From Wikipedia, the free encyclopedia
Phenestrol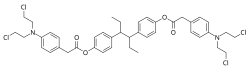 |
|
| Other names | NSC-183736 |
|---|
|
[4-[4-[4-[2-[4-[Bis(2-chloroethyl)amino]phenyl]acetyl]oxyphenyl]hexan-3-yl]phenyl] 2-[4-[bis(2-chloroethyl)amino]phenyl]acetate
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| Formula | C42H48Cl4N2O4 |
|---|
| Molar mass | 786.65352 g/mol g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
CCC(C1=CC=C(C=C1)OC(=O)CC2=CC=C(C=C2)N(CCCl)CCCl)C(CC)C3=CC=C(C=C3)OC(=O)CC4=CC=C(C=C4)N(CCCl)CCCl
|
InChI=1S/C42H48Cl4N2O4/c1-3-39(33-9-17-37(18-10-33)51-41(49)29-31-5-13-35(14-6-31)47(25-21-43)26-22-44)40(4-2)34-11-19-38(20-12-34)52-42(50)30-32-7-15-36(16-8-32)48(27-23-45)28-24-46/h5-20,39-40H,3-4,21-30H2,1-2H3 Key:GTWGWCYJKHSRHO-UHFFFAOYSA-N
|
Phenestrol (developmental code name NSC-183736), or fenestrol, also known as hexestrol bis[4-[bis(2-chloroethyl)amino]phenylacetate, is a synthetic, nonsteroidal estrogen and alkylating antineoplastic agent (i.e., chemotherapy drug) and a chlorphenacyl nitrogen mustard ester of hexestrol that was developed in the early 1960s for the treatment of hormone-dependent tumors but was never marketed.[1][2][3][4]
See also
References
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 163–. ISBN 978-1-4757-2085-3.
- ^ Abraham Goldin; National Cancer Institute (U.S.); Onkologicheskiĭ nauchnyĭ t͡sentr (Akademii͡a medit͡sinskikh nauk SSSR) (1981). Experimental evaluation of antitumor drugs in the USA and USSR and clinical correlations. U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute. p. 17.
- ^ Morozova TM, Merkulova TI, Martynov V, Iaguzhinskaia VP, Shkodinskaia EN (1984). "[Properties of alkylating derivatives of estrogens]". Eksp. Onkol. (in Russian). 6 (2): 50–4. PMID 6510335.
- ^ Lagova ND, Sof'ina ZP, Shkodinskaia EN, Kurdiumova KN, Valueva IM (1988). "[The antineoplastic activity of testiphenon]". Vopr Onkol (in Russian). 34 (11): 1363–8. PMID 3201773.
|
|---|
| ERTooltip Estrogen receptor | | Agonists |
- Steroidal: 2-Hydroxyestradiol
- 2-Hydroxyestrone
- 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
- 3α-Androstanediol
- 3α,5α-Dihydrolevonorgestrel
- 3β,5α-Dihydrolevonorgestrel
- 3α-Hydroxytibolone
- 3β-Hydroxytibolone
- 3β-Androstanediol
- 4-Androstenediol
- 4-Androstenedione
- 4-Fluoroestradiol
- 4-Hydroxyestradiol
- 4-Hydroxyestrone
- 4-Methoxyestradiol
- 4-Methoxyestrone
- 5-Androstenediol
- 7-Oxo-DHEA
- 7α-Hydroxy-DHEA
- 7α-Methylestradiol
- 7β-Hydroxyepiandrosterone
- 8,9-Dehydroestradiol
- 8,9-Dehydroestrone
- 8β-VE2
- 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
- 11β-Chloromethylestradiol
- 11β-Methoxyestradiol
- 15α-Hydroxyestradiol
- 16-Ketoestradiol
- 16-Ketoestrone
- 16α-Fluoroestradiol
- 16α-Hydroxy-DHEA
- 16α-Hydroxyestrone
- 16α-Iodoestradiol
- 16α-LE2
- 16β-Hydroxyestrone
- 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
- 17α-Estradiol (alfatradiol)
- 17α-Dihydroequilenin
- 17α-Dihydroequilin
- 17α-Epiestriol (16α-hydroxy-17α-estradiol)
- 17α-Ethynyl-3α-androstanediol
- 17α-Ethynyl-3β-androstanediol
- 17β-Dihydroequilenin
- 17β-Dihydroequilin
- 17β-Methyl-17α-dihydroequilenin
- Abiraterone
- Abiraterone acetate
- Alestramustine
- Almestrone
- Anabolic steroids (e.g., testosterone and esters, methyltestosterone, metandienone (methandrostenolone), nandrolone and esters, many others; via estrogenic metabolites)
- Atrimustine
- Bolandiol
- Bolandiol dipropionate
- Butolame
- Clomestrone
- Cloxestradiol
- Conjugated estriol
- Conjugated estrogens
- Cyclodiol
- Cyclotriol
- DHEA
- DHEA-S
- ent-Estradiol
- Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
- Epimestrol
- Equilenin
- Equilin
- ERA-63 (ORG-37663)
- Esterified estrogens
- Estetrol
- Estradiol
- Estramustine
- Estramustine phosphate
- Estrapronicate
- Estrazinol
- Estriol
- Estrofurate
- Estrogenic substances
- Estromustine
- Estrone
- Etamestrol (eptamestrol)
- Ethinylandrostenediol
- Ethinylestradiol
- Ethinylestriol
- Ethylestradiol
- Etynodiol
- Etynodiol diacetate
- Hexolame
- Hippulin
- Hydroxyestrone diacetate
- Lynestrenol
- Lynestrenol phenylpropionate
- Mestranol
- Methylestradiol
- Moxestrol
- Mytatrienediol
- Nilestriol
- Norethisterone
- Noretynodrel
- Orestrate
- Pentolame
- Prodiame
- Prolame
- Promestriene
- RU-16117
- Quinestradol
- Quinestrol
- Tibolone
- Xenoestrogens: Anise-related (e.g., anethole, anol, dianethole, dianol, photoanethole)
- Chalconoids (e.g., isoliquiritigenin, phloretin, phlorizin (phloridzin), wedelolactone)
- Coumestans (e.g., coumestrol, psoralidin)
- Flavonoids (incl. 7,8-DHF, 8-prenylnaringenin, apigenin, baicalein, baicalin, biochanin A, calycosin, catechin, daidzein, daidzin, ECG, EGCG, epicatechin, equol, formononetin, glabrene, glabridin, genistein, genistin, glycitein, kaempferol, liquiritigenin, mirificin, myricetin, naringenin, penduletin, pinocembrin, prunetin, puerarin, quercetin, tectoridin, tectorigenin)
- Lavender oil
- Lignans (e.g., enterodiol, enterolactone, nyasol (cis-hinokiresinol))
- Metalloestrogens (e.g., cadmium)
- Pesticides (e.g., alternariol, dieldrin, endosulfan, fenarimol, HPTE, methiocarb, methoxychlor, triclocarban, triclosan)
- Phytosteroids (e.g., digitoxin (digitalis), diosgenin, guggulsterone)
- Phytosterols (e.g., β-sitosterol, campesterol, stigmasterol)
- Resorcylic acid lactones (e.g., zearalanone, α-zearalenol, β-zearalenol, zearalenone, zeranol (α-zearalanol), taleranol (teranol, β-zearalanol))
- Steroid-like (e.g., deoxymiroestrol, miroestrol)
- Stilbenoids (e.g., resveratrol, rhaponticin)
- Synthetic xenoestrogens (e.g., alkylphenols, bisphenols (e.g., BPA, BPF, BPS), DDT, parabens, PBBs, PHBA, phthalates, PCBs)
- Others (e.g., agnuside, rotundifuran)
|
|---|
Mixed
(SERMsTooltip Selective estrogen receptor modulators) | |
|---|
| Antagonists |
- Coregulator-binding modulators: ERX-11
|
|---|
|
|---|
| GPERTooltip G protein-coupled estrogen receptor | | Agonists | |
|---|
| Antagonists | |
|---|
| Unknown | |
|---|
|
|---|
|

