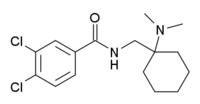
AH-7921
 | |
| Clinical data | |
|---|---|
| Other names | AH-7921 |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H22Cl2N2O |
| Molar mass | 329.265 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AH-7921 is an opioid analgesic drug selective for the µ-opioid receptor, having around 80% the potency of morphine when administered orally.[1][2] It was discovered in the 1970s[3] by a team at Allen and Hanburys.
Dosages have been reported to range from as little as 10 mg to around 200 mg or higher (for opioid-tolerant individuals).[4] This would confirm the previous studies of AH-7921 being roughly 80% as potent as morphine. [citation needed]
Use
Although AH-7921 was extensively studied in vitro and in animals, though not in humans, by the developing company, it was never sold commercially for medical use. In 2013, AH-7921 was discovered to have been used as an active ingredient in "synthetic cannabis" products in Japan.[5]
Legality
AH-7921 was made a Prohibited Substance (Schedule 9 of the Standard for the Uniform Scheduling of Medicines and Poisons) in Australia in May 2014.[6] Although this amendment was repealed in June 2014,[7] which simply means the amendment document ceases, but the actual scheduling is permanent as part of the main document (all SUSMP amendments cease after a few weeks). It may, however, still be a banned import.
AH-7921 has been illegal to distribute in Israel since December 2013.[8]
In the UK, AH-7921 was included as a Class A drug under the 1971 Act in January 2015.[9]
In Brazil, AH-7921 has been considered an illegal drug since May 2015. [10]
As of October 2015 AH-7921 is a controlled substance in China.[11]
Synthesis

See also
References
- ^ Brittain, R. T.; Kellett, D. N.; Neat, M. L.; Stables, R. (1973). "Proceedings: Anti-nociceptive effects in N-substituted cyclohexylmethylbenzamides". British Journal of Pharmacology. 49 (1): 158P–159P. doi:10.1111/j.1476-5381.1973.tb08279.x. PMC 1776456. PMID 4207044.
- ^ Hayes, A. G.; Tyers, M. B. (1983). "Determination of receptors that mediate opiate side effects in the mouse". British Journal of Pharmacology. 79 (3): 731–736. doi:10.1111/j.1476-5381.1983.tb10011.x. PMC 2044905. PMID 6317119.
- ^ US patent 3975443, Harper, N.; Veitch, G., "1-(3,4-DICHLOROBENZAMIDOMETHYL)-CYCLOHEXYLDIMETHYLAMINE", issued 1976-08-17, assigned to Allen & Hanburys
- ^ "AH-7921". Erowid Experience Vaults. Retrieved 8 January 2016.
- ^ Uchiyama, N.; Matsuda, S.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. (2013). "Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products". Forensic Toxicology. doi:10.1007/s11419-013-0182-9.
- ^ http://www.tga.gov.au/pdf/scheduling/scheduling-decisions-1405-final.pdf
- ^ http://www.comlaw.gov.au/Details/F2014L00566
- ^ http://www.health.gov.il/LegislationLibrary/Homarim-12092013.pdf
- ^ https://www.gov.uk/government/publications/circular-0012015-a-change-to-the-misuse-of-drugs-act-1971-control-of-ah-7921-lsd-related-compounds-tryptamines-and-rescheduling-of-ghb/circular-0012015-a-change-to-the-misuse-of-drugs-act-1971-control-of-ah-7921-lsd-related-compounds-tryptamines-and-rescheduling-of-ghb
- ^ http://portal.anvisa.gov.br/wps/wcm/connect/5887f100485fb8f3b2d8bb734e60b39c/44+-+RDC+n%C2%BA+18-2015-DOU.pdf?MOD=AJPERES
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
