Alpha-1 blocker
Alpha-1 blockers (also called alpha-adrenergic blocking agents or alpha-1 antagonists) constitute a variety of drugs that block the effect of catecholamines on alpha-1-adrenergic receptors. They are mainly used to treat benign prostatic hyperplasia (BPH), hypertension and post-traumatic stress disorder.[1] Alpha-1-adrenergic receptors are present in vascular smooth muscle, the central nervous system, and other tissues. When alpha blockers bind to these receptors in vascular smooth muscle, they cause vasodilation.
Over the last 40 years, a variety of drugs have been developed from non-selective alpha-1 receptor antagonists to selective alpha-1 antagonists and alpha-1 receptor inverse agonists.[2][3] The first drug that was used was a non-selective alpha blocker, named phenoxybenzamine and was used to treat BPH.[2] Currently, several relatively selective alpha-1 antagonists are available. As of 2018, prazosin is the only alpha-1 blocker known to act as an inverse agonist at all alpha-1 adrenergic receptor subtypes;[3][4] whereas tamsulosin is a selective antagonist for all alpha-1 subtypes.[3][5] Drugs that act as selective antagonists at specific alpha-1 adrenergic receptor subtypes have also been developed.
Medical uses
[edit]Benign prostatic hyperplasia
[edit]Benign prostatic hyperplasia (BPH) is an enlarged prostate gland. Alpha-1 blockers are the most commonly used medicine to treat BPH.[6] Alpha-1 blockers are first line treatment for the symptoms of BPH in men.[1][2][7][8] Doxazosin, terazosin, alfuzosin, and tamsulosin have all been well established in treatment to reduce lower urine tract symptoms (LUTS) caused by benign prostatic hyperplasia. They are all believed to be similarly effective for this purpose. First generation alpha-1 blockers, like prazosin are not recommended to treat lower urinary tract symptoms because of their blood-pressure-lowering effect. Later generation drugs in this class are used for this purpose.[1][9] In some cases alpha-1 blockers have been used in combined therapy with 5α-reductase inhibitors. Dutasteride and tamsulosin are on the market as combined therapy and results have shown that they improve symptoms significantly versus monotherapy.[9][10]
Hypertension
[edit]Alpha-1 blockers are used as second line treatment for high blood pressure. They are not thought to be good as first line treatment because there are other more selective agents, although they can be good for treating men with hypertension and BPH.[11] Doxazosin has been shown to improve symptoms of BPH in the elderly and reduce blood pressure at the same time. BPH and hypertension are both very common in men over 60 years old.[12]
In patients with neurogenic hypertension who fail in achieving blood pressure control with angiotensin converting enzyme inhibitors (ACEi), Angiotensin receptor blockers (ARB) and calcium channel blockers (CCB), alpha- and beta- adrenergic receptor blockers constitute the main treatment options. This is supported by studies that show surprisingly though consistently that neither alpha- nor beta-blocker mono- therapy reduces sympathetically mediated blood pressure reactivity to acute experimental stressors. Studies of combined oral alpha- and beta-blockade using an alpha-blocker (e.g., doxazosin or terazosin) in combination with a nonlipophilic beta-blocker with more reliable bioavailability (e.g., betaxolol, bisoprolol, atenolol and others) have shown a larger antihypertensive effect.[13][14] [15] This approach also enables separate titration of alpha- and beta-blocking effects. In these studies, doxazosin was prescribed at the low dose of 1–2 mg. [16] [17] [18]
Post-traumatic stress disorder (PTSD) and nightmares
[edit]Post-traumatic stress disorder (PTSD) is a disabling mental condition that can develop following a traumatic experience; for example, it is especially common in military veterans and sexual assault survivors. Prazosin is commonly used as an antihypertensive, but because alpha-1-adrenergic activity has been connected to fear and startle responses, it sees use as a PTSD treatment.[19][20] Prazosin has been established as an effective and safe centrally active alpha-1-adrenergic receptor antagonist. It can be used to treat trauma-related nightmares, sleep disturbance, and other chronic PTSD symptoms.[21]
Adverse effects
[edit]As Alpha-1a blockers affect the symptoms of BPH more specifically than non-selective Alpha-1 blockers, the adverse effects seem to be more linked to the reproductive system while minimizing the effect on the blood pressure system.[22][23] Hypotension and its complications (e.g., weakness, dizziness) are a constant risk, however, even though selective alpha-1a blockers are being used. It is therefore important when starting treatment with an alpha-1 blocker to monitor blood pressure to minimize the risk for adverse effects connected to low blood pressure.
| Selectivity | Adverse effects | Main hepatic pathway | |
|---|---|---|---|
| Prazosin | Alpha-1 | Dizziness, headache, drowsiness, asthenia, weakness, palpitations, nausea, vomiting, diarrhea, constipation, edema, hypotension, dyspnea, vertigo, nervousness, rash, blurred vision | CYP1A1[24] |
| Terazosin | Alpha-1 | Nervousness, vertigo, palpitations, tachycardia, chest pain, hypotension, dyspnoea, nausea, constipation, diarrhoea, vomiting, pruritus, rash, back pain, impotence, dizziness, asthenia, oedema, headache, pain (extreme) | CYP3A1[25] |
| Doxazosin | Alpha-1 | Hypotension (dizziness and weakness), priapism (prolonged erection), floppy iris syndrome during cataract surgery, vertigo, palpitation/tachycardia, bronchitis, cough, dyspnea, pruritus, back pain, cystitis, asthenia, peripheral oedema, retrograde ejaculation | CYP3A4[26] |
| Silodosin | Alpha-1a | Problem with ejaculation/orgasm, hypotension, dizziness, nasal congestion, diarrhoea | CYP3A4[27] |
| Alfuzosin | Alpha-1a | Dizziness (due to hypotension), upper respiratory tract infection, headache, fatigue, impotence, pain in whole body, bronchitis, gastro-intestinal pain, swelling, rash, difficulty swallowing or breathing, chest pain, fainting, hoarsing | CYP3A4[28] |
| Tamsulosin | Alpha-1a | Hypotension, problem with ejaculation/orgasm, disorientation, headache, floppy iris syndrome during cataract surgery | CYP3A4 & CYP2D6[29] |
By reducing alpha-1-adrenergic activity of the blood vessels, these drugs may cause hypotension (low blood pressure) and interrupt the baroreflex response. In doing so, they may cause dizziness, lightheadedness, or fainting when rising from a lying or sitting posture (known as orthostatic hypotension or postural hypotension). For this reason, it is generally recommended that alpha blockers should be taken at bedtime. The risk of first dose phenomenon may be reduced or eliminated by gradual-dose titration, since the adverse effects of Prazosin are dose-related.[7] This is also the case for Tamsulosin and it may be assumed that the others alpha-1 blockers work in a similar manner, since Tamsulosin is an alpha-1-a blocker and Prazosin is an alpha-1 blocker.[30] The risk for floppy iris syndrome during cataract surgery is elevated when the patient is using an alpha-1 blocker. This is especially the case for Tamsulosin and other alpha-1-a blockers, since alpha-1-a receptors are present also in the iris dilator muscle, which allows unopposed action of the parasympathetically innervated iris constrictor muscle and loss of iris tone.[31] This however can be treated if the eye surgeon is experienced and has knowledge of the use of alpha-1 blocker.
Interactions and contraindication
[edit]Contraindication : Allergies or hypersensitivity to alpha-1 blockers or any of the active ingredient, that includes angiodema induced by the drug. Patients with a history of orthostatic hypotension or severe hepatic impairment.[32][33]
Interactions : No interactions were recorded when administered with atenolol (beta blocker), enalapril (ACE inhibitor) and theophylline.[32] Furosemide has drop effect on plasma level for tamsulosin, and a rise in plasma level with cimetidine. No dose adjustment needs to be done when the levels are in normal range. Drugs that inhibit CYP3A4 (for example, itraconazole, ketoconazole, and ritonavir) can increase drug exposure for tamsulosin, alfuzosin, doxazosin, and silodosin. Grapefruit is also a powerful inhibitor of the CYP3A4 enzyme, so concurrent use is not recommended as it may increase the plasma levels of the Alpha-1 blockers which are metabolised by the CYP3A4 enzyme.[34] Some drugs; such as Fluoxetine, Paroxetine and Ritonavir are strong inhibitors of the CYP2D6 enzyme and therefore it is not recommended to use at the same time as tamsulosin, as it may increase plasma levels of tamsulosin and increase the risk of adverse effects.[35]
Warfarin and diclofenac can increase the elimination rate for tamsulosin, but has not shown an effect on alfuzosin hydrochloride. Co-administration of alpha-1 inhibitor can cause hypotension.[32][33]
Since alpha-1 blockers may cause orthostatic hypotension, co-administration with antihypertensives and vasodilators must be evaluated with regards to risk-benefit as the risk for low blood pressure is greatly increased.
By reducing α1-adrenergic activity of the blood vessels, these drugs may cause hypotension (low blood pressure) and interrupt the baroreflex response. In doing so, they may cause dizziness, lightheadedness, or fainting when rising from a lying or sitting posture (known as orthostatic hypotension or postural hypotension). For this reason, it is generally recommended that alpha blockers should be taken at bedtime. Additionally, the risk of first dose phenomenon may be reduced by starting at a low dose and titrating upwards as needed.
Because these medications may cause orthostatic hypotension, as well as low blood pressure in general, these agents may interact with other medications that increase risk for low blood pressure, such as other antihypertensives and vasodilators.
As discussed above, tamsulosin may have less risk for low blood pressure and orthostatic hypotension due to its selectivity for α1a-adrenergic receptors. On the other hand, the drug (a) elevates risk for floppy iris syndrome, and (b) might show adverse drug reactions (ADRs) characteristic of the sulfa related drugs.
List of alpha-1 blockers
[edit]| Alpha-1 inhibitor | Structure | Use | Brand name | Selectivity |
|---|---|---|---|---|
| Prazosin | Hypertension, PTSD and nightmares | Minipress, Vasoflex, Lentopres and Hypovase. | Alpha-1 | |
| Terazosin | Hypertension and BPH | Hytrin, Zaysel and Terazosin | Alpha-1 | |
| Doxazosin | Hypertension and BPH | Cardura and Carduran | Alpha-1 | |
| Silodosin | BPH | Rapaflo, Silodyx, Rapilif, Urief, Trupas, Urorec | Alpha-1a | |
| Alfuzosin | BPH | Uroxatral, Xat, Xatral, Prostetrol and Alfural. | Alpha-1a | |
| Tamsulosin | BPH | Alna, Flomax, Omnic | Alpha-1a |
Silodosin shows high affinity and selectivity for alpha-1a adrenergic receptors found in the prostate which ensures that it works quickly and effectively to relieve the symptoms of BPH. Silodosin's low affinity for alpha-1b receptors in the blood vessels is thought to be reflected in its low incidence of orthostatic and vasodilatory side effects.[36]
The beta blocker Carvedilol is also an alpha-1 blocker.[37]
Pharmacokinetics
[edit]Absorption: Bioavailability of tamsulosin and terazosin is around 90% during oral administration in fasting state. Food can have effect on absorption for tamsulosin if it has been ingested shortly before, Tmax for fasting state is 2,9–5,6 hours compared to 5,2–7 hours in fed state. Food has no effect on absorption of terazosin but can delay plasma level concentration for 1 hour, peak plasma level are around 1–2 hours.[38][32][39] Alfuzosin bioavailability under fed state is around 49%. Tmax is 8 hours in fed state.[40][33] Tamsulosin Cmax range was 13.9–18,6 ng/mL fastest and in fed state 7,2–15,6 ng/mL, Cmax for alfuzion is 13,6 mg/mL.[41][32]
Distribution: Tamsulosin is 99% bound to plasma and distribution volume is low 0.2L/kg.[32] Alfuzosin is 90% bound to plasma and distribution volume is 2.5L/kg.[40][33] Terazosin is 90–94% bound to plasma.[39]
Elimination: Elimination half-life for alfuzosin is around 8 hours, alfuzosin is metabolised mainly via liver. 75–91% is excreted in feces and 35% in unchanged form. Distribution volume and excretion increases with renal impairment due to less protein binding, but the half-life elimination rate is unchanged. therefore no dose adjustment is needed for low to moderate renal impairment. Delay in elimination half-life, peak concentration in plasma is double and bioavailability is changed in hepatic impairment patients. Alfuzosin should not be used for patients with renal impairment.[40][33] Tamsulosin is excreted via urine and 9% of that is unchanged on its active form, elimination half-life for tamsulosin is between 9–13 hours for healthy volunteers. The elimination half-life for target patients is around 14–15 hours. No dose adjustment is needed for patients with renal impairment and moderate hepatic impairment.[38][32] 10–20% of terazosin is excreted unchanged in urine and feces during oral administration. 40% is eliminated in urine and 60% in feces. Eliminations half-life for terazosin is between 8–13 hours. No dose adjustment is needed for patients with renal impairment. Terazosin is metabolised by the liver and is excreted by the biliary tract, so patients with moderate hepatic impairment should receive titrated doses of terazosin witch caution. Patients with severe hepatic impairment should not take terazosin due to lack of clinical data.[39][42]
Mechanism of action
[edit]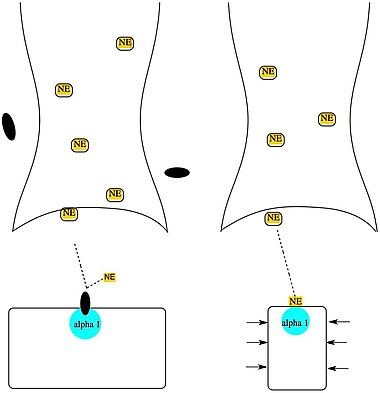
Alpha-1 blockers inhibit the activation of post-synaptic alpha-1 receptors by norepinephrine thus opposing blood vessel contraction. Alpha-1 blockers have no effect on renin release or cardio output.[43]
Benign prostatic hyperplasia
[edit]Alpha-1 blocker, blocks alpha receptors and it relaxes the smooth muscles in the bladder. It helps the urine to flow smoothly and it can lessen the pain caused by the bladder pressing on the prostate.[44][45] Selective alpha-1 blockers are better tolerated than non-selective alpha blockers in the body and therefore works better on BPH.[2] Terazosin, tamsulosin and doxazosin are prime drug for BPH because they have a long half-life and modified release formulation. Tamsulosin is primarily used because it doesn't affect the blood pressure and the side effects of vasodilation is minimum.[44][45][2]
Hypertension
[edit]Alpha-1 blocker lowers the blood pressure by blocking alpha-1 receptors so norepinephrine cannot bind the receptor, causing the blood vessels to dilate. Without the resistance in the blood vessels the blood runs more freely.[46][47] Alpha-1 blockers have a good effect on lipoproteins in plasma, insulin resistance and it causes the glucose levels in blood to lower.[46][47][48]
Structure activity relationship (SAR)
[edit]By changing the furan ring in prazosin to tetrahydrofuran ring (as in alfuzosin) the half-life is greatly increased, allowing once-a-day dosing. Silodosin is the most selective for alpha-1a receptors.[49] The affinity and selectivity for alpha-1 receptors seems to be determined by structure between the quinazoline and the furan ring. Piperazine is present in prazosin, terazosin and doxazosin which seems to contribute to the non-selective inhibition of alpha-1 receptors.[50]

Doxazosin 2,4-diamino-6,7-dimethoxyquinazoline variations for in vitro and in vivo performance. A key factor in these structures were the derivations from 2,4-diamino-6,7-dimethoxyquinazoline nucleus that was replaced for norepinephrine. And N-1 which protonated quinazoline was also a key factor.[51]

Tamsulosin is most potent alpha 1 blocker and has the most selectivity for alpha 1a receptors. It has no beta-blocking activity.[52]
History
[edit]The first effective treatment for benign prostatic hyperplasia (BPH) was a non-selective alpha blocker phenoxybenzamine which was irreversible. Dibenzyline was the first brand name marketed. Today phenoxybenzamine is not the first choice due to many side effects like lowering blood pressure.[2]
First selective alpha-1 blocker that was approved to treat hypertension was prazosin. Prazosin was synthesized in 1974 when Constantin and Hess were trying to discover a vasodilator which had a minimal effect on cardiac activity.[7] Prazosin was a much better tolerated drug than phenoxybenzamine but the problem still remained that it lowered the blood pressure more than desired for a BPH treatment.[2][8]
Terazosin was the first long-lasting alpha 1 blocker approved by FDA to treat BPH. Doxazosin and Tamsulosin were approved after. The first-line treatment choice today to treat BPH is tamsulosin. It is not better tolerated, nor does it have greater efficacy than the previous drugs, however, it requires minimal dose titration in comparison. Alfuzosin SR (sustained release) was the fourth alpha 1 selective blocker to be approved by FDA and requires no dose titration.[2][47]
References
[edit]- ^ a b c Nickel, J. Curtis; Méndez-Probst, Carlos E.; Whelan, Thomas F.; Paterson, Ryan F.; Razvi, Hassan (October 2010). "2010 Update: Guidelines for the management of benign prostatic hyperplasia". Canadian Urological Association Journal. 4 (5): 310–316. doi:10.5489/cuaj.10124. ISSN 1911-6470. PMC 2950766. PMID 20944799.
- ^ a b c d e f g h Lepor, Herbert (2007). "Alpha Blockers for the Treatment of Benign Prostatic Hyperplasia". Reviews in Urology. 9 (4): 181–190. ISSN 1523-6161. PMC 2213889. PMID 18231614.
- ^ a b c "Adrenoceptors". IUPHAR. International Union of Basic and Clinical Pharmacology. Receptors. Retrieved July 7, 2018.
- ^ "Prazosin: Biological activity". IUPHAR. International Union of Basic and Clinical Pharmacology. Retrieved July 7, 2018.
- ^ "Tamsulosin: Summary". IUPHAR. International Union of Basic and Clinical Pharmacology. Retrieved July 7, 2018.
- ^ Sokhal, Ashok Kumar; Sankhwar, Satyanarayan; Goel, Apul; Singh, Kawaljit; Kumar, Manoj; Purkait, Bimalesh; Saini, Durgesh Kumar (August 30, 2017). "A Prospective Study to Evaluate Sexual Dysfunction and Enlargement of Seminal Vesicles in Sexually Active Men Treated for Benign Prostatic Hyperplasia by Alpha Blockers". Urology. 118: 92–97. doi:10.1016/j.urology.2017.08.025. PMID 28860050. S2CID 36531385.
- ^ a b c Stanaszek, W. F.; Kellerman, D.; Brogden, R. N.; Romankiewicz, J. A. (April 1983). "Prazosin update. A review of its pharmacological properties and therapeutic use in hypertension and congestive heart failure". Drugs. 25 (4): 339–384. doi:10.2165/00003495-198325040-00002. ISSN 0012-6667. PMID 6303744. S2CID 46973044.
- ^ a b Carruthers, S. G. (July 1994). "Adverse effects of alpha 1-adrenergic blocking drugs". Drug Safety. 11 (1): 12–20. doi:10.2165/00002018-199411010-00003. ISSN 0114-5916. PMID 7917078. S2CID 195124733.
- ^ a b Tanguay, Simon; Awde, Murray; Brock, Gerald; Casey, Richard; Kozak, Joseph; Lee, Jay; Nickel, J. Curtis; Saad, Fred (June 2009). "Diagnosis and management of benign prostatic hyperplasia in primary care". Canadian Urological Association Journal. 3 (Suppl 2): S92 – S100. doi:10.5489/cuaj.1116. ISSN 1911-6470. PMC 2698785. PMID 19543429.
- ^ Roehrborn, Claus G.; Siami, Paul; Barkin, Jack; Damião, Ronaldo; Major-Walker, Kim; Morrill, Betsy; Montorsi, Francesco (February 1, 2008). "The Effects of Dutasteride, Tamsulosin and Combination Therapy on Lower Urinary Tract Symptoms in Men With Benign Prostatic Hyperplasia and Prostatic Enlargement: 2-Year Results From the CombAT Study". The Journal of Urology. 179 (2): 616–621. doi:10.1016/j.juro.2007.09.084. PMID 18082216.
- ^ Bryson CL, Chris L.; Psaty BM MD PhD, Bruce M. (April 12, 2002). "A Review of the Adverse Effects of Peripheral Alpha-1 Antagonists in Hypertension Therapy". Current Controlled Trials in Cardiovascular Medicine. 3 (1): 7. doi:10.1186/1468-6708-3-7. ISSN 1468-6708. PMC 134479. PMID 12097149.
- ^ Weinberger, M. H.; Fawzy, A. (April 2000). "Doxazosin in elderly patients with hypertension". International Journal of Clinical Practice. 54 (3): 181–189. doi:10.1111/j.1742-1241.2000.tb11878.x. ISSN 1368-5031. PMID 10829361. S2CID 19462674.
- ^ Searle, M.; Dathan, R.; Dean, S.; Christensen, C. C.; Westheim, A. (1990). "Doxazosin in combination with atenolol in essential hypertension: a double-blind placebo-controlled multicentre trial". European Journal of Clinical Pharmacology. 39 (3): 299–300. doi:10.1007/BF00315116. PMID 2147909. S2CID 6750811.
- ^ Pool, J. L. (1991). "Combination antihypertensive therapy with terazosin and other antihypertensive agents: results of clinical trials". American Heart Journal. 122 (3 Pt 2): 926–931. doi:10.1016/0002-8703(91)90813-w. PMID 1678924.
- ^ "Concomitant administration of terazosin and atenolol for the treatment of essential hypertension".
- ^ Pool, J. L. (1991). "Combination antihypertensive therapy with terazosin and other antihypertensive agents: results of clinical trials". American Heart Journal. 122 (3 Pt 2): 926–931. doi:10.1016/0002-8703(91)90813-w. PMID 1678924.
- ^ Holtzman, J. L.; Kaihlanen, P. M.; Rider, J. A.; Lewin, A. J.; Spindler, J. S.; Oberlin, J. A. (1988). "Concomitant administration of terazosin and atenolol for the treatment of essential hypertension". Archives of Internal Medicine. 148 (3): 539–543. doi:10.1001/archinte.1988.00380030045010. PMID 3277569.
- ^ Mann, Samuel J. (2018). "Neurogenic hypertension: pathophysiology, diagnosis and management". Clinical Autonomic Research. 28 (4): 363–374. doi:10.1007/s10286-018-0541-z. PMID 29974290. S2CID 49694037.
- ^ Peskind, Elaine R.; Bonner, Lauren T.; Hoff, David J.; Raskind, Murray A. (September 2003). "Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder". Journal of Geriatric Psychiatry and Neurology. 16 (3): 165–171. doi:10.1177/0891988703256050. ISSN 0891-9887. PMID 12967060. S2CID 44736183.
- ^ Taylor, Fletcher; Raskind, Murray A. (February 2002). "The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder". Journal of Clinical Psychopharmacology. 22 (1): 82–85. doi:10.1097/00004714-200202000-00013. ISSN 0271-0749. PMID 11799347. S2CID 144597560.
- ^ Raskind, Murray A.; Peskind, Elaine R.; Hoff, David J.; Hart, Kimberly L.; Holmes, Hollie A.; Warren, Daniel; Shofer, Jane; O’Connell, James; Taylor, Fletcher (April 15, 2007). "A Parallel Group Placebo Controlled Study of Prazosin for Trauma Nightmares and Sleep Disturbance in Combat Veterans with Post-Traumatic Stress Disorder". Biological Psychiatry. 61 (8): 928–934. doi:10.1016/j.biopsych.2006.06.032. PMID 17069768. S2CID 12694567.
- ^ Yoshida, Masaki; Kudoh, Junzo; Homma, Yukio; Kawabe, Kazuki (2011). "Safety and efficacy of silodosin for the treatment of benign prostatic hyperplasia". Clinical Interventions in Aging. 6: 161–172. doi:10.2147/CIA.S13803. ISSN 1176-9092. PMC 3131986. PMID 21753871.
- ^ Hisasue, Shin-Ichi; Furuya, Ryoji; Itoh, Naoki; Kobayashi, Ko; Furuya, Seiji; Tsukamoto, Taiji (October 1, 2006). "Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission". International Journal of Urology. 13 (10): 1311–1316. doi:10.1111/j.1442-2042.2006.01535.x. ISSN 1442-2042. PMID 17010010. S2CID 31021721.
- ^ Preissner, Saskia; Kroll, Katharina; Dunkel, Mathias; Senger, Christian; Goldsobel, Gady; Kuzman, Daniel; Guenther, Stefan; Winnenburg, Rainer; Schroeder, Michael (January 2010). "SuperCYP: a comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions". Nucleic Acids Research. 38 (Database issue): D237–243. doi:10.1093/nar/gkp970. ISSN 1362-4962. PMC 2808967. PMID 19934256.
- ^ Oh, E Y; Bae, S K; Kwon, J W; You, M; Lee, D C; Lee, M G (May 2007). "Pharmacokinetic and pharmacodynamic consequences of inhibition of terazosin metabolism via CYP3A1 and/or 3A2 by DA-8159, an erectogenic, in rats". British Journal of Pharmacology. 151 (1): 24–34. doi:10.1038/sj.bjp.0707192. ISSN 0007-1188. PMC 2012980. PMID 17351661.
- ^ Hammond, Kyle P.; Nielsen, Craig; Linnebur, Sunny A.; Langness, Jacob A.; Ray, Graham; Maroni, Paul; Kiser, Jennifer J. (January 1, 2014). "Priapism Induced by Boceprevir-CYP3A4 Inhibition and α-Adrenergic Blockade: Case Report". Clinical Infectious Diseases. 58 (1): e35 – e38. doi:10.1093/cid/cit673. ISSN 1058-4838. PMC 4049070. PMID 24092799.
- ^ Rossi, Maxime; Roumeguère, Thierry (October 27, 2010). "Silodosin in the treatment of benign prostatic hyperplasia". Drug Design, Development and Therapy. 4: 291–297. doi:10.2147/DDDT.S10428. ISSN 1177-8881. PMC 2990389. PMID 21116335.
- ^ Doligalski, Christina Teeter; Tong Logan, Angela; Silverman, Andrew (June 2012). "Drug Interactions". Gastroenterology & Hepatology. 8 (6): 376–383. ISSN 1554-7914. PMC 3424472. PMID 22933873.
- ^ Franco-Salinas, Gabriela; de la Rosette, Jean J. M. C. H.; Michel, Martin C. (March 2010). "Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations". Clinical Pharmacokinetics. 49 (3): 177–188. doi:10.2165/11317580-000000000-00000. ISSN 1179-1926. PMID 20170206. S2CID 207298067.
- ^ "General effect of low-dose tamsulosin (0.2mg) as a first-line treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia: A systematic review and meta-analysis (PDF Download Available)". ResearchGate. Retrieved September 26, 2017.
- ^ Brogden, PR; Backhouse, OC; Saldana, M (2007). "Intraoperative floppy iris syndrome associated with tamsulosin". Can Fam Physician. 53 (7): 1148. PMC 1949290. PMID 17872808.
- ^ a b c d e f g "Flomax (Tamsulosin Hydrochloride): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Retrieved September 28, 2017.
- ^ a b c d e Roehrborn, C. G. (December 2001). "Alfuzosin: overview of pharmacokinetics, safety, and efficacy of a clinically uroselective alpha-blocker". Urology. 58 (6 Suppl 1): 55–63, discussion 63–64. doi:10.1016/s0090-4295(01)01322-x. ISSN 1527-9995. PMID 11750253.
- ^ Bailey, David G; Malcolm, J; Arnold, O; David Spence, J (August 1998). "Grapefruit juice–drug interactions". British Journal of Clinical Pharmacology. 46 (2): 101–110. doi:10.1046/j.1365-2125.1998.00764.x. ISSN 0306-5251. PMC 1873672. PMID 9723817.
- ^ "Drug Interactions Indiana University, School of Medicine, Department of Medicine". medicine.iupui.edu. Retrieved September 28, 2017.
- ^ Nomiya, M; Yamaguchi, O (2003). "A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders". J Urol. 170 (2 Pt 1): 649–653. doi:10.1097/01.ju.0000067621.62736.7c. PMID 12853849.
- ^ "Carvedilol Monograph for Professionals". Drugs.com. Retrieved September 13, 2024.
- ^ a b Bird, Steven T; Delaney, Joseph A C; Brophy, James M; Etminan, Mahyar; Skeldon, Sean C; Hartzema, Abraham G (November 5, 2013). "Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40–85 years in the United States: risk window analyses using between and within patient methodology". The BMJ. 347: f6320. doi:10.1136/bmj.f6320. ISSN 0959-8138. PMC 3817852. PMID 24192967.
- ^ a b c "Terazosin 2 mg Tablets - Summary of Product Characteristics (SPC) – (eMC)". www.medicines.org.uk. Retrieved September 28, 2017.
- ^ a b c "Uroxatral (Alfuzosin HCl): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Retrieved September 28, 2017.
- ^ "UROXATRAL® (alfuzosin HCl) extended-release tablets Prescribing Information". products.sanofi.us. Retrieved September 28, 2017.
- ^ Tehranchi, Ali; Rezaei, Yousef; Khalkhali, Hamidreza; Rezaei, Mahdi (November 2013). "Effects of terazosin and tolterodine on ureteral stent related symptoms: a double-blind placebo-controlled randomized clinical trial". International Brazilian Journal of Urology. 39 (6): 832–840. doi:10.1590/s1677-5538.ibju.2013.06.09. ISSN 1677-6119. PMID 24456787.
- ^ Sica, Domenic A. (December 1, 2005). "Alpha1-Adrenergic Blockers: Current Usage Considerations". The Journal of Clinical Hypertension. 7 (12): 757–762. doi:10.1111/j.1524-6175.2005.05300.x. ISSN 1751-7176. PMC 8109532. PMID 16330901. S2CID 25143919.
- ^ a b Chapple, C. R. (1996). "Selective alpha 1-adrenoceptor antagonists in benign prostatic hyperplasia: rationale and clinical experience". European Urology. 29 (2): 129–144. ISSN 0302-2838. PMID 8647139.
- ^ a b Lepor, H. (1990). "Role of alpha-adrenergic blockers in the treatment of benign prostatic hyperplasia". The Prostate. Supplement. 3: 75–84. doi:10.1002/pros.2990170508. ISSN 1050-5881. PMID 1689172. S2CID 25370780.
- ^ a b Kaplan, N. M. (May 23, 1986). "Role of selective alpha 1 blockers in the therapy of hypertension". The American Journal of Medicine. 80 (5B): 100–104. doi:10.1016/0002-9343(86)90861-2. ISSN 0002-9343. PMID 2872800.
- ^ a b c Mathur, R. P.; Nayak, Sumanlata; Sivaramakrishnan, R.; Jain, Vikas (September 2014). "Role of Alpha Blockers in Hypertension with Benign Prostatic Hyperplasia". The Journal of the Association of Physicians of India. 62 (9 Suppl): 40–44. ISSN 0004-5772. PMID 26245042.
- ^ Sever, P. S. (1999). "Alpha 1-blockers in hypertension". Current Medical Research and Opinion. 15 (2): 95–103. doi:10.1185/03007999909113369. ISSN 0300-7995. PMID 10494492. S2CID 71367617.
- ^ Lemke, Thomas L.; Williams, David A. (January 24, 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. ISBN 9781609133450.
- ^ Giardina, Dario; Gulini, Ugo; Massi, Maurizio; Piloni, Maria G.; Pompei, Pierluigi; Rafaiani, Giovanni; Melchiorre, Carlo (March 1, 1993). "Structure-activity relationships in prazosin-related compounds. 2. Role of the piperazine ring on .alpha.-blocking activity". Journal of Medicinal Chemistry. 36 (6): 690–698. doi:10.1021/jm00058a005. ISSN 0022-2623. PMID 8096245.
- ^ Campbell, Simon F.; Hardstone, J. David; Palmer, Michael J. (May 1, 1988). "2,4-Diamino-6,7-dimethoxyquinoline derivatives as .alpha.1-adrenoceptor antagonists and antihypertensive agents". Journal of Medicinal Chemistry. 31 (5): 1031–1035. doi:10.1021/jm00400a025. ISSN 0022-2623. PMID 2896245.
- ^ Ruffolo, Robert R.; Bondinell, William; Hieble, J. Paul (September 1, 1995). ".alpha.- and .beta.-Adrenoceptors: From the Gene to the Clinic. 2. Structure-Activity Relationships and Therapeutic Applications". Journal of Medicinal Chemistry. 38 (19): 3681–3716. doi:10.1021/jm00019a001. ISSN 0022-2623. PMID 7562902.
External links
[edit]- DrugDigest – Alpha blockers
- RxList.com – Tamsulosin
- alpha-Adrenergic+Blockers at the U.S. National Library of Medicine Medical Subject Headings (MeSH)