Methoxy arachidonyl fluorophosphonate: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
No edit summary |
||
| Line 38: | Line 38: | ||
}} |
}} |
||
''' |
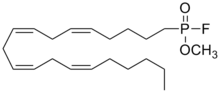
'''Methyl arachidonyl fluorophosphonate''', commonly referred as '''MAFP''', is an irreversible active site-directed [[enzyme inhibitor]] that inhibits nearly all [[serine hydrolase]]s and [[serine protease]]s.<ref name="pmid18657971">{{cite journal |author=Hoover HS, Blankman JL, Niessen S, Cravatt BF |title=Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling |journal=Bioorg. Med. Chem. Lett. |volume= 18|issue= 22|pages= 5838–41|date=July 2008 |pmid=18657971 |doi=10.1016/j.bmcl.2008.06.091 |url= |pmc=2634297}}</ref> It inhibits [[phospholipase A2]] and [[fatty acid amide hydrolase]] with special potency, displaying [[IC50|IC<sub>50</sub>]] values in the low-nanomolar range. In addition, it binds to the [[cannabinoid receptor type 1|CB<sub>1</sub> receptor]] in rat brain membrane preparations ([[IC50|IC<sub>50</sub>]] = 20 nM),<ref name="pmid9065728">{{cite journal | vauthors = Deutsch DG, Omeir R, Arreaza G, Salehani D, Prestwich GD, Huang Z, Howlett A | title = Methyl arachidonyl fluorophosphonate: a potent irreversible inhibitor of anandamide amidase | journal = Biochem. Pharmacol. | volume = 53 | issue = 3 | pages = 255–60 | year = 1997 | pmid = 9065728 | doi = | url = }}</ref> but does not appear to [[agonist|agonize]] or [[receptor antagonist|antagonize]] the receptor.<ref name="pmid14623770">{{cite journal | vauthors = Savinainen JR, Saario SM, Niemi R, Järvinen T, Laitinen JT | title = An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors | journal = Br. J. Pharmacol. | volume = 140 | issue = 8 | pages = 1451–9 | year = 2003 | pmid = 14623770 | pmc = 1574161 | doi = 10.1038/sj.bjp.0705577 | url = }}</ref> |
||
==See also== |
==See also== |
||
Revision as of 12:33, 1 April 2016
| |
| Names | |
|---|---|
| IUPAC name
methyl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-
tetraenylphosphonofluoridate
| |
| Other names
MAFP
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C21H36FO2P | |
| Molar mass | 370.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methyl arachidonyl fluorophosphonate, commonly referred as MAFP, is an irreversible active site-directed enzyme inhibitor that inhibits nearly all serine hydrolases and serine proteases.[1] It inhibits phospholipase A2 and fatty acid amide hydrolase with special potency, displaying IC50 values in the low-nanomolar range. In addition, it binds to the CB1 receptor in rat brain membrane preparations (IC50 = 20 nM),[2] but does not appear to agonize or antagonize the receptor.[3]
See also
- DIFP - diisopropyl fluorophosphate, a related inhibitor
- IDFP - isopropyl dodecylfluorophosphonate, another related inhibitor with selectivity for FAAH and MAGL
- Activity based probes
References
- ^ Hoover HS, Blankman JL, Niessen S, Cravatt BF (July 2008). "Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling". Bioorg. Med. Chem. Lett. 18 (22): 5838–41. doi:10.1016/j.bmcl.2008.06.091. PMC 2634297. PMID 18657971.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Deutsch DG, Omeir R, Arreaza G, Salehani D, Prestwich GD, Huang Z, Howlett A (1997). "Methyl arachidonyl fluorophosphonate: a potent irreversible inhibitor of anandamide amidase". Biochem. Pharmacol. 53 (3): 255–60. PMID 9065728.
- ^ Savinainen JR, Saario SM, Niemi R, Järvinen T, Laitinen JT (2003). "An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors". Br. J. Pharmacol. 140 (8): 1451–9. doi:10.1038/sj.bjp.0705577. PMC 1574161. PMID 14623770.
