Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
April 27
Increase in mean sea level pressure due to global warming
I was wondering if this has been measured. This increase is easy to compute. If we put the original value equal to P = 101325 Pa, then if the CO2 concentration increases by an amount x, the pressure should increase approximately by x P m_C/m_at where m_C is molecular mass of carbon = approximately 12 u and m_at = average molecular mass of the atmosphere = approximately (0.8*32 + 0.2*28) u = 31.2 u. An increase from 0.03% to 0.04% of the CO2 concentration should thus have increased the pressure by about 3.9 Pa.
Around 15 C, the vapor pressure increases at about 110 Pa/K. This means that at about 60% relative humidity, a 0.8 C rise in temperature should have contributed to about 53 Pa increase in pressure, so the total pressure increase is about 57 Pa. This makes the current mean sea level pressure equal to about 101382 Pa, which corresponds to a fictitious barometric height difference of 4.4 meters. Count Iblis (talk) 01:02, 27 April 2014 (UTC)
- That's such a small percentage that it would easily be swamped by normal pressure changes due to storms, etc. So, you'd need to average over a long time period to detect any average change. StuRat (talk) 02:49, 27 April 2014 (UTC)
- Yes, you would expect the standard deviation to be a 100 times larger, this means that you would need about 10,000 independent observations. If air pressure fluctuations are correleted on a typical time scale of a week, then with 100 well separated observation stations, you would need to average the data taken over a two years period. So, it seems to me that it is possible to detect this effect. Count Iblis (talk) 15:20, 27 April 2014 (UTC)
- The definition of "independent measurements" is important here. Since some storms, like hurricanes/typhoons, persist over wide areas and more than a week, they would presumably affect multiple air pressure readings under your schedule. StuRat (talk) 15:31, 27 April 2014 (UTC)
- Another effect is the decrease in the gravitational acceleration at the Earth's surface. The air pressure increase corresponds to there being about 3*10^15 kg of mass more in the atmosphere, which should decrease the gravitational acceleration at the Earth's surface by about 4.9*10^(-9) m/s^2. This decrease of 0.5 ppb is only about 5 times smaller than the standard error of state of the art gravimeters, so it sould be easily detectable with multiple measurements. Count Iblis (talk) 15:20, 27 April 2014 (UTC)
- However, we don't have a good measurement of this when the global temperature was 0.8 C lower than present, but we should be able to measure the decrease in the gravitaional acceleration over the next few decades. Count Iblis (talk) 15:30, 27 April 2014 (UTC)
- There could also be other effects to counter an atmospheric pressure increase. For example, more "air" might well push the atmosphere outwards farther from the Earth's surface, where more of it is lost to space. StuRat (talk) 15:35, 27 April 2014 (UTC)
- You seem to be switching around rather cavalierly between concentrations of CO_2 and mean annual global temperature changes. Of course these are linked, but not in any clear and simple fashion. So, careful with your reasoning there. The seasonal swings in CO_2 are also rather large, and the amplitude of that cycle is also increasing, see e.g. here [1], and here [2]. SemanticMantis (talk) 15:42, 27 April 2014 (UTC)
- This paper isn't exactly what you're talking about, but it does discuss using pressure as an indicator of climate change [3]. You might be able to track the cited literature, or see who has cited this work more recently. SemanticMantis (talk) 15:46, 27 April 2014 (UTC)
- Pressure may be affected by more than CO2 mass in the atmosphere. Any other variations components of the atmosphere would similarly contribute, and would have to be corrected for. I'm thinking here primarily of the total H2O content of the air, which potentially could vary significantly, e.g. with climate change: an increase of temperature might lead to an increase of H2O vapour globally, which would contribute to the pressure increase. An increase of pressure could not then be ascribed wholly to CO2. Another factor that must be taken into account is the change in total O2: most CO2 would be associated with a removal of O2. I see that this balance has been assumed in Count Iblis's initial calculations, but we'd need to consider how exact this is. For example, there are processes that add and remove CO2 without a commensurate change in O2, such as volcanic activity and calcium carbonate precipitation in the ocean. —Quondum 17:47, 27 April 2014 (UTC)
This paper states that the removal of carbon dioxide (and therefore, indirectly, oxygen) by the oceans makes the change of dry air pressure less than 1 Pa, and also says it's likely negative. By the way, the initial calculation of mean molecular mass should have been (0.8*28 + 0.2*32) = 28.8, or more accurately (0.78*28 + 0.21*32 + 0.009*40) = 28.9. Icek (talk) 19:30, 27 April 2014 (UTC)
- Thanks everyone for your comments! Count Iblis (talk) 21:00, 28 April 2014 (UTC)
Liquid and Gas SiO2 ???!!!...
What is the lowest pressure and the temperature range in which the SiO2 is Liquid and Gas:
O=Si=O pressure=… temperature from…. to…. = Liquid temperature from…. to…. = Gas
THANK you
SPYROU Kosta - Greece--85.74.189.98 (talk) 03:43, 27 April 2014 (UTC)
- The article Silicon dioxide says the melting point is 1600 to 1725 C and the boiling point is 2230 C. 24.5.122.13 (talk) 03:51, 27 April 2014 (UTC)
- Cristobalite, the high temperature modification of SiO2, melts at 1986 K (1713 °C), and I don't know why the article Silicon dioxide gives such a large range (maybe someone wrote it using a source about various impure forms of SiO2, and impurities will certainly change the melting point).
- But 85.74.189.98 is more or less asking about the triple point of silicon dioxide. I don't know it, but I guess the triple point temperature isn't much below 1986 K.
- As for the lowest temperature at which SiO2 is a gas, you have to know that in thermal equilibrium at 0 pressure everything is a gas (even if sublimation is often very very slow). So it can in principle be a gas at any temperature, if the pressure is low enough. Whether you can measure it as a gas depends on instruments and experimental procedures.
- Icek (talk) 19:11, 27 April 2014 (UTC)
Amitriptyline
What are its effects on the body? Does it have a sedative/anaesthetic property like sleeping pills? Does it help make muscles relaxed and reduce (or block) stress/pain signals like the stuff they give you before surgery? Money is tight (talk) 06:36, 27 April 2014 (UTC)
- Amitriptyline is our article on the subject. If someone has additional information with a WP:MEDRS-compliant source, please add it there. DMacks (talk) 07:19, 27 April 2014 (UTC)
- Yea I've read the article, it doesn't go into detail nor mention anything about what I asked. If any expert knows please tell me. — Preceding unsigned comment added by Money is tight (talk • contribs) 10:30, 27 April 2014 (UTC)
- Since amitriptyline is a tricyclic antidepressant (which is linked in the first sentence of the article), you might look at that article, which has some information about analgesic properties, etc. Deor (talk) 12:15, 27 April 2014 (UTC)
- Right. It's mainly used as an antidepressant and would not have substantial sedative effects on most people. See Tricyclic antidepressant#Side effects for a list of other effects. Looie496 (talk) 19:00, 27 April 2014 (UTC)
- Since amitriptyline is a tricyclic antidepressant (which is linked in the first sentence of the article), you might look at that article, which has some information about analgesic properties, etc. Deor (talk) 12:15, 27 April 2014 (UTC)
- Yea I've read the article, it doesn't go into detail nor mention anything about what I asked. If any expert knows please tell me. — Preceding unsigned comment added by Money is tight (talk • contribs) 10:30, 27 April 2014 (UTC)
Amitriptyline is a favorite and traditional analgesic as used by primary care physicians. It is fairly sedating and used as such in practice. It is a rather dangerous drug in overdose as well (all tricyclics are). --AboutFace 22 (talk) 23:29, 27 April 2014 (UTC)
In overdose the drug can depress the CNS as an antihistamine antagonizing the Histamine H1 receptor - see [4]. This is among a huge list of other effects such as causing cardiac arrest and delayed seizures. There was a meta-analysis on its use in treating insomnia at [5] but I haven't read the paper; the abstract suggests tolerance develops pretty quickly that reduces antihistamines' effectiveness in inducing sleep. [6] calls it a "sedating antidepressant". [7] classes it as a sedative/hypnotic drug with low abuse potential. Wnt (talk) 17:00, 28 April 2014 (UTC)
Solar tax
Is there more information on Wikipedia about this piece about solar tax? 87.78.128.11 (talk) 07:19, 27 April 2014 (UTC)
- You mean like Political activities of the Koch brothers? Bear in mind that Wikipedia is not the news.--Shantavira|feed me 09:23, 27 April 2014 (UTC)
- You can also inform yourself about the technical parts of this issue by reading about net metering; billing an energy customer for their net use, or, the quantity of energy delivered by the grid, minus the quantity of solar energy produced on site. Nimur (talk) 13:45, 27 April 2014 (UTC)
Sticking out your tongue while doing complicated tasks
Mostly seen in children: sticking out your tongue while doing a complicated task, like coloring between the lines (or playing a violin). Google gives me a wide range of explanations of which "sticking out your tongue reduces the amount of stimuli sent to your brain" sounds reasonable. But then again, besides while eating, my tongue probably sends messages like "yep, still 37 C", "nothing to see here". Taking of your shoes would reduce the amount of messages a lot more. I'd expect a much more direct reason, like where the part of the brain that takes care of complicated stuff has a direct connection to the nerves connected to the tongue. Joepnl (talk) 17:01, 27 April 2014 (UTC)
- Tongue protrusion is one of the earliest behaviors shown by newborn babies. I'm just speculating, but possibly it is a reflex that escapes from inhibition when children are concentrating intensely. I don't recall ever seeing this myself, though, so I wonder how common it really is. Looie496 (talk) 19:36, 27 April 2014 (UTC)
- Maybe you're too young to remember Michael Jordan? ←Baseball Bugs What's up, Doc? carrots→ 20:18, 27 April 2014 (UTC)
- I'm older than Michael Jordan, but I'm not a Pro Basketball fan and don't know what you're talking about. Looie496 (talk) 21:43, 27 April 2014 (UTC)
- Google Image "Michael Jordan tongue" and you'll see many examples. ←Baseball Bugs What's up, Doc? carrots→ 23:04, 27 April 2014 (UTC)
- @Looie496: Google for "tongue concentration" and click images. That must look familiar. Joepnl (talk) 21:41, 27 April 2014 (UTC)
- Sticking one's tongue out while concentrating is a recurring theme in classic cartoons. ←Baseball Bugs What's up, Doc? carrots→ 23:13, 27 April 2014 (UTC)
- Yes, it's a nice visual way to convey "concentrating hard" in a single frame. StuRat (talk) 17:35, 28 April 2014 (UTC)
- Children learning to print letters of the alphabet sometimes stick out there tongues and make small tongue movements not unlike the motion of the pencil. It was funny therefore to observe freshman college students in a psychology lab experiment where they had to look at a star pattern they could only see in a mirror while tracing it with a crayon. Left to right movements have to be the reverse of when they look like. Some of them had their tongues out to somehow aid in the perceptual-motor class, and they laughed when they noticed it, remembering the behavior in kindergarten or first grade and how they had been told back then not to do it. The mirror-drawing task would be a great way to elicit the tongue-sticking-out behavior in adults. I recall people doing the "tongue sticking out" as well while learning to tie a complicated knot. Maybe its just by-play or maybe it helps somehow in doing a new perceptual-motor task. Edison (talk) 19:47, 28 April 2014 (UTC)
How much COLORS in the RAINBOW do see:... (???)
Humans and Primates with three(3) Color-Vision see: 3+3+1 = 7 Colors in the Rainbow...
Animals(Mammals) with two(2) Color-Vision see: 2+1 = 3 Colors in the Rainbow???...
Animals(Birds,Reptiles) with four(4) Color-Vision see: 4+4+4+1 = 13 Colors in the Rainbow???...
The "Mantis-Scrimp" with twelve(12)Color-Vision see: (11*12)+1 = 133 Colors in the Rainbow???...
THANK you VERY-VERY much!!!
and... "Have a nice Day/Night !!!..."
SPYROU Kosta - Greece — Preceding unsigned comment added by Honeycomp (talk • contribs) 22:21, 27 April 2014 (UTC)
- The number of colours we divide the rainbow into is not a physical absolute - it's culturally determined, and the common preference for the number 7 shows itself here. There's no direct connection between that and how our eyes work in reality. AlexTiefling (talk) 23:04, 27 April 2014 (UTC)
- (ec) The article Color vision includes a section about non-human species. Various animals are believed to have monochromatic, dichromatic, trichromatic (the human norm), tetrachromatic or even perhaps pentachromatic color vision, based on the kinds of color recepters in their retinae. We may agree on a set of names for the colors our eyes perceive but that is a subjective process with no obvious limit to how many categories our essentially continuous (analog) sensation has. This article discusses the arbitrariness of our color names. The article Rainbow mentions that a human eye can distinguish in the order of 100 colors in a rainbow spectrum. However this number is not reflected in any language and usually just 7 names suffice: Red, Orange, Yellow, Green, Blue, Indigo, Violet. It is not objectively correct to say any finite number of colors exist in the visible spectrum because it is a (small part of) a continuous range of electromagnetic radiation frequency, nor are colors we see limited to the spectral colors: in addition we see de-saturated (i.e. mixed with white) and impure (mixtures such as red+blue) colors. 84.209.89.214 (talk) 23:14, 27 April 2014 (UTC)
- Back in my day there were six - red, orange, yellow, blue, green, and violet. Now they say seven. Bubba73 You talkin' to me? 23:16, 27 April 2014 (UTC)
- Green has always lain between yellow and blue. 84.209.89.214 (talk) 23:26, 27 April 2014 (UTC)
- The divisions are (of course) completely arbitrary. But, yeah - I was always taught Red/Orange/Yellow/Green/Blue/Indigo/Violet. That doesn't mean that there are no other names..."Cyan", for example, is a color intermediate between green and blue which is clearly visible in a spectrum ("Sky blue" is perhaps a more common name). But some cultures have no separate names for green and blue - check out our article on Distinction of blue and green in various languages. In computer graphics, we talk about the color "Magenta" - which is a mixture of red and blue light. Technically, that doesn't appear in the spectrum because it's a mixture of colors - but it's visually similar to "Violet".
- Color is a subtle business. If you click on the image at right here until you're seeing it at the full size - it contains every single color that a computer screen can display. If you continue to zoom in until the original pixels in the image are big enough to cover a good area of your screen, you'll find that in some areas you can see the difference between adjacent colors - which means that you could have seen a color midway between the two. But in other areas, you can't tell the difference between adjacent pixels - even though they are different colors as far as your computer display is concerned. But even that isn't a complete description of what we can see because there are colors that computer screens and TV's can't display...getting a really good "cherry red" on a computer screen is completely impossible.
- Our perception of color is messy - everything depends on the brightness of the image - the color of the background around it - how bright the room lighting is - how tired you are...many, many things. So I don't think we have a good answer for "the number of colors we can see".
- What our OP is asking is a bit confused. Humans can see three "primary colors" (red, green, blue) and we can perceive three "secondary colors" (cyan, magenta, yellow) and one tertiary color (white). But we know that we see other colors like brown, orange, pink, grey that aren't primary, secondary or tertiary. There is at least one human who can see four primary colors (see: Tetrachromat). She can (presumably) also see seven secondary colors, four tertiary colors and one quaternary color (white)!
- SteveBaker (talk) 23:46, 27 April 2014 (UTC)
- No, normal color vision is based on four psychological primary colors, or six if you count white and black. It's hard to know how tetrachromacy would change that. Anyone with normal color vision can become a tetrachromat—a hexachromat, actually—by holding a broad-spectrum color filter in front of one eye. Congenital tetrachromacy might be like that: certain colors might just look "odd" because the brain is getting conflicting signals. Or it might involve two completely new opponent colors with qualia never experienced by ordinary humans (but it seems unlikely that the visual system is that flexible). I think no one knows. -- BenRG (talk) 04:09, 28 April 2014 (UTC)
- According to Indigo#Classification as a spectral color, Isaac Newton added indigo to the spectrum because for mystical reasons he thought that there ought to be as many colors in the rainbow as notes in the major scale. Given that origin, it's pretty silly that we still teach the seven-color rainbow in grade school. -- BenRG (talk) 04:09, 28 April 2014 (UTC)
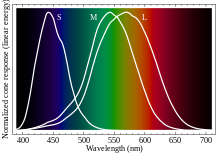
- To explain how our color vision works, look at this graph. That shows the relative strength of the signals coming from each of our 3 color receptors, based on the color. By comparing the signals returned by each of the types of receptors, our brains can determine which color it is. We could even live without the middle receptor, and still determine all the colors of the rainbow, if our brains were set up to use that input. All that we would lose is a bit of fine color resolution between green and red. Similarly, adding more receptor types wouldn't help us see more colors, unless they are further in the infrared or ultraviolet range. They would help us to distinguish close wavelengths more precisely, though. As you can see, those colors between the M and S receptors don't seem to change as much as between the M and L receptors, because those receptors are more widely spaced. If we had a receptor between M and S, say called the "M-S receptor", then we would presumably see more color differences in that range. StuRat (talk) 00:04, 28 April 2014 (UTC)
- Yes, you could "determine all the colors of the rainbow" with only two receptors, so long as the ratio of their responses were strictly monotonic. However, there are colors not in the rainbow: magenta is the most obvious choice, being a mixture from the two ends. With only two receptors you cannot distinguish a magenta that (say) stimulates both receptors equally from some shade of green that also stimulates them equally, or from (an approximate) white that is the sum of the two. (White is also not a spectral color, of course.) Conversely, adding more receptors does let you see more of these non-spectral colors. --Tardis (talk) 07:09, 29 April 2014 (UTC)
SPYROU Kosta: Hi...I unterstand that in the Rainbow Colors someone can see more or less colors from seven(7)... The only think you need is to give Names to the different shades of Colors!!!...
BUT it has to be a Mathematical and Physical FORMULA like:
2 colors A,B : {A},{AB},{B} = 3
3 colors A,B,C : {A},{B},{C},{AB},{AC},{BC},{ABC} = 7
The Eye has the (inner) and the outer Circle: (Red) + anti-red, (Green) + anti-green, (Blue) + anti-blue...
It must to be a way to calculate the Colors-Vision... (You cannot ask your cat-dog-bird how much Colors can they see!!!...)
THANK you and have a nice Day/Night
SPYROU Kosta - Greece - Honeycomp (talk) 02:39, 29 April 2014 (UTC)
- Colors are not finite things, there are an infinite number of colors in the rainbow, each with a slightly different frequency. You can come up with as many colors names and numbers as you would like, even without blending different frequencies of light together. So, how many colors we can see is all a matter of opinion. Now, there is what color resolution you can see, and more color receptors should increase that, but the number of color gradients we can see is in the thousands.
- The mistake you are making is thinking that all color receptors of a given type either fire at full strength or don't fire at all, for a given color. They can also fire at reduced strength or only some can fire. To represent this mathematically, perhaps we can use binary: If you have only two receptor types, and one has a value of "1", while the other has a value of "2", you might think you could only get values of 0, 1, 2, or 3 out of the total of the pair. But the first receptor type can actually have a range of values from 0.01 to 0.99 between 0 and 1, and the second receptor similarly has a range. And it's not just the sum that's significant, as our vision brain cells can distinguish which signal comes from which receptor type.
- So, vectors might be the way to represent the range of inputs to our brain. For example, a brain cell might get 0.87G + 0.93B + 0.02R, and conclude that the color is cyan. In this model, with 100 gradients detectable for each receptor type, and 3 receptor types, we get 1003 or a million possible colors. Our vision works something like that. You might think that with only two receptor types, than such an animal would only have 1002 or 10,000 colors, but they might also have more gradients they can detect at each receptor. If they can detect a thousand gradients, instead of 100, then we are back to 10002 or a million colors they can detect. StuRat (talk) 19:11, 29 April 2014 (UTC)
- Yes, but because most colors are combinations of frequencies, someone with only a red and a blue receptor would be unable to tell the difference between (say) green and magenta! Green light would stimulate both the red and blue receptors to some degree - and so would magenta light. It doesn't matter how sensitive those to receptors are - fundamentally, someone with no green detectors is color-blind and cannot perceive as many color differences as someone with three functional detectors. That's why tetrachromats are able to see more distinct colors than us trichromats. Someone who happened to have a detector for pure yellow light would see the color emitted by a sodium lamp to be a totally different color than the yellow emitted by a computer screen displaying a picture of a sodium lamp. The latter would have both red and green light - but no yellow...so it would look completely different. There is a question of whether a human tetrachromat would develop the necessary brain function to make use of that extra capability - but the study of that lady in the UK who is a tetrachromat proves that (at least in her case) it's perfectly possible. SteveBaker (talk) 19:59, 29 April 2014 (UTC)
- Interesting, do you have any links for that woman ? StuRat (talk) 02:12, 30 April 2014 (UTC)
- The researcher who tracked her down refuses to give her name - she's known in the literature as "subject cDa29" - if you google for that phrase, you'll find a bunch of information about her. I believe she was found as a result of a prediction that a very specific history of parental and grandparent colorblindness and a female subject would (at some probability) yield a tetrachromat - so they tracked down people with that history and tested them. This specific woman was (I believe) the only one they found. SteveBaker (talk) 02:45, 30 April 2014 (UTC)
SPYROU Kosta: THANK you SteveBaker for your Help!!!...Honeycomp (talk) 23:11, 29 April 2014 (UTC)
- SteveBaker made three mistakes. 1) As pointed out by StuRat, the retinal sensing is not binary (on/off), it's graduated. 2) SteveBaker seems to think that all "red" cones have their sensitivity peak at the exact same red wavelength, all "green" peak at the exact same green wavelength, all "blue's" on the exact same blue. That's not correct. In each nominal colour, diferent cones in the same eye peak at AROUND a similar wavelength. That's why you can see more colours than can be reproduced in a three-colour TV display, or even 4-colour printing, though the difference for people with normal colour vision is somewhat subtle. 3) He's not realised that the response of each retinal sensor is not linear - its logarithmic, just like every other sensory system in the animal kingdom, e.g., hearing. As is well known amongst audiologists, to make a sound percieved as twice as loud, you need about 10 times the power, not twice. Same with the light sensisivity of the retina, and the brain can utilise the improved response to weak colours. It means, for example, that the response of "blue" sensors is not just high sensitivity to blue, a bit to green, and none at all to red. The response, which varies for sensor to sensor, is maximum to blue, quite a bit to green, and weakly to red.
- All this means is that you do not get people who are missing (say) green cones and cannot see green at all, or cannot distinguish between different greens. They can, but to reduced degree. Some to greater degree than others.
- Note that individual cones are not wired to the brain. They are wired together in groups, each group sending an agregate signal to the brain. This improves tonal graduation and low light sensitivity at the expense of resolution. It's why I have used the term "sensor" above, because a more smooth logarithmic response to light level is constructed in the aggregation from what an engineer would regard as inputs from noisy erratic pickup in the cones.
- All this means that people can see an immense range of colours, even if only two types of cones are present, and even quite a range of colours if only one type of cone is present.
- SteveBaker is also wrong about not being able to get a good cherry red on TV displays, however this is a common misconception among graphic artists. It came about because in the early days of colour TV, RCA in America was able to make a very good red phosphor for their TV tubes. America and Europe then, very early, standardised the pigment colours for TV cameras and TV tubes on the RCA standards. However, the Japanese, who did everything they could think of to minimise paying royalties on RCA (and Philips) patents, standardised on a slightly brownish red phosphour. Almost all people do not notice the difference, but I used to work in TV repair, and if you adjust a Japanese TV set right beside a European set (using either a Philips tube or a licenced RCA tube), you can clearly see the difference, and you can't get as good a red on the Japanese set. The trouble is, by the 1980's, there was only Japanese picture tubes (made in Japan or Taiwan); RCA and Philips had become uncompetitive. High quality displays with a very good red capability remained available for non-television applications where it mattered, and it all changed when CRT displays were rendered obsolete by plasma and LED displays.
- 121.215.63.226 (talk) 02:02, 30 April 2014 (UTC)
- Nice patent history lesson ! As for those animals with only one cone type, they would be able to detect how far a given color is from the peak for that cone, but couldn't tell on which side of the peak it falls. They'd also be confused by combination colors. StuRat (talk) 02:14, 30 April 2014 (UTC)
- No, you've missed one of the points I made. For an animal with only one cone type, each cone will have its peak on a slightly different wavelength (colour). So, for any colour within the animal's good range, it CAN tell unambiguously what the colour is. The colour may be on one side of the peak for one particular cone (or aggregate of cones) in its retina, but nearby there will be other cones with their peaks on slightly different wavelengths/colours, and one or more of them will be on the same side of the peak of the first mentioned cone. It's quite different to the situation that you could have in a TV camera, where you could have a fault where two of the three colours are faulty or switched off - in such a case it is impossible to tell what the scene colours are, except in so far as how close the colours may be to the operating camera colour, as all the pixels of any of the three colours are the same. 121.221.220.128 (talk) 11:40, 30 April 2014 (UTC)
- But in order for the brain to be able to distinguish whether colors are on one side of the peak or an equal distance on the other side of the peak, using another receptor with a different peak location, the brain would need to know on which side the secondary receptor's peak was offset. How would it know this ? And if it does know this, and is wired to use that info, how is this different from having an additional type of color receptor ?
- In my own experience with cats, they seem to often miss food on the (different colored) floor, until I pick it up and hand feed it to them, so their color vision apparently isn't so good. StuRat (talk) 13:37, 30 April 2014 (UTC)
- How would it know? When born, it will not. But it is well established, that despite a considerable degree of anatomic structure determined by genetics, the brain sorts out what means what by experience. This is why our sense of pain location is exquisitely good on the skin or within limbs, but pain from within the torso is quite unreliable. For example, a heart attack hurts, but the pain is often not from within the appropriate part of the chest - pain in the neck, jaw, or left arm is quite common. That's because when we are babies, there is plenty of instances of contact with the skin and impact with limbs to calibrate the sensory system, but a heart attack occurs for the first time typically in late middle age or later - no prior contact experience. The sensory system knows something is wrong, but not where.
- So how will the brain know which cone peak means what colour? By experience. At first it only knows there something different about one colour versus another. How is this different to how the brain works with additional colour receptors? There is no difference.
- When people who were born deaf with some middle ear problem are fitted with advanced cochlear implants, the macro structure of their nervous system gives them a sense of hearing - but it's all cockeyed. Often not much more than a lot of odd buzzes and high pitched whistles. But over a few months, experience puts things into place, and they hear low frequencies as low frequencies, buzzes as buzzes, and pure tones as pure tones, natural voices as natural voices. There was a famous experiment done in the 1950's - a volunteer wore spectacles with prisms that inverted his view of his environment. Up was down and down was up. After a few weeks, everything looked normal to him - his brain, based on experience, re-wired itself so to speak, to make things right again.
- A friend of mine accidentally sawed off two fingers and the thumb of his hand with a circular saw. The doctors sewed one finger back on, but the other finger and the thumb was ruined and could not be put back. As a thumb is critical to holding and grasping all manner of things, the doctors cut off one of his big toes and with micro-surgery attached it back on where the thumb should be. They joined up the nerves more or less without regard of what nerve was for what. At first his finger and toe/thumb was numb. After a few weeks some feeling came back, but the location sensed was all wrong. After nearly a year, he says his sense of touch is completely normal, and correct as to location.
- I don't know much about cats, but in general, mammals have only 2-colour vision. Humans are an exception to the rule. Birds have 4-colour vision - their extra "colour" is the ultraviolet, which we can't detect. I have performed a number of experiments with my german sheperd dog. He certainly has visual accuity comparable to human vision, but is unable to distinguish between different shades of green. I established this by placing about my yard small balls of different colours, and rewarding him when he brought one to me. But its' not quite as simple as it may seem. Being an inteligent, strong willed animal, he tries to train me as much as I train him. If he's bored with bringing back balls, he simply will not. And of course, for a dog, it matters not how good their sight is, if there is a smell about. It's much more fun to a dog to find things by smell. I grew up on a goat farm. The preference of goats to food handed to them over perfectly good food on the ground is marked - especially if no other goats are within sight. It has nothing to do with any limitation in their vision or any other sense. It is because they crave attention and affection.
- 121.221.220.128 (talk) 15:20, 30 April 2014 (UTC)
- As far as fine-tuning the nerves on your skin, if it feels like a bug bit your palm, but you look at it and see it actually bit your thumb, then you have a secondary source of information to use to correct the primary source. What is the secondary source that will tell your brain whether a particular receptor has a peak on one side or the other of the average ? StuRat (talk) 16:36, 30 April 2014 (UTC)
- I would have thought that obvious. The secondary source is not so much seeing the bug on your thumb, its seeing the bug is on your thumb and not your palm. Ever hurt a toe? We wear shoes and don't manipulate tools with our feet so toes are protected from contact much more than fingers. It's thus hard to know without looking which toe is hurt. Yet the nerve structure is much the same for fingers. Not as fine but the routing is similar. Look at an example: Let's say a person has only "green" receptors. A scene providing (say) 5530 nm (green) would produce strong excitation of cones centred very closely on 530 nm, and less strong excitation in cones peaking at 525 nm (a slightly blueish green) and similarly less strong excitation in cones peaking on 535 nm (a slightly brownish green). But a scene containing 535 nm light would result in strong excitation in the last mentioned cones, a slightly weaker response in the 530 nm cones, and a weaker again response in the 525 nm cones. So the brain gets a different input for 525 nm light, 530 nm light, and 535 nm light. A baby would just learn by experience that the three wavelengths are different, until he can talk and in discussion with others would learn what the colours should be called. Note this: the same person with only "green" cones, would if seeing light on 460 nm (blue) would get a different set of signals again - a weak response in the 525 nm cones, a slightly weaker again response in the 530 nm cones, and the 535 nam cones, an even weaker response again. However the difference in response levels would be a lot less than the differences with various green wavelengths. So, such a person can identify red light, and blue light, and know the difference, but not at all well compared to a person with normal eyes. Scenes of predominantly red and/or blue light would be registered by the eye as quite dark, however the brain has great facility for compensation. — Preceding unsigned comment added by 121.221.220.128 (talk) 08:51, 1 May 2014 (UTC)
- Responding to 121.215.63.226/121.221.220.128 whose posts by content seem by the same person: SB did not make alleged mistakes nos. 1) or 3). SB also has colour theory on his side (this time) in pointing out that there are colors that computer screens and TV's can't display; these colours certainly include saturated spectral reds. Notwithstanding the debateable redness of cherries on TV, this is known to graphic artists. The implication of 121's claim that the brain gains colour discrimination at the expense of resolution by sensing groups of cones with fixed in-group patterns of unequal responses is that one should be able to stimulate different colour perceptions by changing the arrangement on a screen of a set of fixed pixel colours. That would be a significant development in Opponent process theory but has there ever been such an experimental validation? 84.209.89.214 (talk) 20:39, 30 April 2014 (UTC)
- As far as fine-tuning the nerves on your skin, if it feels like a bug bit your palm, but you look at it and see it actually bit your thumb, then you have a secondary source of information to use to correct the primary source. What is the secondary source that will tell your brain whether a particular receptor has a peak on one side or the other of the average ? StuRat (talk) 16:36, 30 April 2014 (UTC)
SPYROU Kosta: From the Book: "The First Steps in Seeing" by R.W.RODIECK - pages 218-219:
"Humans that lack functional L cones or M cones are also Dichromats. Rare human dichromats have this deficit in only one eye. To that eye, a rainbow appears BLUE in the INNER portion of the arc, fades to colorless toward the center, and progresses to YELLOW toward the OUTER edge of the rainbow..."
Kosta: {A}, {B} and White{AB} !!!...
So...my "Theory" has a point(.) !!!
THANK you for your Help - Honeycomp (talk) 00:26, 1 May 2014 (UTC)
Fullerene C24...Space-Filling Fullerite C24...are they only in THEORY???...
The Fullerene C24:
has the shape of the Truncated-Octahedron...with six(6) [Squares] and eight(8) <Hexagons>...
has 12 (C=C) Double Bonds and 24 (C-C) Simple Bonds.
The Fullerite C24:
has the shape of the Space-Filling Truncated-Octahedron...
has only Simple (C-C) Bonds...
Are they REAL or NOT???...
THANK you VERY-VERY much!!!
SPYROU Kosta - GreeceHoneycomp (talk) 22:35, 27 April 2014 (UTC)
- It's my understanding that there are no stable fullerenes smaller than the classic C60 structure, although the existence of cubanes shows that carbon bonds can be made to do much more unusual things. AlexTiefling (talk) 23:03, 27 April 2014 (UTC)
- It seems my recollection was correct - in C60, there are no squares, and no two adjacent pentagons. Either of those features would make a smaller shape unstable. C24H24, using the geometric shape you've described and no double bonds, would be a viable explosive, but there's no reason to make that shape rather than another. AlexTiefling (talk) 14:47, 28 April 2014 (UTC)
- All unstable? That surprises me; I remember reading that someone had isolated C36, whose 15 hexa/penta forms include the two smallest where no three pentagons meet. —Tamfang (talk) 07:28, 29 April 2014 (UTC)
- Interesting - if you can find a source, could it be added to our article on fullerenes? AlexTiefling (talk) 15:37, 30 April 2014 (UTC)
SPYROU Kosta: Why a Space-Filling "Cube(Rook)" of Truncated-Octahedrons-C24 would be EXPLOSIVE???... They are NOT H-Hydrogens in the inner Cube(Rook)!!!... They are only C-Atoms like the Diamond!!!... By the Diamond all the six(6) angles are 109,5o... By the Space-Filling Truncated-Octahedron two(2) angles are 90o
THANK you!!! Honeycomp (talk) 02:22, 29 April 2014 (UTC)
- What do you mean by "rook" here? —Tamfang (talk) 07:28, 29 April 2014 (UTC)
SPYROU Kosta: SORRY I mean a stone: Rock !!! sorry!!!...I play to much Chess!!!...Honeycomp (talk) 14:02, 29 April 2014 (UTC)
- It's my understanding that 90-degree bond angles are pretty unstable - this is part of the reason cubanes are so explosive. You're right that a C24 with no hydrogens would probably be less combustible than one which had hydrogens, but that alone wouldn't make it stable. It might not explode, but it could readily decompose into an assortment of soot and shorter organic molecules.
- One other thing that occurs to me, and this may be hogwash, because it's years since I studied this, but your proposed C24 structure accounts for all the outer-shell electrons of every carbon atom. That rather implies that each molecule would have a very limited capacity to form electrical bonds with its neighbours, so I think it's comparatively unlikely that any theoretical close-packing would be maintained in practice. They'd behave a lot more like ball-bearings than like solid blocks. AlexTiefling (talk) 15:37, 30 April 2014 (UTC)
SPYROU Kosta: "THANK you very much AlexTiefling!!!..." Honeycomp (talk) 19:17, 30 April 2014 (UTC)
Development Evolution-Tree of the 220 Human Cells???...
Humans have 200-220 diferend kind of Cells...
Is there a Development-Tree of them???...
THANK you VERY-VERY much!!!
"Have a nice Day/Night!!!..."
SPYROU Kosta - Greece - Honeycomp (talk) 23:01, 27 April 2014 (UTC)
- In a developing embryo, stem cells differentiate into all the specialized cells—ectoderm, endoderm and mesoderm and thereafter maintain the normal turnover of regenerative organs, such as blood, skin, or intestinal tissues. 84.209.89.214 (talk) 23:23, 27 April 2014 (UTC)
- There are different (related) senses of the word "evolution," so I'm not quite sure if you mean the time evolution of an individual and cell types within, or evolution of species over time (or both), but these links cover a bit of each. Check out embryogenesis for a description of what cell and tissue types form in the development of an individual human. Human_development_(biology) also gives an overview with lots of wiki links. See human evolution for how we think humans evolved. When we study evolution of organisms, and how development changes with evolution (and what the development of different organisms tells us about their evolutionary history), you get the vast topic of evolutionary developmental biology, sometimes shortened to "Evo-devo". SemanticMantis (talk) 00:00, 28 April 2014 (UTC)
- There are about 200 types of uniquely identifiable types of mature cell in the human body. Primitive fungi and algae might have two or three cell types, hyphal, thallus and reproductive, and so forth. For example, and without being specifically correct, there might be a stem blood cell that differentiates into stem white cells and stem red cells, and the stem white cells might differentiate until, after various differentiations, you get the two dozen or so types of immune cell. Same with rods and cones and neurons and glial cells.
- A tree can be drawn which shows which of these cells differentiate from which. That tree evolved slowly over time, and its phylogenetic development is mirrored in onto genetic development. The earliest divisions in animals, the endoderm, mesoderm and ectoderm stem cells have been mentioned above. The user wants a full diagram that shows all 200 as they develop from the zygote. The only charts I have found have been for the blood cells. I did try to see if we had an article on this a couple of years ago, but was unsuccessful. μηδείς (talk) 01:45, 28 April 2014 (UTC)
- Doing a real quick search I stumbled on [8] which led me to [9]. I'm not going to take the time to look over the data and figure out what it means right now, though. We really ought to "emancipate" this data for the good of Wikipedia by some mechanism or other - looking at their topographic map I bet we can do better with a Lua module, if I can find the raw numbers. No promises though! Wnt (talk) 03:52, 28 April 2014 (UTC)
- Thanks, User:Wnt, that first source is an excellent introduction into the topic the OP is looking for. μηδείς (talk) 04:15, 28 April 2014 (UTC)
- A number such as 200-220 strikes me as arbitrary, and dependent on what is counted as a "difference". There are many more distinguishable types of neurons in the brain alone than that. Looie496 (talk) 14:56, 29 April 2014 (UTC)
- The literature does usually say just over 200. It's also a developmental issue. If a neuron in the brain and a neuron in the peripheral nervous system are functionally interchangeable, and could have had their places swapped by manipulation during development, they would be considered the same cell type. It's a matter of differentiation between what genes are expressed (cone cells and liver cells express different genes) rather than just difference in location. Stuart Kauffman addresses the topic and its importance in his book, The Origins of Order.
- That being said, in humans of course there are probably a few dozen known cell types in the brain. But that is conidered in the enumeration. According to this paper Trichoplax has four cell types, Flatworms about a dozen, Pine trees about 30, Earthworms about 60, and Mice and Dogs about 100. That probably implies that much of the difference between humans and other higher mammals does lie in the nervous system, if not also the liver and digestive system, given our more varied diets and tolerance for plant toxins, although that's a guess on my part. μηδείς (talk) 22:13, 29 April 2014 (UTC)
- True, it is sort of arbitrary. To be a bit flippant, but not entirely wrong I don't think, I'd quip that given a source of white blood cells, you probably can take any given hematopoietic cell type and an arbitrary marker CDnnn, and sort out some cells that are CDnnn+ and some that are CDnnn-, and find some meaningful difference between the two. And repeat that as long as you like with one subpopulation of the next, until you run out of FACS time. :) Nonetheless -- even though the cell types recognized by histologists are quite subjective, and subjective standards are kind of bad, classical taxonomy demonstrated that a subjective standard is still relatively level and useful as a guide. It's not perfect but not useless either. Wnt (talk) 11:49, 30 April 2014 (UTC)
April 28
SF2 - SF4 -SF6... Why only H2O and NOT H4O or H6O???...
Sorry but I know that it is a silly Question...
My REAL Question is: If the properties of the Bonds of a Atom changes with the Pressure and Temperature...
So... could we have H4O or H6O under specific circumstances???...
THANK you VERY-VERY much!!!...
"Have a nice Day/Night"
SPYROU Kosta - Greece - Honeycomp (talk) 01:41, 28 April 2014 (UTC)
- Because oxygen is a second-period element and therefore can never be hypervalent -- because the 2nd electron shell only has room for 8 electrons. 24.5.122.13 (talk) 03:00, 28 April 2014 (UTC)
- That said, you can have H3O+, but that is a positively charged cation made by the addition of an H+ cation to H2O, not a neutral molecule. 24.5.122.13 (talk) 03:03, 28 April 2014 (UTC)
- The article about hypervalent molecules has several explanations using more modern theories than the simple "number of electrons in the valance shell" and standard 2-electron valence bonding (i.e., the origins of the octet limit for second-period vs expanded-octet via d orbitals for third and beyond). So much of what's taught in schools is only an approximation or most-common-case in order to give intro students a starting point (but then they forget or never learn the advanced subtitles), or sadly just out-dated! Neutral H3O (CAS #12168-76-2) is known. It's a radical, and indeed requires specific circumstances to synthesize and contain (see doi:10.1021/ja00740a038). Neutral H4O (CAS #37381-92-3) has been studied theoretically. It may have some stability as a tetrahedral structure (doi:10.1016/0022-2860(80)80356-5) or planar/seesaw-shape (International Journal of Quantum Chemistry, Symposium (1972), No. 6, 187–200). Something with this composition, as a covalent network-lattice solid (rather than discrete molecules), is hypothesized to exist at extreme pressure (doi:10.1103/PhysRevB.87.024112). DMacks (talk) 04:00, 28 April 2014 (UTC)
- By "extreme pressure", I presume you mean the kind of pressure that can turn hydrogen into a metal? 24.5.122.13 (talk) 09:48, 28 April 2014 (UTC)
- Simplistically: Atoms like to have their 'electron shells' nicely full of electrons. Oxygen has room for two more electrons than it has - and hydrogen has one electron. So if two hydrogens each share their one electron with a single atom of oxygen, everyone can be happy. If there were more or fewer hydrogens, there would not be the beauty and harmony of a full shell and water wouldn't be such a stable compound.
- That said, H2O2 is hydrogen peroxide - which is very reactive because it wants to dump one of those that extra oxygen atom. SteveBaker (talk) 19:31, 28 April 2014 (UTC)
Excited decay
How does a unimolecular population in a particular excited state decay - linearly, or exponentially, or is it dependent? Plasmic Physics (talk) 07:40, 28 April 2014 (UTC)
- It decay is spontaneous, it will decay exponentially. Ruslik_Zero 12:27, 28 April 2014 (UTC)
- On very short timescales there can be small deviations from exponential decay, see e.g. here. Count Iblis (talk) 21:10, 28 April 2014 (UTC)
- Thank you all. Plasmic Physics (talk) 10:22, 30 April 2014 (UTC)
Mind Gym
Hi all. I'm looking for some scientific review of things like "Mind Gym" (a quick google will show you what it is - we only have an article on it's creator which reads like it was written by the guy himself). It all just seems like it is a load of vague motivational nonsense, which whilst it will increasse motivation, it isn't free and therefore needs to prove it's worth. I remember reading a while ago that UK schools had spent a large amount of money on "workouts" that were supoosed to get different parts of your brain working, but it was later shown to be total nonsense - does anyone have a source for this? Has anything been done to prove whether or not things like mind gym actually work well enough to justify the (probably quite large) cost? 80.254.147.164 (talk) 09:44, 28 April 2014 (UTC)
- Here's a good starting point: Brain Gym®: Building Stronger Brains or Wishful Thinking? (PDF). Basically says it's nonsense. There may also be other good links from some of Ben Goldacre's pages. --OpenToppedBus - Talk to the driver 11:25, 28 April 2014 (UTC)
- It's pretty much a pseudoscientific scam that being peddled to naive/desperate parents and schools in Britain. Much about it in the press over the past few years. Google and you'll find a lot. Dominus Vobisdu (talk) 11:31, 28 April 2014 (UTC)
- Just to be clear; the company I'm querying is called "Mind gym", the one that's been in the news was "brain gym". They have very subtly different claims, with Mind Gym being general vague management promises, whereas Brain Gym is proper psuedoscience. 80.254.147.164 (talk) 11:52, 28 April 2014 (UTC)
- For ease of access, the material in question is Octavius Black#Mind Gym. The material there does look entirely promotional, and really ought to be deleted. Looie496 (talk) 16:10, 28 April 2014 (UTC)
Blackberry Z10 wmv format
Which types of formats of wmv does the Blackberry Z10 play or work on? My dad has one but doesn't play WMV videos and it says that the phone does support the format of wmv. Also, what other formats of other videos like MP4, FLV and etc, does the phone play? Will they work on SDXC card? — Preceding unsigned comment added by 162.219.184.237 (talk) 16:28, 28 April 2014 (UTC)
- The different container file formats and video and audio codecs that the Z10 supports are listed on this page. -- Finlay McWalterᚠTalk 16:51, 28 April 2014 (UTC)
- Keep in mind the extension of a media file does not not tell you all there is to know about how the file is internally formatted. WMV is a digital container format, but inside that is video codec and audio codec formatted data. So your Blackberry might play some WMV files and not others depending on how they are formatted internally. Richard-of-Earth (talk) 18:16, 28 April 2014 (UTC)
- Information about what codecs are used in a specific media file can be discerned with FFmpeg, simply by running ffmpeg -i somevideofile.mpg -- Finlay McWalterᚠTalk 19:07, 28 April 2014 (UTC)
"Deep processing"
I am just wondering to what extent does deep processing would take into account of repetition (aka memory rehearsal). I mean, isn't it plausible to think that the longer you recite something over and over again, you will happen to find some sort of mnemonic? 140.254.226.240 (talk) 20:12, 28 April 2014 (UTC)
- If you are going to put scare quotes around a term like deep processing it might help if you either linked to a WP article or an outside source. And are you looking for actual references, or some sort of approval of your hypothesis? Help us out here. μηδείς (talk) 22:14, 28 April 2014 (UTC)
- In psychology studies I have read "deep processing" means the opposite of mere repetition. It would mean processing letters to see if they are vowels or consonants, for instance, or processing trigrams to the point of determining whether they are words, when only a physical match reaction time task is required. Edison (talk) 23:55, 28 April 2014 (UTC)
- You might like to read The Ravenous Brain by Daniel Bor. In it, he says that countless memory experiments have been skewed by people continually looking for mnemonics to improve their performance. They don't just happen to find mnemonics, they actively search for them. Whatever impact this has on your claim is up to you to decide, because, as Medeis says, you haven't given us a lot of information. IBE (talk) 01:58, 29 April 2014 (UTC)
Big brown-backed bunny with black bordered ears?

The common species of rabbit in NYC and New Jersey is the Florida cottontail, which is somewhat small and uniformly grey in my area. A rabbit which I saw this weekend and which has been around for a month or so at my parents' in NJ is obviously a cottontail, but it has a brownish saddleback with a grey underbelly and has black margins to the inside of its ears, almost like eyeliner. It appears to be about twice the mass of a normal plain grey cottontail of the area. (Indeed, this is the only non-Florida cottontail I have ever seen besides a domestic rabbit.) I have read all our cottontail (quite a few species) articles to no avail.
Can anyone suggest what species this might be? (We have pictures, but they are from the rear with the rabbit crouching low, so you can't see the front margins of the ears or see the difference between the grey belly and the browner back. Thanks. μηδείς (talk) 22:08, 28 April 2014 (UTC)
- "Twice the mass" ? What an odd way to specify a size difference in an animal you clearly haven't put on a balance scale. Are you saying it's 26% longer, wider, and taller, so 1.263, or twice, the volume, assuming similar shapes between the rabbits, and you also assume it has about the same mass density as smaller rabbits, and thus it's twice the mass, too ? StuRat (talk) 23:03, 28 April 2014 (UTC)
- Yes Stu, that's exactly what I meant, which is why I said it. Weird for the science desk, huh? Sorry if it made the space between your ears overheat. I've started a talk page dicussion if you want to explain why my well chosen wording was problematic. μηδείς (talk) 03:09, 29 April 2014 (UTC)
- It's odd because you can't observe the mass directly, but only guess at it, and scientists only report direct observations, not guesses. Feel free to hat this aside, if you wish. StuRat (talk) 18:42, 29 April 2014 (UTC)
- If I were in a position to measure the bunny's length, I'd also be in a position to measure its mass and volume. About twice the mass was chosen as the most economic means of expressing what amounts to the exact same thing, given the shape and density are constant. μηδείς (talk) 17:00, 30 April 2014 (UTC)
- If you had a pic of it up against a brick wall, you could measure how many bricks long it is, then go out with a ruler and determine how long that is. Same with a bush, etc. StuRat (talk) 14:15, 1 May 2014 (UTC)
- Big rabbit with black ear margins? Sounds to me like this guy, but it's got a looong way to hop to New Jersey :) --Dr Dima (talk) 23:42, 28 April 2014 (UTC)
- No, Dr. Dima, it has a nice white cottontail and the same proportions as a cottontail. It's not a hare, but it doeesn't seem to match any of the species native to NJ. I'd estimate the black edges to be less than 1/4", and the ear shape normal for a cottontail. Again the only difference seems to be the noticeably browner back as compared to the rest of the fur, the size, and the black ear margins. I'll post a picture if my dad can get one . μηδείς (talk) 03:09, 29 April 2014 (UTC)
- Could it just be a released/feral/interbreed with a domestic rabbit breed of some sort? --Jayron32 11:05, 29 April 2014 (UTC)
- I came in to suggest a domestic/wild interbreed as well. As a kid, we had Dutch Dwarf bunnies that were tiny, with tiny ears. Several of them roamed the neighborhood. For years later, we would occasionally see "wild" rabbits that were much smaller than average, with tiny ears... SemanticMantis (talk) 13:17, 29 April 2014 (UTC)
- Yes, that occurred to me, and I can't rule it out. But the body form of domesticated rabbits is usually longer than that of cottontails, while this one is normally shaped, and the coloration is drab save the browner saddle on the back and the black "earliner". One would expect some white or some black in the coat if it were a hybrid with a domestic. Plus I am not sure if Sylvilagus hybridizes with other genera, domesticated rabbits being of Eurasian origin. It really does look for all intensive purposes like a larger-than-normal cottontail with noticeably but not strikingly different coloration. I was hoping someone from a nearby state might say-oh that's what all the rabbits here look like. μηδείς (talk) 17:31, 29 April 2014 (UTC)
- Well, you might appreciate more detail from my anecdote: we had two particular dutch dwarf rabbits that roamed around. They were uniform black, a male and female. This was in OH, and I'm pretty sure the wild bunnies around were the S. floridanus that you mentioned. The dutch dwarf is a Oryctolagus variety. I can not claim conclusive proof of interbreeding in my specific case, this paper [10] indicates that Oryctolagus spp. can interbreed with Sylvilagus spp. Anyway, the bunnies that we saw that were presumed hybrids had no noticeable changes in coat, except perhaps a slightly darker hue. The main difference is that their adult size was about 2/3 the weight of the other cottontails, and perhaps 20-30% heavier than the putative dwarf parents. Genetics of coat color are often counterintuitive, see Domestic_rabbit#Genetics and links therein.
- Anyway, pretty tough to say conclusively without genetic tests, but I think that e.g. a 6-8 lb. cinnamon rabbit with dark "earliner" could mate with a 3-4 lb wild cottontail to produce a 7 lb hybrid that would look like you describe. I'm not certain, but I feel like there are no pure wild cottontails in the eastern USA that get to the 7+ pounds that is roughly double the weight of a common eastern cottontail. SemanticMantis (talk) 18:15, 29 April 2014 (UTC)
- Well, it's a shame we don't have a picture of a Cinnamon Rabbit, as they are pretty. The border around the ears is again to broad for the mystery rabbit, but it does match the pattern, if not the width. And I am certainly familiar with the fact that coat color in crosses can be unexpected. We had a blond German Shepherd, and when she mated with a white mle the result was two blond pups, two "Rottweiler" colored pups, and two that looked like silver huskies. I'll buy some carrot friday, and see if we can't get a better picture. μηδείς (talk) 20:59, 29 April 2014 (UTC)
- May or may not be pertinent, but the bog standard British rabbit, although usually grey like your cottontail shown above, will sometimes have black patches, particularly on their ears. Fans of Watership Down might recall a rabbit called "Blackberry" who was distinguished in this way. Totally black wild rabbits are not unknown, like this one. Alansplodge (talk) 20:05, 1 May 2014 (UTC)
Cooler question
I have a portable cooler which comes with something like a hot water bottle, with the idea being to freeze that to provide the cooling to keep everything cold. I've heard that saltwater will hold more "coolth", than freshwater. However, I don't know how much difference it makes. If there's only a minimal benefit, I'd prefer to stick with freshwater, as that makes less of a mess if it spills. So, if we assume I can cool either down to 0 F, how much difference will it make ? StuRat (talk) 22:54, 28 April 2014 (UTC)
- It can make a big difference because you lower the melting temperature. The problem on the long term is that things will heat up eventually. You deal with that by exploiting the fact that the latent heat of melting is large. So, at first the temprature is going to rise steadily, until you hit the melting temperature. When you reach that point the temperature will be stuck there for a long time until everythig is molten. So, by adding salt, you move that melting point downward, therefore the contents will stay a lot cooler for a long time. Count Iblis (talk) 23:29, 28 April 2014 (UTC)
- Right. My understanding is that the difference made will depend on the thermal mass of the (salt) water and the size of the cooler. If the liquid bottle is half the volume of the cooler, the salt/fresh difference will be larger than if the liquid bottle is 1/100 the volume of the cooler. SemanticMantis (talk) 13:14, 29 April 2014 (UTC)
- The water container is about 1/10th the volume of the cooler. But why would that affect the relative value of saltwater versus freshwater ? Is this because the water, once it melts, will no longer provide much cooling, and that would happen quicker with either saltwater or a larger ratio of cooler size to coolant ? StuRat (talk) 18:40, 29 April 2014 (UTC)
- My thinking was just based on thermal mass. If there is x% difference with salt and regular water, then the actual difference in the application of keeping food cool in a cooler will be much larger when you use more water (e.g. if you were measuring minutes until food reaches 40F, then the larger volume of liquid would lead to a larger difference in minutes). SemanticMantis (talk) 21:00, 29 April 2014 (UTC)
- The Count's logic seems backward to me. As he says, the great bulk of the cooling effect comes from the energy it takes to cause a state change from solid to liquid. As I understand it, that energy is actually reduced in salt-water ice (but I might be wrong about that). Disregarding any such difference, the result of using salt-water-ice would be to keep the contents colder while the ice stays frozen, but I think it will melt more quickly, because there will be a larger temperature gradient between the ice and the outside of the cooler. Looie496 (talk) 14:45, 29 April 2014 (UTC)
- Yes, the levelling off of the temperature rise curve will occur at a lower temperature for salt water, but, ultimately, I tend to agree that the total cooling effect over a long period is probably marginally reduced by adding salt. I suppose it depends on the exact configuration of food, container and frozen fluid, and on whether you are trying to keep ice cream at -10C or just ordinary food at below 4C. Dbfirs 07:41, 30 April 2014 (UTC)
- This does seem like more of a practical issue than a scientific one. If you have something that you care about keeping solid rather than liquid, and you are frequently opening the lid, then you're averaging 0 degree ice with plus degree weather to get ... liquid something. But there aren't that many things that this really applies to. Wnt (talk) 11:41, 30 April 2014 (UTC)
- RE: "more of a practical issue than a scientific one", isn't this "applied science" ? StuRat (talk) 17:12, 30 April 2014 (UTC)
- Well, my point is that it depends on what the person wants to do. If you're worried about steaks dripping blood all over the place you might want extra cold. If you want cold beer/soda you can toss some ice in a cooler with the drinks and not give a second thought. (Or in my case omit the cooler since AFAIC they have more flavor warm anyway) Wnt (talk) 19:40, 30 April 2014 (UTC)
- I use it to transport frozen foods home from the grocery store, such as ice cream, which forms ice crystals if it melts on the way home and later refreezes. (At least they stopped selling it in those stupid cardboard boxes that leaked out the corners as soon as it melted.) StuRat (talk) 14:12, 1 May 2014 (UTC)
April 29
How can a universe have properties as a whole, how can it live in a time realm not of its own making?
I miss (in the Big bang lemma) a discussion about the following problem:
A universe only can be said to expand if it has a finite inside dimension –which in increases in time.
Not to mention that Big Bang cosmology in speaking about its age, asserts that the universe lives in a time realm not of its own making, doesn’t a (finite) inside dimension imply a finite outside size –even though it obviously cannot be measured from the outside as space and time, the meter and second aren’t defined outside of it?
Put differently, what is the significance of statements about the size and age of the universe if by definition there is nothing outside of it with respect to which its size and age matters, physically?
Can someone explain how a universe can have particular properties and evolve as a whole if there is nothing outside of it with respect to which it can have properties, can interact with and express such properties? Antonquery (talk) 00:25, 29 April 2014 (UTC)
- You've got a false premise there: the measurement of the size and age of the universe does not imply the existence of any exterior thing relative to which that measurement must be made. All the measurements are made with respect to things inside the universe that are - of necessity - younger and smaller than it. When we say the universe is 13.7 million years old, we do not mean that anything at all happened or existed 14 million years ago - we just mean that the universe has existed a bit more than two and a half times as long as the earth has. And the expansion of the universe means that we can determine that all objects were closer together long ago. It doesn't mean there's a space they've expanded into, and it doesn't mean there's a centre they've expanded from. AlexTiefling (talk) 00:34, 29 April 2014 (UTC)
- Psst... billion, not million, right? ←Baseball Bugs What's up, Doc? carrots→ 02:15, 29 April 2014 (UTC)
- A million years here, a million years there, pretty soon you're talking about a real long time. —Tamfang (talk) 03:42, 29 April 2014 (UTC)
- Ehh, what's a factor of a thousand between colleagues? AlexTiefling (talk) 11:32, 29 April 2014 (UTC)
- A million years here, a million years there, pretty soon you're talking about a real long time. —Tamfang (talk) 03:42, 29 April 2014 (UTC)
- Psst... billion, not million, right? ←Baseball Bugs What's up, Doc? carrots→ 02:15, 29 April 2014 (UTC)
- The OP calls the Big Bang theory a lemma which is a philosophical statement that one accepts as true in order to find out whether another statement is true. This rightly characterizes the theory as a hypothetical model that strives to put an explanation to our observation that the universe is expanding. The idea began with Edwin Hubble's observation that all distant galaxies and clusters have an apparent velocity directly away from our vantage point: the farther away, the higher the apparent velocity, regardless of direction. Assuming that we are not at the center of a giant explosion, it must seem that all observable regions of the universe are receding from each other. Big Bang theory proposes that it has "always" been doing that, where the quotes show that such a simplistic assumption creates the paradox that everything originated from an abstract singular point. The implication is that if one could rewind in time our view of the universe and see it shrink, its outer border would come into view. For the moment, the popularity of the Big Bang model relies on Albert Einstein's general relativity and on simplifying assumptions such as homogeneity and isotropy of space. Since Copernicus, scientific thought has insisted that Earth is not in a central, specially favored position in the universe nor that humans are privileged observers of the universe. Thus we can do no more than theorize about what limits might exist, or existence itself, beyond our particular Cosmological horizon as in the OP's question about the Size of the universe. 84.209.89.214 (talk) 11:26, 29 April 2014 (UTC)
- Lemma can also denote a headword in a dictionary or something similar. That's how the OP was using it—as a (somewhat confusing) way of saying "the article with 'Big Bang' as its title", not to refer to "a philosophical statement that one accepts as true in order to find out whether another statement is true". Deor (talk)
- I'd just like to add a pointer to Mach's principle, which is relevant to this discussion even if it doesn't apply directly. Looie496 (talk) 14:36, 29 April 2014 (UTC)
Is it true that eating burned food is not good for you?
^Topic ScienceApe (talk) 00:54, 29 April 2014 (UTC)
- For refs on WP, see Cooking#Cooking_and_carcinogens, and Heterocyclic_amine_formation_in_meat. SemanticMantis (talk) 13:10, 29 April 2014 (UTC)
Hand Meyer and Ernest Overton in Lund
Greetings. A photo of Hans Meyer and Ernest Overton taken in Lund, Sweden between 1907 and 1911 exists on Wiki. I have seen it twice but cannot find it again. Any suggestions? I have looked on wikis in various languages but I can' locate the page with that photo. — Preceding unsigned comment added by 2602:30A:C08C:F440:0:0:0:41 (talk) 01:42, 29 April 2014 (UTC)
Milk gone off but doesn't smell bad?
Is it possible for milk to curdle without smelling bad? I just poured milk on my cereal and it was all nasty and lumpy but there was no smell. Is that possible naturally or is my housemate trying to kill me? --78.148.106.196 (talk) 07:47, 29 April 2014 (UTC)
- Sure it's possible naturally -- that's how they make cheese! As for smelling bad, that has to do with milk going sour, which is a different process altogether. 24.5.122.13 (talk) 10:15, 29 April 2014 (UTC)
- That curdling is caused by growth of bacteria in the milk. The bacteria produce acids, and the acids cause the milk to curdle. You can also cause milk to curdle by adding acid directly, for example lemon juice or vinegar. Whether the curdling is harmful depends on what type of bacteria are growing. If they are the same types used to produce buttermilk or yogurt, then the milk can be perfectly healthy and good-tasting even after it curdles. But other types of bacteria will make it nasty-smelling and harmful. Looie496 (talk) 14:27, 29 April 2014 (UTC)
- So what happened to my milk? Did I throw away some good cheese? I've never had milk go lumpy and not stink before. --129.215.47.59 (talk) 20:00, 29 April 2014 (UTC) (formerly known as 78.148.106.196 (talk)
- It's probable that you could have made good cheese from your lumpy milk, but commercial cheese is made using rennet and a cultured bacterial mix (so that they know the bacteria are harmless). I think I would have thrown it away as you did, just in case there were some nasties amongst the bacterial mix that came from the environment. Dbfirs 21:20, 29 April 2014 (UTC)
- So what happened to my milk? Did I throw away some good cheese? I've never had milk go lumpy and not stink before. --129.215.47.59 (talk) 20:00, 29 April 2014 (UTC) (formerly known as 78.148.106.196 (talk)
- That curdling is caused by growth of bacteria in the milk. The bacteria produce acids, and the acids cause the milk to curdle. You can also cause milk to curdle by adding acid directly, for example lemon juice or vinegar. Whether the curdling is harmful depends on what type of bacteria are growing. If they are the same types used to produce buttermilk or yogurt, then the milk can be perfectly healthy and good-tasting even after it curdles. But other types of bacteria will make it nasty-smelling and harmful. Looie496 (talk) 14:27, 29 April 2014 (UTC)
- If you drained most of the whey from your curdled milk, and scrambled up what is left, you would have cottage cheese. Looie496 (talk) 01:31, 30 April 2014 (UTC)
Fast walking in the heat
Do people with good levels of fitness not sweat or feel out of breath after walking fast in hot weather? 194.66.246.11 (talk) 11:26, 29 April 2014 (UTC)
- For sweat, it seems to be the opposite of what you suggest. Here are two sources that say that people who are fit will start to sweat sooner, and more easily [11] [12]. No comment there on "out of breath". As for heat, note that humans change when living in different environments, see Acclimatization#Humans, and here [13]. Basically, people who spend more time in heat and exercise at higher heat respond differently than people who are not used to it. SemanticMantis (talk) 13:07, 29 April 2014 (UTC)
- People with good aerobic fitness are less likely to feel out of breath in almost any situation. Regarding sweating, that's more directly a function of weight than aerobic fitness. Light people have a higher ratio of body surface to body mass than heavy people, so they don't need to sweat as much to stay cool. However, there is an additional factor: people who have gotten used to exercising in hot weather are less likely to notice that they are sweating than people who are not adapted to it. Looie496 (talk) 14:32, 29 April 2014 (UTC)
- I'm sure your first sentence is right, but it's a rather tricky think to find a WP:RS to cite, isn't it? If anyone has one, please add. As for the weight, that is surely a big factor as well. My (admittedly not great) links above indicate that for two people of the same weight, the more aerobically fit one will sweat more during the same exercise conditions. SemanticMantis (talk) 14:41, 29 April 2014 (UTC)
- People with good aerobic fitness are less likely to feel out of breath in almost any situation. Regarding sweating, that's more directly a function of weight than aerobic fitness. Light people have a higher ratio of body surface to body mass than heavy people, so they don't need to sweat as much to stay cool. However, there is an additional factor: people who have gotten used to exercising in hot weather are less likely to notice that they are sweating than people who are not adapted to it. Looie496 (talk) 14:32, 29 April 2014 (UTC)
Are been, a telephone links had been a powerful electric volts
Is it been, a powerful electric volts are beening in a telephone links, because a some transformation of a powerful electric volts always had been saved a incoming amplitude frequency of a powerful electric volts after a transaction of it?--Alex Sazonov (talk) 12:11, 29 April 2014 (UTC)
- Landline covers the technical aspects of over-wire telephone service. I think that's what you are asking about. --Jayron32 13:11, 29 April 2014 (UTC)
- Thanks for you. Is it been, a nets of these telephone links which I’m asked may been doing a level of logical as a level of logical of a nets of a computers?--Alex Sazonov (talk) 13:50, 29 April 2014 (UTC)
- There are very complicated switches in a telephone network. See Public_switched_telephone_network and Switching_center#Technologies. There are indeed some analogies to the logic gates that form the basis of computer processing. There are similarities, but they are not the same thing. (I hope that helps. I understand English is not your native language, but you will get better answers here if you can spend a little more time going over your wording.) SemanticMantis (talk) 14:36, 29 April 2014 (UTC)
- A Plain_old_telephone_service uses a nominal 48 volts. "The subscriber loop typically represents an electrical load of about 300 ohms, and does not pose a threat of electrocution to humans, although shorting the loop may be felt as an unpleasant sensation." Modern networking equipment like Router_(computing) are designed to work with these voltages. 196.214.78.114 (talk) 14:12, 29 April 2014 (UTC)
- Thanks for all in our discussion. I been thinking that a logically level of a telephone links of a telephone nets of a powerful electric volts always must been doing as a more much logically level of a telephone links than a simple logically level of a telephone nets of a computers in the USA, thats it they always been be bestly.--Alex Sazonov (talk) 15:19, 29 April 2014 (UTC)
- Thanks welly. Did a computers nets of a telephone links always been use a electrical telephone transformation of a electric telephone signals?--Alex Sazonov (talk) 16:38, 29 April 2014 (UTC)
- Alex, perhaps you want an explanation of the differences between analog communication originally used in telephone links and digital signals used in data networks and inside computers. Please say what is your own language and someone will find a reference for you to read. 84.209.89.214 (talk) 18:56, 29 April 2014 (UTC)
- Thanks. My native language is been Russian, but ofcourse I see that a electrical transformation in telephone links always is been own, but is been not another.--Alex Sazonov (talk) 19:25, 29 April 2014 (UTC)
- Is it been, a telephone electronics always been use a powerful electric volts in a long telephone trunk line of a telephone links?--Alex Sazonov (talk) 06:53, 30 April 2014 (UTC)
- The high voltage (48v) was needed in the old days to transmit signals reliably over miles of copper wire. Modern computer systems usually run on a much lower voltage, but there are still lots of older telephones connected with miles of copper, so the high voltage is still kept. Dbfirs 07:32, 30 April 2014 (UTC)
- Thanks. But I always been mean in our discussion a 220-380 Volts of a powerful electric in a long telephone trunk line of a telephone links or a 550-580 Volts of a powerful electric in a old long telephone trunk line of a telephone links.--Alex Sazonov (talk) 09:33, 30 April 2014 (UTC)
- No one except perhaps the poseur Antonio Meucci ever proposed using high voltage for a telephone. (By the way if you use Google translate the English will probably be far more intelligible than the word salad you have been posting here. It generally makes no sense at all and people try to figure out what you mean based on a word here and there.) Edison (talk) 14:32, 30 April 2014 (UTC)
- Is it been, a tone of a voice speaking by a telephone links of a powerful electric volts been modify from a electrical telephone transformation?--Alex Sazonov (talk) 14:17, 30 April 2014 (UTC)
- Thanks. But I always been mean in our discussion a 220-380 Volts of a powerful electric in a long telephone trunk line of a telephone links or a 550-580 Volts of a powerful electric in a old long telephone trunk line of a telephone links.--Alex Sazonov (talk) 09:33, 30 April 2014 (UTC)
- Why in the USSR always been make a telephone links of a powerful amplitude frequency of a signals but in the USA always been make a telephone links of a none powerful amplitude frequency of a signals?--Alex Sazonov (talk) 14:46, 30 April 2014 (UTC)
- The Tsarist government of Russia issued its first decree on the development of urban telephone networks in 1881 and the first exchanges in the Empire opened the following year. Telephones played a significant role during the upheavals of 1917. According to the last tsarist Chief of Police, 'neither the military authorities nor the mutineers thought of occupying the Telephone Exchange'; consequently it continued to function, serving both sides, until the operators finally left their positions amidst the growing confusion'. In 1919, Sovnarkom nationalized all telephone systems in the Russian Republic. Until the end of the USSR in 1991, the sole fixed-line telephone operator in the country was the Ministry of Communications of the USSR. Today Russian landline telephony employs modern network elements such as digital telephone exchanges, mobile switching centres, media gateways and signalling gateways at the core, interconnected by a wide variety of transmission systems using fibre-optics or Microwave radio relay networks. Our article reports that services are still outdated in rural areas but says nothing about unusual high line voltages that could only create problems for Telephone exchanges. 84.209.89.214 (talk) 16:30, 30 April 2014 (UTC)
- Why in the USSR always been make a telephone links of a powerful amplitude frequency of a signals but in the USA always been make a telephone links of a none powerful amplitude frequency of a signals?--Alex Sazonov (talk) 14:46, 30 April 2014 (UTC)
- I been very thanks for yours explain of a history of the Russian telephone links, and as I been know a telephone links of the USSR always made as a powerful aggregation of a electric volts.--Alex Sazonov (talk) 18:17, 30 April 2014 (UTC)
- Could anyone say me is been a multiplier of a amplitude frequency in a powerful electric volts?--Alex Sazonov (talk) 18:20, 30 April 2014 (UTC)
- Ваш английский язык является недостаточным. Пожалуйста, попросите еще раз в русском языке. 84.209.89.214 (talk) 19:07, 30 April 2014 (UTC)
- (A Google translation of the above is:"Your English is inadequate. Please ask again in the Russian language.") Edison (talk) 01:34, 1 May 2014 (UTC)
- Well. Может ли кто-нибудь сказать мне, существует ли умножение (шаг) амплитудной частоты в силовых электросетях?--Alex Sazonov (talk) 20:22, 30 April 2014 (UTC)
- Unfortunately the Russian version makes no more sense than the English one. 84.209.89.214 (talk) 20:47, 30 April 2014 (UTC)
- Excellent. InedibleHulk (talk) 21:10, April 30, 2014 (UTC)
- A Google translation of Alex Sazonov's Russian text is "Well. Can anyone tell me if there is a multiplication (step) amplitude frequency power electric? " Gibberish? Edison (talk) 01:34, 1 May 2014 (UTC)
- Excellent. InedibleHulk (talk) 21:10, April 30, 2014 (UTC)
- Unfortunately the Russian version makes no more sense than the English one. 84.209.89.214 (talk) 20:47, 30 April 2014 (UTC)
- Ваш английский язык является недостаточным. Пожалуйста, попросите еще раз в русском языке. 84.209.89.214 (talk) 19:07, 30 April 2014 (UTC)
- Could anyone say me is been a multiplier of a amplitude frequency in a powerful electric volts?--Alex Sazonov (talk) 18:20, 30 April 2014 (UTC)
- What a sense always is been mean a contours of a electrical transformation? Same it, in a Russian - Какое значение всегда имеют контуры трансформации электрического тока?--Alex Sazonov (talk) 08:01, 1 May 2014 (UTC)
- Is it been, a ways of a electrical including for a electric transformer are been a contours of a electrical transformation? Same it, in a Russian – Являются ли способы включения трансформатора в электрическую сеть контурами трансформации электрического тока?--Alex Sazonov (talk) 18:38, 1 May 2014 (UTC)
point particle
do elementary particles warp empty space the same way that black holes do? I am imagining two very similar objects with one of their properties in common being "has no empty space 'inside'"?66.87.116.12 (talk) 13:56, 29 April 2014 (UTC)
- All particles that have mass have gravitational fields. Albert Einstein predicted that gravity is due to curvature of the space around masses. In 1919 Arthur_Stanley_Eddington made the first empirical test of Einstein's theory by measuring the deflection of star lights by the sun's gravitational field. However the gravitational attraction between ordinary masses does not accelerate them beyond the speed of light, as occurs entering a black hole. 84.209.89.214 (talk) 15:30, 29 April 2014 (UTC)
- Is that just for rest mass, or do those objects with only relativistic mass also warp space ? StuRat (talk) 16:23, 29 April 2014 (UTC)
- Yes, also. Whenever the stress–energy tensor is nonzero, the geometry of space will be affected according to the Einstein field equations. I have seen a paper on exact solutions for the case of an intense electromagnetic field propagating through otherwise empty space. —Quondum 17:01, 29 April 2014 (UTC)
- You can also see that this must be the case without invoking the intricate details of general relativity. Just consider the fact that a big mass like the Sun will bend light. So, a photon with some initial momentum will after passing close to the Sun hend up having a different momentum (it will have changed in direction). Since total momentum must be conserved, this means that the Sun must have changed its momentum to compensate for the change in the momentum of the photon. Therefore the photon must have a gravitational field. Using just the formula for the bending angle, you can easily compute the effective gravitational potential of a beam of light. Count Iblis (talk) 17:05, 29 April 2014 (UTC)
- because the infinity grab in small scal , because the secend degre of R , it sqewez to a point flip the derection of time and mulltiply by three , and make the three visibel dimention space . Thanks water — Preceding unsigned comment added by 192.116.142.154 (talk) 13:42, 30 April 2014 (UTC)
- Well that explains everything. Justin15w (talk) 15:19, 30 April 2014 (UTC)
- Thanks, water. Thwater. AlexTiefling (talk) 15:42, 30 April 2014 (UTC)
- because the infinity grab in small scal , because the secend degre of R , it sqewez to a point flip the derection of time and mulltiply by three , and make the three visibel dimention space . Thanks water — Preceding unsigned comment added by 192.116.142.154 (talk) 13:42, 30 April 2014 (UTC)
- You can also see that this must be the case without invoking the intricate details of general relativity. Just consider the fact that a big mass like the Sun will bend light. So, a photon with some initial momentum will after passing close to the Sun hend up having a different momentum (it will have changed in direction). Since total momentum must be conserved, this means that the Sun must have changed its momentum to compensate for the change in the momentum of the photon. Therefore the photon must have a gravitational field. Using just the formula for the bending angle, you can easily compute the effective gravitational potential of a beam of light. Count Iblis (talk) 17:05, 29 April 2014 (UTC)
- The classical idea of a black hole does have empty space inside. An event horizon marks a point of return, but is supposed to have nothing locally special visible to someone falling in. Everything gets crushed in a singularity at the center. However, the fuzzball idea does suppose the hole is packed with string theory objects. A particle, by contrast, has a much more poorly defined position (Compton radius) and contains a space within that radius that may be shared with other particles. (See Bose-Einstein condensate, for example) Wnt (talk) 19:35, 30 April 2014 (UTC)
Alien Worlds???...
Do any Biologist or Biochemist know what are the problems for Humans when they find in the Future a Planet with Life???...
First Question: WHAT must be right to be a Planet with Life Human-friendly???...
Second Question: WHAT must be wrong to be a Planet with Life Human-enemy???...("a forbidden Paradise!!!...")
Third Question: Could it be a Si14-World in place of our C6-World???!!!...
THANK you VERY-VERY much!!!...
"Have a nice Day/Night!!!..."
SPYROU Kosta - Greece - Honeycomp (talk) 14:56, 29 April 2014 (UTC)
- As a minimum for life as we understand it, a planet should be in the habitable zone; planets outside it are automatically unfriendly to Earth-based life forms. But beyond that, a lot is speculation - there are so many ways a planet's biome could be harmful to humans, it's hard to know where to start - huge predators, virus- or bacteria-like pathogens, poisonous creatures, all life existing in places (glacial trenches, volcano margins) that are naturally bad for humans... AlexTiefling (talk) 15:03, 29 April 2014 (UTC)
- You might also want to read up on planetary habitability.--Shantavira|feed me 15:30, 29 April 2014 (UTC)
- All scientists, not just bio-scientists, know that an eventual discovery of an extraterrestrial planet with life will force a review of everything we know about our own origins. — Preceding unsigned comment added by 84.209.89.214 (talk) 16:16, 29 April 2014 (UTC)
- That's very dubious. Suppose that 10 years from now, someone takes the spectrum of an Earth-like planet and sees oxygen and methane--a very strong indicator of life. How would that force a review of the African origin of humans, of early conditions on Earth, or of any of the geological record? Suppose that tomorrow, someone discovers a radio signal from an exoplanet that's undeniably artificial. How does that force a review of evolutionary history? Of course discovering life on other planets could radically change everyone's philosophical outlook, but it might have little effect on existing science. --Bowlhover (talk) 16:52, 29 April 2014 (UTC)
- If the philosophy of science were already perfected beyond criticism, there would be no need for the many science fiction writers who have addressed the implications of contact with alien beings. Now on Earth we cannot isolate the scientific hypotheses that we test from the influence of the theories in which our observations are grounded (see Kuhn). Therefore, if someone communicates by radio or other means with life on another planet, they should listen in order to learn, rather than reiterate their favourite theory-laden observations that are circumscribed by their own theoretical presuppositions. 84.209.89.214 (talk) 15:36, 30 April 2014 (UTC)
- That's very dubious. Suppose that 10 years from now, someone takes the spectrum of an Earth-like planet and sees oxygen and methane--a very strong indicator of life. How would that force a review of the African origin of humans, of early conditions on Earth, or of any of the geological record? Suppose that tomorrow, someone discovers a radio signal from an exoplanet that's undeniably artificial. How does that force a review of evolutionary history? Of course discovering life on other planets could radically change everyone's philosophical outlook, but it might have little effect on existing science. --Bowlhover (talk) 16:52, 29 April 2014 (UTC)
- All scientists, not just bio-scientists, know that an eventual discovery of an extraterrestrial planet with life will force a review of everything we know about our own origins. — Preceding unsigned comment added by 84.209.89.214 (talk) 16:16, 29 April 2014 (UTC)
- First Question: The Rare Earth Hypothesis uses the Goldilocks principle in the argument that a planet must neither be too far away from, nor too close to a star and galactic center to support life, while either extreme would result in a planet incapable of supporting life. It considers the likelihood that something we recognize as life might have evolved on a yet unknown planet; however the probability can be greater if we envisage possible Extremophile life forms, and much less probable that we ever find another "human friendly" planet. The prospect of Terraforming a planet to this end has arisen in science fiction.
- Second Question: The more human friendly a planet with life turns out to be, the more likely it is to have predators or parasites that will like to dine on humans, and on Earth our experience has shown unfortunate results for a civilisation that contacts a more advanced one.
- Third Question: This calls for speculation. 84.209.89.214 (talk) 16:00, 29 April 2014 (UTC)
- Two comments:
- A) Habitable zone is defined as distance from a star, but a large planet might provide enough tidal heating to keep a moon warm. So, the habitable zone around a star can be extended outward for some moons around large planets.
- B) Conditions needed for life to begin might be considerable different from those needed for humans to exist there now. The early Earth would have been quite inhospitable to humans, due to lack of atmospheric oxygen, toxic gases in the air, high volcanism and meteor impacts, and obviously a lack of plants and animals to eat. However, using modern technology, we might be able to live in places not well suited to primitive humans. StuRat (talk) 16:20, 29 April 2014 (UTC)
- It occurs to me that question three, i.e. whether it's possible, could be answered by someone smarter than me (which is most of you) looking at what it is that makes Carbon so special as the "anchor" of organic life as we know it. That is, does Silicon have the potential to interact with other elements in pretty much the same way that Carbon does? One possible side issue: The basic organic compounds often include elements with low atomic numbers. Do scientists believe that the larger atomic numbers were created later? That is, would Silicon have turned up well after Carbon, and if so, could primitive life forms or almost-life forms have already gotten started before Silicon came along? ←Baseball Bugs What's up, Doc? carrots→ 16:21, 29 April 2014 (UTC)
- The thing that makes carbon "special" is the ability to form long-chain and complex structures. Silicon can also form chains, but nowhere near as long as carbon can. --Carnildo (talk) 01:43, 30 April 2014 (UTC)
Alien Earth-like World...
SORRY but I made the FALSE Question earlier...
Imagine that we find a Planet with Earth-like life:
1). with Atmosphere like ours...
2). with water H2O...
My Questions are Biology-Questions:
If the D.N.A. is like ours - or NOT...
If the Amino-Acids are like ours - or NOT...
If the Molecules of Life have the same Hirality - or NOT...
a). WHAT of them will be Human-friendly???...
b). WHAT of them will be Human-enemy???...
THANK you VERY-VERY much!!!...
"Have a nice Day/Night!!!..."
SPYROU Kosta - Greece - Honeycomp (talk) 17:38, 29 April 2014 (UTC)
- You may find the Wikipedia articles titled Extraterrestrial life and Astrobiology useful to start your research. --Jayron32 17:42, 29 April 2014 (UTC)
- You mean chirality, which might well be reversed. Also, some evolutionary "mistakes", like the one that causes our blind spot, might not be repeated. And if bipedal locomotion developed earlier there, they might have evolved a spine that actually works properly when walking upright, unlike ours which is prone to all sorts of problems (I think a thick, rubbery spinal cord with no nerves would make sense inside the spine, with the nerves in front of the spine, in a notochord.) StuRat (talk) 18:25, 29 April 2014 (UTC)
There is some evidence that amino acids (Urey-Miller experiment), RNA (RNA world), and metabolism ([14]) date back to the origin of life, or before the origin of life. The solution our cells contain is closely based on the primordial sea. If this is true, then many characteristics of modern Earth life may be very similar to the environment of the early Earth, and the biochemistry of Earth life should not be seen as an evolved optimum. Nonetheless, some important evolution has occurred, most notably the oxygen catastrophe that replaced the primordial reducing atmosphere with a highly oxidizing environment. The presence of oxygen continues to be stressful to life to this day - the activity of genes like superoxide dismutase appears to be important in trying to stave off a process of aging that is partially due to reactive oxygen species. And yet, of course, we now cannot do without oxygen, nor can we tolerate ammonia. To answer your question, you would need to know how many planets have something like Earth's early chemistry, and whether other life is more prone to evolve away from its original biochemistry, but we don't know either of these things. Wnt (talk) 11:35, 30 April 2014 (UTC)
SPYROU Kosta: My Questions are specific!!!:
Same D.N.A. = Human-friendly Or Human-hostile???...
Same Aminoacids = Human-friendly Or Human-hostile???...
Same Chirality = Human-friendly Or Human-hostile???...
THANK you... - Honeycomp (talk) 14:09, 1 May 2014 (UTC)
Math. function
Hi, What function gives 1,7,13,25,49,97,... It begin's with (6+1) then (2*6+1) and (2*12+1) to infinity. It's for Flower of life article. Thx --YB ✍ 20:42, 29 April 2014 (UTC)
- 1 + (2x * 6)
- - EronTalk 20:58, 29 April 2014 (UTC)
- Simple indeed. What if it start at one? --YB ✍ 21:09, 29 April 2014 (UTC)
- The 1 simply doesn't follow your rule; it ought to be (3+1) so that the next term can be (2*3+1). --Tardis (talk) 00:45, 1 May 2014 (UTC)
- Simple indeed. What if it start at one? --YB ✍ 21:09, 29 April 2014 (UTC)
- It might not make that much sense to you, but that sequence has an official name in the OEIS, you can cite this page [15] if/when you add it to the article. SemanticMantis (talk) 21:27, 29 April 2014 (UTC)
- I had the feeling it had something to do with Fibonacci number and fractals. Thanks for the ref. --YB ✍ 23:15, 29 April 2014 (UTC)
- That's not the same sequence; they diverge after 769 (). (Similarly, the 2, 3, 5 part of the standard Fibonacci sequence matches for n 0, 1, and 2, but not afterwards.) --Tardis (talk) 00:45, 1 May 2014 (UTC)
- Why didn't you post this to the Math Desk ? StuRat (talk) 03:39, 30 April 2014 (UTC)
- I haven't examined the connection to Flower of Life but the natural thing would be to include 3+1 = 4 so it goes 1, 4, 7, 13, 25, 49, 97. That's OEIS:A004119: 3×2n+1. A Thabit number is 3×2n−1. PrimeHunter (talk) 17:25, 1 May 2014 (UTC)
- Sorry, the sequence is 1,7,19,37,61,... [16]. Anyway, thanks for the link. --YB ✍ 01:33, 2 May 2014 (UTC)
April 30
Any sources on skin allergies/reactions to vinyl ?
...either inside Wikipedia or outside ? I Googled, but didn't have much luck. StuRat (talk) 03:41, 30 April 2014 (UTC)
- @StuRat: [17] (section Vinyl Gloves and Allergic Reactions) may have what you are looking for. Zhaofeng Li [talk... contribs...] 10:29, 30 April 2014 (UTC)
- This paper has some info [18]. A key search term is contact dermatitis. SemanticMantis (talk) 15:16, 30 April 2014 (UTC)
Thanks so far. Any more info ? StuRat (talk) 14:07, 1 May 2014 (UTC)
This isn't a request for advice, more of a curiosity. Looking at various sources around the web, I've seen all sorts of recommendations on how long you should wait between exercising - some vague, some very specific. At any rate, most of what I've seen seems somewhat contradictory - some sources claiming that exercising every other day can be counterproductive and that you should only exercise twice a week, other sources claiming that you can exercise every day, etc. Most places do not go into any detail about what counts as "exercise" either - obviously an intense session at a gym using your whole body is going to be different that doing some pushups and situps between commercials. So, I wanted to know, if there were any studies that go into greater detail, or if anyone could give a good explanation of what exactly causes overtraining/what exactly it is. --Again, I'm not looking for medical advice, I haven't been to a gym in years, I just stumbled into the topic and find it rather odd and confusing. Thank you for any assistance:-)Phoenixia1177 (talk) 08:11, 30 April 2014 (UTC)
- Basically exercise works by damaging the body, which then needs to be repaired. Where the damage occurs, more muscle cells, blood vessels, etc., are added during the healing process, to prevent future damage when repeating the same exercise. The body will obviously take some time to heal, but how long it takes varies by the amount of damage and other conditions, and also varies by individual. Here focusing on a different part of the body each time a person exercises will help give the previous target more time to heal. If the person's body is sore when they start exercise, then they should probably avoid exercising in that area, so they don't damage it further, until it has time to repair. StuRat (talk) 11:29, 30 April 2014 (UTC)
- It partly depends on the type of exercise, and partly on how intense it is. Top-level athletes train every day, but usually take a break for a couple of days after an event because the exertion is so intense that they need to let the damage heal. There are lots of people who run 5 miles every day, or bicycle 20-30 miles, or walk on a treadmill for an hour, or do Pilates, etc. Looie496 (talk) 13:09, 30 April 2014 (UTC)
- Have you looked through the refs at the article you linked? This paper "Definition, Types, Symptoms, Findings, Underlying Mechanisms, and Frequency of Overtraining and Overtraining Syndrome" seems like a decent overview, but it is not freely accessible. [19]. As usual, you can probably get it via WP:REX. SemanticMantis (talk) 15:13, 30 April 2014 (UTC)
- It also depends on your age see: [20]. I know the Daily Mail is not the most reliable source but one of the quotes from a cardiovascular surgeon says "As we get older the efficiency with which the heart pumps blood round the body and the way both the heart and the muscles use oxygen in the blood changes and becomes less efficient". And from my own original research I can tell you that when you get into your sixties a bit of damage to a muscle by overworking it can take months to repair. Also see this and this. Richerman (talk) 08:46, 1 May 2014 (UTC)
How is the Electron configuration in other Dimensions???:
How is the Electron configuration in other Dimensions???:
Dimension 2: (THAT must be RIGHT!!!...)
x2 [+0]: s2 - p2 - d2 - f2 (2s + 4sp + 6spd + 8spdf...) : [ 2-4-6-4-10-6-16-6-22-8-30-8-38...]
Dimension 2: (THAT must be FALSE!!!...)( "Pauli spin -1/2 +1/2 rule...)
x2 [+2]: s2 - p4 - d6 - f8 (2+6+12+20...) : [ 2-6-8-6-14-12-26-12-38-20-58-20-78...]
Dimension 3:
x3 [+4]: s2 - p6 - d10 - f14 (2s+8sp+18spd+32spdf...) : [ 2-8-10-8-18-18-36-18-54-32-86-32-118...]
Dimension 4:
x4 [+6]: s2 - p8 - d14 - f20 (2+10+24+44...) : [ 2-10-12-10-22-24-46-24-70-44-114-44-158...]
[ Noble Gases ]
2-2-2 = Helium
8-10-12 = Oxygen-Neon-Magnesium
SPYROU Kosta - Greece - Honeycomp (talk) 14:02, 30 April 2014 (UTC)
- There was a Chemistry Olympiad question some years back that asked this question for 2 dimensions; you might like to dig that up. IBE (talk) 02:36, 1 May 2014 (UTC)
SPYROU Kosta: I didn't knew that...I only thought that IF the Physics make calculations in 10 Dimensions... THEN they have to calculate the electron configuration too...(???!!!)... I also ask if the elektron configuration:{s2-p6-d10-f14} says something about our three Dimensional Space???... - Honeycomp (talk) 14:03, 1 May 2014 (UTC)
- I'm no expert, but I think it's more likely to be the other way around - the specific shell sizes are a function of their being embedded in three-dimensional space. 3-space has its basic metric and geometric properties whether or not an atom is present, but the properties of the atom are strictly determined by its embedding in the space. When physicists - usually string theorists - speak of 10 or 26 dimensions, they're not proposing that our space is a 3-dimensional hyperplane through a higher-dimensional Euclidean space. Rather, as our article on string theory explains, they suggest that the additional dimensions are very compact - that the possible extension of anything into them is orders of magnitude smaller than even an electron orbital. AlexTiefling (talk) 14:18, 1 May 2014 (UTC)
SPYROU Kosta: THANKs Alex!!! - Honeycomp (talk) 01:10, 2 May 2014 (UTC)
Extracting neutronium from a neutron star
Say there's a neutron star nearby, and we want to extract some neutronium from it. If we wanted to break off a tiny piece of it, would throwing an asteroid at it do anything, or would it be largely unphased? ScienceApe (talk) 14:23, 30 April 2014 (UTC)
- It would do very little, both because of the low relative mass of the asteroid and because of the low density. It would be like shooting a cotton ball at a mountain. However, if you could accelerate a Jupiter-sized planet to near light speed, that might do something. But, of course, if you can do that, you can probably make your own neutronium with a lot less effort. StuRat (talk) 16:15, 30 April 2014 (UTC)
- If you could, it probably wouldn't stay neutronium long enough for you to use it for any purpose. Once it is taken from the star, and is no longer subject to the extreme pressures that made it neutronium, it would either become free neutrons, or degrade back to protons and electrons. --Jayron32 16:21, 30 April 2014 (UTC)
- Big, common misconception: Neutron stars aren't all neutronium. They have a pretty thick crust of other stuff on top of it. I would, however, like to see more about what happens to neutronium as pressure is reduced... I have a feeling there must be some neat surprises. Wnt (talk) 19:45, 30 April 2014 (UTC)
- A degenerate neutron gas would be a pretty nasty thing to come across at STP conditions. Radioactive, extremely toxic, highly carcinogenic. Plasmic Physics (talk) 06:08, 1 May 2014 (UTC)
Protein and ingestion
Does this link say that only 20 g of protein is utilized by the body and that too it should be taken for every 4-5 hours and anything other than that will be a waste of protein ? http://www.exercisebiology.com/index.php/site/articles/should_you_eat_protein_every_2-3_hours_for_muscle_growth/ — Preceding unsigned comment added by 119.235.54.187 (talk) 17:28, 30 April 2014 (UTC)
- Yes, it seems to say 20 grams of protein at a time is as much as your body can use, however, they only tested with it taken every 2-3 hours, but theorized that an interval twice that long might work, too, as amino acids (the building blocks of protein) remain in the blood after that. Also, taking 20 grams every 2 hours, while awake, would get you to an absurd amount of protein, like 400 grams. That's almost ten times what a normal person needs, and may be too much for your kidneys to process. Myself, I can tell when I've eaten more protein than my body can use, because my urine smells like bacon (which I believe to be amino acids). I don't agree when they say that eating protein every 2-3 hours is inconvenient. Just keep a bag of nuts with you (assuming you're not allergic) and nibble on them all day long. (Make sure they are unsalted, though, or you will get a sodium overdose.) If you find them unpalatable, add in some raisins. StuRat (talk) 19:49, 30 April 2014 (UTC)
- @StuRat So if we assume it works for 4-5 hours then it means even 5*20 i.e 120 gms of protein is optimal for our body ?? — Preceding unsigned comment added by 119.235.54.187 (talk) 05:20, 1 May 2014 (UTC)
- 5x20 = 100, but that's still about twice the amount of protein we normally need. However, a bodybuilder or athlete might need that much. StuRat (talk) 14:05, 1 May 2014 (UTC)
Blended color perception
Based on our discussion earlier on this Desk, it came up that humans are unable to distinguish between an object which reflects the green frequency of light and one which reflects both the blue and yellow frequencies of light. This isn't the case for sound, where we can distinguish a medium frequency from a high and low frequency heard simultaneously. So, do any other animals seem to have the ability to distinguish blended colors ? Do any people have this ability ? If so, how do they describe the difference ? StuRat (talk) 22:02, 30 April 2014 (UTC)
- Light perception is the stimulation of certain rods and cones in your retina right? So green light must stimulate the same receptors as yellow and blue light... This is incorrect by the way, yellow and blue PIGMENTS make green, yellow and blue LIGHT make white. Regardless, I get what you mean. We have Trichromatic vision, this is what lets us distinguish the different colors, different light stimulates our three different receptors, which are obviously deficient because 2 different sources of light can stimulate exactly the same proportion of receptors in our eyes. As might have been mentioned before, there is nothing qualitatively different between the colors, they are different frequencies of a continuously varying spectrum. The mantis shrimp has not three, but SIXTEEN photoreceptor pigments, it would no doubt distinguish between green and "yellow and blue", how would it describe it? I guess the same as you would describe the difference between two different colors. Vespine (talk) 22:59, 30 April 2014 (UTC)
- Also, in respect to other people, at least one person HAS been confirmed as Tetrachromatic, and possibly it's not extremely rare. However it sounds like this makes a measurable but not extreme difference to their color perception, I don't believe it's like they can perceive a whole new color we don't "know", i think it just means they are able to more easily distinguish between more shades of colors than most people. Vespine (talk) 23:06, 30 April 2014 (UTC)
- Actually, functional tetrachromacy has never been validated in a human, though genetically speaking, it is speculated that a tetrachromatic combination of photoreceptors could manifest in a woman, due to variations in a specific allele. However, this would be exceedingly rare and even if that particular combination did exists in the retina itself, there's a high degree of uncertainty that the neurological pathways for color perception within the brain itself would be able to process the additional colors in a way leading to an actual or significant expansion of differentiation between colors. I say uncertain and not impossible because some six or seven years back, a research team genetically modified some mice (naturally dichromats) to be trichromats. They did this with the assumption that they would be confirming that the mice would still be functional dichromats, despite the trichromatic retinas, but to their amazement (and with repercussions to this field of study that are still being weighed today), they discovered they had created true trichromats (these mice, and others in replicated studies) seem to be able to distinguish between colors with near the same level of differentiation found in normal trichromats; the mammalian visual cognition centers are apparently more plastic in this regard than anyone ever suspected. Now, obviously, the fact that this worked with regard to mice moving from the level of dichromat to trichromat does not necessarily mean the principle would hold with humans, or any trichromat, who possesed tetrachromatic retinas, but it is a fascinating area of inquiry. For the record though, the upgrade would not be minor if the neural architecture matched the capabilities of the eye; human vision could go from being able to distinguish roughly one million hues to possibly over 100 million, though this would depend on the specific wavelength of light which the new photoreceptors were sensitive to -- for example, it's worth noting that the theoretical tetracrhomat women who are postulated would actually have a fourth type of photoreceptor that is really not all that far off in the wavelengths detected from that of one of the existing cell types, so the upgrade would be minimal for them, even if the visual centers of their brain were up to the task of processing the information. Snow (talk) 00:04, 1 May 2014 (UTC)
- @Snow Rise: you said they tried to change dichromat mice into trichromats, but actually got tetrachromats. Is that what you meant to say ? StuRat (talk) 01:38, 1 May 2014 (UTC)
- Wooops, no, it was not. Thanks for the catch, Stu. I've edited the passage above to read as it should have -- that is, that they created trichromats. Sheesh. Snow (talk) 01:49, 1 May 2014 (UTC)
- @Snow Rise: Nice try, but you ended up with "tritrachromats". Is that hybrid, or are you editing under the influence ? :-) StuRat (talk) 03:24, 1 May 2014 (UTC)
- LOL, yes - of sleep deprivation. Thanks again! Snow (talk) 04:17, 1 May 2014 (UTC)
- Also, you claimed functional tetrachromacy has never been validated in a human, but even in the article that I linked it states: In June 2012, after 20 years of study of women with four cones (non-functional tetrachromats), neuroscientist Dr. Gabriele Jordan identified a woman (subject cDa29) who was able to detect a greater variety of colors than trichromatic ones, corresponding with a functional tetrachromat (or true tetrachromat). I had actually heard of this case before looking up the article. Vespine (talk) 04:31, 1 May 2014 (UTC)
- Well, I recall that story but to the best of my knowledge the details of that case and the testing procedures were never released to peer-review and were only reported in the general press and I remember my general impression of the details that were released was that they raised more questions as to the verification procedure than they answered. The fact that this case has still not seen review in research literature since then raises further questions as to how verifiable the findings were. That said, Dr. Jordan is known for research in this field. It's possible she did in fact find a functional tetrachromat, but I'd take the claim with a grain of salt until the testing procedure is better validated. I also tend to doubt that, even she did have some increased differentiation, that the subject in this case was seeing a vast new spectrum of colors, as per the question of the ability of the visual modules of the human brain to keep up. Snow (talk) 05:45, 1 May 2014 (UTC)
- As a complete aside, why does everyone only ever talk about green as being a mix of yellow and blue, when some greens are obviously a mix of yellow and indigo? RomanSpa (talk) 09:10, 1 May 2014 (UTC)
- Problem #1 is that people talk about mixing paint/dyes (which is subtractive mixing) versus mixing light (which is additive mixing). In light, green is a primary color (for trichromat humans) - so you can't get it by mixing anything else. In subtractive mixing, it's usually said to be a mixture of yellow and blue.
- Problem #2 is that "blue" is a pretty vague term for most people. The more sciency thing would be to say that in subtractive mixing, green is a mix of yellow and "cyan" (cyan being a pale "sky-blue"). Yellow paint absorbs blue light and reflects red and green. Cyan paint absorbs red light and reflects blue and green. A combination of the two absorbs red and blue and reflects only green. Hence yellow plus cyan makes green.
- Pure blue paint would absorb red AND green and reflect only blue light - mixing that with yellow would result in all three primaries being both reflected and absorbed - so the resulting color is likely to be muddy and the exact color you perceive would be sensitively dependent on the dyes and the ratios of the colors and so forth. "Indigo" is another vague color description...it's hard to say what exactly it is...best description is a very dark blue..which would mean that it absorbs some blue light as well as all of the red and green.
- Problem #3 is that paint can be laid onto a bright white surface or a dark one - and the consequences for light transmission get really complex. Some light is reflected from the surface of the paint - some passes through the paint, reflects off of the backing material and is then selectively absorbed on the way back out. Since some chemicals in the dye reflect color differently than they transmit it - you can get all sorts of complicated effects by mixing them.
- Problem #4 is that when you're mixing light, you have a self-contained system that's easy to describe. With paint and dyes, the color of the incoming light matters. Sunlight is yellowish - and the sky is blue - so you get color changes depending on whether the material is in sunlight or shaded (and therefore lit mostly by blue light from the sky). Interreflections between objects add more complexity - and the fact that our eyes and brain adapt to the prevailing light color and attempt to produce 'perceptual' colors that match what the underlying object's color "should be" if it were not for colored light falling onto it. This mess can get very confused - such as when you view the world lit by old-fashioned orange sodium streetlamps.
- SteveBaker (talk) 16:16, 1 May 2014 (UTC)
- Ah, that might be what's going on: you think indigo is just the same as blue. My father can't tell when something is indigo or not either - you show him something indigo and he just says it's blue. I suppose if other people have this problem (blue/indigo colour-blindness?) it might explain the confusion. (It would also explain why so many people wear clothes that clash horribly with indigo jeans, but that's another story!) If you can't see the difference between blue and indigo I suppose you wouldn't be able to see the difference between yellow-blue and yellow-indigo either. RomanSpa (talk) 05:50, 2 May 2014 (UTC)
- Curious. To me, indigo never seemed like a "real" primary color, just something shoehorned into a list of colors of the rainbow. It's not that I can't tell it from blue, but it seems closer to both blue and to violet than cyan seems to blue or to green. I'd think an "indigo-yellow" would just be a slightly blue green, though not as blue as cyan. I wonder if the difference is all semantics or if there's actually a variation in photoreceptor profiles. Wnt (talk) 12:16, 2 May 2014 (UTC)
- Also, you claimed functional tetrachromacy has never been validated in a human, but even in the article that I linked it states: In June 2012, after 20 years of study of women with four cones (non-functional tetrachromats), neuroscientist Dr. Gabriele Jordan identified a woman (subject cDa29) who was able to detect a greater variety of colors than trichromatic ones, corresponding with a functional tetrachromat (or true tetrachromat). I had actually heard of this case before looking up the article. Vespine (talk) 04:31, 1 May 2014 (UTC)
- LOL, yes - of sleep deprivation. Thanks again! Snow (talk) 04:17, 1 May 2014 (UTC)
May 1
If I can smell something is it losing mass?
If I can smell something I suppose it means that thing is being dissolved into the air. Is it therefore losing mass? Hayttom 11:10, 1 May 2014 (UTC) — Preceding unsigned comment added by Hayttom (talk • contribs)
- Yes. --Jayron32 12:13, 1 May 2014 (UTC)
- Odor implies such. It says "low concentrations", i.e. you're only inhaling a very small quantity of whatever is being emanated, but obviously if you're sensing it then it's no longer "attached to" its source, hence its source is losing some small quantity of mass. ←Baseball Bugs What's up, Doc? carrots→ 12:26, 1 May 2014 (UTC)
- I suppose an exception is if the item modifies existing air in some way, so as to make it have an odor. Air purifiers which create ozone and space heaters which burn dust in the air might qualify. StuRat (talk) 13:55, 1 May 2014 (UTC)
- Also, when you can no longer smell it, that implies that the volatile compounds have all evaporated/sublimated into the air. Of course, grinding off the surface may expose more volatiles. StuRat (talk) 13:59, 1 May 2014 (UTC)
- Supposing you had an open container of a common substance such as rubbing alcohol. Once it "totally" evaporates, isn't there still some residual scent in the container? ←Baseball Bugs What's up, Doc? carrots→ 15:12, 1 May 2014 (UTC)
- In that case, it's highly likely that there are some impurities in the alcohol that are still producing smell - and that some of the alcohol has been absorbed into the container somehow. But if it's literally all gone - then of course there can be no smell.
- Smell is only possible if some of the molecules of the substance made it into your nose somehow...if there are literally no molecules left - then there obviously isn't going to be any smell. But even humans - with our relatively poor sense of smell - can detect quite low concentrations of some substances...so there may not have to be very much left for us to detect it.
- To answer the question though - it may be that the substance is emitting some chemical but absorbing something else. So you could imagine something absorbing water from the air while emitting something else. In that case, the bulk of the material could increase while the smell is being emitted. If there was some kind of chemical reaction with something in the air that produced the smell - then it's quite possible that the result is an increased mass. Imagine something which reacts with air to make nitrous oxide. That stuff smells slightly sweet - but we might imagine some solid that absorbs oxygen and produces nitrous oxide as a byproduct...it would gain mass as time goes on and continue to generate that slightly sweet smell until the reaction is complete and the mass has increased.
- SteveBaker (talk) 15:36, 1 May 2014 (UTC)
- Good point. For example, what about the bloated blue whales discussed in this (fairly gross) story?[21] Could they be gaining mass as they decompose? Or is the decomposition merely re-arranging molecules already existing within the carcass? ←Baseball Bugs What's up, Doc? carrots→ 15:43, 1 May 2014 (UTC)
- I guess mass might be passing from the air to the solid (or semi-solid) parts of the carcass. But the fact that the body is capable of inflating like that suggests that any gases involved in that reaction were wholly contained by the carcass all along. I can't say the same for any decomposition at the outer edge - some substances might be oxidising, in which case they'd be gaining mass from the wider atmosphere, but they might also give off odour and other things at the same time, resulting in mass loss. AlexTiefling (talk) 15:48, 1 May 2014 (UTC)
- I very much doubt that the bloated whales are gaining mass. They are getting bigger - but that's just gasses as a decomposition byproduct. I don't think they are getting any heavier. SteveBaker (talk) 16:36, 1 May 2014 (UTC)
- I guess mass might be passing from the air to the solid (or semi-solid) parts of the carcass. But the fact that the body is capable of inflating like that suggests that any gases involved in that reaction were wholly contained by the carcass all along. I can't say the same for any decomposition at the outer edge - some substances might be oxidising, in which case they'd be gaining mass from the wider atmosphere, but they might also give off odour and other things at the same time, resulting in mass loss. AlexTiefling (talk) 15:48, 1 May 2014 (UTC)
- Good point. For example, what about the bloated blue whales discussed in this (fairly gross) story?[21] Could they be gaining mass as they decompose? Or is the decomposition merely re-arranging molecules already existing within the carcass? ←Baseball Bugs What's up, Doc? carrots→ 15:43, 1 May 2014 (UTC)
- SteveBaker (talk) 15:36, 1 May 2014 (UTC)
Mr Frosty (is such fun; he makes treats for everyone)
Did Nalgene have to make a deal with the toy manufacturer to call their cryogenic storage containers "Mr Frosty"? --129.215.47.59 (talk) 12:48, 1 May 2014 (UTC)
- There's no reference to a "Mr Frosty" in the Nalgene article. Do you have more details you could share with us? ←Baseball Bugs What's up, Doc? carrots→ 13:58, 1 May 2014 (UTC)
- With trade names like that, there are generally only legal problems if the public are likely to confuse the two products. It's a legal minefield though - and these things often end up in court even when the layman would say there was clearly no confusion. For example, I recall a legal battle in the UK between Digital Equipment Corp (DEC) and the company that begat the Dyson vacuum cleaner. DEC had a computer called "VAX" and there was a vacuum cleaner called "Vax". You'd think it was obvious that there was no conflict - but it didn't stop them from winding up in court. In that case, it was ruled that nobody had infinged on anyone's trade name because there was no possibility of confusing a gigantic minicomputer with a domestic vacuum cleaner - so nobody had to rename anything and no money had to change hands. However, such battles do often end up in bloodbaths - the Apple record company (which was owned by the Beatles) wound up in court with Apple computers (the fact that their logos were almost identical didn't help!). But it was ruled to be OK so long as Apple Computers didn't make or sell music...which they didn't back in the era of the Apple 2 computer. Well, you can imagine what went wrong when Apple produced the iPod and started selling music on iTunes...which clearly COULD result in confusion between the record label and the music sales service. The resulting bloody re-run of that legal mess explains why, for the longest time, you couldn't buy Beatles music on iTunes.
- According to Trademark247, "Mr. Frosty" is a registered trademark of Nalge Nunc International Corporation. According to this page, it is also a trademark for Sea Watch International Corporation, for use in labeling seafood. I can't find any information on the use of the name as a trademark for toys. Looie496 (talk) 01:00, 2 May 2014 (UTC)
Using napalm in a car instead of gasoline
What would happen if you used napalm in a car's gasoline tank instead of gasoline? Would it blow up or would the engine just fail to start? ScienceApe (talk) 13:42, 1 May 2014 (UTC)
- Napalm is gasoline - with the addition of substances to make it into a thick gel. The modern form of it is only about 33% gasoline, the rest is polystyrene and benzene. So even if you could somehow get it into the cylinder, there isn't enough gasoline in there to run your engine. Another problem (aside from whether your fuel pump could pump it) is that the fuel injectors in a car engine are designed to spray the fuel as very fine droplets. I doubt that it could do that with something as gelatinous as napalm.
- I strongly disagree with the assertion that gasoline is "highly explosive" - as a liquid, it's really quite hard to even set on fire. Only the vapor from it will burn at all. So you need it sprayed in fine droplets with a large surface area to evaporate from to get it to burn quickly enough. If you toss a lighted match into a bucket of gasoline, the match goes out...there is no exciting "Kaboom!". I'd also argue strongly against the assertion that a car engine is a controlled explosion. Fuel burns in the cylinders - it doesn't explode.
- The significant difference is that most explosives contain their own oxygen. For example - when nitroglycerin explodes:
- 4 C3H5N3O9 ==> 12 CO2 + 10 H2O + 6 N2+O2
- Everything that's needed to make it go "boom!" is right there inside the nitroglycerin molecule...so it's easily able to explode.
- Gasoline, on the other hand, requires LOTS of oxygen to make it burn. Gasoline is a complicated mix of chemicals - but one of the main ones is octane. Here is how octane burns:
- 2 C8H18 + 25 O2 ==> 16 CO2 + 18 H2O
- As you can see, you need 25 oxygen molecules for every two molecules of octane. Octane itself contains no oxygen whatever - so it can't even burn by itself, so it certainly won't explode. You've got to mix it with LOTS of oxygen to get it to combust - every molecule of the octane needs to be close enough to 25 oxygen molecules to react - and that's why it has to be in vapor form. Once that happens, the reaction goes quite quickly - and lots of byproduct means that the volume increases rapidly. You can call that an "explosion" - but it's no different than how wheat flour or sawdust will burn so rapidly that the effect is explosive if you get it in a fine dust up in the air. However, a grain of wheat or a lump of wood isn't "an explosive" - and neither is gasoline.
- That's why people who want their cars to go faster get fixated on turbo-chargers and super-chargers and big air scoops - getting more air into the engine is needed to get more gasoline to burn in order to make the car go faster. If you had a car that ran on nitroglycerin, you wouldn't need an air intake at all.
- SteveBaker (talk) 15:10, 1 May 2014 (UTC)
- If you google "automobile engine controlled explosion" you will find plenty of references, so if you don't like that characterization of the process, go complain to those individual writers, not to me who merely quoted them. Also, what's the answer to the OP's question, "...would the engine just fail to start?" I'm guessing it would indeed fail to start, but you're the expert, so we'll defer to you an that one. ←Baseball Bugs What's up, Doc? carrots→ 15:35, 1 May 2014 (UTC)
- If you google "magnets cure rheumatism" or "obama not US citizen", you'll get lots of hits too...doesn't make it true. SteveBaker (talk) 16:34, 1 May 2014 (UTC)
- One of the links that turns up is this, from the "How Stuff Works" department of the Discovery Channel. Feel free to contact that fringe group and tell them why you're right and they're wrong. ←Baseball Bugs What's up, Doc? carrots→ 18:07, 1 May 2014 (UTC)
- To be somewhat fair to Bugs, sometimes "combustive expansion of gases" is sometimes referred to as a low explosive while those that truly detonate are sometimes referred to as a high explosive. Low explosives occur when any combustible material is confined in a tight space; i.e. what happens in a piston chamber of a car engine. It just depends on what terminology you use. Some people prefer to not use the term "low explosive" because it confuses the very chemically different processes of "deflagration" and detonation. --Jayron32 16:52, 1 May 2014 (UTC)
- As far as I'm aware, a detonation is just an explosion that propagates through the explosive medium at supersonic speeds. That tends to create a much sharper and more damaging shock wave. I don't often disagree with Steve, but I think it's not unreasonable to describe what happens in a combustion engine as a controlled series of fuel-air explosions. --Stephan Schulz (talk) 09:40, 2 May 2014 (UTC)
- If you google "magnets cure rheumatism" or "obama not US citizen", you'll get lots of hits too...doesn't make it true. SteveBaker (talk) 16:34, 1 May 2014 (UTC)
- If you google "automobile engine controlled explosion" you will find plenty of references, so if you don't like that characterization of the process, go complain to those individual writers, not to me who merely quoted them. Also, what's the answer to the OP's question, "...would the engine just fail to start?" I'm guessing it would indeed fail to start, but you're the expert, so we'll defer to you an that one. ←Baseball Bugs What's up, Doc? carrots→ 15:35, 1 May 2014 (UTC)
- SteveBaker (talk) 15:10, 1 May 2014 (UTC)
- In direct answer to your direct question – it is a hydro-carbon, so a compression ignition engine (one that runs on diesel fuel) would run just like it was fueled by any other hydrocarbonous fuel. Don't have any reference to hand but I think you'll find that the naphthoic acid component of napalm is a corrosive by-product of the petroleum industry. It is a very cheap by-product. In modern fuel injected engines, this I think, would cause less problems. However, I it think it corrodes steel pipe lines as well. The combination of naphthoic acid and palmitic acid might also jell, causing the fuel delivery system to gum up. It is slow to vaporize, so a carbureted engine is unlikely to run without with out carb-heat.--Aspro (talk) 19:48, 1 May 2014 (UTC)
vitreous humor
Where does vitreous humor come from? If it's already present at birth, what organ (or other source) secretes it during gestation? Babies' eyeballs are smaller than adults, so as a person grows, where does the extra humor come from? 2001:18E8:2:28CA:F000:0:0:2B89 (talk) 14:00, 1 May 2014 (UTC)
Never mind, the page explains it, and I didn't see it until re-reading. "It is produced by cells in the non-pigmented portion of the ciliary body deriven from embryonic mesenchyme cells which then degenerate after birth." But could you help me understand what this means? I saw ciliary body, but I don't understand what a mesenchyme cell is. 2001:18E8:2:28CA:F000:0:0:2B89 (talk) 14:03, 1 May 2014 (UTC)
- In 1879, Charles Sedgwick Minot, an anatomist based out of Harvard Medical School in Boston, Massachusetts, first described what he termed mesamoeboids, the cellular portion of what would soon come to be recognized as mesenchyme. Minot found these cells in the context of histological studies of mesoderm. He understood the loose, mobile cells of mesenchyme as primitive representatives of the mesoderm, but did not consider these cells as a type of tissue. Two years after Minot’s recognition of mesamoeboids, Oscar and Richard Hertwig, two brothers and doctoral students of Ernst Haeckel at the University of Jena in Jena, Germany, coined the term mesenchyma in their publication Die Coelomtheorie. Versucheiner Erklärung des mittleren Keimblattes (Coelom Theory: An attempt to explain the middle germ layer), and they used it to describe the type of tissue that was comprised of the amoeboid cells that Minot had portrayed. The Hertwig brothers established that mesenchyme originates from mesoderm, and they situated this relationship in the broader context of the development of the coelom, a fluid-filled body cavity. Their Die Coelomtheorie also advanced the idea that the three germ layers maintain separate identities and develop distinct tissues and organs, a concept known as germ-layer theory. (Source: http://embryo.asu.edu/pages/mesenchyme) Basically, mesenchymal cells are the precursors of cells of the mesoderm, which will later become your blood and muscles. 140.254.227.117 (talk) 14:28, 1 May 2014 (UTC)
Andromeda...
The nearest Galaxy to the Earth is Andromeda...
You nead 2,5 Years if you travel in Space with 1.000.000 * c !!!!!!...
(c = 300.000 km/sec)Light speed
I cannot imagine that!!!...
You???...
SPYROU Kosta - Greece - Honeycomp (talk) 14:32, 1 May 2014 (UTC)
- I have more difficulty imagining a plausible faster-than-light propulsion method than I do contemplating how far away the Andromeda galaxy is. For what it's worth, there are several nearer galaxies (apart from the Milky Way) such as the Large Magellanic Cloud However, this 'question' appears to be a request for opinions rather than anything a reference desk can help with - it's best not to ask that sort of question here, as the header explains. AlexTiefling (talk) 14:40, 1 May 2014 (UTC)
- If you mean the cognitive processes that run in the brain when you use the term "imagination", I suppose you - assuming that you are human and have your prefrontal cortex and other areas in tact and normally functioning - can in fact imagine it like any other concept. This may be of interest to you. Source: http://scienceblogs.com/developingintelligence/2007/05/31/the-neuroscience-of-imaginatio-1/ 140.254.227.117 (talk) 14:47, 1 May 2014 (UTC)
SPYROU Kosta: I wanted to point only that IF we could travel faster than Light... faster * 1.000.000 is enormous!!!... Honeycomp (talk) 14:50, 1 May 2014 (UTC)
SPYROU Kosta: THANK you 140.254.227.117 about your consern for my Brain Health!!!... You are so "kind" and "childish"... :-) Honeycomp (talk) 15:04, 1 May 2014 (UTC)
- I think we're coming to the point where this joke isn't funny any more. RomanSpa (talk) 05:53, 2 May 2014 (UTC)
Comets and Aristotle
If Aristotle thought the heavens were unchanging and perfect, how did he account for comets? Looking around on Google produced the sublunary sphere article but nothing else, really. The article notes that Tycho's observation of a nova undermined the Aristotelian position, but it also says that comets likewise undermined it, and I don't understand why, since comets have been well known and rather common throughout human history. 2001:18E8:2:28CA:F000:0:0:2B89 (talk) 14:49, 1 May 2014 (UTC)
- He figured gases rose from Earth to high in the atmosphere, where they ignited and burned either quickly (meteor) or slowly (comet). Meteorology (Aristotle) has a link to the English text to the book; Book I deals with these "fiery exhalations". 88.112.50.121 (talk) 16:14, 1 May 2014 (UTC)
Impedance matching between transmission lines
I have a project that requires good broadband (upto 1GHz say) matching between a 50 ohm parallel plate line and a 350 ohm parallel plate line. Im wondering what would be the better method: a) straight tapered matching section or b) exponential tapered section. Also, what length of section should I use for each of the above methods to get a better than -20dB match upto 1GHz? --109.151.101.168 (talk) 14:53, 1 May 2014 (UTC)
- One issue is that different wavelengths will require different transition lengths. So at 1 MHz it would be 1000 times longer than at 1 GHz. Have you got a lower limit on frequency or do you need to go to DC? Are your two parallel plates sections the same separation and only differ in width? Exponentiation transition will be better. I ran an optimization for you with 4 fixed sections, which gives 73.8 108.9 160.7 and 237.1 Ohms, (values should be at fifth root of 350/50 powers) but this still only gives you 82% power transfer, no-where near the 99% you want.[22] Graeme Bartlett (talk) 23:01, 1 May 2014 (UTC)
- As suggested here, it is the lower end of the matching band that will be the difficult end. Without that specification the problem is ill-defined (or impossible, if you require down to DC). Wouldn't it be great if we could build DC transformers that are 99% efficient out of a simple shaped transmission line? —Quondum 00:07, 2 May 2014 (UTC)
- The problem is most certainly incompletely specified. Power level? Does it need to be bidirectional or are you interested in power flow in only one direction? If you don't need bidirectionality, the simplest, most boardband approach is to use a transistor - if matching from low Z to High Z use a common base circuit. That can go down to DC if necessary. If bidirectionality over a very board band is required, you can use a gyrator. What is important - low return loss and flat response (eg an intrumention problem) or efficient power transfer (eg a transmitter or radio reciever front end)? Most truely boardband applications are instrumentation/measurement applications, so power loss doesn't matter so much, but low reflection is critical. In such cases, use a resistive matching pad - which inherently goes down to DC as well as as high as you need when carefully constructed with chip resistors. Matching with intermediate matching steps and stubs is inherently narrowband as has already been said. 1.122.161.156 (talk) 10:49, 2 May 2014 (UTC)
- In reply to Graeme's posting and helpful calculation of reflection coefficient etc, no, I dont need a dc response as I am sending fast pulses from a low impedance charge line into the high impedance line by means of avalanche switches. To give an indication of the low end response: I can stand, say, about 10% droop on the top of my pulses. What this means as a lower frequency limit of the matching section, I am uncertain.
- In reply to 161.156: Power is pulsed but average is only likely to be milliwatts. I do need biderectionallity as the switches are in the low impedance line. Transistors in normal operation modes are unlikely to work at the sub nanosecond rise times I need. My basic need is to get maximum power transfer from the low Z line to the hiZ line.
- Any more thoughts greatly appreciated.109.151.101.168 (talk) 13:22, 2 May 2014 (UTC)
- PS: parallel plates are same width but different spacings.109.151.101.168 (talk) 13:24, 2 May 2014 (UTC)
- You stated an upper frequency limit of 1 GHz, which will approximately reproduce a step taking no less than 0.5 nS. Transistors are readily available that will give that sort of bandwidth. In any case, at the power level you specified, quite ordinary silicon NPN RF transistors can be used in avalanche mode to provide sub-nanosecond pulse rise times. This technique has been common in sampling oscilloscopes since the 1960's when transistors could barely work to 200 MHz in linear mode. To find the low frequency cutoff required, you need the pulse repetition frequency, unless some sort of DC restoration phenomenum is operational, in which case you need to know the pulse width and calculate on that. Why does the switch need to be in the low impedance line if you actually want the pulses in the high impedance line? It will be a lot simpler to switch in the hi-Z line. 1.122.161.156 (talk) 14:39, 2 May 2014 (UTC)
- The "frequence response" near DC is puzzling. If I apply a very slowly changing voltage to the two conductors why would the output not mirror the input faithfully? Even if I had a length of lamp cord spliced to coax spliced to twinlead? (It's been a long time since the "fields and waves class.) Edison (talk) 13:43, 2 May 2014 (UTC)
- Well, yes, but the OP specified a return loss (reflection) better than 20 dB. And we must presume that the response over the frequency range needs to be flat. Using intermediate impedance sections or matching stubs will result in reflected impedance changing with frequency and thus the return loss and power level must change with frequency. Putting it another way, yes, at low frequencies, the voltage on the high impedance line must faithfully copy the input, but it is the wrong voltage, as required voltage for a given power rises with impedance. 1.122.161.156 (talk) 14:39, 2 May 2014 (UTC)
Male and female bell peppers?
According to a perhaps unreliable source, some bell pepper fruits (aka sweet peppers) are male and some are female. Moreover, the "sex" of each pepper can be determined by counting the lobes: 4 lobes = female, 3 lobes = male.
Further, female peppers = sweeter and better for eating raw, male peppers = firmer and better for cooking.
Is there any truth to this? Either the part about two sexes of pepper fruits or the part about the shape of peppers being related to their sweetness and consistency.
I suspect it is pure nonsense but... how does one know for sure? Thanks, CBHA (talk) 19:33, 1 May 2014 (UTC)
- I am not a botanist, but I found a knowledgeable-looking blog post that debunks the idea. Capsicum annuum should be relevant, but it doesn't have much information on the morphology of the plant. AndrewWTaylor (talk) 20:09, 1 May 2014 (UTC)
- This claim is total nonsense. A bell pepper is the fruit of the plant. All botanical fruits (of which bell peppers are an example) are developed from the plants ovary- making them as definitively female as I can imagine. The whole complex of female plant parts, including the ovary, is called the Gynoecium. Most fruits that we eat come from "perfect" flowers, meaning each flower has male and female parts. Fruit_anatomy also has some good descriptions. It is true that some plants come in male and female versions, but in that case, the male bears no fruit. Holly is a well-known example of such a Dioecious plant (other popular examples of dioecy are ginko and cannabis). Finally, some plants, like birch have both sex organs on a plant, but each individual flower can be male or female. If you want to get more into the details of how all this works, see plant reproductive morphology. SemanticMantis (talk) 20:24, 1 May 2014 (UTC)
- I will add that in my WP:OR experience, smaller peppers tend to have fewer lobes, and tend to be firmer. SemanticMantis (talk) 20:25, 1 May 2014 (UTC)
May 2
Did the Inflationary epoch create time?
As we all know, the arrow of time is driven by the growing spread of quantum entanglement. Since any Cosmic egg would be fully entangled, it would be a state where no forward motion in time could occur.
Ergo it was the Inflationary epoch when the universe got dis-entangled that laid the groundwork for Entropy's clock, and no time could have occurred during any previous hypothetical fully connected phase.
Hcobb (talk) 03:55, 2 May 2014 (UTC)
- We do? It was? Eh? It would? I don't see that at all, however using Paradoxes of material implication I think I would have to agree that your conclusion does follow your premises. Dmcq (talk) 09:07, 2 May 2014 (UTC)
- You don't really seem to be asking a question here, but rather trying to persuade us of a handful of highly questionable cosmological claims. AlexTiefling (talk) 09:14, 2 May 2014 (UTC)
- The arrow of time article doesn't mention the word "entanglement". However, entanglement does mention this idea, citing [23]. Unfortunately, I don't think I know anything about quantum physics that isn't in Wikipedia, and half of that people will say is wrong when it's discussed here. Wnt (talk) 12:05, 2 May 2014 (UTC)
If we've got it wrong let's straighten it out, but it's the first solidish looking theory anybody's had to explain where time comes from.
Perception of time => Entropy => Quantum entanglement => Initial non-entangled state.
Hcobb (talk) 12:31, 2 May 2014 (UTC)
- You're second => does not follow, really. Also, your initial question treats time as a "substance" It isn't. --Jayron32 12:34, 2 May 2014 (UTC)
- You're clearly not the first person to propose a theory of time. Don't give yourself airs. Come back when you've spotted the contradiction in your original proposal. AlexTiefling (talk) 12:43, 2 May 2014 (UTC)
- No, don't come back, unless you have a question to ask. This is not a place for debating issues or propounding theories, and the above editors should not have engaged the OP. -- Jack of Oz [pleasantries] 12:48, 2 May 2014 (UTC)
Did been in a time of a virtual realism a time been during as in a real time?--Alex Sazonov (talk) 12:50, 2 May 2014 (UTC)
- Huh? What is the Russian text for your question? It makes no sense in English. Edison (talk) 13:36, 2 May 2014 (UTC)
Backup CF card
When travelling, I used to back up pictures from a CF card to a portable hard disk like this one. This worked perfectly also in remote places. Now I am planning a trip to a country where there is free wifi at every corner (or at least at any hotel) and I was wondering whether there is a better (smaller/fail proof) solution that allows me to upload the pictures to a server (e.g. my own server). Maybe something that connects to a smartphone or a standalone wifi/card-reader device? bamse (talk) 09:19, 2 May 2014 (UTC)
- If your phone has USB OTG you may be able to just plug your CF card into a cheap USB CF card reader, then plug the card reader into the phone. Main issue is whether the card consumes more power than the phone can supply. This question should actually be on the computer reference desk, by the way. 70.36.142.114 (talk) 14:02, 2 May 2014 (UTC)
Water in solar system planets
If some comets are made up of water, and if they collide with planets every now and then, isn't it obvious that at some time they have collided against any planet in the solar system and therefore, there is water in any of them? Maybe the smallish monds won't have enough gravity to keep it, but how could giants as Jupiter or Mars lose it? OsmanRF34 (talk) 13:26, 2 May 2014 (UTC)
- Indeed, there's plenty of water on both Jupiter and Mars (Frank Sinatra singing in my head there...). I might be wrong, but I don't think there's a single planet or minor planet that has been shown to be entirely devoid of water. Fgf10 (talk) 13:32, 2 May 2014 (UTC)
- I thought it was considered surprising when water was discovered on earth's moon. Of course water-bearing stuff hits the moon now and then, but one would expect the water to evaporate on exposure to the sun. The moon's known water (iirc) is at the poles, where the sunlight exposure is minimal, and I think Mars's water is the same way. 70.36.142.114 (talk) 14:05, 2 May 2014 (UTC)
- Does evaporation defy gravity? Water cannot escape the Earth's atmosphere that way, so why should it escape the moon?--Shantavira|feed me 14:30, 2 May 2014 (UTC)
- For the same reason there's so little helium (second most abundant element in the solar system!) in Earth's atmosphere. Or, for that matter, why there's so little of anything in the Moon's atmosphere. TenOfAllTrades(talk) 14:40, 2 May 2014 (UTC)
- See atmospheric escape. The Earth loses water all the time - or the hydrogen part of it anyway. (We should be ok for drinks until the Sun goes nova; there is water inside the Earth, and geological processes bring it up at roughly the same rate it is lost to space.)
- Incidentally, we have an article on origin of water on Earth - it's not as clear cut as SimEarth taught us. 88.112.50.121 (talk) 15:11, 2 May 2014 (UTC)
- Does evaporation defy gravity? Water cannot escape the Earth's atmosphere that way, so why should it escape the moon?--Shantavira|feed me 14:30, 2 May 2014 (UTC)
Plant Intelligent/ brain theory
Charles Darwin first proposed that plants root system was a "brain-like" organ and a few studies have been done on plant intelligence. The question is 1. is the science sound enough to be put onto Wikipedia, and 2. should it get its own page or be a section on the plant page. Manofgun (talk) 14:17, 2 May 2014 (UTC) forgot to log in
Maintain Hot Water
When I travel I have been lowering the temperature on my water heater. Is this necessary?
Does it take more energy to maintain a higher temperature than a lower one?
I know it takes more energy to achieve higher temperatures but, once achieved, does it also take more to keep them there?
Thank you — Preceding unsigned comment added by ChrisIsFromCanada (talk • contribs) 14:38, 2 May 2014 (UTC)
- To maintain the temperature of something, you have to put in as much heat energy as it is losing. How much heat energy something loses is proportional to the difference in temperature between that thing, and the things around it. Hotter water loses more heat energy to its surroundings than cooler water, because there is a bigger difference between the temperature of the hot water and the temperature of the surroundings.
- So, if your water heater is actually keeping a volume of hot water perpetually hot, then it will take more energy to keep it at a higher temperature. However, is this actually what it does? When you travel, does your heater at home really keep a tank of water hot for the whole time you're away? If so, do you really want to use that water when you get back? 86.146.28.229 (talk) 15:12, 2 May 2014 (UTC)



