From Wikipedia, the free encyclopedia
Dimethylethanolamine
Names
IUPAC name
2-(Dimethylamino)ethanol
Other names
deanol, dimethylaminoethanol, dimethylaminoethanol
Identifiers
Abbreviations
DMAE, DMEA
1209235
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.003.221
EC Number
KEGG
MeSH
Deanol
RTECS number
UNII
UN number
2051
InChI=1S/C4H11NO/c1-5(2)3-4-6/h6H,3-4H2,1-2H3
N Key: UEEJHVSXFDXPFK-UHFFFAOYSA-N
N
Properties
C 4 H 11 N O
Molar mass
−1
Appearance
Colourless liquid
Odor
Fishy, ammoniacal
Density
890 mg mL−1
Melting point
−59.00 °C; −74.20 °F; 214.15 K
Boiling point
134.1 °C; 273.3 °F; 407.2 K
log P
−0.25
Vapor pressure
816 Pa (at 20 °C)
Acidity (pK a )
9.23 (at 20 °C)[ 1]
Basicity (pK b )
4.77 (at 20 °C)
1.4294
Pharmacology
N06BX04 (WHO
Hazards
GHS labelling
Danger
H226 , H302 , H312 , H314 , H332
P280 , P305+P351+P338 , P310
Flash point
39 °C (102 °F; 312 K)
Explosive limits
1.4–12.2%
Lethal dose or concentration (LD, LC):
1.214 g kg−1 (dermal, rabbit) 2 g kg−1 (oral, rat)
Related compounds
Related alkanols
Related compounds
Diethylhydroxylamine
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
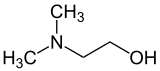
Dimethylethanolamine (DMAE or DMEA) is an organic compound with the formula (CH3 )2 NCH2 CH2 OH. It is bifunctional , containing both a tertiary amine and primary alcohol functional groups . It is a colorless viscous liquid. It is used in skin care products. It is prepared by the ethoxylation of dimethylamine .[ 2]
Industrial uses
It is a precursor to other chemicals, such as the nitrogen mustard 2-dimethylaminoethyl chloride.[ 3] acrylate ester is used as a flocculating agent.
Related compounds are used in gas purification, e.g. removal of hydrogen sulfide from sour gas streams.
Neutraceutical uses
The bitartrate salt of DMAE, i.e. 2-dimethylaminoethanol (+)-bitartrate, is sold as a dietary supplement.[ 4] [ 5]
See also
References
^ Littel, RJ; Bos, M; Knoop, GJ (1990). "Dissociation constants of some alkanolamines at 293, 303, 318, and 333 K" (Submitted manuscript) . Journal of Chemical and Engineering Data . 35 (3): 276–77. doi :10.1021/je00061a014 . INIST 19352048 . ^ "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry . Weinheim: Wiley-VCH. 2002. doi :10.1002/14356007.a10_001 . ^ Ashford's Dictionary of Industrial Chemicals, 3rd edition, 2011, ISBN 978-0-9522674-3-0 , p. 3294.
^ Karen E. Haneke & Scott Masten, 2002, "Dimethylethanolamine (DMAE) [108-01-0] and Selected Salts and Esters: Review of Toxicological Literature (Update)," Report on National Institute of Environmental Health Sciences Contract No. N01-ES-65402, November 2002, from Contractee Integrated Laboratory Systems, Research Triangle Park, North Carolina 27709, see [1] , accessed 30 April 2015.
^ Sigma Aldrich: Safety Data Sheet: 2-Dimethylaminoethanol (+)-bitartrate
Template:Nootropics
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists Antagonists
3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g., brompheniramine , buclizine , captodiame , chlorphenamine (chlorpheniramine) , cinnarizine , clemastine , cyproheptadine , dimenhydrinate , dimetindene , diphenhydramine , doxylamine , meclizine , mequitazine , perlapine , phenindamine , pheniramine , phenyltoloxamine , promethazine , propiomazine , triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g., clozapine , fluperlapine , olanzapine (+fluoxetine ), rilapine , quetiapine , tenilapine , zotepine )Benactyzine Benzatropine (benztropine) Benzilone Benzilylcholine mustard Benzydamine Bevonium BIBN 99 Biperiden Bornaprine Camylofin CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cimetropium bromide Clidinium bromide Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Desfesoterodine Dexetimide DIBD Dicycloverine (dicyclomine) Dihexyverine Difemerine Diphemanil metilsulfate Ditran Drofenine EA-3167 EA-3443 EA-3580 EA-3834 Emepronium bromide Etanautine Etybenzatropine (ethybenztropine) Fenpiverinium Fentonium bromide Fesoterodine Flavoxate Glycopyrronium bromide (+beclometasone/formoterol , +indacaterol , +neostigmine )Hexahydrodifenidol Hexahydrosiladifenidol Hexbutinol Hexocyclium Himbacine HL-031,120 Homatropine Imidafenacin Ipratropium bromide (+salbutamol )Isopropamide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Meladrazine Mepenzolate Methantheline Methoctramine Methylatropine Methylhomatropine Methylscopolamine Metixene Muscarinic toxin 7 N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Nefopam Octatropine methylbromide (anisotropine methylbromide) Orphenadrine Otenzepad (AF-DX 116) Otilonium bromide Oxapium iodide Oxitropium bromide Oxybutynin Oxyphencyclimine Oxyphenonium bromide PBID PD-102,807 PD-0298029 Penthienate Pethidine pFHHSiD Phenglutarimide Phenyltoloxamine Pipenzolate bromide Piperidolate Pirenzepine Piroheptine Pizotifen Poldine Pridinol Prifinium bromide Procyclidine Profenamine (ethopropazine) Propantheline bromide Propiverine Quinidine 3-Quinuclidinyl thiochromane-4-carboxylate Revefenacin Rociverine RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Scopolamine butylbromide (hyoscine butylbromide) Silahexacyclium Sofpironium bromide Solifenacin SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g., femoxetine , paroxetine )Telenzepine Terodiline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tiemonium iodide Timepidium bromide Tiotropium bromide Tiquizium bromide Tofenacin Tolterodine Tricyclic antidepressants (e.g., amitriptyline (+perphenazine ), amitriptylinoxide , butriptyline , cidoxepin , clomipramine , desipramine , desmethyldesipramine , dibenzepin , dosulepin (dothiepin) , doxepin , imipramine , lofepramine , nitroxazepine , northiaden (desmethyldosulepin) , nortriptyline , protriptyline , quinupramine , trimipramine )Tridihexethyl Trihexyphenidyl Trimebutine Tripitamine (tripitramine) Tropacine Tropatepine Tropicamide Trospium chloride Typical antipsychotics (e.g., chlorpromazine , chlorprothixene , cyamemazine (cyamepromazine) , loxapine , mesoridazine , thioridazine )Umeclidinium bromide (+vilanterol )WIN-2299 Xanomeline Zamifenacin
Precursors (and prodrugs )
nAChRs Tooltip Nicotinic acetylcholine receptors
Agonists PAMs Tooltip positive allosteric modulators )
5-HIAA 6-Chloronicotine A-84,543 A-366,833 A-582,941 A-867,744 ABT-202 ABT-418 ABT-560 ABT-894 Acetylcholine Altinicline Anabasine Anatabine Anatoxin-a AR-R17779 Bephenium hydroxynaphthoate Butinoline Butyrylcholine Carbachol Choline Cotinine Cytisine Decamethonium Desformylflustrabromine Dianicline Dimethylphenylpiperazinium Epibatidine Epiboxidine Ethanol (alcohol) Ethoxysebacylcholine EVP-4473 EVP-6124 Galantamine GTS-21 Ispronicline Ivermectin JNJ-39393406 Levamisole Lobeline MEM-63,908 (RG-3487) Morantel Nicotine (tobacco )NS-1738 PHA-543,613 PHA-709,829 PNU-120,596 PNU-282,987 Pozanicline Pyrantel Rivanicline RJR-2429 Sazetidine A SB-206553 Sebacylcholine SIB-1508Y SIB-1553A SSR-180,711 Suberyldicholine Suxamethonium (succinylcholine) Suxethonium (succinyldicholine) TC-1698 TC-1734 TC-1827 TC-2216 TC-5214 TC-5619 TC-6683 Tebanicline Tribendimidine Tropisetron UB-165 Varenicline WAY-317,538 XY-4083 Antagonists NAMs Tooltip negative allosteric modulators )
Precursors (and prodrugs )