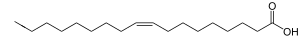
Fat

In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds; most commonly those that occur in living beings or in food.[1]
The term often refers specifically to triglycerides (triple esters of glycerol), that are the main components of vegetable oils and of fatty tissue in animals and humans;[2] or, even more narrowly, to triglycerides that are solid or semisolid at room temperature, thus excluding oils. The term may also be used more broadly as a synonym of lipid -- any substance of biological relevance, composed of carbon, hydrogen, or oxygen, that is insoluble in water but soluble in non-polar solvents.[1] In this sense, besides the triglycerides, the term would include several other types of compounds like mono- and diglycerides, phospholipids (such as lecithin), sterols (such as cholesterol), waxes (such as beeswax),[1] and free fatty acids, which are usually present in human diet in smaller amounts.[2]
Fats are one of the three main macronutrient groups in human diet, along with carbohydrates and proteins,[1][3] and the main components of common food products like milk, butter, tallow, lard, bacon, and cooking oils. They are a major and dense source of food energy for many animals and play important structural and metabolic functions, in most living beings, including energy storage, waterproofing, and thermal insulation.[4] The human body can produce the fat that it needs from other food ingredients, except for a few essential fatty acids that must be included in the diet. Dietary fats are also the carriers of some flavor and aroma ingredients and vitamins that are not water-soluble.[2]
Chemical structure
The most important elements in the chemical makeup of fats are the fatty acids. The molecule of a fatty acid consists of a carboxyl group HO(O=)C− connected to an unbranched alkyl group –(CH
x)
nH: namely, a chain of carbon atoms, joined by single, double, or (more rarely) triple bonds, with all remaining free bonds filled by hydrogen atoms[5]
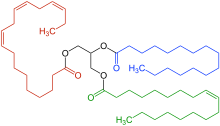
The most common type of fat, in human diet and most living beings, is a triglyceride, an ester of the triple alcohol glycerol H(–CHOH–)
3H and three fatty acids. The molecule of a triglyceride can be described as resulting from a condensation reaction (specifically, esterification) between each of glycerol's –OH groups and the HO– part of the carboxyl group HO(O=)C− of each fatty acid, forming an ester bridge −O−(O=)C− with elimination of a water molecule H
2O.
Other less common types of fats include diglycerides and monoglycerides, where the esterification is limited to two or just one of glycerol's –OH groups. Other alcohols, such as cetyl alcohol (predominant in spermaceti), may replace glycerol. In the phospholipids, one of the fatty acids is replaced by phosphoric acid or a monoester thereof.
Conformation
The shape of fat and fatty acid molecules is usually not well-defined. Any two parts of a molecule that are connected by just one single bond are free to rotate about that bond. Thus a fatty acid molecule with n simple bonds can be deformed in n-1 independent ways (counting also rotation of the terminal methyl group).
Such rotation cannot happen across a double bond, except by breaking and then reforming it with one of the halves of the molecule rotated by 180 degrees, which requires crossing a significant energy barrier. Thus a fat or fatty acid molecule with double bonds (excluding at the very end of the chain) can have multiple cis-trans isomers with significantly different chemical and biological properties. Each double bond reduces the number of conformational degrees of freedom by one. Each triple bond forces the four nearest carbons to lie in a straight line, removing two degrees of freedom.
It follows that depictions of "saturated" fatty acids with no double bonds (like stearic) having a "straight zig-zag" shape, and those with one cis bond (like oleic) being bent in an "elbow" shape are somewhat misleading. While the latter are a little less flexible, both can be twisted to assume similar straight or elbow shapes. In fact, outside of some specific contexts like crystals or bilayer membranes, both are more likely to be found in randomly contorted configurations than in either of those two shapes.
Examples
| Stearic acid saturated |
|
|---|---|
| Oleic acid unsaturated cis-8 |

|
| Elaidic acid unsaturated trans-8 |
|
| Vaccenic acid unsaturated trans-11 |
Stearic acid is a saturated fatty acid (with only single bonds) found in animal fats, and is the intended product in full hydrogenation.
Oleic acid has a double bond (thus being "unsaturated") with cis geometry about midway in the chain; it makes up 55–80% of olive oil.
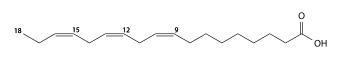
Elaidic acid is its trans isomer; it may be present in partially hydrogenated vegetable oils, and also occurs in the fat of the durian fruit (about 2%) and in milk fat (less than 0.1%).
Vaccenic acid is another trans acid that differs from elaidic only in the position of the double bond; it also occurs in milk fat (about 1-2%).
Nomenclature
Common fat names
Fats are usually named after their source (like olive oil, cod liver oil, shea butter, tail fat) or have traditional names of their own (like butter, lard, ghee, and margarine). Some of these names refer to products that contain substantial amounts of other components besides fats proper.
Chemical fatty acid names
In chemistry and biochemistry, dozens of saturated fatty acids and of hundreds of unsaturated ones have traditional scientific/technical names usually inspired by their source fats (butyric, caprylic, stearic, oleic, palmitic, and nervonic), but sometimes their discoverer (mead, osbond).
A triglyceride would then be named as an ester of those acids, such as "glyceryl 1,2-dioleate 3-palmitate".[6]
IUPAC
In the general chemical nomenclature developed by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of a fatty acid, derived from the name of the corresponding hydrocarbon, completely describes its structure, by specifying the number of carbons and the number and position of the double bonds. Thus, for example, oleic acid would be called "(9Z)-octadec-9-enoic acid", meaning that it has a 18 carbon chain ("octadec") with a carboxyl at one end ("oic") and a double bound at carbon 9 counting from the carboxyl ("9-en"), and that the configuration of the single bonds adjacent to that double bond is cis ("(9Z)") The IUPAC nomenclature can also handle branched chains and derivatives where hydrogen atoms are replaced by other chemical groups.
A triglyceride would then be named according to general ester rules as, for example, "propane-1,2,3-tryl 1,2-bis((9Z)-octadec-9-enoate) 3-(hexadecanoate)".
Fatty acid code
A notation specific for fatty acids with unbranched chain, that is as precise as the IUPAC one but easier to parse, is a code of the form "{N}:{D} cis-{CCC} trans-{TTT}", where {N} is the number of carbons (including the carboxyl one), {D} is the number of double bonds, {CCC} is a list of the positions of the cis double bonds, and {TTT} is a list of the postions of the trans bounds. Either list and the label is omitted if there are no bounds of that type.
Thus, for example, the codes for stearic, oleic, elaidic, and vaccenic acids would be "18:0", "18:1 cis-9", "18:1 trans-9", and "18:1 trans-11", respectively. The code for α-oleostearic acid, which is "(9E,11E,13Z)-octadeca-9,11,13-trienoic acid" in the IUPAC nomenclature, has the code "18:3 trans-9,11 cis-13"
Classification
By chain length
Fats can be classified according to the lengths of the carbon chains of their constituent fatty acids. Most chemical properties, such as melting point and acidity, vary gradually with this parameter, so there is no sharp division. Chemically, formic acid (1 carbon) and acetic acid (2 carbons) could be viewed as the shortest fatty acids; then triformin would be the simplest triglyceride. However, the terms "fatty acid" and "fat" are usually reserved for compounds with substantially longer chains.[citation needed]
A division commonly made in biochemistry and nutrition is:[citation needed]
- Short-chain fatty acid (SCFA) with less than six carbons (e. g. butyric acid).
- Medium-chain fatty acid (MCFA) with 6 to 12 carbons (e.g. capric acid).
- Long-chain fatty acids (LCFA) with 13 to 21 carbons (e.g. petroselinic acid).
- Very long chain fatty acids (VLCFA) with 22 or more carbons (e. g. cerotic acid with 26)
A triglyceride molecule may have fatty acid elements of different lengths, and a fat product will often be a mix of various triglycerides. Most fats found in food, whether vegetable or animal, are made up of medium to long-chain fatty acids, usually of equal or nearly equal length.
Saturated and unsaturated fats
For human nutrition, an important classification of fats is based on the number and position of double bonds in the constituent fatty acids. Saturated fat has a predominance of saturated fatty acids, without any double bonds, while unsaturated fat has predominantly unsaturated acids with double bonds. (The names refer to the fact that each double bond means two fewer hydrogen atoms in the chemical formula. Thus, a saturated fatty acid, having no double bonds, has the maximum number of hydrogen atoms for a given number of carbon atoms — that is, it is "saturated" with hydrogen atoms.)[7][8]
Unsaturated fatty acids are further classified into monounsaturated (MUFAs), with a single double bond, and polyunsaturated (PUFAs), with two or more.[7][8] Natural fats usually contain several different saturated and unsaturated acids, even on the same molecule. For example, in most vegetable oils, the saturated palmitic (C16:0) and stearic (C18:0) acid residues are usually attached to positions 1 and 3 (sn1 and sn3) of the glycerol hub, whereas the middle position (sn2) is usually occupied by an unsaturated one, such as oleic (C18:1, ω–9) or linoleic (C18:2, ω–6).[9])
| Stearic acid (saturated, C18:0) | |
| Palmitoleic acid (mono-unsaturated, C16:1 cis-9, omega-7) | |
| Oleic acid (mono-unsaturated, C18:1 cis-9, omega-9) | |
 |
α-Linolenic acid (polyunsaturated, C18:3 cis-9,12,15, omega-3) |
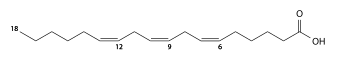 |
γ-Linolenic acid (polyunsaturated, C18:3 cis-6,9,12, omega-6) |
While it is the nutritional aspects of polyunsaturated fatty acids that are generally of greatest interest, these materials also have non-food applications. They include the drying oils, such as linseed (flax seed), tung, poppy seed, perilla, and walnut oil, which polymerize on exposure to oxygen to form solid films, and are used to make paints and varnishes.
Saturated fats generally have a higher melting point than unsaturated ones with the same molecular weight, and thus are more likely to be solid at room temperature. For example, the animal fats tallow and lard are high in saturated fatty acid content and are solids. Olive and linseed oils on the other hand are unsaturated and liquid. Unsaturated fats are prone to oxidation by air, which causes them to become rancid and inedible.
The double bonds in unsaturated fats can be converted into single bonds by reaction with hydrogen effected by a catalyst. This process, called hydrogenation, is used to turn vegetable oils into solid or semisolid vegetable fats like margarine, which can substitute for tallow and butter and (unlike unsaturated fats) can be stored indefinitely without becoming rancid. However, partial hydrogenation also creates some unwanted trans acids from cis acids.[citation needed]
In cellular metabolism, unsaturated fat molecules yield slightly less less energy (i.e., fewer calories) than an equivalent amount of saturated fat. The heats of combustion of saturated, mono-, di-, and tri-unsaturated 18-carbon fatty acid esters have been measured as 2859, 2828, 2794, and 2750 kcal/mol, respectively; or, on a weight basis, 10.75, 10.71, 10.66, and 10.58 kcal/g — a decrease of about 0.6% for each additional double bond.[10]
The greater the degree of unsaturation in a fatty acid (i.e., the more double bonds in the fatty acid) the more vulnerable it is to lipid peroxidation (rancidity). Antioxidants can protect unsaturated fat from lipid peroxidation.
Cis and trans fats
Another important classification of unsaturated fatty acids considers the cis-trans isomerism, the spatial arrangement of the C–C single bonds adjacent to the double bonds. Most unsaturated fatty acids that occur in nature have those bonds in the cis ("same side") configuration. Partial hydrogenation of cis fats can turn some of their fatty acids into trans ("opposite sides") variety.
Elaidic acid is the trans isomer of oleic acid, one of the most common fatty acids in human diet. The single change of configuration in one double bond causes them to have different chemical and physical properties. Elaidic acid has a much higher melting point than oleic acid, 45 °C instead of 13.4 °C. This difference is commonly attributed to the supposed ability of the trans molecules to pack more tightly, forming a solid that is more difficult to break apart.[11]
Omega number
Another classification considers the position of the double bonds relative to the end of the chain (opposite to the carboxyl group). The position is denoted by "ω−k" or "n−k", meaning that there is a double bond between carbons k and k+1 counted from 1 at that end. For example, alpha-Linolenic acid is a "ω−3" or "n−3" acid, meaning that there is a double bond between the third and fourth carbons, counted from that end; that is, its structural formula ends with –CH=CH–CH
2–CH
3.[12]
Examples of saturated fatty acids
Some common examples of fatty acids:
- Butyric acid with 4 carbon atoms (contained in butter)
- Lauric acid with 12 carbon atoms (contained in coconut oil, palm kernel oil, and breast milk)
- Myristic acid with 14 carbon atoms (contained in cow's milk and dairy products)
- Palmitic acid with 16 carbon atoms (contained in palm oil and meat)
- Stearic acid with 18 carbon atoms (also contained in meat and cocoa butter)
Examples of unsaturated fatty acids
- Myristoleic acid C14:1, ω−5, cis-9-tetradecenoic acid
- Sapienic acid C16:1 ω−10, cis-6-Hexadecenoic acid
- Palmitoleic acid C16:1, ω−7 , cis-9-hexadecenoic acid
- Oleic acid C18:1 ω−9, cis-9-octadecenoic acid
- Petroselinic acid C18:1 ω−12, cis-Octadec-6-enoic acid
- cis-Vaccenic acid, C18:1 ω−7), cis-11-octadecenoic acid
- Vaccenic acid C18:1 ω−7, trans-11-octadecenoic acid
- Elaidic acid 18:1 ω−9, trans-9-octadecenoic acid (trans-oleic acid)
- Linoleic acid
- Linolenic acid
- Paullinic acid C20:1 ω−7, cis-13-eicosenoic acid
- Gadoleic acid C20:1 ω−11, cis-9-icosenoic acid
- Gondoic acid 20:1 ω−9, cis-11-eicosenoic acid
- Erucic acid C22:1 ω−9, cis-15-tetracosenoic acid
- Brassidic acid C22:1 ω−9, trans-15-tetracosenoic acid
- Nervonic acid C24:1 ω−9, | cis-15-tetracosenoic acid
- Arachidonic acid
Biological importance
In humans and many animals, fats serve both as energy sources and as stores for energy in excess of what the body needs immediately. Each gram of fat when burned or metabolized releases about 9 food calories (37 kJ = 8.8 kcal).[13]
Fats are also sources of essential fatty acids, an important dietary requirement. Vitamins A, D, E, and K are fat-soluble, meaning they can only be digested, absorbed, and transported in conjunction with fats.
Fats play a vital role in maintaining healthy skin and hair, insulating body organs against shock, maintaining body temperature, and promoting healthy cell function. Fat also serves as a useful buffer against a host of diseases. When a particular substance, whether chemical or biotic, reaches unsafe levels in the bloodstream, the body can effectively dilute—or at least maintain equilibrium of—the offending substances by storing it in new fat tissue.[citation needed] This helps to protect vital organs, until such time as the offending substances can be metabolized or removed from the body by such means as excretion, urination, accidental or intentional bloodletting, sebum excretion, and hair growth.
Adipose tissue
In animals, adipose tissue, or fatty tissue is the body's means of storing metabolic energy over extended periods of time. Adipocytes (fat cells) store fat derived from the diet and from liver metabolism. Under energy stress these cells may degrade their stored fat to supply fatty acids and also glycerol to the circulation. These metabolic activities are regulated by several hormones (e.g., insulin, glucagon and epinephrine). Adipose tissue also secretes the hormone leptin.[14]
The location of the tissue determines its metabolic profile: visceral fat is located within the abdominal wall (i.e., beneath the wall of abdominal muscle) whereas subcutaneous fat is located beneath the skin (and includes fat that is located in the abdominal area beneath the skin but above the abdominal muscle wall). Visceral fat was recently discovered to be a significant producer of signaling chemicals (i.e., hormones), among which several are involved in inflammatory tissue responses. One of these is resistin which has been linked to obesity, insulin resistance, and Type 2 diabetes. This latter result is currently controversial, and there have been reputable studies supporting all sides on the issue.[citation needed]
Production and processing
A variety of chemical and physical techniques are used for the production and processing of fats, both industrially and in cottage or home settings. They include:
- Pressing to extract liquid fats from fruits, seeds, or algae, e.g. olive oil from olives;
- Solvent extraction using solvents like hexane or supercritical carbon dioxide.
- Rendering, the melting of fat in adipose tissue, e.g. to produce tallow, lard, fish oil, and whale oil.
- Churning of milk to produce butter.
- Hydrogenation to reduce the degree of unsaturation of the fatty acids.
- Interesterification, the rearrangement of fatty acids across different triglycerides.
- Winterization to remove oil components with higher melting points.
- Clarification of butter.
Nutritional and health aspects
| Types of fats in food |
|---|
| Components |
| Manufactured fats |
The benefits and risks of various amounts and types of dietary fats have been the object of much study, and are still highly controversial topics.[15][16][17][18]
Essential fatty acids
There are two essential fatty acids (EFAs) in human nutrition: alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid).[19][13] Other lipids needed by the body can be synthesized from these and other fats.
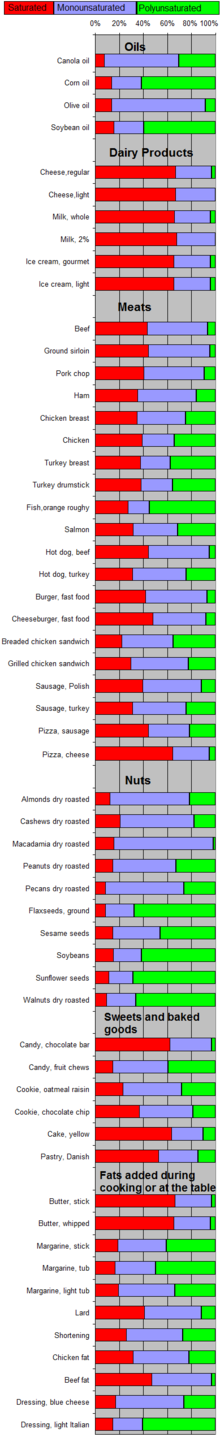
Saturated vs. unsaturated fats
Different foods contain different amounts of fat with different proportions of saturated and unsaturated fatty acids. Most animal fats, such as lard, schmaltz and sausages, fatty meats and dairy products made with whole or reduced fat milk like yogurt, ice cream, cheese and butter have mostly saturated fatty acids (and some have significant contents of dietary cholesterol). Industrialized baked goods may use fats with high unsaturated fat contents as well, especially those containing partially partially hydrogenated oils, and processed foods that are deep-fried in hydrogenated oil are high in saturated fat content..[20][21][22]
Plants and fish oil generally contain a higher proportion of unsaturated acids, although there are exceptions such as coconut oil and palm kernel oil.[23][24] Foods containing unsaturated fats include avocado, nuts, olive oils, and vegetable oils such as canola.
| Food | Lauric acid | Myristic acid | Palmitic acid | Stearic acid |
|---|---|---|---|---|
| Coconut oil | 47% | 18% | 9% | 3% |
| Palm kernel oil | 48% | 1% | 44% | 5% |
| Butter | 3% | 11% | 29% | 13% |
| Ground beef | 0% | 4% | 26% | 15% |
| Salmon | 0% | 1% | 29% | 3% |
| Egg yolks | 0% | 0.3% | 27% | 10% |
| Cashews | 2% | 1% | 10% | 7% |
| Soybean oil | 0% | 0% | 11% | 4% |
Many careful studies have found that replacing saturated fats with cis unsaturated fats in the diet reduces risk of risks of cardiovascular diseases,[26][27] diabetes, or death.[28] These studies prompted many medical organizations and public health departments, including the World Health Organization,[29][30] to officially issue that advice. Some countries with such recommendations include:
- United Kingdom [31][32][33][34][35]
- United States [28][36][37][38][39]
- India [40][41]
- Canada [42]
- Australia [43]
- Singapore [44]
- New Zealand [45]
- Hong Kong [46]
A 2004 review concluded that "no lower safe limit of specific saturated fatty acid intakes has been identified" and recommended that the influence of varying saturated fatty acid intakes against a background of different individual lifestyles and genetic backgrounds should be the focus in future studies.[47]
This advice is often oversimplified by labeling the two kinds of fats as bad fats and good fats, respectively. However, since the fats and oils in most natural and traditionally processed foods contain both unsaturated and saturated fatty acids,[48] the complete exclusion of saturated fat is unrealistic and possibly unwise. For instance, some foods rich in saturated fat, such as coconut and palm oil, are an important source of cheap dietary calories for a large fraction of the population in developing countries.[49]
Concerns were also expressed at a 2010 conference of the American Dietetic Association that a blanket recommendation to avoid saturated fats could drive people to also reduce the amount of polyunsaturated fats, which may have health benefits, and/or replace fats by refined carbohydrates — which carry a high risk of obesity and heart disease.[50]
For these reasons, the United States Food and Drug Administration (FDA), for example, does not advise the complete elimination of saturated fat, but only recommends that it does not exceed 30% of one's daily caloric intake.[citation needed] A 2003 report by the World Health Organization and the Food and Agriculture Organization (FAO) recommends limiting the saturated fatty acids to less than 10% of daily energy intake and less than 7% for high-risk groups.[49] A general 7% limit was recommended also by the American Heart Association in 2006.[51][52]
The WHO/FAO report also recommended replacing fats so as to reduce the content of myristic and palmitic acids, specifically.[49]
The so-called Mediterranean diet, prevalent in many countries in the Mediterranean Sea area, includes more total fat than the diet of Northern European countries, but most of it is in the form of unsaturated fatty acids (specifically, monounsaturated and omega-3) from olive oil and fish, vegetables, and certain meats like lamb, while consumption of saturated fat is minimal in comparison. A 2017 review found evidence that a Mediterranean-style diet could reduce the risk of cardiovascular diseases, overall cancer incidence, neurodegenerative diseases, diabetes, and mortality rate.[53] A 2018 review showed that a Mediterranean-like diet may improve overall health status, such as reduced risk of non-communicable diseases. It also may reduce the social and economic costs of diet-related illnesses.[54]
A small number of contemporary reviews have challenged this negative view of saturated fats. For example, an evaluation of evidence from 1966-1973 of the observed health impact of replacing dietary saturated fat with linoleic acid found that it increased rates of death from all causes, coronary heart disease, and cardiovascular disease.[55] These studies have been disputed by many scientists,[56] and the consensus in the medical community is that saturated fat and cardiovascular disease are closely related.[57][58][59] Still, these discordant studies fueled debate over the merits of substituting polyunsaturated fats for saturated fats.[60]
Cardiovascular disease
The effect of saturated fat on cardiovascular disease has been extensively studied.[61] The general consensus is that there is evidence of moderate-quality of a strong, consistent, and graded relationship between saturated fat intake, blood cholesterol levels, and the incidence of cardiovascular disease.[28][61] The relationships are accepted as causal,[62][63] including by many government and medical organizations.[49][64][65][28][66][67][68][69]
A 2017 review by the American Heart Association estimated that replacement of saturated fat with polyunsaturated fat in the American diet could reduce the risk of cardiovascular diseases by 30%.[28]
The consumption of saturated fat is generally considered a risk factor for dyslipidemia — abnormal blood lipid levels, including high total cholesterol, high levels of triglycerides, high levels of low-density lipoprotein (LDL, "bad" cholesterol) or low levels of high-density lipoprotein (HDL, "good" cholesterol). These parameters in turn are believed to be risk indicators for some types of cardiovascular disease.[70][71][72][73][74][66].[75][76][77] These effects were observed in children too.[78]
Several meta-analyses (reviews and consolidations of multiple previously published experimental studies) have confirmed a significant relationship between saturated fat and high serum cholesterol levels,[28][79] which in turn have been claimed to have a causal relation with increased risk of cardiovascular disease (the so-called lipid hypothesis).[80][81] However, high cholesterol may be caused by many factors. Other indicators, such as high LDL/HDL ratio, have proved to be more predictive.[81] In a study of myocardial infarction in 52 countries, the ApoB/ApoA1 (related to LDL and HDL, respectively) ratio was the strongest predictor of CVD among all risk factors.[82] There are other pathways involving obesity, triglyceride levels, insulin sensitivity, endothelial function, and thrombogenicity, among others, that play a role in CVD, although it seems, in the absence of an adverse blood lipid profile, the other known risk factors have only a weak atherogenic effect.[83] Different saturated fatty acids have differing effects on various lipid levels.[84]
Cancer
The evidence for a relation between saturated fat intake and cancer is significantly weaker, and there does not seem to be a clear medical consensus about it.
- A meta-analysis published in 2003 found a epidemiology and etiology of breast cancer#Specific dietary fatty acidssignificant positive relationship between saturated fat and breast cancer.[85] However two subsequent reviews have found weak or insignificant relation,[86][87] and noted the prevalence of confounding factors.[86][88]
- Another review found limited evidence for a positive relationship between consuming animal fat and incidence of colorectal cancer.[89]
- Other meta-analyses found evidence for increased risk of ovarian cancer by high consumption of saturated fat.[90]
- Some studies have indicated that serum myristic acid[91][92] and palmitic acid[92] and dietary myristic[93] and palmitic[93] saturated fatty acids and serum palmitic combined with alpha-tocopherol supplementation[91] are associated with increased risk of prostate cancer in a dose-dependent manner. These associations may, however, reflect differences in intake or metabolism of these fatty acids between the precancer cases and controls, rather than being an actual cause.[92]
Bones
Various animal studies have indicated that the intake of saturated fat has a negative effect on effects on the mineral density of bones. One study suggested that men may be particularly vulnerable.[94]
Disposition and overall health
Studies have shown that substituting monounsaturated fatty acids for saturated ones is associated with increased daily physical activity and resting energy expenditure. More physical activity, less anger, and less irritability were associated with a higher-oleic acid diet than one of a palmitic acid diet.[95]

Monounsaturated vs. polyunsaturated fat

Assuming given that unsaturated fatty acids (UFAs) are generally healthier than saturated ones (SFAs), another question that has gained attention in recent decades is the risks and benefits of monounsaturated fatty acids (MUFAs, with a single double bond) versus polyunsaturated fatty acids (PUFAs, with two or more double bonds).
The most common fatty acids in human diet are unsaturated or mono-unsaturated. Monounsaturated fats are found in animal flesh such as red meat, whole milk products, nuts, and high fat fruits such as olives and avocados. Algal oil is about 92% monounsaturated fat.[96] Olive oil is about 75% monounsaturated fat.[97] The high oleic variety sunflower oil contains at least 70% monounsaturated fat.[98] Canola oil and cashews are both about 58% monounsaturated fat.[citation needed] Tallow (beef fat) is about 50% monounsaturated fat.[99] and lard is about 40% monounsaturated fat.[citation needed] Other sources include hazelnut, avocado oil, macadamia nut oil, grapeseed oil, groundnut oil (peanut oil), sesame oil, corn oil, popcorn, whole grain wheat, cereal, oatmeal, almond oil, sunflower oil, hemp oil, and tea-oil Camellia.[100]
Polyunsaturated fatty acids can be found mostly in nuts, seeds, fish, seed oils, and oysters.[7]
Food sources of polyunsaturated fats include:[7][101]
| Food source (100g) | Polyunsaturated fat (g) |
|---|---|
| Walnuts | 47 |
| Canola Oil | 34 |
| Sunflower seeds | 33 |
| Sesame Seeds | 26 |
| Chia Seeds | 23.7 |
| Unsalted Peanuts | 16 |
| Peanut Butter | 14.2 |
| Avocado Oil | 13.5 [102] |
| Olive Oil | 11 |
| Safflower Oil | 12.82[103] |
| Seaweed | 11 |
| Sardines | 5 |
| Soybeans | 7 |
| Tuna | 14 |
| Wild Salmon | 17.3 |
| Whole Grain Wheat | 9.7 |
Cardiovascular disease
Studies have given conflicting indications about the effect of MUFA/PUFA intake and cardiovascular disease. Although PUFAs seem to protect against cardiac arrhythmias, a study concluded that PUFA intake is positively associated coronary atherosclerosis progression in a group of post-menopauseal women, whereas MUFA intake is not.[104] This probably is an indication of the greater vulnerability of polyunsaturated fats to lipid peroxidation, against which vitamin E has been shown to be protective.[105]
Insulin resistance and sensitivity
MUFAs (especially oleic acid) have been found to lower the incidence of insulin resistance PUFAs (especially large amounts of arachidonic acid) and SFAs (such as arachidic acid) increased it. These ratios can be indexed in the phospholipids of human skeletal muscle and in other tissues as well. This relationship between dietary fats and insulin resistance is presumed secondary to the relationship between insulin resistance and inflammation, which is partially modulated by dietary fat ratios (Omega-3/6/9) with both omega 3 and 9 thought to be anti-inflammatory, and omega 6 pro-inflammatory (as well as by numerous other dietary components, particularly polyphenols and exercise, with both of these anti-inflammatory). Although both pro- and anti-inflammatory types of fat are biologically necessary, fat dietary ratios in most US diets are skewed towards Omega 6, with subsequent disinhibition of inflammation and potentiation of insulin resistance.[48] But this is contrary to the suggestion of more recent studies, in which polyunsaturated fats are shown as protective against insulin resistance.
The large scale KANWU study found that increasing MUFA and decreasing SFA intake could improve insulin sensitivity, but only when the overall fat intake of the diet was low.[106] However, some MUFAs may promote insulin resistance (like the SFAs), whereas PUFAs may protect against it.[107][108][clarification needed]
Cancer
Levels of oleic acid along with other MUFAs in red blood cell membranes were positively associated with breast cancer risk. The saturation index (SI) of the same membranes was inversely associated with breast cancer risk. MUFAs and low SI in erythrocyte membranes are predictors of postmenopausal breast cancer. Both of these variables depend on the activity of the enzyme delta-9 desaturase (Δ9-d).[109]
Results from observational clinical trials on PUFA intake and cancer have been inconsistent and vary by numerous factors of cancer incidence, including gender and genetic risk.[110] Some studies have shown associations between higher intakes and/or blood levels of omega-3 PUFAs and a decreased risk of certain cancers, including breast and colorectal cancer, while other studies found no associations with cancer risk.[110][111]
Pregnancy disorders
Polyunsaturated fat supplementation was found to have no effect on the incidence of pregnancy-related disorders, such as hypertension or preeclampsia, but may increase the length of gestation slightly and decreased the incidence of early premature births.[7]
Expert panels in the United States and Europe recommend that pregnant and lactating women consume higher amounts of polyunsaturated fats than the general population to enhance the DHA status of the fetus and newborn.[7]
"Cis fat" vs. "trans fat"
In nature, unsaturated fatty acids generally have double bonds in cis configuration (with the adjacent C–C bonds on the same side) as opposed to trans.[112] Nevertheless, trans fatty acids (TFAs) occur in small amounts in meat and milk of ruminants (such as cattle and sheep),[113] typically 2–5% of total fat.[114] Natural TFAs, which include conjugated linoleic acid (CLA) and vaccenic acid, originate in the rumen of these animals. CLA has two double bonds, one in the cis configuration and one in trans, which makes it simultaneously a cis- and a trans-fatty acid.[115]
| Food type | Trans fat content |
|---|---|
| butter | 2g to 7 g |
| whole milk | 0.07g to 0.1 g |
| animal fat | 0g to 5 g[114] |
| ground beef | 1 g |



Concerns about trans fatty acids in human diet were raised when they were found to be an unintentional byproduct of the partial hydrogenation of vegetable and fish oils. While these trans fatty acids (popularly called "trans fats") are edible, they have been implicated in many health problems.[117]
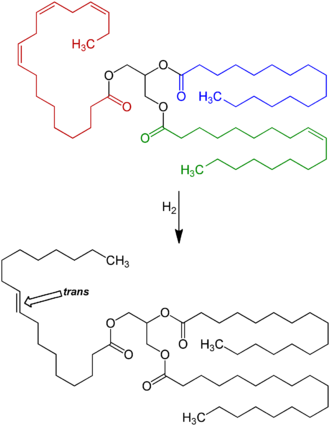
The hydrogenation process, invented and patented by Wilhelm Normann in 1902, made it possible to turn relatively cheap liquid fats such as whale or fish oil into more solid fats and to extend their shelf-life by preventing rancidification. (The source fat and the process were initially kept secret to avoid consumer distaste.[118]) This process was widely adopted by the food industry already in the early 1900s; first for the production of margarine, a replacement for butter and shortening,[119] and eventually for various other fats used in snack food, packaged baked goods, and deep fried products.[120][121]
Full hydrogenation of a fat or oil produces a fully saturated fat. However, hydrogenation generally was interrupted before completion, to yield a fat product with specific melting point, hardness, and other properties. Unfortunately, partial hydrogenation turns some of the cis double bonds into trans bonds by an isomerization reaction.[120][121] [122] The trans configuration is favored [citation needed] because it is the lower energy form.
This side reaction accounts for most of the trans fatty acids consumed today, by far.[123][124] An analysis of some industrialized foods in 2006 found up to 30% "trans fats" in artificial shortening, 10% in breads and cake products, 8% in cookies and crackers, 4% in salty snacks, 7% in cake frostings and sweets, and 26% in margarine and other processed spreads. [116] Another 2010 analysis however found only 0.2% of trans fats in margarine and other processed spreads. [125] Up to 45% of the total fat in those foods containing man-made trans fats formed by partially hydrogenating plant fats may be trans fat.[114] Baking shortenings, unless reformulated, contain around 30% trans fats compared to their total fats. High-fat dairy products such as butter contain about 4%. Margarines not reformulated to reduce trans fats may contain up to 15% trans fat by weight,[126] but some reformulated ones are less than 1% trans fat.
High levels of TFAs have been recorded in popular "fast food" meals.[124] An analysis of samples of McDonald's French fries collected in 2004 and 2005 found that fries served in New York City contained twice as much trans fat as in Hungary, and 28 times as much as in Denmark, where trans fats are restricted. For Kentucky Fried Chicken products, the pattern was reversed: the Hungarian product containing twice the trans fat of the New York product. Even within the United States there was variation, with fries in New York containing 30% more trans fat than those from Atlanta.[127]
Cardiovascular disease
Numerous studies have found that consumption of TFAs increases risk of cardiovascular disease.[19][13] The Harvard School of Public Health advises that replacing TFAs and saturated fats with cis monounsaturated and polyunsaturated fats is beneficial for health.[128]
Consuming trans fats has been shown to increase the risk of coronary artery disease in part by raising levels of low-density lipoprotein (LDL, often termed "bad cholesterol"), lowering levels of high-density lipoprotein (HDL, often termed "good cholesterol"), increasing triglycerides in the bloodstream and promoting systemic inflammation.[129][130][131]
The primary health risk identified for trans fat consumption is an elevated risk of coronary artery disease (CAD).[132] A 1994 study estimated that over 30,000 cardiac deaths per year in the United States are attributable to the consumption of trans fats.[133] By 2006 upper estimates of 100,000 deaths were suggested.[134] A comprehensive review of studies of trans fats published in 2006 in the New England Journal of Medicine reports a strong and reliable connection between trans fat consumption and CAD, concluding that "On a per-calorie basis, trans fats appear to increase the risk of CAD more than any other macronutrient, conferring a substantially increased risk at low levels of consumption (1 to 3% of total energy intake)".[135][135]
The major evidence for the effect of trans fat on CAD comes from the Nurses' Health Study – a cohort study that has been following 120,000 female nurses since its inception in 1976. In this study, Hu and colleagues analyzed data from 900 coronary events from the study's population during 14 years of followup. He determined that a nurse's CAD risk roughly doubled (relative risk of 1.93, CI: 1.43 to 2.61) for each 2% increase in trans fat calories consumed (instead of carbohydrate calories). By contrast, for each 5% increase in saturated fat calories (instead of carbohydrate calories) there was a 17% increase in risk (relative risk of 1.17, CI: 0.97 to 1.41). "The replacement of saturated fat or trans unsaturated fat by cis (unhydrogenated) unsaturated fats was associated with larger reductions in risk than an isocaloric replacement by carbohydrates."[136] Hu also reports on the benefits of reducing trans fat consumption. Replacing 2% of food energy from trans fat with non-trans unsaturated fats more than halves the risk of CAD (53%). By comparison, replacing a larger 5% of food energy from saturated fat with non-trans unsaturated fats reduces the risk of CAD by 43%.[136]
Another study considered deaths due to CAD, with consumption of trans fats being linked to an increase in mortality, and consumption of polyunsaturated fats being linked to a decrease in mortality.[132][137]
Trans fat has been found to act like saturated in raising the blood level of LDL ("bad cholesterol"); but, unlike saturated fat, it also decreases levels of HDL ("good cholesterol"). The net increase in LDL/HDL ratio with trans fat, a widely accepted indicator of risk for coronary artery, is approximately double that due to saturated fat.[138][139][140] One randomized crossover study published in 2003 comparing the effect of eating a meal on blood lipids of (relatively) cis and trans fat rich meals showed that cholesteryl ester transfer (CET) was 28% higher after the trans meal than after the cis meal and that lipoprotein concentrations were enriched in apolipoprotein(a) after the trans meals.[141]
The citokyne test is a potentially more reliable indicator of CAD risk, although is still being studied.[132] A study of over 700 nurses showed that those in the highest quartile of trans fat consumption had blood levels of C-reactive protein (CRP) that were 73% higher than those in the lowest quartile.[142]
Breast feeding
It has been established that trans fats in human breast milk fluctuate with maternal consumption of trans fat, and that the amount of trans fats in the bloodstream of breastfed infants fluctuates with the amounts found in their milk. In 1999, reported percentages of trans fats (compared to total fats) in human milk ranged from 1% in Spain, 2% in France, 4% in Germany, and 7% in Canada and the United States.[143]
Other health risks
There are suggestions that the negative consequences of trans fat consumption go beyond the cardiovascular risk. In general, there is much less scientific consensus asserting that eating trans fat specifically increases the risk of other chronic health problems:
- Alzheimer's Disease: A study published in Archives of Neurology in February 2003 suggested that the intake of both trans fats and saturated fats promote the development of Alzheimer disease,[144] although not confirmed in an animal model.[145] It has been found that trans fats impaired memory and learning in middle-age rats. The trans-fat eating rats' brains had fewer proteins critical to healthy neurological function. Inflammation in and around the hippocampus, the part of the brain responsible for learning and memory. These are the exact types of changes normally seen at the onset of Alzheimer's, but seen after six weeks, even though the rats were still young.[146]
- Cancer: There is no scientific consensus that consuming trans fats significantly increases cancer risks across the board.[132] The American Cancer Society states that a relationship between trans fats and cancer "has not been determined."[147] One study has found a positive connection between trans fat and prostate cancer.[148] However, a larger study found a correlation between trans fats and a significant decrease in high-grade prostate cancer.[149] An increased intake of trans fatty acids may raise the risk of breast cancer by 75%, suggest the results from the French part of the European Prospective Investigation into Cancer and Nutrition.[150][151]
- Diabetes: There is a growing concern that the risk of type 2 diabetes increases with trans fat consumption.[132][152] However, consensus has not been reached.[135] For example, one study found that risk is higher for those in the highest quartile of trans fat consumption.[153] Another study has found no diabetes risk once other factors such as total fat intake and BMI were accounted for.[154]
- Obesity: Research indicates that trans fat may increase weight gain and abdominal fat, despite a similar caloric intake.[155] A 6-year experiment revealed that monkeys fed a trans fat diet gained 7.2% of their body weight, as compared to 1.8% for monkeys on a mono-unsaturated fat diet.[156][157] Although obesity is frequently linked to trans fat in the popular media,[158] this is generally in the context of eating too many calories; there is not a strong scientific consensus connecting trans fat and obesity, although the 6-year experiment did find such a link, concluding that "under controlled feeding conditions, long-term TFA consumption was an independent factor in weight gain. TFAs enhanced intra-abdominal deposition of fat, even in the absence of caloric excess, and were associated with insulin resistance, with evidence that there is impaired post-insulin receptor binding signal transduction."[157]
- Infertility in women: One 2007 study found, "Each 2% increase in the intake of energy from trans unsaturated fats, as opposed to that from carbohydrates, was associated with a 73% greater risk of ovulatory infertility...".[159]
- Major depressive disorder: Spanish researchers analysed the diets of 12,059 people over six years and found that those who ate the most trans fats had a 48 per cent higher risk of depression than those who did not eat trans fats.[160] One mechanism may be trans-fats' substitution for docosahexaenoic acid (DHA) levels in the orbitofrontal cortex (OFC). Very high intake of trans-fatty acids (43% of total fat) in mice from 2 to 16 months of age was associated with lowered DHA levels in the brain (p=0.001).[145] When the brains of 15 major depressive subjects who had committed suicide were examined post-mortem and compared against 27 age-matched controls, the suicidal brains were found to have 16% less (male average) to 32% less (female average) DHA in the OFC. The OFC controls reward, reward expectation, and empathy (all of which are reduced in depressive mood disorders) and regulates the limbic system.[161]
- Behavioral irritability and aggression: a 2012 observational analysis of subjects of an earlier study found a strong relation between dietary trans fat acids and self-reported behavioral aggression and irritability, suggesting but not establishing causality.[162]
- Diminished memory: In a 2015 article, researchers re-analyzing results from the 1999-2005 UCSD Statin Study argue that "greater dietary trans fatty acid consumption is linked to worse word memory in adults during years of high productivity, adults age <45".[163]
- Acne: According to a 2015 study, trans fats are one of several components of Western pattern diets which promote acne, along with carbohydrates with high glycemic load such as refined sugars or refined starches, milk and dairy products, and saturated fats, while omega-3 fatty acids, which reduce acne, are deficient in Western pattern diets.[164]
Biochemical mechanisms
The exact biochemical process by which trans fats produce specific health problems are a topic of continuing research. Intake of dietary trans fat perturbs the body's ability to metabolize essential fatty acids (EFAs, including Omega-3) leading to changes in the phospholipid fatty acid composition of the arterial walls, thereby raising risk of coronary artery disease.[165]
Trans double bonds are claimed to induce a linear conformation to the molecule, favoring its rigid packing as in plaque formation. The geometry of the cis double bond, in contrast, is claimed to create a bend in the molecule, thereby precluding rigid formations.[citation needed].
While the mechanisms through which trans fatty acids contribute to coronary artery disease are fairly well understood, the mechanism for their effects on diabetes is still under investigation. They may impair the metabolism of long-chain polyunsaturated fatty acids (LCPUFAs).[166] However, maternal pregnancy trans fatty acid intake has been inversely associated with LCPUFAs levels in infants at birth thought to underlie the positive association between breastfeeding and intelligence.[167]
Trans fats are processed by the liver differently than other fats. They may cause liver dysfunction by interfering with delta 6 desaturase, an enzyme involved in converting essential fatty acids to arachidonic acid and prostaglandins, both of which are important to the functioning of cells.[168]
Natural "trans fats" in dairy products
Some trans fatty acids occur in natural fats and traditionally processed foods. Vaccenic acid occurs in breast milk, and some isomers of conjugated linoleic acid (CLA) are found in meat and dairy products from ruminants. Butter, for example, contains about 3% trans fat.[169]
The US National Dairy Council has asserted that the trans fats present in animal foods are of a different type than those in partially hydrogenated oils, and do not appear to exhibit the same negative effects.[170] While a recent scientific review agrees with the conclusion (stating that "the sum of the current evidence suggests that the Public health implications of consuming trans fats from ruminant products are relatively limited"), it cautions that this may be due to the low consumption of trans fats from animal sources compared to artificial ones.[135]
More recent inquiry (independent of the dairy industry) has found in a 2008 Dutch meta-analysis that all trans fats, regardless of natural or artificial origin equally raise LDL and lower HDL levels.[171] Other studies though have shown different results when it comes to animal based trans fats like conjugated linoleic acid (CLA). Although CLA is known for its anticancer properties, researchers have also found that the cis-9, trans-11 form of CLA can reduce the risk for cardiovascular disease and help fight inflammation.[172][173]
Two Canadian studies have shown that vaccenic acid, a TFA that naturally occurs in dairy products, could be beneficial compared to hydrogenated vegetable shortening, or a mixture of pork lard and soy fat, by lowering total LDL and triglyceride levels.[174][175][176][175] A study by the US Department of Agriculture showed that vaccenic acid raises both HDL and LDL cholesterol, whereas industrial trans fats only raise LDL with no beneficial effect on HDL.[177]
Official recommendations
In light of recognized evidence and scientific agreement, nutritional authorities consider all trans fats equally harmful for health and recommend that their consumption be reduced to trace amounts.[178][179][180][181][182] The World Health Organization recommended that trans fats make up no more than 0.9% of a person's diet in 2003[114] and, in 2018, introduced a 6-step guide to eliminate industrially-produced trans-fatty acids from the global food supply.[183]
The National Academy of Sciences (NAS) advises the United States and Canadian governments on nutritional science for use in public policy and product labeling programs. Their 2002 Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids[184] contains their findings and recommendations regarding consumption of trans fat (summary).
Their recommendations are based on two key facts. First, "trans fatty acids are not essential and provide no known benefit to human health",[129] whether of animal or plant origin.[185] Second, given their documented effects on the LDL/HDL ratio,[130] the NAS concluded "that dietary trans fatty acids are more deleterious with respect to coronary artery disease than saturated fatty acids". A 2006 review published in the New England Journal of Medicine (NEJM) that states "from a nutritional standpoint, the consumption of trans fatty acids results in considerable potential harm but no apparent benefit."[135]
Because of these facts and concerns, the NAS has concluded there is no safe level of trans fat consumption. There is no adequate level, recommended daily amount or tolerable upper limit for trans fats. This is because any incremental increase in trans fat intake increases the risk of coronary artery disease.[130]
Despite this concern, the NAS dietary recommendations have not included eliminating trans fat from the diet. This is because trans fat is naturally present in many animal foods in trace quantities, and thus its removal from ordinary diets might introduce undesirable side effects and nutritional imbalances. The NAS has, thus, "recommended that trans fatty acid consumption be as low as possible while consuming a nutritionally adequate diet".[186] Like the NAS, the World Health Organization has tried to balance public health goals with a practical level of trans fat consumption, recommending in 2003 that trans fats be limited to less than 1% of overall energy intake.[114]
Regulatory action
In the last few decades, there has been substantial amount of regulation in many countries, limiting trans fat contents of industrialized and commercial food products.
Alternatives to hydrogenation
In recent years, the negative public image and strict regulations have driven many fat processing industries to replace partial hydrogenation by fat interesterification, a process that chemically scrambles the fatty acids among a mix of triglycerides. When applied to a suitable bend of oils and saturated fats, possibly followed by separation of unwanted solid or liquid triglycerides, this process can achieve results similar to those of partial hydrogenation without affecting the fatty acids themselves; in particular, without creating any new "trans fat".
Researchers at the United States Department of Agriculture have investigated whether hydrogenation can be achieved without the side effect of trans fat production. They varied the pressure under which the chemical reaction was conducted – applying 1400 kPa (200 psi) of pressure to soybean oil in a 2-liter vessel while heating it to between 140 °C and 170 °C. The standard 140 kPa (20 psi) process of hydrogenation produces a product of about 40% trans fatty acid by weight, compared to about 17% using the high-pressure method. Blended with unhydrogenated liquid soybean oil, the high-pressure-processed oil produced margarine containing 5 to 6% trans fat. Based on current U.S. labeling requirements (see below), the manufacturer could claim the product was free of trans fat.[187] The level of trans fat may also be altered by modification of the temperature and the length of time during hydrogenation.
A University of Guelph research group has found a way to mix oils (such as olive, soybean, and canola), water, monoglycerides, and fatty acids to form a "cooking fat" that acts the same way as trans and saturated fats.[188][189]
Omega-three and omega-six fatty acids
The ω−3 fatty acids have received substantial atterntion in recent years.
In preliminary research, omega-3 fatty acids in algal oil, fish oil, fish and seafood have been shown to lower the risk of heart attacks.[110] Other preliminary research indicates that omega-6 fatty acids in sunflower oil and safflower oil may also reduce the risk of cardiovascular disease.[190]
Among omega-3 fatty acids, neither long-chain nor short-chain forms were consistently associated with breast cancer risk. High levels of docosahexaenoic acid (DHA), however, the most abundant omega-3 polyunsaturated fatty acid in erythrocyte (red blood cell) membranes, were associated with a reduced risk of breast cancer.[109] The DHA obtained through the consumption of polyunsaturated fatty acids is positively associated with cognitive and behavioral performance.[191] In addition DHA is vital for the grey matter structure of the human brain, as well as retinal stimulation and neurotransmission.[7]
Interesterification
Some studies have investigated the health effects of insteresterified (IE) fats, by comparing diets with IE and non-IE fats with the same overall fatty acid composition.[192]
Several experimental studies in humans found no statistical difference on fasting blood lipids between a with large amounts of IE fat, having 25-40% C16:0 or C18:0 on the 2-position, and a similar diet with non-IE fat, having only 3-9% C16:0 or C18:0 on the 2-position.[193][194][195] A negative result was obtained also in a study that compared the effects on blood cholesterol levels of an IE fat product mimicking cocoa butter and the real non-IE product.[196][197][198][199][200][201][202]
A 2007 study funded by the Malaysian Palm Oil Board[203] claimed that replacing natural palm oil by other interesterified or partial hydrogenated fats caused adverse health effects, such as higher LDL/ HDL ratio and plasma glucose levels. However, these effects could be attributed to the higher percentage of saturated acids in the IE and partially hydrogenated fats, rather than to the IE process itself.[204][205]
Fat digestion and metabolism
Fats are broken down in the healthy body to release their constituents, glycerol and fatty acids. Glycerol itself can be converted to glucose by the liver and so become a source of energy. Fats and other lipids are broken down in the body by enzymes called lipases produced in the pancreas.
Many cell types can use either glucose or fatty acids as a source of energy for metabolism. In particular, heart and skeletal muscle prefer fatty acids.[citation needed] Despite long-standing assertions to the contrary, fatty acids can also be used as a source of fuel for brain cells through mitochondrial oxidation. [206]
See also
|
|
Further reading
Further information is available. [207] [208] [209] [210] [211] [212] [213] [214] [215] [216] [217] [218] [219]
References
- ^ a b c d Entry for "fat" in the online Merriam-Webster disctionary, sense 3.2. Accessed on 2020-08-09
- ^ a b c Thomas A. B. Sanders (2016): "The Role of Fats in Human Diet". Pages 1-20 of Functional Dietary Lipids. Woodhead/Elsevier, 332 pages. ISBN 978-1-78242-247-1doi:10.1016/B978-1-78242-247-1.00001-6
- ^ "Macronutrients: the Importance of Carbohydrate, Protein, and Fat". McKinley Health Center. University of Illinois at Urbana–Champaign. Retrieved 20 September 2014.
- ^ "Introduction to Energy Storage". Khan Academy.
- ^ Anna Ohtera, Yusaku Miyamae, Naomi Nakai, Atsushi Kawachi, Kiyokazu Kawada, Junkyu Han, Hiroko Isoda, Mohamed Neffati, Toru Akita, Kazuhiro Maejima, Seiji Masuda, Taiho Kambe, Naoki Mori, Kazuhiro Irie, and Masaya Nagao (2013): "Identification of 6-octadecynoic acid from a methanol extract of Marrubium vulgare L. as a peroxisome proliferator-activated receptor γ agonist". Biochemical and Biophysical Research Communications, volume 440, issue 2, pages 204-209. doi:10.1016/j.bbrc.2013.09.003
- ^ N. Koeniger and H. J. Veith (1983): "Glyceryl-1,2-dioleate-3-palmitate, a brood pheromone of the honey bee (Apis mellifera L.)". Experientia, volume 39, pages 1051–1052 doi:10.1007/BF01989801
- ^ a b c d e f g "Essential Fatty Acids". Micronutrient Information Center, Oregon State University, Corvallis, OR. May 2014. Retrieved 24 May 2017.
- ^ a b "Omega-3 fatty acids, fish oil, alpha-linolenic acid". Mayo Clinic. 2017. Retrieved 24 May 2017.
- ^ Institute of Shortenings and Edible oils (2006). "Food Fats and oils" (PDF). Archived from the original (PDF) on 2007-03-26. Retrieved 2009-02-19.
- ^ Krisnangkura, Kanit (1991). "Estimation of heat of combustion of triglycerides and fatty acid methyl esters". Journal of the American Oil Chemists' Society. 68: 56–58. doi:10.1007/BF02660311. S2CID 84433984.
- ^ "Section 7: Biochemistry". Handbook of chemistry and physics. 2007–2008 (88th ed.). Taylor and Francis. 2007. Retrieved 19 November 2007.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Karen Dooley (2008): "Omega-three fatty acids and diabetes". Online article at the University of Florida's UFHealt website. Accessed on 2020-08-30.
- ^ a b c Government of the United Kingdom (1996): "Schedule 7: Nutrition labelling". In Food Labelling Regulations 1996'. Accessed on 2020-08-09.
- ^ "The human proteome in adipose - The Human Protein Atlas". www.proteinatlas.org. Retrieved 2017-09-12.
- ^ Rebecca J. Donatelle (2005): Health, the Basics, 6th edition. Pearson Education, San Francisco; ISBN 978-0-13-120687-8
- ^ Frank B. Hu, JoAnn E. Manson, and Walter C. Willett (2001): "Types of dietary fat and risk of coronary heart disease: A critical review". Journal of the American College of Nutrition, volume 20, issue 1, pages 5-19. doi:10.1080/07315724.2001.10719008
- ^ Lee Hooper, Carolyn D. Summerbell, Julian P. T. Higgins, Rachel L. Thompson, Nigel E. Capps, George Davey Smith, Rudolph A. Riemersma, and Shah Ebrahim (2001): "Dietary fat intake and prevention of cardiovascular disease: systematic review". The BMJ, volume 322, pages 757-. doi:10.1136/bmj.322.7289.757
- ^ George A. Bray, Sahasporn Paeratakul, Barry M. Popkin (2004): "Dietary fat and obesity: a review of animal, clinical and epidemiological studies". Physiology & Behavior, volume 83, issue 4, pages 549-555. doi:10.1016/j.physbeh.2004.08.039
- ^ a b Dariush Mozaffarian, Martijn B. Katan, Alberto Ascherio, Meir J. Stampfer, and Walter C. Willett (2006): "Trans fatty acids and cardiovascular disease". New England Journal of Medicine, volume 354, issue 15, pages 1601–1613. doi:10.1056/NEJMra054035 PMID 16611951
- ^ "Saturated fats". American Heart Association. 2014. Retrieved 1 March 2014.
- ^ "Top food sources of saturated fat in the US". Harvard University School of Public Health. 2014. Retrieved 1 March 2014.
- ^ "Saturated, Unsaturated, and Trans Fats". choosemyplate.gov. 2020.
- ^ Reece, Jane; Campbell, Neil (2002). Biology. San Francisco: Benjamin Cummings. pp. 69–70. ISBN 978-0-8053-6624-2.
- ^ "What are "oils"?". ChooseMyPlate.gov, US Department of Agriculture. 2015. Archived from the original on 9 June 2015. Retrieved 13 June 2015.
- ^ "USDA National Nutrient Database for Standard Reference, Release 20". United States Department of Agriculture. 2007. Archived from the original on 2016-04-14.
- ^ Hooper L, Martin N, Abdelhamid A, Davey Smith G (June 2015). "Reduction in saturated fat intake for cardiovascular disease". The Cochrane Database of Systematic Reviews. 6 (6): CD011737. doi:10.1002/14651858.CD011737. PMID 26068959.
- ^ Hooper, L; Martin, N; Jimoh, OF; Kirk, C; Foster, E; Abdelhamid, AS (21 August 2020). "Reduction in saturated fat intake for cardiovascular disease". The Cochrane Database of Systematic Reviews. 8: CD011737. doi:10.1002/14651858.CD011737.pub3. PMID 32827219. Retrieved 6 October 2020.
- ^ a b c d e f Sacks FM, Lichtenstein AH, Wu JH, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ, Van Horn LV (July 2017). "Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association". Circulation. 136 (3): e1–e23. doi:10.1161/CIR.0000000000000510. PMID 28620111. S2CID 367602.
- ^ "Healthy diet Fact sheet N°394". May 2015. Retrieved 12 August 2015.
- ^ World Health Organization: Food pyramid (nutrition)
- ^ "Fats explained" (PDF). HEART UK – The Cholesterol Charity. Retrieved 20 February 2019.
- ^ "Live Well, Eat well, Fat: the facts". NHS. Retrieved 20 February 2019.
- ^ "Fat: the facts". United Kingdom's National Health Service. 2018-04-27. Retrieved 2019-09-24.
- ^ Eat less saturated fat
- ^ Fats explained
- ^ "Key Recommendations: Components of Healthy Eating Patterns". Dietary Guidelines 2015-2020. Retrieved 20 February 2019.
- ^ "Cut Down on Saturated Fats" (PDF). United States Department of Health and Human Services. Retrieved 2019-09-24.
- ^ "Trends in Intake of Energy, Protein, Carbohydrate, Fat, and Saturated Fat — United States, 1971–2000". Centers for Disease Control. 2004. Archived from the original on 2008-12-01.
- ^ "Dietary Guidelines for Americans" (PDF). United States Department of Agriculture. 2005.
- ^ "Dietary Guidelines for Indians - A Manual" (PDF). Indian Council of Medical Research, National Institute of Nutrition. Archived from the original (PDF) on 2018-12-22. Retrieved 2019-02-20.
- ^ "Health Diet". India's Ministry of Health and Family Welfare. Retrieved 2019-09-24.
- ^ "Choosing foods with healthy fats". Health Canada. 2018-10-10. Retrieved 2019-09-24.
- ^ "Fat". Australia's National Health and Medical Research Council and Department of Health and Ageing. 2012-09-24. Retrieved 2019-09-24.
- ^ "Getting the Fats Right!". Singapore's Ministry of Health. Retrieved 2019-09-24.
- ^ "Eating and Activity Guidelines for New Zealand Adults" (PDF). New Zealand's Ministry of Health. Retrieved 2019-09-24.
- ^ "Know More about Fat". Hong Kong's Department of Health. Retrieved 2019-09-24.
- ^ German JB, Dillard CJ (September 2004). "Saturated fats: what dietary intake?". American Journal of Clinical Nutrition. 80 (3): 550–559. doi:10.1093/ajcn/80.3.550. PMID 15321792.
- ^ a b Storlien LH, Baur LA, Kriketos AD, Pan DA, Cooney GJ, Jenkins AB, et al. (June 1996). "Dietary fats and insulin action". Diabetologia. 39 (6): 621–31. doi:10.1007/BF00418533. PMID 8781757. S2CID 33171616.
- ^ a b c d Joint WHO/FAO Expert Consultation (2003). Diet, Nutrition and the Prevention of Chronic Diseases (WHO technical report series 916) (PDF). World Health Organization. pp. 81–94. ISBN 978-92-4-120916-8. Retrieved 2016-04-04.
- ^ Zelman K (2011). "The Great Fat Debate: A Closer Look at the Controversy—Questioning the Validity of Age-Old Dietary Guidance". Journal of the American Dietetic Association. 111 (5): 655–658. doi:10.1016/j.jada.2011.03.026. PMID 21515106.
- ^ Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J (July 2006). "Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee". Circulation. 114 (1): 82–96. doi:10.1161/CIRCULATIONAHA.106.176158. PMID 16785338. S2CID 647269.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Smith SC, Jackson R, Pearson TA, Fuster V, Yusuf S, Faergeman O, Wood DA, Alderman M, Horgan J, Home P, Hunn M, Grundy SM (June 2004). "Principles for national and regional guidelines on cardiovascular disease prevention: a scientific statement from the World Heart and Stroke Forum" (PDF). Circulation. 109 (25): 3112–21. doi:10.1161/01.CIR.0000133427.35111.67. PMID 15226228.
- ^ Dinu M, Pagliai G, Casini A, Sofi F (January 2018). "Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials". European Journal of Clinical Nutrition. 72 (1): 30–43. doi:10.1038/ejcn.2017.58. PMID 28488692. S2CID 7702206.
- ^ Martinez-Lacoba R, Pardo-Garcia I, Amo-Saus E, Escribano-Sotos F (October 2018). "Mediterranean diet and health outcomes: a systematic meta-review". European Journal of Public Health. 28 (5): 955–961. doi:10.1093/eurpub/cky113. PMID 29992229.
- ^ Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR (February 2013). "Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis". BMJ. 346: e8707. doi:10.1136/bmj.e8707. PMC 4688426. PMID 23386268.
- ^ Interview: Walter Willett (2017). "Research Review: Old data on dietary fats in context with current recommendations: Comments on Ramsden et al. in the British Medical Journal". TH Chan School of Public Health, Harvard University, Boston. Retrieved 24 May 2017.
- ^ de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J, Anand SS (August 2015). "Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies". BMJ. 351 (Aug 11): h3978. doi:10.1136/bmj.h3978. PMC 4532752. PMID 26268692.
- ^ Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, et al. (February 2013). "Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis". BMJ. 346: e8707. doi:10.1136/bmj.e8707. PMC 4688426. PMID 23386268.
- ^ Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR (April 2016). "Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73)". BMJ. 353: i1246. doi:10.1136/bmj.i1246. PMC 4836695. PMID 27071971.
- ^ Weylandt KH, Serini S, Chen YQ, Su HM, Lim K, Cittadini A, Calviello G (2015). "Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence". BioMed Research International. 2015: 143109. doi:10.1155/2015/143109. PMC 4537707. PMID 26301240.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS (2020). "Reduction in saturated fat intake for cardiovascular disease". Cochrane Database of Systematic Reviews (Systematic review). 5: CD011737. doi:10.1002/14651858.CD011737.pub2. ISSN 1465-1858. PMC 7388853. PMID 32428300.
- ^ Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. (2007). "European guidelines on cardiovascular disease prevention in clinical practice: executive summary". European Heart Journal. 28 (19): 2375–2414. doi:10.1093/eurheartj/ehm316. PMID 17726041.
- ^ Labarthe D (2011). "Chapter 17 What Causes Cardiovascular Diseases?". Epidemiology and prevention of cardiovascular disease: a global challenge (2nd ed.). Jones and Bartlett Publishers. ISBN 978-0-7637-4689-6.
- ^ Kris-Etherton PM, Innis S (September 2007). "Position of the American Dietetic Association and Dietitians of Canada: Dietary Fatty Acids". Journal of the American Dietetic Association. 107 (9): 1599–1611 [1603]. doi:10.1016/j.jada.2007.07.024. PMID 17936958.
- ^ "Food Fact Sheet - Cholesterol" (PDF). British Dietetic Association. Retrieved 3 May 2012.
- ^ a b "Cardiovascular Disease Risk Factors". World Heart Federation. 30 May 2017. Retrieved 2012-05-03.
- ^ "Lower your cholesterol". National Health Service. Retrieved 2012-05-03.
- ^ "Nutrition Facts at a Glance - Nutrients: Saturated Fat". Food and Drug Administration. 2009-12-22. Retrieved 2012-05-03.
- ^ "Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol". European Food Safety Authority. 2010-03-25. Retrieved 3 May 2012.
- ^ Faculty of Public Health of the Royal Colleges of Physicians of the United Kingdom. Position Statement on Fat [Retrieved 2011-01-25].
- ^ Report of a Joint WHO/FAO Expert Consultation (2003). "Diet, Nutrition and the Prevention of Chronic Diseases" (PDF). World Health Organization. Retrieved 2011-03-11.
- ^ "Cholesterol". Irish Heart Foundation. Retrieved 2011-02-28.
- ^ U.S. Department of Agriculture and U.S. Department of Health and Human Services (December 2010). Dietary Guidelines for Americans, 2010 (PDF) (7th ed.). Washington, DC: U.S. Government Printing Office.
- ^ Cannon C, O'Gara P (2007). Critical Pathways in Cardiovascular Medicine (2nd ed.). Lippincott Williams & Wilkins. p. 243.
- ^ Catapano AL, Reiner Z, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. (July 2011). "ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS)". Atherosclerosis. 217 Suppl 1 (14): S1-44. doi:10.1016/j.atherosclerosis.2011.06.012. hdl:10138/307445. PMID 21723445.
- ^ "Monounsaturated Fat". American Heart Association. Archived from the original on 2018-03-07. Retrieved 2018-04-19.
- ^ "You Can Control Your Cholesterol: A Guide to Low-Cholesterol Living". MerckSource. Archived from the original on 2009-03-03. Retrieved 2018-01-02.
- ^ Sanchez-Bayle M, Gonzalez-Requejo A, Pelaez MJ, Morales MT, Asensio-Anton J, Anton-Pacheco E (February 2008). "A cross-sectional study of dietary habits and lipid profiles. The Rivas-Vaciamadrid study". European Journal of Pediatrics. 167 (2): 149–54. doi:10.1007/s00431-007-0439-6. PMID 17333272. S2CID 8798248.
- ^ Clarke R, Frost C, Collins R, Appleby P, Peto R (1997). "Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies". BMJ (Clinical Research Ed.). 314 (7074): 112–7. doi:10.1136/bmj.314.7074.112. PMC 2125600. PMID 9006469.
- ^ Bucher HC, Griffith LE, Guyatt GH (February 1999). "Systematic review on the risk and benefit of different cholesterol-lowering interventions". Arteriosclerosis, Thrombosis, and Vascular Biology. 19 (2): 187–195. doi:10.1161/01.atv.19.2.187. PMID 9974397.
- ^ a b Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R (December 2007). "Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths". Lancet. 370 (9602): 1829–39. doi:10.1016/S0140-6736(07)61778-4. PMID 18061058. S2CID 54293528.
- ^ Labarthe D (2011). "Chapter 11 Adverse Blood Lipid Profile". Epidemiology and prevention of cardiovascular disease: a global challenge (2 ed.). Jones and Bartlett Publishers. p. 290. ISBN 978-0-7637-4689-6.
- ^ Labarthe D (2011). "Chapter 11 Adverse Blood Lipid Profile". Epidemiology and prevention of cardiovascular disease: a global challenge (2nd ed.). Jones and Bartlett Publishers. p. 277. ISBN 978-0-7637-4689-6.
- ^ Thijssen MA, Mensink RP (2005). "Fatty acids and atherosclerotic risk". Atherosclerosis: Diet and Drugs. Handbook of Experimental Pharmacology. Vol. 170. Springer. pp. 165–94. doi:10.1007/3-540-27661-0_5. ISBN 978-3-540-22569-0. PMID 16596799.
- ^ Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin S (November 2003). "Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature". British Journal of Cancer. 89 (9): 1672–1685. doi:10.1038/sj.bjc.6601314. PMC 2394401. PMID 14583769.
- ^ a b Hanf V, Gonder U (2005-12-01). "Nutrition and primary prevention of breast cancer: foods, nutrients and breast cancer risk". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 123 (2): 139–149. doi:10.1016/j.ejogrb.2005.05.011. PMID 16316809.
- ^ Lof M, Weiderpass E (February 2009). "Impact of diet on breast cancer risk". Current Opinion in Obstetrics and Gynecology. 21 (1): 80–85. doi:10.1097/GCO.0b013e32831d7f22. PMID 19125007. S2CID 9513690.
- ^ Freedman LS, Kipnis V, Schatzkin A, Potischman N (Mar–Apr 2008). "Methods of Epidemiology: Evaluating the Fat–Breast Cancer Hypothesis – Comparing Dietary Instruments and Other Developments". Cancer Journal (Sudbury, Mass.). 14 (2): 69–74. doi:10.1097/PPO.0b013e31816a5e02. PMC 2496993. PMID 18391610.
- ^ Lin OS (2009). "Acquired risk factors for colorectal cancer". Cancer Epidemiology. Methods in Molecular Biology. Vol. 472. pp. 361–72. doi:10.1007/978-1-60327-492-0_16. ISBN 978-1-60327-491-3. PMID 19107442.
- ^ Huncharek M, Kupelnick B (2001). "Dietary fat intake and risk of epithelial ovarian cancer: a meta-analysis of 6,689 subjects from 8 observational studies". Nutrition and Cancer. 40 (2): 87–91. doi:10.1207/S15327914NC402_2. PMID 11962260. S2CID 24890525.
- ^ a b Männistö S, Pietinen P, Virtanen MJ, Salminen I, Albanes D, Giovannucci E, Virtamo J (December 2003). "Fatty acids and risk of prostate cancer in a nested case-control study in male smokers". Cancer Epidemiology, Biomarkers & Prevention. 12 (12): 1422–8. PMID 14693732.
- ^ a b c Crowe FL, Allen NE, Appleby PN, Overvad K, Aardestrup IV, Johnsen NF, Tjønneland A, Linseisen J, Kaaks R, Boeing H, Kröger J, Trichopoulou A, Zavitsanou A, Trichopoulos D, Sacerdote C, Palli D, Tumino R, Agnoli C, Kiemeney LA, Bueno-de-Mesquita HB, Chirlaque MD, Ardanaz E, Larrañaga N, Quirós JR, Sánchez MJ, González CA, Stattin P, Hallmans G, Bingham S, Khaw KT, Rinaldi S, Slimani N, Jenab M, Riboli E, Key TJ (November 2008). "Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition". The American Journal of Clinical Nutrition. 88 (5): 1353–63. doi:10.3945/ajcn.2008.26369 (inactive 2020-10-19). PMID 18996872.
{{cite journal}}: CS1 maint: DOI inactive as of October 2020 (link) - ^ a b Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS (April 2008). "Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men". Cancer Epidemiology, Biomarkers & Prevention. 17 (4): 930–7. doi:10.1158/1055-9965.EPI-07-2681. PMID 18398033. S2CID 551427.
- ^ Corwin RL, Hartman TJ, Maczuga SA, Graubard BI (2006). "Dietary saturated fat intake is inversely associated with bone density in humans: Analysis of NHANES III". The Journal of Nutrition. 136 (1): 159–165. doi:10.1093/jn/136.1.159. PMID 16365076. S2CID 4443420.
- ^ Kien CL, Bunn JY, Tompkins CL, Dumas JA, Crain KI, Ebenstein DB, Koves TR, Muoio DM (April 2013). "Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood". The American Journal of Clinical Nutrition. 97 (4): 689–97. doi:10.3945/ajcn.112.051730. PMC 3607650. PMID 23446891.
- ^ https://www.thrivealgae.com/our-product/. Retrieved 7 January 2019.
{{cite web}}: Missing or empty|title=(help) - ^ Abdullah MM, Jew S, Jones PJ (February 2017). "Health benefits and evaluation of healthcare cost savings if oils rich in monounsaturated fatty acids were substituted for conventional dietary oils in the United States". Nutrition Reviews. 75 (3): 163–174. doi:10.1093/nutrit/nuw062. PMC 5914363. PMID 28158733.
- ^ Huth PJ, Fulgoni VL, Larson BT (November 2015). "A systematic review of high-oleic vegetable oil substitutions for other fats and oils on cardiovascular disease risk factors: implications for novel high-oleic soybean oils". Advances in Nutrition. 6 (6): 674–93. doi:10.3945/an.115.008979. PMC 4642420. PMID 26567193.
- ^ Board on Agriculture and Renewable Resources Commission on Natural Resources and Food and Nutrition Board, Assembly of Life Sciences, National Research Council (1976). Fat content and composition of animal products: proceedings of a symposium, Washington, D.C., December 12-13, 1974. Washington: National Academy of Sciences. ISBN 978-0-309-02440-2. PMID 25032409.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Aizpurua-Olaizola O, Ormazabal M, Vallejo A, Olivares M, Navarro P, Etxebarria N, Usobiaga A (January 2015). "Optimization of supercritical fluid consecutive extractions of fatty acids and polyphenols from Vitis vinifera grape wastes". Journal of Food Science. 80 (1): E101-7. doi:10.1111/1750-3841.12715. PMID 25471637.
- ^ "National nutrient database for standard reference, release 23". United States Department of Agriculture, Agricultural Research Service. 2011. Archived from the original on 2015-03-03. Retrieved 2009-02-22.
- ^ "Vegetable oil, avocado Nutrition Facts & Calories". nutritiondata.self.com.
- ^ "United States Department of Agriculture – National Nutrient Database". 8 September 2015.
- ^ Mozaffarian D, Rimm EB, Herrington DM (November 2004). "Dietary fats, carbohydrate, and progression of coronary atherosclerosis in postmenopausal women". The American Journal of Clinical Nutrition. 80 (5): 1175–84. doi:10.1093/ajcn/80.5.1175. PMC 1270002. PMID 15531663.
- ^ Leibovitz B, Hu ML, Tappel AL (January 1990). "Dietary supplements of vitamin E, beta-carotene, coenzyme Q10 and selenium protect tissues against lipid peroxidation in rat tissue slices". The Journal of Nutrition. 120 (1): 97–104. doi:10.1093/jn/120.1.97. PMID 2303916.
- ^ Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nälsén C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH (March 2001). "Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study". Diabetologia. 44 (3): 312–9. doi:10.1007/s001250051620. PMID 11317662.
- ^ Lovejoy JC (October 2002). "The influence of dietary fat on insulin resistance". Current Diabetes Reports. 2 (5): 435–40. doi:10.1007/s11892-002-0098-y. PMID 12643169. S2CID 31329463.
- ^ Fukuchi S, Hamaguchi K, Seike M, Himeno K, Sakata T, Yoshimatsu H (June 2004). "Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats". Experimental Biology and Medicine. 229 (6): 486–93. doi:10.1177/153537020422900606. PMID 15169967. S2CID 20966659.
- ^ a b "Erythrocyte membrane fatty acids and subsequent breast cancer: a prospective Italian study". Journal of the National Cancer Institute. 93 (14): 1088–95. July 2001. doi:10.1093/jnci/93.14.1088. PMID 11459870.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c "Omega-3 Fatty Acids and Health: Fact Sheet for Health Professionals". US National Institutes of Health, Office of Dietary Supplements. 2 November 2016. Retrieved 5 April 2017.
- ^ Patterson RE, Flatt SW, Newman VA, Natarajan L, Rock CL, Thomson CA, Caan BJ, Parker BA, Pierce JP (February 2011). "Marine fatty acid intake is associated with breast cancer prognosis". The Journal of Nutrition. 141 (2): 201–6. doi:10.3945/jn.110.128777. PMC 3021439. PMID 21178081.
- ^ Martin CA, Milinsk MC, Visentainer JV, Matsushita M, de-Souza NE (June 2007). "Trans fatty acid-forming processes in foods: a review". Anais da Academia Brasileira de Ciencias. 79 (2): 343–50. doi:10.1590/S0001-37652007000200015. PMID 17625687.
- ^ Kuhnt K, Baehr M, Rohrer C, Jahreis G (October 2011). "Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods". European Journal of Lipid Science and Technology. 113 (10): 1281–1292. doi:10.1002/ejlt.201100037. PMC 3229980. PMID 22164125.
- ^ a b c d e Trans Fat Task Force (June 2006). TRANSforming the Food Supply. ISBN 0-662-43689-X. Retrieved 7 January 2007.
- ^ trans-fatty acid
- ^ a b Tarrago-Trani MT, Phillips KM, Lemar LE, Holden JM (June 2006). "New and existing oils and fats used in products with reduced trans-fatty acid content". Journal of the American Dietetic Association. 106 (6): 867–80. doi:10.1016/j.jada.2006.03.010. PMID 16720128.
- ^ Menaa F, Menaa A, Menaa B, Tréton J (June 2013). "Trans-fatty acids, dangerous bonds for health? A background review paper of their use, consumption, health implications and regulation in France". European Journal of Nutrition. 52 (4): 1289–302. doi:10.1007/s00394-012-0484-4. PMID 23269652. S2CID 206968361.
- ^ "Wilhelm Normann und die Geschichte der Fetthärtung von Martin Fiedler, 2001". 20 December 2011. Archived from the original on 1 October 2011. Retrieved 14 August 2007.
- ^ Gormley JJ, Juturu V (2010). "Partially Hydrogenated Fats in the US Diet and Their Role in Disease". In De Meester F, Zibadi S, Watson RR (eds.). Modern Dietary Fat Intakes in Disease Promotion. Nutrition and Health. Totowa, NJ: Humana Press. pp. 85–94. doi:10.1007/978-1-60327-571-2_5. ISBN 9781603275712.
- ^ a b "About Trans Fat and Partially Hydrogenated Oils" (PDF). Center for Science in the Public Interest.
- ^ a b "Tentative Determination Regarding Partially Hydrogenated Oils". Federal Register. 8 November 2013. 2013-26854, Vol. 78, No. 217. Archived from the original on 6 April 2014. Retrieved 8 November 2013.
- ^ Hill JW, Kolb DK (2007). Chemistry for changing times. Pearson / Prentice Hall. ISBN 978-0136054498.
- ^ Ashok C, Ajit V (2009). "Chapter 4: Fatty acids". A Textbook of Molecular Biotechnology. p. 181. ISBN 978-93-80026-37-4.
- ^ a b Valenzuela A, Morgado N (1999). "Trans fatty acid isomers in human health and in the food industry". Biological Research. 32 (4): 273–87. doi:10.4067/s0716-97601999000400007. PMID 10983247.
- ^ "Heart Foundation: Butter has 20 times the trans fats of marg | Australian Food News". www.ausfoodnews.com.au.
- ^ Hunter JE (2005). "Dietary levels of trans fatty acids" basis for health concerns and industry efforts to limit use". Nutrition Research. 25 (5): 499–513. doi:10.1016/j.nutres.2005.04.002.
- ^ "What's in that french fry? Fat varies by city". NBC News. 12 April 2006. Retrieved 7 January 2007. AP story concerning Stender, S; Dyerberg, J; Astrup, A (April 2006). "High levels of industrially produced trans fat in popular fast foods". N. Engl. J. Med. 354 (15): 1650–2. doi:10.1056/NEJMc052959. PMID 16611965.
- ^ "Fats and Cholesterol", Harvard School of Public Health. Retrieved 02-11-16.
- ^ a b Food and nutrition board, institute of medicine of the national academies (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academies Press. pp. 423. doi:10.17226/10490. ISBN 978-0-309-08525-0.
- ^ a b c Food and nutrition board, institute of medicine of the national academies (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academies Press. p. 504.[permanent dead link]
- ^ "Trans fat: Avoid this cholesterol double whammy". Mayo Foundation for Medical Education and Research (MFMER). Retrieved 10 December 2007.
- ^ a b c d e Trans Fat Task Force (June 2006). "TRANSforming the Food Supply (Appendix 9iii)". Archived from the original on 25 February 2007. Retrieved 9 January 2007.
{{cite journal}}: Cite journal requires|journal=(help) (Consultation on the health implications of alternatives to trans fatty acids: Summary of Responses from Experts) - ^ Willett WC, Ascherio A (May 1994). "Trans fatty acids: are the effects only marginal?". American Journal of Public Health. 84 (5): 722–4. doi:10.2105/AJPH.84.5.722. PMC 1615057. PMID 8179036.
- ^ Zaloga GP, Harvey KA, Stillwell W, Siddiqui R (October 2006). "Trans fatty acids and coronary heart disease". Nutrition in Clinical Practice. 21 (5): 505–12. doi:10.1177/0115426506021005505. PMID 16998148.
- ^ a b c d e Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC (April 2006). "Trans fatty acids and cardiovascular disease". The New England Journal of Medicine. 354 (15): 1601–13. doi:10.1056/NEJMra054035. PMID 16611951. S2CID 35121566.
- ^ a b Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, et al. (November 1997). "Dietary fat intake and the risk of coronary heart disease in women". The New England Journal of Medicine. 337 (21): 1491–9. doi:10.1056/NEJM199711203372102. PMID 9366580.
- ^ Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC (April 2005). "Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses' health study". American Journal of Epidemiology. 161 (7): 672–9. doi:10.1093/aje/kwi085. PMID 15781956.
- ^ Ascherio A, Katan MB, Zock PL, Stampfer MJ, Willett WC (June 1999). "Trans fatty acids and coronary heart disease". The New England Journal of Medicine. 340 (25): 1994–8. doi:10.1056/NEJM199906243402511. PMID 10379026. S2CID 30165590.
- ^ Mensink RP, Katan MB (August 1990). "Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects". The New England Journal of Medicine. 323 (7): 439–45. doi:10.1056/NEJM199008163230703. PMID 2374566.
- ^ Mensink RP, Zock PL, Kester AD, Katan MB (May 2003). "Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials". The American Journal of Clinical Nutrition. 77 (5): 1146–55. doi:10.1093/ajcn/77.5.1146. PMID 12716665.
- ^ Gatto LM, Sullivan DR, Samman S (May 2003). "Postprandial effects of dietary trans fatty acids on apolipoprotein(a) and cholesteryl ester transfer". The American Journal of Clinical Nutrition. 77 (5): 1119–24. doi:10.1093/ajcn/77.5.1119. PMID 12716661.
- ^ Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, et al. (March 2005). "Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction". The Journal of Nutrition. 135 (3): 562–6. doi:10.1093/jn/135.3.562. PMID 15735094.
- ^ Innis SM, King DJ (September 1999). "trans Fatty acids in human milk are inversely associated with concentrations of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants". The American Journal of Clinical Nutrition. 70 (3): 383–90. doi:10.1093/ajcn/70.3.383. PMID 10479201.
- ^ Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. (February 2003). "Dietary fats and the risk of incident Alzheimer disease". Archives of Neurology. 60 (2): 194–200. doi:10.1001/archneur.60.2.194. PMID 12580703.
- ^ a b Phivilay A, Julien C, Tremblay C, Berthiaume L, Julien P, Giguère Y, Calon F (March 2009). "High dietary consumption of trans fatty acids decreases brain docosahexaenoic acid but does not alter amyloid-beta and tau pathologies in the 3xTg-AD model of Alzheimer's disease". Neuroscience. 159 (1): 296–307. doi:10.1016/j.neuroscience.2008.12.006. PMID 19135506. S2CID 35748183.
- ^ Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K (June 2008). "Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat". Journal of Alzheimer's Disease. 14 (2): 133–45. doi:10.3233/JAD-2008-14202. PMC 2670571. PMID 18560126.
- ^ American Cancer Society. "Common questions about diet and cancer". Retrieved 9 January 2007.
- ^ Chavarro J, Stampfer M, Campos H, Kurth T, Willett W, Ma J (1 April 2006). "A prospective study of blood trans fatty acid levels and risk of prostate cancer". Proc. Amer. Assoc. Cancer Res. 47 (1): 943. Retrieved 9 January 2007.
- ^ Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, et al. (June 2011). "Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial". American Journal of Epidemiology. 173 (12): 1429–39. doi:10.1093/aje/kwr027. PMC 3145396. PMID 21518693.
- ^ "Breast cancer: a role for trans fatty acids?". World Health Organization (Press release). 11 April 2008. Archived from the original on 13 April 2008.
- ^ Chajès V, Thiébaut AC, Rotival M, Gauthier E, Maillard V, Boutron-Ruault MC, et al. (June 2008). "Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC Study". American Journal of Epidemiology. 167 (11): 1312–20. doi:10.1093/aje/kwn069. PMC 2679982. PMID 18390841.
- ^ Riserus U (2006). "Trans fatty acids, insulin sensitivity and type 2 diabetes". Scandinavian Journal of Food and Nutrition. 50 (4): 161–165. doi:10.1080/17482970601133114.
- ^ Hu FB, van Dam RM, Liu S (July 2001). "Diet and risk of Type II diabetes: the role of types of fat and carbohydrate". Diabetologia. 44 (7): 805–17. doi:10.1007/s001250100547. PMID 11508264.
- ^ van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB (March 2002). "Dietary fat and meat intake in relation to risk of type 2 diabetes in men". Diabetes Care. 25 (3): 417–24. doi:10.2337/diacare.25.3.417. PMID 11874924.
- ^ Gosline A (12 June 2006). "Why fast foods are bad, even in moderation". New Scientist. Retrieved 9 January 2007.
- ^ "Six years of fast-food fats supersizes monkeys". New Scientist (2556): 21. 17 June 2006.
- ^ a b Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, Rudel LL (July 2007). "Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys". Obesity. 15 (7): 1675–84. doi:10.1038/oby.2007.200. PMID 17636085. S2CID 4835948.
- ^ Thompson TG. "Trans Fat Press Conference". Archived from the original on 9 July 2006., US Secretary of health and human services
- ^ Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC (January 2007). "Dietary fatty acid intakes and the risk of ovulatory infertility". The American Journal of Clinical Nutrition. 85 (1): 231–7. doi:10.1093/ajcn/85.1.231. PMID 17209201.
- ^ Roan S (28 January 2011). "Trans fats and saturated fats could contribute to depression". The Sydney Morning Herald. Retrieved 8 February 2011.
- ^ McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM (July 2007). "Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder". Biological Psychiatry. 62 (1): 17–24. doi:10.1016/j.biopsych.2006.08.026. PMID 17188654. S2CID 32898004.
- ^ Golomb BA, Evans MA, White HL, Dimsdale JE (2012). "Trans fat consumption and aggression". PLOS ONE. 7 (3): e32175. Bibcode:2012PLoSO...732175G. doi:10.1371/journal.pone.0032175. PMC 3293881. PMID 22403632.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Golomb BA, Bui AK (2015). "A Fat to Forget: Trans Fat Consumption and Memory". PLOS ONE. 10 (6): e0128129. Bibcode:2015PLoSO..1028129G. doi:10.1371/journal.pone.0128129. PMC 4470692. PMID 26083739.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Melnik BC (15 July 2015). Weinberg J (ed.). "Linking diet to acne metabolomics, inflammation, and comedogenesis: an update". Clinical, Cosmetic and Investigational Dermatology. 8: 371–88. doi:10.2147/CCID.S69135. PMC 4507494. PMID 26203267.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kummerow FA, Zhou Q, Mahfouz MM, Smiricky MR, Grieshop CM, Schaeffer DJ (April 2004). "Trans fatty acids in hydrogenated fat inhibited the synthesis of the polyunsaturated fatty acids in the phospholipid of arterial cells". Life Sciences. 74 (22): 2707–23. doi:10.1016/j.lfs.2003.10.013. PMID 15043986.
- ^ Mojska H (2003). "Influence of trans fatty acids on infant and fetus development". Acta Microbiologica Polonica. 52 Suppl: 67–74. PMID 15058815.
- ^ Koletzko B, Decsi T (October 1997). "Metabolic aspects of trans fatty acids". Clinical Nutrition. 16 (5): 229–37. doi:10.1016/s0261-5614(97)80034-9. PMID 16844601.
- ^ Mahfouz M (1981). "Effect of dietary trans fatty acids on the delta 5, delta 6 and delta 9 desaturases of rat liver microsomes in vivo". Acta Biologica et Medica Germanica. 40 (12): 1699–1705. PMID 7345825.
- ^ "National Nutrient Database for Standard Reference Release 28". United States Department of Agriculture.[permanent dead link]
- ^ National Dairy Council (18 June 2004). "comments on 'Docket No. 2003N-0076 Food Labeling: Trans Fatty Acids in Nutrition Labeling'" (PDF). Retrieved 7 January 2007.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Brouwer IA, Wanders AJ, Katan MB (March 2010). Reitsma PH (ed.). "Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans--a quantitative review". PLOS ONE. 5 (3): e9434. Bibcode:2010PLoSO...5.9434B. doi:10.1371/journal.pone.0009434. PMC 2830458. PMID 20209147.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Jones EL, et al. (September 2004). "Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans". The American Journal of Clinical Nutrition. 80 (3): 614–20. doi:10.1093/ajcn/80.3.614. PMID 15321800.
- ^ Zulet MA, Marti A, Parra MD, Martínez JA (September 2005). "Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health". Journal of Physiology and Biochemistry. 61 (3): 483–94. doi:10.1007/BF03168454. PMID 16440602. S2CID 32082565.
- ^ Trans Fats From Ruminant Animals May Be Beneficial – Health News. redOrbit (8 September 2011). Retrieved 22 January 2013.
- ^ a b Bassett CM, Edel AL, Patenaude AF, McCullough RS, Blackwood DP, Chouinard PY, et al. (January 2010). "Dietary vaccenic acid has antiatherogenic effects in LDLr-/- mice". The Journal of Nutrition. 140 (1): 18–24. doi:10.3945/jn.109.105163. PMID 19923390.
- ^ Wang Y, Jacome-Sosa MM, Vine DF, Proctor SD (20 May 2010). "Beneficial effects of vaccenic acid on postprandial lipid metabolism and dyslipidemia: Impact of natural trans-fats to improve CVD risk". Lipid Technology. 22 (5): 103–106. doi:10.1002/lite.201000016.
- ^ David J. Baer, PhD. US Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Laboratory. New Findings on Dairy Trans Fat and Heart Disease Risk, IDF World Dairy Summit 2010, 8–11 November 2010. Auckland, New Zealand
- ^ EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) (2010). "Scientific opinion on dietary reference values for fats". EFSA Journal. 8 (3): 1461. doi:10.2903/j.efsa.2010.1461.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ UK Scientific Advisory Committee on Nutrition (2007). "Update on trans fatty acids and health, Position Statement" (PDF). Archived from the original (PDF) on 10 December 2010.
- ^ Brouwer IA, Wanders AJ, Katan MB (March 2010). "Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans--a quantitative review". PLOS ONE. 5 (3): e9434. Bibcode:2010PLoSO...5.9434B. doi:10.1371/journal.pone.0009434. PMC 2830458. PMID 20209147.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Trans fat". It's your health. Health Canada. Dec 2007. Archived from the original on 20 April 2012.
- ^ "EFSA sets European dietary reference values for nutrient intakes" (Press release). European Food Safety Authority. 26 March 2010.
- ^ "WHO plan to eliminate industrially-produced trans-fatty acids from global food supply" (Press release). World Health Organization. 14 May 2018.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. pp. i. Archived from the original on 18 September 2006.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 447.[permanent dead link]
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 424.[permanent dead link]
- ^ Eller FJ, List GR, Teel JA, Steidley KR, Adlof RO (July 2005). "Preparation of spread oils meeting U.S. Food and Drug Administration Labeling requirements for trans fatty acids via pressure-controlled hydrogenation". Journal of Agricultural and Food Chemistry. 53 (15): 5982–4. doi:10.1021/jf047849+. PMID 16028984.
- ^ Hadzipetros P (25 January 2007). "Trans Fats Headed for the Exit". CBC News.
- ^ Spencelayh M (9 January 2007). "Trans fat free future". Royal Society of Chemistry.
- ^ Willett WC (September 2007). "The role of dietary n-6 fatty acids in the prevention of cardiovascular disease". Journal of Cardiovascular Medicine. 8 Suppl 1: S42-5. doi:10.2459/01.JCM.0000289275.72556.13. PMID 17876199. S2CID 1420490.
- ^ van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP (August 2008). "Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial". Neurology. 71 (6): 430–8. doi:10.1212/01.wnl.0000324268.45138.86. PMID 18678826. S2CID 45576671.
- ^ Mensink, Ronald P.; Sanders, Thomas A.; Baer, David J.; Hayes, K. C.; Howles, Philip N.; Marangoni, Alejandro (2016-07-01). "The Increasing Use of Interesterified Lipids in the Food Supply and Their Effects on Health Parameters". Advances in Nutrition. 7 (4): 719–729. doi:10.3945/an.115.009662. ISSN 2161-8313. PMC 4942855. PMID 27422506.
- ^ Zock PJ, de Vries JH, de Fouw NJ, Katan MB (1995), "Positional distribution of fatty acids in dietary triglycerides: effects on fasting blood lipoprotein concentrations in humans." (PDF), Am J Clin Nutr, vol. 61, no. 1, pp. 48–551, doi:10.1093/ajcn/61.1.48, hdl:1871/11621, PMID 7825538
- ^ Nestel PJ, Noakes M, Belling GB, et al. (1995), "Effect on plasma lipids of interesterifying a mix of edible oils." (PDF), Am J Clin Nutr, vol. 62, no. 5, pp. 950–55, doi:10.1093/ajcn/62.5.950, PMID 7572740
- ^ Meijer GW, Weststrate JA (1997), "Interesterification of fats in margarine: effect on blood lipids, blood enzymes and hemostasis parameters.", Eur J Clin Nutr, vol. 51, no. 8, pp. 527–34, doi:10.1038/sj.ejcn.1600437, PMID 11248878
- ^ Grande F, Anderson JT, Keys A (1970), "Comparison of effects of palmitic and stearic acids in the diet on serum cholesterol in man." (PDF), Am J Clin Nutr, vol. 23, no. 9, pp. 1184–93, doi:10.1093/ajcn/23.9.1184, PMID 5450836
- ^ Berry SE, Miller GJ, Sanders TA (2007), "The solid fat content of stearic acid-rich fats determines their postprandial effects." (PDF), Am J Clin Nutr, vol. 85, no. 6, pp. 1486–94, doi:10.1093/ajcn/85.6.1486, PMID 17556683
- ^ Zampelas A, Williams CM, Morgan LM, et al. (1994), "The effect of triacylglycerol fatty acids positional distribution on postprandial plasma metabolite and hormone responses in normal adult men.", Br J Nutr, vol. 71, no. 3, pp. 401–10, doi:10.1079/bjn19940147, PMID 8172869
- ^ Yli-Jokipii K, Kallio H, Schwab U, et al. (2001), "Effects of palm oil and transesterified palm oil on chylomicron and VLDL triacylglycerol structures and postprandial lipid response." (PDF), J Lipid Res, vol. 42, no. 10, pp. 1618–25, PMID 11590218
- ^ Berry SE, Woodward R, Yeoh C, Miller GJ, Sanders TA (2007), "Effect of interesterification of palmitic-acid rich tryacylglycerol on postprandial lipid and factor VII response", Lipids, 42 (4): 315–323, doi:10.1007/s11745-007-3024-x, PMID 17406926, S2CID 3986807
- ^ Summers LK, Fielding BA, Herd SL, et al. (1999), "Use of structured triacylglycerols containing predominantly stearic and oleic acids to probe early events in metabolic processing of dietary fat" (PDF), J Lipid Res, vol. 40, no. 10, pp. 1890–98, PMID 10508209
- ^ Christophe AB, De Greyt WF, Delanghe JR, Huyghebaert AD (2000), "Substituting enzymically interesterified butter for native butter has no effect on lipemia or lipoproteinemia in man", Annals of Nutrition and Metabolism, 44 (2): 61–67, doi:10.1159/000012822, PMID 10970994, S2CID 22276158
- ^ Sundram K, Karupaiah T, Hayes K (2007). "Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans" (PDF). Nutr Metab. 4: 3. doi:10.1186/1743-7075-4-3. PMC 1783656. PMID 17224066. Retrieved 2007-01-19.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Destaillats F, Moulin J, Bezelgues JB (2007), "Letter to the editor: healthy alternatives to trans fats", Nutr Metab, vol. 4, p. 10, doi:10.1186/1743-7075-4-10, PMC 1867814, PMID 17462099
{{citation}}: CS1 maint: unflagged free DOI (link) - ^ Mensink RP, Zock PL, Kester AD, Katan MB (2003), "Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials." (PDF), Am J Clin Nutr, vol. 77, no. 5, pp. 1146–1155, doi:10.1093/ajcn/77.5.1146, PMID 12716665
- ^ Panov, Alexander; Orynbayeva, Zulfiya; Vavilin, Valentin; Lyakhovich, Vyacheslav (2014). Baranova, Ancha (ed.). "Fatty Acids in Energy Metabolism of the Central Nervous System". BioMed Research International. 2014 (The Roads to Mitochondrial Dysfunction). Hindawa: 472459. doi:10.1155/2014/472459. PMC 4026875. PMID 24883315.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Feinman RD (October 2010). "Saturated fat and health: recent advances in research". Lipids. 45 (10): 891–2. doi:10.1007/s11745-010-3446-8. PMC 2974200. PMID 20827513.
- ^ Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. (2006). "Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial". Journal of the American Medical Association. 295 (6): 655–66. doi:10.1001/jama.295.6.655. PMID 16467234.
- ^ Zelman K (May 2011). "The great fat debate: a closer look at the controversy-questioning the validity of age-old dietary guidance". Journal of the American Dietetic Association. 111 (5): 655–8. doi:10.1016/j.jada.2011.03.026. PMID 21515106.
- ^ Dijkstra A, Hamilton RJ, Wolf H, eds. (2008). Trans Fatty Acids. Blackwell. ISBN 978-1-4051-5691-2.
- ^ Jang ES, Jung MY, Min DB (2005). "Hydrogenation for Low Trans and High Conjugated Fatty Acids" (PDF). Comprehensive Reviews in Food Science and Food Safety. 1. Archived from the original (PDF) on 17 December 2008.
- ^ "Ban the Trans: These Sorry Lipids Should Go Away"
- ^ Center for Science in the Public Interest Trans Fat Page
- ^ Harvard School of Public Health webpage on trans-fat
- ^ "Labeling & Nutrition – Guidance for Industry: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, Health Claims; Small Entity Compliance Guide". Center for Food Safety and Applied Nutrition. August 2003. Archived from the original on 26 October 2013. Retrieved 6 April 2014.
- ^ Federal Register – 68 FR 41433 11 July 2003: Food Labeling: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, and Health Claims
- ^ Fats (Mayo Clinic)
- ^ The Chemistry of Unsaturated Fats
- ^ Hayes, K.C. (May 2005). Dietary fat and blood lipids. Retrieved March 10, 2011.