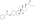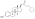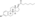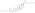Estradiol (medication): Difference between revisions
| Line 156: | Line 156: | ||
In addition to the [[route of administration]], the type of estrogen influences VTE risk.<ref name="pmid31465627" /><ref name="pmid30008249" /> Oral [[conjugated estrogens]] are associated with a higher risk of VTE than oral estradiol.<ref name="pmid26444994">{{cite journal | vauthors = Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ | title = Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline | journal = J. Clin. Endocrinol. Metab. | volume = 100 | issue = 11 | pages = 3975–4011 | date = November 2015 | pmid = 26444994 | doi = 10.1210/jc.2015-2236 | url = }}</ref><ref name="pmid26327865" /><ref name="pmid24081194">{{cite journal | vauthors = Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS, Hwang M, Bis JC, McKnight B, Rice KM, Lumley T, Rosendaal FR, Heckbert SR, Psaty BM | title = Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens | journal = JAMA Intern Med | volume = 174 | issue = 1 | pages = 25–31 | date = January 2014 | pmid = 24081194 | pmc = 4636198 | doi = 10.1001/jamainternmed.2013.11074 | url = }}</ref> [[Estradiol-containing birth control pill|Estradiol- and estradiol valerate-containing birth control pill]]s are associated with a lower risk of VTE than birth control pills containing [[ethinylestradiol]].<ref name="pmid30519125" /><ref name="pmid30008249" /> The relative risk of VTE is thought to be highest with oral ethinylestradiol, intermediate with oral conjugated estrogens, low with oral estradiol and parenteral estradiol valerate, and very low with transdermal estradiol.<ref name="pmid31465627">{{cite journal | vauthors = Connors JM, Middeldorp S | title = Transgender patients and the role of the coagulation clinician | journal = J. Thromb. Haemost. | volume = 17 | issue = 11 | pages = 1790–1797 | date = November 2019 | pmid = 31465627 | doi = 10.1111/jth.14626 | url = }}</ref> Conjugated estrogens and ethinylestradiol are thought to have a higher risk of VTE than estradiol because they are resistant to [[liver|hepatic]] [[metabolism]] and have a disproportionate influence on liver production of coagulation factors.<ref name="pmid16112947" /><ref name="pmid31465627" /><ref name="pmid30008249" /> |
In addition to the [[route of administration]], the type of estrogen influences VTE risk.<ref name="pmid31465627" /><ref name="pmid30008249" /> Oral [[conjugated estrogens]] are associated with a higher risk of VTE than oral estradiol.<ref name="pmid26444994">{{cite journal | vauthors = Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ | title = Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline | journal = J. Clin. Endocrinol. Metab. | volume = 100 | issue = 11 | pages = 3975–4011 | date = November 2015 | pmid = 26444994 | doi = 10.1210/jc.2015-2236 | url = }}</ref><ref name="pmid26327865" /><ref name="pmid24081194">{{cite journal | vauthors = Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS, Hwang M, Bis JC, McKnight B, Rice KM, Lumley T, Rosendaal FR, Heckbert SR, Psaty BM | title = Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens | journal = JAMA Intern Med | volume = 174 | issue = 1 | pages = 25–31 | date = January 2014 | pmid = 24081194 | pmc = 4636198 | doi = 10.1001/jamainternmed.2013.11074 | url = }}</ref> [[Estradiol-containing birth control pill|Estradiol- and estradiol valerate-containing birth control pill]]s are associated with a lower risk of VTE than birth control pills containing [[ethinylestradiol]].<ref name="pmid30519125" /><ref name="pmid30008249" /> The relative risk of VTE is thought to be highest with oral ethinylestradiol, intermediate with oral conjugated estrogens, low with oral estradiol and parenteral estradiol valerate, and very low with transdermal estradiol.<ref name="pmid31465627">{{cite journal | vauthors = Connors JM, Middeldorp S | title = Transgender patients and the role of the coagulation clinician | journal = J. Thromb. Haemost. | volume = 17 | issue = 11 | pages = 1790–1797 | date = November 2019 | pmid = 31465627 | doi = 10.1111/jth.14626 | url = }}</ref> Conjugated estrogens and ethinylestradiol are thought to have a higher risk of VTE than estradiol because they are resistant to [[liver|hepatic]] [[metabolism]] and have a disproportionate influence on liver production of coagulation factors.<ref name="pmid16112947" /><ref name="pmid31465627" /><ref name="pmid30008249" /> |
||
The combination of oral or transdermal estradiol and a progestin is associated with a higher risk of VTE than estradiol alone.<ref name="pmid29570359">{{cite journal | vauthors = Scarabin PY | title = Progestogens and venous thromboembolism in menopausal women: an updated oral versus transdermal estrogen meta-analysis | journal = Climacteric | volume = 21 | issue = 4 | pages = 341–345 | date = August 2018 | pmid = 29570359 | doi = 10.1080/13697137.2018.1446931 | url = }}</ref> [[Dydrogesterone]] is associated with a lower risk than other progestins such as [[medroxyprogesterone acetate]] and [[norethisterone]], while oral [[progesterone (medication)|progesterone]] is associated with no increase in risk of VTE.<ref name="pmid29570359" /><ref name="pmid30626577" /> Older [[ageing|age]], higher [[body weight]], lower [[physical activity]], and [[smoking]] are all associated with a higher risk of VTE with oral estrogen therapy.<ref name="pmid30008249">{{cite journal | vauthors = Beyer-Westendorf J, Bauersachs R, Hach-Wunderle V, Zotz RB, Rott H | title = Sex hormones and venous thromboembolism - from contraception to hormone replacement therapy | journal = VASA | volume = 47 | issue = 6 | pages = 441–450 | date = October 2018 | pmid = 30008249 | doi = 10.1024/0301-1526/a000726 | url = }}</ref><ref name="pmid29526116">{{cite journal | vauthors = Davey DA | title = Menopausal hormone therapy: a better and safer future | journal = Climacteric | volume = 21| issue = 5| pages = 454–461 | date = March 2018 | pmid = 29526116 | doi = 10.1080/13697137.2018.1439915 | url = }}</ref><ref name="pmid23136837">{{cite journal | vauthors = Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A | title = The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy | journal = J. Thromb. Haemost. | volume = 11 | issue = 1 | pages = 124–31 | date = January 2013 | pmid = 23136837 | doi = 10.1111/jth.12060 | url = }}</ref> Risk of VTE with estrogen therapy is highest at the start of treatment, particularly during the first year, and decreases over time.<ref name="pmid30008249" /> |
The combination of oral or transdermal estradiol and a progestin is associated with a higher risk of VTE than estradiol alone.<ref name="pmid29570359">{{cite journal | vauthors = Scarabin PY | title = Progestogens and venous thromboembolism in menopausal women: an updated oral versus transdermal estrogen meta-analysis | journal = Climacteric | volume = 21 | issue = 4 | pages = 341–345 | date = August 2018 | pmid = 29570359 | doi = 10.1080/13697137.2018.1446931 | url = }}</ref><ref name="pmid31372078" /> [[Dydrogesterone]] is associated with a lower risk than other progestins such as [[medroxyprogesterone acetate]] and [[norethisterone]], while oral [[progesterone (medication)|progesterone]] is associated with no increase in risk of VTE.<ref name="pmid29570359" /><ref name="pmid30626577" /> Older [[ageing|age]], higher [[body weight]], lower [[physical activity]], and [[smoking]] are all associated with a higher risk of VTE with oral estrogen therapy.<ref name="pmid30008249">{{cite journal | vauthors = Beyer-Westendorf J, Bauersachs R, Hach-Wunderle V, Zotz RB, Rott H | title = Sex hormones and venous thromboembolism - from contraception to hormone replacement therapy | journal = VASA | volume = 47 | issue = 6 | pages = 441–450 | date = October 2018 | pmid = 30008249 | doi = 10.1024/0301-1526/a000726 | url = }}</ref><ref name="pmid29526116">{{cite journal | vauthors = Davey DA | title = Menopausal hormone therapy: a better and safer future | journal = Climacteric | volume = 21| issue = 5| pages = 454–461 | date = March 2018 | pmid = 29526116 | doi = 10.1080/13697137.2018.1439915 | url = }}</ref><ref name="pmid31372078" /><ref name="pmid23136837">{{cite journal | vauthors = Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A | title = The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy | journal = J. Thromb. Haemost. | volume = 11 | issue = 1 | pages = 124–31 | date = January 2013 | pmid = 23136837 | doi = 10.1111/jth.12060 | url = }}</ref> Risk of VTE with estrogen therapy is highest at the start of treatment, particularly during the first year, and decreases over time.<ref name="pmid30008249" /><ref name="pmid31372078" /> |
||
The [[absolute risk]] of VTE with |
The [[absolute risk]] of VTE with estrogen and/or progestin therapy is small.<ref name="pmid27051991">{{cite journal | vauthors = Bateson D, Butcher BE, Donovan C, Farrell L, Kovacs G, Mezzini T, Raynes-Greenow C, Pecoraro G, Read C, Baber R | title = Risk of venous thromboembolism in women taking the combined oral contraceptive: A systematic review and meta-analysis | journal = Aust Fam Physician | volume = 45 | issue = 1 | pages = 59–64 | date = 2016 | pmid = 27051991 | doi = | url = https://www.racgp.org.au/afp/2016/januaryfebruary/risk-of-venous-thromboembolism-in-women-taking-the-combined-oral-contraceptive-a-systematic-review-and-meta-analysis/}}</ref><ref name="FDA2018" /><ref name="pmid31372078">{{cite journal | vauthors = Goldstein Z, Khan M, Reisman T, Safer JD | title = Managing the risk of venous thromboembolism in transgender adults undergoing hormone therapy | journal = J Blood Med | volume = 10 | issue = | pages = 209–216 | date = 2019 | pmid = 31372078 | pmc = 6628137 | doi = 10.2147/JBM.S166780 | url = }}</ref> Women who are not on a birth control pill or hormone therapy have a risk of VTE of about 1 to 5 out of 10,000 women per year.<ref name="pmid27051991" /><ref name="FDA2018" /><ref name="pmid26327865" /><ref name="pmid31372078" /> In women taking a birth control pill containing ethinylestradiol and a progestin, the risk of VTE is in the range of 3 to 10 out of 10,000 women per year.<ref name="pmid27051991" /><ref name="FDA2018" /><ref name="pmid31372078" /> Birth control pills containing estradiol valerate and a progestin are associated with about half the risk of VTE of ethinylestradiol/progestin-containing birth control pills.<ref name="pmid30519125" /> [[Transgender hormone therapy (male-to-female)|Hormone therapy]] for [[transgender women]] likewise is associated with a lower risk of VTE than birth control pills containing ethinylestradiol and a progestin.<ref name="pmid30602475">{{cite journal | vauthors = Khan J, Schmidt RL, Spittal MJ, Goldstein Z, Smock KJ, Greene DN | title = Venous Thrombotic Risk in Transgender Women Undergoing Estrogen Therapy: A Systematic Review and Metaanalysis | journal = Clin. Chem. | volume = 65 | issue = 1 | pages = 57–66 | date = January 2019 | pmid = 30602475 | doi = 10.1373/clinchem.2018.288316 | url = }}</ref><ref name="pmid31465627" /> The risk of VTE during [[pregnancy]], when estrogens and progesterone increase to very high levels, is 5 to 20 in 10,000 women per year, while the risk is 40 to 65 per 10,000 women per year during the [[postpartum period]].<ref name="FDA2018" /><ref name="pmid31372078" /> |
||
===Long-term effects=== |
===Long-term effects=== |
||
Revision as of 20:54, 26 December 2019
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛstrəˈdaɪoʊl/ ES-trə-DY-ohl[1][2] |
| Trade names | Numerous |
| Other names | Oestradiol; E2; 17β-Estradiol; Estra-1,3,5(10)-triene-3,17β-diol |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | • By mouth (tablet) • Sublingual (tablet) • Intranasal (nasal spray) • Transdermal (patch, gel, cream, emulsion, spray) • Vaginal (tablet, cream, suppository, insert, ring) • IM injection (oil solution) • SC injection (aq. soln.) • Subcutaneous implant |
| Drug class | Estrogen |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: <5%[3] IM: 100%[4] |
| Protein binding | ~98%:[3][5] • Albumin: 60% • SHBG: 38% • Free: 2% |
| Metabolism | Liver (via hydroxylation, sulfation, glucuronidation) |
| Metabolites | Major (90%):[3] • Estrone • Estrone sulfate • Estrone glucuronide • Estradiol glucuronide |
| Elimination half-life | Oral: 13–20 hours[3] Sublingual: 8–18 hours[6] Transdermal (gel): 37 hours[7] IM (as EV): 4–5 days[4] IM (as EC): 8–10 days[8] IV (as E2): 1–2 hours[4] |
| Excretion | Urine: 54%[3] Feces: 6%[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H24O2 |
| Molar mass | 272.388 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Estradiol (E2) is a medication and naturally occurring steroid hormone.[9][10][11] It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women.[9][12] It is also used in hormonal birth control for women, in hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses.[13][14][15][16][17] Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.[9]
Side effects of estradiol in women include breast tenderness, breast enlargement, headache, fluid retention, and nausea among others.[9][18] Men and children who are exposed to estradiol may develop symptoms of feminization, such as breast development and a feminine pattern of fat distribution, and men may also experience low testosterone levels and infertility.[19][20] Estradiol may increase the risk of endometrial hyperplasia and endometrial cancer in women with intact uteruses if it is not taken together with a progestogen such as progesterone.[9] The combination of estradiol with a progestin, though not with oral progesterone, may increase the risk of breast cancer.[21][22] Estradiol should not be used in women who are pregnant or breastfeeding or who have breast cancer, among other contraindications.[18]
Estradiol is a naturally occurring and bioidentical estrogen, or an agonist of the estrogen receptor, the biological target of estrogens like endogenous estradiol.[9] Due to its estrogenic activity, estradiol has antigonadotropic effects and can inhibit fertility and suppress sex hormone production in both women and men.[23][24] Estradiol differs from non-bioidentical estrogens like conjugated estrogens and ethinylestradiol in various ways, with implications for tolerability and safety.[9]
Estradiol was discovered in 1933.[25][26] It became available as a medication that same year, in an injectable form known as estradiol benzoate.[27][28][29] Forms that were more useful by mouth, estradiol valerate and micronized estradiol, were introduced in the 1960s and 1970s and increased its popularity by this route.[30][31][32] Estradiol is also used as other prodrugs, like estradiol cypionate.[9] Related estrogens such as ethinylestradiol, which is the most common estrogen in birth control pills, and conjugated estrogens (brand name Premarin), which is used in menopausal hormone therapy, are used as medications as well.[9] In 2016, estradiol was the 59th most prescribed medication in the United States, with more than 13 million prescriptions.[33]
Medical uses
Hormone therapy
Menopause
Estradiol is used in menopausal hormone therapy to prevent and treat moderate to severe menopausal symptoms such as hot flashes, vaginal dryness and atrophy, and osteoporosis (bone loss).[9] As unopposed estrogen therapy (using estrogen alone without progesterone) increases the risk of endometrial hyperplasia and endometrial cancer in women with intact uteruses, estradiol is usually combined with a progestogen like progesterone or medroxyprogesterone acetate to prevent the effects of estradiol on the endometrium.[9][34] This is not necessary if the woman has undergone a hysterectomy (surgical removal of the uterus).[9] A 2017 meta-analysis found that estradiol had no effect on depressive symptoms in peri- and postmenopausal women.[35]
| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | 2.5–10 μg/day | 5–20 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
| Transdermal gel | Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
| Vaginal | Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
| IM or SC injection | Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
Hypogonadism
Estrogen is responsible for the mediation of puberty in females, and in girls with delayed puberty due to hypogonadism (low-functioning gonads, which can result in low sex hormone levels) such as in Turner syndrome, estradiol is used to induce the development of and maintain female secondary sexual characteristics such as breasts, wide hips, and a female fat distribution.[36][12][37] It is also used to restore estradiol levels in adult premenopausal women with hypogonadism, for instance those with premature ovarian failure or who have undergone oophorectomy.[12][37] It is used to treat women with hypogonadism due to hypopituitarism as well.[37][12]
Transgender women
Estradiol is used as part of feminizing hormone therapy for transgender women.[38][15] The drug is used in higher dosages prior to sex reassignment surgery or orchiectomy to help suppress testosterone levels; after this procedure, estradiol continues to be used at lower dosages to maintain estradiol levels in the normal premenopausal female range.[38][15]
Birth control
Although almost all combined oral contraceptives contain the synthetic estrogen ethinylestradiol,[39] natural estradiol itself is also used in some hormonal contraceptives, including in estradiol-containing oral contraceptives and combined injectable contraceptives.[13][14] It is formulated in combination with a progestin such as dienogest, nomegestrol acetate, or medroxyprogesterone acetate, and is often used in the form of an ester prodrug like estradiol valerate or estradiol cypionate.[13][14] Hormonal contraceptives contain a progestin and/or estrogen and prevent ovulation and thus the possibility of pregnancy by suppressing the secretion of the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the peak of which around the middle of the menstrual cycle causes ovulation to occur.[40]
Hormonal cancer
Prostate cancer
Estradiol is used as a form of high-dose estrogen therapy to treat prostate cancer and is similarly effective to other therapies such as androgen deprivation therapy with castration and antiandrogens.[16][11][41][42] It is used in the form of long-lasting injected estradiol prodrugs like polyestradiol phosphate, estradiol valerate, and estradiol undecylate,[11][41][43] and has also more recently been assessed in the form of transdermal estradiol patches.[41][44] Estrogens are effective in the treatment of prostate cancer by suppressing testosterone levels into the castrate range, increasing levels of sex hormone-binding globulin (SHBG) and thereby decreasing the fraction of free testosterone, and possibly also via direct cytotoxic effects on prostate cancer cells.[45][46][47] Parenteral estradiol is largely free of the cardiovascular side effects of the high oral dosages of synthetic estrogens like diethylstilbestrol ad ethinylestradiol that were used previously.[41][48][49] In addition, estrogens may have advantages relative to castration in terms of hot flashes, sexual interest and function, osteoporosis, cognitive function, and quality of life.[41][49][46][50] However, side effects such as gynecomastia and feminization in general may be difficult to tolerate and unacceptable for many men.[41]
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Breast cancer
High-dose estrogen therapy is effective in the treatment of about 35% of cases of breast cancer in women who are at least 5 years menopausal and has comparable effectiveness to antiestrogen therapy with medications like the selective estrogen receptor modulator (SERM) tamoxifen.[17][51][52] Although estrogens are rarely used in the treatment of breast cancer today and synthetic estrogens like diethylstilbestrol and ethinylestradiol have most commonly been used, estradiol itself has been used in the treatment of breast cancer as well.[17][18][53] It has been used orally at very high doses (30 mg/day) in the treatment of therapy-naive breast cancer and orally at low doses (2 to 6 mg/day) in the treatment of breast cancer in women who were previously treated with and benefited from but acquired resistance to aromatase inhibitors.[17][54][18] Polyestradiol phosphate is also used to treat breast cancer.[55][56]
| Route/form | Estrogen | Dosage | Ref(s) |
|---|---|---|---|
| Oral | Estradiol | 10 mg 3x/day AI-resistant: 2 mg 1–3x/day |
[17][57] [17][54] |
| Estradiol valerate | AI-resistant: 2 mg 1–3x/day | [17][54] | |
| Conjugated estrogens | 10 mg 3x/day | [58][59][60][61] | |
| Ethinylestradiol | 0.5–1 mg 3x/day | [59][17][62][61] | |
| Diethylstilbestrol | 5 mg 3x/day | [59][63][64] | |
| Dienestrol | 5 mg 3x/day | [62][61][64] | |
| Dimestrol | 30 mg/day | [58][61][64] | |
| Chlorotrianisene | 24 mg/day | [58][64] | |
| IM or SC injection | Estradiol benzoate | 5 mg 2–3x/week | [62][65][63][66] |
| Estradiol dipropionate | 5 mg 2–3x/week | [62][63][67][66] | |
| Estradiol valerate | 30 mg 1x/2 weeks | [65] | |
| Polyestradiol phosphate | 40–80 mg 1x/4 weeks | [68][56] | |
| Estrone | 5 mg ≥3x/week | [69] | |
| Notes: (1) Only in women who are at least 5 years postmenopausal.[17] (2) Dosages are not necessarily equivalent. | |||
Other uses
Infertility
Estrogens may be used in treatment of infertility in women when there is a need to develop sperm-friendly cervical mucous or an appropriate uterine lining.[70][71]
It is also commonly used during in vitro fertilization (IVF). Estrogen helps maintains the endometrial lining of the uterus and help prepare for pregnancy. Research shows higher pregnancy rate if the mother takes estrogen in addition to progesterone.[72] Estradiol is the predominant form of estrogen during reproductive years and is most commonly prescribed.[72]
Lactation suppression
Estrogens can be used to suppress and cease lactation and breast engorgement in postpartum women who do not wish to breastfeed.[73][51] They do this by directly decreasing the sensitivity of the alveoli of the mammary glands to the lactogenic hormone prolactin.[51]
Tall stature
Estrogens have been used to limit final height in adolescent girls with tall stature.[74] They do this by inducing epiphyseal closure and suppressing growth hormone-induced hepatic production and by extension circulating levels of insulin-like growth factor-1 (IGF-1), a hormone that causes the body to grow and increase in size.[74] Although ethinylestradiol and conjugated estrogens have mainly been used for this purpose, estradiol can also be employed.[75][76]
Breast enhancement
Estrogens are involved in breast development and estradiol may be used as a form of hormonal breast enhancement to increase the size of the breasts.[77][78][79][80][81] Both polyestradiol phosphate monotherapy and pseudopregnancy with a combination of high-dosage intramuscular estradiol valerate and hydroxyprogesterone caproate have been assessed for this purpose in clinical studies.[77][78][79][80] However, acute or temporary breast enlargement is a well-known side effect of estrogens, and increases in breast size tend to regress following discontinuation of treatment.[77][79][80] Aside from those without prior established breast development, evidence is lacking for a sustained increases in breast size with estrogens.[77][79][80]
Schizophrenia
Estradiol has been found to be effective in the adjunctive treatment of schizophrenia in women.[82][83][84] It has been found to significantly reduce positive, negative, and cognitive symptoms, with particular benefits on positive symptoms.[82][83][84][85] Other estrogens, as well as selective estrogen receptor modulators (SERMs) like raloxifene, have been found to be effective in the adjunctive treatment of schizophrenia in women similarly.[82][86][87] Estrogens may be useful in the treatment of schizophrenia in men as well, but their use in this population is limited by feminizing side effects.[88] SERMs, which have few or no feminizing side effects, have been found to be effective in the adjunctive treatment of schizophrenia in men similarly to in women and may be more useful than estrogens in this sex.[86][87]
Sexual deviance
Estradiol has been used at high doses in the treatment of sexual deviance, such as paraphilias, in men.[89][90] It has specifically been used for this indication in the form of subcutaneous pellet implants of estradiol and intramuscular injections of estradiol undecylate.[89][90]
Available forms
Estradiol is available in a variety of different formulations, including oral, intranasal, transdermal/topical, vaginal, injectable, and implantable preparations.[9][91] An ester may be attached to one or both of the hydroxyl groups of estradiol to improve its oral bioavailability and/or duration of action with injection.[9] Such modifications give rise to forms such as estradiol acetate (oral and vaginal), estradiol valerate (oral and injectable), estradiol cypionate (injectable), estradiol benzoate (injectable), estradiol undecylate (injectable), and polyestradiol phosphate (injectable; a polymerized ester of estradiol), which are all prodrugs of estradiol.[9][91][92]
| Route | Ingredient | Form | Dose[b] | Brand names[c] |
|---|---|---|---|---|
| Oral | Estradiol | Tablet | 0.1, 0.2, 0.5, 1, 2, 4 mg | Estrace, Ovocyclin |
| Estradiol valerate | Tablet | 0.5, 1, 2, 4 mg | Progynova | |
| Transdermal | Estradiol | Patch | 14, 25, 37.5, 50, 60, 75, 100 µg/d | Climara, Vivelle |
| Gel pump | 0.06% (0.52, 0.75 mg/pump) | Elestrin, EstroGel | ||
| Gel packet | 0.1% (0.25, 0.5, 1.0 mg/pk.) | DiviGel, Sandrena | ||
| Emulsion | 0.25% (25 µg/pouch) | Estrasorb | ||
| Spray | 1.53 mg/spray | Evamist, Lenzetto | ||
| Vaginal | Estradiol | Tablet | 10, 25 µg | Vagifem |
| Cream | 0.01% (0.1 mg/gram) | Estrace | ||
| Insert | 4, 10 µg | Imvexxy | ||
| Ring | 2 mg/ring (7.5 µg/d, 3 mon.) | Estring | ||
| Estradiol acetate | Ring | 50, 100 µg/d, 3 months | Femring | |
| Injection[d] | Estradiol | Microspheres | 1 mg/mL | Juvenum E |
| Estradiol benzoate | Oil solution | 0.167, 0.2, 0.333, 1, 1.67, 2, 5, 10, 20, 25 mg/mL | Progynon-B | |
| Estradiol cypionate | Oil solution | 1, 3, 5 mg/mL | Depo-Estradiol | |
| Estradiol valerate | Oil solution | 5, 10, 20, 40 mg/mL | Progynon Depot | |
| Implant | Estradiol | Pellet | 20, 25, 50, 100 mg, 6 mon. | Estradiol Implants |
Notes and sources:
| ||||
| Brand name | Dose (µg/day) |
DOA (d) | Size[b][c] (cm2) |
Levels (pg/mL) |
Intro. |
|---|---|---|---|---|---|
| Alora | 25, 50, 75, 100 | 3–4 | 9, 18, 27, 36 | 43–144 | 1996 |
| Climara[d] | 25, 37.5, 50, 60, 75, 100 |
7 | 6.5, 9.375, 12.5, 15, 18.75, 25 |
17–174 | 1994 |
| Climara Pro[e] | E2 (45) LNG (15) |
7 | 22 | 27–54 | 2003 |
| CombiPatch[e] | E2 (50) NETA (14, 25) |
3–4 | 9, 16 | 27–71 | 1998 |
| Menostar | 14 | 7 | 3.25 | 13–21 | 2004 |
| Minivelle | 25, 37.5, 50, 75, 100 |
3–4 | 1.65, 2.48, 3.3, 4.95, 6.6 |
30–117 | 2012 |
| Vivelle | 50, 100 | 3–4 | 14.5, 29 | 30–145 | 2000 |
| Vivelle-Dot[d] | 25, 37.5, 50, 75, 100 |
3–4 | 2.5, 3.75, 5, 7.5, 10 |
30–145 | 1996 |
Template:Combined injectable contraceptives marketed for clinical use
Contraindications
Estrogens like estradiol have a number of contraindications.[109][26][110][111] Estradiol should be avoided when there is undiagnosed abnormal vaginal bleeding, known, suspected or a history of breast cancer, current treatment for metastatic disease, known or suspected estrogen-dependent neoplasia, deep vein thrombosis, pulmonary embolism or history of these conditions, active or recent arterial thromboembolic disease such as stroke, myocardial infarction, liver dysfunction or disease. Estradiol should not be taken by people with a hypersensitivity/allergy or those who are pregnant or are suspected pregnant.[18]
Side effects
Common side effects of estradiol in women include headache, breast pain or tenderness, breast enlargement, irregular vaginal bleeding or spotting, abdominal cramps, bloating, fluid retention, and nausea.[18][112][3] Other possible side effects of estrogens may include high blood pressure, high blood sugar, enlargement of uterine fibroids, melasma, vaginal yeast infections, and liver problems.[18] In men, estrogens can cause breast pain or tenderness, gynecomastia (male breast development), feminization, demasculinization, sexual dysfunction (decreased libido and erectile dysfunction), hypogonadism, testicular atrophy, and infertility.[19][20]
Blood clots
Oral estradiol and estradiol valerate, for instance in menopausal hormone therapy or birth control pills, are associated with a significantly higher risk of venous thromboembolism (VTE) than non-use.[113][114][115][116] Higher doses of oral estrogens are associated with higher risks of VTE.[115][117][118] In contrast to oral estradiol, transdermal and vaginal estradiol at menopausal replacement dosages are not associated with a higher incidence of VTE.[113][114][119][115] Low doses (e.g., 50 μg/day) and high doses (e.g., 100 μg/day) of transdermal estradiol for menopausal replacement do not differ in terms of VTE risk.[120][119][121][115] The higher risk of VTE with oral estradiol can be attributed to the first pass and a disproportionate effect on liver synthesis of coagulation factors.[9][122] Even high doses of parenteral estradiol, such as high-dose polyestradiol phosphate, have minimal influence on coagulation factors, in contrast to oral estrogen therapy.[49][41][123] However, sufficient doses of parenteral estradiol, for instance very high doses of estradiol valerate by intramuscular injection, can nonetheless activate coagulation, presumably increasing VTE risk.[124][125]
In addition to the route of administration, the type of estrogen influences VTE risk.[126][122] Oral conjugated estrogens are associated with a higher risk of VTE than oral estradiol.[127][121][128] Estradiol- and estradiol valerate-containing birth control pills are associated with a lower risk of VTE than birth control pills containing ethinylestradiol.[116][122] The relative risk of VTE is thought to be highest with oral ethinylestradiol, intermediate with oral conjugated estrogens, low with oral estradiol and parenteral estradiol valerate, and very low with transdermal estradiol.[126] Conjugated estrogens and ethinylestradiol are thought to have a higher risk of VTE than estradiol because they are resistant to hepatic metabolism and have a disproportionate influence on liver production of coagulation factors.[9][126][122]
The combination of oral or transdermal estradiol and a progestin is associated with a higher risk of VTE than estradiol alone.[114][129] Dydrogesterone is associated with a lower risk than other progestins such as medroxyprogesterone acetate and norethisterone, while oral progesterone is associated with no increase in risk of VTE.[114][115] Older age, higher body weight, lower physical activity, and smoking are all associated with a higher risk of VTE with oral estrogen therapy.[122][130][129][117] Risk of VTE with estrogen therapy is highest at the start of treatment, particularly during the first year, and decreases over time.[122][129]
The absolute risk of VTE with estrogen and/or progestin therapy is small.[131][132][129] Women who are not on a birth control pill or hormone therapy have a risk of VTE of about 1 to 5 out of 10,000 women per year.[131][132][121][129] In women taking a birth control pill containing ethinylestradiol and a progestin, the risk of VTE is in the range of 3 to 10 out of 10,000 women per year.[131][132][129] Birth control pills containing estradiol valerate and a progestin are associated with about half the risk of VTE of ethinylestradiol/progestin-containing birth control pills.[116] Hormone therapy for transgender women likewise is associated with a lower risk of VTE than birth control pills containing ethinylestradiol and a progestin.[133][126] The risk of VTE during pregnancy, when estrogens and progesterone increase to very high levels, is 5 to 20 in 10,000 women per year, while the risk is 40 to 65 per 10,000 women per year during the postpartum period.[132][129]
Long-term effects
Uncommon but serious possible side effects of estrogens associated with long-term therapy may include breast cancer, uterine cancer, stroke, heart attack, blood clots, dementia, gallbladder disease, and ovarian cancer.[32] Warning signs of these serious side effects include breast lumps, unusual vaginal bleeding, dizziness, faintness, changes in speech, severe headaches, chest pain, shortness of breath, pain in the legs, changes in vision, and vomiting.[32]
Due to health risks observed with the combination of conjugated estrogens and medroxyprogesterone acetate in the Women's Health Initiative (WHI) studies (see below), the United States Food and Drug Administration (FDA) label for Estrace (estradiol) advises that estrogens should be used in menopausal hormone therapy only for the shortest time possible and at the lowest effective dose.[18] While the FDA states that is unknown if these risks generalize to estradiol (alone or in combination with progesterone or a progestin), it advises that in the absence of comparable data, the risks should be assumed to be similar.[18] When used to treat menopausal symptoms, the FDA recommends that discontinuation of estradiol should be attempted every three to six months via a gradual dose taper.[18]
The combination of bioidentical transdermal or vaginal estradiol and oral or vaginal progesterone appears to be a safer form of hormone therapy than the combination of oral conjugated estrogens and medroxyprogesterone acetate and may not share the same health risks.[134][135][136][137][138][139][140][141][130] Advantages may include reduced or no risk of venous thromboembolism, cardiovascular disease, and breast cancer, among others.[134][135][136][137][138][139][140][141][130]
| Clinical outcome | Hypothesized effect on risk |
Estrogen and progestogen (CEs 0.625 mg/day p.o. + MPA 2.5 mg/day p.o.) (n = 16,608, with uterus, 5.2–5.6 years follow up) |
Estrogen alone (CEs 0.625 mg/day p.o.) (n = 10,739, no uterus, 6.8–7.1 years follow up) | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | AR | HR | 95% CI | AR | ||
| Coronary heart disease | Decreased | 1.24 | 1.00–1.54 | +6 / 10,000 PYs | 0.95 | 0.79–1.15 | −3 / 10,000 PYs |
| Stroke | Decreased | 1.31 | 1.02–1.68 | +8 / 10,000 PYs | 1.37 | 1.09–1.73 | +12 / 10,000 PYs |
| Pulmonary embolism | Increased | 2.13 | 1.45–3.11 | +10 / 10,000 PYs | 1.37 | 0.90–2.07 | +4 / 10,000 PYs |
| Venous thromboembolism | Increased | 2.06 | 1.57–2.70 | +18 / 10,000 PYs | 1.32 | 0.99–1.75 | +8 / 10,000 PYs |
| Breast cancer | Increased | 1.24 | 1.02–1.50 | +8 / 10,000 PYs | 0.80 | 0.62–1.04 | −6 / 10,000 PYs |
| Colorectal cancer | Decreased | 0.56 | 0.38–0.81 | −7 / 10,000 PYs | 1.08 | 0.75–1.55 | +1 / 10,000 PYs |
| Endometrial cancer | – | 0.81 | 0.48–1.36 | −1 / 10,000 PYs | – | – | – |
| Hip fractures | Decreased | 0.67 | 0.47–0.96 | −5 / 10,000 PYs | 0.65 | 0.45–0.94 | −7 / 10,000 PYs |
| Total fractures | Decreased | 0.76 | 0.69–0.83 | −47 / 10,000 PYs | 0.71 | 0.64–0.80 | −53 / 10,000 PYs |
| Total mortality | Decreased | 0.98 | 0.82–1.18 | −1 / 10,000 PYs | 1.04 | 0.91–1.12 | +3 / 10,000 PYs |
| Global index | – | 1.15 | 1.03–1.28 | +19 / 10,000 PYs | 1.01 | 1.09–1.12 | +2 / 10,000 PYs |
| Diabetes | – | 0.79 | 0.67–0.93 | 0.88 | 0.77–1.01 | ||
| Gallbladder disease | Increased | 1.59 | 1.28–1.97 | 1.67 | 1.35–2.06 | ||
| Stress incontinence | – | 1.87 | 1.61–2.18 | 2.15 | 1.77–2.82 | ||
| Urge incontinence | – | 1.15 | 0.99–1.34 | 1.32 | 1.10–1.58 | ||
| Peripheral artery disease | – | 0.89 | 0.63–1.25 | 1.32 | 0.99–1.77 | ||
| Probable dementia | Decreased | 2.05 | 1.21–3.48 | 1.49 | 0.83–2.66 | ||
| Abbreviations: CEs = conjugated estrogens. MPA = medroxyprogesterone acetate. p.o. = per oral. HR = hazard ratio. AR = attributable risk. PYs = person–years. CI = confidence interval. Notes: Sample sizes (n) include placebo recipients, which were about half of patients. "Global index" is defined for each woman as the time to earliest diagnosis for coronary heart disease, stroke, pulmonary embolism, breast cancer, colorectal cancer, endometrial cancer (estrogen plus progestogen group only), hip fractures, and death from other causes. Sources: See template. | |||||||
Overdose
Estrogens are relatively safe in overdose.[92] During pregnancy, levels of estradiol increase to very high concentrations that are as much as 100-fold normal levels.[142][143][144] In late pregnancy, the body produces and secretes approximately 100 mg of estrogens, including estradiol, estrone, and estriol, per day.[142] Serious adverse effects have not been described following acute overdose of large doses of estrogen- and progestogen-containing birth control pills by small children.[92] Symptoms of estrogen overdosage may include nausea, vomiting, bloating, increased weight, water retention, breast tenderness, vaginal discharge, vaginal bleeding, heavy legs, and leg cramps.[109][92] These side effects can be diminished by reducing the estrogen dosage.[109]
Interactions
Inducers of cytochrome P450 enzymes like CYP3A4 such as St. John's wort, phenobarbital, carbamazepine and rifampicin decrease the circulating levels of estradiol by accelerating its metabolism, whereas inhibitors of cytochrome P450 enzymes like CYP3A4 such as erythromycin, cimetidine,[145] clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice[146] may slow its metabolism resulting in increased levels of estradiol in the circulation.[18] There is an interaction between estradiol and alcohol such that alcohol considerably increases circulating levels of estradiol during oral estradiol therapy and also increases estradiol levels in normal premenopausal women and with parenteral estradiol therapy.[147][11][148][149] This appears to be due to a decrease in hepatic 17β-hydroxysteroid dehydrogenase type 2 (17β-HSD2) activity and hence estradiol inactivation into estrone due to an alcohol-mediated increase in the ratio of NADH to NAD in the liver.[148][149] Spironolactone can reduce the bioavailability of oral estradiol.[150]
Pharmacology
Pharmacodynamics
Estradiol is an estrogen, or an agonist of the estrogen receptors (ERs), the ERα and ERβ.[9] It is also an agonist of membrane estrogen receptors (mERs), including the GPER, Gq-mER, ER-X, and ERx.[151][152] Estradiol is highly selective for these ERs and mERs, and does not interact importantly with other steroid hormone receptors.[153][154][155] It is far more potent as an estrogen than are other bioidentical estrogens like estrone and estriol.[9][156] Given by subcutaneous injection in mice, estradiol is about 10-fold more potent than estrone and about 100-fold more potent than estriol.[156]
The ERs are expressed widely throughout the body, including in the breasts, uterus, vagina, fat, skin, bone, liver, pituitary gland, hypothalamus, and other parts of the brain.[25] In accordance, estradiol has numerous effects throughout the body.[25][157][158][159][160][161][162][11][45][163][164][165][166] Among other effects, estradiol produces breast development, feminization, changes in the female reproductive system, changes in liver protein synthesis, and changes in brain function.[161][162][11][45][163][164][165][166] The effects of estradiol can influence health in both positive and negative ways.[9] In addition to the aforementioned effects, estradiol has antigonadotropic effects due to its estrogenic activity, and can inhibit ovulation and suppress gonadal sex hormone production.[162][11][45][46][47][23][24] At sufficiently high dosages, estradiol is a powerful antigonadotropic, capable of suppressing testosterone levels into the castrate/female range in men.[45][46][47][23][24]
There are differences between estradiol and other estrogens, such as non-bioidentical estrogens like natural conjugated estrogens and synthetic estrogens like ethinylestradiol and diethylstilbestrol, with implications for pharmacodynamics and pharmacokinetics as well as efficacy, tolerability, and safety.[9]
Pharmacokinetics
Estradiol can be taken by a variety of different routes of administration.[9] These include oral, buccal, sublingual, intranasal, transdermal (gels, creams, patches), vaginal (tablets, creams, rings, suppositories), rectal, by intramuscular or subcutaneous injection (in oil or aqueous), and as a subcutaneous implant.[9] The pharmacokinetics of estradiol, including its bioavailability, metabolism, biological half-life, and other parameters, differ by route of administration.[9] Likewise, the potency of estradiol, and its local effects in certain tissues, most importantly the liver, differ by route of administration as well.[9] In particular, the oral route is subject to a high first-pass effect, which results in high levels of estradiol and consequent estrogenic effects in the liver and low potency due to first-pass hepatic and intestinal metabolism into metabolites like estrone and estrogen conjugates.[9] Conversely, this is not the case for parenteral (non-oral) routes, which bypass the intestines and liver.[9]
Different estradiol routes and dosages can achieve widely varying circulating estradiol levels.[9] For purposes of comparison with normal physiological circumstances, menstrual cycle circulating levels of estradiol in premenopausal women are 40 pg/mL in the early follicular phase, 250 pg/mL at the middle of the cycle, and 100 pg/mL during the mid-luteal phase.[165] Mean integrated levels of circulating estradiol in premenopausal women across the whole menstrual cycle have been reported to be in the range of 80 and 150 pg/mL, according to some sources.[167][168][169]
Chemistry
Estradiol is a naturally occurring estrane steroid.[9][170] It is also known as 17β-estradiol (to distinguish it from 17α-estradiol) or as estra-1,3,5(10)-triene-3,17β-diol.[171][172][9] It has two hydroxyl groups, one at the C3 position and the other at the C17β position, as well as three double bonds in the A ring (the estra-1,3,5(10)-triene core).[170][173] Due to its two hydroxyl groups, estradiol is often abbreviated as E2.[170] The structurally related estrogens, estrone (E1), estriol (E3), and estetrol (E4) have one, three, and four hydroxyl groups, respectively.[170][174]
Hemihydrate
A hemihydrate form of estradiol, estradiol hemihydrate, is widely used medically under a large number of brand names similarly to estradiol.[172] In terms of activity and bioequivalence, estradiol and its hemihydrate are identical, with the only disparities being an approximate 3% difference in potency by weight (due to the presence of water molecules in the hemihydrate form of the substance) and a slower rate of release with certain formulations of the hemihydrate.[175][176] This is because estradiol hemihydrate is more hydrated than anhydrous estradiol, and for this reason, is more insoluble in water in comparison, which results in slower absorption rates with specific formulations of the drug such as vaginal tablets.[176] Estradiol hemihydrate has also been shown to result in less systemic absorption as a vaginal tablet formulation relative to other topical estradiol formulations such as vaginal creams.[177] Estradiol hemihydrate is used in place of estradiol in some estradiol products.[108][178][179]
Derivatives
A variety of C17β and/or C3 ester prodrugs of estradiol, such as estradiol acetate, estradiol benzoate, estradiol cypionate, estradiol dipropionate, estradiol enantate, estradiol undecylate, estradiol valerate, and polyestradiol phosphate (an estradiol ester in polymeric form), among many others, have been developed and introduced for medical use as estrogens.[171][172][9][180] Estramustine phosphate is also an estradiol ester, but with a nitrogen mustard moiety attached, and is used as a cytostatic antineoplastic agent in the treatment of prostate cancer.[171][172][181] Cloxestradiol acetate and promestriene are ether prodrugs of estradiol that have been introduced for medical use as estrogens as well, although they are little known and rarely used.[171][172]
Synthetic derivatives of estradiol used as estrogens include ethinylestradiol, ethinylestradiol sulfonate, mestranol, methylestradiol, moxestrol, and quinestrol, all of which are 17α-substituted estradiol derivatives.[171][172][9] Synthetic derivatives of estradiol used in scientific research include 8β-VE2 and 16α-LE2.[182]
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
Estradiol was first discovered and synthesized in 1933 via reduction of estrone.[26] Subsequently, estradiol was isolated for the first time in 1935.[25][183] It was also originally known as dihydroxyestrin, dihydrofolliculin, or alpha-estradiol.[173][184] The medication was first introduced for medical use, in the form of estradiol benzoate, a short-acting ester prodrug of estradiol administered by intramuscular injection in oil solution, under the brand name Progynon-B in 1933.[27][28][29][185] Another short-acting ester of estradiol, estradiol dipropionate, was also marketed by 1940.[186][187] Non-esterified estradiol itself was marketed in the mid-to-late 1930s in oral and other formulations under brand names such as Progynon-DH, Ovocylin, Gynoestryl, and Dimenformon as well.[173][184][188][100] Sublingual administration of estradiol was first described in the early 1940s.[189][190][191] Estradiol tablets for use by the sublingual route were marketed under the brand name Estradiol Membrettes in 1950,[192][193][194][195] as well as under the brand name Diogynets by 1952.[196][197][198] Longer-acting esters of estradiol like estradiol valerate, estradiol cypionate, estradiol undecylate, as well as the polymeric ester polyestradiol phosphate, were developed and introduced for use by intramuscular injection in the 1950s.[172][171][199][200]
Due to poor absorption and low potency relative to other estrogens, oral estradiol was not widely used as late as the early 1970s.[201] Instead, estrogens like conjugated estrogens, ethinylestradiol, and diethylstilbestrol were used by the oral route.[201] In 1966, oral estradiol valerate was introduced by Schering for medical use under the brand name Progynova.[30][31][202][203] Esterification of estradiol, as in estradiol valerate, improved its metabolic stability with oral administration.[9][4] Studies in the 1960s showed that micronization of steroids such as spironolactone and norethisterone acetate improved their absorption and oral potency by several-fold.[204][205][206][207][208] In 1972, micronization of estradiol was studied in women and was likewise found to improve the absorption and potency of estradiol by the oral route.[201] Subsequently, oral micronized estradiol was introduced for medical use in the United States under the brand name Estrace in 1975.[32] Oral micronized estradiol and oral estradiol valerate have similar bioavailability and are both now widely used throughout the world.[9][4]
After the introduction of oral micronized estradiol, vaginal and intranasal micronized estradiol were evaluated in 1977 and both subsequently introduced.[209][9]
The first transdermal estradiol gel, a hydroalcoholic gel known as EstroGel, was initially described in 1980 and was introduced in Europe around 1981.[210] Transdermal estradiol gel did not become available in the United States until 2004, when EstroGel was introduced in this country as well.[210] A transdermal estradiol emulsion, Estrasorb, was marketed in the United States in 2003 as well.[210] One of the earliest reports of transdermal estradiol patches was published in 1983.[210][211] Estraderm, a reservoir patch and the first transdermal estradiol patch to be marketed, was introduced in Europe in 1985 and in the United States in 1986.[212][213] The first transdermal matrix estradiol patches to be introduced were Climara and Vivelle between 1994 and 1996, and were followed by many others.[210][214]
Ethinylestradiol, a synthetic derivative of estradiol, was synthesized from estradiol by Inhoffen and Hohlweg in 1938 and was introduced for oral use by Schering in the United States under the brand name Estinyl in 1943.[215][216] It remains widely used in combined birth control pills.[215]
Society and culture
Generic names
Estradiol is the generic name of estradiol in American English and its INN, USAN, USP, BAN, DCF, and JAN.[102][172][171][217][218] Estradiolo is the name of estradiol in Italian and the DCIT[102] and estradiolum is its name in Latin, whereas its name remains unchanged as estradiol in Spanish, Portuguese, French, and German.[102][172] Oestradiol was the former BAN of estradiol and its name in British English,[217] but the spelling was eventually changed to estradiol.[102] When estradiol is provided in its hemihydrate form, its INN is estradiol hemihydrate.[172]
Brand names
Estradiol is marketed under a large number of brand names throughout the world.[172][102] Examples of major brand names in which estradiol has been marketed in include Climara, Climen, Dermestril, Divigel, Estrace, Natifa, Estraderm, Estraderm TTS, Estradot, Estreva, Estrimax, Estring, Estrofem, EstroGel, Evorel, Fem7 (or FemSeven), Imvexxy, Menorest, Oesclim, OestroGel, Sandrena, Systen, and Vagifem.[172][102] Estradiol valerate is marketed mainly as Progynova and Progynon-Depot, while it is marketed as Delestrogen in the U.S.[172][108] Estradiol cypionate is used mainly in the U.S. and is marketed under the brand name Depo-Estradiol.[172][108] Estradiol acetate is available as Femtrace, Femring, and Menoring.[108]
Estradiol is also widely available in combination with progestogens.[102] It is available in combination with norethisterone acetate under the major brand names Activelle, Cliane, Estalis, Eviana, Evorel Conti, Evorel Sequi, Kliogest, Novofem, Sequidot, and Trisequens; with drospirenone as Angeliq; with dydrogesterone as Femoston, Femoston Conti; and with nomegestrol acetate as Zoely.[102] Estradiol valerate is available with cyproterone acetate as Climen; with dienogest as Climodien and Qlaira; with norgestrel as Cyclo-Progynova and Progyluton; with levonorgestrel as Klimonorm; with medroxyprogesterone acetate as Divina and Indivina; and with norethisterone enantate as Mesigyna and Mesygest.[102] Estradiol cypionate is available with medroxyprogesterone acetate as Cyclo-Provera, Cyclofem, Feminena, Lunelle, and Novafem;[14] estradiol enantate with algestone acetophenide as Deladroxate and Topasel;[102][219][220] and estradiol benzoate is marketed with progesterone as Mestrolar and Nomestrol.[102]
Estradiol valerate is also widely available in combination with prasterone enantate (DHEA enantate) under the brand name Gynodian Depot.[102]
Availability
Estradiol and/or its esters are widely available in countries throughout the world in a variety of formulations.[102][221][222][172][108]
United States

As of November 2016[update], estradiol is available in the United States in the following forms:[108]
- Oral tablets (Femtrace (as estradiol acetate), Gynodiol, Innofem, generics)
- Transdermal patches (Alora, Climara, Esclim, Estraderm, FemPatch, Menostar, Minivelle, Vivelle, Vivelle-Dot, generics)
- Topical gels (Divigel, Elestrin, EstroGel, Sandrena), emulsions (Estrasorb), and sprays (Evamist)
- Vaginal tablets (Vagifem, generics), creams (Estrace), inserts (Imvexxy), and rings (Estring, Femring (as estradiol acetate))
- Oil solution for intramuscular injection (Delestrogen (as estradiol valerate), Depo-Estradiol (as estradiol cypionate))
Oral estradiol valerate (Progynova) and other esters of estradiol that are used by injection like estradiol benzoate, estradiol enantate, and estradiol undecylate all are not marketed in the U.S.[108] Polyestradiol phosphate (Estradurin) was marketed in the U.S. previously but is no longer available.[108]
Estradiol is also available in the U.S. in combination with progestogens for the treatment of menopausal symptoms and as a combined hormonal contraceptive:[108]
- Oral oil-filled capsules with progesterone (Bijuva)[223][224]
- Oral tablets with drospirenone (Angeliq) and norethisterone acetate (Activella, Amabelz) and as estradiol valerate with dienogest (Natazia)
- Transdermal patches with levonorgestrel (Climara Pro) and norethisterone acetate (Combipatch)
Estradiol and estradiol esters are also available in custom preparations from compounding pharmacies in the U.S.[225] This includes subcutaneous pellet implants, which are not available in the United States as FDA-approved pharmaceutical drugs.[226] In addition, topical creams that contain estradiol are generally regulated as cosmetics rather than as drugs in the U.S. and hence are also sold over-the-counter and may be purchased without a prescription on the Internet.[227]
Other countries
Pharmaceutical estradiol subcutaneous pellet implants were formerly available in the United Kingdom and Australia under the brand name Estradiol Implants or Oestradiol Implants (Organon; 25, 50, or 100 mg), but have been discontinued.[172][228][229][230][231] However, an estradiol subcutaneous implant with the brand name Meno-Implant (Organon; 20 mg) continues to be available in the Netherlands.[102][172][232][233] Previously, for instance in the 1970s and 1980s, other subcutaneous estradiol implant products such as Progynon Pellets (Schering; 25 mg) and Estropel Pellets (25 mg; Bartor Pharmacol) were marketed.[234][235][236] It has been said that pharmaceutical estradiol implants have been almost exclusively used in the United Kingdom.[237] Subcutaneous estradiol implants are also available as custom compounded products in some countries.[238][226][239] In 2019 estradiol become “long-term out of stock” in the United Kingdom.[240]
Usage
Besides ethinylestradiol used in birth control pills, estradiol was the most used estrogen in the U.S. in 2016, with 13.4 million total prescriptions filled.[33] The next most used estrogen in the U.S. in 2016 was conjugated estrogens, with 4.2 million total prescriptions filled.[33]
Cost
Generic oral estradiol tablets are much less expensive than other forms of estradiol such as transdermal gel and patches and vaginal rings.[241]
Research
Estradiol has been studied in the treatment of postpartum depression and postpartum psychosis.[242][243][244][245][246]
References
- ^ Susan M. Ford; Sally S. Roach (7 October 2013). Roach's Introductory Clinical Pharmacology. Lippincott Williams & Wilkins. pp. 525–. ISBN 978-1-4698-3214-2.
- ^ Maryanne Hochadel; Mosby (1 April 2015). Mosby's Drug Reference for Health Professions. Elsevier Health Sciences. pp. 602–. ISBN 978-0-323-31103-8.
- ^ a b c d e f g Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- ^ a b c d e Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ^ Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 978-0-323-03309-1.
- ^ Price, T; Blauer, K; Hansen, M; Stanczyk, F; Lobo, R; Bates, G (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17-estradiol". Obstetrics & Gynecology. 89 (3): 340–345. doi:10.1016/S0029-7844(96)00513-3. ISSN 0029-7844. PMID 9052581.
- ^ Naunton, Mark; Al Hadithy, Asmar F. Y.; Brouwers, Jacobus R. B. J.; Archer, David F. (2006). "Estradiol gel". Menopause. 13 (3): 517–527. doi:10.1097/01.gme.0000191881.52175.8c. ISSN 1072-3714. PMID 16735950.
- ^ Sierra-Ramírez JA, Lara-Ricalde R, Lujan M, Velázquez-Ramírez N, Godínez-Victoria M, Hernádez-Munguía IA, Padilla A, Garza-Flores J (2011). "Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg)". Contraception. 84 (6): 565–70. doi:10.1016/j.contraception.2011.03.014. PMID 22078184.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ^ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 121, 226, 235–237. ISBN 978-3-642-58616-3.
- ^ a b c d e f g Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 163–178, 235–237, 252–253, 261–276, 538–543. ISBN 978-3-642-60107-1.
- ^ a b c d Christin-Maitre S (2017). "Use of Hormone Replacement in Females with Endocrine Disorders". Horm Res Paediatr. 87 (4): 215–223. doi:10.1159/000457125. PMID 28376481.
- ^ a b c Christin-Maitre S, Laroche E, Bricaire L (January 2013). "A new contraceptive pill containing 17β-estradiol and nomegestrol acetate". Womens Health (Lond). 9 (1): 13–23. doi:10.2217/whe.12.70. PMID 23241152.
- ^ a b c d Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- ^ a b c Wesp LM, Deutsch MB (2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". Psychiatr. Clin. North Am. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
- ^ a b Ali Shah SI (2015). "Emerging potential of parenteral estrogen as androgen deprivation therapy for prostate cancer". South Asian J Cancer. 4 (2): 95–7. doi:10.4103/2278-330X.155699. PMC 4418092. PMID 25992351.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g h i Coelingh Bennink HJ, Verhoeven C, Dutman AE, Thijssen J (January 2017). "The use of high-dose estrogens for the treatment of breast cancer". Maturitas. 95: 11–23. doi:10.1016/j.maturitas.2016.10.010. PMID 27889048. Cite error: The named reference "pmid27889048" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i j k Warner Chilcott (March 2005). "ESTRACE TABLETS, (estradiol tablets, USP)" (PDF). fda.gov. Retrieved 27 November 2016.
- ^ a b Richard P. Pohanish (2011). Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens. William Andrew. pp. 1167–. ISBN 978-1-4377-7869-4.
- ^ a b Russell La Fayette Cecil; J. Claude Bennett; Fred Plum (1996). Cecil Textbook of Medicine. Saunders. ISBN 978-0-7216-3575-0.
Estrogen excess in men causes inhibition of gonadotropin secretion and secondary hypogonadism. Estrogen excess may result from either exogenous administration of estrogens or estrogenic substances (e.g., diethylstilbestrol administration [...]
- ^ Yang Z, Hu Y, Zhang J, Xu L, Zeng R, Kang D (February 2017). "Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis". Gynecol. Endocrinol. 33 (2): 87–92. doi:10.1080/09513590.2016.1248932. PMID 27898258.
- ^ Lambrinoudaki I (April 2014). "Progestogens in postmenopausal hormone therapy and the risk of breast cancer". Maturitas. 77 (4): 311–7. doi:10.1016/j.maturitas.2014.01.001. PMID 24485796.
- ^ a b c Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384.
- ^ a b c Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD (May 2003). "Transdermal estradiol therapy for advanced prostate cancer--forward to the past?". J. Urol. 169 (5): 1735–7. doi:10.1097/01.ju.0000061024.75334.40. PMID 12686820.
- ^ a b c d Fritz F. Parl (2000). Estrogens, Estrogen Receptor and Breast Cancer. IOS Press. pp. 4, 111. ISBN 978-0-9673355-4-4.
- ^ a b c Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. pp. 44, 95–98, 488. ISBN 978-0-203-48612-2.
- ^ a b Kaufman, C. (1933). "Die Behandlung der Amenorrhöe mit Hohen Dosen der Ovarialhormone". Klinische Wochenschrift. 12 (40): 1557–1562. doi:10.1007/BF01765673. ISSN 0023-2173.
- ^ a b Buschbeck, Herbert (2009). "Neue Wege der Hormontherapie in der Gynäkologie" [New ways of hormonal therapy in gynecology]. Deutsche Medizinische Wochenschrift. 60 (11): 389–393. doi:10.1055/s-0028-1129842. ISSN 0012-0472.
- ^ a b Biskind, Morton S. (1935). "Commercial Glandular Products". Journal of the American Medical Association. 105 (9): 667. doi:10.1001/jama.1935.92760350007009a. ISSN 0002-9955.
Progynon-B, Schering Corporation: This is crystalline hydroxyestrin benzoate obtained by hydrogenation of theelin and subsequent conversion to the benzoate. [...] Progynon-B is marketed in ampules containing 1 cc. of a sesame oil solution of hydroxyestrin benzoate of either 2,500, 5,000, 10,000 or 50,000 international units.
- ^ a b "Neue Spezialitäten". Klinische Wochenschrift. 44 (23): 1381. 1966. doi:10.1007/BF01747900. ISSN 0023-2173.
NEUE SPEZIALITATEN [...] Progynova. 1 Dragee enthält 2 mg Oestradiolvalerinat (Klimakterium). Hersteller: Schering AG, Berlin 65.
- ^ a b Dapunt O (September 1967). "Behandlung klimakterischer Beschwerden mit Östradiolvalerianat (Progynova)" [The management of climacteric disorders using estradiol valerate (Progynova)]. Med Klin (in German). 62 (35): 1356–61 passim. ISSN 0025-8458. PMID 5593020.
- ^ a b c d http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Set_Current_Drug&ApplNo=084499&DrugName=ESTRACE&ActiveIngred=ESTRADIOL&SponsorApplicant=BRISTOL%20MYERS%20SQUIBB&ProductMktStatus=3&goto=Search.DrugDetails
- ^ a b c "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- ^ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 434, 444. ISBN 978-3-8047-1763-3.
- ^ Whedon JM, KizhakkeVeettil A, Rugo NA, Kieffer KA (January 2017). "Bioidentical Estrogen for Menopausal Depressive Symptoms: A Systematic Review and Meta-Analysis". J Womens Health (Larchmt). 26 (1): 18–28. doi:10.1089/jwh.2015.5628. PMID 27603786.
- ^ Matthews D, Bath L, Högler W, Mason A, Smyth A, Skae M (October 2017). "Hormone supplementation for pubertal induction in girls" (PDF). Arch. Dis. Child. 102 (10): 975–980. doi:10.1136/archdischild-2016-311372. PMID 28446424.
- ^ a b c Laura Rosenthal; Jacqueline Burchum (17 February 2017). Lehne's Pharmacotherapeutics for Advanced Practice Providers - E-Book. Elsevier Health Sciences. pp. 524–. ISBN 978-0-323-44779-9.
- ^ a b World Professional Association for Transgender Health (September 2011), Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Seventh Version (PDF), archived from the original (PDF) on 6 January 2016
- ^ Evans G, Sutton EL (May 2015). "Oral contraception". Med Clin North Am. 99 (3): 479–503. doi:10.1016/j.mcna.2015.01.004. PMID 25841596.
- ^ Glasier, Anna (2010). "Contraception". In Jameson, J. Larry; De Groot, Leslie J. (eds.). Endocrinology (6th ed.). Philadelphia: Saunders Elsevier. pp. 2417–2427. ISBN 978-1-4160-5583-9.
- ^ a b c d e f g Lycette JL, Bland LB, Garzotto M, Beer TM (2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- ^ Cox RL, Crawford ED (1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/S0022-5347(01)66670-9. PMID 7500443.
- ^ Altwein, J. (1983). "Controversial Aspects of Hormone Manipulation in Prostatic Carcinoma". Cancer of the Prostate and Kidney. pp. 305–316. doi:10.1007/978-1-4684-4349-3_38. ISBN 978-1-4684-4351-6.
- ^ Ockrim JL; Lalani el-N; Kakkar AK; Abel PD (August 2005). "Transdermal estradiol therapy for prostate cancer reduces thrombophilic activation and protects against thromboembolism". J. Urol. 174 (2): 527–33, discussion 532–3. doi:10.1097/01.ju.0000165567.99142.1f. PMID 16006886.
- ^ a b c d e Waun Ki Hong; James F. Holland (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 753–. ISBN 978-1-60795-014-1.
- ^ a b c d Scherr DS, Pitts WR (2003). "The nonsteroidal effects of diethylstilbestrol: the rationale for androgen deprivation therapy without estrogen deprivation in the treatment of prostate cancer". J. Urol. 170 (5): 1703–8. doi:10.1097/01.ju.0000077558.48257.3d. PMID 14532759.
- ^ a b c Coss, Christopher C.; Jones, Amanda; Parke, Deanna N.; Narayanan, Ramesh; Barrett, Christina M.; Kearbey, Jeffrey D.; Veverka, Karen A.; Miller, Duane D.; Morton, Ronald A.; Steiner, Mitchell S.; Dalton, James T. (2012). "Preclinical Characterization of a Novel Diphenyl Benzamide Selective ERα Agonist for Hormone Therapy in Prostate Cancer". Endocrinology. 153 (3): 1070–1081. doi:10.1210/en.2011-1608. ISSN 0013-7227. PMID 22294742.
- ^ von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function--metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738.
- ^ a b c Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- ^ Wibowo E, Schellhammer P, Wassersug RJ (2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". J. Urol. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- ^ a b c John A. Thomas; Edward J. Keenan (6 December 2012). Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 148–. ISBN 978-1-4684-5036-1.
- ^ William R. Miller; James N. Ingle (8 March 2002). Endocrine Therapy in Breast Cancer. CRC Press. pp. 49–52. ISBN 978-0-203-90983-6.
- ^ Ellis, MJ; Dehdahti, F; Kommareddy, A; Jamalabadi-Majidi, S; Crowder, R; Jeffe, DB; Gao, F; Fleming, G; Silverman, P; Dickler, M; Carey, L; Marcom, PK (2014). "A randomized phase 2 trial of low dose (6 mg daily) versus high dose (30 mg daily) estradiol for patients with estrogen receptor positive aromatase inhibitor resistant advanced breast cancer". Cancer Research. 69 (2 Supplement): 16. doi:10.1158/0008-5472.SABCS-16. ISSN 0008-5472.
- ^ a b c Palmieri C, Patten DK, Januszewski A, Zucchini G, Howell SJ (January 2014). "Breast cancer: current and future endocrine therapies". Mol. Cell. Endocrinol. 382 (1): 695–723. doi:10.1016/j.mce.2013.08.001. PMID 23933149. Cite error: The named reference "pmid23933149" was defined multiple times with different content (see the help page).
- ^ http://pharmanovia.com/product/estradurin/
- ^ a b Ostrowski MJ, Jackson AW (1979). "Polyestradiol phosphate: a preliminary evaluation of its effect on breast carcinoma". Cancer Treat Rep. 63 (11–12): 1803–7. PMID 393380. Cite error: The named reference "pmid393380" was defined multiple times with different content (see the help page).
- ^ "ESTRACE® TABLETS (estradiol tablets, USP) FDA label" (PDF). 2005.
- ^ a b c Green RB, Sethi RS, Lindner HH (July 1964). "Treatment of advanced carcinoma of the breast: Progress in therapy during the past decade". Am. J. Surg. 108: 107–21. doi:10.1016/0002-9610(64)90094-7. PMID 14182428.
- ^ a b c Thomas, John A.; Keenan, Edward J. (6 December 1986). "Estrogens and Antiestrogenic Drugs". Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 135–165. doi:10.1007/978-1-4684-5036-1_7. ISBN 978-1-4684-5036-1.
- ^ "Premarin® (conjugated estrogens tablets, USP) FDA label" (PDF). 2003.
- ^ a b c d Van Winkle, Walton (1949). "Council on Pharmacy and Chemistry. Estrogens and Androgens in Mammary Cancer". JAMA: The Journal of the American Medical Association. 140 (15): 1214. doi:10.1001/jama.1949.02900500022007. ISSN 0098-7484.
- ^ a b c d Kahr, Ernst (1966). "Die Allgemeinbehandlung" [General Treatment]. Der Inoperable Krebskranke: Möglichkeiten der Therapie in Klinik und Praxis [The Inoperable Cancer Patient: Possibilities of Therapy in Clinic and Practice]. Springer-Verlag. pp. 104–155. doi:10.1007/978-3-642-86140-6_4. ISBN 978-3-642-86140-6.
- ^ a b c Dao, Thomas L. (1975). "Pharmacology and Clinical Utility of Hormones in Hormone Related Neoplasms". In Alan C. Sartorelli; David G. Johns (eds.). Antineoplastic and Immunosuppressive Agents. pp. 170–192. doi:10.1007/978-3-642-65806-8_11. ISBN 978-3-642-65806-8.
- ^ a b c d Nathanson IT, Kelley RM (January 1952). "Hormonal treatment of cancer". N. Engl. J. Med. 246 (5): 180–9, concl. doi:10.1056/NEJM195201312460505. PMID 14890833.
- ^ a b Dobson L (August 1962). "The management of metastatic breast cancer". Surg. Clin. North Am. 42: 861–76. doi:10.1016/S0039-6109(16)36728-7. PMID 13886800.
- ^ a b Martz G (13 March 2013). Die hormonale Therapie maligner Tumoren: Endokrine Behandlungsmethoden des metastasierenden Mamma-, Prostata- und Uterus-Corpuscarcinoms. Springer-Verlag. pp. 39–. ISBN 978-3-642-86282-3.
- ^ Committee on Research, AMA (1960). "Androgens and estrogens in the treatment of disseminated mammary carcinoma. Retrospective study of nine hundred forty-four patients". Journal of the American Medical Association. 172 (12): 1271. doi:10.1001/jama.1960.03020120049010. ISSN 0002-9955.
- ^ "Estradurin® (polyestradiol phosphate) information and labels". Pharmanovia.
- ^ "Estrone suspension FDA review" (PDF). 1979.
- ^ J. Aiman (6 December 2012). Infertility: Diagnosis and Management. Springer Science & Business Media. pp. 133–134. ISBN 978-1-4613-8265-2.
- ^ Glenn L. Schattman; Sandro Esteves; Ashok Agarwal (12 May 2015). Unexplained Infertility: Pathophysiology, Evaluation and Treatment. Springer. pp. 266–. ISBN 978-1-4939-2140-9.
- ^ a b Pinheiro, Lanna Marla Andrade; Cândido, Priscilla da Silva; Moreto, Tássia Camila; Almeida, Wanessa Gonzaga Di; de Castro, Eduardo Camelo (2017). "Estradiol use in the luteal phase and its effects on pregnancy rates in IVF cycles with GnRH antagonist: a systematic review". JBRA Assisted Reproduction. 21 (3): 247–250. doi:10.5935/1518-0557.20170046. ISSN 1517-5693. PMC 5574648. PMID 28837035.
- ^ A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 512, 696. ISBN 978-3-642-96158-8.
- ^ a b Juul A (2001). "The effects of oestrogens on linear bone growth". Hum. Reprod. Update. 7 (3): 303–13. doi:10.1093/humupd/7.3.303. PMID 11392377.
- ^ Albuquerque EV, Scalco RC, Jorge AA (2017). "Management of Endocrine Disease: Diagnostic and therapeutic approach of tall stature". Eur. J. Endocrinol. 176 (6): R339–R353. doi:10.1530/EJE-16-1054. PMID 28274950.
- ^ Upners EN, Juul A (2016). "Evaluation and phenotypic characteristics of 293 Danish girls with tall stature: effects of oral administration of natural 17β-estradiol". Pediatr. Res. 80 (5): 693–701. doi:10.1038/pr.2016.128. PMID 27410906.
- ^ a b c d Gunther Göretzlehner; Christian Lauritzen; Thomas Römer; Winfried Rossmanith (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 385–. ISBN 978-3-11-024568-4.
- ^ a b R.E. Mansel; Oystein Fodstad; Wen G. Jiang (14 June 2007). Metastasis of Breast Cancer. Springer Science & Business Media. pp. 217–. ISBN 978-1-4020-5866-0.
- ^ a b c d Hartmann BW, Laml T, Kirchengast S, Albrecht AE, Huber JC (1998). "Hormonal breast augmentation: prognostic relevance of insulin-like growth factor-I". Gynecol. Endocrinol. 12 (2): 123–7. doi:10.3109/09513599809024960. PMID 9610425.
- ^ a b c d Lauritzen, C (1980). "Hormonkur kann hypoplastischer Mamma aufhelfen". Selecta (in German) (43): 3798–3801.
- ^ Kaiser, Rolf; Leidenberger, Freimut A. (1991). Hormonbehandlung in der gynäkologischen Praxis (6 ed.). Stuttgart, New York: Georg Thieme Verlag. pp. 138–139. ISBN 978-3133574075.
- ^ a b c Begemann MJ, Dekker CF, van Lunenburg M, Sommer IE (November 2012). "Estrogen augmentation in schizophrenia: a quantitative review of current evidence". Schizophr. Res. 141 (2–3): 179–84. doi:10.1016/j.schres.2012.08.016. PMID 22998932.
- ^ a b Kulkarni J, Gavrilidis E, Wang W, Worsley R, Fitzgerald PB, Gurvich C, Van Rheenen T, Berk M, Burger H (June 2015). "Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age". Mol. Psychiatry. 20 (6): 695–702. doi:10.1038/mp.2014.33. PMID 24732671.
- ^ a b Brzezinski A, Brzezinski-Sinai NA, Seeman MV (May 2017). "Treating schizophrenia during menopause". Menopause. 24 (5): 582–588. doi:10.1097/GME.0000000000000772. PMID 27824682.
- ^ McGregor C, Riordan A, Thornton J (October 2017). "Estrogens and the cognitive symptoms of schizophrenia: Possible neuroprotective mechanisms". Front Neuroendocrinol. 47: 19–33. doi:10.1016/j.yfrne.2017.06.003. PMID 28673758.
- ^ a b de Boer J, Prikken M, Lei WU, Begemann M, Sommer I (January 2018). "The effect of raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a systematic review and meta-analysis". NPJ Schizophr. 4 (1): 1. doi:10.1038/s41537-017-0043-3. PMC 5762671. PMID 29321530.
- ^ a b Khan MM (July 2016). "Neurocognitive, Neuroprotective, and Cardiometabolic Effects of Raloxifene: Potential for Improving Therapeutic Outcomes in Schizophrenia". CNS Drugs. 30 (7): 589–601. doi:10.1007/s40263-016-0343-6. PMID 27193386.
- ^ Owens SJ, Murphy CE, Purves-Tyson TD, Weickert TW, Shannon Weickert C (February 2018). "Considering the role of adolescent sex steroids in schizophrenia". J. Neuroendocrinol. 30 (2): e12538. doi:10.1111/jne.12538. PMID 28941299.
- ^ a b Guay, David R.P. (2009). "Drug treatment of paraphilic and nonparaphilic sexual disorders". Clinical Therapeutics. 31 (1): 1–31. doi:10.1016/j.clinthera.2009.01.009. ISSN 0149-2918. PMID 19243704.
- ^ a b Howard Gethin Morgan; Margaret Hilary Morgan (1984). Aids to Psychiatry. Churchill Livingstone. p. 75. ISBN 978-0-443-02613-3.
Treatment of sexual offenders. Hormone therapy. [...] Oestrogens may cause breast hypertrophy, testicular atrophy, osteoporosis (oral ethinyl oestradiol 0.01-0.05 mg/day causes least nausea). Depot preparation: oestradiol [undecyleate] 50-100mg once every 3–4 weeks. Benperidol or butyrophenone and the antiandrogen cyproterone acetate also used.
- ^ a b Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1419–. ISBN 978-1-60913-345-0.
- ^ a b c d Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. PMID 10230677.
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- ^ Lobo RA (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- ^ Falcone T, Hurd WW (14 June 2017). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer. pp. 179–. ISBN 978-3-319-52210-4.
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- ^ Kleemann A, Engel J, Kutscher B, Reichert D (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1167–1174. ISBN 978-3-13-179525-0.
- ^ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 276, 454–455, 566–567. ISBN 978-3-7692-2114-5.
- ^ Krishna UR, Sheriar NK (1996). Menopause. Orient Blackswan. pp. 70–. ISBN 978-81-250-0910-8.
- ^ a b "NNR: Products Recently Accepted by the A. M. A. Council on Pharmacy and Chemistry". Journal of the American Pharmaceutical Association (Practical Pharmacy ed.). 10 (11): 692–694. 1949. doi:10.1016/S0095-9561(16)31995-8. ISSN 0095-9561. Cite error: The named reference "NNR1949" was defined multiple times with different content (see the help page).
- ^ "AERODIOL (Oestradiol hemihydrate 150 micrograms/actuation)" (PDF). Servier Laboratories (Aust) Pty Ltd.
- ^ a b c d e f g h i j k l m n o "Estradiol". Drugs.com. Cite error: The named reference "Drugs.com" was defined multiple times with different content (see the help page).
- ^ Sahin FK, Koken G, Cosar E, Arioz DT, Degirmenci B, Albayrak R, Acar M (2008). "Effect of Aerodiol administration on ocular arteries in postmenopausal women". Gynecol. Endocrinol. 24 (4): 173–7. doi:10.1080/09513590701807431. PMID 18382901.
300 μg 17β-estradiol (Aerodiol®; Servier, Chambrayles-Tours, France) was administered via the nasal route by a gynecologist. This product is unavailable after March 31, 2007 because its manufacturing and marketing are being discontinued.
- ^ Plouffe Jr L, Ravnikar VA, Speroff L, Watts NB (6 December 2012). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 271–. ISBN 978-1-4612-4330-4.
- ^ Hospital Formulary and Compendium of Useful Information. University of California Press. 1952. pp. 49–. GGKEY:2UAAZRZ5LN0.
- ^ Leidenberger FA (17 April 2013). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 527–. ISBN 978-3-662-08110-5.
- ^ Henzl MR, Loomba PK (July 2003). "Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice". J Reprod Med. 48 (7): 525–40. PMID 12953327.
- ^ a b c d e f g h i j "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018. Cite error: The named reference "Drugs@FDA" was defined multiple times with different content (see the help page).
- ^ a b c Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ Laurtizen, Christian (2001). "Hormone Substitution Before, During and After Menopause" (PDF). In Fisch, Franz H. (ed.). Menopause – Andropause: Hormone Replacement Therapy Through the Ages. Krause & Pachernegg: Gablitz. pp. 67–88. ISBN 978-3-901299-34-6.
- ^ Midwinter, Audrey (1976). "Contraindications to estrogen therapy and management of the menopausal syndrome in these cases". In Campbell, Stuart (ed.). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. MTP Press Limited. pp. 377–382. doi:10.1007/978-94-011-6165-7_33. ISBN 978-94-011-6167-1.
- ^ Pfizer (August 2008). "ESTRING (estradiol vaginal ring)" (PDF).
- ^ a b Rovinski D, Ramos RB, Fighera TM, Casanova GK, Spritzer PM (August 2018). "Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: A systematic review and meta-analysis". Thromb. Res. 168: 83–95. doi:10.1016/j.thromres.2018.06.014. PMID 29936403.
- ^ a b c d Scarabin PY (August 2018). "Progestogens and venous thromboembolism in menopausal women: an updated oral versus transdermal estrogen meta-analysis". Climacteric. 21 (4): 341–345. doi:10.1080/13697137.2018.1446931. PMID 29570359.
- ^ a b c d e Vinogradova Y, Coupland C, Hippisley-Cox J (January 2019). "Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases". BMJ. 364: k4810. doi:10.1136/bmj.k4810. PMC 6326068. PMID 30626577.
- ^ a b c Fruzzetti F, Cagnacci A (2018). "Venous thrombosis and hormonal contraception: what's new with estradiol-based hormonal contraceptives?". Open Access J Contracept. 9: 75–79. doi:10.2147/OAJC.S179673. PMC 6239102. PMID 30519125.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A (January 2013). "The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy". J. Thromb. Haemost. 11 (1): 124–31. doi:10.1111/jth.12060. PMID 23136837.
- ^ Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M (2016). "Oral Contraceptives and HRT Risk of Thrombosis". Clin. Appl. Thromb. Hemost. 24 (2): 217–225. doi:10.1177/1076029616683802. PMC 6714678. PMID 28049361.
- ^ a b Scarabin PY (December 2014). "Hormones and venous thromboembolism among postmenopausal women". Climacteric. 17 Suppl 2: 34–7. doi:10.3109/13697137.2014.956717. PMID 25223916.
- ^ Olié V, Canonico M, Scarabin PY (February 2011). "Postmenopausal hormone therapy and venous thromboembolism". Thromb. Res. 127 Suppl 3: S26–9. doi:10.1016/S0049-3848(11)70008-1. PMID 21262434.
- ^ a b c Bińkowska M (October 2014). "Menopausal hormone therapy and venous thromboembolism". Prz Menopauzalny. 13 (5): 267–72. doi:10.5114/pm.2014.46468. PMC 4520375. PMID 26327865.
- ^ a b c d e f Beyer-Westendorf J, Bauersachs R, Hach-Wunderle V, Zotz RB, Rott H (October 2018). "Sex hormones and venous thromboembolism - from contraception to hormone replacement therapy". VASA. 47 (6): 441–450. doi:10.1024/0301-1526/a000726. PMID 30008249.
- ^ Phillips I, Shah SI, Duong T, Abel P, Langley RE (2014). "Androgen Deprivation Therapy and the Re-emergence of Parenteral Estrogen in Prostate Cancer". Oncol Hematol Rev. 10 (1): 42–47. doi:10.17925/ohr.2014.10.1.42. PMC 4052190. PMID 24932461.
- ^ Kohli M (January 2006). "Phase II study of transdermal estradiol in androgen-independent prostate carcinoma". Cancer. 106 (1): 234–5, author reply 235. doi:10.1002/cncr.21528. PMID 16284988.
- ^ Horský, Jan; Presl, Jiří (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In J. Horsky; J. Presl (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ a b c d Connors JM, Middeldorp S (November 2019). "Transgender patients and the role of the coagulation clinician". J. Thromb. Haemost. 17 (11): 1790–1797. doi:10.1111/jth.14626. PMID 31465627.
- ^ Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ (November 2015). "Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline". J. Clin. Endocrinol. Metab. 100 (11): 3975–4011. doi:10.1210/jc.2015-2236. PMID 26444994.
- ^ Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS, Hwang M, Bis JC, McKnight B, Rice KM, Lumley T, Rosendaal FR, Heckbert SR, Psaty BM (January 2014). "Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens". JAMA Intern Med. 174 (1): 25–31. doi:10.1001/jamainternmed.2013.11074. PMC 4636198. PMID 24081194.
- ^ a b c d e f g Goldstein Z, Khan M, Reisman T, Safer JD (2019). "Managing the risk of venous thromboembolism in transgender adults undergoing hormone therapy". J Blood Med. 10: 209–216. doi:10.2147/JBM.S166780. PMC 6628137. PMID 31372078.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Davey DA (March 2018). "Menopausal hormone therapy: a better and safer future". Climacteric. 21 (5): 454–461. doi:10.1080/13697137.2018.1439915. PMID 29526116.
- ^ a b c Bateson D, Butcher BE, Donovan C, Farrell L, Kovacs G, Mezzini T, Raynes-Greenow C, Pecoraro G, Read C, Baber R (2016). "Risk of venous thromboembolism in women taking the combined oral contraceptive: A systematic review and meta-analysis". Aust Fam Physician. 45 (1): 59–64. PMID 27051991.
- ^ a b c d Cite error: The named reference
FDA2018was invoked but never defined (see the help page). - ^ Khan J, Schmidt RL, Spittal MJ, Goldstein Z, Smock KJ, Greene DN (January 2019). "Venous Thrombotic Risk in Transgender Women Undergoing Estrogen Therapy: A Systematic Review and Metaanalysis". Clin. Chem. 65 (1): 57–66. doi:10.1373/clinchem.2018.288316. PMID 30602475.
- ^ a b L'hermite M, Simoncini T, Fuller S, Genazzani AR (2008). "Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review". Maturitas. 60 (3–4): 185–201. doi:10.1016/j.maturitas.2008.07.007. PMID 18775609.
- ^ a b Holtorf K (January 2009). "The bioidentical hormone debate: are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy?". Postgrad Med. 121 (1): 73–85. doi:10.3810/pgm.2009.01.1949. PMID 19179815.
- ^ a b Conaway E (March 2011). "Bioidentical hormones: an evidence-based review for primary care providers". J Am Osteopath Assoc. 111 (3): 153–64. PMID 21464264.
- ^ a b Simon JA (April 2012). "What's new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone". Climacteric. 15 Suppl 1: 3–10. doi:10.3109/13697137.2012.669332. PMID 22432810.
- ^ a b Mueck AO (April 2012). "Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone". Climacteric. 15 Suppl 1: 11–7. doi:10.3109/13697137.2012.669624. PMID 22432811.
- ^ a b L'Hermite M (August 2013). "HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT". Climacteric. 16 Suppl 1: 44–53. doi:10.3109/13697137.2013.808563. PMID 23848491.
- ^ a b Simon JA (July 2014). "What if the Women's Health Initiative had used transdermal estradiol and oral progesterone instead?". Menopause. 21 (7): 769–83. doi:10.1097/GME.0000000000000169. PMID 24398406.
- ^ a b L'Hermite M (August 2017). "Bioidentical menopausal hormone therapy: registered hormones (non-oral estradiol ± progesterone) are optimal". Climacteric. 20 (4): 331–338. doi:10.1080/13697137.2017.1291607. PMID 28301216.
- ^ a b Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- ^ http://www.ilexmedical.com/files/PDF/Estradiol_ARC.pdf
- ^ Roger Smith (Prof.) (1 January 2001). The Endocrinology of Parturition: Basic Science and Clinical Application. Karger Medical and Scientific Publishers. pp. 89–. ISBN 978-3-8055-7195-1.
- ^ Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (February 2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacol. Sin. 22 (2): 148–54. PMID 11741520.
- ^ Schubert W, Cullberg G, Edgar B, Hedner T (December 1994). "Inhibition of 17 beta-estradiol metabolism by grapefruit juice in ovariectomized women". Maturitas. 20 (2–3): 155–63. doi:10.1016/0378-5122(94)90012-4. PMID 7715468.
- ^ Hormones, Brain and Behavior. Elsevier. 18 June 2002. pp. 759–761. ISBN 978-0-08-053415-2.
- ^ a b Ginsburg ES, Mello NK, Mendelson JH, Barbieri RL, Teoh SK, Rothman M, Gao X, Sholar JW (December 1996). "Effects of alcohol ingestion on estrogens in postmenopausal women". JAMA. 276 (21): 1747–51. doi:10.1001/jama.1996.03540210055034. PMID 8940324.
- ^ a b Sarkola T, Mäkisalo H, Fukunaga T, Eriksson CJ (June 1999). "Acute effect of alcohol on estradiol, estrone, progesterone, prolactin, cortisol, and luteinizing hormone in premenopausal women" (PDF). Alcohol. Clin. Exp. Res. 23 (6): 976–82. doi:10.1111/j.1530-0277.1999.tb04215.x. PMID 10397281.
- ^ Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgend Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- ^ Soltysik K, Czekaj P (April 2013). "Membrane estrogen receptors - is it an alternative way of estrogen action?". J. Physiol. Pharmacol. 64 (2): 129–42. PMID 23756388.
- ^ Prossnitz ER, Barton M (May 2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Mol. Cell. Endocrinol. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ^ Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Res. 38 (11 Pt 2): 4186–98. PMID 359134.
- ^ Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ^ Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". J. Steroid Biochem. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- ^ a b A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548–. ISBN 978-3-642-96158-8.
- ^ Jennifer E. Dietrich (18 June 2014). Female Puberty: A Comprehensive Guide for Clinicians. Springer. pp. 53–. ISBN 978-1-4939-0912-4.
- ^ Randy Thornhill; Steven W. Gangestad (25 September 2008). The Evolutionary Biology of Human Female Sexuality. Oxford University Press. pp. 145–. ISBN 978-0-19-988770-5.
- ^ Raine-Fenning NJ, Brincat MP, Muscat-Baron Y (2003). "Skin aging and menopause : implications for treatment". Am J Clin Dermatol. 4 (6): 371–8. doi:10.2165/00128071-200304060-00001. PMID 12762829.
- ^ Chris Hayward (31 July 2003). Gender Differences at Puberty. Cambridge University Press. pp. 22–. ISBN 978-0-521-00165-6.
- ^ a b Shlomo Melmed; Kenneth S. Polonsky; P. Reed Larsen; Henry M. Kronenberg (11 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 1105–. ISBN 978-0-323-34157-8.
- ^ a b c Richard E. Jones; Kristin H. Lopez (28 September 2013). Human Reproductive Biology. Academic Press. pp. 19–. ISBN 978-0-12-382185-0.
- ^ a b Ethel Sloane (2002). Biology of Women. Cengage Learning. pp. 496–. ISBN 978-0-7668-1142-3.
- ^ a b Tekoa L. King; Mary C. Brucker (25 October 2010). Pharmacology for Women's Health. Jones & Bartlett Learning. pp. 1022–. ISBN 978-0-7637-5329-0.
- ^ a b c Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- ^ a b David Warshawsky; Joseph R. Landolph Jr. (31 October 2005). Molecular Carcinogenesis and the Molecular Biology of Human Cancer. CRC Press. pp. 457–. ISBN 978-0-203-50343-0.
- ^ M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28–November 2, 1984. Springer Science & Business Media. pp. 397, 399. ISBN 978-94-009-4145-8.
[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/ml to only 13 pg/ml.
- ^ C. Christian; B. von Schoultz (15 March 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 9–16, 60. ISBN 978-1-85070-545-1.
The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/ml.
- ^ Eugenio E. Müller; Robert M. MacLeod (6 December 2012). Neuroendocrine Perspectives. Springer Science & Business Media. pp. 121–. ISBN 978-1-4612-3554-5.
[...] [premenopausal] mean [estradiol] concentration of 150 pg/ml [...]
- ^ a b c d Botros R M B Rizk; Hassan N Sallam (15 June 2012). Clinical Infertility and In Vitro Fertilization. JP Medical Ltd. pp. 11–. ISBN 978-93-5025-095-2.
- ^ a b c d e f g J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 897–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g h i j k l m n o p q Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. pp. 404–406. ISBN 978-3-88763-075-1. Retrieved 13 September 2012.
- ^ a b c Fluhmann CF (1938). "Estrogenic Hormones: Their Clinical Usage". Cal West Med. 49 (5): 362–6. PMC 1659459. PMID 18744783.
- ^ James R. Givens; Garland D. Anderson (1981). Endocrinology of Pregnancy: Based on the Proceedings of the Fifth Annual Symposium on Gynecologic Endocrinology, Held March 3-5, 1980 at the University of Tennessee, Memphis, Tennessee. Year Book Medical Publishers. p. 158. ISBN 978-0-8151-3529-6.
Estetrol (E4) is an estrogen with four hydroxyl groups. More specifically, E4, is 15α-hydroxyestriol.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research On Cancer (2007). Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. World Health Organization. p. 384. ISBN 978-92-832-1291-1. Retrieved 13 September 2012.
- ^ a b Archana Desai; Mary Lee (7 May 2007). Gibaldi's Drug Delivery Systems in Pharmaceutical Care. ASHP. p. 337. ISBN 978-1-58528-136-7. Retrieved 13 September 2012.
- ^ Rebekah Wang-Cheng; Joan M. Neuner; Vanessa M. Barnabei (2007). Menopause. ACP Press. pp. 91–. ISBN 978-1-930513-83-9.
- ^ Mary Lee; Archana Desai (2007). Gibaldi's Drug Delivery Systems in Pharmaceutical Care. ASHP. pp. 336–. ISBN 978-1-58528-136-7.
- ^ Nursing2013 Drug Handbook. Lippincott Williams & Wilkins. 2012. pp. 528–. ISBN 978-1-4511-5023-0.
- ^ Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- ^ Ravery V, Fizazi K, Oudard S, Drouet L, Eymard JC, Culine S, Gravis G, Hennequin C, Zerbib M (December 2011). "The use of estramustine phosphate in the modern management of advanced prostate cancer". BJU Int. 108 (11): 1782–6. doi:10.1111/j.1464-410X.2011.10201.x. PMID 21756277.
- ^ Hubert Vaudry; Olivier Kah (25 January 2018). Trends in Comparative Endocrinology and Neurobiology. Frontiers Media SA. pp. 115–. ISBN 978-2-88945-399-3.
- ^ Shoupe, Donna; Haseltine, Florence P. (6 December 2012). Contraception. Springer Science & Business Media. pp. 2–. ISBN 978-1-4612-2730-4.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Reilly WA (1941). "Estrogens: Their Use in Pediatrics". Cal West Med. 55 (5): 237–9. PMC 1634235. PMID 18746057.
- ^ Novak, Emil (1935). "The Therapeutic Use of Estrogenic Substances". JAMA: The Journal of the American Medical Association. 104 (20): 1815. doi:10.1001/jama.1935.92760200002012. ISSN 0098-7484.
Progynon B (Schering), in 1 cc. ampules, of 10,000 or 50,000 international units of hydroxyestrin benzoate in sesame oil.
- ^ Escamilla, Roberto F.; Lisserf, H. (1940). "Induction of menarche and development of secondary sexual characteristics in a woman aged 34 by injections of estradiol dipropionate". Endocrinology. 27 (1): 153. doi:10.1210/endo-27-1-153. ISSN 0013-7227.
The estradiol dipropionate used in this case was furnished by the Ciba Co. Their trade name for this product is Di-Ovocylin.
- ^ Shorr, E. (1940). "Effect of Concomitant Administration of Estroens and Proesterone on Vainal Smear in Man". Experimental Biology and Medicine. 43 (3): 501–506. doi:10.3181/00379727-43-11244. ISSN 1535-3702.
Grateful acknowledgment is made to Dr. Erwin Schwenk of the Schering Corporation for the estradiol benzoate (Progynon B), estradiol dipropionate (Progynon DP), progesterone (Proluton), and pregneninolone (Pranone) used in these experiments;
- ^ Macpherson AS (June 1940). "The Use of Œstrogens in Obstetrics and Gynæcology". Edinb Med J. 47 (6): 406–424. PMC 5306594. PMID 29646930.
- ^ Walton, Robert P. (1944). "Sublingual Administration of Drugs". Journal of the American Medical Association. 124 (3): 138. doi:10.1001/jama.1944.02850030006002. ISSN 0002-9955.
- ^ Corner, George W. (1944). "The Absorption of Steroid Hormones from the Oral Mucous Membranes, with Special Reference to the Sublingual Administration of Progesterone". American Journal of Obstetrics and Gynecology. 47 (5): 670–677. doi:10.1016/S0002-9378(16)40321-2. ISSN 0002-9378.
- ^ Lisser, H.; Gordan, Gilbert S.; Aird, R. B.; Arrick, M. S.; Craig, Leela S.; Escamilla, R. F.; Goldberg, Minnie B. (1950). "Sublingual or Buccal Administration of Steroidal Hormones". Postgraduate Medicine. 8 (5): 393–400. doi:10.1080/00325481.1950.11694030. ISSN 0032-5481.
- ^ Oil, Paint and Drug Reporter. April 1950.
Trade Briefs Wyeth, Inc., Philadelphia, has commenced marketing "Estradiol Membrettes, 0.25 mg." as an addition to its hormone line.
- ^ American Professional Pharmacist. American Professional Pharmacist, Incorporated. 1950. p. 647.
- ^ Medical Times. Romaine Pierson Pub. 1950. p. 248.
- ^ Ashton Leroy Welsh (1951). Dermatological Formulary: A Guide for Medical Students and Resident Physicians in Dermatology. Educational Publishers. p. 155.
- ^ Omaha Midwest Clinical Society (1952). Journal.
DIOGYNETS*. Estradiol, U.S.P., Transmucosal Tablets 0.125 mg., 0.25 mg. and 1.0 mg.
- ^ General Practitioner. American Academy of General Practice. April 1954. pp. 168–170.
Diogynets* [...] * brand of estradiol transmucosal tablets, scored: 0.125 mg., 0.25 mg. and 1.0 mg., bottles of 50 and 100.
- ^ Allan William Spence (1953). Clinical Endocrinology. Cassell. p. 547.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. ISBN 978-0-8155-1856-3.
- ^ Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
- ^ a b c Martin PL, Burnier AM, Greaney MO (1972). "Oral menopausal therapy using 17- micronized estradiol. A preliminary study of effectiveness, tolerance and patient preference". Obstet Gynecol. 39 (5): 771–4. doi:10.1097/00006250-197205000-00022 (inactive 2019-12-09). PMID 5023261.
{{cite journal}}: CS1 maint: DOI inactive as of December 2019 (link) - ^ Velikay L (March 1968). "Die perorale Behandlung des klimakterischen Syndroms mit Ostradiolvalerianat" [The peroral treatment of the climacteric syndrome with estradiol valerate]. Wien. Klin. Wochenschr. (in German). 80 (12): 229–33. ISSN 0043-5325. PMID 5728263.
- ^ Koed J (May 1972). "Zur Behandlung klimakterischer Ausfallserscheinungen mit Progynova" [Therapy of climacteric deficiency symptoms using Progynova]. Med Welt (in German). 23 (22): 834–6. ISSN 0025-8512. PMID 5045321.
- ^ Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 392–. ISBN 978-1-4832-7299-3.
- ^ J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 313–. ISBN 978-94-009-8195-9.
- ^ Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. p. 34. ISBN 978-0-12-137250-7.
- ^ Gibian H, Kopp R, Kramer M, Neumann F, Richter H (1968). "Effect of particle size on biological activity of norethisterone acetate". Acta Physiol Lat Am. 18 (4): 323–6. PMID 5753386.
- ^ He CH, Shi YE, Liao DL, Zhu YH, Xu JQ, Matlin SA, Vince PM, Fotherby K, Van Look PF (May 1990). "Comparative cross-over pharmacokinetic study on two types of postcoital contraceptive tablets containing levonorgestrel". Contraception. 41 (5): 557–67. doi:10.1016/0010-7824(90)90064-3. PMID 2112080.
- ^ Rigg LA, Milanes B, Villanueva B, Yen SS (1977). "Efficacy of intravaginal and intranasal administration of micronized estradiol-17beta". J. Clin. Endocrinol. Metab. 45 (6): 1261–4. doi:10.1210/jcem-45-6-1261. PMID 591620.
- ^ a b c d e Yoo JW, Lee CH (May 2006). "Drug delivery systems for hormone therapy". J Control Release. 112 (1): 1–14. doi:10.1016/j.jconrel.2006.01.021. PMID 16530874.
Transdermal gels. The first system used for estrogen delivery through skin was the application of estrogen dissolved into a water–alcohol solvent in a form of gel for the treatment of postmenopausal symptoms [80] [...] EstroGel® (Solvay) has been in the Europe market for more than 25 years, but approved in the U.S. only in 2004. EstroGel® contains 17β-estradiol in a hydro-alcoholic gel base which renders a controlled release profile. [...] Estrasorb® (Novavax, Malvern, PA) is launched in 2003 as the first topical, lotion-like nanoemulsion for the treatment of vasomotor symptoms. [...] Conventional reservoir patches. The first transdermal patch for HT was Estraderm® (Novartis, Switzerland) which was launched in Europe in 1985 and has been widely used ever since. [...] Transdermal matrix patches. [...] Climara® (Berlex, Montville, NJ) was first introduced as the matrix patch in 1995. A year later, Vivelle® (Novogyne, Miami, FL) was introduced in the market [...]
- ^ Davis SR, Dinatale I, Rivera-Woll L, Davison S (May 2005). "Postmenopausal hormone therapy: from monkey glands to transdermal patches". J. Endocrinol. 185 (2): 207–22. doi:10.1677/joe.1.05847. PMID 15845914.
One of the earliest reports of the novel transdermal patch delivery system for oestradiol was published in 1983 (Laufer et al. 1983).
- ^ Benson, Heather A. E.; Watkinson, Adam C. (2011). Transdermal and Topical Drug Delivery Today. doi:10.1002/9781118140505. ISBN 9781118140505.
Table 18.1 Passive Transdermal Drugs for Systemic Drug Delivery Launched in the United States and Europe [...] Drug: Estradiol. Indication: Female HRT. U.S. approval: 1986. Marketed in the EU: Yes.
- ^ Prausnitz MR, Mitragotri S, Langer R (February 2004). "Current status and future potential of transdermal drug delivery". Nat Rev Drug Discov. 3 (2): 115–24. doi:10.1038/nrd1304. PMID 15040576.
Timeline: Important events in transdermal drug delivery: [...] 1986: Estraderm (17β-oestradiol) patch, FDA approval for hormone replacement.
- ^ Pastore MN, Kalia YN, Horstmann M, Roberts MS (May 2015). "Transdermal patches: history, development and pharmacology". Br. J. Pharmacol. 172 (9): 2179–209. doi:10.1111/bph.13059. PMC 4403087. PMID 25560046.
- ^ a b Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 7–8. ISBN 978-3-642-58616-3.
- ^ Mosby's GenRx: A Comprehensive Reference for Generic and Brand Prescription Drugs. Mosby. 2001. p. 944. ISBN 978-0-323-00629-3.
- ^ a b I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 206–. ISBN 978-94-011-4439-1.
- ^ http://www.kegg.jp/entry/D00105
- ^ http://www.wjpps.com/download/article/1412071798.pdf
- ^ Rowlands, S (2009). "New technologies in contraception" (PDF). BJOG: An International Journal of Obstetrics & Gynaecology. 116 (2): 230–239. doi:10.1111/j.1471-0528.2008.01985.x. ISSN 1470-0328. PMID 19076955.
- ^ Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2097. ISBN 978-0-85369-840-1.
- ^ http://www.micromedexsolutions.com
- ^ http://adisinsight.springer.com/drugs/800038089
- ^ Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B (2015). "Pharmacokinetics of the first combination 17β-estradiol/progesterone capsule in clinical development for menopausal hormone therapy". Menopause. 22 (12): 1308–16. doi:10.1097/GME.0000000000000467. PMC 4666011. PMID 25944519.
- ^ Kaunitz AM, Kaunitz JD (2015). "Compounded bioidentical hormone therapy: time for a reality check?". Menopause. 22 (9): 919–20. doi:10.1097/GME.0000000000000484. PMID 26035149.
- ^ a b Pinkerton JV, Pickar JH (February 2016). "Update on medical and regulatory issues pertaining to compounded and FDA-approved drugs, including hormone therapy". Menopause. 23 (2): 215–23. doi:10.1097/GME.0000000000000523. PMC 4927324. PMID 26418479.
- ^ Fugh-Berman A, Bythrow J (2007). "Bioidentical hormones for menopausal hormone therapy: variation on a theme". J Gen Intern Med. 22 (7): 1030–4. doi:10.1007/s11606-007-0141-4. PMC 2219716. PMID 17549577.
- ^ Catriona Melville (22 September 2015). Sexual and Reproductive Health at a Glance. Wiley. pp. 108–. ISBN 978-1-119-23516-3.
- ^ Colin T. Dollery (1991). Therapeutic Drugs. Churchill Livingstone.
Parenteral preparations 1. Oestradiol implants (Organon, UK) are sterilized pellets containing 25, 50 or 100 mg. They are supplied individually in glass tubes.
- ^ Ashutosh Kar (2005). Medicinal Chemistry. New Age International. pp. 614–. ISBN 978-81-224-1565-0.
Oestradiol Implants(R) (Organon, U.K.). Dose. [...] implantation, 20 to 100 mg.
- ^ William A. W. Walters (10 July 1986). Transsexualism and sex reassignment. Oxford University Press. p. 159. ISBN 978-0-19-554462-6.
2. Injections or implants [...] Oestradiol Implants (Organon) 20 mg pellets. 50 mg pellets. 100 mg pellets.
- ^ Wiebe J. Braam (2006). Broze botten. Inmerc. pp. 79–. ISBN 978-90-6611-844-7.
- ^ https://www.gezondheidsnet.nl/medicijnen/meno-implantr
- ^ Bernard A. Eskin (1988). The Menopause: Comprehensive Management. Macmillan. p. 278. ISBN 978-0-02-334230-1.
Estradiol Pellets — (Progynon Pellets® — Progynon Associates), 25 mg (Estropel Pellets® — Bartor Pharmacal).
- ^ American Medical Association. Dept. of Drugs; Council on Drugs (American Medical Association); American Society for Clinical Pharmacology and Therapeutics (1 February 1977). "Estrogens, Progestagens, Oral Contraceptives, and Ovulatory Agents". AMA drug evaluations. Publishing Sciences Group. pp. 540–572. ISBN 978-0-88416-175-2.
Subcutaneous implantation: (Estradiol) One 25 mg pellet every three to four months or two 25 mg pellets every four to six months. Preparations. Progynon (Schering). Implantation: Pellets 25 mg.
- ^ John Morgan Jones (1979). Physicians' Desk Reference. Medical Economics Company. p. 1508.
PROGYNON Pellets for subcutaneous implantation are cylindrical in shape with an approximate diameter of 3.2 mm. and length of 3.5 mm. Each PROGYNON Pellet contains 25 mg. estradiol.
- ^ Martin Birkhauser; David Barlow; Morris Notelovitz; Margaret Rees (12 August 2005). Health Plan for the Adult Woman: Management Handbook. CRC Press. pp. 27–. ISBN 978-0-203-49009-9.
- ^ Pinkerton JV (December 2014). "What are the concerns about custom-compounded "bioidentical" hormone therapy?". Menopause. 21 (12): 1298–300. doi:10.1097/GME.0000000000000376. PMID 25387347.
- ^ Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (April 2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". J. Clin. Endocrinol. Metab. 101 (4): 1318–43. doi:10.1210/jc.2016-1271. PMID 27032319.
- ^ "HRT stock update shows that supply problems are set to continue into 2020". Pharmaceutical Journal. 29 August 2019. Retrieved 5 October 2019.
- ^ "Estradiol/Progesterone (Bijuva) for Menopausal Vasomotor Symptoms". JAMA. 322 (12): 1206. 2019. doi:10.1001/jama.2019.10692. ISSN 0098-7484.
- ^ Gentile S (December 2005). "The role of estrogen therapy in postpartum psychiatric disorders: an update". CNS Spectr. 10 (12): 944–52. doi:10.1017/S1092852900010518. PMID 16344831.
- ^ Ng RC, Hirata CK, Yeung W, Haller E, Finley PR (September 2010). "Pharmacologic treatment for postpartum depression: a systematic review". Pharmacotherapy. 30 (9): 928–41. doi:10.1592/phco.30.9.928. PMID 20795848.
- ^ di Scalea TL, Wisner KL (November 2009). "Pharmacotherapy of postpartum depression". Expert Opin Pharmacother. 10 (16): 2593–607. doi:10.1517/14656560903277202. PMC 2929691. PMID 19874247.
- ^ Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL (September 2009). "Transdermal estradiol for postpartum depression: a promising treatment option". Clin Obstet Gynecol. 52 (3): 516–29. doi:10.1097/GRF.0b013e3181b5a395. PMC 2782667. PMID 19661765.
- ^ Sharma V (October 2003). "Pharmacotherapy of postpartum psychosis". Expert Opin Pharmacother. 4 (10): 1651–8. doi:10.1517/14656566.4.10.1651. PMID 14521476.
Further reading
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. ISBN 978-3-642-58616-3.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. ISBN 978-3-642-60107-1.
- Fruzzetti F, Trémollieres F, Bitzer J (2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- Stanczyk FZ, Archer DF, Bhavnani BR (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–27. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.