Meprylcaine: Difference between revisions
Content deleted Content added
Adding template |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox |
|||
{{unsourced|date=June 2015}} |
|||
| ⚫ | |||
{{chembox |
|||
| image = Epirocaine.svg |
|||
| Verifiedfields = changed |
|||
| width = 220 |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 444176138 |
|||
<!--Clinical data--> |
|||
| ImageFile=Meprylcaine.png |
|||
| tradename = |
|||
| ImageSize=200px |
|||
| routes_of_administration = |
|||
| ⚫ | |||
| legal_status = |
|||
| OtherNames= |
|||
|Section1={{Chembox Identifiers |
|||
<!--Identifiers--> |
|||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| |
| CAS_number = 495-70-5 |
||
| ATC_suffix = |
|||
| PubChem=4065 |
| PubChem = 4065 |
||
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
|||
| ChEMBL = 127810 |
| ChEMBL = 127810 |
||
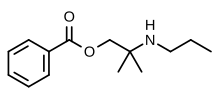
| SMILES=CCCNC(C)(C)COC(=O)C1=CC=CC=C1 |
|||
| EINECS = 213-475-6 |
|||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
| ChemSpiderID = 3925 |
| ChemSpiderID = 3925 |
||
| InChI = 1/C14H21NO2/c1-4-10-15-14(2,3)11-17-13(16)12-8-6-5-7-9-12/h5-9,15H,4,10-11H2,1-3H3 |
|||
<!--Chemical data--> |
|||
| InChIKey = VXJABHHJLXLNMP-UHFFFAOYAB |
|||
| C=14 | H=21 | N=1 | O=2 |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| smiles = CC(COC(C1=CC=CC=C1)=O)(C)NCCC |
|||
| StdInChI = 1S/C14H21NO2/c1-4-10-15-14(2,3)11-17-13(16)12-8-6-5-7-9-12/h5-9,15H,4,10-11H2,1-3H3 |
| StdInChI = 1S/C14H21NO2/c1-4-10-15-14(2,3)11-17-13(16)12-8-6-5-7-9-12/h5-9,15H,4,10-11H2,1-3H3 |
||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChIKey = VXJABHHJLXLNMP-UHFFFAOYSA-N |
| StdInChIKey = VXJABHHJLXLNMP-UHFFFAOYSA-N |
||
}} |
|||
|Section2={{Chembox Properties |
|||
| Formula=C<sub>14</sub>H<sub>21</sub>NO<sub>2</sub> |
|||
| MolarMass=235.32 g/mol |
|||
| Appearance= |
|||
| Density= |
|||
| MeltingPt= |
|||
| BoilingPt= |
|||
| Solubility= |
|||
}} |
|||
|Section3={{Chembox Hazards |
|||
| MainHazards= |
|||
| FlashPt= |
|||
| AutoignitionPt = |
|||
}} |
|||
}} |
}} |
||
'''Meprylcaine''' (also known as '''Epirocaine''' and '''Oracaine''') is a [[local anesthetic]] with stimulant properties that is structurally related to [[dimethocaine]].<ref>{{cite journal | url=http://link.springer.com/article/10.1007/s002109900184 | title=Selective inhibition of monoamine neurotransmitter transporters by synthetic local anesthetics | author=T. Sato, S. Kitayama, C. Mitsuhata, T. Ikeda, K. Morita, T. Dohi | journal=Naunyn-Schmiedeberg's Archives of Pharmacology | date=February 2000 | volume=361 | issue=2 | pages=214-220 | doi=10.1007/s002109900184 | pmid=10685879}}</ref> |
|||
'''Meprylcaine''' ('''Epirocain''') is a [[local anesthetic]]. |
|||
Meprylcaine has a relatively potent inhibitory action on the monoamine transporter and inhibits the reuptake of [[dopamine]], [[norepinephrine]] and [[serotonin]].<ref>{{cite journal | url=http://www.sciencedirect.com/science/article/pii/S0006899302040684 | title=Chronic inhibition of the norepinephrine transporter in the brain participates in seizure sensitization to cocaine and local anesthetics | author=Shigeaki Arai, Katsuya Morita, Shigeo Kitayama, Kei Kumagai, Michio Kumagai, Kenji Kihira, Toshihiro Dohi | journal=Brain Research | date=February 2003 | volume=964 | issue=1 | pages=83–90 | doi=10.1016/S0006-8993(02)04068-4 | pmid=12573515}}</ref><ref>{{cite journal | url=http://www.sciencedirect.com/science/article/pii/S0006899305011169 | title=Inhibition of serotonin transporters by cocaine and meprylcaine through 5-HT<sub>2C</sub> receptor stimulation facilitates their seizure activities | author=Katsuya Morita, Masahiro Hamamoto, Shigeaki Arai, Shigeo Kitayama, Masahiro Irifune, Michio Kawahara, Kenji Kihira, Toshihiro Dohi | journal=Brain Research | date=September 2005 | volume=1057 | issue=1–2 | pages=153–160 | doi=10.1016/j.brainres.2005.07.049 | pmid=16125150}}</ref> |
|||
==References== |
|||
{{reflist}} |
|||
{{Stimulants}} |
|||
{{Local anesthetics}} |
{{Local anesthetics}} |
||
[[Category:Local anesthetics]] |
[[Category:Local anesthetics]] |
||
[[Category:Benzoates]] |
[[Category:Benzoates]] |
||
[[Category:Serotonin-norepinephrine-dopamine reuptake inhibitors]] |
|||
[[Category:Stimulants]] |
|||
Revision as of 14:20, 17 December 2015
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H21NO2 |
| Molar mass | 235.327 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Meprylcaine (also known as Epirocaine and Oracaine) is a local anesthetic with stimulant properties that is structurally related to dimethocaine.[1]
Meprylcaine has a relatively potent inhibitory action on the monoamine transporter and inhibits the reuptake of dopamine, norepinephrine and serotonin.[2][3]
References
- ^ T. Sato, S. Kitayama, C. Mitsuhata, T. Ikeda, K. Morita, T. Dohi (February 2000). "Selective inhibition of monoamine neurotransmitter transporters by synthetic local anesthetics". Naunyn-Schmiedeberg's Archives of Pharmacology. 361 (2): 214–220. doi:10.1007/s002109900184. PMID 10685879.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shigeaki Arai, Katsuya Morita, Shigeo Kitayama, Kei Kumagai, Michio Kumagai, Kenji Kihira, Toshihiro Dohi (February 2003). "Chronic inhibition of the norepinephrine transporter in the brain participates in seizure sensitization to cocaine and local anesthetics". Brain Research. 964 (1): 83–90. doi:10.1016/S0006-8993(02)04068-4. PMID 12573515.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Katsuya Morita, Masahiro Hamamoto, Shigeaki Arai, Shigeo Kitayama, Masahiro Irifune, Michio Kawahara, Kenji Kihira, Toshihiro Dohi (September 2005). "Inhibition of serotonin transporters by cocaine and meprylcaine through 5-HT2C receptor stimulation facilitates their seizure activities". Brain Research. 1057 (1–2): 153–160. doi:10.1016/j.brainres.2005.07.049. PMID 16125150.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
