Dextroamphetamine
 | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /ˌdɛkstroʊæmˈfɛtəmiːn/ | ||
| Trade names | Dexedrine, Zenzedi, Xelstrym, others | ||
| Other names | d-Amphetamine, (S)-Amphetamine, S(+)-Amphetamine | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a605027 | ||
| License data | |||
| Pregnancy category |
| ||
| Dependence liability | Moderate[1][2] - high[3][4][5] | ||
| Addiction liability | Moderate[1][2] - high[3][4][5] | ||
| Routes of administration | By mouth, transdermal | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Bioavailability | Oral: ~90%[13] | ||
| Protein binding | 15–40%[14] | ||
| Metabolism | CYP2D6,[19] DBH,[25] FMO3[26] | ||
| Onset of action | IR dosing: 0.5–1.5 hours[15][16] XR dosing: 1.5–2 hours[17][18] | ||
| Elimination half-life | 9–11 hours[19][20] pH-dependent: 7–34 hours[21] | ||
| Duration of action | IR dosing: 3–6 hours[17][22] XR dosing: 8–12 hours[23][17][22] | ||
| Excretion | Kidney (45%);[24] urinary pH-dependent | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.000.103 | ||
| Chemical and physical data | |||
| Formula | C9H13N | ||
| Molar mass | 135.210 g·mol−1 | ||
| 3D model (JSmol) | |||
| Density | 0.913 g/cm3 | ||
| Boiling point | 201.5 °C (394.7 °F) | ||
| Solubility in water | 20 | ||
| |||
| |||
| | |||
Dextroamphetamine (INN:dexamfetamine) is a potent central nervous system (CNS) stimulant and enantiomer[note 1] of amphetamine that is prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[10][27] It is also used as an athletic performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant.
The amphetamine molecule exists as two enantiomers,[note 1] levoamphetamine and dextroamphetamine. Dextroamphetamine is the dextrorotatory, or 'right-handed', enantiomer and exhibits more pronounced effects on the central nervous system than levoamphetamine. Pharmaceutical dextroamphetamine sulfate is available as both a brand name and generic drug in a variety of dosage forms. Dextroamphetamine is sometimes prescribed as the inactive prodrug lisdexamfetamine dimesylate, which is converted into dextroamphetamine after absorption.
Dextroamphetamine, like other amphetamines, elicits its stimulating effects via several distinct actions: it inhibits or reverses the transporter proteins for the monoamine neurotransmitters (namely the serotonin, norepinephrine and dopamine transporters) either via trace amine-associated receptor 1 (TAAR1) or in a TAAR1 independent fashion when there are high cytosolic concentrations of the monoamine neurotransmitters[29] and it releases these neurotransmitters from synaptic vesicles via vesicular monoamine transporter 2.[30] It also shares many chemical and pharmacological properties with human trace amines, particularly phenethylamine and N-methylphenethylamine, the latter being an isomer of amphetamine produced within the human body. It is available as a generic medication.[31] In 2021, it was the 17th most commonly prescribed medication in the United States, with more than 30.3 million prescriptions.[32][33]
Uses[edit]
Medical[edit]
Dextroamphetamine is used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy (a sleep disorder),[10] and is sometimes prescribed off-label for depression and obesity.[27] Long-term amphetamine exposure at sufficiently high doses in some animal species is known to produce abnormal dopamine system development or nerve damage,[34][35] but, in humans with ADHD, long-term use of pharmaceutical amphetamines at therapeutic doses appears to improve brain development and nerve growth.[36][37][38] Reviews of magnetic resonance imaging (MRI) studies suggest that long-term treatment with amphetamine decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleus of the basal ganglia.[36][37][38]
Reviews of clinical stimulant research have established the safety and effectiveness of long-term continuous amphetamine use for the treatment of ADHD.[39][40][41] Randomized controlled trials of continuous stimulant therapy for the treatment of ADHD spanning 2 years have demonstrated treatment effectiveness and safety.[39][40] Two reviews have indicated that long-term continuous stimulant therapy for ADHD is effective for reducing the core symptoms of ADHD (i.e., hyperactivity, inattention, and impulsivity), enhancing quality of life and academic achievement, and producing improvements in a large number of functional outcomes[note 2] across 9 categories of outcomes related to academics, antisocial behavior, driving, non-medicinal drug use, obesity, occupation, self-esteem, service use (i.e., academic, occupational, health, financial, and legal services), and social function.[39][41] One review highlighted a nine-month randomized controlled trial of amphetamine treatment for ADHD in children that found an average increase of 4.5 IQ points, continued increases in attention, and continued decreases in disruptive behaviors and hyperactivity.[40] Another review indicated that, based upon the longest follow-up studies conducted to date, lifetime stimulant therapy that begins during childhood is continuously effective for controlling ADHD symptoms and reduces the risk of developing a substance use disorder as an adult.[39]
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems;[42] these functional impairments involve impaired dopamine neurotransmission in the mesocorticolimbic projection and norepinephrine neurotransmission in the noradrenergic projections from the locus coeruleus to the prefrontal cortex.[42] Psychostimulants like methylphenidate and amphetamine are effective in treating ADHD because they increase neurotransmitter activity in these systems.[43][42][44] Approximately 80% of those who use these stimulants see improvements in ADHD symptoms.[45] Children with ADHD who use stimulant medications generally have better relationships with peers and family members, perform better in school, are less distractible and impulsive, and have longer attention spans.[46][47] The Cochrane reviews[note 3] on the treatment of ADHD in children, adolescents, and adults with pharmaceutical amphetamines stated that short-term studies have demonstrated that these drugs decrease the severity of symptoms, but they have higher discontinuation rates than non-stimulant medications due to their adverse side effects.[49][50] A Cochrane review on the treatment of ADHD in children with tic disorders such as Tourette syndrome indicated that stimulants in general do not make tics worse, but high doses of dextroamphetamine could exacerbate tics in some individuals.[51]
Enhancing performance[edit]
Cognitive performance[edit]
In 2015, a systematic review and a meta-analysis of high quality clinical trials found that, when used at low (therapeutic) doses, amphetamine produces modest yet unambiguous improvements in cognition, including working memory, long-term episodic memory, inhibitory control, and some aspects of attention, in normal healthy adults;[52][53] these cognition-enhancing effects of amphetamine are known to be partially mediated through the indirect activation of both dopamine receptor D1 and adrenoceptor α2 in the prefrontal cortex.[43][52] A systematic review from 2014 found that low doses of amphetamine also improve memory consolidation, in turn leading to improved recall of information.[54] Therapeutic doses of amphetamine also enhance cortical network efficiency, an effect which mediates improvements in working memory in all individuals.[43][55] Amphetamine and other ADHD stimulants also improve task saliency (motivation to perform a task) and increase arousal (wakefulness), in turn promoting goal-directed behavior.[43][56][57] Stimulants such as amphetamine can improve performance on difficult and boring tasks and are used by some students as a study and test-taking aid.[43][57][58] Based upon studies of self-reported illicit stimulant use, 5–35% of college students use diverted ADHD stimulants, which are primarily used for enhancement of academic performance rather than as recreational drugs.[59][60][61] However, high amphetamine doses that are above the therapeutic range can interfere with working memory and other aspects of cognitive control.[43][57]
Physical performance[edit]
Amphetamine is used by some athletes for its psychological and athletic performance-enhancing effects, such as increased endurance and alertness;[62][63] however, non-medical amphetamine use is prohibited at sporting events that are regulated by collegiate, national, and international anti-doping agencies.[64][65] In healthy people at oral therapeutic doses, amphetamine has been shown to increase muscle strength, acceleration, athletic performance in anaerobic conditions, and endurance (i.e., it delays the onset of fatigue), while improving reaction time.[62][66][67] Amphetamine improves endurance and reaction time primarily through reuptake inhibition and release of dopamine in the central nervous system.[66][67][68] Amphetamine and other dopaminergic drugs also increase power output at fixed levels of perceived exertion by overriding a "safety switch", allowing the core temperature limit to increase in order to access a reserve capacity that is normally off-limits.[67][69][70] At therapeutic doses, the adverse effects of amphetamine do not impede athletic performance;[62][66] however, at much higher doses, amphetamine can induce effects that severely impair performance, such as rapid muscle breakdown and elevated body temperature.[71][66]
Recreational[edit]
Dextroamphetamine is also used recreationally as a euphoriant and aphrodisiac, and like other amphetamines is used as a club drug for its energetic and euphoric high. Dextroamphetamine is considered to have a high potential for misuse in a recreational manner since individuals typically report feeling euphoric, more alert, and more energetic after taking the drug.[72][73][74] Dextroamphetamine's dopaminergic (rewarding) properties affect the mesocorticolimbic circuit; a group of neural structures responsible for incentive salience (i.e., "wanting"; desire or craving for a reward and motivation), positive reinforcement and positively-valenced emotions, particularly ones involving pleasure.[75] Large recreational doses of dextroamphetamine may produce symptoms of dextroamphetamine overdose.[74] Recreational users sometimes open dexedrine capsules and crush the contents in order to insufflate (snort) it or subsequently dissolve it in water and inject it.[74] Immediate-release formulations have higher potential for abuse via insufflation (snorting) or intravenous injection due to a more favorable pharmacokinetic profile and easy crushability (especially tablets).[76][77]
The reason for using crushed spansules for insufflation and injection methods is evidently due to the "instant-release" forms of the drug seen in tablet preparations often containing a sizable amount of inactive binders and fillers alongside the active d-amphetamine, such as dextrose.[78] Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[74] Chronic overuse of dextroamphetamine can lead to severe drug dependence, resulting in withdrawal symptoms when drug use stops.[74]
Contraindications[edit]
According to the International Programme on Chemical Safety (IPCS) and the United States Food and Drug Administration (USFDA),[note 4] amphetamine is contraindicated in people with a history of drug abuse,[note 5] cardiovascular disease, severe agitation, or severe anxiety.[80][71][81] It is also contraindicated in individuals with advanced arteriosclerosis (hardening of the arteries), glaucoma (increased eye pressure), hyperthyroidism (excessive production of thyroid hormone), or moderate to severe hypertension.[80][71][81] These agencies indicate that people who have experienced allergic reactions to other stimulants or who are taking monoamine oxidase inhibitors (MAOIs) should not take amphetamine,[80][71][81] although safe concurrent use of amphetamine and monoamine oxidase inhibitors has been documented.[82][83] These agencies also state that anyone with anorexia nervosa, bipolar disorder, depression, hypertension, liver or kidney problems, mania, psychosis, Raynaud's phenomenon, seizures, thyroid problems, tics, or Tourette syndrome should monitor their symptoms while taking amphetamine.[71][81] Evidence from human studies indicates that therapeutic amphetamine use does not cause developmental abnormalities in the fetus or newborns (i.e., it is not a human teratogen), but amphetamine abuse does pose risks to the fetus.[81] Amphetamine has also been shown to pass into breast milk, so the IPCS and the USFDA advise mothers to avoid breastfeeding when using it.[71][81] Due to the potential for reversible growth impairments,[note 6] the USFDA advises monitoring the height and weight of children and adolescents prescribed an amphetamine pharmaceutical.[71]
Adverse effects[edit]
Physical[edit]
Cardiovascular side effects can include hypertension or hypotension from a vasovagal response, Raynaud's phenomenon (reduced blood flow to the hands and feet), and tachycardia (increased heart rate).[71][63][84] Sexual side effects in males may include erectile dysfunction, frequent erections, or prolonged erections.[71] Gastrointestinal side effects may include abdominal pain, constipation, diarrhea, and nausea.[5][71][85] Other potential physical side effects include appetite loss, blurred vision, dry mouth, excessive grinding of the teeth, nosebleed, profuse sweating, rhinitis medicamentosa (drug-induced nasal congestion), reduced seizure threshold, tics (a type of movement disorder), and weight loss.[sources 1] Dangerous physical side effects are rare at typical pharmaceutical doses.[63]
Amphetamine stimulates the medullary respiratory centers, producing faster and deeper breaths.[63] In a normal person at therapeutic doses, this effect is usually not noticeable, but when respiration is already compromised, it may be evident.[63] Amphetamine also induces contraction in the urinary bladder sphincter, the muscle which controls urination, which can result in difficulty urinating.[63] This effect can be useful in treating bed wetting and loss of bladder control.[63] The effects of amphetamine on the gastrointestinal tract are unpredictable.[63] If intestinal activity is high, amphetamine may reduce gastrointestinal motility (the rate at which content moves through the digestive system);[63] however, amphetamine may increase motility when the smooth muscle of the tract is relaxed.[63] Amphetamine also has a slight analgesic effect and can enhance the pain relieving effects of opioids.[5][63]
USFDA-commissioned studies from 2011 indicate that in children, young adults, and adults there is no association between serious adverse cardiovascular events (sudden death, heart attack, and stroke) and the medical use of amphetamine or other ADHD stimulants.[sources 2] However, amphetamine pharmaceuticals are contraindicated in individuals with cardiovascular disease.[sources 3]
Psychological[edit]
At normal therapeutic doses, the most common psychological side effects of amphetamine include increased alertness, apprehension, concentration, initiative, self-confidence and sociability, mood swings (elated mood followed by mildly depressed mood), insomnia or wakefulness, and decreased sense of fatigue.[71][63] Less common side effects include anxiety, change in libido, grandiosity, irritability, repetitive or obsessive behaviors, and restlessness;[sources 4] these effects depend on the user's personality and current mental state.[63] Amphetamine psychosis (e.g., delusions and paranoia) can occur in heavy users.[71][93][94] Although very rare, this psychosis can also occur at therapeutic doses during long-term therapy.[71][94][95] According to the USFDA, "there is no systematic evidence" that stimulants produce aggressive behavior or hostility.[71]
Amphetamine has also been shown to produce a conditioned place preference in humans taking therapeutic doses,[49][96] meaning that individuals acquire a preference for spending time in places where they have previously used amphetamine.[96][97]
Reinforcement disorders[edit]
Addiction[edit]
| Addiction and dependence glossary[97][98][99] | |
|---|---|
| |
| Transcription factor glossary | |
|---|---|
| |
Addiction is a serious risk with heavy recreational amphetamine use, but is unlikely to occur from long-term medical use at therapeutic doses;[39][107][108] in fact, lifetime stimulant therapy for ADHD that begins during childhood reduces the risk of developing substance use disorders as an adult.[39] Pathological overactivation of the mesolimbic pathway, a dopamine pathway that connects the ventral tegmental area to the nucleus accumbens, plays a central role in amphetamine addiction.[109][110] Individuals who frequently self-administer high doses of amphetamine have a high risk of developing an amphetamine addiction, since chronic use at high doses gradually increases the level of accumbal ΔFosB, a "molecular switch" and "master control protein" for addiction.[98][111][112] Once nucleus accumbens ΔFosB is sufficiently overexpressed, it begins to increase the severity of addictive behavior (i.e., compulsive drug-seeking) with further increases in its expression.[111][113] While there are currently no effective drugs for treating amphetamine addiction, regularly engaging in sustained aerobic exercise appears to reduce the risk of developing such an addiction.[114][115] Exercise therapy improves clinical treatment outcomes and may be used as an adjunct therapy with behavioral therapies for addiction.[114][116][sources 5]
Biomolecular mechanisms[edit]
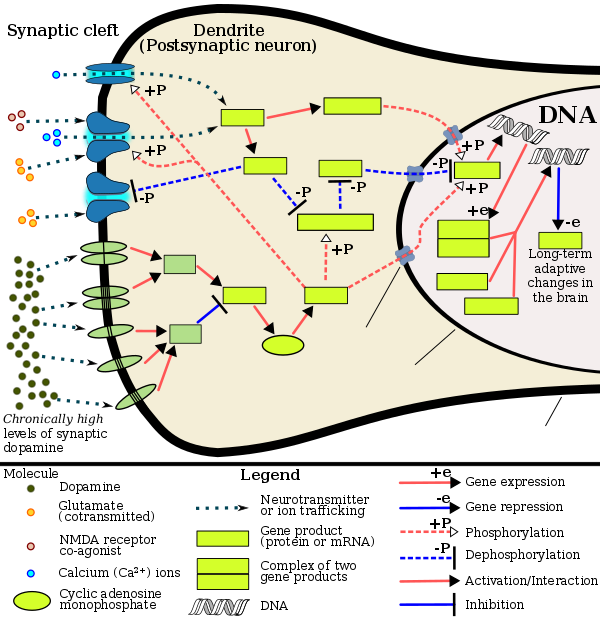
Chronic use of amphetamine at excessive doses causes alterations in gene expression in the mesocorticolimbic projection, which arise through transcriptional and epigenetic mechanisms.[112][117][118] The most important transcription factors[note 7] that produce these alterations are Delta FBJ murine osteosarcoma viral oncogene homolog B (ΔFosB), cAMP response element binding protein (CREB), and nuclear factor-kappa B (NF-κB).[112] ΔFosB is the most significant biomolecular mechanism in addiction because ΔFosB overexpression (i.e., an abnormally high level of gene expression which produces a pronounced gene-related phenotype) in the D1-type medium spiny neurons in the nucleus accumbens is necessary and sufficient[note 8] for many of the neural adaptations and regulates multiple behavioral effects (e.g., reward sensitization and escalating drug self-administration) involved in addiction.[98][111][112] Once ΔFosB is sufficiently overexpressed, it induces an addictive state that becomes increasingly more severe with further increases in ΔFosB expression.[98][111] It has been implicated in addictions to alcohol, cannabinoids, cocaine, methylphenidate, nicotine, opioids, phencyclidine, propofol, and substituted amphetamines, among others.[sources 6]
ΔJunD, a transcription factor, and G9a, a histone methyltransferase enzyme, both oppose the function of ΔFosB and inhibit increases in its expression.[98][112][122] Sufficiently overexpressing ΔJunD in the nucleus accumbens with viral vectors can completely block many of the neural and behavioral alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[112] Similarly, accumbal G9a hyperexpression results in markedly increased histone 3 lysine residue 9 dimethylation (H3K9me2) and blocks the induction of ΔFosB-mediated neural and behavioral plasticity by chronic drug use,[sources 7] which occurs via H3K9me2-mediated repression of transcription factors for ΔFosB and H3K9me2-mediated repression of various ΔFosB transcriptional targets (e.g., CDK5).[112][122][123] ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[113][112][126] Since both natural rewards and addictive drugs induce the expression of ΔFosB (i.e., they cause the brain to produce more of it), chronic acquisition of these rewards can result in a similar pathological state of addiction.[113][112] Consequently, ΔFosB is the most significant factor involved in both amphetamine addiction and amphetamine-induced sexual addictions, which are compulsive sexual behaviors that result from excessive sexual activity and amphetamine use.[113][127][128] These sexual addictions are associated with a dopamine dysregulation syndrome which occurs in some patients taking dopaminergic drugs.[113][126]
The effects of amphetamine on gene regulation are both dose- and route-dependent.[118] Most of the research on gene regulation and addiction is based upon animal studies with intravenous amphetamine administration at very high doses.[118] The few studies that have used equivalent (weight-adjusted) human therapeutic doses and oral administration show that these changes, if they occur, are relatively minor.[118] This suggests that medical use of amphetamine does not significantly affect gene regulation.[118]
Pharmacological treatments[edit]
As of December 2019,[update] there is no effective pharmacotherapy for amphetamine addiction.[129][130][131] Reviews from 2015 and 2016 indicated that TAAR1-selective agonists have significant therapeutic potential as a treatment for psychostimulant addictions;[132][133] however, as of February 2016,[update] the only compounds which are known to function as TAAR1-selective agonists are experimental drugs.[132][133] Amphetamine addiction is largely mediated through increased activation of dopamine receptors and co-localized NMDA receptors[note 9] in the nucleus accumbens;[110] magnesium ions inhibit NMDA receptors by blocking the receptor calcium channel.[110][134] One review suggested that, based upon animal testing, pathological (addiction-inducing) psychostimulant use significantly reduces the level of intracellular magnesium throughout the brain.[110] Supplemental magnesium[note 10] treatment has been shown to reduce amphetamine self-administration (i.e., doses given to oneself) in humans, but it is not an effective monotherapy for amphetamine addiction.[110]
A systematic review and meta-analysis from 2019 assessed the efficacy of 17 different pharmacotherapies used in randomized controlled trials (RCTs) for amphetamine and methamphetamine addiction;[130] it found only low-strength evidence that methylphenidate might reduce amphetamine or methamphetamine self-administration.[130] There was low- to moderate-strength evidence of no benefit for most of the other medications used in RCTs, which included antidepressants (bupropion, mirtazapine, sertraline), antipsychotics (aripiprazole), anticonvulsants (topiramate, baclofen, gabapentin), naltrexone, varenicline, citicoline, ondansetron, prometa, riluzole, atomoxetine, dextroamphetamine, and modafinil.[130]
Behavioral treatments[edit]
A 2018 systematic review and network meta-analysis of 50 trials involving 12 different psychosocial interventions for amphetamine, methamphetamine, or cocaine addiction found that combination therapy with both contingency management and community reinforcement approach had the highest efficacy (i.e., abstinence rate) and acceptability (i.e., lowest dropout rate).[135] Other treatment modalities examined in the analysis included monotherapy with contingency management or community reinforcement approach, cognitive behavioral therapy, 12-step programs, non-contingent reward-based therapies, psychodynamic therapy, and other combination therapies involving these.[135]
Additionally, research on the neurobiological effects of physical exercise suggests that daily aerobic exercise, especially endurance exercise (e.g., marathon running), prevents the development of drug addiction and is an effective adjunct therapy (i.e., a supplemental treatment) for amphetamine addiction.[sources 5] Exercise leads to better treatment outcomes when used as an adjunct treatment, particularly for psychostimulant addictions.[114][116][136] In particular, aerobic exercise decreases psychostimulant self-administration, reduces the reinstatement (i.e., relapse) of drug-seeking, and induces increased dopamine receptor D2 (DRD2) density in the striatum.[113][136] This is the opposite of pathological stimulant use, which induces decreased striatal DRD2 density.[113] One review noted that exercise may also prevent the development of a drug addiction by altering ΔFosB or c-Fos immunoreactivity in the striatum or other parts of the reward system.[115]
| Form of neuroplasticity or behavioral plasticity |
Type of reinforcer | Sources | |||||
|---|---|---|---|---|---|---|---|
| Opiates | Psychostimulants | High fat or sugar food | Sexual intercourse | Physical exercise (aerobic) |
Environmental enrichment | ||
| ΔFosB expression in nucleus accumbens D1-type MSNs |
↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [113] |
| Behavioral plasticity | |||||||
| Escalation of intake | Yes | Yes | Yes | [113] | |||
| Psychostimulant cross-sensitization |
Yes | Not applicable | Yes | Yes | Attenuated | Attenuated | [113] |
| Psychostimulant self-administration |
↑ | ↑ | ↓ | ↓ | ↓ | [113] | |
| Psychostimulant conditioned place preference |
↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [113] |
| Reinstatement of drug-seeking behavior | ↑ | ↑ | ↓ | ↓ | [113] | ||
| Neurochemical plasticity | |||||||
| CREB phosphorylation in the nucleus accumbens |
↓ | ↓ | ↓ | ↓ | ↓ | [113] | |
| Sensitized dopamine response in the nucleus accumbens |
No | Yes | No | Yes | [113] | ||
| Altered striatal dopamine signaling | ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [113] | |
| Altered striatal opioid signaling | No change or ↑μ-opioid receptors |
↑μ-opioid receptors ↑κ-opioid receptors |
↑μ-opioid receptors | ↑μ-opioid receptors | No change | No change | [113] |
| Changes in striatal opioid peptides | ↑dynorphin No change: enkephalin |
↑dynorphin | ↓enkephalin | ↑dynorphin | ↑dynorphin | [113] | |
| Mesocorticolimbic synaptic plasticity | |||||||
| Number of dendrites in the nucleus accumbens | ↓ | ↑ | ↑ | [113] | |||
| Dendritic spine density in the nucleus accumbens |
↓ | ↑ | ↑ | [113] | |||
Dependence and withdrawal[edit]
Drug tolerance develops rapidly in amphetamine abuse (i.e., recreational amphetamine use), so periods of extended abuse require increasingly larger doses of the drug in order to achieve the same effect.[137][138] According to a Cochrane review on withdrawal in individuals who compulsively use amphetamine and methamphetamine, "when chronic heavy users abruptly discontinue amphetamine use, many report a time-limited withdrawal syndrome that occurs within 24 hours of their last dose."[139] This review noted that withdrawal symptoms in chronic, high-dose users are frequent, occurring in roughly 88% of cases, and persist for 3–4 weeks with a marked "crash" phase occurring during the first week.[139] Amphetamine withdrawal symptoms can include anxiety, drug craving, depressed mood, fatigue, increased appetite, increased movement or decreased movement, lack of motivation, sleeplessness or sleepiness, and lucid dreams.[139] The review indicated that the severity of withdrawal symptoms is positively correlated with the age of the individual and the extent of their dependence.[139] Mild withdrawal symptoms from the discontinuation of amphetamine treatment at therapeutic doses can be avoided by tapering the dose.[5]
Overdose[edit]
An amphetamine overdose can lead to many different symptoms, but is rarely fatal with appropriate care.[5][81][140] The severity of overdose symptoms increases with dosage and decreases with drug tolerance to amphetamine.[63][81] Tolerant individuals have been known to take as much as 5 grams of amphetamine in a day, which is roughly 100 times the maximum daily therapeutic dose.[81] Symptoms of a moderate and extremely large overdose are listed below; fatal amphetamine poisoning usually also involves convulsions and coma.[71][63] In 2013, overdose on amphetamine, methamphetamine, and other compounds implicated in an "amphetamine use disorder" resulted in an estimated 3,788 deaths worldwide (3,425–4,145 deaths, 95% confidence).[note 11][141]
| System | Minor or moderate overdose[71][63][81] | Severe overdose[sources 8] |
|---|---|---|
| Cardiovascular |
| |
| Central nervous system |
|
|
| Musculoskeletal |
| |
| Respiratory |
|
|
| Urinary |
|
|
| Other |
|
|
Toxicity[edit]
In rodents and primates, sufficiently high doses of amphetamine cause dopaminergic neurotoxicity, or damage to dopamine neurons, which is characterized by dopamine terminal degeneration and reduced transporter and receptor function.[144][145] There is no evidence that amphetamine is directly neurotoxic in humans.[146][147] However, large doses of amphetamine may indirectly cause dopaminergic neurotoxicity as a result of hyperpyrexia, the excessive formation of reactive oxygen species, and increased autoxidation of dopamine.[sources 9] Animal models of neurotoxicity from high-dose amphetamine exposure indicate that the occurrence of hyperpyrexia (i.e., core body temperature ≥ 40 °C) is necessary for the development of amphetamine-induced neurotoxicity.[145] Prolonged elevations of brain temperature above 40 °C likely promote the development of amphetamine-induced neurotoxicity in laboratory animals by facilitating the production of reactive oxygen species, disrupting cellular protein function, and transiently increasing blood–brain barrier permeability.[145]
Psychosis[edit]
An amphetamine overdose can result in a stimulant psychosis that may involve a variety of symptoms, such as delusions and paranoia.[93][94] A Cochrane review on treatment for amphetamine, dextroamphetamine, and methamphetamine psychosis states that about 5–15% of users fail to recover completely.[93][150] According to the same review, there is at least one trial that shows antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[93] Psychosis rarely arises from therapeutic use.[71][94][95]
Interactions[edit]
Many types of substances are known to interact with amphetamine, resulting in altered drug action or metabolism of amphetamine, the interacting substance, or both.[19][151][152] Inhibitors of the enzymes that metabolize amphetamine (e.g., CYP2D6 and FMO3) will prolong its elimination half-life, meaning that its effects will last longer.[26][151][152] Amphetamine also interacts with MAOIs, particularly monoamine oxidase A inhibitors, since both MAOIs and amphetamine increase plasma catecholamines (i.e., norepinephrine and dopamine);[151][152] therefore, concurrent use of both is dangerous.[151][152] Amphetamine modulates the activity of most psychoactive drugs. In particular, amphetamine may decrease the effects of sedatives and depressants and increase the effects of stimulants and antidepressants.[151][152] Amphetamine may also decrease the effects of antihypertensives and antipsychotics due to its effects on blood pressure and dopamine respectively.[151][152] Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 12][156]
Pharmacology[edit]
Pharmacodynamics[edit]
Pharmacodynamics of amphetamine in a dopamine neuron
|
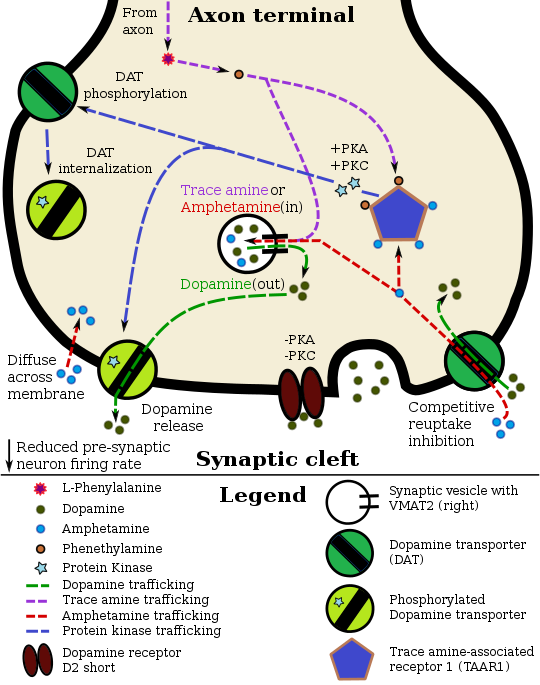
Amphetamine and its enantiomers have been identified as potent full agonists of trace amine-associated receptor 1 (TAAR1), a GPCR, discovered in 2001, that is important for regulation of monoaminergic systems in the brain.[162][163] Activation of TAAR1 increases cAMP production via adenylyl cyclase activation and inhibits the function of the dopamine transporter, norepinephrine transporter, and serotonin transporter, as well as inducing the release of these monoamine neurotransmitters (effluxion).[29][162][164] Amphetamine enantiomers are also substrates for a specific neuronal synaptic vesicle uptake transporter called VMAT2.[30] When amphetamine is taken up by VMAT2, the vesicle releases (effluxes) dopamine, norepinephrine, and serotonin, among other monoamines, into the cytosol in exchange.[30]
Dextroamphetamine (the dextrorotary enantiomer) and levoamphetamine (the levorotary enantiomer) have identical pharmacodynamics, but their binding affinities to their biomolecular targets vary.[163][165] Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[163] Consequently, dextroamphetamine produces roughly three to four times more central nervous system (CNS) stimulation than levoamphetamine;[163][165] however, levoamphetamine has slightly greater cardiovascular and peripheral effects.[165]
Related endogenous compounds[edit]
Amphetamine has a very similar structure and function to the endogenous trace amines, which are naturally occurring neuromodulator molecules produced in the human body and brain.[29][166][167] Among this group, the most closely related compounds are phenethylamine, the parent compound of amphetamine, and N-methylphenethylamine, a structural isomer of amphetamine (i.e., it has an identical molecular formula).[29][166][168] In humans, phenethylamine is produced directly from L-phenylalanine by the aromatic amino acid decarboxylase (AADC) enzyme, which converts L-DOPA into dopamine as well.[166][168] In turn, N-methylphenethylamine is metabolized from phenethylamine by phenylethanolamine N-methyltransferase, the same enzyme that metabolizes norepinephrine into epinephrine.[166][168] Like amphetamine, both phenethylamine and N-methylphenethylamine regulate monoamine neurotransmission via TAAR1;[29][167][168] unlike amphetamine, both of these substances are broken down by monoamine oxidase B, and therefore have a shorter half-life than amphetamine.[166][168]
Pharmacokinetics[edit]
The oral bioavailability of amphetamine varies with gastrointestinal pH;[71] it is well absorbed from the gut, and bioavailability is typically 90%.[13] Amphetamine is a weak base with a pKa of 9.9;[19] consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium.[19][71] Conversely, an acidic pH means the drug is predominantly in a water-soluble cationic (salt) form, and less is absorbed.[19] Approximately 20% of amphetamine circulating in the bloodstream is bound to plasma proteins.[14] Following absorption, amphetamine readily distributes into most tissues in the body, with high concentrations occurring in cerebrospinal fluid and brain tissue.[21]
The half-lives of amphetamine enantiomers differ and vary with urine pH.[19] At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively.[19] Highly acidic urine will reduce the enantiomer half-lives to 7 hours;[21] highly alkaline urine will increase the half-lives up to 34 hours.[21] The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively.[19] Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH.[19] When the urinary pH is basic, amphetamine is in its free base form, so less is excreted.[19] When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively.[19] Following oral administration, amphetamine appears in urine within 3 hours.[21] Roughly 90% of ingested amphetamine is eliminated 3 days after the last oral dose.[21]
CYP2D6, dopamine β-hydroxylase (DBH), flavin-containing monooxygenase 3 (FMO3), butyrate-CoA ligase (XM-ligase), and glycine N-acyltransferase (GLYAT) are the enzymes known to metabolize amphetamine or its metabolites in humans.[sources 10] Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone.[19][169] Among these metabolites, the active sympathomimetics are 4-hydroxyamphetamine,[170] 4-hydroxynorephedrine,[171] and norephedrine.[172] The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination.[19][173] The known metabolic pathways, detectable metabolites, and metabolizing enzymes in humans include the following:
Metabolic pathways of amphetamine in humans[sources 10]
|
History, society, and culture[edit]
Racemic amphetamine was first synthesized under the chemical name "phenylisopropylamine" in Berlin, 1887 by the Romanian chemist Lazăr Edeleanu. It was not widely marketed until 1932, when the pharmaceutical company Smith, Kline & French (now known as GlaxoSmithKline) introduced it in the form of the Benzedrine inhaler for use as a bronchodilator. Notably, the amphetamine contained in the Benzedrine inhaler was the liquid free-base,[note 14] not a chloride or sulfate salt.
Three years later, in 1935, the medical community became aware of the stimulant properties of amphetamine, specifically the dextroamphetamine isomer, and in 1937 Smith, Kline, and French introduced tablets under the brand name Dexedrine.[182] In the United States, Dexedrine was approved to treat narcolepsy and attention disorders.[10] In Canada indications once included epilepsy and parkinsonism.[183] Dextroamphetamine was marketed in various other forms in the following decades, primarily by Smith, Kline, and French, such as several combination medications including a mixture of dextroamphetamine and amobarbital (a barbiturate) sold under the tradename Dexamyl and, in the 1950s, an extended release capsule (the "Spansule").[184] Preparations containing dextroamphetamine were also used in World War II as a treatment against fatigue.[185]
It quickly became apparent that dextroamphetamine and other amphetamines had a high potential for misuse, although they were not heavily controlled until 1970, when the Comprehensive Drug Abuse Prevention and Control Act was passed by the United States Congress. Dextroamphetamine, along with other sympathomimetics, was eventually classified as Schedule II, the most restrictive category possible for a drug with a government-sanctioned, recognized medical use.[186] Internationally, it has been available under the names AmfeDyn (Italy), Curban (US), Obetrol (Switzerland), Simpamina (Italy), Dexedrine/GSK (US & Canada), Dexedrine/UCB (United Kingdom), Dextropa (Portugal), and Stild (Spain).[187] It became popular on the mod scene in England in the early 1960s, and carried through to the Northern Soul scene in the north of England to the end of the 1970s.
In October 2010, GlaxoSmithKline sold the rights for Dexedrine Spansule to Amedra Pharmaceuticals (a subsidiary of CorePharma).[188]
The U.S. Air Force uses dextroamphetamine as one of its "go pills", given to pilots on long missions to help them remain focused and alert. Conversely, "no-go pills" are used after the mission is completed, to combat the effects of the mission and "go-pills".[189][190][191] The Tarnak Farm incident was linked by media reports to the use of this drug on long term fatigued pilots. The military did not accept this explanation, citing the lack of similar incidents. Newer stimulant medications or awakeness promoting agents with different side effect profiles, such as modafinil, are being investigated and sometimes issued for this reason.[190]
Formulations[edit]
| Brand name |
United States Adopted Name |
(D:L) ratio | Dosage form |
Marketing start date |
Sources |
|---|---|---|---|---|---|
| Adderall | Mixed amphetamine salts | 3:1 (salts) | tablet | 1996 | [27][200] |
| Adderall XR | Mixed amphetamine salts | 3:1 (salts) | capsule | 2001 | [27][200] |
| Mydayis | Mixed amphetamine salts | 3:1 (salts) | capsule | 2017 | [201] |
| Adzenys XR-ODT | amphetamine | 3:1 (base) | ODT | 2016 | [202][203] |
| Dyanavel XR | amphetamine | 3.2:1 (base) | suspension | 2015 | [85][204] |
| Evekeo | amphetamine sulfate | 1:1 (salts) | tablet | 2012 | [80] [205] |
| Dexedrine | dextroamphetamine sulfate | 1:0 (salts) | capsule | 1976 | [27][200] |
| Zenzedi | dextroamphetamine sulfate | 1:0 (salts) | tablet | 2013 | [200] |
| Vyvanse | lisdexamfetamine dimesylate | 1:0 (prodrug) | capsule | 2007 | [27][206] |
| tablet | |||||
| Xelstrym | dextroamphetamine | 1:0 (base) | patch | 2022 | [11] |
Transdermal Dextroamphetamine Patches[edit]
Dextroamphetamine is available as a transdermal patch containing dextroamphetamine base under the brand name Xelstrym.[11]
Dextroamphetamine sulfate[edit]
In the United States, immediate release (IR) formulations of dextroamphetamine sulfate are available generically as 5 mg and 10 mg tablets, marketed by Barr (Teva Pharmaceutical Industries), Mallinckrodt Pharmaceuticals, Wilshire Pharmaceuticals, Aurobindo Pharmaceutical USA and CorePharma. Previous IR tablets sold under the brand names Dexedrine and Dextrostat have been discontinued but in 2015, IR tablets became available by the brand name Zenzedi, offered as 2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg and 30 mg tablets.[207] Dextroamphetamine sulfate is also available as a controlled-release (CR) capsule preparation in strengths of 5 mg, 10 mg, and 15 mg under the brand name Dexedrine Spansule, with generic versions marketed by Barr and Mallinckrodt. A bubblegum flavored oral solution is available under the brand name ProCentra, manufactured by FSC Pediatrics, which is designed to be an easier method of administration in children who have difficulty swallowing tablets, each 5 mL contains 5 mg dextroamphetamine.[208] The conversion rate between dextroamphetamine sulfate to amphetamine free base is .728.[209]
In Australia, dexamfetamine is available in bottles of 100 instant release 5 mg tablets as a generic drug[210] or slow release dextroamphetamine preparations may be compounded by individual chemists.[211] In the United Kingdom, it is available in 5 mg instant release sulfate tablets under the generic name dexamfetamine sulfate as well as 10 mg and 20 mg strength tablets under the brand name Amfexa. It is also available in generic dexamfetamine sulfate 5 mg/ml oral sugar-free syrup.[212] The brand name Dexedrine was available in the United Kingdom prior to UCB Pharma disinvesting the product to another pharmaceutical company (Auden Mckenzie).[213]
Lisdexamfetamine[edit]
Dextroamphetamine is the active metabolite of the prodrug lisdexamfetamine (L-lysine-dextroamphetamine), available by the brand name Vyvanse (Elvanse in the European market) (Venvanse in the Brazil market) (lisdexamfetamine dimesylate). Dextroamphetamine is liberated from lisdexamfetamine enzymatically following contact with red blood cells. The conversion is rate-limited by the enzyme, which prevents high blood concentrations of dextroamphetamine and reduces lisdexamfetamine's drug liking and abuse potential at clinical doses.[214][215] Vyvanse is marketed as once-a-day dosing as it provides a slow release of dextroamphetamine into the body. Vyvanse is available as capsules, and chewable tablets, and in seven strengths; 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, and 70 mg. The conversion rate between lisdexamfetamine dimesylate (Vyvanse) to dextroamphetamine base is 29.5%.[216][217][218]
Adderall[edit]

Another pharmaceutical that contains dextroamphetamine is commonly known by the brand name Adderall.[151][152] It is available as immediate release (IR) tablets and extended release (XR) capsules.[151][152] Adderall contains equal amounts of four amphetamine salts:[151][152]
- One-quarter racemic (d,l-)amphetamine aspartate monohydrate
- One-quarter dextroamphetamine saccharate
- One-quarter dextroamphetamine sulfate
- One-quarter racemic (d,l-)amphetamine sulfate
Adderall has a total amphetamine base equivalence of 63%.[151][152] While the enantiomer ratio by dextroamphetamine salts to levoamphetamine salts is 3:1, the amphetamine base content is 75.9% dextroamphetamine, 24.1% levoamphetamine. [note 16]
| drug | formula | molar mass [note 17] |
amphetamine base [note 18] |
amphetamine base in equal doses |
doses with equal base content [note 19] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[220][221] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
73.38%
|
—
|
22.0 mg
|
—
|
30.0 mg
| |
| amphetamine sulfate[222] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
36.69%
|
36.69%
|
11.0 mg
|
11.0 mg
|
30.0 mg
| |
| Adderall | 62.57%
|
47.49%
|
15.08%
|
14.2 mg
|
4.5 mg
|
35.2 mg
| ||||
| 25% | dextroamphetamine sulfate[220][221] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
73.38%
|
—
|
|||
| 25% | amphetamine sulfate[222] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
36.69%
|
36.69%
|
|||
| 25% | dextroamphetamine saccharate[223] | (C9H13N)2•C6H10O8 | 480.55
|
270.41
|
56.27%
|
56.27%
|
—
|
|||
| 25% | amphetamine aspartate monohydrate[224] | (C9H13N)•C4H7NO4•H2O | 286.32
|
135.21
|
47.22%
|
23.61%
|
23.61%
|
|||
| lisdexamfetamine dimesylate[206] | C15H25N3O•(CH4O3S)2 | 455.49
|
135.21
|
29.68%
|
29.68%
|
—
|
8.9 mg
|
—
|
74.2 mg
| |
| amphetamine base suspension[85] | C9H13N | 135.21
|
135.21
|
100%
|
76.19%
|
23.81%
|
22.9 mg
|
7.1 mg
|
22.0 mg
| |
Notes[edit]
- ^ a b Enantiomers are molecules that are mirror images of one another; they are structurally identical, but of the opposite orientation.[28]
- ^ The ADHD-related outcome domains with the greatest proportion of significantly improved outcomes from long-term continuous stimulant therapy include academics (≈55% of academic outcomes improved), driving (100% of driving outcomes improved), non-medical drug use (47% of addiction-related outcomes improved), obesity (≈65% of obesity-related outcomes improved), self-esteem (50% of self-esteem outcomes improved), and social function (67% of social function outcomes improved).[41]
The largest effect sizes for outcome improvements from long-term stimulant therapy occur in the domains involving academics (e.g., grade point average, achievement test scores, length of education, and education level), self-esteem (e.g., self-esteem questionnaire assessments, number of suicide attempts, and suicide rates), and social function (e.g., peer nomination scores, social skills, and quality of peer, family, and romantic relationships).[41]
Long-term combination therapy for ADHD (i.e., treatment with both a stimulant and behavioral therapy) produces even larger effect sizes for outcome improvements and improves a larger proportion of outcomes across each domain compared to long-term stimulant therapy alone.[41] - ^ Cochrane reviews are high quality meta-analytic systematic reviews of randomized controlled trials.[48]
- ^ The statements supported by the USFDA come from prescribing information, which is the copyrighted intellectual property of the manufacturer and approved by the USFDA. USFDA contraindications are not necessarily intended to limit medical practice but limit claims by pharmaceutical companies.[79]
- ^ According to one review, amphetamine can be prescribed to individuals with a history of abuse provided that appropriate medication controls are employed, such as requiring daily pick-ups of the medication from the prescribing physician.[27]
- ^ In individuals who experience sub-normal height and weight gains, a rebound to normal levels is expected to occur if stimulant therapy is briefly interrupted.[39][40][84] The average reduction in final adult height from 3 years of continuous stimulant therapy is 2 cm.[84]
- ^ Transcription factors are proteins that increase or decrease the expression of specific genes.[119]
- ^ In simpler terms, this necessary and sufficient relationship means that ΔFosB overexpression in the nucleus accumbens and addiction-related behavioral and neural adaptations always occur together and never occur alone.
- ^ NMDA receptors are voltage-dependent ligand-gated ion channels that requires simultaneous binding of glutamate and a co-agonist (D-serine or glycine) to open the ion channel.[134]
- ^ The review indicated that magnesium L-aspartate and magnesium chloride produce significant changes in addictive behavior;[110] other forms of magnesium were not mentioned.
- ^ The 95% confidence interval indicates that there is a 95% probability that the true number of deaths lies between 3,425 and 4,145.
- ^ The human dopamine transporter contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[153][154][155] The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[155]
- ^ 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[174][177] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[177][179] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[180][181]
- ^ Free-base form amphetamine is a volatile oil, hence the efficacy of the inhalers.
- ^ These represent the current brands in the United States, except Dexedrine instant release tablets. Dexedrine tablets, introduced in 1937, is discontinued but available as Zenzedi and generically;[192][193] Dexedrine listed here represents the extended release "Spansule" capsule which was approved in 1976.[194][195] Amphetamine sulfate tablets, now sold as Evekeo (brand), were originally sold as Benzedrine (brand) sulfate in 1935[196][197] and discontinued sometime after 1982.[198][199]
- ^ Calculated by dextroamphetamine base percent / total amphetamine base percent = 47.49/62.57 = 75.90% from table: Amphetamine base in marketed amphetamine medications. The remainder is levoamphetamine.
- ^ For uniformity, molar masses were calculated using the Lenntech Molecular Weight Calculator[219] and were within 0.01 g/mol of published pharmaceutical values.
- ^ Amphetamine base percentage = molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- ^ dose = (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- Image legend
- ^ (Text color) Transcription factors
Reference notes[edit]
References[edit]
- ^ a b Vitiello B (April 2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child and Adolescent Psychiatric Clinics of North America. 17 (2): 459–74, xi. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ^ a b Graham J, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, et al. (January 2011). "European guidelines on managing adverse effects of medication for ADHD". European Child & Adolescent Psychiatry. 20 (1): 17–37. doi:10.1007/s00787-010-0140-6. eISSN 1435-165X. PMC 3012210. PMID 21042924.
- ^ a b Kociancic T, Reed MD, Findling RL (March 2004). "Evaluation of risks associated with short- and long-term psychostimulant therapy for treatment of ADHD in children". Expert Opinion on Drug Safety. 3 (2): 93–100. doi:10.1517/14740338.3.2.93. eISSN 1744-764X. PMID 15006715. S2CID 31114829.
- ^ a b Clemow DB, Walker DJ (September 2014). "The potential for misuse and abuse of medications in ADHD: a review". Postgraduate Medicine. 126 (5): 64–81. doi:10.3810/pgm.2014.09.2801. eISSN 1941-9260. PMID 25295651. S2CID 207580823.
- ^ a b c d e f g Stahl SM (March 2017). "Amphetamine (D,L)". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. pp. 45–51. ISBN 9781108228749. Retrieved 5 August 2017.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Therapeutic Goods (Poisons Standard—February 2023) Instrument 2022". Australian Government Federal Register of Legislation. 26 September 2022. Retrieved 9 January 2023.
- ^ Fuller K (20 February 2022). "ADHD Stimulant Prescribing Regulations & Authorities in Australia & New Zealand". AADPA. Retrieved 9 January 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b c d "Dexedrine spansule- dextroamphetamine sulfate capsule, extended release". DailyMed. 10 January 2022. Retrieved 28 March 2022.
- ^ a b c "Xelstrym- dextroamphetamine patch, extended release". DailyMed. 6 January 2023. Retrieved 21 January 2023.
- ^ "List of nationally authorised medicinal products : Active substance(s): dexamfetamine : Procedure No. PSUSA/00000986/202109" (PDF). Ema.europa.eu. Retrieved 5 June 2022.
- ^ a b Patel VB, Preedy VR, eds. (2022). Handbook of Substance Misuse and Addictions. Cham: Springer International Publishing. p. 2006. doi:10.1007/978-3-030-92392-1. ISBN 978-3-030-92391-4.
Amphetamine is usually consumed via inhalation or orally, either in the form of a racemic mixture (levoamphetamine and dextroamphetamine) or dextroamphetamine alone (Childress et al. 2019). In general, all amphetamines have high bioavailability when consumed orally, and in the specific case of amphetamine, 90% of the consumed dose is absorbed in the gastrointestinal tract, with no significant differences in the rate and extent of absorption between the two enantiomers (Carvalho et al. 2012; Childress et al. 2019). The onset of action occurs approximately 30 to 45 minutes after consumption, depending on the ingested dose and on the degree of purity or on the concomitant consumption of certain foods (European Monitoring Centre for Drugs and Drug Addiction 2021a; Steingard et al. 2019). It is described that those substances that promote acidification of the gastrointestinal tract cause a decrease in amphetamine absorption, while gastrointestinal alkalinization may be related to an increase in the compound's absorption (Markowitz and Patrick 2017).
- ^ a b Wishart DS, Djombou Feunang Y, Guo AC, Lo EJ, Marcu A, Grant JR, et al. "Amphetamine | DrugBank Online". DrugBank. 5.0.
- ^ Green-Hernandez C, Singleton JK, Aronzon DZ (1 January 2001). Primary Care Pediatrics. Lippincott Williams & Wilkins. p. 243. ISBN 978-0-7817-2008-3.|quote = Table 21.2 Medications for ADHD ... D-amphetamine ... Onset: 30 min.
- ^ "Dexedrine, ProCentra(dextroamphetamine) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 4 October 2015.
Onset of action: 1–1.5 hr
- ^ a b c Millichap JG (2010). "Chapter 9: Medications for ADHD". In Millichap JG (ed.). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. p. 112. ISBN 978-1-4419-1396-8.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - ^ Brams M, Mao AR, Doyle RL (September 2008). "Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder". Postgrad. Med. 120 (3): 69–88. doi:10.3810/pgm.2008.09.1909. PMID 18824827. S2CID 31791162.
Onset of efficacy was earliest for d-MPH-ER at 0.5 hours, followed by d, l-MPH-LA at 1 to 2 hours, MCD at 1.5 hours, d, l-MPH-OR at 1 to 2 hours, MAS-XR at 1.5 to 2 hours, MTS at 2 hours, and LDX at approximately 2 hours. ... MAS-XR, and LDX have a long duration of action at 12 hours postdose
- ^ a b c d e f g h i j k l m n o p q "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ^ "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". DailyMed. 27 February 2022. Retrieved 21 January 2023.
- ^ a b c d e f "Metabolism/Pharmacokinetics". Amphetamine. Hazardous Substances Data Bank. United States National Library of Medicine – Toxicology Data Network. Archived from the original on 2 October 2017. Retrieved 2 October 2017.
Duration of effect varies depending on agent and urine pH. Excretion is enhanced in more acidic urine. Half-life is 7 to 34 hours and is, in part, dependent on urine pH (half-life is longer with alkaline urine). ... Amphetamines are distributed into most body tissues with high concentrations occurring in the brain and CSF. Amphetamine appears in the urine within about 3 hours following oral administration. ... Three days after a dose of (+ or -)-amphetamine, human subjects had excreted 91% of the (14)C in the urine
- ^ a b Mignot EJ (October 2012). "A practical guide to the therapy of narcolepsy and hypersomnia syndromes". Neurotherapeutics. 9 (4): 739–752. doi:10.1007/s13311-012-0150-9. PMC 3480574. PMID 23065655.
- ^ Stahl SM (March 2017). "Amphetamine (D)". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. pp. 39–44. ISBN 978-1-108-22874-9. Retrieved 8 August 2017.
- ^ "dextrostat (dextroamphetamine sulfate) tablet [Shire US Inc.]". DailyMed. Wayne, PA: Shire US Inc. August 2006. Retrieved 8 November 2013.
- ^ Lemke TL, Williams DA, Roche VF, Zito W (2013). Foye's Principles of Medicinal Chemistry (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 648. ISBN 978-1-60913-345-0.
Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ a b c Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ a b c d e f g Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomer". doi:10.1351/goldbook.E02069
- ^ a b c d e f g h i j Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ^ a b c d e Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Annals of the New York Academy of Sciences. 1216 (1): 86–98. Bibcode:2011NYASA1216...86E. doi:10.1111/j.1749-6632.2010.05906.x. PMC 4183197. PMID 21272013.
- ^ "Dextroamphetamine Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 3 February 2019. Retrieved 2 February 2019.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Dextroamphetamine; Dextroamphetamine Saccharate; Amphetamine; Amphetamine Aspartate - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ a b Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, et al. (August 2012). "Toxicity of amphetamines: an update". Archives of Toxicology. 86 (8): 1167–1231. doi:10.1007/s00204-012-0815-5. PMID 22392347. S2CID 2873101.
- ^ Berman S, O'Neill J, Fears S, Bartzokis G, London ED (October 2008). "Abuse of amphetamines and structural abnormalities in the brain". Annals of the New York Academy of Sciences. 1141 (1): 195–220. doi:10.1196/annals.1441.031. PMC 2769923. PMID 18991959.
- ^ a b Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- ^ a b Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, et al. (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". The Journal of Clinical Psychiatry. 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
- ^ a b Frodl T, Skokauskas N (February 2012). "Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects". Acta Psychiatrica Scandinavica. 125 (2): 114–126. doi:10.1111/j.1600-0447.2011.01786.x. PMID 22118249. S2CID 25954331.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- ^ a b c d e f g Huang YS, Tsai MH (July 2011). "Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge". CNS Drugs. 25 (7): 539–554. doi:10.2165/11589380-000000000-00000. PMID 21699268. S2CID 3449435.
Several other studies,[97-101] including a meta-analytic review[98] and a retrospective study,[97] suggested that stimulant therapy in childhood is associated with a reduced risk of subsequent substance use, cigarette smoking and alcohol use disorders. ... Recent studies have demonstrated that stimulants, along with the non-stimulants atomoxetine and extended-release guanfacine, are continuously effective for more than 2-year treatment periods with few and tolerable adverse effects. The effectiveness of long-term therapy includes not only the core symptoms of ADHD, but also improved quality of life and academic achievements. The most concerning short-term adverse effects of stimulants, such as elevated blood pressure and heart rate, waned in long-term follow-up studies. ... The current data do not support the potential impact of stimulants on the worsening or development of tics or substance abuse into adulthood. In the longest follow-up study (of more than 10 years), lifetime stimulant treatment for ADHD was effective and protective against the development of adverse psychiatric disorders.
- ^ a b c d Millichap JG (2010). "Chapter 9: Medications for ADHD". In Millichap JG (ed.). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, US: Springer. pp. 121–123, 125–127. ISBN 9781441913968.
Ongoing research has provided answers to many of the parents' concerns, and has confirmed the effectiveness and safety of the long-term use of medication.
- ^ a b c d e Arnold LE, Hodgkins P, Caci H, Kahle J, Young S (February 2015). "Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review". PLOS ONE. 10 (2): e0116407. doi:10.1371/journal.pone.0116407. PMC 4340791. PMID 25714373.
The highest proportion of improved outcomes was reported with combination treatment (83% of outcomes). Among significantly improved outcomes, the largest effect sizes were found for combination treatment. The greatest improvements were associated with academic, self-esteem, or social function outcomes.
Figure 3: Treatment benefit by treatment type and outcome group - ^ a b c Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, US: McGraw-Hill Medical. pp. 154–157. ISBN 9780071481274.
- ^ a b c d e f Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, US: McGraw-Hill Medical. pp. 318, 321. ISBN 9780071481274.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in normal subjects and those with ADHD. ... stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks ... through indirect stimulation of dopamine and norepinephrine receptors. ...
Beyond these general permissive effects, dopamine (acting via D1 receptors) and norepinephrine (acting at several receptors) can, at optimal levels, enhance working memory and aspects of attention. - ^ Bidwell LC, McClernon FJ, Kollins SH (August 2011). "Cognitive enhancers for the treatment of ADHD". Pharmacology Biochemistry and Behavior. 99 (2): 262–274. doi:10.1016/j.pbb.2011.05.002. PMC 3353150. PMID 21596055.
- ^ Parker J, Wales G, Chalhoub N, Harpin V (September 2013). "The long-term outcomes of interventions for the management of attention-deficit hyperactivity disorder in children and adolescents: a systematic review of randomized controlled trials". Psychology Research and Behavior Management. 6: 87–99. doi:10.2147/PRBM.S49114. PMC 3785407. PMID 24082796.
Only one paper53 examining outcomes beyond 36 months met the review criteria. ... There is high level evidence suggesting that pharmacological treatment can have a major beneficial effect on the core symptoms of ADHD (hyperactivity, inattention, and impulsivity) in approximately 80% of cases compared with placebo controls, in the short term.
- ^ Millichap JG (2010). "Chapter 9: Medications for ADHD". In Millichap JG (ed.). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, US: Springer. pp. 111–113. ISBN 9781441913968.
- ^ "Stimulants for Attention Deficit Hyperactivity Disorder". WebMD. Healthwise. 12 April 2010. Retrieved 12 November 2013.
- ^ Scholten RJ, Clarke M, Hetherington J (August 2005). "The Cochrane Collaboration". European Journal of Clinical Nutrition. 59 (Suppl 1): S147–S149, discussion S195–S196. doi:10.1038/sj.ejcn.1602188. PMID 16052183. S2CID 29410060.
- ^ a b Castells X, Blanco-Silvente L, Cunill R (August 2018). "Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults". Cochrane Database of Systematic Reviews. 2018 (8): CD007813. doi:10.1002/14651858.CD007813.pub3. PMC 6513464. PMID 30091808.
- ^ Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, et al. (February 2016). "Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents". Cochrane Database of Systematic Reviews. 2016 (2): CD009996. doi:10.1002/14651858.CD009996.pub2. PMC 10329868. PMID 26844979.
- ^ Osland ST, Steeves TD, Pringsheim T (June 2018). "Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders". Cochrane Database of Systematic Reviews. 2018 (6): CD007990. doi:10.1002/14651858.CD007990.pub3. PMC 6513283. PMID 29944175.
- ^ a b Spencer RC, Devilbiss DM, Berridge CW (June 2015). "The Cognition-Enhancing Effects of Psychostimulants Involve Direct Action in the Prefrontal Cortex". Biological Psychiatry. 77 (11): 940–950. doi:10.1016/j.biopsych.2014.09.013. PMC 4377121. PMID 25499957.
The procognitive actions of psychostimulants are only associated with low doses. Surprisingly, despite nearly 80 years of clinical use, the neurobiology of the procognitive actions of psychostimulants has only recently been systematically investigated. Findings from this research unambiguously demonstrate that the cognition-enhancing effects of psychostimulants involve the preferential elevation of catecholamines in the PFC and the subsequent activation of norepinephrine α2 and dopamine D1 receptors. ... This differential modulation of PFC-dependent processes across dose appears to be associated with the differential involvement of noradrenergic α2 versus α1 receptors. Collectively, this evidence indicates that at low, clinically relevant doses, psychostimulants are devoid of the behavioral and neurochemical actions that define this class of drugs and instead act largely as cognitive enhancers (improving PFC-dependent function). ... In particular, in both animals and humans, lower doses maximally improve performance in tests of working memory and response inhibition, whereas maximal suppression of overt behavior and facilitation of attentional processes occurs at higher doses.
- ^ Ilieva IP, Hook CJ, Farah MJ (June 2015). "Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis". Journal of Cognitive Neuroscience. 27 (6): 1069–1089. doi:10.1162/jocn_a_00776. PMID 25591060. S2CID 15788121.
Specifically, in a set of experiments limited to high-quality designs, we found significant enhancement of several cognitive abilities. ... The results of this meta-analysis ... do confirm the reality of cognitive enhancing effects for normal healthy adults in general, while also indicating that these effects are modest in size.
- ^ Bagot KS, Kaminer Y (April 2014). "Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review". Addiction. 109 (4): 547–557. doi:10.1111/add.12460. PMC 4471173. PMID 24749160.
Amphetamine has been shown to improve consolidation of information (0.02 ≥ P ≤ 0.05), leading to improved recall.
- ^ Devous MD, Trivedi MH, Rush AJ (April 2001). "Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers". Journal of Nuclear Medicine. 42 (4): 535–542. PMID 11337538.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, US: McGraw-Hill Medical. p. 266. ISBN 9780071481274.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ^ a b c Wood S, Sage JR, Shuman T, Anagnostaras SG (January 2014). "Psychostimulants and cognition: a continuum of behavioral and cognitive activation". Pharmacological Reviews. 66 (1): 193–221. doi:10.1124/pr.112.007054. PMC 3880463. PMID 24344115.
- ^ Twohey M (26 March 2006). "Pills become an addictive study aid". JS Online. Archived from the original on 15 August 2007. Retrieved 2 December 2007.
- ^ Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ (October 2006). "Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration". Pharmacotherapy. 26 (10): 1501–1510. doi:10.1592/phco.26.10.1501. PMC 1794223. PMID 16999660.
- ^ Weyandt LL, Oster DR, Marraccini ME, Gudmundsdottir BG, Munro BA, Zavras BM, et al. (September 2014). "Pharmacological interventions for adolescents and adults with ADHD: stimulant and nonstimulant medications and misuse of prescription stimulants". Psychology Research and Behavior Management. 7: 223–249. doi:10.2147/PRBM.S47013. PMC 4164338. PMID 25228824.
misuse of prescription stimulants has become a serious problem on college campuses across the US and has been recently documented in other countries as well. ... Indeed, large numbers of students claim to have engaged in the nonmedical use of prescription stimulants, which is reflected in lifetime prevalence rates of prescription stimulant misuse ranging from 5% to nearly 34% of students.
- ^ Clemow DB, Walker DJ (September 2014). "The potential for misuse and abuse of medications in ADHD: a review". Postgraduate Medicine. 126 (5): 64–81. doi:10.3810/pgm.2014.09.2801. PMID 25295651. S2CID 207580823.
Overall, the data suggest that ADHD medication misuse and diversion are common health care problems for stimulant medications, with the prevalence believed to be approximately 5% to 10% of high school students and 5% to 35% of college students, depending on the study.
- ^ a b c Liddle DG, Connor DJ (June 2013). "Nutritional supplements and ergogenic AIDS". Primary Care: Clinics in Office Practice. 40 (2): 487–505. doi:10.1016/j.pop.2013.02.009. PMID 23668655.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training ...
Physiologic and performance effects
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - ^ a b c d e f g h i j k l m n o p q r s Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York, US: McGraw-Hill. ISBN 9780071624428.
- ^ Bracken NM (January 2012). "National Study of Substance Use Trends Among NCAA College Student-Athletes" (PDF). NCAA Publications. National Collegiate Athletic Association. Archived (PDF) from the original on 9 October 2022. Retrieved 8 October 2013.
- ^ Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". British Journal of Pharmacology. 154 (3): 606–622. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- ^ a b c d Parr JW (July 2011). "Attention-deficit hyperactivity disorder and the athlete: new advances and understanding". Clinics in Sports Medicine. 30 (3): 591–610. doi:10.1016/j.csm.2011.03.007. PMID 21658550.
In 1980, Chandler and Blair47 showed significant increases in knee extension strength, acceleration, anaerobic capacity, time to exhaustion during exercise, pre-exercise and maximum heart rates, and time to exhaustion during maximal oxygen consumption (VO2 max) testing after administration of 15 mg of dextroamphetamine versus placebo. Most of the information to answer this question has been obtained in the past decade through studies of fatigue rather than an attempt to systematically investigate the effect of ADHD drugs on exercise.
- ^ a b c Roelands B, de Koning J, Foster C, Hettinga F, Meeusen R (May 2013). "Neurophysiological determinants of theoretical concepts and mechanisms involved in pacing". Sports Medicine. 43 (5): 301–311. doi:10.1007/s40279-013-0030-4. PMID 23456493. S2CID 30392999.
In high-ambient temperatures, dopaminergic manipulations clearly improve performance. The distribution of the power output reveals that after dopamine reuptake inhibition, subjects are able to maintain a higher power output compared with placebo. ... Dopaminergic drugs appear to override a safety switch and allow athletes to use a reserve capacity that is 'off-limits' in a normal (placebo) situation.
- ^ Parker KL, Lamichhane D, Caetano MS, Narayanan NS (October 2013). "Executive dysfunction in Parkinson's disease and timing deficits". Frontiers in Integrative Neuroscience. 7: 75. doi:10.3389/fnint.2013.00075. PMC 3813949. PMID 24198770.
Manipulations of dopaminergic signaling profoundly influence interval timing, leading to the hypothesis that dopamine influences internal pacemaker, or "clock," activity. For instance, amphetamine, which increases concentrations of dopamine at the synaptic cleft advances the start of responding during interval timing, whereas antagonists of D2 type dopamine receptors typically slow timing;... Depletion of dopamine in healthy volunteers impairs timing, while amphetamine releases synaptic dopamine and speeds up timing.
- ^ Rattray B, Argus C, Martin K, Northey J, Driller M (March 2015). "Is it time to turn our attention toward central mechanisms for post-exertional recovery strategies and performance?". Frontiers in Physiology. 6: 79. doi:10.3389/fphys.2015.00079. PMC 4362407. PMID 25852568.
Aside from accounting for the reduced performance of mentally fatigued participants, this model rationalizes the reduced RPE and hence improved cycling time trial performance of athletes using a glucose mouthwash (Chambers et al., 2009) and the greater power output during a RPE matched cycling time trial following amphetamine ingestion (Swart, 2009). ... Dopamine stimulating drugs are known to enhance aspects of exercise performance (Roelands et al., 2008)
- ^ Roelands B, De Pauw K, Meeusen R (June 2015). "Neurophysiological effects of exercise in the heat". Scandinavian Journal of Medicine & Science in Sports. 25 (Suppl 1): 65–78. doi:10.1111/sms.12350. PMID 25943657. S2CID 22782401.
This indicates that subjects did not feel they were producing more power and consequently more heat. The authors concluded that the "safety switch" or the mechanisms existing in the body to prevent harmful effects are overridden by the drug administration (Roelands et al., 2008b). Taken together, these data indicate strong ergogenic effects of an increased DA concentration in the brain, without any change in the perception of effort.
- ^ a b c d e f g h i j k l m n o p q r s t u v w "Adderall XR- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate capsule, extended release". DailyMed. Shire US Inc. 17 July 2019. Retrieved 22 December 2019.
- ^ "Commonly Abused Prescription Drugs Chart". National Institute on Drug Abuse. Retrieved 7 May 2012.
- ^ "Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. Archived from the original on 2 May 2012. Retrieved 7 May 2012.
- ^ a b c d e "National Institute on Drug Abuse. 2009. Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. Retrieved 27 February 2013.
- ^ Schultz W (2015). "Neuronal reward and decision signals: from theories to data". Physiological Reviews. 95 (3): 853–951. doi:10.1152/physrev.00023.2014. PMC 4491543. PMID 26109341.
Rewards in operant conditioning are positive reinforcers. ... Operant behavior gives a good definition for rewards. Anything that makes an individual come back for more is a positive reinforcer and therefore a reward. Although it provides a good definition, positive reinforcement is only one of several reward functions. ... Rewards are attractive. They are motivating and make us exert an effort. ... Rewards induce approach behavior, also called appetitive or preparatory behavior, sexual behavior, and consummatory behavior. ... Thus any stimulus, object, event, activity, or situation that has the potential to make us approach and consume it is by definition a reward. ... Rewarding stimuli, objects, events, situations, and activities consist of several major components. First, rewards have basic sensory components (visual, auditory, somatosensory, gustatory, and olfactory) ... Second, rewards are salient and thus elicit attention, which are manifested as orienting responses. The salience of rewards derives from three principal factors, namely, their physical intensity and impact (physical salience), their novelty and surprise (novelty/surprise salience), and their general motivational impact shared with punishers (motivational salience). A separate form not included in this scheme, incentive salience, primarily addresses dopamine function in addiction and refers only to approach behavior (as opposed to learning) ... Third, rewards have a value component that determines the positively motivating effects of rewards and is not contained in, nor explained by, the sensory and attentional components. This component reflects behavioral preferences and thus is subjective and only partially determined by physical parameters. Only this component constitutes what we understand as a reward. It mediates the specific behavioral reinforcing, approach generating, and emotional effects of rewards that are crucial for the organism's survival and reproduction, whereas all other components are only supportive of these functions. ... Rewards can also be intrinsic to behavior. They contrast with extrinsic rewards that provide motivation for behavior and constitute the essence of operant behavior in laboratory tests. Intrinsic rewards are activities that are pleasurable on their own and are undertaken for their own sake, without being the means for getting extrinsic rewards. ... Intrinsic rewards are genuine rewards in their own right, as they induce learning, approach, and pleasure, like perfectioning, playing, and enjoying the piano. Although they can serve to condition higher order rewards, they are not conditioned, higher order rewards, as attaining their reward properties does not require pairing with an unconditioned reward. ... These emotions are also called liking (for pleasure) and wanting (for desire) in addiction research and strongly support the learning and approach generating functions of reward.
- ^ Canadian ADHD Practice Guidelines (PDF) (Fourth ed.). Canadian ADHD Resource Alliance. 2018. p. 67. Archived from the original (PDF) on 2 May 2023. Retrieved 2 May 2023.
- ^ Bright GM (May 2008). "Abuse of medications employed for the treatment of ADHD: results from a large-scale community survey". Medscape Journal of Medicine. 10 (5): 111. PMC 2438483. PMID 18596945.
- ^ Childs E, de Wit H (November 2013). "Contextual conditioning enhances the psychostimulant and incentive properties of d-amphetamine in humans". Addict Biology. 18 (6): 985–992. doi:10.1111/j.1369-1600.2011.00416.x. PMC 4242554. PMID 22129527.
- ^ Kessler S (January 1996). "Drug therapy in attention-deficit hyperactivity disorder". Southern Medical Journal. 89 (1): 33–38. doi:10.1097/00007611-199601000-00005. PMID 8545689. S2CID 12798818.
statements on package inserts are not intended to limit medical practice. Rather they are intended to limit claims by pharmaceutical companies. ... the FDA asserts explicitly, and the courts have upheld that clinical decisions are to be made by physicians and patients in individual situations.
- ^ a b c d "Evekeo- amphetamine sulfate tablet". DailyMed. Arbor Pharmaceuticals, LLC. 14 August 2019. Retrieved 22 December 2019.
- ^ a b c d e f g h i j k Heedes G, Ailakis J. "Amphetamine (PIM 934)". INCHEM. International Programme on Chemical Safety. Retrieved 24 June 2014.
- ^ Feinberg SS (November 2004). "Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication". The Journal of Clinical Psychiatry. 65 (11): 1520–1524. doi:10.4088/jcp.v65n1113. PMID 15554766.
- ^ Stewart JW, Deliyannides DA, McGrath PJ (June 2014). "How treatable is refractory depression?". Journal of Affective Disorders. 167: 148–152. doi:10.1016/j.jad.2014.05.047. PMID 24972362.
- ^ a b c d Vitiello B (April 2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child and Adolescent Psychiatric Clinics of North America. 17 (2): 459–474. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ^ Ramey JT, Bailen E, Lockey RF (2006). "Rhinitis medicamentosa" (PDF). Journal of Investigational Allergology & Clinical Immunology. 16 (3): 148–155. PMID 16784007. Retrieved 29 April 2015.
Table 2. Decongestants Causing Rhinitis Medicamentosa
– Nasal decongestants:
– Sympathomimetic:
• Amphetamine - ^ a b "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in children and young adults". United States Food and Drug Administration. 1 November 2011. Archived from the original on 25 August 2019. Retrieved 24 December 2019.
- ^ Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. (November 2011). "ADHD drugs and serious cardiovascular events in children and young adults". New England Journal of Medicine. 365 (20): 1896–1904. doi:10.1056/NEJMoa1110212. PMC 4943074. PMID 22043968.
- ^ a b "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults". United States Food and Drug Administration. 12 December 2011. Archived from the original on 14 December 2019. Retrieved 24 December 2013.
- ^ Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, et al. (December 2011). "ADHD medications and risk of serious cardiovascular events in young and middle-aged adults". JAMA. 306 (24): 2673–2683. doi:10.1001/jama.2011.1830. PMC 3350308. PMID 22161946.
- ^ Montgomery KA (June 2008). "Sexual desire disorders". Psychiatry. 5 (6): 50–55. PMC 2695750. PMID 19727285.
- ^ O'Connor PG (February 2012). "Amphetamines". Merck Manual for Health Care Professionals. Merck. Retrieved 8 May 2012.
- ^ a b c d Shoptaw SJ, Kao U, Ling W (January 2009). Shoptaw SJ, Ali R (ed.). "Treatment for amphetamine psychosis". Cochrane Database of Systematic Reviews. 2009 (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMC 7004251. PMID 19160215.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis.
psychotic symptoms of individuals with amphetamine psychosis may be due exclusively to heavy use of the drug or heavy use of the drug may exacerbate an underlying vulnerability to schizophrenia. - ^ a b c d Bramness JG, Gundersen ØH, Guterstam J, Rognli EB, Konstenius M, Løberg EM, et al. (December 2012). "Amphetamine-induced psychosis—a separate diagnostic entity or primary psychosis triggered in the vulnerable?". BMC Psychiatry. 12: 221. doi:10.1186/1471-244X-12-221. PMC 3554477. PMID 23216941.
In these studies, amphetamine was given in consecutively higher doses until psychosis was precipitated, often after 100–300 mg of amphetamine ... Secondly, psychosis has been viewed as an adverse event, although rare, in children with ADHD who have been treated with amphetamine
- ^ a b Greydanus D. "Stimulant Misuse: Strategies to Manage a Growing Problem" (PDF). American College Health Association (Review Article). ACHA Professional Development Program. p. 20. Archived from the original (PDF) on 3 November 2013. Retrieved 2 November 2013.
- ^ a b Childs E, de Wit H (May 2009). "Amphetamine-induced place preference in humans". Biological Psychiatry. 65 (10): 900–904. doi:10.1016/j.biopsych.2008.11.016. PMC 2693956. PMID 19111278.
This study demonstrates that humans, like nonhumans, prefer a place associated with amphetamine administration. These findings support the idea that subjective responses to a drug contribute to its ability to establish place conditioning.
- ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–375. ISBN 9780071481274.
- ^ a b c d e Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues in Clinical Neuroscience. 15 (4): 431–443. PMC 3898681. PMID 24459410.
Despite the importance of numerous psychosocial factors, at its core, drug addiction involves a biological process: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type [nucleus accumbens] neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement ... Another ΔFosB target is cFos: as ΔFosB accumulates with repeated drug exposure it represses c-Fos and contributes to the molecular switch whereby ΔFosB is selectively induced in the chronic drug-treated state.41. ... Moreover, there is increasing evidence that, despite a range of genetic risks for addiction across the population, exposure to sufficiently high doses of a drug for long periods of time can transform someone who has relatively lower genetic loading into an addict.
- ^ Volkow ND, Koob GF, McLellan AT (January 2016). "Neurobiologic Advances from the Brain Disease Model of Addiction". New England Journal of Medicine. 374 (4): 363–371. doi:10.1056/NEJMra1511480. PMC 6135257. PMID 26816013.
Substance-use disorder: A diagnostic term in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) referring to recurrent use of alcohol or other drugs that causes clinically and functionally significant impairment, such as health problems, disability, and failure to meet major responsibilities at work, school, or home. Depending on the level of severity, this disorder is classified as mild, moderate, or severe.
Addiction: A term used to indicate the most severe, chronic stage of substance-use disorder, in which there is a substantial loss of self-control, as indicated by compulsive drug taking despite the desire to stop taking the drug. In the DSM-5, the term addiction is synonymous with the classification of severe substance-use disorder. - ^ a b c Renthal W, Nestler EJ (September 2009). "Chromatin regulation in drug addiction and depression". Dialogues in Clinical Neuroscience. 11 (3): 257–268. doi:10.31887/DCNS.2009.11.3/wrenthal. PMC 2834246. PMID 19877494.
[Psychostimulants] increase cAMP levels in striatum, which activates protein kinase A (PKA) and leads to phosphorylation of its targets. This includes the cAMP response element binding protein (CREB), the phosphorylation of which induces its association with the histone acetyltransferase, CREB binding protein (CBP) to acetylate histones and facilitate gene activation. This is known to occur on many genes including fosB and c-fos in response to psychostimulant exposure. ΔFosB is also upregulated by chronic psychostimulant treatments, and is known to activate certain genes (eg, cdk5) and repress others (eg, c-fos) where it recruits HDAC1 as a corepressor. ... Chronic exposure to psychostimulants increases glutamatergic [signaling] from the prefrontal cortex to the NAc. Glutamatergic signaling elevates Ca2+ levels in NAc postsynaptic elements where it activates CaMK (calcium/calmodulin protein kinases) signaling, which, in addition to phosphorylating CREB, also phosphorylates HDAC5.
Figure 2: Psychostimulant-induced signaling events - ^ Broussard JI (January 2012). "Co-transmission of dopamine and glutamate". The Journal of General Physiology. 139 (1): 93–96. doi:10.1085/jgp.201110659. PMC 3250102. PMID 22200950.
Coincident and convergent input often induces plasticity on a postsynaptic neuron. The NAc integrates processed information about the environment from basolateral amygdala, hippocampus, and prefrontal cortex (PFC), as well as projections from midbrain dopamine neurons. Previous studies have demonstrated how dopamine modulates this integrative process. For example, high frequency stimulation potentiates hippocampal inputs to the NAc while simultaneously depressing PFC synapses (Goto and Grace, 2005). The converse was also shown to be true; stimulation at PFC potentiates PFC–NAc synapses but depresses hippocampal–NAc synapses. In light of the new functional evidence of midbrain dopamine/glutamate co-transmission (references above), new experiments of NAc function will have to test whether midbrain glutamatergic inputs bias or filter either limbic or cortical inputs to guide goal-directed behavior.
- ^ Kanehisa Laboratories (10 October 2014). "Amphetamine – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
Most addictive drugs increase extracellular concentrations of dopamine (DA) in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), projection areas of mesocorticolimbic DA neurons and key components of the "brain reward circuit". Amphetamine achieves this elevation in extracellular levels of DA by promoting efflux from synaptic terminals. ... Chronic exposure to amphetamine induces a unique transcription factor delta FosB, which plays an essential role in long-term adaptive changes in the brain.
- ^ Cadet JL, Brannock C, Jayanthi S, Krasnova IN (2015). "Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat". Molecular Neurobiology. 51 (2): 696–717 (Figure 1). doi:10.1007/s12035-014-8776-8. PMC 4359351. PMID 24939695.
- ^ a b c Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews Neuroscience. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB serves as one of the master control proteins governing this structural plasticity. ... ΔFosB also represses G9a expression, leading to reduced repressive histone methylation at the cdk5 gene. The net result is gene activation and increased CDK5 expression. ... In contrast, ΔFosB binds to the c-fos gene and recruits several co-repressors, including HDAC1 (histone deacetylase 1) and SIRT 1 (sirtuin 1). ... The net result is c-fos gene repression.
Figure 4: Epigenetic basis of drug regulation of gene expression - ^ a b c Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clinical Psychopharmacology and Neuroscience. 10 (3): 136–143. doi:10.9758/cpn.2012.10.3.136. PMC 3569166. PMID 23430970.
The 35-37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives. ... As a result of its stability, the ΔFosB protein persists in neurons for at least several weeks after cessation of drug exposure. ... ΔFosB overexpression in nucleus accumbens induces NFκB ... In contrast, the ability of ΔFosB to repress the c-Fos gene occurs in concert with the recruitment of a histone deacetylase and presumably several other repressive proteins such as a repressive histone methyltransferase
- ^ Nestler EJ (October 2008). "Transcriptional mechanisms of addiction: Role of ΔFosB". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1507): 3245–3255. doi:10.1098/rstb.2008.0067. PMC 2607320. PMID 18640924.
Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure
- ^ Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 16: Reinforcement and Addictive Disorders". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). New York: McGraw-Hill Medical. ISBN 9780071827706.
Such agents also have important therapeutic uses; cocaine, for example, is used as a local anesthetic (Chapter 2), and amphetamines and methylphenidate are used in low doses to treat attention deficit hyperactivity disorder and in higher doses to treat narcolepsy (Chapter 12). Despite their clinical uses, these drugs are strongly reinforcing, and their long-term use at high doses is linked with potential addiction, especially when they are rapidly administered or when high-potency forms are given.
- ^ Kollins SH (May 2008). "A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders". Current Medical Research and Opinion. 24 (5): 1345–1357. doi:10.1185/030079908X280707. PMID 18384709. S2CID 71267668.
When oral formulations of psychostimulants are used at recommended doses and frequencies, they are unlikely to yield effects consistent with abuse potential in patients with ADHD.
- ^ Kanehisa Laboratories (10 October 2014). "Amphetamine – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
- ^ a b c d e f Nechifor M (March 2008). "Magnesium in drug dependences". Magnesium Research. 21 (1): 5–15. doi:10.1684/mrh.2008.0124 (inactive 31 January 2024). PMID 18557129.
{{cite journal}}: CS1 maint: DOI inactive as of January 2024 (link) - ^ a b c d e Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–437. doi:10.3109/00952990.2014.933840. PMID 25083822. S2CID 19157711.
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure.
- ^ a b c d e f g h i j k Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews Neuroscience. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB has been linked directly to several addiction-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure14,22–24. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption14,26–30. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states. ... ΔFosB serves as one of the master control proteins governing this structural plasticity.
- ^ a b c d e f g h i j k l m n o p q r s t u v Olsen CM (December 2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology. 61 (7): 1109–1122. doi:10.1016/j.neuropharm.2011.03.010. PMC 3139704. PMID 21459101.
Similar to environmental enrichment, studies have found that exercise reduces self-administration and relapse to drugs of abuse (Cosgrove et al., 2002; Zlebnik et al., 2010). There is also some evidence that these preclinical findings translate to human populations, as exercise reduces withdrawal symptoms and relapse in abstinent smokers (Daniel et al., 2006; Prochaska et al., 2008), and one drug recovery program has seen success in participants that train for and compete in a marathon as part of the program (Butler, 2005). ... In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al., 2006; Aiken, 2007; Lader, 2008).
- ^ a b c d Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA (September 2013). "Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis". Neuroscience & Biobehavioral Reviews. 37 (8): 1622–1644. doi:10.1016/j.neubiorev.2013.06.011. PMC 3788047. PMID 23806439.
These findings suggest that exercise may "magnitude"-dependently prevent the development of an addicted phenotype possibly by blocking/reversing behavioral and neuroadaptive changes that develop during and following extended access to the drug. ... Exercise has been proposed as a treatment for drug addiction that may reduce drug craving and risk of relapse. Although few clinical studies have investigated the efficacy of exercise for preventing relapse, the few studies that have been conducted generally report a reduction in drug craving and better treatment outcomes ... Taken together, these data suggest that the potential benefits of exercise during relapse, particularly for relapse to psychostimulants, may be mediated via chromatin remodeling and possibly lead to greater treatment outcomes.
- ^ a b c Zhou Y, Zhao M, Zhou C, Li R (July 2015). "Sex differences in drug addiction and response to exercise intervention: From human to animal studies". Frontiers in Neuroendocrinology. 40: 24–41. doi:10.1016/j.yfrne.2015.07.001. PMC 4712120. PMID 26182835.
Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. ... The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation.
- ^ a b c Linke SE, Ussher M (January 2015). "Exercise-based treatments for substance use disorders: evidence, theory, and practicality". The American Journal of Drug and Alcohol Abuse. 41 (1): 7–15. doi:10.3109/00952990.2014.976708. PMC 4831948. PMID 25397661.
The limited research conducted suggests that exercise may be an effective adjunctive treatment for SUDs. In contrast to the scarce intervention trials to date, a relative abundance of literature on the theoretical and practical reasons supporting the investigation of this topic has been published. ... numerous theoretical and practical reasons support exercise-based treatments for SUDs, including psychological, behavioral, neurobiological, nearly universal safety profile, and overall positive health effects.
- ^ Hyman SE, Malenka RC, Nestler EJ (July 2006). "Neural mechanisms of addiction: the role of reward-related learning and memory" (PDF). Annual Review of Neuroscience. 29: 565–598. doi:10.1146/annurev.neuro.29.051605.113009. PMID 16776597. S2CID 15139406. Archived from the original (PDF) on 19 September 2018.
- ^ a b c d e Steiner H, Van Waes V (January 2013). "Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants". Progress in Neurobiology. 100: 60–80. doi:10.1016/j.pneurobio.2012.10.001. PMC 3525776. PMID 23085425.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 4: Signal Transduction in the Brain". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, US: McGraw-Hill Medical. p. 94. ISBN 9780071481274.
- ^ Kanehisa Laboratories (29 October 2014). "Alcoholism – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
- ^ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (February 2009). "Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens". Proceedings of the National Academy of Sciences. 106 (8): 2915–2920. Bibcode:2009PNAS..106.2915K. doi:10.1073/pnas.0813179106. PMC 2650365. PMID 19202072.
- ^ a b Nestler EJ (January 2014). "Epigenetic mechanisms of drug addiction". Neuropharmacology. 76 (Pt B): 259–268. doi:10.1016/j.neuropharm.2013.04.004. PMC 3766384. PMID 23643695.
- ^ a b Biliński P, Wojtyła A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studziński T (2012). "Epigenetic regulation in drug addiction". Annals of Agricultural and Environmental Medicine. 19 (3): 491–496. PMID 23020045.
- ^ Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, et al. (April 2013). "Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation". Nature Neuroscience. 16 (4): 434–440. doi:10.1038/nn.3354. PMC 3609040. PMID 23475113.
- ^ Whalley K (December 2014). "Psychiatric disorders: a feat of epigenetic engineering". Nature Reviews. Neuroscience. 15 (12): 768–769. doi:10.1038/nrn3869. PMID 25409693. S2CID 11513288.
- ^ a b Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, et al. (March 2012). "Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms". Journal of Psychoactive Drugs. 44 (1): 38–55. doi:10.1080/02791072.2012.662112. PMC 4040958. PMID 22641964.
It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. ... these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance. ... both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry.
- ^ Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (February 2013). "Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator". The Journal of Neuroscience. 33 (8): 3434–3442. doi:10.1523/JNEUROSCI.4881-12.2013. PMC 3865508. PMID 23426671.
- ^ Beloate LN, Weems PW, Casey GR, Webb IC, Coolen LM (February 2016). "Nucleus accumbens NMDA receptor activation regulates amphetamine cross-sensitization and deltaFosB expression following sexual experience in male rats". Neuropharmacology. 101: 154–164. doi:10.1016/j.neuropharm.2015.09.023. PMID 26391065. S2CID 25317397.
- ^ Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 16: Reinforcement and Addictive Disorders". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). New York: McGraw-Hill Medical. ISBN 9780071827706.
Pharmacologic treatment for psychostimulant addiction is generally unsatisfactory. As previously discussed, cessation of cocaine use and the use of other psychostimulants in dependent individuals does not produce a physical withdrawal syndrome but may produce dysphoria, anhedonia, and an intense desire to reinitiate drug use.
- ^ a b c d Chan B, Freeman M, Kondo K, Ayers C, Montgomery J, Paynter R, et al. (December 2019). "Pharmacotherapy for methamphetamine/amphetamine use disorder-a systematic review and meta-analysis". Addiction. 114 (12): 2122–2136. doi:10.1111/add.14755. PMID 31328345. S2CID 198136436.
- ^ Stoops WW, Rush CR (May 2014). "Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research". Expert Review of Clinical Pharmacology. 7 (3): 363–374. doi:10.1586/17512433.2014.909283. PMC 4017926. PMID 24716825.
Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved.
- ^ a b Grandy DK, Miller GM, Li JX (February 2016). ""TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference". Drug and Alcohol Dependence. 159: 9–16. doi:10.1016/j.drugalcdep.2015.11.014. PMC 4724540. PMID 26644139.
When considered together with the rapidly growing literature in the field a compelling case emerges in support of developing TAAR1-selective agonists as medications for preventing relapse to psychostimulant abuse.
- ^ a b Jing L, Li JX (August 2015). "Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction". European Journal of Pharmacology. 761: 345–352. doi:10.1016/j.ejphar.2015.06.019. PMC 4532615. PMID 26092759.
Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction.
- ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 5: Excitatory and Inhibitory Amino Acids". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, US: McGraw-Hill Medical. pp. 124–125. ISBN 9780071481274.
- ^ a b c Carroll ME, Smethells JR (February 2016). "Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments". Frontiers in Psychiatry. 6: 175. doi:10.3389/fpsyt.2015.00175. PMC 4745113. PMID 26903885.
Physical Exercise
There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction ... In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. ... The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in [laboratory animals and humans] regarding exercise as a treatment for drug addiction supports this hypothesis. ... Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon. - ^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M (September 2013). "Efficacy of psychostimulant drugs for amphetamine abuse or dependence". Cochrane Database of Systematic Reviews. 9 (9): CD009695. doi:10.1002/14651858.CD009695.pub2. PMID 23996457.
- ^ "Amphetamines: Drug Use and Abuse". Merck Manual Home Edition. Merck. February 2003. Archived from the original on 17 February 2007. Retrieved 28 February 2007.
- ^ a b c d Shoptaw SJ, Kao U, Heinzerling K, Ling W (April 2009). Shoptaw SJ (ed.). "Treatment for amphetamine withdrawal". Cochrane Database of Systematic Reviews. 2009 (2): CD003021. doi:10.1002/14651858.CD003021.pub2. PMC 7138250. PMID 19370579.
The prevalence of this withdrawal syndrome is extremely common (Cantwell 1998; Gossop 1982) with 87.6% of 647 individuals with amphetamine dependence reporting six or more signs of amphetamine withdrawal listed in the DSM when the drug is not available (Schuckit 1999) ... The severity of withdrawal symptoms is greater in amphetamine dependent individuals who are older and who have more extensive amphetamine use disorders (McGregor 2005). Withdrawal symptoms typically present within 24 hours of the last use of amphetamine, with a withdrawal syndrome involving two general phases that can last 3 weeks or more. The first phase of this syndrome is the initial "crash" that resolves within about a week (Gossop 1982;McGregor 2005) ...
- ^ a b Spiller HA, Hays HL, Aleguas A (June 2013). "Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management". CNS Drugs. 27 (7): 531–543. doi:10.1007/s40263-013-0084-8. PMID 23757186. S2CID 40931380.
Amphetamine, dextroamphetamine, and methylphenidate act as substrates for the cellular monoamine transporter, especially the dopamine transporter (DAT) and less so the norepinephrine (NET) and serotonin transporter. The mechanism of toxicity is primarily related to excessive extracellular dopamine, norepinephrine, and serotonin.
- ^ Collaborators (2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". The Lancet. 385 (9963): 117–171. doi:10.1016/S0140-6736(14)61682-2. hdl:11655/15525. PMC 4340604. PMID 25530442.
Amphetamine use disorders ... 3,788 (3,425–4,145)
- ^ Greene SL, Kerr F, Braitberg G (October 2008). "Review article: amphetamines and related drugs of abuse". Emergency Medicine Australasia. 20 (5): 391–402. doi:10.1111/j.1742-6723.2008.01114.x. PMID 18973636. S2CID 20755466.
- ^ Albertson TE (2011). "Amphetamines". In Olson KR, Anderson IB, Benowitz NL, Blanc PD, Kearney TE, Kim-Katz SY, Wu AH (eds.). Poisoning & Drug Overdose (6th ed.). New York: McGraw-Hill Medical. pp. 77–79. ISBN 9780071668330.
- ^ Advokat C (July 2007). "Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD". Journal of Attention Disorders. 11 (1): 8–16. doi:10.1177/1087054706295605. PMID 17606768. S2CID 7582744.
- ^ a b c d Bowyer JF, Hanig JP (November 2014). "Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity". Temperature. 1 (3): 172–182. doi:10.4161/23328940.2014.982049. PMC 5008711. PMID 27626044.
Hyperthermia alone does not produce amphetamine-like neurotoxicity but AMPH and METH exposures that do not produce hyperthermia (≥40 °C) are minimally neurotoxic. Hyperthermia likely enhances AMPH and METH neurotoxicity directly through disruption of protein function, ion channels and enhanced ROS production. ... The hyperthermia and the hypertension produced by high doses amphetamines are a primary cause of transient breakdowns in the blood-brain barrier (BBB) resulting in concomitant regional neurodegeneration and neuroinflammation in laboratory animals. ... In animal models that evaluate the neurotoxicity of AMPH and METH, it is quite clear that hyperthermia is one of the essential components necessary for the production of histological signs of dopamine terminal damage and neurodegeneration in cortex, striatum, thalamus and hippocampus.
- ^ "Amphetamine". United States National Library of Medicine – Toxicology Data Network. Hazardous Substances Data Bank. Archived from the original on 2 October 2017. Retrieved 2 October 2017.
Direct toxic damage to vessels seems unlikely because of the dilution that occurs before the drug reaches the cerebral circulation.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and addictive disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, US: McGraw-Hill Medical. p. 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
- ^ Sulzer D, Zecca L (February 2000). "Intraneuronal dopamine-quinone synthesis: a review". Neurotoxicity Research. 1 (3): 181–195. doi:10.1007/BF03033289. PMID 12835101. S2CID 21892355.
- ^ Miyazaki I, Asanuma M (June 2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself" (PDF). Acta Medica Okayama. 62 (3): 141–150. doi:10.18926/AMO/30942. PMID 18596830.
- ^ Hofmann FG (1983). A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects (2nd ed.). New York, US: Oxford University Press. p. 329. ISBN 9780195030570.
- ^ a b c d e f g h i j "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". DailyMed. 27 February 2022. Retrieved 28 March 2022.
- ^ a b c d e f g h i j "Adderall XR- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate capsule, extended release". DailyMed. 3 March 2022. Retrieved 28 March 2022.
- ^ Krause J (April 2008). "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Rev. Neurother. 8 (4): 611–625. doi:10.1586/14737175.8.4.611. PMID 18416663. S2CID 24589993.
Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.
- ^ Sulzer D (February 2011). "How addictive drugs disrupt presynaptic dopamine neurotransmission". Neuron. 69 (4): 628–649. doi:10.1016/j.neuron.2011.02.010. PMC 3065181. PMID 21338876.
They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).
- ^ a b Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH (June 2002). "The role of zinc ions in reverse transport mediated by monoamine transporters". J. Biol. Chem. 277 (24): 21505–21513. doi:10.1074/jbc.M112265200. PMID 11940571.
- ^ Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (October 2012). "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". J. Am. Acad. Child Adolesc. Psychiatry. 51 (10): 1003–1019.e20. doi:10.1016/j.jaac.2012.08.015. PMID 23021477.
- ^ Sulzer D, Cragg SJ, Rice ME (August 2016). "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia. 6 (3): 123–148. doi:10.1016/j.baga.2016.02.001. PMC 4850498. PMID 27141430.
Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.
- ^ Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (July 2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148. PMID 21772817.
Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
- ^ "TAAR1". GenAtlas. University of Paris. 28 January 2012. Retrieved 29 May 2014.
• tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)
- ^ Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (July 2014). "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron. 83 (2): 404–416. doi:10.1016/j.neuron.2014.05.043. PMC 4159050. PMID 25033183.
AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH
- ^ Vaughan RA, Foster JD (September 2013). "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. doi:10.1016/j.tips.2013.07.005. PMC 3831354. PMID 23968642.
AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].
- ^ a b Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, et al. (December 2001). "Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor". Molecular Pharmacology. 60 (6): 1181–1188. doi:10.1124/mol.60.6.1181. PMID 11723224. S2CID 14140873.
- ^ a b c d Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ^ Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, et al. (July 2001). "Trace amines: identification of a family of mammalian G protein-coupled receptors". Proceedings of the National Academy of Sciences of the United States of America. 98 (16): 8966–8971. Bibcode:2001PNAS...98.8966B. doi:10.1073/pnas.151105198. PMC 55357. PMID 11459929.
- ^ a b c Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 978-0-07-162442-8.
- ^ a b c d e Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ a b Khan MZ, Nawaz W (October 2016). "The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system". Biomedicine & Pharmacotherapy. 83: 439–449. doi:10.1016/j.biopha.2016.07.002. PMID 27424325.
- ^ a b c d e Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ a b c Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ^ "Compound Summary". p-Hydroxyamphetamine. PubChem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- ^ "Compound Summary". p-Hydroxynorephedrine. PubChem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- ^ "Compound Summary". Phenylpropanolamine. PubChem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- ^ "Pharmacology and Biochemistry". Amphetamine. Pubchem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 12 October 2013.
- ^ a b Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. doi:10.1016/S0021-9258(19)43051-2. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- ^ a b c Sjoerdsma A, von Studnitz W (April 1963). "Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate". British Journal of Pharmacology and Chemotherapy. 20 (2): 278–284. doi:10.1111/j.1476-5381.1963.tb01467.x. PMC 1703637. PMID 13977820.
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
- ^ a b Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation". Expert Opinion on Drug Metabolism & Toxicology. 9 (9): 1139–1153. doi:10.1517/17425255.2013.796929. PMID 23650932. S2CID 23738007.
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
- ^ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circulation Research. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201. S2CID 28641000.
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study, hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
- ^ Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine". Neuropharmacology. 13 (12): 1187–1190. doi:10.1016/0028-3908(74)90069-0. PMID 4457764.
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway, p-hydroxyamphetamine (POH) and p-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the p-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN. - ^ Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems". Journal of Pharmacology and Experimental Therapeutics. 251 (3): 901–908. PMID 2600821.
The metabolism of p-OHA to p-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert p-OHA to p-OHNor after intraventricular administration.
- ^ "Dexedrine". Medic8. Archived from the original on 19 December 2009. Retrieved 27 November 2013.
- ^ "Dextroamphetamine [monograph]". Internet Mental Health. Archived from the original on 27 April 2006. Retrieved 6 September 2015.
- ^ "Information on Dexedrine: A Quick Review | Weitz & Luxenberg". Weitzlux.com. 31 August 2013. Retrieved 5 January 2017.
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present—a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–96. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ King DG (4 January 2017). "Prescription Forgery". Handwriting Services International. Archived from the original on 5 July 2008.
- ^ Sittig M (ed.). Pharmaceutical Manufacturing Encyclopedia. Vol. 1 (2nd ed.). Noyes Publications. ISBN 978-0-8155-1144-1.
- ^ "Dexedrine FAQs". Archived from the original on 17 June 2011.
- ^ Bonné J (9 January 2003). "'Go pills': A war on drugs?". NBC News. Retrieved 5 January 2017.
- ^ a b Woodring JC. "Air Force scientists battle aviator fatigue". Archived from the original on 14 October 2012. Retrieved 5 January 2017.
- ^ Emonson DL, Vanderbeek RD (1995). "The use of amphetamines in U.S. Air Force tactical operations during Desert Shield and Storm". Aviation, Space, and Environmental Medicine. 66 (3): 260–3. PMID 7661838.
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
Smith, Kline and French synthesised both isomers, and in 1937 commenced marketing of d-amphetamine, which was the more potent of the two isomers, under the trade name of Dexedrine.
- ^ "Drugs@FDA: Dexedrine". U.S. Food and Drug Administration (FDA). Retrieved 28 March 2022.
- ^ "Drugs@FDA: Dexedrine". U.S. Food and Drug Administration (FDA). Retrieved 28 March 2022.
- ^ "Drugs@FDA: Dexedrine: Label and Approval History". U.S. Food and Drug Administration (FDA). Archived from the original on 28 August 2021. Retrieved 30 December 2015.
08/02/1976 ... Approval
- ^ Strohl MP (March 2011). "Bradley's Benzedrine studies on children with behavioral disorders". The Yale Journal of Biology and Medicine. 84 (1): 27–33. PMC 3064242. PMID 21451781.
Bradley experimented with Benzedrine sulfate, a drug marketed to doctors by the company Smith, Kline & French (SKF) between 1935 and 1937...
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
Smith, Kline and French introduced Benzedrine onto the market in 1935 as a treatment for narcolepsy (for which it is still used today), mild depression, post-encephalitic Parkinsonism and a raft of other disorders.
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
The use of Benzedrine to treat ADHD declined dramatically after Gross (1976) reported that the racemate was significantly less clinically effective than Dexedrine. Currently, the only use of l-amphetamine in ADHD medications is in mixed salts/mixed enantiomers amphetamine...
- ^ "FDA Approved Drug Products: Label and Approval History (Benzedrine)". U.S. Food and Drug Administration (FDA). Retrieved 11 March 2016.
Action Date 5/11/1982, Supplement Number 007, Approval Type Chemistry
- ^ a b c d "National Drug Code Amphetamine Search Results". National Drug Code Directory. U.S. Food and Drug Administration (FDA). Archived from the original on 16 December 2013. Retrieved 16 December 2013.
- ^ "Mydayis- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine aspartate monohydrate, and amphetamine sulfate capsule, extended release". DailyMed. 28 October 2022. Retrieved 21 January 2023.
- ^ "Adzenys XR-ODT- amphetamine tablet, orally disintegrating". DailyMed. 10 March 2022. Retrieved 21 January 2023.
- ^ "Drug Approval Package: Adzenys XR-ODT (amphetamine)". U.S. Food and Drug Administration (FDA). 27 January 2016. Retrieved 21 January 2023.
- ^ "Evekeo". U.S. Food and Drug Administration (FDA). Retrieved 11 August 2015.
- ^ a b "Vyvanse Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. May 2017. pp. 17–21. Retrieved 10 July 2017.
- ^ "Zenzedi (dextroamphetamine sulfate, USP)". Zenzedi.com. Retrieved 5 January 2017.
- ^ "ProCentra (dextroamphetamine sulfate 5 mg/5 mL Oral Solution)". FSC Laboratories. Archived from the original on 5 October 2010.
- ^ US 7655630, Mickle T, Krishnan S, Bishop B, Lauderback C, Moncrief JS, Oberlender R, Piccariello T, Paul BJ, Verbicky CD, "Abuse-resistant amphetamine prodrugs", issued 2010, assigned to Takeda Pharmaceutical Co Ltd
- ^ Hazell P (1995). "Stimulant treatment for attention deficit hyperactivity disorder". Australian Prescriber. 18 (3): 60–63. doi:10.18773/austprescr.1995.064.
- ^ "Pharmaceutical Services". .health.nsw.gov.au. Archived from the original on 5 May 2013. Retrieved 5 January 2017.
- ^ "Dexamfetamine sulphate - Medicinal forms". British National Formulary. BMJ Group and Pharmaceutical Press (Royal Pharmaceutical Society). Retrieved 9 November 2019.
- ^ "Dexamfetamine – Prescribe Generically" (PDF). Red/Amber News (22). Interface Pharmacist Network Specialist Medicines (IPNSM): 2. November 2010. Archived from the original (PDF) on 18 May 2013. Retrieved 20 April 2012.
- ^ Hutson PH, Pennick M, Secker R (December 2014). "Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: a novel d-amphetamine pro-drug". Neuropharmacology. 87: 41–50. doi:10.1016/j.neuropharm.2014.02.014. PMID 24594478. S2CID 37893582.
- ^ Elayan I (2006). "NRP-104 (lisdexamphetamine dimesylate)" (PDF). Pharmacology/Toxicology Review and Evaluation. U.S. Food and Drug Administration. pp. 18–19.
- ^ Mohammadi M, Akhondzadeh S (September 2011). "Advances and considerations in attention-deficit/hyperactivity disorder pharmacotherapy". Acta Medica Iranica. 49 (8): 487–498. PMID 22009816. Retrieved 12 March 2014.
- ^ Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D (October 2013). "A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to D-amfetamine, methylphenidate and modafinil". Neuropharmacology. 73: 348–358. doi:10.1016/j.neuropharm.2013.05.021. PMID 23748096. S2CID 25343254.
- ^ Rowley HL, Kulkarni R, Gosden J, Brammer R, Hackett D, Heal DJ (November 2012). "Lisdexamfetamine and immediate release d-amfetamine - differences in pharmacokinetic/pharmacodynamic relationships revealed by striatal microdialysis in freely-moving rats with simultaneous determination of plasma drug concentrations and locomotor activity". Neuropharmacology. 63 (6): 1064–1074. doi:10.1016/j.neuropharm.2012.07.008. PMID 22796358. S2CID 29702399.
- ^ "Molecular Weight Calculator". Lenntech. Retrieved 19 August 2015.
- ^ a b "Dextroamphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ a b "D-amphetamine sulfate". Tocris. 2015. Retrieved 19 August 2015.
- ^ a b "Amphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ "Dextroamphetamine Saccharate". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ "Amphetamine Aspartate". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
External links[edit]
- "PIM 178: Dexamphetamine Sulphate)". Poison Information Monograph. International Programme on Chemical Safety (IPCS) Chemical Safety Information from Intergovernmental organizations (INCHEM).
- Amphetamine
- Anorectics
- Aphrodisiacs
- Antihypotensive agents
- Drugs acting on the nervous system
- Enantiopure drugs
- Ergogenic aids
- Euphoriants
- Excitatory amino acid reuptake inhibitors
- Nootropics
- Norepinephrine-dopamine releasing agents
- Phenethylamines
- Stimulants
- Substituted amphetamines
- TAAR1 agonists
- Attention deficit hyperactivity disorder management
- VMAT inhibitors
- World Anti-Doping Agency prohibited substances