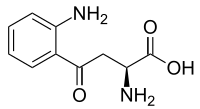
Kynurenine
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
(2S)-2-Amino-4-(2-aminophenyl)-4-oxo-butanoic acid | |
| Other names
(S)-Kynurenine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| MeSH | Kynurenine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H12N2O3 | |
| Molar mass | 208.217 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
l-Kynurenine is a metabolite of the amino acid l-tryptophan used in the production of niacin.
Kynurenine is synthesized by the enzyme tryptophan dioxygenase, which is made primarily but not exclusively in the liver, and indoleamine 2,3-dioxygenase, which is made in many tissues in response to immune activation.[1] Kynurenine and its further breakdown products carry out diverse biological functions, including dilating blood vessels during inflammation[2] and regulating the immune response.[3] Some cancers increase kynurenine production, which increases tumor growth.[1]
Kynurenine protects the eye by absorbing UV light, especially in the UVA region (315-400 nm).[4] Kynurenine is present in the lens and retina as one of multiple tryptophan derivatives produced in the eye, including 3-hydroxykynurenine, that together provide UV protection and aid in enhancing visual acuity.[5][6] The use of kynurenine as a UV filter is consistent with its photostability and low photosensitization, owing to its efficient relaxation from the UV-induced excited state.[7] The concentration of this UV filter decreases with age,[8] and this loss of free kynurenine and the concomitant formation of relatively more photosensitizing kynurenine derivatives and kynurenine-protein conjugates may contribute to the formation of cataracts.[9][10][11]
Evidence suggests that increased kynurenine production may precipitate depressive symptoms associated with interferon treatment for hepatitis C.[12] Cognitive deficits in schizophrenia are associated with imbalances in the enzymes that break down kynurenine.[13] Blood levels of kynurenine are reduced in people with bipolar disorder.[14] Kynurenine production is increased in Alzheimer's disease[15] and cardiovascular disease[16] where its metabolites are associated with cognitive deficits[17] and depressive symptoms.[18] Kynurenine is also associated with tics.[19][20]
Kynureninase catabolizes the conversion of kynurenine into anthranilic acid[21] while kynurenine-oxoglutarate transaminase catabolizes its conversion into kynurenic acid. Kynurenine 3-hydroxylase converts kynurenine to 3-hydroxykynurenine.[22]
Kynurenine has also been identified as one of two compounds that makes up the pigment that gives the goldenrod crab spider its yellow color.[23]

Kynurenine pathway dysfunction[edit]
Dysfunctional states of distinct steps of the kynurenine pathway (such as kynurenine, kynurenic acid, quinolinic acid, anthranilic acid, 3-hydroxykynurenine) have been described for a number of disorders, including:[25]
- HIV dementia
- Tourette syndrome
- Tic disorders
- Psychiatric disorders (such as schizophrenia, bipolar disorder,[14] major depression,[26] anxiety disorders)
- Multiple sclerosis
- Huntington's disease
- Encephalopathies
- Lipid metabolism
- Liver fat metabolism
- Systemic lupus erythematosus
- Glutaric aciduria
- Vitamin B6 deficiency
- Eosinophilia-myalgia syndrome
- Myalgic encephalomyelitis/chronic fatigue syndrome[27]
Downregulation of kynurenine-3-monooxygenase (KMO) can be caused by genetic polymorphisms, cytokines, or both.[28][29] KMO deficiency leads to an accumulation of kynurenine and to a shift within the tryptophan metabolic pathway towards kynurenine acid and anthranilic acid.[30] Kynurenine-3-monooxygenase deficiency is associated with disorders of the brain (e.g. major depressive disorder, bipolar disorder, schizophrenia, tic disorders) [31] and of the liver.[19][32][33][34][35]
Drug development[edit]
It is hypothesized that the kynurenine pathway is partly responsible for the therapeutic effect of lithium on bipolar disorder. If that is the case, it could be a target of drug discovery.[36][37]
See also[edit]
References[edit]
- ^ a b Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M (2011). "An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor". Nature. 478 (7368): 197–203. Bibcode:2011Natur.478..197O. doi:10.1038/nature10491. PMID 21976023.
- ^ Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF, Hunt NH, Stocker R (2010). "Kynurenine is an endothelium-derived relaxing factor produced during inflammation". Nature Medicine. 16 (3): 279–85. doi:10.1038/nm.2092. PMC 3556275. PMID 20190767.
- ^ Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T (2010). "Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism". Proceedings of the National Academy of Sciences. 107 (46): 19961–6. Bibcode:2010PNAS..10719961N. doi:10.1073/pnas.1014465107. PMC 2993339. PMID 21041655.
- ^ Sherin, Peter S.; Grilj, Jakob; Tsentalovich, Yuri P.; Vauthey, Eric (2009-04-09). "Ultrafast Excited-State Dynamics of Kynurenine, a UV Filter of the Human Eye". The Journal of Physical Chemistry B. 113 (14): 4953–4962. doi:10.1021/jp900541b. ISSN 1520-6106.
- ^ Wood, Andrew M.; Truscott, Roger J. W. (1993-03-01). "UV Filters in Human Lenses: Tryptophan Catabolism". Experimental Eye Research. 56 (3): 317–325. doi:10.1006/exer.1993.1041. ISSN 0014-4835.
- ^ Truscott, Roger J. W.; Wood, Andrew M.; Carver, John A.; Sheil, Margaret M.; Stutchbury, Glen M.; Zhu, Jiulin; Kilby, Greg W. (1994-07-11). "A new UV-filter compound in human lenses". FEBS Letters. 348 (2): 173–176. doi:10.1016/0014-5793(94)00601-6. ISSN 0014-5793.
- ^ Tuna, Deniz; Došlić, Nađa; Mališ, Momir; Sobolewski, Andrzej L.; Domcke, Wolfgang (2015-02-12). "Mechanisms of Photostability in Kynurenines: A Joint Electronic-Structure and Dynamics Study". The Journal of Physical Chemistry B. 119 (6): 2112–2124. doi:10.1021/jp501782v. ISSN 1520-6106.
- ^ Bova, L. M.; Sweeney, M. H.; Jamie, J. F.; Truscott, R. J. (January 2001). "Major changes in human ocular UV protection with age". Investigative Ophthalmology & Visual Science. 42 (1): 200–205. ISSN 0146-0404. PMID 11133868.
- ^ Tsentalovich, Yuri P.; Sherin, Peter S.; Kopylova, Lyudmila V.; Cherepanov, Ivan V.; Grilj, Jakob; Vauthey, Eric (2011-09-29). "Photochemical properties of UV Filter molecules of the human eye". Investigative Ophthalmology & Visual Science. 52 (10): 7687–7696. doi:10.1167/iovs.11-8120. ISSN 1552-5783. PMID 21873681.
- ^ Vazquez, Santiago; Aquilina, J. Andrew; Sheil, Margaret M.; Truscott, Roger J. W.; Jamie, Joanne F. (2002-02-15). "Novel Protein Modification by Kynurenine in Human Lenses*". Journal of Biological Chemistry. 277 (7): 4867–4873. doi:10.1074/jbc.M107529200. ISSN 0021-9258.
- ^ Sherin, Peter S.; Grilj, Jakob; Kopylova, Lyudmila V.; Yanshole, Vadim V.; Tsentalovich, Yuri P.; Vauthey, Eric (2010-09-16). "Photophysics and Photochemistry of the UV Filter Kynurenine Covalently Attached to Amino Acids and to a Model Protein". The Journal of Physical Chemistry B. 114 (36): 11909–11919. doi:10.1021/jp104485k. ISSN 1520-6106.
- ^ Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH (2003). "Interferon-alpha–induced changes in tryptophan metabolism". Biological Psychiatry. 54 (9): 906–14. doi:10.1016/S0006-3223(03)00173-2. PMID 14573318. S2CID 24079984.
- ^ Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R (2011). "Downregulated Kynurenine 3-Monooxygenase Gene Expression and Enzyme Activity in Schizophrenia and Genetic Association with Schizophrenia Endophenotypes". Archives of General Psychiatry. 68 (7): 665–74. doi:10.1001/archgenpsychiatry.2011.71. PMC 3855543. PMID 21727251.
- ^ a b Bartoli, F; Misiak, B; Callovini, T; Cavaleri, D; Cioni, RM; Crocamo, C; Savitz, JB; Carrà, G (19 October 2020). "The kynurenine pathway in bipolar disorder: a meta-analysis on the peripheral blood levels of tryptophan and related metabolites". Molecular Psychiatry. 26 (7): 3419–3429. doi:10.1038/s41380-020-00913-1. PMID 33077852. S2CID 224314102.
- ^ Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM (2005). "Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer's disease hippocampus". Neuropathology and Applied Neurobiology. 31 (4): 395–404. doi:10.1111/j.1365-2990.2005.00655.x. PMID 16008823. S2CID 7754894.
- ^ Wirleitner B, Rudzite V, Neurauter G, Murr C, Kalnins U, Erglis A, Trusinskis K, Fuchs D (2003). "Immune activation and degradation of tryptophan in coronary heart disease". European Journal of Clinical Investigation. 33 (7): 550–4. doi:10.1046/j.1365-2362.2003.01186.x. PMID 12814390. S2CID 10300941.
- ^ Gulaj E, Pawlak K, Bien B, Pawlak D (2010). "Kynurenine and its metabolites in Alzheimer's disease patients". Advances in Medical Sciences. 55 (2): 204–11. doi:10.2478/v10039-010-0023-6. PMID 20639188.
- ^ Swardfager W, Herrmann N, Dowlati Y, Oh PI, Kiss A, Walker SE, Lanctôt KL (2009). "Indoleamine 2,3-dioxygenase activation and depressive symptoms in patients with coronary artery disease". Psychoneuroendocrinology. 34 (10): 1560–6. doi:10.1016/j.psyneuen.2009.05.019. PMID 19540675. S2CID 36687413.
- ^ a b Hoekstra PJ, Anderson GM, Troost PW, Kallenberg CG, Minderaa RB (2007). "Plasma kynurenine and related measures in tic disorder patients". European Child & Adolescent Psychiatry. 16: 71–7. doi:10.1007/s00787-007-1009-1. PMID 17665285. S2CID 39150343.
- ^ McCreary AC, Handley SL (1995). "Kynurenine potentiates the DOI head shake in mice". Journal of Psychopharmacology. 9 (1): 69–70. doi:10.1177/026988119500900112. PMID 22298697. S2CID 28700510.
- ^ Kynureninase, European Bioinformatics Institute
- ^ Saito Y, Hayaishi O, Rothberg S (1957-12-01). "Studies on Oxygenases". The Journal of Biological Chemistry. 229 (2): 921–34. doi:10.1016/S0021-9258(19)63696-3. PMID 13502353.[permanent dead link]
- ^ Oxford, G. S.; Gillespie, R. G. (January 1998). "Evolution and Ecology of Spider Coloration". Annual Review of Entomology. 43 (1): 619–643. doi:10.1146/annurev.ento.43.1.619. ISSN 0066-4170. PMID 15012400. S2CID 6963733.
- ^ Schwarcz, Robert; John P. Bruno; Paul J. Muchowski; Hui-Qiu Wu (July 2012). "Kynurenines in the Mammalian Brain: When Physiology Meets Pathology". Nature Reviews Neuroscience. 13 (7): 465–477. doi:10.1038/nrn3257. PMC 3681811. PMID 22678511.
- ^ Stone TW (2001). "Kynurenines in the CNS: from endogenous obscurity to therapeutic importance". Progress in Neurobiology. 64 (2): 185–218. doi:10.1016/s0301-0082(00)00032-0. PMID 11240212. S2CID 6446144.
- ^ Liu, Duan; Ray, Balmiki; Neavin, Drew R.; Zhang, Jiabin; Athreya, Arjun P.; Biernacka, Joanna M.; Bobo, William V.; Hall-Flavin, Daniel K.; Skime, Michelle K.; Zhu, Hongjie; Jenkins, Gregory D. (January 10, 2018). "Beta-defensin 1, aryl hydrocarbon receptor and plasma kynurenine in major depressive disorder: metabolomics-informed genomics". Translational Psychiatry. 8 (1): 10. doi:10.1038/s41398-017-0056-8. ISSN 2158-3188. PMC 5802574. PMID 29317604.
- ^ Kashi, Alex A.; Davis, Ronald W.; Phair, Robert D. (2019). "The IDO Metabolic Trap Hypothesis for the Etiology of ME/CFS". Diagnostics. 9 (3): 82. doi:10.3390/diagnostics9030082. PMC 6787624. PMID 31357483.
- ^ "Neurobiochemie" (in German).
- ^ Müller N, Myint AM, Schwarz MJ (2011). "Inflammatory biomarkers and depression". Neurotox Res. 19 (2): 308–18. doi:10.1007/s12640-010-9210-2. PMID 20658274. S2CID 3225744.
- ^ Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R (2011). "Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes". Arch. Gen. Psychiatry. 68 (7): 665–74. doi:10.1001/archgenpsychiatry.2011.71. PMC 3855543. PMID 21727251.
- ^ Marx W, McGuinness AJ, Rocks T, Ruusunen A, Cleminson J, Walker AJ, Gomes-da-Costa S, Lane M, Sanches M, Diaz AP, Tseng PT, Lin PY, Berk M, Clarke G, O'Neil A, Jacka F, Stubbs B, Carvalho AF, Quevedo J, Soares JC, Fernandes BS (2020). "The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies". Molecular Psychiatry. 26 (8): 4158–4178. doi:10.1038/s41380-020-00951-9. PMID 33230205. S2CID 227132820 – via doi: 10.1038/s41380-020-00951-9. PMID 33230205.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holtze M, Saetre P, Engberg G, Schwieler L, Werge T, Andreassen OA, Hall H, Terenius L, Agartz I, Jönsson EG, Schalling M, Erhardt S (2012). "Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls". J Psychiatry Neurosci. 37 (1): 53–7. doi:10.1503/jpn.100175. PMC 3244499. PMID 21693093.
- ^ Campbell BM, Charych E, Lee AW, Möller T (2014). "Kynurenines in CNS disease: regulation by inflammatory cytokines". Front Neurosci. 8: 12. doi:10.3389/fnins.2014.00012. PMC 3915289. PMID 24567701.
- ^ Buness A, Roth A, Herrmann A, Schmitz O, Kamp H, Busch K, Suter L (2014). "Identification of metabolites, clinical chemistry markers and transcripts associated with hepatotoxicity". PLOS ONE. 9 (5): e97249. Bibcode:2014PLoSO...997249B. doi:10.1371/journal.pone.0097249. PMC 4023975. PMID 24836604.
- ^ Hirata Y, Kawachi T, Sugimura T (1967). "Fatty liver induced by injection of L-tryptophan". Biochim. Biophys. Acta. 144 (2): 233–41. doi:10.1016/0005-2760(67)90153-1. PMID 4168935.
- ^ Bartoli, Francesco; Cioni, Riccardo M.; Cavaleri, Daniele; Callovini, Tommaso; Crocamo, Cristina; Misiak, Błażej; Savitz, Jonathan B.; Carrà, Giuseppe (January 2022). "The association of kynurenine pathway metabolites with symptom severity and clinical features of bipolar disorder: An overview". European Psychiatry. 65 (1): e82. doi:10.1192/j.eurpsy.2022.2340. ISSN 0924-9338.
- ^ Fornaro, Michele; Kardash, Lubna; Novello, Stefano; Fusco, Andrea; Anastasia, Annalisa; De Berardis, Domenico; Perna, Giampaolo; Carta, Mauro Giovanni (4 March 2018). "Progress in bipolar disorder drug design toward the development of novel therapeutic targets: a clinician's perspective". Expert Opinion on Drug Discovery. 13 (3): 221–228. doi:10.1080/17460441.2018.1428554.
