From Wikipedia, the free encyclopedia
Panomifene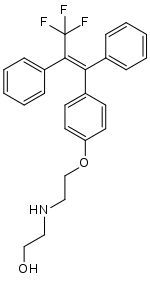 |
|
| Other names | GYKI-13504; EGIS-5650 |
|---|
|
2-[2-[4-[(E)-3,3,3-trifluoro-1,2-diphenylprop-1-enyl]phenoxy]ethylamino]ethanol
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| Formula | C25H24F3NO2 |
|---|
| Molar mass | 427.467 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
C1=CC=C(C=C1)/C(=C(/C2=CC=CC=C2)\C(F)(F)F)/C3=CC=C(C=C3)OCCNCCO
|
InChI=1S/C25H24F3NO2/c26-25(27,28)24(21-9-5-2-6-10-21)23(19-7-3-1-4-8-19)20-11-13-22(14-12-20)31-18-16-29-15-17-30/h1-14,29-30H,15-18H2/b24-23+ Key:MHXVDXXARZCVRK-WCWDXBQESA-N
|
Panomifene (INN; developmental codes GYKI 13504 and EGIS 5650) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group related to tamoxifen that was under development as an antineoplastic agent by Egis Pharmaceuticals and IVAX Drug Research Institute in the 1990s for the treatment of breast cancer, but it was never marketed.[1][2][3][4][5][6] It reached phase II clinical trials before development was terminated.[2] The drug was described in 1981.[1]
References
|
|---|
| ERTooltip Estrogen receptor | | Agonists |
- Steroidal: 2-Hydroxyestradiol
- 2-Hydroxyestrone
- 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
- 3α-Androstanediol
- 3α,5α-Dihydrolevonorgestrel
- 3β,5α-Dihydrolevonorgestrel
- 3α-Hydroxytibolone
- 3β-Hydroxytibolone
- 3β-Androstanediol
- 4-Androstenediol
- 4-Androstenedione
- 4-Fluoroestradiol
- 4-Hydroxyestradiol
- 4-Hydroxyestrone
- 4-Methoxyestradiol
- 4-Methoxyestrone
- 5-Androstenediol
- 7-Oxo-DHEA
- 7α-Hydroxy-DHEA
- 7α-Methylestradiol
- 7β-Hydroxyepiandrosterone
- 8,9-Dehydroestradiol
- 8,9-Dehydroestrone
- 8β-VE2
- 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
- 11β-Chloromethylestradiol
- 11β-Methoxyestradiol
- 15α-Hydroxyestradiol
- 16-Ketoestradiol
- 16-Ketoestrone
- 16α-Fluoroestradiol
- 16α-Hydroxy-DHEA
- 16α-Hydroxyestrone
- 16α-Iodoestradiol
- 16α-LE2
- 16β-Hydroxyestrone
- 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
- 17α-Estradiol (alfatradiol)
- 17α-Dihydroequilenin
- 17α-Dihydroequilin
- 17α-Epiestriol (16α-hydroxy-17α-estradiol)
- 17α-Ethynyl-3α-androstanediol
- 17α-Ethynyl-3β-androstanediol
- 17β-Dihydroequilenin
- 17β-Dihydroequilin
- 17β-Methyl-17α-dihydroequilenin
- Abiraterone
- Abiraterone acetate
- Alestramustine
- Almestrone
- Anabolic steroids (e.g., testosterone and esters, methyltestosterone, metandienone (methandrostenolone), nandrolone and esters, many others; via estrogenic metabolites)
- Atrimustine
- Bolandiol
- Bolandiol dipropionate
- Butolame
- Clomestrone
- Cloxestradiol
- Conjugated estriol
- Conjugated estrogens
- Cyclodiol
- Cyclotriol
- DHEA
- DHEA-S
- ent-Estradiol
- Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
- Epimestrol
- Equilenin
- Equilin
- ERA-63 (ORG-37663)
- Esterified estrogens
- Estetrol
- Estradiol
- Estramustine
- Estramustine phosphate
- Estrapronicate
- Estrazinol
- Estriol
- Estrofurate
- Estrogenic substances
- Estromustine
- Estrone
- Etamestrol (eptamestrol)
- Ethinylandrostenediol
- Ethinylestradiol
- Ethinylestriol
- Ethylestradiol
- Etynodiol
- Etynodiol diacetate
- Hexolame
- Hippulin
- Hydroxyestrone diacetate
- Lynestrenol
- Lynestrenol phenylpropionate
- Mestranol
- Methylestradiol
- Moxestrol
- Mytatrienediol
- Nilestriol
- Norethisterone
- Noretynodrel
- Orestrate
- Pentolame
- Prodiame
- Prolame
- Promestriene
- RU-16117
- Quinestradol
- Quinestrol
- Tibolone
- Xenoestrogens: Anise-related (e.g., anethole, anol, dianethole, dianol, photoanethole)
- Chalconoids (e.g., isoliquiritigenin, phloretin, phlorizin (phloridzin), wedelolactone)
- Coumestans (e.g., coumestrol, psoralidin)
- Flavonoids (incl. 7,8-DHF, 8-prenylnaringenin, apigenin, baicalein, baicalin, biochanin A, calycosin, catechin, daidzein, daidzin, ECG, EGCG, epicatechin, equol, formononetin, glabrene, glabridin, genistein, genistin, glycitein, kaempferol, liquiritigenin, mirificin, myricetin, naringenin, penduletin, pinocembrin, prunetin, puerarin, quercetin, tectoridin, tectorigenin)
- Lavender oil
- Lignans (e.g., enterodiol, enterolactone, nyasol (cis-hinokiresinol))
- Metalloestrogens (e.g., cadmium)
- Pesticides (e.g., alternariol, dieldrin, endosulfan, fenarimol, HPTE, methiocarb, methoxychlor, triclocarban, triclosan)
- Phytosteroids (e.g., digitoxin (digitalis), diosgenin, guggulsterone)
- Phytosterols (e.g., β-sitosterol, campesterol, stigmasterol)
- Resorcylic acid lactones (e.g., zearalanone, α-zearalenol, β-zearalenol, zearalenone, zeranol (α-zearalanol), taleranol (teranol, β-zearalanol))
- Steroid-like (e.g., deoxymiroestrol, miroestrol)
- Stilbenoids (e.g., resveratrol, rhaponticin)
- Synthetic xenoestrogens (e.g., alkylphenols, bisphenols (e.g., BPA, BPF, BPS), DDT, parabens, PBBs, PHBA, phthalates, PCBs)
- Others (e.g., agnuside, rotundifuran)
|
|---|
Mixed
(SERMsTooltip Selective estrogen receptor modulators) | |
|---|
| Antagonists |
- Coregulator-binding modulators: ERX-11
|
|---|
|
|---|
| GPERTooltip G protein-coupled estrogen receptor | | Agonists | |
|---|
| Antagonists | |
|---|
| Unknown | |
|---|
|
|---|
|

