Phosphofructokinase 2: Difference between revisions
→Isozymes: per WP:HEAD, no reason to keep repeating phrase in subheading that is included in an heading |
consistent citation formatting |
||
| Line 30: | Line 30: | ||
}} |
}} |
||
'''Phosphofructokinase-2''' ([[6-phosphofructo-2-kinase]], '''PFK-2''') or '''fructose bisphosphatase-2''' ('''FBPase-2'''), is an [[enzyme]] indirectly responsible for regulating the rates of [[glycolysis]] and [[gluconeogenesis]] in cells. It catalyzes formation and degradation of a significant allosteric regulator, [[Fructose 2,6-bisphosphate|fructose-2,6-bisphosphate]] (Fru-2,6-P<sub>2</sub>) from substrate [[Fructose 6-phosphate|fructose-6-phosphate]]. Fru-2,6-P<sub>2</sub> contributes to the rate-determining step of glycolysis as it activates enzyme [[Phosphofructokinase 1]] in the glycolysis pathway, and inhibits [[Fructose 1,6-bisphosphatase|fructose-1,6-bisphosphatase 1]] in gluconeogenesis.<ref name="Kurland_1995">{{cite journal | vauthors = Kurland IJ, Pilkis SJ | title = Covalent control of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: insights into autoregulation of a bifunctional enzyme | journal = Protein Science | volume = 4 | issue = 6 | pages = 1023–37 | date = June 1995 | pmid = 7549867 | pmc = 2143155 | doi = 10.1002/pro.5560040601 }}</ref> Since Fru-2,6-P<sub>2</sub> differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways.<ref name="Kurland_1995" /> Because PFK-2 produces Fru-2,6-P<sub>2</sub> in response to hormonal signaling, [[metabolism]] can be more sensitively and efficiently controlled to align with the organism's glycolytic needs.<ref>Lenzen |
'''Phosphofructokinase-2''' ([[6-phosphofructo-2-kinase]], '''PFK-2''') or '''fructose bisphosphatase-2''' ('''FBPase-2'''), is an [[enzyme]] indirectly responsible for regulating the rates of [[glycolysis]] and [[gluconeogenesis]] in cells. It catalyzes formation and degradation of a significant allosteric regulator, [[Fructose 2,6-bisphosphate|fructose-2,6-bisphosphate]] (Fru-2,6-P<sub>2</sub>) from substrate [[Fructose 6-phosphate|fructose-6-phosphate]]. Fru-2,6-P<sub>2</sub> contributes to the rate-determining step of glycolysis as it activates enzyme [[Phosphofructokinase 1]] in the glycolysis pathway, and inhibits [[Fructose 1,6-bisphosphatase|fructose-1,6-bisphosphatase 1]] in gluconeogenesis.<ref name="Kurland_1995">{{cite journal | vauthors = Kurland IJ, Pilkis SJ | title = Covalent control of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: insights into autoregulation of a bifunctional enzyme | journal = Protein Science | volume = 4 | issue = 6 | pages = 1023–37 | date = June 1995 | pmid = 7549867 | pmc = 2143155 | doi = 10.1002/pro.5560040601 }}</ref> Since Fru-2,6-P<sub>2</sub> differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways.<ref name="Kurland_1995" /> Because PFK-2 produces Fru-2,6-P<sub>2</sub> in response to hormonal signaling, [[metabolism]] can be more sensitively and efficiently controlled to align with the organism's glycolytic needs.<ref name="pmid24637025">{{cite journal | vauthors = Lenzen S | title = A fresh view of glycolysis and glucokinase regulation: history and current status | journal = The Journal of Biological Chemistry | volume = 289 | issue = 18 | pages = 12189–94 | date = May 2014 | pmid = 24637025 | pmc = 4007419 | doi = 10.1074/jbc.R114.557314 }}</ref> |
||
PFK-2 is known as the "bifunctional enzyme" because of its notable structure: though both are located on one protein [[Protein dimer|homodimer]], its two domains act as independently functioning enzymes.<ref name="Rider_2004">{{cite journal | vauthors = Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L | title = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis | journal = The Biochemical Journal | volume = 381 | issue = Pt 3 | pages = 561–79 | date = August 2004 | pmid = 15170386 | pmc = 1133864 | doi = 10.1042/BJ20040752 }}</ref> One terminus serves as a [[kinase]] domain (for PFK-2) while the other terminus acts as a [[phosphatase]] domain (FBPase-2).<ref name="Hasemann_1996">{{cite journal | vauthors = Hasemann CA, Istvan ES, Uyeda K, Deisenhofer J | title = The crystal structure of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase reveals distinct domain homologies | journal = Structure | volume = 4 | issue = 9 | pages = 1017–29 | date = September 1996 | pmid = 8805587 | doi =10.1016/S0969-2126(96)00109-8 }}</ref> |
PFK-2 is known as the "bifunctional enzyme" because of its notable structure: though both are located on one protein [[Protein dimer|homodimer]], its two domains act as independently functioning enzymes.<ref name="Rider_2004">{{cite journal | vauthors = Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L | title = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis | journal = The Biochemical Journal | volume = 381 | issue = Pt 3 | pages = 561–79 | date = August 2004 | pmid = 15170386 | pmc = 1133864 | doi = 10.1042/BJ20040752 }}</ref> One terminus serves as a [[kinase]] domain (for PFK-2) while the other terminus acts as a [[phosphatase]] domain (FBPase-2).<ref name="Hasemann_1996">{{cite journal | vauthors = Hasemann CA, Istvan ES, Uyeda K, Deisenhofer J | title = The crystal structure of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase reveals distinct domain homologies | journal = Structure | volume = 4 | issue = 9 | pages = 1017–29 | date = September 1996 | pmid = 8805587 | doi =10.1016/S0969-2126(96)00109-8 }}</ref> |
||
In mammals, genetic mechanisms encode different PFK-2 [[isoforms]] to accommodate tissue specific needs. While general function remains the same, isoforms feature slight differences in enzymatic properties and are controlled by different methods of regulation; these differences are discussed below.<ref>Atsumi |
In mammals, genetic mechanisms encode different PFK-2 [[isoforms]] to accommodate tissue specific needs. While general function remains the same, isoforms feature slight differences in enzymatic properties and are controlled by different methods of regulation; these differences are discussed below.<ref name="pmid16306349">{{cite journal | vauthors = Atsumi T, Nishio T, Niwa H, Takeuchi J, Bando H, Shimizu C, Yoshioka N, Bucala R, Koike T | title = Expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase/PFKFB3 isoforms in adipocytes and their potential role in glycolytic regulation | journal = Diabetes | volume = 54 | issue = 12 | pages = 3349–57 | date = December 2005 | pmid = 16306349 | doi = 10.2337/diabetes.54.12.3349 }}</ref> |
||
== Structure == |
== Structure == |
||
| Line 41: | Line 41: | ||
On the other hand, the phosphatase domain is located on the C-terminal.<ref name="pmid1328239">{{cite journal | vauthors = Li L, Lin K, Pilkis J, Correia JJ, Pilkis SJ | title = Hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. The role of surface loop basic residues in substrate binding to the fructose-2,6-bisphosphatase domain | journal = The Journal of Biological Chemistry | volume = 267 | issue = 30 | pages = 21588–94 | date = October 1992 | pmid = 1328239 | doi = }}</ref> It resembles the family of proteins that include phosphoglycerate mutases and acid phosphatases.<ref name="Jedrzejas_2000" /><ref name="Stryer_2008">{{cite book | last1 = Stryer | first1 = Lubert | last2 = Berg | first2 = Jeremy Mark | last3 = Tymoczko | first3 = John L. |name-list-format = vanc | title = Biochemistry (Looseleaf) | edition = | publisher = W. H. Freeman | location = San Francisco | year = 2008 | chapter = The Balance Between Glycolysis and Gluconeogenesis in the Liver Is Sensitive to Blood-Glucose Concentration | pages = 466–467 | isbn = 978-1-4292-3502-0 }}</ref> The domain has a mixed α/ β structure, with a six-stranded central β sheet, plus an additional α-helical subdomain that covers the presumed active site of the molecule.<ref name="Hasemann_1996" /> Finally, the N-terminal region modulates PFK-2 and FBPase2 activities, and stabilizes the dimer form of the enzyme.<ref name="Stryer_2008" /><ref name="Tominaga_1993">{{cite journal | vauthors = Tominaga N, Minami Y, Sakakibara R, Uyeda K | title = Significance of the amino terminus of rat testis fructose-6-phosphate, 2-kinase:fructose-2,6-bisphosphatase | journal = The Journal of Biological Chemistry | volume = 268 | issue = 21 | pages = 15951–7 | date = July 1993 | pmid = 8393455 | doi = }}</ref> |
On the other hand, the phosphatase domain is located on the C-terminal.<ref name="pmid1328239">{{cite journal | vauthors = Li L, Lin K, Pilkis J, Correia JJ, Pilkis SJ | title = Hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. The role of surface loop basic residues in substrate binding to the fructose-2,6-bisphosphatase domain | journal = The Journal of Biological Chemistry | volume = 267 | issue = 30 | pages = 21588–94 | date = October 1992 | pmid = 1328239 | doi = }}</ref> It resembles the family of proteins that include phosphoglycerate mutases and acid phosphatases.<ref name="Jedrzejas_2000" /><ref name="Stryer_2008">{{cite book | last1 = Stryer | first1 = Lubert | last2 = Berg | first2 = Jeremy Mark | last3 = Tymoczko | first3 = John L. |name-list-format = vanc | title = Biochemistry (Looseleaf) | edition = | publisher = W. H. Freeman | location = San Francisco | year = 2008 | chapter = The Balance Between Glycolysis and Gluconeogenesis in the Liver Is Sensitive to Blood-Glucose Concentration | pages = 466–467 | isbn = 978-1-4292-3502-0 }}</ref> The domain has a mixed α/ β structure, with a six-stranded central β sheet, plus an additional α-helical subdomain that covers the presumed active site of the molecule.<ref name="Hasemann_1996" /> Finally, the N-terminal region modulates PFK-2 and FBPase2 activities, and stabilizes the dimer form of the enzyme.<ref name="Stryer_2008" /><ref name="Tominaga_1993">{{cite journal | vauthors = Tominaga N, Minami Y, Sakakibara R, Uyeda K | title = Significance of the amino terminus of rat testis fructose-6-phosphate, 2-kinase:fructose-2,6-bisphosphatase | journal = The Journal of Biological Chemistry | volume = 268 | issue = 21 | pages = 15951–7 | date = July 1993 | pmid = 8393455 | doi = }}</ref> |
||
While this central catalytic core remains conserved in all forms of PFK-2, slight structural variations exist in isoforms as a result of different amino acid sequences or alternative splicing.<ref name="El-Maghrabi_2001">{{cite journal | vauthors = El-Maghrabi MR, Noto F, Wu N, Manes N | title = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: suiting structure to need, in a family of tissue-specific enzymes | journal = Current Opinion in Clinical Nutrition and Metabolic Care | volume = 4 | issue = 5 | pages = 411–8 | date = September 2001 | pmid = 11568503 | doi |
While this central catalytic core remains conserved in all forms of PFK-2, slight structural variations exist in isoforms as a result of different amino acid sequences or alternative splicing.<ref name="El-Maghrabi_2001">{{cite journal | vauthors = El-Maghrabi MR, Noto F, Wu N, Manes N | title = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: suiting structure to need, in a family of tissue-specific enzymes | journal = Current Opinion in Clinical Nutrition and Metabolic Care | volume = 4 | issue = 5 | pages = 411–8 | date = September 2001 | pmid = 11568503 | doi = }}</ref> With some minor exceptions, the size of PFK-2 enzymes is typically around 55 kDa.<ref name="Kurland_1995" /> |
||
Researchers hypothesize that the unique bifunctional structure of this enzyme arose from a gene fusion event between a primordial bacterial PFK-1 and a primordial mutase/phosphatase.<ref> |
Researchers hypothesize that the unique bifunctional structure of this enzyme arose from a gene fusion event between a primordial bacterial PFK-1 and a primordial mutase/phosphatase.<ref name="pmid2557623">{{cite journal | vauthors = Bazan JF, Fletterick RJ, Pilkis SJ | title = Evolution of a bifunctional enzyme: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 86 | issue = 24 | pages = 9642–6 | date = December 1989 | pmid = 2557623 | pmc = 298557 | doi = }}</ref> |
||
== Function == |
== Function == |
||
| Line 50: | Line 50: | ||
In [[enzymology]], a 6-phosphofructo-2-kinase ({{EC number|2.7.1.105}}) is an [[enzyme]] that [[catalysis|catalyzes]] the [[chemical reaction]]: |
In [[enzymology]], a 6-phosphofructo-2-kinase ({{EC number|2.7.1.105}}) is an [[enzyme]] that [[catalysis|catalyzes]] the [[chemical reaction]]: |
||
:ATP + beta-D-fructose 6-phosphate <math>\rightleftharpoons</math> ADP + beta-D-fructose 2,6-bisphosphate<ref name=":0">{{ |
:ATP + beta-D-fructose 6-phosphate <math>\rightleftharpoons</math> ADP + beta-D-fructose 2,6-bisphosphate<ref name=":0">{{cite web | url = https://enzyme.expasy.org/EC/2.7.1.105 | title = ENZYME entry 2.7.1.105 | website = enzyme.expasy.org | access-date = 2018-03-24 }}</ref> |
||
Thus, the kinase domain hydrolyzes ATP to phosphorylate the carbon-2 of fructose-6-phosphate, producing Fru-2,6-P<sub>2</sub> and ADP<sub>.</sub> A phosphohistidine intermediate is formed within the reaction.<ref>{{ |
Thus, the kinase domain hydrolyzes ATP to phosphorylate the carbon-2 of fructose-6-phosphate, producing Fru-2,6-P<sub>2</sub> and ADP<sub>.</sub> A phosphohistidine intermediate is formed within the reaction.<ref>{{cite web | url = https://www.ebi.ac.uk/interpro/entry/IPR013079|title=6-phosphofructo-2-kinase (IPR013079) | work = InterPro | publisher = EMBL-EBI | access-date = 2018-03-25 }}</ref> |
||
:At the other terminal, the fructose-2,6-bisphosphate 2-phosphatase ([[Enzyme Commission number|EC]] [https://enzyme.expasy.org/EC/3.1.3.46 3.1.3.46]) domain dephosphorylates Fru-2,6-P<sub>2</sub> with the addition of water. This opposing chemical reaction is: |
:At the other terminal, the fructose-2,6-bisphosphate 2-phosphatase ([[Enzyme Commission number|EC]] [https://enzyme.expasy.org/EC/3.1.3.46 3.1.3.46]) domain dephosphorylates Fru-2,6-P<sub>2</sub> with the addition of water. This opposing chemical reaction is: |
||
:beta-D-fructose 2,6-bisphosphate + H<sub>2</sub>O <math>\rightleftharpoons</math> D-fructose 6-phosphate + phosphate<ref name=":1">{{ |
:beta-D-fructose 2,6-bisphosphate + H<sub>2</sub>O <math>\rightleftharpoons</math> D-fructose 6-phosphate + phosphate<ref name=":1">{{cite web | url = https://enzyme.expasy.org/EC/3.1.3.46 | title = ENZYME entry 3.1.3.46 | website = enzyme.expasy.org | access-date = 2018-03-25 }}</ref> |
||
: |
: |
||
| Line 63: | Line 63: | ||
== Regulation == |
== Regulation == |
||
In almost all isoforms, PFK-2 undergoes covalent modification through phosphorylation/dephosphorylation based on the cell's hormonal signaling. Phosphorylation of a specific residue may prompt a shift that stabilizes either kinase or phosphatase domain function. This regulation signal thus controls whether F-2,6-P<sub>2</sub> will be synthesized or degraded.<ref> |
In almost all isoforms, PFK-2 undergoes covalent modification through phosphorylation/dephosphorylation based on the cell's hormonal signaling. Phosphorylation of a specific residue may prompt a shift that stabilizes either kinase or phosphatase domain function. This regulation signal thus controls whether F-2,6-P<sub>2</sub> will be synthesized or degraded.<ref name="Okar_2001">{{cite journal | vauthors = Okar DA, Manzano A, Navarro-Sabatè A, Riera L, Bartrons R, Lange AJ | title = PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate | journal = Trends in Biochemical Sciences | volume = 26 | issue = 1 | pages = 30–5 | date = January 2001 | pmid = 11165514 | doi = }}</ref> |
||
Furthermore, the allosteric regulation of PFK2 is very similar to the regulation of [[PFK1]].<ref name="Van Schaftingen_1981">{{cite journal|vauthors=Van Schaftingen E, Hers HG |
Furthermore, the allosteric regulation of PFK2 is very similar to the regulation of [[PFK1]].<ref name="Van Schaftingen_1981">{{cite journal | vauthors = Van Schaftingen E, Hers HG | title = Phosphofructokinase 2: the enzyme that forms fructose 2,6-bisphosphate from fructose 6-phosphate and ATP | journal = Biochemical and Biophysical Research Communications | volume = 101 | issue = 3 | pages = 1078–84 | date = August 1981 | pmid = 6458291 | doi = 10.1016/0006-291X(81)91859-3 }}</ref> High levels of [[Adenosine monophosphate|AMP]] or phosphate group signifies a low energy charge state and thus stimulates PFK2. On the other hand, a high concentration of [[phosphoenolpyruvate]](PEP) and [[citrate]] signifies that there is a high level of biosynthetic precursor and hence inhibits PFK2. Interestingly and unlike PFK1, PFK2 is not affected by ATP concentration.<ref name="pmid24280138">{{cite journal | vauthors = Ros S, Schulze A | title = Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases in cancer metabolism | journal = Cancer & Metabolism | volume = 1 | issue = 1 | pages = 8 | date = February 2013 | pmid = 24280138 | pmc = 4178209 | doi = 10.1186/2049-3002-1-8 }}</ref> |
||
# |
# |
||
| Line 90: | Line 90: | ||
* L-Type: liver tissue |
* L-Type: liver tissue |
||
** [[Insulin]] activates liver PFK-2 function to indicate a high abundance of blood glucose is available for glycolysis. Insulin activates a [[protein phosphatase]] which dephosphorylates the PFK-2 complex and causes favored PFK-2 activity. PFK-2 then increases production of F-2,6-P<sub>2.</sub> As this product allosterically activates PFK-1, it activates glycolysis.<ref>Hue, L., Rider, M. H. and Rousseau, G. G. (1990) Fructose-2,6-bisphosphate in extra hepatic tissues. In Fructose-2,6-bisphosphate (Pilkis, S. J., ed.), pp. 173–193, CRC Press, Boca Raton </ref> |
** [[Insulin]] activates liver PFK-2 function to indicate a high abundance of blood glucose is available for glycolysis. Insulin activates a [[protein phosphatase]] which dephosphorylates the PFK-2 complex and causes favored PFK-2 activity. PFK-2 then increases production of F-2,6-P<sub>2.</sub> As this product allosterically activates PFK-1, it activates glycolysis.<ref>Hue, L., Rider, M. H. and Rousseau, G. G. (1990) Fructose-2,6-bisphosphate in extra hepatic tissues. In Fructose-2,6-bisphosphate (Pilkis, S. J., ed.), pp. 173–193, CRC Press, Boca Raton </ref> |
||
** In contrast, [[glucagon]] increases FBPase-2 activity. At low blood glucose concentrations, glucagon activates a [[Cyclic adenosine monophosphate|cAMP]]-dependent pathway and in turn, [[Protein kinase A|Protein Kinase A]] (PKA) phosphorylates Serine 32 near the N-terminus. Therefore, glucagon decreases concentrations of F-2,6-P<sub>2</sub> and slows rates of glycolysis.<ref>Pilkis |
** In contrast, [[glucagon]] increases FBPase-2 activity. At low blood glucose concentrations, glucagon activates a [[Cyclic adenosine monophosphate|cAMP]]-dependent pathway and in turn, [[Protein kinase A|Protein Kinase A]] (PKA) phosphorylates Serine 32 near the N-terminus. Therefore, glucagon decreases concentrations of F-2,6-P<sub>2</sub> and slows rates of glycolysis.<ref name="pmid3052289">{{cite journal | vauthors = Pilkis SJ, el-Maghrabi MR, Claus TH | title = Hormonal regulation of hepatic gluconeogenesis and glycolysis | journal = Annual Review of Biochemistry | volume = 57 | issue = | pages = 755–83 | date = 1988 | pmid = 3052289 | doi = 10.1146/annurev.bi.57.070188.003543 }}</ref><ref name="Marker_1989">{{cite journal | vauthors = Marker AJ, Colosia AD, Tauler A, Solomon DH, Cayre Y, Lange AJ, el-Maghrabi MR, Pilkis SJ | title = Glucocorticoid regulation of hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene expression | journal = The Journal of Biological Chemistry | volume = 264 | issue = 12 | pages = 7000–4 | date = April 1989 | pmid = 2540168 | doi = }}</ref> |
||
[[File:Liver Tissue PFK-2 Regulation.png|center|thumb|714x714px|Liver-Tissue PFK-2 Regulation: Concentrations of hormones glucagon and insulin activate proteins which change phosphorylation state of PFK-2. Depending on which domain is stabilized, PFK-2 will synthesize or degrade fructose-2,6-bisphosphate, which impacts rates of glycolysis. ]] |
[[File:Liver Tissue PFK-2 Regulation.png|center|thumb|714x714px|Liver-Tissue PFK-2 Regulation: Concentrations of hormones glucagon and insulin activate proteins which change phosphorylation state of PFK-2. Depending on which domain is stabilized, PFK-2 will synthesize or degrade fructose-2,6-bisphosphate, which impacts rates of glycolysis. ]] |
||
* M-Type: skeletal muscle tissue; F-Type: fibroblast and fetal tissue<ref>Cosin-Roger |
* M-Type: skeletal muscle tissue; F-Type: fibroblast and fetal tissue<ref name="Cosin-Roger_2013">{{cite journal | vauthors = Cosin-Roger J, Vernia S, Alvarez MS, Cucarella C, Boscá L, Martin-Sanz P, Fernández-Alvarez AJ, Casado M | title = Identification of a novel Pfkfb1 mRNA variant in rat fetal liver | journal = Biochemical and Biophysical Research Communications | volume = 431 | issue = 1 | pages = 36–40 | date = February 2013 | pmid = 23291237 | doi = 10.1016/j.bbrc.2012.12.109 }}</ref> |
||
** In contrast to most other PFK-2 tissues, PFK-2 in both skeletal muscle and fetal tissue is solely regulated by concentrations of Fructose-6-phosphate. Within their first exon, there are no regulatory sites that require phosphorylation/dephosphorylation to provoke a change in function. High concentrations of F-6-P will activate kinase function and increase rates of glycolysis, whereas low concentrations of F-6-P will stabilize phosphatase action.<ref name="Salway_2017" />{{enzyme |
** In contrast to most other PFK-2 tissues, PFK-2 in both skeletal muscle and fetal tissue is solely regulated by concentrations of Fructose-6-phosphate. Within their first exon, there are no regulatory sites that require phosphorylation/dephosphorylation to provoke a change in function. High concentrations of F-6-P will activate kinase function and increase rates of glycolysis, whereas low concentrations of F-6-P will stabilize phosphatase action.<ref name="Salway_2017" /> |
||
{{enzyme |
|||
| Name = 6-phosphofructo-2-kinase |
| Name = 6-phosphofructo-2-kinase |
||
| EC_number = 2.7.1.105 |
| EC_number = 2.7.1.105 |
||
| Line 106: | Line 107: | ||
=== PFKB2: cardiac === |
=== PFKB2: cardiac === |
||
This PFKB2 gene is located on chromosome 1.<ref>Darville |
This PFKB2 gene is located on chromosome 1.<ref name="pmid1652483">{{cite journal | vauthors = Darville MI, Chikri M, Lebeau E, Hue L, Rousseau GG | title = A rat gene encoding heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase | journal = FEBS Letters | volume = 288 | issue = 1-2 | pages = 91–4 | date = August 1991 | pmid = 1652483 | doi = }}</ref> When greater concentrations of [[adrenaline]] or insulin hormone are circulated, a Protein Kinase A pathway is activated which phosphorylates either Serine 466 or Serine 483 in the C-terminus, which is part of the FBPase-2 domain.<ref name="pmid9652401">{{cite journal | vauthors = Heine-Suñer D, Díaz-Guillén MA, Lange AJ, Rodríguez de Córdoba S | title = Sequence and structure of the human 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase heart isoform gene (PFKFB2) | journal = European Journal of Biochemistry | volume = 254 | issue = 1 | pages = 103–10 | date = May 1998 | pmid = 9652401 | doi = }}</ref> Alternatively, [[Protein kinase B|Protein Kinase B]] may also phosphorylate these regulatory sites.<ref name="pmid11069105">{{cite journal | vauthors = Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L | title = Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia | journal = Current Biology : CB | volume = 10 | issue = 20 | pages = 1247–55 | date = October 2000 | pmid = 11069105 | doi = }}</ref> When this serine residue is phosphorylated, FBPase-2 function is inactivated and greater PFK-2 activity is stabilized.<ref name="Salway_2017" /> |
||
=== PFKB3: brain, placental, and inducible === |
=== PFKB3: brain, placental, and inducible === |
||
| Line 114: | Line 115: | ||
* |
* |
||
=== PFKB4: testis === |
=== PFKB4: testis === |
||
Gene PFKB4, located on chromosome 3, expresses PFK-2 in human testis tissue.<ref>Manzano |
Gene PFKB4, located on chromosome 3, expresses PFK-2 in human testis tissue.<ref name="pmid10095107">{{cite journal | vauthors = Manzano A, Pérez JX, Nadal M, Estivill X, Lange A, Bartrons R | title = Cloning, expression and chromosomal localization of a human testis 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene | journal = Gene | volume = 229 | issue = 1-2 | pages = 83–9 | date = March 1999 | pmid = 10095107 | doi = }}</ref> PFK-2 enzymes encoded by PFK-4 are comparable to the liver enzyme in size at around 54kDa, and like the muscle tissue, do not contain a protein kinase phosphorylation site.<ref name="Manzano_1998">{{cite journal | vauthors = Manzano A, Rosa JL, Ventura F, Pérez JX, Nadal M, Estivill X, Ambrosio S, Gil J, Bartrons R | title = Molecular cloning, expression, and chromosomal localization of a ubiquitously expressed human 6-phosphofructo-2-kinase/ fructose-2, 6-bisphosphatase gene (PFKFB3) | journal = Cytogenetics and Cell Genetics | volume = 83 | issue = 3-4 | pages = 214–7 | date = 1998 | pmid = 10072580 | doi = 10.1159/000015181 }}</ref> While less research has clarified regulation mechanisms for this isoform, studies have confirmed that modification from multiple transcription factors in the 5' flanking region regulates the amount of PFK-2 expression in developing testis tissue.<ref name="Gómez_2005">{{cite journal | vauthors = Gómez M, Manzano A, Navarro-Sabaté A, Duran J, Obach M, Perales JC, Bartrons R | title = Specific expression of pfkfb4 gene in spermatogonia germ cells and analysis of its 5'-flanking region | journal = FEBS Letters | volume = 579 | issue = 2 | pages = 357–62 | date = January 2005 | pmid = 15642344 | doi = 10.1016/j.febslet.2004.11.096 }}</ref> This isoform has been particularly implicated as being modified and hyperexpressed for prostate cancer cell survival.<ref name="Novellasdemunt_2013">{{cite journal | vauthors = Novellasdemunt L, Tato I, Navarro-Sabate A, Ruiz-Meana M, Méndez-Lucas A, Perales JC, Garcia-Dorado D, Ventura F, Bartrons R, Rosa JL | title = Akt-dependent activation of the heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) isoenzyme by amino acids | journal = The Journal of Biological Chemistry | volume = 288 | issue = 15 | pages = 10640–51 | date = April 2013 | pmid = 23457334 | pmc = 3624444 | doi = 10.1074/jbc.M113.455998 }}</ref> |
||
[[File:PFKB4 Testis isozyme.png|center|thumb|300x300px|6-phosphofructo-2-kinase structure, testis tissue]] |
[[File:PFKB4 Testis isozyme.png|center|thumb|300x300px|6-phosphofructo-2-kinase structure, testis tissue]] |
||
Revision as of 07:29, 25 March 2018
| 6-phosphofructo-2-kinase/fructose-bisphosphatase-2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 Structure of PFK2. Shown: kinase domain (cyan) and the phosphatase domain (green). | |||||||||||
| Identifiers | |||||||||||
| Symbol | 6PF2K | ||||||||||
| Pfam | PF01591 | ||||||||||
| InterPro | IPR013079 | ||||||||||
| PROSITE | PDOC00158 | ||||||||||
| SCOP2 | 1bif / SCOPe / SUPFAM | ||||||||||
| |||||||||||
| fructose-bisphosphatase-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | FBPase-2 | ||||||||
| Pfam | PF00316 | ||||||||
| InterPro | IPR028343 | ||||||||
| PROSITE | PDOC00114 | ||||||||
| |||||||||
Phosphofructokinase-2 (6-phosphofructo-2-kinase, PFK-2) or fructose bisphosphatase-2 (FBPase-2), is an enzyme indirectly responsible for regulating the rates of glycolysis and gluconeogenesis in cells. It catalyzes formation and degradation of a significant allosteric regulator, fructose-2,6-bisphosphate (Fru-2,6-P2) from substrate fructose-6-phosphate. Fru-2,6-P2 contributes to the rate-determining step of glycolysis as it activates enzyme Phosphofructokinase 1 in the glycolysis pathway, and inhibits fructose-1,6-bisphosphatase 1 in gluconeogenesis.[1] Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways.[1] Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs.[2]
PFK-2 is known as the "bifunctional enzyme" because of its notable structure: though both are located on one protein homodimer, its two domains act as independently functioning enzymes.[3] One terminus serves as a kinase domain (for PFK-2) while the other terminus acts as a phosphatase domain (FBPase-2).[4]
In mammals, genetic mechanisms encode different PFK-2 isoforms to accommodate tissue specific needs. While general function remains the same, isoforms feature slight differences in enzymatic properties and are controlled by different methods of regulation; these differences are discussed below.[5]
Structure
The monomers of the bifunctional protein are clearly divided into two functional domains. The kinase domain is located on the N-terminal.[6] It consists of a central six-stranded β sheet, with five parallel strands and an antiparallel edge strand, surrounded by seven α helices.[4] The domain contains nucleotide-binding fold (nbf) at the C-terminal end of the first β-strand.[7] The PFK-2 domain appears to be closely related to the superfamily of mononucleotide binding proteins including adenylate cyclase.[8]
On the other hand, the phosphatase domain is located on the C-terminal.[9] It resembles the family of proteins that include phosphoglycerate mutases and acid phosphatases.[8][10] The domain has a mixed α/ β structure, with a six-stranded central β sheet, plus an additional α-helical subdomain that covers the presumed active site of the molecule.[4] Finally, the N-terminal region modulates PFK-2 and FBPase2 activities, and stabilizes the dimer form of the enzyme.[10][11]
While this central catalytic core remains conserved in all forms of PFK-2, slight structural variations exist in isoforms as a result of different amino acid sequences or alternative splicing.[12] With some minor exceptions, the size of PFK-2 enzymes is typically around 55 kDa.[1]
Researchers hypothesize that the unique bifunctional structure of this enzyme arose from a gene fusion event between a primordial bacterial PFK-1 and a primordial mutase/phosphatase.[13]
Function
This enzyme's main function is to synthesize or degrade allosteric regulator Fru-2,6-P2 in response to glycolytic needs of the cell or organism, as depicted in the accompanying diagram.
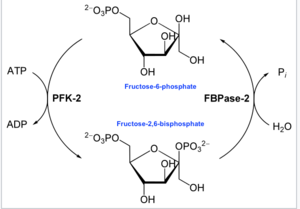
In enzymology, a 6-phosphofructo-2-kinase (EC 2.7.1.105) is an enzyme that catalyzes the chemical reaction:
- ATP + beta-D-fructose 6-phosphate ADP + beta-D-fructose 2,6-bisphosphate[14]
Thus, the kinase domain hydrolyzes ATP to phosphorylate the carbon-2 of fructose-6-phosphate, producing Fru-2,6-P2 and ADP. A phosphohistidine intermediate is formed within the reaction.[15]
- At the other terminal, the fructose-2,6-bisphosphate 2-phosphatase (EC 3.1.3.46) domain dephosphorylates Fru-2,6-P2 with the addition of water. This opposing chemical reaction is:
- beta-D-fructose 2,6-bisphosphate + H2O D-fructose 6-phosphate + phosphate[16]
Because of the enzyme's dual functions, it can be categorized into multiple families. Through categorization by the kinase reaction, this enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. [14] On the other hand, the phosphatase reaction is characteristic of the family of hydrolases, specifically those acting on phosphoric monoester bonds.[16]
Regulation
In almost all isoforms, PFK-2 undergoes covalent modification through phosphorylation/dephosphorylation based on the cell's hormonal signaling. Phosphorylation of a specific residue may prompt a shift that stabilizes either kinase or phosphatase domain function. This regulation signal thus controls whether F-2,6-P2 will be synthesized or degraded.[17]
Furthermore, the allosteric regulation of PFK2 is very similar to the regulation of PFK1.[18] High levels of AMP or phosphate group signifies a low energy charge state and thus stimulates PFK2. On the other hand, a high concentration of phosphoenolpyruvate(PEP) and citrate signifies that there is a high level of biosynthetic precursor and hence inhibits PFK2. Interestingly and unlike PFK1, PFK2 is not affected by ATP concentration.[19]
Isozymes
Protein isozymes are enzymes that catalyze the same reaction but are encoded with different amino acid sequences and as such, display slight differences in protein characteristics. In humans, the four genes that encode phosphofructokinase 2 proteins include PFKFB-1, PFKFB2, PFKB3 and PFKB4.[3]
Multiple mammalian isozymes of the protein have been reported to date, difference rising by either the transcription of different enzymes or alternative splicing.[20] [21][22] While the structural core that catalyzes the PFK-2/FBPase-2 reaction is highly conserved across isoforms, the major isozyme differences arise from highly variable flanking sequences in the isoform amino and carboxyl terminals.[12] Because these areas often contain phosphorylation sites, changes in amino acid composition or terminal length may result in vastly different enzyme kinetics and characteristics.[1][12] Each variant differs in their primary tissue of expression, response to protein kinase regulation, and ratio of kinase/phosphatase domain activity.[23] While multiple types of isozymes may consist in a tissue, isozymes are identified by their primary tissue expression and tissue of discovery below.[24]
PFKB1: Liver, muscle, and fetal
| 6-phosophofructo-2-kinase: PFKB1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystal structure of human liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase | |||||||||
| Identifiers | |||||||||
| EC no. | 2.7.1.105 | ||||||||
| CAS no. | 78689-77-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Located on the X chromosome, this gene is the most well-known of the four genes particularly because it encodes the highly researched liver enzyme.[20]Variable mRNA splicing of PFKB1 yields three different promoters (L, M and F) and therefore, three tissue-specific variants that differ in regulation:[25]
- L-Type: liver tissue
- Insulin activates liver PFK-2 function to indicate a high abundance of blood glucose is available for glycolysis. Insulin activates a protein phosphatase which dephosphorylates the PFK-2 complex and causes favored PFK-2 activity. PFK-2 then increases production of F-2,6-P2. As this product allosterically activates PFK-1, it activates glycolysis.[26]
- In contrast, glucagon increases FBPase-2 activity. At low blood glucose concentrations, glucagon activates a cAMP-dependent pathway and in turn, Protein Kinase A (PKA) phosphorylates Serine 32 near the N-terminus. Therefore, glucagon decreases concentrations of F-2,6-P2 and slows rates of glycolysis.[27][28]
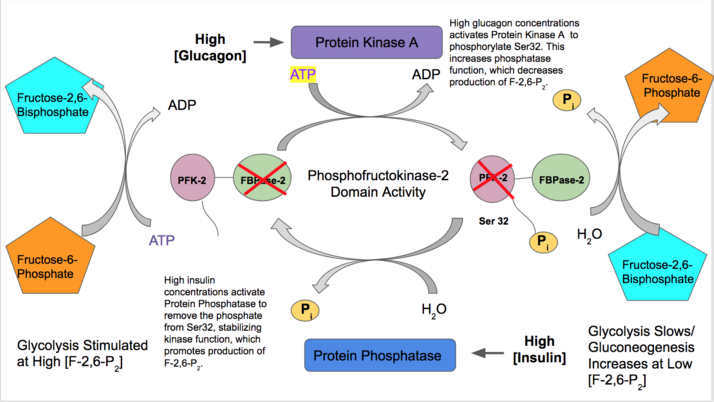
- M-Type: skeletal muscle tissue; F-Type: fibroblast and fetal tissue[29]
- In contrast to most other PFK-2 tissues, PFK-2 in both skeletal muscle and fetal tissue is solely regulated by concentrations of Fructose-6-phosphate. Within their first exon, there are no regulatory sites that require phosphorylation/dephosphorylation to provoke a change in function. High concentrations of F-6-P will activate kinase function and increase rates of glycolysis, whereas low concentrations of F-6-P will stabilize phosphatase action.[25]
| 6-phosophofructo-2-kinase: PFKB2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 6-phosphofructo-2-kinase dimer, Human heart tissue | |||||||||
| Identifiers | |||||||||
| EC no. | 2.7.1.105 | ||||||||
| CAS no. | 78689-77-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
PFKB2: cardiac
This PFKB2 gene is located on chromosome 1.[30] When greater concentrations of adrenaline or insulin hormone are circulated, a Protein Kinase A pathway is activated which phosphorylates either Serine 466 or Serine 483 in the C-terminus, which is part of the FBPase-2 domain.[31] Alternatively, Protein Kinase B may also phosphorylate these regulatory sites.[32] When this serine residue is phosphorylated, FBPase-2 function is inactivated and greater PFK-2 activity is stabilized.[25]
PFKB3: brain, placental, and inducible
Located on chromosome 10.
- Inducible (I-type)
- Increased kinase activity in response to phosphorylation of Ser-461 residue.
PFKB4: testis
Gene PFKB4, located on chromosome 3, expresses PFK-2 in human testis tissue.[33] PFK-2 enzymes encoded by PFK-4 are comparable to the liver enzyme in size at around 54kDa, and like the muscle tissue, do not contain a protein kinase phosphorylation site.[34] While less research has clarified regulation mechanisms for this isoform, studies have confirmed that modification from multiple transcription factors in the 5' flanking region regulates the amount of PFK-2 expression in developing testis tissue.[24] This isoform has been particularly implicated as being modified and hyperexpressed for prostate cancer cell survival.[23]
Clinical significance
Because this enzyme family maintains rates of glycolysis and gluconeogenesis, it presents potential for therapeutic action and control of metabolism particularly in diabetes and cancer cells. Many cancer cell types including leukemia, lung, breast, colon, pancreatic, ovarian cancers demonstrate overexpression of PFK3 and/or PFK4.[23] Furthermore, the control of PFK-2/FBP-ase2 activity was found to be linked to heart functioning and the control against hypoxia.[35]
Lastly, the Pfkfb2 gene encoding PFK2/FBPase2 protein is linked to the predisposition to schizophrenia.[36]
Structural studies
As of late 2007, 8 structures have been solved for the phosphofructokinase class of enzymes, with PDB accession codes 1BIF, 1K6M, 2AXN, 2BIF, 2DWO, 2DWP, 2I1V, and 3BIF.
This article incorporates text from the public domain Pfam and InterPro IPR013079
References
- ^ a b c d Kurland IJ, Pilkis SJ (June 1995). "Covalent control of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: insights into autoregulation of a bifunctional enzyme". Protein Science. 4 (6): 1023–37. doi:10.1002/pro.5560040601. PMC 2143155. PMID 7549867.
- ^ Lenzen S (May 2014). "A fresh view of glycolysis and glucokinase regulation: history and current status". The Journal of Biological Chemistry. 289 (18): 12189–94. doi:10.1074/jbc.R114.557314. PMC 4007419. PMID 24637025.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L (August 2004). "6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis". The Biochemical Journal. 381 (Pt 3): 561–79. doi:10.1042/BJ20040752. PMC 1133864. PMID 15170386.
- ^ a b c Hasemann CA, Istvan ES, Uyeda K, Deisenhofer J (September 1996). "The crystal structure of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase reveals distinct domain homologies". Structure. 4 (9): 1017–29. doi:10.1016/S0969-2126(96)00109-8. PMID 8805587.
- ^ Atsumi T, Nishio T, Niwa H, Takeuchi J, Bando H, Shimizu C, Yoshioka N, Bucala R, Koike T (December 2005). "Expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase/PFKFB3 isoforms in adipocytes and their potential role in glycolytic regulation". Diabetes. 54 (12): 3349–57. doi:10.2337/diabetes.54.12.3349. PMID 16306349.
- ^ Kurland I, Chapman B, Lee YH, Pilkis S (August 1995). "Evolutionary reengineering of the phosphofructokinase active site: ARG-104 does not stabilize the transition state in 6-phosphofructo-2-kinase". Biochemical and Biophysical Research Communications. 213 (2): 663–72. doi:10.1006/bbrc.1995.2183. PMID 7646523.
- ^ Walker JE, Saraste M, Runswick MJ, Gay NJ (1982). "Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold". The EMBO Journal. 1 (8): 945–51. PMC 553140. PMID 6329717.
- ^ a b Jedrzejas MJ (2000). "Structure, function, and evolution of phosphoglycerate mutases: comparison with fructose-2,6-bisphosphatase, acid phosphatase, and alkaline phosphatase". Progress in Biophysics and Molecular Biology. 73 (2–4): 263–87. doi:10.1016/S0079-6107(00)00007-9. PMID 10958932.
- ^ Li L, Lin K, Pilkis J, Correia JJ, Pilkis SJ (October 1992). "Hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. The role of surface loop basic residues in substrate binding to the fructose-2,6-bisphosphatase domain". The Journal of Biological Chemistry. 267 (30): 21588–94. PMID 1328239.
- ^ a b Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2008). "The Balance Between Glycolysis and Gluconeogenesis in the Liver Is Sensitive to Blood-Glucose Concentration". Biochemistry (Looseleaf). San Francisco: W. H. Freeman. pp. 466–467. ISBN 978-1-4292-3502-0.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Tominaga N, Minami Y, Sakakibara R, Uyeda K (July 1993). "Significance of the amino terminus of rat testis fructose-6-phosphate, 2-kinase:fructose-2,6-bisphosphatase". The Journal of Biological Chemistry. 268 (21): 15951–7. PMID 8393455.
- ^ a b c El-Maghrabi MR, Noto F, Wu N, Manes N (September 2001). "6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: suiting structure to need, in a family of tissue-specific enzymes". Current Opinion in Clinical Nutrition and Metabolic Care. 4 (5): 411–8. PMID 11568503.
- ^ Bazan JF, Fletterick RJ, Pilkis SJ (December 1989). "Evolution of a bifunctional enzyme: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase". Proceedings of the National Academy of Sciences of the United States of America. 86 (24): 9642–6. PMC 298557. PMID 2557623.
- ^ a b "ENZYME entry 2.7.1.105". enzyme.expasy.org. Retrieved 2018-03-24.
- ^ "6-phosphofructo-2-kinase (IPR013079)". InterPro. EMBL-EBI. Retrieved 2018-03-25.
- ^ a b "ENZYME entry 3.1.3.46". enzyme.expasy.org. Retrieved 2018-03-25.
- ^ Okar DA, Manzano A, Navarro-Sabatè A, Riera L, Bartrons R, Lange AJ (January 2001). "PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate". Trends in Biochemical Sciences. 26 (1): 30–5. PMID 11165514.
- ^ Van Schaftingen E, Hers HG (August 1981). "Phosphofructokinase 2: the enzyme that forms fructose 2,6-bisphosphate from fructose 6-phosphate and ATP". Biochemical and Biophysical Research Communications. 101 (3): 1078–84. doi:10.1016/0006-291X(81)91859-3. PMID 6458291.
- ^ Ros S, Schulze A (February 2013). "Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases in cancer metabolism". Cancer & Metabolism. 1 (1): 8. doi:10.1186/2049-3002-1-8. PMC 4178209. PMID 24280138.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Darville MI, Crepin KM, Hue L, Rousseau GG (September 1989). "5' flanking sequence and structure of a gene encoding rat 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase". Proceedings of the National Academy of Sciences of the United States of America. 86 (17): 6543–7. doi:10.1073/pnas.86.17.6543. PMC 297880. PMID 2549541.
- ^ Tsuchiya Y, Uyeda K (May 1994). "Bovine heart fructose 6-P,2-kinase:fructose 2,6-bisphosphatase mRNA and gene structure". Archives of Biochemistry and Biophysics. 310 (2): 467–74. doi:10.1006/abbi.1994.1194. PMID 8179334.
- ^ Sakata J, Abe Y, Uyeda K (August 1991). "Molecular cloning of the DNA and expression and characterization of rat testes fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase". The Journal of Biological Chemistry. 266 (24): 15764–70. PMID 1651918.
- ^ a b c Novellasdemunt L, Tato I, Navarro-Sabate A, Ruiz-Meana M, Méndez-Lucas A, Perales JC, Garcia-Dorado D, Ventura F, Bartrons R, Rosa JL (April 2013). "Akt-dependent activation of the heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) isoenzyme by amino acids". The Journal of Biological Chemistry. 288 (15): 10640–51. doi:10.1074/jbc.M113.455998. PMC 3624444. PMID 23457334.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Gómez M, Manzano A, Navarro-Sabaté A, Duran J, Obach M, Perales JC, Bartrons R (January 2005). "Specific expression of pfkfb4 gene in spermatogonia germ cells and analysis of its 5'-flanking region". FEBS Letters. 579 (2): 357–62. doi:10.1016/j.febslet.2004.11.096. PMID 15642344.
- ^ a b c Salway JG (2017). Metabolism at a Glance. Wiley-Blackwell. ISBN 978-0-470-67471-0.
- ^ Hue, L., Rider, M. H. and Rousseau, G. G. (1990) Fructose-2,6-bisphosphate in extra hepatic tissues. In Fructose-2,6-bisphosphate (Pilkis, S. J., ed.), pp. 173–193, CRC Press, Boca Raton
- ^ Pilkis SJ, el-Maghrabi MR, Claus TH (1988). "Hormonal regulation of hepatic gluconeogenesis and glycolysis". Annual Review of Biochemistry. 57: 755–83. doi:10.1146/annurev.bi.57.070188.003543. PMID 3052289.
- ^ Marker AJ, Colosia AD, Tauler A, Solomon DH, Cayre Y, Lange AJ, el-Maghrabi MR, Pilkis SJ (April 1989). "Glucocorticoid regulation of hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene expression". The Journal of Biological Chemistry. 264 (12): 7000–4. PMID 2540168.
- ^ Cosin-Roger J, Vernia S, Alvarez MS, Cucarella C, Boscá L, Martin-Sanz P, Fernández-Alvarez AJ, Casado M (February 2013). "Identification of a novel Pfkfb1 mRNA variant in rat fetal liver". Biochemical and Biophysical Research Communications. 431 (1): 36–40. doi:10.1016/j.bbrc.2012.12.109. PMID 23291237.
- ^ Darville MI, Chikri M, Lebeau E, Hue L, Rousseau GG (August 1991). "A rat gene encoding heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase". FEBS Letters. 288 (1–2): 91–4. PMID 1652483.
- ^ Heine-Suñer D, Díaz-Guillén MA, Lange AJ, Rodríguez de Córdoba S (May 1998). "Sequence and structure of the human 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase heart isoform gene (PFKFB2)". European Journal of Biochemistry. 254 (1): 103–10. PMID 9652401.
- ^ Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L (October 2000). "Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia". Current Biology : CB. 10 (20): 1247–55. PMID 11069105.
- ^ Manzano A, Pérez JX, Nadal M, Estivill X, Lange A, Bartrons R (March 1999). "Cloning, expression and chromosomal localization of a human testis 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene". Gene. 229 (1–2): 83–9. PMID 10095107.
- ^ Manzano A, Rosa JL, Ventura F, Pérez JX, Nadal M, Estivill X, Ambrosio S, Gil J, Bartrons R (1998). "Molecular cloning, expression, and chromosomal localization of a ubiquitously expressed human 6-phosphofructo-2-kinase/ fructose-2, 6-bisphosphatase gene (PFKFB3)". Cytogenetics and Cell Genetics. 83 (3–4): 214–7. doi:10.1159/000015181. PMID 10072580.
- ^ Wang Q, Donthi RV, Wang J, Lange AJ, Watson LJ, Jones SP, Epstein PN (June 2008). "Cardiac phosphatase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase increases glycolysis, hypertrophy, and myocyte resistance to hypoxia". American Journal of Physiology. Heart and Circulatory Physiology. 294 (6): H2889-97. doi:10.1152/ajpheart.91501.2007. PMID 18456722.
- ^ Stone WS, Faraone SV, Su J, Tarbox SI, Van Eerdewegh P, Tsuang MT (May 2004). "Evidence for linkage between regulatory enzymes in glycolysis and schizophrenia in a multiplex sample". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 127B (1): 5–10. doi:10.1002/ajmg.b.20132. PMID 15108172.
External links
- Fructose+2,6-bisphosphatase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- 6-phosphofructokinase of Arabidopsis thaliana at genome.jp

