Hydromorphinol: Difference between revisions
ClueBot NG (talk | contribs) m Reverting possible vandalism by 78.79.38.85 to version by Nagelfar. False positive? Report it. Thanks, ClueBot NG. (1019922) (Bot) |
No edit summary |
||
| Line 63: | Line 63: | ||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
{{Opioids}} |
|||
[[Category:Opioids]] |
|||
[[Category:Morphinans]] |
[[Category:Morphinans]] |
||
Revision as of 13:15, 20 October 2012
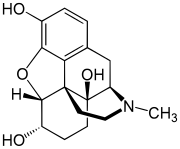 | |
| Clinical data | |
|---|---|
| Other names | Hydromorphinol, 14-hydroxy-7,8-dihydromorphine, RAM-320 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.878 |
| Chemical and physical data | |
| Formula | C17H21NO4 |
| Molar mass | 303.35 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| | |
Hydromorphinol (RAM-320, 14-Hydroxydihydromorphine)[1] is an opiate analogue that is a derivative of morphine, where the 14-position has been hydroxylated and the 7,8- double bond saturated.[2] It has similar effects to morphine such as sedation, analgesia and respiratory depression, but is more potent and has a steeper dose-response curve and longer half-life.[3] It is used in medicine as the bitartrate salt (free base conversion ratio 0.643, molecular weight 471.5) and hydrochloride (free base conversion ratio 0.770, molecular weight 393.9)
Hydromorphinol was developed in Austria in 1932. It is available, and commonly used, as a medicine in Sweden; in the United States, it was never available and is classified as a Schedule I drug.
Hydromorphinol is metabolised mainly in the liver in the same fashion as many other opioids and is itself a minor active metabolite of 14-Hydroxydihydrocodeine, an uncommonly-used opiate (but is also therefore an active metabolite too of a first order active metabolite of oxycodone).
It is distributed under the trade name Numorphan in some countries, whereas this trade name is used for oxymorphone much more commonly. It is controlled under the Single Convention On Narcotic Drugs.
See also
- Oxymorphol
- N-Phenethylhydromorphinol (RAM-378)
References
- ^ US 2960505, Weiss U., published 11/15/1960
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 14287245, please use {{cite journal}} with
|pmid=14287245instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 2326098, please use {{cite journal}} with
|pmid=2326098instead.
