Promegestone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 49: | Line 49: | ||
<!-- History, society, and culture --> |
<!-- History, society, and culture --> |
||
Promegestone was first described in 1973 and was introduced for medical use in [[France]] in 1983.<ref name="pmid4353432" /><ref name="Publishing2013" /> It has only been marketed in a few countries, including France, [[Portugal]], [[Tunisia]], and [[Argentina]].<ref name="Drugs.com" /><ref name="IndexNominum2000" /> In addition to its use as a medication, promegestone has been used widely throughout the world as a [[radioligand]] for the progesterone receptor.<ref name="pmid6366037" /> |
Promegestone was first described in 1973 and was introduced for medical use in [[France]] in 1983.<ref name="pmid4353432" /><ref name="Publishing2013" /> It has only been marketed in a few countries, including France, [[Portugal]], [[Tunisia]], and [[Argentina]].<ref name="Drugs.com" /><ref name="IndexNominum2000" /> In addition to its use as a medication, promegestone has been used widely throughout the world as a [[radioligand]] for the progesterone receptor.<ref name="pmid6366037" /><ref name="RaynaudOjasoo1981">{{cite journal|last1=Raynaud|first1=Jean-Pierre|last2=Ojasoo|first2=Tiiu|last3=Vaché|first3=Viviane|title=Stable and Specific Tracers|year=1981|pages=163–179|doi=10.1007/978-1-4684-3824-6_7}}</ref> |
||
==Medical uses== |
==Medical uses== |
||
Revision as of 19:22, 22 March 2018
 | |
| Clinical data | |
|---|---|
| Trade names | Surgestone |
| Other names | PMG; R-5020; RU-5020; 17α,21-Dimethyl-δ9-19-norprogesterone; 17α,21-Dimethyl-19-norpregna-4,9-diene-3,20-dione |
| Routes of administration | By mouth[1] |
| Drug class | Progestin; Progestogen |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | To albumin[1] |
| Metabolism | Liver (hydroxylation)[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.681 |
| Chemical and physical data | |
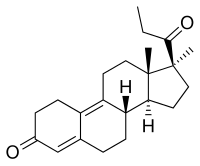
| Formula | C22H30O2 |
| Molar mass | 326.472 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders.[3][1][4][5] It is taken by mouth.[1]
Promegestone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] It has weak glucocorticoid activity and no other important hormonal activity.[1]
Promegestone was first described in 1973 and was introduced for medical use in France in 1983.[6][7] It has only been marketed in a few countries, including France, Portugal, Tunisia, and Argentina.[5][8] In addition to its use as a medication, promegestone has been used widely throughout the world as a radioligand for the progesterone receptor.[3][9]
Medical uses
Promegestone is used in menopausal hormone therapy and in the treatment of gynecological conditions caused by luteal insufficiency, including premenopausal disorders, dysmenorrhea, and premenstrual syndrome.[1][4] It has also been used to treat benign breast disorders.[10]
Side effects
Promegestone has no androgenic side effects.[3][4]
Pharmacology
Pharmacodynamics
Promegestone is a progestogen, or an agonist of the progesterone receptor.[1][2] It has about 200% of the affinity of progesterone for the PR.[1][2] The endometrial transformation dosage is 10 mg per cycle and the ovulation-inhibiting dosage is 0.5 mg/day.[1][2] Promegestone has weak glucocorticoid activity in addition to its progestogenic activity.[1][2] Conversely, it has no androgenic, antiandrogenic, estrogenic, mineralocorticoid, or other hormonal activity.[1][2][4] However, promegestone has been found to possess some neurosteroid activity by acting as a non-competitive antagonist of the nicotinic acetylcholine receptor, similarly to progesterone.[11]
Pharmacokinetics
Following oral administration, peak serum levels of promegestone are reached after 1 to 2 hours.[1][2] The medication is mainly bound to albumin; it does not bind to sex hormone-binding globulin, and binds only weakly to corticosteroid-binding globulin.[1][2][12] The metabolism of promegestone is mainly via hydroxylation at the C21 position and at other positions.[1][2]
Chemistry
Promegestone, also known as 17α,21-dimethyl-δ9-19-norprogesterone or as 17α,21-dimethyl-19-norpregna-4,9-diene-3,20-dione, is a synthetic norpregnane steroid and a derivative of progesterone.[13][8][7][1] It is specifically a combined derivative of 17α-methylprogesterone and 19-norprogesterone, or of 17α-methyl-19-norprogesterone.[13][7][1] Related derivatives of 17α-methyl-19-norprogesterone include demegestone and trimegestone.[13][8][1]
History
Promegestone was first described in the literature in 1973 and was introduced for medical use in France in 1983.[6][7][4] It was developed by Roussel Uclaf in France.[4]
Society and culture
Generic names
Promegestone is the generic name of the drug and its INN, while promégestone is its DCF.[5][13][8] It is also known by its developmental code name R-5020 or RU-5020.[5][13][8]
Brand names
Promegestone is marketed exclusively under the brand name Surgestone.[5][8]
Availability
Promegestone is or has been marketed in France, Portugal, Tunisia, and Argentina.[5][8]
References
- ^ a b c d e f g h i j k l m n o p q r s Kuhl, H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (sup1): 3–63. doi:10.1080/13697130500148875. ISSN 1369-7137. PMID 16112947.
- ^ a b c d e f g h i Kuhl H (2011). "Pharmacology of progestogens" (PDF). Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology. 8 (Special Issue 1): 157–176.
- ^ a b c Raynaud JP, Ojasoo T (1983). "[Promegestone, a new progestin]". J Gynecol Obstet Biol Reprod (Paris) (in French). 12 (7): 697–710. PMID 6366037.
- ^ a b c d e f Cain (11 September 1984). ANNUAL REPORTS IN MED CHEMISTRY V19 PPR. Academic Press. pp. 323–. ISBN 978-0-08-058363-1.
- ^ a b c d e f https://www.drugs.com/international/promegestone.html
- ^ a b Philibert D, Raynaud JP (July 1973). "Progesterone binding in the immature mouse and rat uterus". Steroids. 22 (1): 89–98. PMID 4353432.
- ^ a b c d William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 2935–2936. ISBN 978-0-8155-1856-3.
- ^ a b c d e f g Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 883–. ISBN 978-3-88763-075-1.
- ^ Raynaud, Jean-Pierre; Ojasoo, Tiiu; Vaché, Viviane (1981). "Stable and Specific Tracers": 163–179. doi:10.1007/978-1-4684-3824-6_7.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Uzan S, Denis C, Pomi V, Varin C (February 1992). "Double-blind trial of promegestone (R 5020) and lynestrenol in the treatment of benign breast disease". Eur. J. Obstet. Gynecol. Reprod. Biol. 43 (3): 219–27. PMID 1563574.
- ^ Blanton MP, Xie Y, Dangott LJ, Cohen JB (February 1999). "The steroid promegestone is a noncompetitive antagonist of the Torpedo nicotinic acetylcholine receptor that interacts with the lipid-protein interface". Mol. Pharmacol. 55 (2): 269–78. PMID 9927618.
- ^ Chan DW, Slaunwhite WR (May 1977). "The binding of a synthetic progestin, R5020 to transcortin and serum albumin". J. Clin. Endocrinol. Metab. 44 (5): 983–5. doi:10.1210/jcem-44-5-983. PMID 858781.
- ^ a b c d e J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1026–. ISBN 978-1-4757-2085-3.
