:2000PNAS...9712503O. doi:10.1073/pnas.220406697. PMC 18793. PMID 11058157.
- ^ a b Mulkidjanian, A. Y. (2009). "On the origin of life in the zinc world: 1. Photosynthesizing, porous edifices built of hydrothermally precipitated zinc sulfide as cradles of life on Earth". Biol Direct. 4: 1–39. doi:10.1186/1745-6150-4-26.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wächtershäuser, G. (1988). "Before enzymes and templates: theory of surface metabolism". Microbiol Rev. 52 (4): 452–84. PMC 373159. PMID 3070320.
- ^ Mulkidjanian, A. Y.; Galperin, M. Y. (2009). "On the origin of life in the zinc world. 2. Validation of the hypothesis on the photosynthesizing zinc sulfide edifices as cradles of life on Earth". Biol Direct. 4: 1–27. doi:10.1186/1745-6150-4-27.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Macallum, A. B. (1926). "The Paleochemistry of the body fluids and tissues". Physiol. Rev. 6: 316–357.
- ^ Mulkidjanian, A. Y.; Bychkov, A. Y.; Dibrova, D. V.; Galperin, M. Y.; Koonin, E. V. (2012). "Origin of first cells at terrestrial, anoxic geothermal fields". Proceedings of the National Academy of Sciences of the United States of America. 109 (14): E821–30. Bibcode:2012PNAS..109E.821M. doi:10.1073/pnas.1117774109. PMC 3325685. PMID 22331915.
- ^ For a deeper integrative version of this hypothesis see Egel, R. (2011). Lankenau, D.-H.; Mulkidjanian,, A. Y. (eds.). Origins of Life: The Primal Self-Organization. Springer. ISBN 978-3-642-21625-1.
{{cite book}}: CS1 maint: extra punctuation (link), in particular Lankenau, D.-H. (2011). "Two RNA Worlds: Toward the Origin of Replication, Genes, Recombination and Repair". Origins of Life: The Primal Self-Organization. Springer. pp. 225–286. ISBN 978-3-642-21625-1., interconnecting the "Two RNA worlds" concept and other detailed aspects; and Davidovich, C.; Belousoff, M.; Bashan, A.; Yonath, A. (2009). "The evolving ribosome: from non-coded peptide bond formation to sophisticated translation machinery". Res Microbiol. 160 (7): 487–492. doi:10.1016/j.resmic.2009.07.004. - ^ Schirber, Michael (24 June 2014). "Hydrothermal Vents Could Explain Chemical Precursors to Life". AstroBioNet. NASA Astrobiology. Retrieved 13 August 2014.
- ^ Martin, William; Russell, Michael J. (2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Philosophical Transactions of the Royal Society B. 358 (1429): 59–83, discussion 83–5. doi:10.1098/rstb.2002.1183. PMC 1693102. PMID 12594918.
- ^ Ignatov, I.; Mosin, O.V. (2013). "Possible Processes for Origin of Life and Living Matter with modeling of Physiological Processes of Bacterium Bacillus Subtilis in Heavy Water as Model System". Journal of Natural Sciences Research. 9 (3): 65–76.
- ^ Calvin, M. (1969). Chemical Evolution. Oxford: Clarendon. pp. 1–278.
- ^ Ward, D. (2010). "First fossil-makers in hot water". Astrobiology magazine.
- ^ Kurihara, K.; Tamura, M.; Shohda, K.; Toyota, T.; Suzuki, K.; Sugawara, T. (2011). "Self-Reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA". Nature Chemistry. 10 (4): 775–781. doi:10.1038/nchem.1127.
- ^ Chemistry of seabed’s hot vents could explain emergence of life. Astrobiology Magazine 27 April 2015.
- ^ "Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions. (PDF) Nora H. de Leeuw, et. al. Chemical Communications, 2015, 51, 7501-7504. DOI: 10.1039/C5CC02078F. 24 March 2015.
- ^ Muller, A.W.J. (1985). "Thermosynthesis by biomembranes: energy gain from cyclic temperature changes". Journal of Theoretical Biology. 115 (3): 429–53. doi:10.1016/S0022-5193(85)80202-2. PMID 3162066.
- ^ Muller, A.W.J. (1995). "Were the first organisms heat engines? A new model for biogenesis and the early evolution of biological energy conversion". Progress in Biophysics and Molecular Biology. 63 (2): 193–231. doi:10.1016/0079-6107(95)00004-7. PMID 7542789.
- ^ Dyson 1999
- ^ Muller, A.W.J.; Schulze-Makuch, D. (2006). "Sorption heat engines: simple inanimate negative entropy generators". Physica A. 362 (2): 369–381. arXiv:physics/0507173. Bibcode:2006PhyA..362..369M. doi:10.1016/j.physa.2005.12.003.
- ^ Orgel, L. (1987). "Evolution of the genetic apparatus: a review". Cold Spring Harbor Symposia on Quantitative Biology. 52: 9–16. doi:10.1101/sqb.1987.052.01.004. PMID 2456886.
- ^ Clay-armored bubbles may have formed first protocells: Minerals could have played a key role in the origins of life February 7, 2011. Harvard University.
- ^ Dawkins, Richard (1996) [1986]. The Blind Watchmaker. New York: W. W. Norton & Company, Inc. pp. 148–161. ISBN 0-393-31570-3.
- ^ Huang, W; Ferris, JP (July 2006). "One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis". J. Am. Chem. Soc. 128 (27): 8914–9. doi:10.1021/ja061782k. PMID 16819887.
- ^ Bullard, T; Freudenthal, J; Avagyan, S; Kahr, B (2007). "Test of Cairns-Smith's crystals-as-genes hypothesis". Faraday Discuss. 136: 231–45. Bibcode:2007FaDi..136..231B. doi:10.1039/b616612c.
- ^ Moore, Caroline (16 July 2007). "Crystals as genes?". Chemical Science.
- ^ "Tough Earth bug may be from Mars". New Scientist. 25 September 2002.
- ^ "Exobiology and Radiation Assembly (ERA)". ESA. NASA. 1992.
- ^ Horneck, G.; Klaus, D. M.; Mancinelli, R. L. (2010). "Space Microbiology". Microbiology and Molecular Biology Reviews. 74 (1): 121–56. doi:10.1128/MMBR.00016-09. PMC 2832349. PMID 20197502.
- ^ Paul Clancy (23 June 2005). Looking for Life, Searching the Solar System. Cambridge University Press.[page needed]
- ^ Rabbow, Elke; Horneck, Gerda; Rettberg, Petra; Schott, Jobst-Ulrich; Panitz, Corinna; L'Afflitto, Andrea; von Heise-Rotenburg,, Ralf; Willnecker, Reiner; Baglioni, Pietro; Hatton,, Jason; Dettmann, Jan; Demets, René; Reitz, Günther (9 July 2009). "EXPOSE, an Astrobiological Exposure Facility on the International Space Station – from Proposal to Flight". Orig Life Evol Biosph. 39 (6): 581–98. Bibcode:2009OLEB...39..581R. doi:10.1007/s11084-009-9173-6. PMID 19629743.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Onofri, Silvano; de la Torre, Rosa; de Vera, Jean-Pierre; Ott, Sieglinde; Zucconi, Laura; Selbmann, Laura; Scalzi, Giuliano; Venkateswaran, Kasthuri J.; Rabbow,, Elke; Sánchez Iñigo, Francisco J.; Horneck, Gerda (May 2012). "Survival of Rock-Colonizing Organisms After 1.5 Years in Outer Space". Astrobiology. 12 (5): 508–516. Bibcode:2012AsBio..12..508O. doi:10.1089/ast.2011.0736. PMID 22680696.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Amos, Jonathan (23 August 2010). "Beer microbes live 553 days outside ISS". Science and Technology. BBC News.
- ^ "Biological Abundance of Elements". The Internet Encyclopedia of Science. Retrieved 9 October 2008.
- ^ a b c d e Hoover, Rachel (21 February 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". NASA.
- ^ Chang, Kenneth (18 August 2009). "From a Distant Comet, a Clue to Life". Space & Cosmos. New York Times. p. A18.
- ^ V. V. Goncharuk and O. V. Zui. "Water and carbon dioxide as the main precursors of organic matter on Earth and in space." Journal of Water Chemistry and Technology. February 2015, Volume 37, Issue 1, pp 2-3 Date: 19 March 2015.
- ^ "Laboratory experimental simulations: Chemicalevolution of the organic matter from interstellar and cometary ice analogs". Bulletin de la Société Royale des Sciences de Liège,. 84: 21–32. 2015. Retrieved 6 April 2015.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ a b Gallori, Enzo (November 2010). "Astrochemistry and the origin of genetic material". Rendiconti Lincei. 22 (2): 113–118. doi:10.1007/s12210-011-0118-4.
- ^ Martins, Zita (February 2011). "Organic Chemistry of Carbonaceous Meteorites". Elements. 7 (1): 35–40. doi:10.2113/gselements.7.1.35.
- ^ Martins, Zita; Botta, Oliver; Fogel, Marilyn L.; Sephton, Mark A.; Glavin, Daniel P.; Watson, Jonathan S.; Dworkin, Jason P.; Schwartz, Alan W.; Ehrenfreund, Pascale (15 June 2008). "Extraterrestrial nucleobases in the Murchison meteorite". Earth and Planetary Science Letters. 270 (1–2): 130–136. arXiv:0806.2286. Bibcode:2008E&PSL.270..130M. doi:10.1016/j.epsl.2008.03.026.
- ^ AFP Staff (20 August 2009). "We may all be space aliens: study". AFP.
- ^ Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzica, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. (11 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". PNAS. doi:10.1073/pnas.1106493108.
- ^ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA.
- ^ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily.
- ^ a b Chow, Denise (26 October 2011). "Discovery: Cosmic Dust Contains Organic Matter from Stars". Space.com.
- ^ ScienceDaily Staff (26 October 2011). "Astronomers Discover Complex Organic Matter Exists Throughout the Universe". ScienceDaily.
- ^ Kwok, Sun; Zhang, Yong (26 October 2011). "Mixed aromatic–aliphatic organic nanoparticles as carriers of unidentified infrared emission features". Nature. 479 (7371): 80–3. Bibcode:2011Natur.479...80K. doi:10.1038/nature10542. PMID 22031328.
- ^ a b Clemence, Lara (7 February 2005). "Space Sugar's a Sweet Find". Goddard Space Flight Center. NASA.
- ^ Than, Ker (29 August 2012). "Sugar Found in Space". National Geographic.
- ^ Staff (29 August 2012). "Sweet! Astronomers spot sugar molecule near star". Associated Press.
- ^ "Building blocks of life found around young star". Retrieved 11 December 2013.
- ^ Jørgensen, J. K.; Favre; Bisschop; Bourke; Van Dishoeck; Schmalzl; Favre, C.; Bisschop, S.; Bourke, T.; Dishoeck, E.; Schmalzl, M. (2012). "Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA" (PDF). The Astrophysical Journal Letters. eprint. 757: L4. arXiv:1208.5498. Bibcode:2012ApJ...757L...4J. doi:10.1088/2041-8205/757/1/L4.
- ^ "'Life chemical' detected in comet". BBC News. 18 August 2009.
- ^ Thompson, WR; Murray, BG; Khare, BN; Sagan, C (December 1987). "Coloration and darkening of methane clathrate and other ices by charged particle irradiation: applications to the outer solar system". Journal of geophysical research. 92 (A13): 14933–47. Bibcode:1987JGR....9214933T. doi:10.1029/JA092iA13p14933. PMID 11542127.
- ^ Stark, Anne M. (20 June 2013). "Life on Earth shockingly comes from out of this world". Lawrence Livermore National Laboratory. Retrieved 5 August 2014.
- ^ Goldman, Nir; Tamblyn, Isaac (2013). "Prebiotic Chemistry within a Simple Impacting Icy Mixture". The Journal of Physical Chemistry. 117 (24): 5124–31. doi:10.1021/jp402976n.
- ^ a b c Carey, Bjorn (18 October 2005). "Life's Building Blocks 'Abundant in Space'". Space.com.
- ^ a b c Hudgins, Douglas M.; Bauschlicher,Jr, Charles W.; Allamandola, L. J. (10 October 2005). "Variations in the Peak Position of the 6.2 μm Interstellar Emission Feature: A Tracer of N in the Interstellar Polycyclic Aromatic Hydrocarbon Population". Astrophysical Journal. 632 (1): 316–332. Bibcode:2005ApJ...632..316H. doi:10.1086/432495.
- ^ a b c Allamandola; Louis; et al. (13 April 2011). "Cosmic Distribution of Chemical Complexity". NASA.
- ^ García-Hernández, D. A.; Manchado, A.; García-Lario, P.; Stanghellini, L.; Villaver, E.; Shaw, R. A.; Szczerba, R.; Perea-Calderón, J. V. (28 October 2010). "Formation of Fullerenes in H-Containing Planetary Nebulae". The Astrophysical Journal Letters. 724: L39–L43. arXiv:1009.4357. Bibcode:2010ApJ...724L..39G. doi:10.1088/2041-8205/724/1/L39.
- ^ Atkinson, Nancy (27 October 2010). "Buckyballs Could Be Plentiful in the Universe". Universe Today.
- ^ Staff (3 April 2013). "NASA team investigates complex chemistry at Titan". Phys.Org.
- ^ a b Grotzinger, John P. (24 January 2014). "Introduction to Special Issue – Habitability, Taphonomy, and the Search for Organic Carbon on Mars". Science. 343 (6169): 386–387. Bibcode:2014Sci...343..386G. doi:10.1126/science.1249944.
- ^ "Special Issue – Table of Contents – Exploring Martian Habitability". Science. 343 (6169): 345–452. 24 January 2014.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Special Collection – Curiosity – Exploring Martian Habitability". Science. 24 January 2014.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Grotzinger, J.P.; et al. (24 January 2014). "A Habitable Fluvio-Lacustrine Environment at Yellowknife Bay, Gale Crater, Mars". Science. 343 (6169): 1242777. doi:10.1126/science.1242777.
- ^ Science Daily. "Did Mars once have a nitrogen cycle?".
- ^ David Szong,GizMag. "Curiosity rover finds nitrogen on Mars".
- ^ Marlaire, Ruth (3 March 2015). "NASA Ames Reproduces the Building Blocks of Life in Laboratory". NASA. Retrieved 5 March 2015.
- ^ "Origin of Life at the Weizmann Institute". Ool.weizmann.ac.il. 6 January 2008.
- ^ Segré, D.; Ben-Eli, D.; Deamer, D; Lancet, D. (February–April 2001). "The Lipid World" (PDF). Origins of Life and Evolution of Biospheres. 31 (1–2): 119–45. doi:10.1023/A:1006746807104. PMID 11296516. Retrieved 11 September 2008.
- ^ Chen, I. A.; Walde, P (2 June 2010). "From Self-Assembled Vesicles to Protocells". Cold Spring Harbor Perspectives in Biology. 2 (7): a002170–a002170. doi:10.1101/cshperspect.a002170. PMC 2890201. PMID 20519344.
- ^ Eigen, Manfred; Schuster, Peter (November 1977). "The Hypercycle. A Principle of Natural Self-Organization. Part A: Emergence of the Hypercycle" (PDF). Naturwissenschaften. 64 (11). Berlin: Springer-Verlag: 541–565. ISSN 0028-1042. PMID 593400. Retrieved 13 June 2015.
- Eigen, Manfred; Schuster, Peter (1978). "The Hypercycle. A Principle of Natural Self-Organization. Part B: The Abstract Hypercycle" (PDF). Naturwissenschaften. 65. Berlin: Springer-Verlag: 7–41. ISSN 0028-1042. Retrieved 13 June 2015.
- Eigen, Manfred; Schuster, Peter (July 1978). "The Hypercycle. A Principle of Natural Self-Organization. Part C: The Realistic Hypercycle" (PDF). Naturwissenschaften. 65 (7). Berlin: Springer-Verlag: 341–369. ISSN 0028-1042. Retrieved 13 June 2015.
- ^ Markovitch, O.; Lancet, D. (2012). "Excess Mutual Catalysis Is Required for Effective Evolvability". Artificial Life. 18 (3): 243–266. doi:10.1162/artl_a_00064. PMID 22662913.
- ^ Tessera, Marc (1 June 2011). "Origin of Evolution versus Origin of Life: A Shift of Paradigm". International Journal of Molecular Sciences. 12 (6): 3445–3458. doi:10.3390/ijms12063445. PMC 3131571. PMID 21747687.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Brown, MR; Kornberg, A (November 2004). "Inorganic polyphosphate in the origin and survival of species". Proceedings of the National Academy of Sciences of the United States of America. 101 (46): 16085–7. Bibcode:2004PNAS..10116085B. doi:10.1073/pnas.0406909101. PMC 528972. PMID 15520374.
- ^ "The Origin of Life". Archived from the original on 2 October 2000.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Pasek, MA (January 2008). "Rethinking early Earth phosphorus geochemistry". Proceedings of the National Academy of Sciences of the United States of America. 105 (3): 853–8. Bibcode:2008PNAS..105..853P. doi:10.1073/pnas.0708205105. PMC 2242691. PMID 18195373.
- ^ Witt, AN; Vijh, UP; Gordon, KD (2003). "Discovery of Blue Fluorescence by Polycyclic Aromatic Hydrocarbon Molecules in the Red Rectangle". Bulletin of the American Astronomical Society. 35: 1381. Bibcode:2003AAS...20311017W. Archived from the original on 19 December 2003.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ García-Hernández, D. A.; Manchado, A.; García-Lario, P.; Stanghellini, L.; Villaver, E.; Shaw, R. A.; Szczerba, R.; Perea-Calderón, J. V. (28 October 2010). "Formation of Fullerenes in H-Containing Planetary Nebulae". The Astrophysical Journal Letters. 724: L39. arXiv:1009.4357. Bibcode:2010ApJ...724L..39G. doi:10.1088/2041-8205/724/1/L39.
- ^ a b Staff (20 September 2012). "NASA Cooks Up Icy Organics to Mimic Life's Origins". Space.com.
- ^ a b Gudipati, Murthy S.; Yang, Rui (1 September 2012). "In-Situ Probing of Radiation-Induced Processing of Organics in Astrophysical Ice Analogs—Novel Laser Desorption Laser Ionization Time-Of-Flight Mass Spectroscopic Studies". The Astrophysical Journal Letters. 756 (1): L24. Bibcode:2012ApJ...756L..24G. doi:10.1088/2041-8205/756/1/L24.
- ^ The PAH IR Spectral Database
- ^ Hoover, Rachel (24 February 2014). "Online Database Tracks Organic Nano-Particles Across the Universe". Sci Tech Daily. Retrieved 10 March 2015.
- ^ Dartnell, Lewis (12 January 2008). "Life's a beach on planet Earth". New Scientist.
- ^ Adam, Zachary (2007). "Actinides and Life's Origins". Astrobiology. 7 (6): 852–72. Bibcode:2007AsBio...7..852A. doi:10.1089/ast.2006.0066. PMID 18163867.
- ^ Parnell, John (2004). "Mineral Radioactivity in Sands as a Mechanism for Fixation of Organic Carbon on the Early Earth" (PDF). Origins of Life and Evolution of Biospheres. 34 (6): 533–547. Bibcode:2004OLEB...34..533P. doi:10.1023/B:ORIG.0000043132.23966.a1.
- ^ Michaelian, Karo (2009). "Thermodynamic Function of Life". arXiv:0907.0040 [physics.gen-ph].
- ^ Michaelian, Karo (2011). "Biological Catalysis of the Hydrological Cycle: Life's Thermodynamic Function" (PDF). Hydrol. Earth Syst. Sci. Discuss. 8: 1093–1123. Bibcode:2011HESSD...8.1093M. doi:10.5194/hessd-8-1093-2011.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Michaelian, Karo (2010). "Thermodynamic Origin of Life" (PDF). Earth Syst. Dynam. Discuss. 1: 1–39. doi:10.5194/esdd-1-1-2010.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Michaelian, Karo (2011). "Thermodynamic Dissipation Theory for the Origin of Life" (PDF). Earth Syst. Dynam. 2: 37–51. Bibcode:2011ESD.....2...37M. doi:10.5194/esd-2-37-2011.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cnossen, I.; et al. (2007). "The habitat of early life: Solar X-ray and UV radiation at Earth's surface 4-3.5 billion years ago". J. Geophys. Research. 112: E02008. arXiv:astro-ph/0702529. Bibcode:2007JGRE..11202008C. doi:10.1029/2006JE002784.
- ^ Sagan, Carl (1973). "Ultraviolet Selection Pressure on the Earliest Organisms". J. Theor. Biol. 39: 195–200. doi:10.1016/0022-5193(73)90216-6.
- ^ Cnossen, I.; Sanz-Forcada, J.; Favata, F.; Witasse, O.; Zegers, T.; Arnold, N.F. (2007). "The habitat of early life: Solar X-ray and UV radiation at Earth's surface 4–3.5 billion years ago". J. Geophys. Res. 112: E02008. Bibcode:2007JGRE..112.2008C. doi:10.1029/2006JE002784.
- ^ Michaelian, K.; Simeonov, A. "Fundamental Molecules of Life are Pigments which Arose and Evolved to Dissipate the Solar Spectrum". ArXiv. Retrieved 17 September 2014.
- ^ Michaelian, Karo (2013). "A non-linear irreversible thermodynamic perspective on organic pigment proliferation and biological evolution". J. Phys. Conf. Ser. 475: 012010. doi:10.1088/1742-6596/475/1/012010.
- ^ Knauth, L.P. (1992). .: Isotopic Signatures and Sedimentary Records, in: Lecture Notes in Earth Sciences #43. Berlin: Springer-Verlag. pp. 123–152.
- ^ Knauth, L.P; Lowe, D.R. (2003). "High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland group, South Africa". Geol. Soc. Am. Bull. 115: 566–580. doi:10.1130/0016-7606(2003)115<0566:hactif>2.0.co;2.
- ^ Lowe, Donald R.; Tice, Michael M. (2004). "Geologic evidence for Archean atmospheric and climatic evolution: Fluctuating levels of CO2, CH4, and O2 with an overriding tectonic control". Geology. 32 (6): 493–496. Bibcode:2004Geo....32..493L. doi:10.1130/G20342.1.
- ^ Michaelian, K.; Santillán Padilla, N. "DNA Denaturing through UV-C Photon Dissipation: A Possible Route to Archean Non-enzymatic Replication". ResearchGate. Retrieved 17 September 2014.
- ^ Michaelian, Karo; Santillán Padilla, Norberto. "DNA Denaturing through UV-C Photon Dissipation: A Possible Route to Archean Non-enzymatic Replication". BioRxiv. Cold Spring Harbor Labs Journals. doi:10.1101/009126. Retrieved 23 December 2014.
{{cite web}}:|archive-date=requires|archive-url=(help) - ^ Michaelian, Karo (2009). "Thermodynamic Origin of Life". arXiv:0907.0042 [physics.gen-ph].
- ^ Michaelian, Karo (2010). "Homochirality Through Photon-Induced Melting of RNA/DNA: Thermodynamic Dissipation Theory Of The Origin Of Life" (PDF). WebmedCentral Biochemistry. 1: WMC00924.
- ^ Davies, P (19 November 2007). "Are Aliens Among Us?". Scientific American.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Hartman, Hyman (October 1998). Photosynthesis and the Origin of Life. Vol. 28. pp. 4–6.
{{cite book}}:|work=ignored (help)
Bibliography
- Altermann, Wladyslaw (2009). "From Fossils to Astrobiology – A Roadmap to Fata Morgana?". In Seckbach, Joseph; Walsh, Maud (eds.). From Fossils to Astrobiology: Records of Life on Earth and the Search for Extraterrestrial Biosignatures. Cellular Origin, Life in Extreme Habitats and Astrobiology. Vol. 12. Dordrecht, the Netherlands; London: Springer Science+Business Media. ISBN 978-1-4020-8836-0. LCCN 2008933212.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|ref=harv(help); Unknown parameter|chapterurl=ignored (|chapter-url=suggested) (help) - Bada, Jeffrey L.; Lazcano, Antonio (2009). "The Origin of Life". In Ruse, Michael; Travis, Joseph (eds.). Evolution: The First Four Billion Years. Foreword by Edward O. Wilson. Cambridge, MA: Belknap Press of Harvard University Press. ISBN 978-0-674-03175-3. LCCN 2008030270. OCLC 225874308.
{{cite book}}: Invalid|ref=harv(help) - Barton, Nicholas H.; Briggs, Derek E. G.; Eisen, Jonathan A.; et al. (2007). Evolution. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-684-9. LCCN 2007010767. OCLC 86090399.
{{cite book}}: Invalid|ref=harv(help) - Bastian, H. Charlton (1871). The Modes of Origin of Lowest Organisms. London; New York: Macmillan and Company. LCCN 11004276. OCLC 42959303. Retrieved 6 June 2015.
{{cite book}}: Invalid|ref=harv(help) - Bernal, J. D. (1951). The Physical Basis of Life. London: Routledge & Kegan Paul. LCCN 51005794.
{{cite book}}: Invalid|ref=harv(help) - Bernal, J. D. (1960). "The Problem of Stages in Biopoesis". In Florkin, M. (ed.). Aspects of the Origin of Life. International Series of Monographs on Pure and Applied Biology. Oxford, UK; New York: Pergamon Press. ISBN 978-1-4831-3587-8. LCCN 60013823.
{{cite book}}: Invalid|ref=harv(help) - Bernal, J. D. (1967) [Reprinted work by A. I. Oparin originally published 1924; Moscow: The Moscow Worker]. The Origin of Life. The Weidenfeld and Nicolson Natural History. Translation of Oparin by Ann Synge. London: Weidenfeld & Nicolson. LCCN 67098482.
{{cite book}}: Invalid|ref=harv(help) - Bock, Gregory R.; Goode, Jamie A., eds. (1996). Evolution of Hydrothermal Ecosystems on Earth (and Mars?). Ciba Foundation Symposium. Vol. 202. Chichester, UK; New York: John Wiley & Sons. ISBN 0-471-96509-X. LCCN 96031351.
{{cite book}}: Invalid|ref=harv(help) - Bondeson, Jan (1999). The Feejee Mermaid and Other Essays in Natural and Unnatural History. Ithaca, NY: Cornell University Press. ISBN 0-8014-3609-5. LCCN 98038295.
{{cite book}}: Invalid|ref=harv(help) - Bryson, Bill (2004). A Short History of Nearly Everything. London: Black Swan. ISBN 978-0-552-99704-1. OCLC 55589795.
{{cite book}}: Invalid|ref=harv(help) - Dalrymple, G. Brent (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". In Lewis, C. L. E.; Knell, S. J. (eds.). The Age of the Earth: from 4004 BC to AD 2002. Geological Society Special Publication. Vol. 190. London: Geological Society of London. Bibcode:2001GSLSP.190..205D. doi:10.1144/gsl.sp.2001.190.01.14. ISBN 1-86239-093-2. ISSN 0305-8719. LCCN 2003464816. OCLC 48570033.
{{cite book}}: Invalid|ref=harv(help) - Darwin, Charles (1887). Darwin, Francis (ed.). The Life and Letters of Charles Darwin, Including an Autobiographical Chapter. Vol. 3 (3rd ed.). London: John Murray. OCLC 834491774.
{{cite book}}: Invalid|ref=harv(help) - Davies, Geoffrey F. (2007). "Chapter 2.3 Dynamics of the Hadean and Archaean Mantle". In van Kranendonk, Martin J.; Smithies, R. Hugh; Bennett, Vickie C. (eds.). Earth's Oldest Rocks. Developments in Precambrian Geology. Vol. 15. Amsterdam, the Netherlands; Boston: Elsevier. doi:10.1016/S0166-2635(07)15023-4. ISBN 978-0-444-52810-0. LCCN 2009525003.
{{cite book}}: Invalid|ref=harv(help) - Davies, Paul (1999). The Fifth Miracle: The Search for the Origin of Life. London: Penguin Books. ISBN 0-14-028226-2.
{{cite book}}: Invalid|ref=harv(help) - Dawkins, Richard (2004). The Ancestor's Tale: A Pilgrimage to the Dawn of Evolution. Boston, MA: Houghton Mifflin. ISBN 0-618-00583-8. LCCN 2004059864. OCLC 56617123.
{{cite book}}: Invalid|ref=harv(help) - Dobell, Clifford (1960) [Originally published 1932; New York: Harcourt, Brace & Company]. Antony van Leeuwenhoek and His 'Little Animals'. New York: Dover Publications. LCCN 60002548.
{{cite book}}: Invalid|ref=harv(help) - Dyson, Freeman (1999). Origins of Life (Revised ed.). Cambridge, UK; New York: Cambridge University Press. ISBN 0-521-62668-4. LCCN 99021079.
{{cite book}}: Invalid|ref=harv(help) - Eigen, M.; Schuster, P. (1979). The Hypercycle: A Principle of Natural Self-Organization. Berlin; New York: Springer-Verlag. ISBN 0-387-09293-5. LCCN 79001315. OCLC 4665354.
{{cite book}}: Invalid|ref=harv(help) - Fesenkov, V. G. (1959). "Some Considerations about the Primaeval State of the Earth". In Oparin, A. I.; et al. (eds.). The Origin of Life on the Earth. I.U.B. Symposium Series. Vol. 1. Edited for the International Union of Biochemistry by Frank Clark and R. L. M. Synge (English-French-German ed.). London; New York: Pergamon Press. ISBN 978-1-4832-2240-0. LCCN 59012060. Retrieved 3 June 2015.
{{cite book}}: Invalid|ref=harv(help) International Symposium on the Origin of Life on the Earth (held at Moscow, 19–24 August 1957) - Huxley, Thomas Henry (1968) [Originally published 1897]. "VIII Biogenesis and Abiogenesis [1870]". Discourses, Biological and Geological. Collected Essays. Vol. VIII (Reprint ed.). New York: Greenwood Press. LCCN 70029958.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|ref=harv(help); Unknown parameter|chapterurl=ignored (|chapter-url=suggested) (help) - Kauffman, Stuart (1993). The Origins of Order: Self-Organization and Selection in Evolution. New York: Oxford University Press. ISBN 978-0-19-507951-7. LCCN 91011148. OCLC 23253930.
{{cite book}}: Invalid|ref=harv(help) - Kauffman, Stuart (1995). At Home in the Universe: The Search for Laws of Self-Organization and Complexity. New York: Oxford University Press. ISBN 0-19-509599-5. LCCN 94025268.
{{cite book}}: Invalid|ref=harv(help) - Klyce, Brig (22 January 2001). Kingsley, Stuart A.; Bhathal, Ragbir (eds.). Panspermia Asks New Questions. The Search for Extraterrestrial Intelligence (SETI) in the Optical Spectrum III. Vol. 4273. Bellingham, WA: SPIE. doi:10.1117/12.435366. ISBN 0-8194-3951-7. LCCN 2001279159. Retrieved 9 June 2015.
{{cite conference}}: Invalid|ref=harv(help) Proceedings of the SPIE held at San Jose, CA, 22–24 January 2001 - Lennox, James G. (2001). Aristotle's Philosophy of Biology: Studies in the Origins of Life Science. Cambridge Studies in Philosophy and Biology. Cambridge, UK; New York: Cambridge University Press. ISBN 0-521-65976-0. LCCN 00026070.
{{cite book}}: Invalid|ref=harv(help) - McKinney, Michael L. (1997). "How do rare species avoid extinction? A paleontological view". In Kunin, William E.; Gaston, Kevin J. (eds.). The Biology of Rarity: Causes and consequences of rare—common differences (1st ed.). London; New York: Chapman & Hall. ISBN 0-412-63380-9. LCCN 96071014. OCLC 36442106.
{{cite book}}: Invalid|ref=harv(help) - Miller, G. Tyler; Spoolman, Scott E. (2012). Environmental Science (14th ed.). Belmont, CA: Brooks/Cole. ISBN 978-1-111-98893-7. LCCN 2011934330. OCLC 741539226.
{{cite book}}: Invalid|ref=harv(help) - Oparin, A. I. (1953) [Originally published 1938; New York: The Macmillan Company]. The Origin of Life. Translation and new introduction by Sergius Morgulis (2nd ed.). Mineola, NY: Dover Publications. ISBN 0-486-49522-1. LCCN 53010161.
{{cite book}}: Invalid|ref=harv(help) - Raven, Peter H.; Johnson, George B. (2002). Biology (6th ed.). Boston, MA: McGraw-Hill. ISBN 0-07-112261-3. LCCN 2001030052. OCLC 45806501.
{{cite book}}: Invalid|ref=harv(help) - Russell, Michael, ed. (2010). Origins, Abiogenesis and the Search for Life. Cambridge, MA: Cosmology Science Publishers. ISBN 978-0-9829552-1-5.
{{cite book}}: Invalid|ref=harv(help) - Shapiro, Robert (1987). Origins: A Skeptic's Guide to the Creation of Life on Earth. Toronto; New York: Bantam Books. ISBN 0-553-34355-6.
{{cite book}}: Invalid|ref=harv(help) - Sheldon, Robert B. (22 September 2005). Hoover, Richard B.; Levin, Gilbert V.; Rozanov, Alexei Y.; Gladstone, G. Randall (eds.). Historical Development of the Distinction between Bio- and Abiogenesis (PDF). Astrobiology and Planetary Missions. Vol. 5906. Bellingham, WA: SPIE. doi:10.1117/12.663480. ISBN 978-0-8194-5911-4. LCCN 2005284378. Retrieved 13 April 2015.
{{cite conference}}: Invalid|ref=harv(help) Proceedings of the SPIE held at San Diego, CA, 31 July–2 August 2005 - Stearns, Beverly Peterson; Stearns, Stephen C. (1999). Watching, from the Edge of Extinction. New Haven, CT: Yale University Press. ISBN 0-300-07606-1. LCCN 98034087. OCLC 47011675.
{{cite book}}: Invalid|ref=harv(help) - Tyndall, John (1905) [Originally published 1871; London; New York: Longmans, Green & Co.; D. Appleton and Company]. Fragments of Science. Vol. 2 (6th ed.). New York: P.F. Collier & Sons. OCLC 726998155. Retrieved 6 June 2015.
{{cite book}}: Invalid|ref=harv(help) - Vartanian, Aram (1973). "Spontaneous Generation". In Wiener, Philip P. (ed.). Dictionary of the History of Ideas. Vol. IV. New York: Charles Scribner's Sons. ISBN 0-684-13293-1. LCCN 72007943.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|ref=harv(help); Unknown parameter|chapterurl=ignored (|chapter-url=suggested) (help) - Voet, Donald; Voet, Judith G. (2004). Biochemistry. Vol. 1 (3rd ed.). New York: John Wiley & Sons. ISBN 0-471-19350-X. LCCN 2003269978.
{{cite book}}: Invalid|ref=harv(help) - Woodward, Robert J., ed. (1969). Our Amazing World of Nature: Its Marvels & Mysteries. Pleasantville, NY: Reader's Digest Association. ISBN 0-340-13000-8. LCCN 69010418.
{{cite book}}: Invalid|ref=harv(help) - Yarus, Michael (2010). Life from an RNA World: The Ancestor Within. Cambridge, MA: Harvard University Press. ISBN 978-0-674-05075-4. LCCN 2009044011.
{{cite book}}: Invalid|ref=harv(help)
Further reading
- Arrhenius, Gustaf; et al. (1997). "Entropy and Charge in Molecular Evolution—the Case of Phosphate". Journal of Theoretical Biology. 187 (4): 503–22. doi:10.1006/jtbi.1996.0385. PMID 9299295.
- Buehler, Lukas K. (2000–2005) The physico-chemical basis of life, http://www.whatislife.com/about.html accessed 27 October 2005.
- De Duve, Christian (January 1996). Vital Dust: The Origin and Evolution of Life on Earth. Basic Books. ISBN 0-465-09045-1.
- Egel, R.; Lankenau, D.-H.; Mulkidjanian, A. Y. (2011). Origins of Life: The Primal Self-Organization. Berlin Heidelberg: Springer-Verlag. pp. 1–366, . doi:10.1007/978-3-642-21625-1. ISBN 978-3-642-21624-4.
{{cite book}}: CS1 maint: extra punctuation (link) - Fernando, CT; Rowe, J (2007). "Natural selection in chemical evolution". Journal of Theoretical Biology. 247 (1): 152–67. doi:10.1016/j.jtbi.2007.01.028. PMID 17399743.
- Roy, Debjani; Schleyer, Paul (2010). Chapter 6:Chemical Origin of Life: How do Five HCN Molecules Combine to form Adenine under Prebiotic and Interstellar Conditions. Verlag: Wiley-VCH. doi:10.1002/9783527629213.ch6. ISBN 9783527629213.
- Hartman, Hyman (1998). "Photosynthesis and the Origin of Life". Origins of Life and Evolution of Biospheres. 28 (4–6): 515–521. Bibcode:1998OLEB...28..515H. doi:10.1023/A:1006548904157.
- Harris, Henry (2002). Things come to life. Spontaneous generation revisited. Oxford: Oxford University Press. ISBN 0-19-851538-3.
- Hazen, Robert M. (December 2005). Genesis: The Scientific Quest for Life's Origins. Joseph Henry Press. ISBN 0-309-09432-1.
- Gribbon, John (1998). The Case of the Missing Neutrinos and other Curious Phenomena of the Universe. Penguin Science, London. ISBN 0-14-028734-5.
- Horgan, J (1991). "In the beginning". Scientific American. 264 (6): 100–109. Bibcode:1991SciAm.264..100P. doi:10.1038/scientificamerican0691-100. (Cited on p. 108).
- Huber, C; Wächtershäuser, G. (1998). "Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: implications for the origin of life". Science. 281 (5377): 670–2. Bibcode:1998Sci...281..670H. doi:10.1126/science.281.5377.670. PMID 9685253. (Cited on p. 108).
- Ignatov, I.; Mosin, O. V. (2013). "Modeling of Possible Processes for Origin of Life and Living Matter in Hot Mineral and Seawater with Deuterium". Journal of Environment and Earth Science. 3 (14): 103–118.
- Klotz, I., Did Life Start in a Pond, Not Oceans? – opinion of Jack Szostak in Discovery News http://news.discovery.com/earth/oceans/life-pond-ocean-122402.htm
- Knoll, Andrew H. (2003). Life on a Young Planet: The First Three Billion Years of Evolution on Earth. Princeton University Press. ISBN 0-691-00978-3.
- Kurihara, K.; Tamura, M.; Shohda, K.; Toyota, T.; Suzuki, K.; Sugawara, T. (2011). "Self-Reproduction of Supramolecular Giant Vesicles Combined with the Amplification of Encapsulated DNA". Nature Chemistry. 4 (10): 775–781.
- Luisi, Pier Luigi (2006). The Emergence of Life: From Chemical Origins to Synthetic Biology. Cambridge University Press. ISBN 0-521-82117-7.
- Martin, W.; Russell, M.J. (2002). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Philosophical Transactions of the Royal Society B. 358 (1429): 59–83, discussion 83–5. doi:10.1098/rstb.2002.1183. PMC 1693102. PMID 12594918.
- Maynard Smith, John; Szathmary, Eors (16 March 2000). The Origins of Life: From the Birth of Life to the Origin of Language. Oxford Paperbacks. ISBN 0-19-286209-X.
- Morowitz, Harold J. (1992) "Beginnings of Cellular Life: Metabolism Recapitulates Biogenesis". Yale University Press. ISBN 0-300-05483-1
- NASA Astrobiology Institute: Earth's Early Environment and Life
- NASA Specialized Center of Research and Training in Exobiology: Gustaf O. Arrhenius
- Pitsch, Stefan; Krishnamurthy, Ramanarayanan; Arrhenius, Gustaf (2000). "Concentration of Simple Aldehydes by Sulfite-Containing Double-Layer Hydroxide Minerals: Implications for Biopoesis". Helvetica Chimica Acta. 83 (9): 2398 2411. doi:10.1002/1522-2675(20000906)83:9<2398::AID-HLCA2398>3.0.CO;2-5.
- Pons, M.L.; Quitte, G.; Fujii, T.; et al. (2011). "Early Archean Serpentine Mud Volcanoes at Isua, Greenland, as a Niche for Early Life". PNAS. 108 (43): 17639–17643. doi:10.1073/pnas.1108061108.
{{cite journal}}: Explicit use of et al. in:|last4=(help) - Pross, Addy (2012). What is Life?: How chemistry becomes biology. Oxford University Press. ISBN 0-19-964101-3.
- Russell, MJ; Hall, AJ; Cairns-Smith, AG; Braterman, PS (1988). "Submarine hot springs and the origin of life". Nature. 336 (6195): 117. Bibcode:1988Natur.336..117R. doi:10.1038/336117a0.
- Shock, E (1997). "High Temperature Life without Photosyntesis as a Model for Mars" (PDF). Journal of Geophysical Research. 102 (E10): 23687–23694. Bibcode:1997JGR...10223687S. doi:10.1029/97je01087.
- Ward, D. (2010) First Fossil-makers in Hot Water. Astrobiology Magazine. http://www.astrobio.net/exclusive/3418/first-fossil-makers-in-hot-water
- Dedicated issue of Philosophical Transactions B on Major Steps in Cell Evolution freely available.
- Dedicated issue of Philosophical Transactions B on the Emergence of Life on the Early Earth freely available.
External links
- Zlobin, A.E. (2014). "Symmetry infringement in mathematical metrics of hydrogen atom as illustration of ideas by V.I.Vernadsky concerning origin of life and biosphere" (PDF). Acta Naturae (Special Issue 1): 48.
- A.E.Zlobin, Tunguska similar impacts and origin of life (mathematical theory of origin of life, incoming of pattern recognition algorithm due to comets)
- The Origin of Life Video by John Maynard Smith[dead link]
- "Harvard Team Creates the World's 1st Synthesized Cells"
- Martin A. Nowak and Hisashi Ohtsuki. Prevolutionary dynamics and the origin of evolution. Proceedings of the National Academy of Sciences 2008
- "Exploring Life's Origins: a Virtual Exhibit"
- "SELF-REPLICATION: Even peptides do it" by Stuart A. Kauffman (web archive version as original page no longer accessible)
- Origins of Life website including papers, resources, by Dr. Michael Russell at the U. of Glasgow
- Possible Connections Between Interstellar Chemistry and the Origin of Life on the Earth[dead link]
- Scientists Find Clues That Life Began in Deep Space—NASA Astrobiology Institute[dead link]
- Self-organizing biochemical cycles—by Leslie Orgel
- How Life Began: New Research Suggests Simple Approach
- Primordial Soup's On: Scientists Repeat Evolution's Most Famous Experiment–an article in Scientific American. 28 March 2007[dead link]
- Illustrations from Evolution (textbook)
- An abiogenesis primer for laymen[dead link]
- Vasas, Vera; et al. (2012). "Evolution before genes". Biology Direct. 7: 1. doi:10.1186/1745-6150-7-1.
{{cite journal}}: Explicit use of et al. in:|last2=(help)CS1 maint: unflagged free DOI (link) - How life began on Earth
- Early Archean Serpentine mud Volcanoes at Isua, Greenland, as a Niche for Early Life Marie-Laure Pons et al. PNAS
- The Origins of Life Smithsonian magazine
- Minerals and the Origins of Life (Robert Hazen, NASA) (video, 60m, April 2014).
- Search for Life in the Universe on YouTube (NASA) (video, 87m, 14 July 2014).


