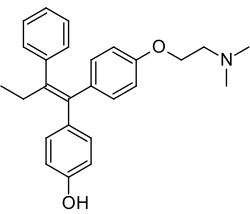
Afimoxifene
 | |
| Clinical data | |
|---|---|
| Other names | 4-Hydroxytamoxifen; 4-OHT; 4-HT; OHTAM; TamoGel |
| Routes of administration | Topical (gel) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.163.120 |
| Chemical and physical data | |
| Formula | C26H29NO2 |
| Molar mass | 387.5139 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Afimoxifene, also known as 4-hydroxytamoxifen (4-OHT, 4-HT, OHTAM, others), is a selective estrogen receptor modulator (SERM) of the triphenylethylene group and the major active metabolite of tamoxifen.[1][2][3] The drug is under development under the tentative brand name TamoGel as a topical gel for the treatment of hyperplasia of the breast.[1][4] It has completed a phase II clinical trial for cyclical mastalgia,[5] but further studies are required before afimoxifene can be approved for this indication and marketed.[4]
A study in France on 55 women showed that rubbing afimoxifene on the skin was as effective as oral tamoxifen at slowing breast cancer growth. A United States trial will compare 6 weeks use before breast cancer surgery. Skin application can reduce systemic levels by a factor of nine and this is expected to reduce the unpleasant side-effects of tamoxifen.[citation needed]
In addition to its antiestrogenic and estrogenic activity, afimoxifene has been found to act as an antagonist of the estrogen-related receptors (ERRs) ERRβ and ERRγ.[6][7]
See also
- List of investigational hormonal agents § Estrogenics
- List of selective estrogen receptor modulators
References
- ^ a b http://adisinsight.springer.com/drugs/800019175
- ^ Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004). "Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6". J Pharmacol Exp Ther. 310 (3): 1062–1075. doi:10.1124/jpet.104.065607. PMID 15159443.
- ^ "Statement on a nonproprietary name adopted by the USAN council: Afimoxifene" (PDF). American Medical Association. Retrieved 2008-03-26.
- ^ a b Ismail Jatoi; Achim Rody (16 November 2016). Management of Breast Diseases. Springer. pp. 77–. ISBN 978-3-319-46356-8.
- ^ Mansel R, Goyal A, Nestour EL, Masini-Etévé V, O'Connell K (2007). "A phase II trial of Afimoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal women". Breast Cancer Res. Treat. 106 (3): 389–397. doi:10.1007/s10549-007-9507-x. PMID 17351746.
- ^ Alice C. Levine (3 October 2011). Hormones and Cancer: Breast and Prostate, An Issue of Endocrinology and Metabolism Clinics of North America,. Elsevier Health Sciences. pp. 271–. ISBN 1-4557-1239-6.
- ^ Sushil K. Khetan (23 May 2014). Endocrine Disruptors in the Environment. Wiley. pp. 104–. ISBN 978-1-118-89115-5.
External links
- 4-hydroxytamoxifen at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Afimoxifene - AdisInsight
