Wikipedia:Reference desk/Science: Difference between revisions
Count Iblis (talk | contribs) |
|||
| Line 656: | Line 656: | ||
:It should never be forgoten that with drugs such as heroin, that while the addict has a good supply, he/she thinks life is really good and their perception of themself and their life is distorted. Thinks like Western Govt "Just say no" programms sound about as sensible as saying the moon is made of cheese. |
:It should never be forgoten that with drugs such as heroin, that while the addict has a good supply, he/she thinks life is really good and their perception of themself and their life is distorted. Thinks like Western Govt "Just say no" programms sound about as sensible as saying the moon is made of cheese. |
||
:Opiates are disease masking. When heroin addicts came to us expressing a wish to get off it (usually when their supply has become a little unreliable), the fisrt thing we did was take then to a doctor for a really go checkout. Occaisonally they had broken bones and/or serious kidney or liver disease - and they didn't know. |
:Opiates are disease masking. When heroin addicts came to us expressing a wish to get off it (usually when their supply has become a little unreliable), the fisrt thing we did was take then to a doctor for a really go checkout. Occaisonally they had broken bones and/or serious kidney or liver disease - and they didn't know. |
||
:I hope that, if you read through all this, you have some understanding that the graph the OP posted is about right. I've simplified |
:I hope that, if you read through all this, you have some understanding that the graph the OP posted is roughly about right. I've simplified the story somewhat. I would move some drugs about on the graph to a certain extent. Barbituate addiction is pretty nasty. I smoked as a teenager but found the habit easy to break. :Wickwack[[Special:Contributions/124.182.14.180|124.182.14.180]] ([[User talk:124.182.14.180|talk]]) 01:20, 25 May 2012 (UTC) |
||
:Wickwack[[Special:Contributions/124.182.14.180|124.182.14.180]] ([[User talk:124.182.14.180|talk]]) 01:20, 25 May 2012 (UTC) |
|||
= May 25 = |
= May 25 = |
||
Revision as of 01:28, 25 May 2012
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
May 20
Locomotives
There are 0-4-0s, 2-2-0s, and 0-2-2s. Why aren't there 0-2-0s? Whoop whoop pull up Bitching Betty | Averted crashes 01:57, 20 May 2012 (UTC)
- In France an 0-4-0 would be an 020. Sticking to American notation, an 0-2-0 would have a single axle, which seems unsatisfactory, rather like a car with one axle. Acroterion (talk) 02:03, 20 May 2012 (UTC)
- Although, strangely, there have been 0-3-0s. Gandalf61 (talk) 08:51, 20 May 2012 (UTC)
- And 1-1-2-1-1's. Tevildo (talk) 12:40, 20 May 2012 (UTC)
- The answer is, therefore, simple statics. It would be possible to have a 0-2-0 or 0-1-0 locomotive on a Lartigue-style monorail, with the centre of gravity of the loco below the rail. However, the minimum stable configuration for a loco with all its wheels on the ground would be a tricycle 1-2-0 or 0-2-1 arrangement, but this would require three rails rather than two. Tevildo (talk) 12:51, 20 May 2012 (UTC)
- There is an important confusion to be resolved here. In Europe, an 0-4-0 engine has 8 wheels, 4 on each side - the number '4' refers to the number of axles. In the US, the numbers count wheels instead so an 0-4-0 has only 4 wheels, 2 on each side. Hence, in US notation, an 0-2-0 would be silly - an engine with just one wheel on each side! Obviously such a thing cannot exist on a conventional railroad. In Europe, an 0-2-0 is a simple 4-wheeled, 2 axle vehicle with all four wheels being driven...like a 4WD car. I strongly suspect that our questioner is using European notation and asking why there aren't any engines with just four driven wheels on two axles. The answer probably relates to stability on the track or something...but I don't know details. SteveBaker (talk) 15:03, 20 May 2012 (UTC)
- There were plenty of two-axled locomotives (0-4-0 in American (Whyte) notation, 0-2-0 in French) during the age of steam after the initial era of development: they were most typically small shunting engines, though they might occasionally be used for pulling small, local freight or passenger trains. In the subsequent diesel and electric era, 0-6-0s tended to be the minimum, probably because railway vehicles in general became larger and heavier, and train lengths on average greater: the locos themselves therefore also had to become more powerful and heavier, and a heavy 0-4-0 would exert too great an axle load on the track, whereas the same loco in 0-6-0 format would have a maximum axle load 33% less.
- (Early in locomotive evolution, of course, 0-2-2s and 2-2-0s also existed – Stephenson's Rocket was an 0-2-2.)
- To further avoid confusion, it should be mentioned that the UK (and hence the Empire/Commonwealth countries) also employed the Whyte notation system rather than the Continental.
- By the way – Welcome back, Steve! {The poster formerly known as 87.81.230.195} 90.197.66.211 (talk) 22:49, 20 May 2012 (UTC)
Geiger tube continuation
I calculated the potential difference between the wire and the tube of a Geiger tube to be approximately. The power-supply voltage is slowly increased until you see a glow in the air very near the inner wire. This means that the air breakdown potential has been reached (and they provide a value for this and for all the other variables). Calculate this power-supply voltage. How do you do this calculation? Do you just substitute the numbers into the above equation or is it more involved than that? --150.203.114.37 (talk) 04:54, 20 May 2012 (UTC
- It would help if you told us what L, Q, R, & r signify. Operating a geiger tube at a voltage high enough to cause a gas discharge (as indicated by a glow) is harmful to it. Normally, you just set the voltage to the value stipulated by the gieger tube manufacturer. This is at a "plateau" of maximum sensitivity that occures just below the glow point. Ratbone60.230.203.253 (talk) 07:23, 20 May 2012 (UTC)
- Maybe; I didn't actually observe this; it's just a theoretical problem. Q = charge on the wire, R = radius of the tube, r = radius of the wire, L = length of the wire/tube. --150.203.114.37 (talk) 07:30, 20 May 2012 (UTC)
- Then the formula given is the relation between the potential difference and charge as for any coaxial pair of conductors separated by a perfect insulator of permitivity e0. It is a concept useful in calculating the capacitance http://en.wikipedia.org/wiki/Capacitance and has no relavence whatsowver to the operating voltage of a gieger tube. Under operating conditions,gieger tubes are operated with DC voltage, and no current flows in the tube capacitance, except for the very brief recharging current after each detected particle. Note that the term eo, strictly speaking, being the symbol for the permitivity of free space, is in any case incorrect. It should be the permitivity of the gas used (e0.k, which will however be sensibly close to e0). Keit120.145.31.247 (talk) 09:22, 20 May 2012 (UTC)
- Ah. What is the correct furmula then, and how do you derive it? --150.203.114.37 (talk) 10:44, 20 May 2012 (UTC)
- Then the formula given is the relation between the potential difference and charge as for any coaxial pair of conductors separated by a perfect insulator of permitivity e0. It is a concept useful in calculating the capacitance http://en.wikipedia.org/wiki/Capacitance and has no relavence whatsowver to the operating voltage of a gieger tube. Under operating conditions,gieger tubes are operated with DC voltage, and no current flows in the tube capacitance, except for the very brief recharging current after each detected particle. Note that the term eo, strictly speaking, being the symbol for the permitivity of free space, is in any case incorrect. It should be the permitivity of the gas used (e0.k, which will however be sensibly close to e0). Keit120.145.31.247 (talk) 09:22, 20 May 2012 (UTC)
- Maybe; I didn't actually observe this; it's just a theoretical problem. Q = charge on the wire, R = radius of the tube, r = radius of the wire, L = length of the wire/tube. --150.203.114.37 (talk) 07:30, 20 May 2012 (UTC)
- As Ratbone said, this is not something you would normally calculate. The manufacturer of the geiger tube will tell you what voltage to use. The manufacturer determines the optimum voltage by testing. However, an approximate calculation of the optimum operating voltage can be found by using the formula for breakdown voltage of a gas under partial vacuum, given in http://en.wikipedia.org/wiki/Breakdown_voltage. As Ratbone also said, the optimum voltage is just a bit below the breakdown voltage. If you are intending to make your own geiger tube, say so, we can then give you information of more practical benefit. Keit60.230.198.136 (talk) 11:41, 20 May 2012 (UTC)
- This is just a homework question; I am not trying to make one of these. I have never seen this formula before. Presumably you are required to use some of the parameters given. It's possible you also need some other well-known constants, but I wouldn't know. This is the exact text of the question: The power-supply voltage is slowly increased until you see a glow in the air very near the inner wire. This means that the air breakdown potential of has been reached. Calculate this power-supply voltage (give a numerical value) and explain your calculation. The length L = 80 cm, the inner radius r = 0.7 mm, and the outer radius R = 3 cm.--150.203.114.37 (talk) 13:20, 20 May 2012 (UTC)
- In which case you have attempted a $10 answer to a 10 cent question, unless the orginal question gave you the gas pressure, and you can ignore the answers given previously by Ratbone and myself. Whoever wrote this question has a very simplified view of geiger tubes. All you need to answer the question is given in the question, plus a bit of the most elementary algebra. You can ignore the length, as a long tube has the gas under the same voltage gradient stress as a short one. The answer, fitting the data given, is 88 kV. As it is a homework question, I won't say any more than that. Real geiger tubes have an operating voltage of around 500V, because radius of the tube is smaller, and because the gas inside, which isn't air, is under partial vacuum, but this is no concern to you - you need to write what the teacher wants. The moral of this posting is that if you have a homework question, gives us the exact question, and what you have attempted in order to solve it. Then you may get an answer from us appropriate to your needs. Keit60.230.198.136 (talk) 15:14, 20 May 2012 (UTC)60.230.198.136 (talk) 14:57, 20 May 2012 (UTC)
- The problem is I don't know where to start. Is there an equation which relates all these variables that I have to manipulate? --150.203.114.37 (talk) 15:49, 20 May 2012 (UTC)
- Are you pulling my leg? This is very simple for a chap prepared to get involved with integrals etc as in your first version of this posted question. You don't need no fancy formula - its just proportion. As stated in your assignemnt question, the breakdown voltage of air at standard atmostpheric pressure is approx 3 x 106 V / m. So if you have a breakdown distance of 2 m, you'd need 2 x 3 x 106 V (ie 6 MV); if you have 0.1 m, you'd need 0.1 x 3 x 106 V (300 kV). And the breakdown is between the 0.7 mm radius wire and the 3 cm radius cylinder.... Go for it tiger. Keit58.167.225.195 (talk) 16:10, 20 May 2012 (UTC)
- I see. But how do you know that that is the breakdown distance? There being a glow "very near" the wire may imply that the breakdown distance is less than that. Or is the breakdown distance necessarily the distance between the wire and the tube in a Geiger tube? --150.203.114.37 (talk) 17:45, 20 May 2012 (UTC)
- (edit conflict) I think that is missleading, the field is not homogenius, the glow was just closest to the inner conductor. You need to integrate the field along a radius or use a ready formula for this case. Due to rules regarding homework i do not want to give it here but you could calculate the linear charge that gives the desired field at the surface of the inner conductor and then use the formula you gave. Gr8xoz (talk) 17:49, 20 May 2012 (UTC)
- Does this matter? Is the power-supply voltage not still 87900 V? What calculation am I supposed to do? I don't understand. --150.203.114.37 (talk) 21:38, 20 May 2012 (UTC)
- Are you pulling my leg? This is very simple for a chap prepared to get involved with integrals etc as in your first version of this posted question. You don't need no fancy formula - its just proportion. As stated in your assignemnt question, the breakdown voltage of air at standard atmostpheric pressure is approx 3 x 106 V / m. So if you have a breakdown distance of 2 m, you'd need 2 x 3 x 106 V (ie 6 MV); if you have 0.1 m, you'd need 0.1 x 3 x 106 V (300 kV). And the breakdown is between the 0.7 mm radius wire and the 3 cm radius cylinder.... Go for it tiger. Keit58.167.225.195 (talk) 16:10, 20 May 2012 (UTC)
- The problem is I don't know where to start. Is there an equation which relates all these variables that I have to manipulate? --150.203.114.37 (talk) 15:49, 20 May 2012 (UTC)
- In which case you have attempted a $10 answer to a 10 cent question, unless the orginal question gave you the gas pressure, and you can ignore the answers given previously by Ratbone and myself. Whoever wrote this question has a very simplified view of geiger tubes. All you need to answer the question is given in the question, plus a bit of the most elementary algebra. You can ignore the length, as a long tube has the gas under the same voltage gradient stress as a short one. The answer, fitting the data given, is 88 kV. As it is a homework question, I won't say any more than that. Real geiger tubes have an operating voltage of around 500V, because radius of the tube is smaller, and because the gas inside, which isn't air, is under partial vacuum, but this is no concern to you - you need to write what the teacher wants. The moral of this posting is that if you have a homework question, gives us the exact question, and what you have attempted in order to solve it. Then you may get an answer from us appropriate to your needs. Keit60.230.198.136 (talk) 15:14, 20 May 2012 (UTC)60.230.198.136 (talk) 14:57, 20 May 2012 (UTC)
- This is just a homework question; I am not trying to make one of these. I have never seen this formula before. Presumably you are required to use some of the parameters given. It's possible you also need some other well-known constants, but I wouldn't know. This is the exact text of the question: The power-supply voltage is slowly increased until you see a glow in the air very near the inner wire. This means that the air breakdown potential of has been reached. Calculate this power-supply voltage (give a numerical value) and explain your calculation. The length L = 80 cm, the inner radius r = 0.7 mm, and the outer radius R = 3 cm.--150.203.114.37 (talk) 13:20, 20 May 2012 (UTC)
- As Ratbone said, this is not something you would normally calculate. The manufacturer of the geiger tube will tell you what voltage to use. The manufacturer determines the optimum voltage by testing. However, an approximate calculation of the optimum operating voltage can be found by using the formula for breakdown voltage of a gas under partial vacuum, given in http://en.wikipedia.org/wiki/Breakdown_voltage. As Ratbone also said, the optimum voltage is just a bit below the breakdown voltage. If you are intending to make your own geiger tube, say so, we can then give you information of more practical benefit. Keit60.230.198.136 (talk) 11:41, 20 May 2012 (UTC)
- When the glow is seen, breakdown has already occurred. Any non-linearity in electric field strength as alluded to by Gr8xoz has no relavence to your assignment question, because you want the breakdown initiating voltage, which is under conditions existing just before breakdown actually occurs. In practice, as you slowly increase the voltage, breakdown occurs suddenly with only a very small increase. So this additional small voltage, between no breakdown and breakdown started, can be neglected. Yes, obviously, the electric field stress on the gas/air (ie a field which accelerates the odd free electron fast enough to strike a gas molecule with enough kinetic energy to knock off another electron) is between the wire and the cylinder. As it is homework I don't want to put it any plainer than that - what you learn and remember is proportional to the mental effort YOU put in, not what I put in. If you want to understand the breakdown phenomena, and thus why the breakdown voltage is proportional to the breakdown distance, FOR THE DIMENSIONS AS GIVEN, start with our article on Paschen's Law, http://en.wikipedia.org/wiki/Paschen%27s_law. You could have found this yourself if you checked the Wiki link I gave you previously. The relationship between distance and voltage becomes very non-linear at small distances, but this is at distances very tiny compared to the dimensions given in your question. Given that whoever wrote the question has a very simpilfied view of geiger tubes (the dimensions are very innappropriate, and geiger tubes use particular gases under partial vacuum, not air at standard pressure, tubes are operated just below breakdown, not at breakdown), I suspect only a very simple answer is required. Is this from a high school math assignment? What year? Or a university physics course? Keit124.182.148.14 (talk) 01:32, 21 May 2012 (UTC)
Domestic Heat Pump connections
I am planning to install an air to water domestic heat pump for heating and am looking at the manuals. The 12 kW heat pump works best when there is a 35 litres/minute flow through it (it then heats the water by about 5C each sweep through the exchanger). If the flow rate falls below this, the manual says it becomes inefficient and cuts in and out. The circuit I wish to heat is already partly structural to the house and would not take that flow rate (indeed I am guessing even approaching that flow rate would be rather noisy), but the heat loss on that circuit is more than 5C. So is there any reason I cannot just "short" part of the outflow from the heat pump directly into the inflow for the heat pump mixing with the return? It seems a little too obvious as a solution...obviously the later part of the heating circuit will be a little cooler from having a high temp drop across them (thats ok, the later parts of the circuit will be the larger surface area ones), but if the heat pump is happy I know the actual heat is being transferred into the house a reasonable efficiency, right? --BozMo talk 07:22, 20 May 2012 (UTC)
- Heat pumps are more efficient when the heat exchanger is at a lower geometric mean temperature throughout the exchanger, and more efficient if the hottest part of the exchanger is lower in temperature. So, if you reduce the water flow, the hottest point, and the geometric mean temperature, must increase, lowering efficiency. If you try and get around this by providing a bypass flow, what you are doing is increasing (by mixing cold with warm) the inlet water temperature, again increasing the mean exchange temperature. So efficiency is still lost, but not necessarily at quite the same degree. Ratbone58.167.225.195 (talk) 16:36, 20 May 2012 (UTC)
- I'd think just lowering the flow rate through the entire system would lower efficiency less than the bypass. I don't quite understand what they mean when they say it will "cut in and out", though. StuRat (talk) 16:45, 20 May 2012 (UTC)
- A heat pump water heater is essentially the same technology as a heat pump (ie compressor type) airconditioner. A temperature sensor controlls when the compressor runs - if the water is too hot, the sensor turns the compressor off. Thus the system cycles just like an airconditioner does. If frequent cylcing occurs, effieciency drops for 2 reasons: a) a high exchanger temperature itself reduces efficiency, b) the system spends a higher fraction of time in running up and stabilising. Ratbone124.182.19.67 (talk) 01:05, 21 May 2012 (UTC)
- Right, but none of that explains why a lower flow rate would cause it to cut off more often. On the contrary, a lower flow rate should allow the water inside the cooling part of the loop to get cooler, since it stays in there longer, and the house should remain hotter than at a higher flow rate. Both of these should make it stay on longer. StuRat (talk) 02:59, 21 May 2012 (UTC)
- Stu, it seems like you are talking about an airconditioner as in some domestic airconditioners that use a chilled water system, like large commercial buildings - the commpressor (termed a chiller) at a convenient location chills water, which is then piped to one or more fan-coil units within the dwelling. I assumed that the OP was asking about the more common heat pump water heater, ie a compressor unit, which "pumps" heat from outside air to heat water for your kitchen, bathroom, etc. On reviewing the OP's wording, it does seem that he's talking about an airconditioner. In which case, my explanation was the wrong one. I'm not familiar with domestic grade chilled water systems, but assuming they are much the same as commercial systems, the on-exchanger water temp in cooling mode will be about 12 to 14 C, and the off-exchanger water temperature will be a design temperature of around 6 to 8 C. If you halve the flow rate, the compressor (somewhat simplifying) in moving the same amount of heat, will cool the water to 0 C. The water must not be allowed to freeze - that will stop functionality and damage the system. So a sensor on the off-exchanger temperature will prevent it by shutting down the compressor until the water warms up again. If the OP installs a water bypass, the water will still get too cold, because some of it doesn't go thru the fan-coil unit and get warmed up, and will cause cycling. This problem sometimes occurs in commercial buildings, where (say) a 10-storey building has been constructed, but so far there are tenents on only one or 2 floors, so the fan-coil units on the empty floors are not functioning. A common solution is to temporily use electric heating, or pump in outside air thru some fan-coils, to make the chiller work cycle more slowly. This of course wastes energy. In heating mode, there shouldn't be much of a problem, though the chiller will cycle on and off within a short time frame for the same reason a standard aircon will, if way too large for the space conditioned. When its running, it will heat the room(s) too quickly, and the thermsotat will turn it off. Installing a water bypass won't fix that either, except that the higher exchanger temperature will make the system a little less efficient. Ratbone120.145.172.175 (talk) 03:33, 21 May 2012 (UTC)
- I did consider the idea that water inside the cooling loop might freeze, but thought that they could just add anti-freeze to fix that problem. Or is the issue that frost will build up around the cooling loop ? I can see why that would reduce efficiency, but would it damage the system ? StuRat (talk) 03:52, 21 May 2012 (UTC)
- I have never heard of adding antifreeze to chiller water, though that does not say it's not done. I note that common antifreeze (glycol) has a lot less specific heat than water, so adding it will promote short cycling and reduce heat & cooling capacity. You are right about frost on the exchanger though - I should have remembered that. Frost is, compared to moving air, a thermal insulator, so once frost forms, the exchanger temerature drops, forming more frost, ending up with bulk ice and no efficeincy. When I mentioned damage, I was refering to the fact, that at freezing, water expands. Ratbone120.145.172.175 (talk) 04:26, 21 May 2012 (UTC)
- Well, yes, of course ice inside the pipes would damage them. You normally only need a small portion of antifreeze, depending on how low the temperature protection needs to go. StuRat (talk) 01:27, 22 May 2012 (UTC)
Hexahydrogen sulfide
There is hydrogen sulfide (H2S). There is sulfur hexafluoride (SF6). Why isn't there hexahydrogen sulfide (H6S)? Whoop whoop pull up Bitching Betty | Averted crashes 12:34, 20 May 2012 (UTC)
- Hydrogen does not hybridize its orbitals. So many hydrogens can't quite overlap with all six of sulfur's hybridized orbitals.--Jasper Deng (talk) 16:35, 20 May 2012 (UTC)
- I fail to see how hydrogen orbitals come into it. It's probably because hydrogen isn't electronegative enough to stabilise sulfur in the +6 oxidation state, whereas fluorine is electronegative enough. However, a few molecules are known with sulfur bonded to six carbons (Organosulfur compounds#Sulfuranes and persulfuranes) which casts doubt on the electronegativity argument. --Ben (talk) 16:40, 20 May 2012 (UTC)
- Well, hypervalency tends to occur when you have enough stretched-out orbitals that can link many atoms.--Jasper Deng (talk) 18:26, 20 May 2012 (UTC)
- See Hypervalent molecule; one of the models of hypervalency is the Three-center four-electron bond, which can be predicted and explained using molecular orbital theory. To put things simply, a 3c-4e bond requires p-p-p mixing between the two colinear ligands and central atom. Hydrogen doesn't have any electrons in p orbitals availible to create the 3c-4e bond, so it won't bond like SF6 will. There's even a nice picture of the type of bonding that happens in SF6 in the hypervalent molecule article which shows the p-p-p interactions in forming the 3-center bond. Hydrogen doesn't do this. --Jayron32 18:36, 20 May 2012 (UTC)
- If hydrogen couldn't form 3c-4e bonds, then the bifluoride anion wouldn't exist either—but it does. Whoop whoop pull up Bitching Betty | Averted crashes 19:32, 20 May 2012 (UTC)
- It does so only when it is the central atom, not when it is the ligand. Hydrogen can form such multi-center bonds as the central atom, and does so in the 2-electron 3-center bond in diborane as well. The difference is that hydrogen isn't the central atom in the hypothetical SH6, sulfur is. --Jayron32 19:44, 20 May 2012 (UTC)
- If hydrogen couldn't form 3c-4e bonds, then the bifluoride anion wouldn't exist either—but it does. Whoop whoop pull up Bitching Betty | Averted crashes 19:32, 20 May 2012 (UTC)
- See Hypervalent molecule; one of the models of hypervalency is the Three-center four-electron bond, which can be predicted and explained using molecular orbital theory. To put things simply, a 3c-4e bond requires p-p-p mixing between the two colinear ligands and central atom. Hydrogen doesn't have any electrons in p orbitals availible to create the 3c-4e bond, so it won't bond like SF6 will. There's even a nice picture of the type of bonding that happens in SF6 in the hypervalent molecule article which shows the p-p-p interactions in forming the 3-center bond. Hydrogen doesn't do this. --Jayron32 18:36, 20 May 2012 (UTC)
- Well, hypervalency tends to occur when you have enough stretched-out orbitals that can link many atoms.--Jasper Deng (talk) 18:26, 20 May 2012 (UTC)
- I fail to see how hydrogen orbitals come into it. It's probably because hydrogen isn't electronegative enough to stabilise sulfur in the +6 oxidation state, whereas fluorine is electronegative enough. However, a few molecules are known with sulfur bonded to six carbons (Organosulfur compounds#Sulfuranes and persulfuranes) which casts doubt on the electronegativity argument. --Ben (talk) 16:40, 20 May 2012 (UTC)
Ingredients of paint primer?
Two questions:
- I need to know what ingredients are used in paint primers (the kind used for wood and plaster - not metals) - our article Primer (paint) has no indication of ingredients and Paint discusses paint ingredients in general but doesn't explain what's different about primers.
- Some interior paints say that they include a primer. From the description in our two articles of what a primer does, this seems unlikely to be true. Can anyone explain what's really going on here? (And again, what are the ingredients in primer+paint products that aren't in the paint-only stuff?)
Background: I'm using a 100 watt CO2 laser-cutter to cut 5mm plywood - when the plywood is painted with some kinds of paint (I'm using and off-white glydden interior eggshell), the laser has a hard time cutting it - with other kinds of paint (acrylic craft paint), it's no problem. Shinier paints seem to cut worse than flat paint and our paint article suggests that shinier paints have silica, glass and metal flakes in them...glass and metal can't be cut with such a low power CO2 laser, so this explains a lot. However, I still want to use primer on my plywood...and I'd like to find a way to put a shiney/waterproof surface on my plywood without impeding the laser too much. (No, I can't paint the plywood after it's cut because I'm also etching a design into the paint using the laser.) SteveBaker (talk) 14:48, 20 May 2012 (UTC)
- Have you seen the Wikipedia article specifically on paint primers? The main purpose of a primer for wood is to seal it, so that the colour coat is not absorbed into the wood, leaving it pale & uneven and permeable to moisture. Paints intended for wood that incorporate a primer are quite common. Primer ingredients commonly are linseed or synthetic oils, or synthetic latex (such as PVA). In at least some cases, the only difference between (for wood) a primer and a topcoat is the proportions of the ingredients, the primer having a higher percentage of oil or latex. See http://www.buildings.com/tabid/3334/ArticleID/2846/Default.aspx. Why not use a matt paint compatible with the laser, then spray on a clear varnish to seal and make shiny. Ratbone58.167.225.195 (talk) 15:53, 20 May 2012 (UTC)
- One thing to keep in mind: paints are not subject to ingredients disclosure laws like, say, foods are. Many paints and pigments are proprietary formulas whose exact compositiona and processes for making them are kept highly secretive as trade secrets. Actually getting the specific composition of a specific primer may be impossible. I had a friend that was a research chemist worked in the industry, and he had a ten-year non-competition agreement with the company he worked for; that is if he ever left the company he worked for, he couldn't work for any company in the pigments industry for ten years. They take that secrecy pretty seriously. --Jayron32 17:43, 20 May 2012 (UTC)
Electricity of x cm³ of water at y m
Can you calculate how much electricity can be generated by x cm³ of water at y m.88.9.109.2 (talk) 15:04, 20 May 2012 (UTC)
- Yes, by determining the potential energy of that mass at that height, then applying an efficiency factor to determine the percentage of that energy actually converted to electricity by your dynamo. Do you want the formula ? StuRat (talk) 15:10, 20 May 2012 (UTC)
- Looks like Stu thinks you want a hydro-electric (water-fall) answer, which is most likley the case. But you might want an answer appropriate to ocean thermal power (OTEC). Which is it? Is this homework? Keit60.230.198.136 (talk) 15:31, 20 May 2012 (UTC)
- No homework. Just curiosity. Can you give me two formulas: 1. water is flowing through a dynamo/turbine. 2. water is enclosed, hanging in a cable attached to pulley connected to a dynamo. 88.9.109.2 (talk) 15:36, 20 May 2012 (UTC)
- Water has a density of 1 g/cm³ (depending on temperature, impurities, pressure, etc.). So, we have X grams, at Y meters. The formula for gravitational potential energy is U = mgh, where m = Xg, g (on Earth) = 9.8m/s², h = Ym. This gives us U = (9.8)XY gm²/s². That's 1/1000th of joule. We also need to apply the efficiency, let's say 0.0 < E < 1.0. So, the amount of electricity generated, in joules, is (9.8)EXY/1000. The formula would be the same for the water in a container, although there the mass of the container must also be considered, and the cable is more complicated, because each portion has a different gravitational potential energy. StuRat (talk) 17:13, 20 May 2012 (UTC)
Would radon be a gas if its radioactivity were neglected? Astatine and francium both would be vaporized by their own decay.--Jasper Deng (talk) 18:33, 20 May 2012 (UTC)
- Quite likely it would, since the boiling point trends displayed by the noble gases don't have an obvious discontinuity between Xenon and Radon. I don't think radioactivity has anything to do with phase of matter at room temperature, which is a function of an atom's electronic properties, not its nuclear properties. That is, a substance is a gas because it has weak intermolecular forces, the strength of which can be preicted solely by the structure of the electron cloud around the molecules/atoms that make up the substance. --Jayron32 18:42, 20 May 2012 (UTC)
- Well, radioactivity does tend to change temperature, so there's an indirect effect there. Francium's article said it would be liquid especially because it's radioactive.--Jasper Deng (talk) 18:46, 20 May 2012 (UTC)
- Yes, but it doesn't change the boiling point or melting point. You're confusing the two temperatures. The boiling point of a substance is the expected temperature for it to boil, NOT the temperature it exists at. That is, Radon will boil at 211 K regardless of where the source of the heat making it 211 K comes from. If it gets to 211, it boils, and that has nothing to do with radioactivity. Likewise with Francium, the sentence in the article is misleading. What it is saying isn't that the 27 Celsius MP quoted in the article is effected by its radioactivity, it is saying that the extra heat generated by its radioactivity means that in room temperature air (nominally 25 degrees Celsius), the excess heat from the radiation is more than sufficient to raise Francium's temperature the extra 2 or so degrees to melt it. That is, sitting in the average indoor room, Francium will be a liquid because it will always be somewhate warmer than that room. Again, the radiactivity of the Francium doesn't affect the temperature it should melt at, it affects the temperature it is. Two different ideas. --Jayron32 19:01, 20 May 2012 (UTC)
- Radon's boiling point is −61.85 °C so any radiative self-heating wouldn't be significant re: the state in a cozy room (or lab). Maybe if you had some radon at Vostok Station where the air temp was -62 °C radioactivity might push the temperature of your radon just above the boiling point. 19:19, 20 May 2012 (UTC)
- Well, radioactivity does tend to change temperature, so there's an indirect effect there. Francium's article said it would be liquid especially because it's radioactive.--Jasper Deng (talk) 18:46, 20 May 2012 (UTC)
Ultra High power mechanical HVDC-breakers?
I am doing some calculations on the feasibility of a ultra high voltage ultra high current world wide HVDC-grid. I speculate about 2000 kV to ground and 25 kA nominal current, 100 GW per bi-pole. The fault current after a few ms short circuit would be in the range of 100 kA. One of the big problems in constructing such a system are of course the selective removal of faulty components while maintaining operations of the rest of the system.
My question is if the needed size and cost of DC-breakers capable of breaking 2 000 kV and 100 kA can be estimated?
The challenge are of course to contain the blast and to cool the arc (generating >200 GW?) to temperatures below the temperature were significant ionization occurs while maintaining the isolation.
Today no HVDC-breakers are in operation, HVAC-breakers in the range of 800 kV and 50 kA exists, as I understand it they can only break a few kV so they will only interrupt the current near a zero crossing in the AC-current. ABB have developed a "Hybrid" HVDC-breaker, using semiconductors. Proactive Hybrid HVDC Breakers. It seems that it would be extremely expensive to use semiconductors in all the breakers at this level. Could large fuses in parallel with a smaller mechanical breaker be used? Fuses normally break the current before the zero crossing so it should work equally well with DC. Smaller fuses at for example 36 kV and 20 kA breaking current has a mass of about 2 kg, giving about 3 kg per gigawatt short circuit power, would it scale so that a fuse for 2000 kV and 100 kA would have a mass around 0.003*200 000 kg= 600 kg? (It seems small) Could it be done with explosives for fast and synchronized operation as in some MV Is-limiters? How can the breaking capacity of a given breaker chamber be approximated without complicated fluid dynamics and FEM-simulations? Gr8xoz (talk) 20:32, 20 May 2012 (UTC)
- By "of curse", do you mean "of course" ? StuRat (talk) 20:43, 20 May 2012 (UTC)
- Yes, corrected that. Gr8xoz (talk) 20:54, 20 May 2012 (UTC)
- ABB Group, Siemens and Alstom likely have the information you seek, though they may consider it proprietary. Do you really want to be replacing extremely expensivbe fuses whenever lightning hits a line, as opposed to a breaker opening and reclosing? There are fuses with explosive charges to open the circuit and programmable sensors and actuators to provide any desired operating curve, for a price. If you want to de-energize a HVDC line, could you do it via the "valve hall" or electronics which are transforming AC to DC at the sending end? Limiting fault current is inherently easier with HVDC than with HVAC. Edison (talk) 03:10, 21 May 2012 (UTC)
- Yes someone in the suggested companies has probably looked in to this. It is well outside their current product lines. The most powerful HVDC-liks built today are around 7 GW, this are 100 GW, almost twice the maximum consumption in the UK. For point to point HVDC-links the converter station ("valve hall") or HVAC-breaker on the AC side can be used to interrupt the fault current. The need for HVDC-breakers comes when building large redundant HVDC-grids with many converter stations. You do not want to shutdown the whole world wide HVDC-grid in this example as soon as there is a fault anywhere. Why do you think it is easier to limit the fault current in HVDC-systems? That is not the impression I got.
- If a fully reusable low maintenance breakers can be made at a reasonable price, that is of course better but I get the impression that some consider it almost impossible. That is the reason ABB introduces semiconductor based solutions. I think one time controlled fuses could be competitive if they can be made at 10 % of the price of a reusable breaker. I think it would be possible to repair used fuses.
- Lighting is not a likely source of faults as the system I think of are built with Gas Insulated lines. Gr8xoz (talk) 06:52, 21 May 2012 (UTC)
- You link to gas insulated lines did not work, but please look at the cost per km for gas insulated bus versus overhead HVDC. Look at the meantime between failure for a 100 km HVDC overhead line, versus that length of gas insulated line, with a leak anywhere in one of the zones causing a flashover and line failure. Lightning will be a frequent cause of overhead HVDC momentary outages, since it can be a way higher voltage than the insulators are designed for. It is corrected by tripping and quickly reclosing the line. The metal shell outside a gas insulated line would seem to protect better than the static line above an overhead HVDC line, but I expect that any elevated section of gas insulated transmission could also be knocked out by a sufficiently intense lightning strike. When a fuse operated, the silver strip in the center basically vaporized and is dispersed into the silica sand around it (some fuses are oil filled, same idea). If it has a pyrotechnic operation, then even more disturbance of the conductor and its surrounding nonconductor would take place. Maybe you could reuse the shell and the endcaps, or reprocess the sand to recover the silver. I sawed open a blown 12ky fuse and did not find any silver droplets (alas). In HVAC, the impedance of the source (generators, lines, transformers, series inductors) along with the fault impedance is all that limits the current. IN HVDC, the valves can limit the current much more easily when a fault occurs. It is more actively than passively sourced. Edison (talk) 19:05, 22 May 2012 (UTC)
- Lighting is not a likely source of faults as the system I think of are built with Gas Insulated lines. Gr8xoz (talk) 06:52, 21 May 2012 (UTC)
- Thank you for your answers, I have corrected the link now. I have limited time now so I will be brief. According to my link GIL are most reliable, 87 km installed world wide over 35 years and not a single fault so far. Of course this are like the Concorde before the accident it was the safest airliner in the world and after the accident it was the worst. I expect that the probability of a leakage should be at least as low as in a pipe line and they seem to be reliable. The transported power are larger than a gas pipe line such as Nord_Stream and I expect the cost to be lower due to smaller pipe, 800 mm vs 1200 mm, if produced in large quantities. I think overhead lines will be cheaper over uninhabited land but it is increasingly hard to get permits for new overhead lines in many places and most of the Earth are sea and there overhead lines are very expensive. I expect the cost advantage of overhead lines to be lower the larger the conducting area is since the construction will start to approach that of a suspension bridge, in the case of 100 GW I have calculated with about 0.1 m^2 cross section per conductor.
- Lightning do normally not have currents above 300 kA, in the same range as the normal fault current so I do not expect it to give significant voltage rise on the outer pipe. In Is-limiters the pyrotechnic is used to blow away a conductor parallel to the fuse and then the fuse operates as normal, in this way the operation can be computer controlled. I think of something similar or a pyrotechnically operated reusable breaker. If the fault current can be controlled by the converter station depends on the converter topology, in a normal voltage source converter it can not be controlled due to the free wheeling diodes in parallel with the IGBTs. In thyristor based HVDC it is probably possible. Anyhow it is not possible in large HVDC grids were selectivity are needed. On long lines you would like to interrupt the current even before a signal at the speed of light could reach the other end.
- I think my initial question could be phrased as follow: what is needed to cool a high density plasma with about 200 GW during a few milliseconds down to a temperature where the ionization stops. Breakers often contains the plasma between close surfaces (conducting or non conducting) to improve heat conduction. If we assume 5 mm between surfaces and 10 MPa SF6, how big area would be needed? How does this depend on gas speed?
- Gr8xoz (talk) 10:46, 23 May 2012 (UTC)
Planetary Resources, Inc.
This may belong on the Language help desk but I think I get better answers here. Planetary Resources, Inc. are a company that plans to mine asteroids. (NEOs) Are there any logic behind the naming? To my it sounds like they are one of very few mining companies that plan to use non planetary resources. Almost all other use resources on the planet Earth while the NEOs are not planets. The IAU states: "the term 'minor planet' may still be used, but generally the term 'Small Solar System body' will be preferred." Minor planet. So are they basing their name on the obsolete term "minor planet" or are there any other rationale. Gr8xoz (talk) 20:51, 20 May 2012 (UTC)
- Planetoid is another name for a minor planet. But perhaps they mean they will provide resources to a planet (Earth), rather than from one. StuRat (talk) 20:56, 20 May 2012 (UTC)
- (ec) I doubt if anybody can give you an authoritative answer, but my take on it is that "planetary" is a word that to most people has nothing to do with the Earth, but just conjures up "space". --ColinFine (talk) 20:58, 20 May 2012 (UTC)
electric car batteries
If there were a way to charge a battery while driving without hydrocarbons, how many kwh would be needed to supply the electric motor with the needed energy? — Preceding unsigned comment added by 70.162.248.221 (talk) 21:12, 20 May 2012 (UTC)
- Well, there have been solar powered cars, but that requires an extremely light car (dangerous in an accident) completely covered with solar cells, and a sunny day with the Sun high in the sky. A more practical solar-powered car might be able to charge up while parked in the Sun (assuming it spends at least 90% of the time parked), with a range slightly increased by driving in sunlight. I assume the reason no manufacturer offers such a car is that a solar-cell covered car is rather ugly. StuRat (talk) 21:17, 20 May 2012 (UTC)
- Then, of course, there's regenerative braking. This only charges the car battery while braking, so the net effect is to reduce the overall rate at which it discharges. StuRat (talk) 21:25, 20 May 2012 (UTC)
- The Chevy Volt has a 111 kW motor [1]. So, if you want to be able to drive it continuously, you would need to provide that much. If you wanted to be able to drive it for 10 hours using the batteries, they would need to hold 1110 kWh. Of course, these both assume maximum power consumption. If you are driving more conservatively, you can get by with less. StuRat (talk) 21:37, 20 May 2012 (UTC)
- Depending on a loot of factors but generaly around 20 kW in 100 km/h :Electric_car#Running_costs_and_maintenance
- see also Road-powered electric vehicle.
- A car uses the full motor power a very small fraction of the time so the rated power of the motor is not a useful approximation.
- Gr8xoz (talk) 22:03, 20 May 2012 (UTC)
Are these "proper" moobs?
On the gynecomastia article, the representative and first image is File:Gynecomastia_001.jpg.
Without trying to show false sensitivity - are we sure that's not just a fatty? Is there proper diagnosis that that image is actually Gynecomastia? Obviously a doctor or medical specialist cannot diagnose over the internet but does it "look right"? I've actually started a very similar discussion on the representative talk page but makes sense to come here too. Egg Centric 21:47, 20 May 2012 (UTC)
- It seems to me that there is no sharp line. All men have breasts, and those on the overweight are larger, as are those with men suffering from certain problems, like hormone imbalances. I wouldn't expect them to look much different. I suppose a thin man with large breasts might be a better example, though. StuRat (talk) 22:00, 20 May 2012 (UTC)
- Is a breast that is large purely cause the guy is fat, and which will shrink to normal if he loses weight, actually gynecomastia though? Indeed, I don't think any of the images on that page are very great as displaying tits - something like this is more like it...Egg Centric 22:10, 20 May 2012 (UTC)
- On a mere fatty (pseudogynecomastia), I'd expect more continuity between the upper slope of the "breast" and the surface of the flesh above it. —Tamfang (talk) 22:50, 20 May 2012 (UTC)
- I will ask the original uploader although they haven't edited since 2010 so it's a long shot... Egg Centric 11:32, 22 May 2012 (UTC)
- In that photo the nipples and areolae seem unusually large; if so this would argue for a bit of extra estrogen in the mix somewhere. That's not particularly rare. Certainly anyone who appreciated a recent Zane Donovan shower scene will have noted how much smaller these can be in other individuals. Wnt (talk) 02:20, 23 May 2012 (UTC)
- However, I believe obese men tend to have a higher estrogen level, so, again, it's difficult to draw a dark line between normal and abnormal male breast development. StuRat (talk) 05:47, 23 May 2012 (UTC)
Wood as an electric conductor
In Jurassic Park, the Dr. Alan Grant Sam Neill is walking with John Hammond's Richard Attenborough grandkids and comes upon an electric fence that appears to have been deactivated. To check it, Grant throws a stick at the fence. If wood doesn't conduct electricity, what good would this do? DRosenbach (Talk | Contribs) 22:52, 20 May 2012 (UTC)
- I've forgotten where this is in the narrative but wasn't it raining just before this? A wet stick would possibly spark if the voltage were high enough. Dismas|(talk) 23:06, 20 May 2012 (UTC)
- It makes for good cinematography. It was just a film. Would an american audience comprehend SIDE?--Aspro (talk) 23:10, 20 May 2012 (UTC)
- (edit conflict) x2 Wet wood is a good conductor of electricity, and even dry wood conducts to a certain extent, especially at the high voltage (but low current) generated by most electric fences. Allowing the wood to rest against a modern animal electric fence would short the current to earth. I'm not sure whether the fence in Jurassic Park was designed to kill. If so, then it might have been both high voltage and high current, producing an electric arc when shorted (or perhaps that's what the director wanted viewers to think.) Dbfirs 23:14, 20 May 2012 (UTC)
- Think I might have to explain that. SIDE =Switch off, Isolate, Dump & Earth. Until then then an electric fence is not to be trusted. It was just a film for the Hoi polloi -not a science lesson... Dinosaurs... I ask you! It was on par with the utter hogwash that came out of seaQuest DSV.--Aspro (talk) 23:30, 20 May 2012 (UTC)
- Also, Some EF's pulse -such as cow fences. So the stick might might just hit between energising and thus show no observable effect.--Aspro (talk) 23:38, 20 May 2012 (UTC)
- A random piece of wood cannot be counted on as either a good conductor or a good insulator. Oven dried wood has resistivity of about 10E14 to 10E16 ohm-meter , while damp wood has resistivity of 10E3 to 10E4 ohm-meter. The conductivity increases with temperature. If an electric fence in Jurassic Park were built to kill dinosaurs who touched it, it might well be energized at 480 or even 2400 volts, with a high current, low impedance source, in contrast to the high voltage, low current pulses of a typical farm electric fence. A piece of tree limb wood connected from the high voltage J park fence to ground (such as the nonenergized metal of the fence) might blacken, then catch fire. It might or might not pop the breaker. When a tree limb touches 2 utility conductors at 4kv or higher, the current may be sufficient to cause a fuse or breaker to open the circuit. A carefully dried wooden stick might not conduct as much current. Edison (talk) 02:54, 21 May 2012 (UTC)
- "SIDE =Switch off, Isolate, Dump & Earth." Thanks for the explanation of the acronym. I think I understand S, I, and E. But what is "Dump"? Wanderer57 (talk) 04:32, 21 May 2012 (UTC)
- D = Dump = Discharge to earth. 84.209.89.214 (talk) 23:32, 21 May 2012 (UTC)
- Thanks. It seems that dump and earth mean the same thing, which is what is called "ground" in this neck of the woods. Wanderer57 (talk) 05:22, 22 May 2012 (UTC)
- D = Dump = Discharge to earth. 84.209.89.214 (talk) 23:32, 21 May 2012 (UTC)
- "SIDE =Switch off, Isolate, Dump & Earth." Thanks for the explanation of the acronym. I think I understand S, I, and E. But what is "Dump"? Wanderer57 (talk) 04:32, 21 May 2012 (UTC)
- Dump: this refers to a controlled discharge -usually via a 'dump resistor'. Inductors and capacitors (re: electric fence) can store a lot of energy and so an immediate short to earth can cause very high currents to flow. Thus, dumping prevents these high currents from wreaking the the equipment. After 'dump' a small -low current- earthing lead can be attached. As I stated: this was a just cheap B movie and a stick on a real electric fence might show nothing. Yet... you just touch it and the nearest velociraptor will likely be perforated by your molten dental fillings flying through the primeval jungle (poetic-licence added for emphasis only). As Michael Caine says:”Not a lot of people know that”. --Aspro (talk) 19:49, 22 May 2012 (UTC)
- This of course depend on regulation but normaly the earthing cable need to be thick enugh to saftly handle any short circut current if the object would be energized by misstake.Gr8xoz (talk) 08:20, 23 May 2012 (UTC)
- Think you way miss the point. Earthing cables that can carry high surges are expensive and heavy. So why use one, when a cheaper lead can be used -incorporating a dump resistor? Second: the equipment being earthed may not be able to handle the surge that results from suddenly being shorted to earth. One's finger can discharge several thousand volts with barely a tingle felt -if the energy transferred is low enough. Yet, try putting a screwdriver across the terminals of a very large capacitor and then ask yourself it that was a very good idea! However, just small earthing lead can leak away the incoming energy faster than it can be stored -so no large conductor required. So and therefore, to get a very large critter to think (when encountering an electric fence) WHAT THE ∆˚®ß¥ø≤††† was the THAT? a fence needs a power feed at common human safe supply levels and then an energy storage system so that when the critter makes contact, s/he thinks ∆˚®ß¥ø≤†††; was the hell was THAT! Its not 'regulations' but practicalities that tend to determine these SIDE procedures. As Michael Caine says... etc.--Aspro (talk) 20:40, 23 May 2012 (UTC)
- I have not heard about SIDE before, the steps you describe seems appropriate when dealing with high voltage capacitors that can only be charged by a relatively low powered source. My reference frame is power distribution and transmission systems. If the power line you are working on are connected by mistake in such systems the earthing cable can get a current of 50 kA, then you probably are happy that the cable are thick. Electric fences for dinosaurs does probably have lower power but I do not know. Gr8xoz (talk) 22:57, 23 May 2012 (UTC)
- Thanks for the explanation of SIDE. Live and learn.
- As for the movie, I thought the dinosaurs were very well done but I kept getting distracted by details which seemed to me scientifically very inept. Wanderer57 (talk) 03:39, 24 May 2012 (UTC)
Asthma meds for otherwise healthy people
What would puffing on an inhaler do for someone who does not have asthma? Would it basically be the list of adverse side effects listed at the Salbutamol page?
Note: I don't have asthma, don't have anyone in my immediate circle of friends/family with it and therefore don't have easy access to an inhaler. I don't plan on finding one to experiment with no matter what the answers I get here are. I have not died and come back to see if maybe an inhaler caused my death. I'm not asking for medical advice. I'm simply asking out of curiosity. Dismas|(talk) 23:27, 20 May 2012 (UTC)
- The first effect of overdoses might be hands trembling. However, puffing only once won't have any noticeable effect for sure. It's quite difficult to overdose on the inhaler (which is different from taking a pill or taking salbutamol intravenously). OsmanRF34 (talk) 00:10, 21 May 2012 (UTC)
- I've had a few puffs of friend's inhalers in the past, just for a laugh and to see what would happen, I noticed no effect. Vespine (talk) 04:44, 21 May 2012 (UTC)
- Albuterol is often prescribed to people who do not have asthma. (I have had it prescribed to me a number of times to just clear up bronchial weirdness and phlegm that occasionally trails behind long after the cold has gone.) In my experience (just anecdotal), a single deep pull of albuterol (expel all air from lungs, inhale a puff and suck it as deep into lungs as possible) usually makes one feel a little jittery and makes the lungs feels a little "funny." (But it does seem to work wonders at "clearing them out".) Which are some but definitely not all of the adverse effects listed there. I would expect those effects to be there whether you are "otherwise healthy" or not; they are the effect of inhaling that kind of drug into your lungs, not an interaction produced between the drug and your injury/disease. I know, not super helpful, but I thought I'd offer it up. Not medical advice, etc. etc., all of what I am describing was prescribed to me personally by a doctor, please do not replicate without a doctor, etc. --Mr.98 (talk) 13:12, 21 May 2012 (UTC)
- Some asthma meds are widely used as quasi-legal (with a prescription) doping for endurance athletes. I think 40% of the Tour de France participants are certified asthmatics, and up to 80% of UCI professional cyclists share this fate [2]. --Stephan Schulz (talk) 21:24, 21 May 2012 (UTC)
- (See also exercise-induced asthma). Wnt (talk) 02:12, 23 May 2012 (UTC)
- Some asthma meds are widely used as quasi-legal (with a prescription) doping for endurance athletes. I think 40% of the Tour de France participants are certified asthmatics, and up to 80% of UCI professional cyclists share this fate [2]. --Stephan Schulz (talk) 21:24, 21 May 2012 (UTC)
Thanks for the info, all. And Mr.98, I don't mind the WP:OR at all! Dismas|(talk) 03:21, 22 May 2012 (UTC)
May 21
Oxidation state trend
Why is it that the vertical trend of stable oxidation states varies so much horizontally across the periodic table? Why is that carbon(IV) is more stable than lead(IV), but osmium(VI) is more stable than iron(VI)? Is there an underlying trend, or is it completely unpredictable? Plasmic Physics (talk) 00:50, 21 May 2012 (UTC)
- These particular cases are due to relativistic effects. Normally, oxidation states increase going down a group because of the progressively looser hold by the atoms on their valence electrons. However, relativistic effects may counter-act that in the cases of Tl, Pb, Bi, and 7th-period elements. They reduce the effect of lower effective nuclear charge by stabilizing particular electron configurations more than in lighter members of the same group.--Jasper Deng (talk) 01:10, 21 May 2012 (UTC)
- I would expect that the stability for high oxidation states should increase down the periods for every group. What is so special about the electron configurations for those cases? Plasmic Physics (talk) 02:28, 21 May 2012 (UTC)
- It seems that the s orbitals for Tl, Pb, Bi are mostly inert, and removing their electrons has less of a stabilizing effect. I don't understand the core reason - since I don't understand relativistic effects. But what is clear is that as the electrons are further and further from the nucleus, the nucleus cares less and less about a full octet than about keeping all those electrons, it appears.--Jasper Deng (talk) 02:53, 21 May 2012 (UTC)
- I would expect that the stability for high oxidation states should increase down the periods for every group. What is so special about the electron configurations for those cases? Plasmic Physics (talk) 02:28, 21 May 2012 (UTC)
Could obesity be caused by a lack of magnesium in the diet?
Let me explain why I think magnesium could be a factor in obesity. First, the obvious explanation, i.e. too much calorie intake is, I think, not so plausible, because obese people actually don't eat that much calorie-wise, at least not the obese people who I know. Also, most people will be at some constant weight, so they are in dynamical equilibrium between calorie intake and calorie use. Then that dynamical equilibrium could in theory be reached at any weight, there is no good reason why at an intake of say, 3000 Kcal/day you have to weigh 100 kg, and not 70 kg or 150 kg. Most of the calories are burned by muscles, the fat tissue doesn't use a lot of energy, so you could just as well be at the same equilibrium of 3000 Kcal energy intake and energy use, but at a much lower body weight.
In fact, I weigh only 60 kg, yet I eat on average 3600 Kcal per day. Some of my obese family members eat way less than I do, but they are also eating a lot less healthy foods. Now, some time ago I posted here about magnesium, when I checked my diet I found that my magnesium intake was way too high (I get about 1 gram of magnesium per day from eating whole grain bread, potatoes, brown rice, whole grain pasta, and bananas). However, since then I've read that my magnesium intake may be normal, and that most people are actually magnesium deficient. Now, magnesium plays an important role in metabolism, so I thought that perhaps one can explain why some people are obese as follows.
If I eat X calories a day then I could gain or lose weight until I reach dynamical equilibrium where I burn X calories per day. If my food does not contain enough magnesium, then the metabolism becomes less efficient, it would take a cell longer to burn energy, so for my body to burn X calories per day would take a larger store of energy in my body, therefore I would be a lot fatter. Count Iblis (talk) 03:10, 21 May 2012 (UTC)
- It's always possible, but magnesium is a rather basic nutrient, and if a deficiency caused obesity, I rather think they'd have noticed it by now. I suspect it's a different aspect of their unhealthy diets that are to blame. Also note that it's not always easy to tell how much someone eats by casual observation. I knew an obese woman who seemed to eat modestly at each meal. However, if I went back later for, say, a 2nd piece of pie as a snack, I'd discover that the entire pie had somehow disappeared. :-) StuRat (talk) 03:19, 21 May 2012 (UTC)
- A confounding factor: Most obese people I know may not eat that much more, but what they do that's different, is do a lot less physical work. For example, for quite a while I worked in a company that occupied two floors of a building, and the way the company laid out their operations, and the type of work done, meant that most empoyees had to frequently walk from one floor to the other. Most of us took the stairs as that was quicker. But, with no exceptions, obese folks used the lift every time. Also you could use free public carparks about 6 block away, or you could park in the basement at $20 per day. Guess which folks paid the $20/day for their own cars! We ran construction projects. I've never seen an obese labouror, and seldom seen a fat one. Ratbone120.145.172.175 (talk) 03:44, 21 May 2012 (UTC)
- You don't live in the UK do you! I've seen plenty of obese brickies here in my time... --TammyMoet (talk) 09:50, 21 May 2012 (UTC)
- No, I live in Australia. Brickies are paid per number of bricks laid and use labourors/assistants to keep them constantly supplied with bricks and fresh mortar. So the faster they go, the more money they make. Are they paid by the hour, or at a flat rate, in the UK? Bricklaying is damm hard work. Ratbone60.230.230.72 (talk) 10:57, 21 May 2012 (UTC)
- It is more complicated than that. As Tammy says, manual labourers can still be obese. I know women who have struggled with their weight their whole lives, and are definitely well into the obese range by any metric (not just BMI), but who regularly take the stairs, dance for hours at proper dance classes, walk miles most days (one is building up to a huge walking holiday), and so on. They wear pedometers everywhere, every day, so that they can make sure they keep up their distance. When their doctor gives them the same patronising advice they give everyone, the doctor flatly disbelieves that they do this exercise. This will change, as I have seen the data from recent studies starting to be properly propagated. Exercise makes you hungry. And if you've been struggling with your weight all your life, dieting from a young age, then your body gets really efficient at laying down fat and holding onto it. 86.161.213.137 (talk) 11:02, 22 May 2012 (UTC)
- You don't live in the UK do you! I've seen plenty of obese brickies here in my time... --TammyMoet (talk) 09:50, 21 May 2012 (UTC)
- Is it even possible to avoid ingesting magnesium to the extent that one becomes deficient? Roger (talk) 09:55, 21 May 2012 (UTC)
- Yes, actually. 57% of the US populace has inadequate magnesium intake according to the USDA. Also, certain medications affect magnesium absorption: transplant patients are often put on supplements because ciclosporin (an important anti-rejection drug) impedes magnesium absorption. --NellieBlyMobile (talk) 22:41, 21 May 2012 (UTC)
- There are many things written about this, but see [3] for a good publication pointing at obesity-induced type II diabetes as the cause of lowered magnesium levels, rather than the other way around. Wnt (talk) 02:10, 23 May 2012 (UTC)
- Yes, actually. 57% of the US populace has inadequate magnesium intake according to the USDA. Also, certain medications affect magnesium absorption: transplant patients are often put on supplements because ciclosporin (an important anti-rejection drug) impedes magnesium absorption. --NellieBlyMobile (talk) 22:41, 21 May 2012 (UTC)
Cartography - resolution?
Is there a concept in cartography that is analogous to resolution? By this I mean, will a particular map have a property that, say, features smaller that 10m2 are ignored? I just want to know what this is called so that I can read about it. ike9898 (talk) 14:04, 21 May 2012 (UTC)
- You may find the Coastline paradox article interesting. hydnjo (talk) 14:22, 21 May 2012 (UTC)
- I think, as a practical matter, a map will have a higher resolution in areas which are "interesting". Thus, a street map of the US will show more detail in cities than, say, the Alaskan interior. This would apply to street maps, while maps for geologists might have more detail in areas with interesting geology. The increased detail is sometimes handled with an inset map at increased scale, but not always. On a Google map, for example, you may find that you can't zoom in as far in uninteresting areas, or, if you can, you don't see any additional detail when you do. StuRat (talk) 06:07, 22 May 2012 (UTC)
- There might not be a standard name for the concept, but Googling 'mapping detail threshold' gets a relevant result. In Mapnik (the standard renderer used by OSM) they call it 'detail level'. --Heron (talk) 20:23, 22 May 2012 (UTC)
steam engine plans
I want to make a working 1/12 scale model of this: http://upload.wikimedia.org/wikipedia/commons/f/f3/TrevithicksEngine.jpg or something much like it, any ideas where I can find plans of how it's made?
Kitutal (talk) 16:12, 21 May 2012 (UTC)
- 10 seconds of google revealed this thread http://www.model-engineer.co.uk/forums/postings.asp?th=47132 which might be of use. There are also companies that sell live steam models. --TrogWoolley (talk) 16:27, 21 May 2012 (UTC)
- If this is to be a working model, make sure that you realize that, if the thickness of the steam tank is 1/12th the original, then the pressure differential with the atmosphere must be much less, as well (I'm not sure if it's 1/12th, though). You don't want an exploding tank. However, if the volume is 1/12³ or 1/1728, and the pressure differential is 1/12, this will do something like 1/20736 times as much work. This might not even be enough to make it move (specifically, to overcome the static coefficient of friction and rolling resistance). So, you might want to keep the tank thickness the same as the original, rather than scale it down, so it can handle higher pressures. StuRat (talk) 06:00, 22 May 2012 (UTC)
- While safety of course are very important when building a steam engine theoretical the maximum pressure is the same for an exact scale model. This can be seen by looking on any cut trough the boiler. The force are the inside area multiplied by pressure and the needed material strength are proportional to the force divided by the cross section area of the boiler wall. The areas will scale by the same factor so the maximum pressure will be the same. Gr8xoz (talk) 08:15, 23 May 2012 (UTC)
- Sorry, but that doesn't make any sense. The pressure must be reduced in a tank with reduced thickness, or else all tanks would be made of a thin foil. StuRat (talk) 17:33, 23 May 2012 (UTC)
- Would you be so kind to actually read what I write and think about it before you try to contradict me. I wrote that if you scale down a big tank so that all proportions are kept the same then it can withstand the same pressure. If you need a big tank you still need thick walls. A hot coke can has about the same pressure as a workshop compressor tank but the coke can is 0.1 mm thick while the compressor tank is several millimetre thick.
- Lets take an example tank A has an outer diameter of 2000 mm and an inner diameter of 1900 mm, the wall material has an allowed average tensile stress of 100 N/mm^2 in the axial direction. What is the allowed pressure? The total wall cross section area is 2000^2*pi/4-1900^2*pi/4= 306 000 mm^2 the allowed force are 100 N/mm^2*306 000 mm^2=30.6 MN. The inner cross section area of the tank is 1900^2*pi/4 mm^2 = 2.8 m^2 The allowed pressure is 30.6 MN/2.8 m^2= 10.8 MPa. Very similar calculations needs to be done in the tangential direction.
- Lets scale it down by a factor 100, tank B has an outer diameter of 20 mm and an inner diameter of 19 mm, the wall material has an allowed average tensile stress of 100 N/mm^2 in the axial direction. What is the allowed pressure? The total wall cross section area is 20^2*pi/4-19^2*pi/4= 30.6 mm^2 the allowed force are 100 N/mm^2*30.6 mm^2=3060 N. The inner cross section area of the tank is 19^2*pi/4 mm^2 = 0.00028 m^2 The allowed pressure is 3060 MN/0.00028 m^2= 10.8 MPa.
- Both tanks can withstand the same pressure even if the wall in the big tank is 50 mm thick and the wall in the small tank is 0.5 mm thick. If you do not agree with this then you need to show some REAL ARGUMENTS. Gr8xoz (talk) 21:58, 23 May 2012 (UTC)
- Just checked with an expert (he volunteers at the Southeastern Railway Museum, working on steam engine locomotives). They are currently building a scale model (half scale, though, not 1/12th scale). He agrees that the thinner the wall thickness, the less pressure the tank can hold, regardless of the size of the tank. StuRat (talk) 01:34, 24 May 2012 (UTC)
- That contradicts what our Pressure tank article says here: "(for a given pressure) the thickness of the walls scales with the radius of the tank" (which agrees with what Gr8xoz wrote). -84user (talk) 18:56, 26 May 2012 (UTC)
Yea, or just hide a little battery powered motor inside Kitutal (talk) 15:57, 22 May 2012 (UTC)
Purple lightning
I was watching this video, http://www.youtube.com/watch?v=RDDfkKEa2ls&feature=related
And some of the lightning looks quite purple. What causes that? Is it just the camera, or does it really look purple? ScienceApe (talk) 16:27, 21 May 2012 (UTC)
- If the camera is using a whitebalance for fluorescent lighting, which is greenish, then other real white stuff like lightning can look purplish. Graeme Bartlett (talk) 21:47, 21 May 2012 (UTC)
- Also, lightning, like all other sources (or reflectors) of light, is subject to atmospheric effects. Thus, distant lightning, like distant mountains, will tend to look more purple. StuRat (talk) 05:49, 22 May 2012 (UTC)
- Distant mountains appear more blue, not more purple. If that was the reason for lightning appearing not quite white, the lightning would appear blue, not purple. Whoop whoop pull up Bitching Betty | Averted crashes 04:44, 24 May 2012 (UTC)
- The author of America the Beautiful disagrees: [4]. StuRat (talk) 05:07, 25 May 2012 (UTC)
- Well, then, that guy is wrong. Whoop whoop pull up Bitching Betty | Averted crashes 22:10, 25 May 2012 (UTC)
- Distant lightning would only be made more blue if it was daylight with sky colour adding to it. If it was at night time, blue would be removed, making the colour more yellow or even red if it goes through smoke. Graeme Bartlett (talk) 22:37, 25 May 2012 (UTC)
- Well, then, that guy is wrong. Whoop whoop pull up Bitching Betty | Averted crashes 22:10, 25 May 2012 (UTC)
Comparing climate between places in Australia & North America
Is there somewhere in North America that would have a very similar climate to that of Sydney, Australia for example? Or Perth, Australia?
More generally, are there tables that answer such questions?
Eg, is the climate of Portugal very similar to that of Northern California (both being on the west coast of a continent and at the same latitude)? Thanks, CBHA (talk) 19:43, 21 May 2012 (UTC)
- Well, there's the Köppen climate classification system. Per that map, Portugal and coastal California are both Mediterranean climates (Csa or Csb on the Koppen map). Sydney appears to be in an oceanic climate region, of which there is little in North America (perhaps around Seattle or Vancouver) but loads in Europe. It may also be useful to pull up single-factor maps such as average temperature or rainfall. — Lomn 21:03, 21 May 2012 (UTC)
- I think I see a 2nd area around Knoxville, Tennessee with an oceanic climate, similar to Sydney. As for Perth, it's Mediterranean climate is, indeed, common on the US West coast, including California, Oregon, and Washington (state). StuRat (talk) 05:32, 22 May 2012 (UTC)
Diversity of sexuality
I've sometimes heard people claim that the diverse sexual tastes of humans is a feature that distinguishes us from all the lower animals. However, I'm heavily skeptical of any claim that separates humans from the natural world and declares "we are unique amongst all species". So, do other animals have unusual sexual interests? Are there chimps, for example, who enjoy sadism, necrophilia, pedophilia, or foot fetishes (assuming these aren't typical amongst the species)? --140.180.5.169 (talk) 19:57, 21 May 2012 (UTC)
- The concept of sexuality is something which, in itself, is a human-created idea. To assign sexuality to an animal is to disrepect it by anthropomorphizing it. Animal behavior is to be understood on its own terms, not by analogy to human behavior. Animals are not incomplete people, and we cannot hope to understand them properly by starting with the premise that they are. Assuming that animal behavior has human analogues, or that the classifications of animal behavior fit in the same schema as we have created for human behavior is a wrong-headed tack. --Jayron32 23:01, 21 May 2012 (UTC)
- (ec) I don't accept the validity of the artificial wall you're putting up between human and animal sexual behavior. While the word "sexuality" was invented by humans, sex itself predates the first multicellular organism and is several orders of magnitude older than all of human history. To say that human sexuality is unique in a profound way, instead of being just a variant of one of the oldest and most widespread biological phenomena, seems very anthropocentric and anti-Copernician. — Preceding unsigned comment added by 140.180.5.169 (talk) 23:31, 21 May 2012 (UTC)
- The problem is, how do you ask questions about animals and their internal thought processes? Where is your evidence that animals experience the sort of metacognition necessary to experience sexuality in the same way that humans do (this as being distinct from sexual behavior or the act of sex). That is, animals have sex, and they have sexual behaviors, but they do not have the same values that are assigned to those behaviors that human culture does. How entirely presumptuous of you that animals should have the same values that humans do, and how disrespectful to those animals to meet them on your own terms, and not on their own terms. How can you consider humanity to be so superior to animals that you can judge what animals do solely on the motivations, values, and culture of human? What a horribly anthropocentric view of the world you have. --Jayron32 00:20, 22 May 2012 (UTC)
- My question was entirely about animal and human behavior, not about internal thought processes or values. It is empirically difficult with current technology to determine whether a chimp believes that a foot fetish is socially acceptable or jibes with his beliefs, but that's not what I'm interested in. I only care about whether chimps have foot fetishes in the first place, precisely because internal thought processes are hard to determine. --140.180.5.169 (talk) 02:16, 22 May 2012 (UTC)
- Bonobos, dolphins, and chimpanzees are known to engage in sexual intercourse even when the female is not in estrus, and to engage in sex acts with same-sex partners. Like humans engaging in sex primarily for pleasure, this behaviour in the above mentioned animals is also presumed to be for pleasure, and a contributing factor to strengthening their social bonds. 84.209.89.214 (talk) 23:23, 21 May 2012 (UTC)
| Veering way off-topic into non-scientific discussion and personal attacks. — The Hand That Feeds You:Bite 12:47, 23 May 2012 (UTC) |
|---|
| The following discussion has been closed. Please do not modify it. |
I mean just the obvious with 'intended by nature'. For example, one could ask the question "Why do some snakes have venomous teeth?". I would answer: "In order to kill their prey". If I take your approach I would have to say: "There is no answer, nature has no purpose, it just is". - Lindert (talk) 13:41, 22 May 2012 (UTC)
|
- Yes, but they don't have complex language with a distinction between signified, signifier and referent. Chimpanzees enjoy injuring other chimpanzees, they don't experience sadism. Animals copulate with animals that lack reproductive maturity. Animals don't have "children" as a unique category of social being, and so are incapable of "pedophilia" as sex with a child. Animals do not have sexuality, they have sex and sexual behaviours. Animals lack culture, because they lack language. Animals lack sexual taste, because they lack language. Fifelfoo (talk) 23:33, 21 May 2012 (UTC)
- I don't understand your distinction. If the same neural mechanism in both chimps and humans cause the organism to enjoy hurting others, why do you call one sadism but not the other? If certain neurons fire to arouse a human when he sees a young child, and the same neurons fire to arouse a chimp when he sees a young chimp child, why is one pedophilia but not the other? I also question your claim that animals don't have "children" as a unique category. Animals certainly behave differently towards children than they do towards adults, and many social species have a rigid social structure where children have a well-defined place. --140.180.5.169 (talk) 02:16, 22 May 2012 (UTC)
- Dogs hump legs HiLo48 (talk) 23:34, 21 May 2012 (UTC)
- Animals are, well, animals. For example: this chimp with a toad fetish. Also, here is an example of chimp fellatio. Of course, people are sexual animals too... :-) Agentundertables (talk) 00:06, 22 May 2012 (UTC)
- Based on some of the above responses, it seems to me that the editor who asked this question stepped into a minefield of something comparable to "political correctness" (or incorrectness) because of the way they worded the question.
- If the question is reworded in a more "neutral" way, what are the answers? My attempt at rewording follows.
- Do non-human species engage in the following sorts of activities? And if so, is there evidence that sexual arousal is occasioned by these activities?
- -- Hurting/torturing other animals for no apparent good reason
- -- sexual "intercourse" with dead animals, i.e., penetration of dead bodies with a penis?
- -- sexual "intercourse" with immature members of the same species?
- -- sexual "intercourse" with animals of another species?
- Kind of a tacky subject but I understand that Wikipedia is not censored. (Just for the record, I am not asking here for medical or legal advice. :o) Thanks, CBHA (talk) 02:43, 22 May 2012 (UTC)
- Dolphins will maim and kill other animals for no apparent porpoise. Animals that were not threatening, and that the dolphins have no intention of eating. Is there a purpose to it? You could argue that they are "practicing", I suppose, or fulfilling an evolutionary urge, but nobody knows. Certain species of frog will have intercourse with very obviously dead (re: squashed, rotting) frogs. But once again, is that for fun, or is it fulfilling an urge, or what? I can't think of any animals that have sex with juveniles. As for interspecies sex, that definitely happens a lot. Take a mule, for instance. If the animals are closely related enough, you don't even need to coax the two to get it on. And I'm reminding of that hilarious picture of a dog getting it on with a duck. But like everyone else is saying, who knows why these animals are acting this way. In some cases we can definitely tie a weird sexual behavior to biology. There is a mutant strain of mice in which all the males are bisexual, and we know that's because in that strain, males cannot smell the difference between a male and a female. But in most cases we really have no clue. It's possible the animal just likes it that way, but anyone who tells you that is guessing. Someguy1221 (talk) 05:01, 22 May 2012 (UTC)
- My ex has nice feet, and I'm thinking that foot fetishes are possibly more of a unique behavior, or are manifested more strongly, with us humans, on account of our much greater dependency on needing superb feet for upright walking. Although the OP did ask about a minefield of human taboos, they also asked a much more general question regarding "unusual sexual interests" and most large primates and odd birds don't seem need to ever need to shop for attractive shoes though. Agentundertables (talk) 03:33, 22 May 2012 (UTC)
Wow, could you guys pontificate about your woodshop notions of anthroprocentrism a little bit more? We're all animals. There are plenty of animals with non-procreative sexual drives, but they seem to be the minority. However higher level social creatures have reasons for a lot of the things they do, and no, it's not something made up by the ministry of culture. But somehow I suspect the OP just wants to hear people talk about the subject. That tends to be what we do here at the reference desk, but it'd be nice if we didn't. Any specific question? Shadowjams (talk) 04:45, 22 May 2012 (UTC)
- My question was very specific: "So, do other animals have unusual sexual interests? Are there chimps, for example, who enjoy sadism, necrophilia, pedophilia, or foot fetishes (assuming these aren't typical amongst the species)?" If you don't understand that, or think that it needs to be more specific, feel free to ask for clarification. --140.180.5.169 (talk) 06:25, 22 May 2012 (UTC)
- I'm aware of instances of animal pedophilia (which I define as an adult attempting to mate with a juvenile too young to reproduce) and a documented case of a duck performing a homosexual and necrophiliac act on another. Sadism might include female grasshoppers who behead the male during intercourse, although the motivation is likely not to cause pain. I'm not aware of any foot fetish, perhaps because feet are only covered and hence "taboo", in humans. Other unusual sexual practices include sex with other species and sex with inanimate objects. Masturbation is also common, although this may actually serve a reproductive purpose (to exercise the equipment and keep it working until needed). In social animals, non-reproductive sex may also serve a social purpose, as in the case of bonobos. StuRat (talk) 06:34, 22 May 2012 (UTC)
- Again with the allure of feet, because beauty is in the genes of the beholder too, and these can be more attractive by making them unavailable to viewing. In addition, our visual processes, like those of other animals, do operate upon abstract features of the physique which we often think of as beautiful, which various dress can also accentuate. --Agentundertables (talk) 13:50, 22 May 2012 (UTC)
- I'm aware of instances of animal pedophilia (which I define as an adult attempting to mate with a juvenile too young to reproduce) and a documented case of a duck performing a homosexual and necrophiliac act on another. Sadism might include female grasshoppers who behead the male during intercourse, although the motivation is likely not to cause pain. I'm not aware of any foot fetish, perhaps because feet are only covered and hence "taboo", in humans. Other unusual sexual practices include sex with other species and sex with inanimate objects. Masturbation is also common, although this may actually serve a reproductive purpose (to exercise the equipment and keep it working until needed). In social animals, non-reproductive sex may also serve a social purpose, as in the case of bonobos. StuRat (talk) 06:34, 22 May 2012 (UTC)
- I think we have to distinguish between sexually mature and physically mature when talking about whether adult animals have sex with juvenile animals. For example, it is quite possible for a 5 month old female kitten to come into oestrus and get pregnant and have kittens, yet the kitten itself will not become fully grown until it is about 3 years old. Male tomcats will not make the distinction between "kitten" and "cat", merely a cat that is in oestrus or one that isn't. --TammyMoet (talk) 09:41, 22 May 2012 (UTC)
- Any polemics about "unnatural" sexuality suffer from a double fallacy. First, homosexuality is natural; second, nature is devoid of morality anyway. Watch any nature show about grizzly bears, where the mothers spend much of their lives watching out for males who will kill their cubs so that the mother will stop nursing and go into estrus for them. Wnt (talk) 02:03, 23 May 2012 (UTC)
Odd notation in genetics (greater than symbol?)
In this paper (on royal jelly), specifically in the section "Royalactin changes Drosophila phenotypes via Egfr," there is some form of gene notation using greater than (>) symbols that I am not familiar with and cannot find a non-dense reference on google, or indeed really any useful information at all. I think it may be describing something to do with gene silencing but I'm not sure. Could anyone shed some light on this for me? Examples are:
- P0206>dPI3K
- P0206>dEpgrRNAi
- ppl>dPI3KDN (I know the DN part means dominant-negative as thats noted in the text, but thats it)
Specifically, I'm wondering what the > and d stand for, and what relationship the > symbol denotes for the genes either side of it. Hoping someone who knows about genetics will recognise it. Thanks!! -Zynwyx (talk) 20:37, 21 May 2012 (UTC)
- I'm not sure exactly, but I can tell you that the thing on the left is not a gene, it's a line -- i.e., a group of animals that are either identical genetically or at least very similar. Looie496 (talk) 02:27, 22 May 2012 (UTC)
- P0206 is a p-element insertion site, and the greater-than symbol is used to indicate that gal4 is present at that site (I have no idea who came up with that notation or why). The "d" simply indicates that the gene that follows is the Drosophila version. Someguy1221 (talk) 04:48, 22 May 2012 (UTC)
- Thanks for the responses. Now that I read closer it mentions it in the text Aug21-Gal4 and ppl-Gal4 as two lines used with specific Gal4 tissue expression. I knew ppl was a gene (pumpless), and all of these are examples are italicised in the text so I was assuming they stood for with genes. Some more examples:
- P0206 is a p-element insertion site, and the greater-than symbol is used to indicate that gal4 is present at that site (I have no idea who came up with that notation or why). The "d" simply indicates that the gene that follows is the Drosophila version. Someguy1221 (talk) 04:48, 22 May 2012 (UTC)
- Aug21>dEfgrRNAi
- ppl>royalactin
- act>royalactin
- rho>royalactin
- So would I be right in assuming the letters on the left denote a genetic line with a Gal4 UAS at/near that locus? And would the > imply then that the gene on the right replaces the gene on the left, so that the gene on the right is expressed in a Gal4 study instead of the gene on the left, or are both genes present? Also would anyone know where I could find more notation on genetic notation (including this >d stuff)? I'm guessing the >d is specific to Gal4 studies though now. Cheers -Zynwyx (talk) 08:39, 22 May 2012 (UTC)
- A much more common notation would be rho::royalactin, which would indicate that the RNA-coding sequence for royalactin is being driven by the promoter for rho. So to me this is reminiscent of that notation except > is used instead of :: to indicate that the driving is occurring indirectly through Gal4/UAS. Now, there's a question of how the thing was made, and for that you have to dig through the methods and references, because the notation does not make it obvious. In the case of P0206>, gal4 was shoved into that position and possibly knocked out whatever gene was original driven by that promoter. But in the case of rho>, I'm going to assume they constructed it denovo and did not do anything to the original gene, since rho knockouts have severe, sometimes lethal phenotypes. No idea where you'd learn more about notations though, aside from asking here and other places. Someguy1221 (talk) 16:04, 22 May 2012 (UTC)
- d stands for Drosophila (abstract: "Drosophila Egfr interference (dEgfri)"). I think Someguy dealt with the rest. Wnt (talk) 21:53, 23 May 2012 (UTC)
May 22
Rotating in space
If an electric motor was allowed to run while floating free in space, then presumably the armature would rotate one way and the casing the other, but at what relative speeds? I'm guessing there must be an equation relating the two rotational speeds, that somehow also involves the masses of the components. For example if the casing was very heavy compared to the armature then it it seems intuitive that it would rotate more slowly. But what exactly would that equation be? 86.177.105.243 (talk) 00:05, 22 May 2012 (UTC)
- It would be "whatever is necessary to conserve angular momentum." In the general case, that means solving the constrained equations such that the torque satisfies the elecromagnetic characteristics of the motor windings; and then solving for the two angular velocities for the case and the loaded-axle (respectively). In the most general case, the angular momentum is computed using a tensor formulation; but for simple cylindrically-symmetric motors, a scalar moment is usually a good approximation. The torque produced by the motor is governed by Faraday's law; or one of its engineering approximations like the solenoid equation or an empirical motor equation.
- So, we have:
- torque τ = (some engineering approximation related to the input current or voltage)
- total angular momentum L = ΣLi = ΣIiωi (if you've got a non-serif font, you're surely finding L's and i's and 1s troublesome by now)...
- ...for the sign convention I've arbitrarily chosen (angular velocity always positive and direction indicated by sign). Analytical calculation for the moment of inertia I of any non-ideal shape is quite difficult; but it can be measured.
- Incidentally, this is the physics behind a reaction wheel for spacecraft stabilization and orientation. Nimur (talk) 01:41, 22 May 2012 (UTC)
- Thank you for your reply. This is about at the limit of my ability to understand. Can we say that if the motor is initially switched off and has no overall angular momentum, then, when the motor is subsequently switched on, regardless of the torque, it remains that (assuming no external forces applied)? Does this lead to , regardless of torque? That equation appears to make some intuitive sense to me. 86.177.105.243 (talk) 01:56, 22 May 2012 (UTC)
- Actually I noticed that when I converted to MathML I introduced a serious typo in my system of equations that changed the meaning of my torque equation. I have just corrected that mistake; sorry for the error. Yes, you're correct: angular momentum is conserved, regardless of the torque of the motor. And, the torque will impart an angular momentum to the load, where the moment of inertia must account for the total load. The equations need to be solved simultaneously to solve for the rotation rate for either component. Nimur (talk) 03:34, 22 May 2012 (UTC)
- Thank you for your reply. This is about at the limit of my ability to understand. Can we say that if the motor is initially switched off and has no overall angular momentum, then, when the motor is subsequently switched on, regardless of the torque, it remains that (assuming no external forces applied)? Does this lead to , regardless of torque? That equation appears to make some intuitive sense to me. 86.177.105.243 (talk) 01:56, 22 May 2012 (UTC)
- Wouldn't they rotate in opposite directions at the same speed? They should just have a different acceleration?
_
/ \
| _
| -----____
| -----------
|
---------------------------
\______________
-----------
- I think that the armature should initially turn much faster than the terminal velocity; as the casing accelerates the armature should slow-down, and tend towards an asymtote. The casing also works towards an asymtote. Plasmic Physics (talk) 03:42, 22 May 2012 (UTC)
- No, that is not correct. That would violate conservation of momentum, and it's neither what our mathematical formula predicts, nor what we observe to happen in experiment. Nimur (talk) 03:48, 22 May 2012 (UTC)
- Oh yeah, nevermind. I didn't think it through. Plasmic Physics (talk) 03:53, 22 May 2012 (UTC)
- No, that is not correct. That would violate conservation of momentum, and it's neither what our mathematical formula predicts, nor what we observe to happen in experiment. Nimur (talk) 03:48, 22 May 2012 (UTC)
Gravitational time dilation at the centre of a large mass
How does gravitational time dilation operate at the centre of a large mass (not a black hole though)? Does gravity affect time when it pulls in all directions? And on a related note, what time dilation effects would happen if you have a clock in between two massive objects, and is therefore pulled in two opposing directions (equally, and let's pretend the two objects will not fall into each other)? Would the opposing gravitational fields cancel out the effects of time-dilation on the clock or would the two sum together? 137.111.13.167 (talk) 02:49, 22 May 2012 (UTC)
- Yes, gravitational time dilation does occur in symmetrical situations such as when a clock is midway between two identical massive objects, or when the clock is at the center of a spherically symmetrical massive object. In the weak field limit, the amount of the time dilation is proportional to the difference in gravitational potential, so the effect due to two objects is additive in a scalar sense, not additive in a vector sense. Red Act (talk) 04:24, 22 May 2012 (UTC)
Physics: Aerodynamics of Pigeon Wing.
A normal Racing pigeon has ten primary flight feathers in each wing. There is, however, an uncommon gene that duplicates the second primary flight feather. This can be seen here. Compare that to the regular, 10-flighted bird seen here.
You'll notice the first few flight feathers of the regular bird are separated at their tips, however this is not the case in the bird with the duplicated second flight feather.
What do you think will be the impact on flight efficiency and speed caused by the extra flight feather? Will the bird fly more efficiently due to the extra feather, or less efficiently due to the lack of a gap? Or another reason?
Thanks, Abbott75
- A very interesting and difficult question! The second link to Wing.jpg is broken ("remote linking forbidden"). If you can fix or replace that link, you might get better answers. SemanticMantis (talk) 13:21, 22 May 2012 (UTC)
- I would suspect that separated feathers create more drag, but also allow for greater control, as they can then be moved independently. For an analogue, think of separate fingers versus webbed fingers. The separate fingers are more versatile, while the webbed version makes for more efficient swimming. StuRat (talk) 18:49, 22 May 2012 (UTC)
"Whoa!" moments
I hope this is not an inappropriate question for the Science Desk, but there are so many smart people here I'd love to see the answers. Delete this question if necessary.
I was thinking this morning about "Whoa!" moments; the moments when you learn a new fact that rocks your world. Two for me I could think of right off were: 1) When I learned every bit of me used to be inside a star; and 2) When I learned the "double slit" experiment worked with only one electron.
What were your "Whoa!" moments? Tdjewell (talk) 11:29, 22 May 2012 (UTC)
- Wheeler's delayed choice experiment's produced a definite "whoa," for me, especially the astronomical variant of the experiment. Some of the cognitive science experiments on consciousness (do I think, "I will extend my hand and pick up that pie," or does the hand extend and then the conscious brain rationalizes it?) are pretty mind-blowing, if true. (I hold back much judgment on the truth of brain experiments at this point — there's still a lot of fundamental unknowns out there.) --Mr.98 (talk) 12:02, 22 May 2012 (UTC)
- When I heard about the experiment in Walter Jakob Gehring's lab "to introduce the mouse Small eye gene into flies ... Fly tissues were induced to form eye structures. However. it is important to emphasize that the tissues formed were fly eye structures, not mouse eye structures." [1] When I first heard this, I was sure the person who told me it must have made a mistake. --ColinFine (talk) 14:39, 22 May 2012 (UTC)
- ^ Carroll, Sean B (2006). Endless Forms Most Beautiful. Phoenix. ISBN 0 7548 2182 4.
- In a more mathematical vein, I was pretty amazed to learn the Banach–Tarski paradox, and that there are exactly 17 wallpaper groups. Another fun math fact is that if you throw a dart at a [0,1] interval, the probability of hitting any number you've ever heard of is zero.SemanticMantis (talk) 15:53, 22 May 2012 (UTC)
- Alain Aspect's experiment testing Bell's theorem. I second Mr.98 on those cognitive science experiments. I can't find a link to an article that discusses them; I would have thought they would be mentioned at Mental chronometry, but I don't see any mention of them there. Perhaps they didn't pan out on further research?--Srleffler (talk) 17:02, 22 May 2012 (UTC)
- Relativity of simultaneity. The idea that a sequence of events happens in a specific order is a fundamental aspect of intuitive understanding in the world. If A precedes B for me, then A precedes B for you. This seems like an axiomatically true idea, and seems to be basically necessary given our intuitive understanding of how the universe works. It is also fundamentally wrong. There is no privileged frame of reference really means that: that there is no where in the universe that has a perspective which is universally true for all other locations. For any sequence of events, there is a place in the universe where that sequence can be seen to occur in a different order. If that doesn't shock your basic core, nothing will. --Jayron32 17:20, 22 May 2012 (UTC)
- That quantum tunneling experiments seem to be able to transmit information faster than the speed of light, when the theory says this is impossible. StuRat (talk) 18:34, 22 May 2012 (UTC)
- That inside a black hole, matter can collapse into an infinitely small gravitational singularity. StuRat (talk) 18:34, 22 May 2012 (UTC)
- Superconductivity, superfluidity, and a Bose–Einstein condensate. StuRat (talk) 18:41, 22 May 2012 (UTC)
When I took the derivative of e^x... and alsothe first time I heard Miles Davis. Shadowjams (talk) 21:46, 22 May 2012 (UTC)
- More math than science, but after not believing it for the longest time, the moment the solution to the Monty Hall problem finally made sense. -RunningOnBrains(talk) 00:14, 23 May 2012 (UTC)
- Actually that's a good one... in light of that i'll update mine... understanding how RSA works. Shadowjams (talk) 01:13, 23 May 2012 (UTC)
- More math than science, but after not believing it for the longest time, the moment the solution to the Monty Hall problem finally made sense. -RunningOnBrains(talk) 00:14, 23 May 2012 (UTC)
- I had a "Whoa!" moment a couple of months ago when reading a short article in New Scientist about the unusual reproduction of some starfish (our article doesn't mention it). Starfish initially live as free-swimming larvae, which usually (per our article) settle on a substratum and become sessile. However (per the NS article), in some species, the adult form grows within the abdomen of the larva, detaches from it and settles down, while the larva continues to swim, feed, etc for weeks or months – almost as if the adult starfish had been a parasite, or two previously separate species' genomes and development had merged. I believe Darwin touched upon the latter possibility in On the Origin of Species . . . when discussing metamorphosis. {The poster formerly known as 87.81.230.195} 84.21.143.150 (talk) 12:30, 23 May 2012 (UTC)
- A few more from biology: birds are dinosaurs. Several parasites can control their host's behavior, a.k.a "mind control", see zombie ants or Emerald cockroach wasp. Bdelloidea have only female individuals. They don't have sex per se, but they can uptake DNA and incorporate from the environment, from dead sisters, or even other species! SemanticMantis (talk) 13:37, 23 May 2012 (UTC)
- It's easy to look for all the grander ones, but as a kid, I remember finding out that the identity of an element is determined solely by the number of protons. The elegant simplicity of it kind of shocked me. We're all used to it now, but cast your mind back....right? Throw in colour vision for me (like, how come brown and pink aren't in the rainbow?) and my recent reading of Steven Pinker's The Blank Slate. Whoa - ! so all that political correctness is just plain wrong (or thereabouts)? IBE (talk) 14:03, 23 May 2012 (UTC)
Many such moments. I learned a lot about science in primary school myself from books, and I realized that religion had to be nonsense despite many grown up people telling stories about the Bible and God as if it were fact. So, the first (and I guess most important) whoa moment was that you could actually not trust grown ups to know the facts even on points they were very sure about.
But I get whoa moments almost every week when I read about some intneresting facts. E.g. just last weeks on a t.v. documentary about the evolution of plants, it was mentioned that grass evolved in such a way as to be able to start fires that would destroy trees, making more room for them. Also, I found out rather late in my life that many vegetables didn't exist more than a few thousand years ago, being the result of selective breeding. Count Iblis (talk) 18:04, 23 May 2012 (UTC)
Fun examples of body parts that go on living
Here (youtube link) is an example of a turtle heart that keeps on beating half an hour after the turtle died - and a few minutes after being cut out of the body.
What are other examples of zombie body parts that just keep on going? Are there any human examples? Egg Centric 11:44, 22 May 2012 (UTC)
- I presume you know about Mike the headless chicken? --TammyMoet (talk) 14:05, 22 May 2012 (UTC)
- This article, published a few days ago, is an interesting read on the subject. --Jayron32 17:26, 22 May 2012 (UTC)
- Ooops, I should have mentioned - that's where I got the link from! Any other good 'uns? Especially interested in human bits here.... Egg Centric 17:55, 22 May 2012 (UTC)
- A snake can bite you after it has been decapitated.. Vespine (talk) 22:56, 22 May 2012 (UTC)
- Ooops, I should have mentioned - that's where I got the link from! Any other good 'uns? Especially interested in human bits here.... Egg Centric 17:55, 22 May 2012 (UTC)
F-22 Raptor's deadly toxic stealth goo
What sort of chemicals would cause these problems? Hcobb (talk) 15:47, 22 May 2012 (UTC)
- As the article notes, the US government is particularly cagey about the composition of the stealthy materials used in the construction of low-observable aircraft. They successfully resisted court efforts to uncover the materials used when building the B2, and (as Area 51#Environmental lawsuit notes) materials associated with the earlier Have Blue / F117 programme. But the lawsuit discussed in that last article does reveal, broadly, some of the chemicals detected in the tissues of plaintiffs, listing dioxin, dibenzofuran, and trichloroethylene. Materials data sheets for these list dizziness as a side effect, but in fairness dizziness, vertigo, and similar rather non-specific effects are in the materials safety sheets for a huge swathe of chemicals used in or with adhesives, paints, synthetic polymers, cleaning solvents, and other modern materials. You would think that the aircrew and groundcrew would be exposed to quite different things, as the aircrew mostly breathe a mixture of scrubbed recycled air and engine bleed air (that's what you breathe on an airliner, with people occasionally complaining that the air smells of engine degreaser); whereas it seems the groundcrew are crawling about on the outside, rubbing magic space slime into the aerosurfaces. Given USAF/DoD's success in persuading courts that even disclosing the broad chemical nature of materials to which workers have been exposed is a threat to national security, I think you can count on not getting a concrete answer to this question for many decades. As the Russians, and probably the Chinese, have big chunks of F117 (ref) surely they've got a general idea of what (last generation) stealth aircraft were made of. The Area 51 article cites 60 Minutes interview with Congressman Lee H. Hamilton who ascribes the secrecy in this regards to a USAF attempt "to protect themselves from a lawsuit." -- Finlay McWalterჷTalk 17:01, 22 May 2012 (UTC)
- I don't think you have to look too hard for anything exotic. Since they reapply the stealth materials frequently, volatile compounds, present in any glue, must out-gas. This is why the safety label on just about any glue says "only use in a well ventilated area". I suspect that the hangars where they apply the stuff are not particularly well ventilated, since they don't want dust particles getting in (and people being able to view the process). These planes also bring in outside air and use that for the pilots, and that outside air must contain the toxins, especially when waiting on the ground to launch.
- I suggest that both the maintenance crew and pilots use a completely contained oxygen source (oxygen bottles). I wonder if they have tried this yet. They do represent a potential fire danger, however, so a more sophisticated system could also be designed, where, instead of trying to filter out contaminants, and apparently missing at least one, they just extract the oxygen (and maybe some nitrogen) from the outside air, effectively only filtering in those gases, and nothing else. I've also wondered why they don't just take air samples and analyze them to find the problem component(s). I can see why they may not want to release this info to the public, but, for their own use, this would be quite valuable info. StuRat (talk) 18:17, 22 May 2012 (UTC)
- It's hard to know what to think about this sort of thing. For all I know some foreign power has stoked the fires of these media reports simply in order to get more of the chemical data about the stealth material going through less-secure channels they can spy on. Or for all I know truthout is right. A mystery like this (e.g. "sick building syndrome") can be hard enough to crack in the best and most open of circumstances. Wnt (talk) 01:52, 23 May 2012 (UTC)
- Could be as simple as variations in oxygen content. Without a buffer, any fluctuation would reach the pilots. How does the system cope with temperature variations? What's the humidity? Could there be high concentrations of ionic compounds? How are transients in pressure handled by the system? In a passenger airplane you'll probably won't notice short interrupts in air supply when valves open or close, like when the system switches between low and high pressure bleed ports. There's enough air in the plane to "dilute" or dampen any transients. In the F-22, the buffer will be much smaller, and the system will have to cope with much larger and more rapid variations in temperature and pressure. Taking in air at 300°C, cooling, feeding it at high pressure to the zeolite adsorbents, pump the remaining oxygen further, switching to the other sieve while lowering the pressure so the adsorbed nitrogen is released, dump that nitrogen and start the next cycle, and controlling all this to maintain a stable output under varying conditions could be a challenge. Resolution of oxygen level monitoring may not be optimal, I doubt paramagnetic sensors can be used in planes, and electrochemical sensors have long response times. Given the hypoxia and decompression incidents, a faulty system seems more likely than toxic chemicals imo. Ssscienccce (talk) 23:15, 24 May 2012 (UTC)
Quick weather question
The National Weather Service in Tuscon has issued a Red Flag Warning for my area. I was reading the warning and had a question about some terminology used within:
.LOW PRESSURE PASSING NORTH OF ARIZONA WILL CREATE STRONG AND GUSTY WINDS ON WEDNESDAY...WITH SUSTAINED 20-FOOT WIND SPEEDS UP TO 30 MPH AND GUSTS TO NEAR 50 MPH. THESE WINDS...COUPLED WITH CONTINUED HOT AND DRY CONDITIONS...WILL CREATE CRITICAL FIRE WEATHER CONDITIONS THAT MAY RESULT IN RAPID FIRE GROWTH AND SPREAD. ... * WINDS...SUSTAINED 20-FOOT SOUTHWEST WINDS OF 25 TO 35 MPH ARE EXPECTED WITH GUSTS TO NEAR 50 MPH. THE STRONGEST WINDS ARE EXPECTED IN EASTERN PORTIONS OF THE WARNED AREA.
The warning is archived here.
What does "20-foot winds" mean? Thanks! -- 143.85.199.242 (talk) 17:53, 22 May 2012 (UTC)
- WAG, but maybe this is the standard height off the ground that winds are measured from; that is the anemometer is located at 20 feet off the ground. It would make sense, for standardization purposes, to make the wind speed measurement at some standard height, as wind speeds will be quite different depending on where they are measured. That's just a guess tho. --Jayron32 18:02, 22 May 2012 (UTC)
- Per Rachelle Willgren, writing in the "Blanco County News" in April 2011, the "Red flag warnings" for fire danger define "20 foot winds" as "the average 10-minute wind speed (mph) and direction 20-feet above the vegetation." Flames and smoke from burning brush or trees rise this high, and it is useful to know which way and how far the wind will carry them. [8] here is the National Weather Service definition. Edison (talk) 18:45, 22 May 2012 (UTC)
- Your confusion is certainly justified; I had never heard this term before even working as an atmospheric scientist. It appears to be used solely for fire-weather-related purposes [9]; almost all other atmospheric science fields measure "surface" winds at 10 meters above the ground, regardless of vegetation [10]. -RunningOnBrains(talk) 00:32, 23 May 2012 (UTC)
do most mainstream water-resistant sunscreens have dangerous ingredients?
I've read that according to the sunscreen controversy that many sunscreens contain avobenzone and octocrylene which absorb into the skin and are potent free radical generators. I would certainly like to avoid this kind of thing while protecting my skin. Where can I find waterproof sunscreens that judiciously prefer to use titanium dioxide or zinc oxide as stabilisers rather than octocrylene, as well as other dangerous ingredients? 137.54.7.13 (talk) 18:24, 22 May 2012 (UTC)
- I'd expect any store which sells organic products might have such sunscreens. And don't forget about just covering up, with loose-fitting white clothes and wide-brimmed hats. Also, sit in the shade. StuRat (talk) 18:56, 22 May 2012 (UTC)
- This is for looking good at the beach. There'd be no reason to have really good skin if one wasn't showing off. 76.104.28.221 (talk) 18:03, 24 May 2012 (UTC)
- "Organic" isn't always a good sign that something is safe, though. Nightshade, deathcap mushrooms, and anthrax are all organic. --Carnildo (talk) 02:32, 23 May 2012 (UTC)
Gravitational Blueshift Equation
I have noticed that on the Gravitational Redshift Page there exists an equation for calculating gravitational redshift. There is no Equation on their for calculating Gravitational Blueshift, there is no Gravitational Blueshift Page, and there is a Blueshift page, but it also lacks an equation for calculating Gravitational Blueshift. The Equation:
I also failed to locate such a blueshift equation anywhere else on the internet. So I then tried to figure it out on my own. I went about this by plugging variables into the redshift equation and then attempting to discern the blueshift one through the results. I am not entirely sure I did this correctly. Please tell me if I didn't.
First off the variables:
R* = 5m
r = 10m
M = 1x1027kg
G (used the compatible one) = 6.673848x10-11 N (m/kg)2
c (not sure if this is the compatible version) = 3x108m/s
λI = 100m
So I solved it step by step:
2GM: 2 x G x (1x1027) = 1.3347696x1017
R*c2: 5 x (3x108)2 = 4.5x1017
2GM/R*c2: 1.3347696x1017 / 4.5x1017 = .296615467
1 - 2GM/R*c2: 1 - .296615467 = .703384533
Square Root of 1 - 2GM/R*c2: Square Root of .703384533 = .838680233
1/(Square Root of 1 - 2GM/R*c2): 1 / .838680233 = 1.192349552
1/(Square Root of 1 - 2GM/R*c2) - 1: .192349552
z(r) = 1/(Square Root of 1 - 2GM/R*c2) - 1 --> z = [1/(Square Root of 1 - 2GM/R*c2) - 1]/r:
z(10) = .192349552 --> z = .192349552 / 10 = .0192349552
Now as the page said z is a fractional change of the wavelength, I came up with this equation:
λF = λI - (λIz)
When plugging this in I got: λF = 98.07650448
Now to figure out the Blueshift Equation I figured I had to keep the same variables (except R* and r swap values, since R* "is the radial coordinate of the point of emission (which is analogous to the classical distance from the center of the object, but is actually a Schwarzschild coordinate)." And r is "the radial coordinate of the observer").
I came up with the equation one would need after one figures out z:
λF = λI + (λIz) Which in this case would be: 100 = 98.07650448 + (98.07650448z) Because for the sake of Energy Conservation the amount of potential gravitational blueshift must be equivalent to the potential gravitational redshift and so 98.07650448m would have to return to being 100m.
I then calculated what z would have to equal for such a thing to happen through these steps:
λF - λI = Required Increase: 100 - 98.07650448 = 1.92349552
Required Increase/λI = z: 1.92349552 / 98.07650448 = .019612195
And so, I arrived at z = .019612195
I even plugged it in to the equation to be sure:
λF = λI + (λI x z): 98.07650448 + (98.07650448 x .019612195) = 100
I then could not figure out a Gravitational Blueshift Equation that yielded said z value. I found many incorrect solutions. In fact, I even tried plugging into the Gravitational Redshift Equation (which I knew would not work) in desperation.
Could someone who is more experienced with this sort of calculations please help? Some of the problems I have been working on simply require a Gravitational Blueshift Equation.
Doctor Condensate (talk) 22:46, 22 May 2012 (UTC)
- I'm not more experienced, but this source [11] seems to touch on it. Two things which I don't know to be true: a) a gravitational redshifted photon, reflected back from a mirror, should arrive at its source at its original frequency, implying a simple inverse relationship; b) the frequency change should match the amount of gravitational potential energy gained or lost as the mass of the photon is moved from one position to the other. (Though both would apply only in constant static gravitational fields, if even that often...) Wnt (talk) 02:41, 23 May 2012 (UTC)
- These two things are true (in a static gravitational field, like the Schwarzschild solution used for the common formula Doctor Condensate has mentioned). In the Schwarzschild solution, the observed frequency fo (observed at radius ro) and the emitted frequency fe (emitted at radius re) are related as follows:
- If you want a "z value" as it's commonly used in astronomy for redshift, you will get a negative z for blueshift. In general:
- Substitute the ratio of fe and fo from the eqation above and you get your z. (I don't know any field of study where it's common to use z for blueshifts; and personally I think the ratio of frequencies is the more convenient number anyway)
- Icek (talk) 03:35, 23 May 2012 (UTC)
- First of all, z(r) doesn't mean z times r. It means that z is a function of r. The equation at the top only gives you the value of z in the limit where r is very large.
- There's no such thing as the redshift (or blueshift) formula; it depends on the situation. The equation you gave is only valid in one specific case: the emitter is hovering at a fixed distance from a spherical object of mass M, and the receiver is hovering "at infinity" (i.e., very far away). If you want to calculate the blueshift in some situation you need to be specific about what situation that is. Note that physicists often use the word "redshift" in a way that includes blueshift, just as they use "acceleration" in a way that includes deceleration. A blueshift is just a redshift by a factor of less than one. -- BenRG (talk) 20:38, 23 May 2012 (UTC)
- God, I read "Gravitational Bullshit Equation," glad I didn't see this question until now or the first reply may have been very different. RMoD (talk) 23:17, 23 May 2012 (UTC)
Thank you for the quick responses. Well, BenRG. My current work requires the following. First, an equation that calculates the redshift of light emitted from a point at a specific distance from the center of a spherical object with mass, and observed at a point directly above the point of emission (which of course is also at a specific distance from the center).
Second, I need an equation that calculates the inverse. I need to be able to calculate the blueshift of light emitted from a point at a specific distance from the center of a spherical object with mass, and observed at a point directly below it. Which, I think Icek provided for me.
For both of these, a line that passes through both the point of emission and the point of observation should also pass directly though the center of the spherical object with mass.
Doctor Condensate (talk) 00:16, 24 May 2012 (UTC)
May 23
Diversity of sexuality #2
I feel bad for reposting this question, but even though the original discussion had a few high-quality answers, it has gone way off track. I think this was because my question wasn't sufficiently clear, so let me clarify:
"Do other animals have unusual sexual interests? Are there chimps, for example, who enjoy sadism, necrophilia, pedophilia, or foot fetishes (assuming these aren't typical amongst the species)?" I'm only interested in objective descriptions of observable behavior, not in any moral judgements made by either humans or animals.
Note that I carefully avoided using any terms that imply a normative judgement. I used "unusual" instead of deviant, unnatural, or abhorrent because unusual is a positive statement, whereas the others imply a moral judgement I refuse to make. Also, the only reason I omitted homosexuality from my list is because I already know many animals are homosexual. It isn't because homosexuality is not unusual (it obviously is), nor is it related to any opinions I have about sexual behavior. If possible, I'd prefer the answers to similarly refrain from all normative judgement. Thanks in advance. --140.180.5.169 (talk) 04:17, 23 May 2012 (UTC)
- Because you included the word "enjoy", an answer must require some judgement, normative or whatever. For instance, dogs humping legs are commonly seen. I would assume this is NOT because they have a leg fetish - its more likely they are feeling horny, but can't find a female of compatible size. But how are we to know? Maybe dogs enjoy humping legs, or maybe its frustrating for them. Its common for dogs to lick their genitals - because they can. Do they enjoy it as masturbation? It certainly seems so. Male spiders tend to get eaten after they've done the deed. Is this part of sex for the female? Who knows, put probably not. Its probably just a case of the sexual stimulation of the males's courtship dance stopped, and the female's brain now recognises food. Ratbone120.145.20.154 (talk) 05:43, 23 May 2012 (UTC)
- You're still assuming animals possess language and culture, "sadism, necrophilia, pedophilia, or foot fetishes" are specific human cultural features in English. Your comment above regarding neuronal analysis of behaviour is specious, as you can't neuronally analyse human sadism. Do animals injure other animals during coitus: yes. Do animals construct the injury of other animals as part of a psychosexual structure of symbols: no. Do animals engage in coitus with dead things: yes. Do animals construct "death," "permissable sexual activity," "impermissable sexual activity," and the "specific impermissable sexual activity of sex with dead things" as cultural meanings: no. Do animals engage in coitus with sexually immature animals: yes. Do animals construct a social and cultural category of a "child" and construct the impermissable sexual activity of sex with a child: no. Animals do not socially construct "children," and any different behaviour towards juveniles is purely different behaviour towards juveniles. Do animals have foot fetishes: no. Animals do not construct cultural or psychosexual fetish objects, animals do not have fetishes, they have behaviours. Are there stereotyped behavioural examples of animals attempting to copulate with limbs, yes. Do dogs have an interior construction of a socially ideal leg: no. You're persisting in inflicting anthropocentric categories of social, cultural and psychosexual analysis on animal behaviours. Answer me this: Who was dog Hitler? Fifelfoo (talk) 08:23, 23 May 2012 (UTC)
- Are you sure this is true? I know that the tendency to anthropomorphism is a much condemned excess; nonetheless, I find it very hard to believe that there is not some literal homology between the bass defending his nest and the farmer with the rock salt shotgun watching over his field, or indeed the elephant seal or the sultan defending their harem. Animal culture exists to some extent, and I'd be more surprised if dogs didn't have some way to agree that some females are particularly attractive. I'll agree, of course, that anthropomorphizing is often dubious and can quickly stray into fantasy, but it is a rich source of plausible hypotheses, and I doubt many of them have actually been disproved conclusively. Wnt (talk) 12:54, 23 May 2012 (UTC)
- One of the tests we can briefly create for "culture" is the reversal of behaviour structure in certain circumstances—the festival or jubilee. Sultans allow their harems to be cuckolded for specific reasons, farmers allow members of their religious community to share in their excess. These contradictions don't arise in animal behaviour because animals aren't subject of language. Even when we can credit behavioural dominance over consciousness in human behaviour structures—such as new human males killing their female's old juveniles and similar animal behaviour structures—the animals never experience the human cultural elements: marriage, cuckoldry, bastardy, infanticide, murder, the cycle of the Titans, etc. So while we can draw behavioural metaphors in some circumstances (and I'd limit them far more than your examples), even when the behaviours are demonstrably pre-conscious and not related to the sub-conscious, the human experience of these behaviours is radically different to animal experience. Cats ejaculate, I ejaculate, cats never stare at the ceiling wondering if their ejaculate was sufficiently pleasing for their partner. Fifelfoo (talk) 23:31, 23 May 2012 (UTC)
- Something resembling a source for this discussion can be found at [12]; on thinking in general it raises the example that scrub-jays which cache food when observed will re-cache it when unobserved, reflecting their awareness that the other bird will consider taking it. Maybe it would be interesting to have three scrub-jays, two conditioned to cache red balls for a food reward, a third conditioned to cache blue balls, each marked in some way for recognizability within some vast experimental enclosure with limited domains of visibility. Would the blue-caching scrub-jays recognize that the red-ball cacher is no threat to them, while taking precautions against one another? Or what if they had a chance to observe that one scrub-jay were conditioned, or had a natural propensity, to steal others' caches, and the other didn't? Would they recognize the bird by personality? Wnt (talk) 20:52, 24 May 2012 (UTC)
- One of the problems with the scrub-jay experiment you outline, is that it would demonstrate that the blue caching scrub-jay carries an interior model of the thought process of the red caching scrub-jay, but it wouldn't demonstrate an internal symbol system sufficiently complex to get that contradictory kind of thought process that plagues our days. I like the discussion in the Handbook of Jealousy, that would be really interesting to conduct vasopressin antagonist studies on humans during sex, and then prompt them with threats and ask them about their interiority. Fifelfoo (talk) 23:25, 24 May 2012 (UTC)
- Something resembling a source for this discussion can be found at [12]; on thinking in general it raises the example that scrub-jays which cache food when observed will re-cache it when unobserved, reflecting their awareness that the other bird will consider taking it. Maybe it would be interesting to have three scrub-jays, two conditioned to cache red balls for a food reward, a third conditioned to cache blue balls, each marked in some way for recognizability within some vast experimental enclosure with limited domains of visibility. Would the blue-caching scrub-jays recognize that the red-ball cacher is no threat to them, while taking precautions against one another? Or what if they had a chance to observe that one scrub-jay were conditioned, or had a natural propensity, to steal others' caches, and the other didn't? Would they recognize the bird by personality? Wnt (talk) 20:52, 24 May 2012 (UTC)
- One of the tests we can briefly create for "culture" is the reversal of behaviour structure in certain circumstances—the festival or jubilee. Sultans allow their harems to be cuckolded for specific reasons, farmers allow members of their religious community to share in their excess. These contradictions don't arise in animal behaviour because animals aren't subject of language. Even when we can credit behavioural dominance over consciousness in human behaviour structures—such as new human males killing their female's old juveniles and similar animal behaviour structures—the animals never experience the human cultural elements: marriage, cuckoldry, bastardy, infanticide, murder, the cycle of the Titans, etc. So while we can draw behavioural metaphors in some circumstances (and I'd limit them far more than your examples), even when the behaviours are demonstrably pre-conscious and not related to the sub-conscious, the human experience of these behaviours is radically different to animal experience. Cats ejaculate, I ejaculate, cats never stare at the ceiling wondering if their ejaculate was sufficiently pleasing for their partner. Fifelfoo (talk) 23:31, 23 May 2012 (UTC)
- Are you sure this is true? I know that the tendency to anthropomorphism is a much condemned excess; nonetheless, I find it very hard to believe that there is not some literal homology between the bass defending his nest and the farmer with the rock salt shotgun watching over his field, or indeed the elephant seal or the sultan defending their harem. Animal culture exists to some extent, and I'd be more surprised if dogs didn't have some way to agree that some females are particularly attractive. I'll agree, of course, that anthropomorphizing is often dubious and can quickly stray into fantasy, but it is a rich source of plausible hypotheses, and I doubt many of them have actually been disproved conclusively. Wnt (talk) 12:54, 23 May 2012 (UTC)
- The 2003 Ignobel Prize for Biology was won by Kees Moeliker for his investigations into Homosexual necrophilia in the mallard duck [13]. You may be interested in the rest of the Animal sexual behaviour article as well. LukeSurl t c 09:46, 23 May 2012 (UTC)
- The fundamental error in the question is the term "unusual". It amounts to applying human standards to non-humans. Morality is a human concept. Animals don't have "morality". They do what they do. ←Baseball Bugs What's up, Doc? carrots→ 12:05, 23 May 2012 (UTC)
- Meh. I've known pets with a greater sense of morality, as in knowing right from wrong, than many humans. --76.182.93.85 (talk) 12:13, 23 May 2012 (UTC)
- I think it's fairly obvious what the OP is asking for [i.e. interesting animal sex facts]. I'm not sure what purpose scrutinising the exact wording of his inquiry serves. LukeSurl t c 12:33, 23 May 2012 (UTC)
- It serves for clarification. Your assumption may be correct, but that's not entirely clear from the question. — The Hand That Feeds You:Bite 12:57, 23 May 2012 (UTC)
- Meh. I've known pets with a greater sense of morality, as in knowing right from wrong, than many humans. --76.182.93.85 (talk) 12:13, 23 May 2012 (UTC)
- The issue is that we cannot be certain of the motive behind the act. As an example, there was a humorous meme image that involved a mouse having sex with a female who was dead in a mousetrap. There's no way of knowing if the male was sexually attracted because she was dead, or if he was simply taking advantage of the fact that the female didn't rebuff him (not realizing she was dead). There's no way of knowing if this was necrophilia in the psychological sense, or if it was just sex with a corpse because it was available. — The Hand That Feeds You:Bite 13:14, 23 May 2012 (UTC)
- Ha, if it were that simple and not entirely relevant, because even with people its not likely to be an either/or motivation, for its likely that a very high level of availability, such as being an ugly frustrated son of an undertaker, leads to the necrophilia of ending up with "unusual" macabre acts of loving the dead, so they are not behaving any better than some poor rodents. --76.182.93.85 (talk) 13:35, 23 May 2012 (UTC)
- The other thing being ignored here is that no non-Human animal shows signs of true metacognition and theory of mind. That is what Fifelfoo is getting at above. Do animals display sexual behaviors which, if humans did them, other humans may view as deviant? Absolutely. I have seen animals fuck anything. But to assign human values to those behaviors is meaningless, as those human values require that the animals are capable of sapience or self-actualization, or metacognition, or theory of mind, which no evidence has been shown that they can or do. A dog doesn't spend a lot of time worrying about if his sexual partner enjoyed the experience, a dog doesn't have the ability to project its own values on the minds of others, or even to think about its own values. It gets horny, it fucks something. It will fuck the nearest female dog in heat, if availible. But it doesn't spend a whole lot of effort considering the implications of its sexual act in culturally acceptable ways. It just doesn't. The OP has been provided with ample evidence of sexual behaviors which, when compared to human sexual standards, would be deviant in humans. It doesn't mean anything that animals display these behaviors, however, because animals don't have the expectations within themselves over what constitutes acceptable sexual boundaries. --Jayron32 17:40, 23 May 2012 (UTC)
- I think there is evidence that some animals consider how others will view their sexual activity. In many cases a secret sexual encounter occurs, when making it known would have negative consequences. StuRat (talk) 17:54, 23 May 2012 (UTC)
- Avoidance of consequences is not a sign of metacognition. If I hit my dog on the nose with newspaper every time he pees on the carpet, he stops peeing on the carpet. It doesn't mean he has an understanding of socially correct bathroom usage. He just doesn't like getting hit with a newspaper. --Jayron32 18:08, 23 May 2012 (UTC)
- Certainly avoidance of consequences are apart of metacognition, because operant conditioning is a major part of our own human social conditioning. --76.182.93.85 (talk) 18:17, 23 May 2012 (UTC)
- It isn't a part of metacognition though. You don't have to be able to understand your own internal thought processes to avoid consequences. You just do it without thinking about it. The important parts of metacognition is being able to think about your own thoughts, and think about the thoughts of others. Dogs can't do this. --Jayron32 19:33, 23 May 2012 (UTC)
- Saying "Dogs can't do this." is simply meaningless chopping off the head of the dog (which are quite intelligent) to make people seem more cognitive. Instead of advocating on their terms, whatever that may be, we get unfounded assertions. (and I am the 76. IP, because wasn't logged in earlier) --Modocc (talk) 19:39, 23 May 2012 (UTC)
- I suppose you have evidence that dogs can do it then. We don't just accept a random assertion without evidence, or I can claim anything I want and you need to prove me wrong. I can assert that a dog wrote all of the Homeric epics, and you haven't proven that it didn't. On the contrary, we have no evidence that any form of life other than humans is capable of metacognitive thought on anything close to what humans can do. There just isn't. There are occasional studies that show maybe, kinda, if you look at it in a certain way, that an animal displays a behavior that with enough wishful thinking and hope that it shows metacognitive thought. But as capable as dogs are of doing very complex things, there is simply no evidence that they have internal monologues or have a theory of mind or anything like that. --Jayron32 20:27, 23 May 2012 (UTC)
- I am not the one claiming they can't have cognitive thoughts (which BTW, contrary to what one might conclude from scripted forms of communications do not require internal symbolic monologues) that associate actors with emotions, actions and potential actions and emotions (their own as with well as with others), as well as various strategies involving those things. To the contrary, both dogs and people show a wide range of variation in their individual abilities to socialize and/or to problem solve. --Modocc (talk) 20:42, 23 May 2012 (UTC)
- I have never made that claim either. You should read what I wrote, and learn the definitions of words before you disagree with someone over something they haven't said. --Jayron32 20:59, 23 May 2012 (UTC)
- "The important parts of metacognition is being able to think about your own thoughts, and think about the thoughts of others." which is the cognition of thoughts, which might be as simple as whether or not when I pick up the car keys we might be going for a car ride (Teachy runs for the door in anticipation). Call this metacognition or simply a cognition or whatever you like, its absurd to set up a model of thought or a theory of mind and use these as litmus test to make unfounded claims. Its a typical strawman fallacy. --Modocc (talk) 21:19, 23 May 2012 (UTC)
- It isn't a strawman fallacy. There's a difference between reacting to a stimuli and understanding what another is thinking. Your dog knows that when you pick up the keys, you're going for a car ride. He doesn't analyze what you are thinking about. He's responding to training. --Jayron32 01:06, 24 May 2012 (UTC)
- How do you know my dog knows? What does he know or thinking about? Teachy also does her best to convince me its a great idea. She is uncertain because I don't always leave or take her with me. Of course I figured you would jump to the training bit as if people never take their own such experiences in to account. Models should fit facts, because facts do not fit models unless the facts are true. Its not hard to believe anything if you base conclusions on poorly conceived models of reality which is what strawmen generally are. --Modocc (talk) 01:13, 24 May 2012 (UTC)
- When you say "How do you know my dog knows? What does he know or thinking about?", that is my whole point. You can't make positive assertions without evidence. You can't declare that your dog has some ability simply because no one has yet found evidence that she does not. Can you prove that she isn't busy translating Shakespeare into idiomatic Quebecois French in her mind or solving Maxwell's equations for fun as an intellectual exercise? No, you can't. But just because you can't prove she's not doing those things doesn't mean you should demand that the world accepts that she is doing them. That's the whole point: there is no way to access the internal thought processes of a dog (or any similar animal), and so to make outrageous claims about their abilities based on the fact that you cannot prove they don't have them is just patently rediculous. --Jayron32 19:44, 24 May 2012 (UTC)
- How do you know my dog knows? What does he know or thinking about? Teachy also does her best to convince me its a great idea. She is uncertain because I don't always leave or take her with me. Of course I figured you would jump to the training bit as if people never take their own such experiences in to account. Models should fit facts, because facts do not fit models unless the facts are true. Its not hard to believe anything if you base conclusions on poorly conceived models of reality which is what strawmen generally are. --Modocc (talk) 01:13, 24 May 2012 (UTC)
- It isn't a strawman fallacy. There's a difference between reacting to a stimuli and understanding what another is thinking. Your dog knows that when you pick up the keys, you're going for a car ride. He doesn't analyze what you are thinking about. He's responding to training. --Jayron32 01:06, 24 May 2012 (UTC)
- "The important parts of metacognition is being able to think about your own thoughts, and think about the thoughts of others." which is the cognition of thoughts, which might be as simple as whether or not when I pick up the car keys we might be going for a car ride (Teachy runs for the door in anticipation). Call this metacognition or simply a cognition or whatever you like, its absurd to set up a model of thought or a theory of mind and use these as litmus test to make unfounded claims. Its a typical strawman fallacy. --Modocc (talk) 21:19, 23 May 2012 (UTC)
- I have never made that claim either. You should read what I wrote, and learn the definitions of words before you disagree with someone over something they haven't said. --Jayron32 20:59, 23 May 2012 (UTC)
- I am not the one claiming they can't have cognitive thoughts (which BTW, contrary to what one might conclude from scripted forms of communications do not require internal symbolic monologues) that associate actors with emotions, actions and potential actions and emotions (their own as with well as with others), as well as various strategies involving those things. To the contrary, both dogs and people show a wide range of variation in their individual abilities to socialize and/or to problem solve. --Modocc (talk) 20:42, 23 May 2012 (UTC)
- I suppose you have evidence that dogs can do it then. We don't just accept a random assertion without evidence, or I can claim anything I want and you need to prove me wrong. I can assert that a dog wrote all of the Homeric epics, and you haven't proven that it didn't. On the contrary, we have no evidence that any form of life other than humans is capable of metacognitive thought on anything close to what humans can do. There just isn't. There are occasional studies that show maybe, kinda, if you look at it in a certain way, that an animal displays a behavior that with enough wishful thinking and hope that it shows metacognitive thought. But as capable as dogs are of doing very complex things, there is simply no evidence that they have internal monologues or have a theory of mind or anything like that. --Jayron32 20:27, 23 May 2012 (UTC)
- I agree that dogs can't, but there are more intelligent animals around. For other evidence of their intelligence, there's the mirror test, where the most intelligent animals realize they are seeing themselves and are fascinated by their reflections. StuRat (talk) 20:10, 23 May 2012 (UTC)
- Such tests should be evaluated with caution. In other words, they can rule in or out certain recognition tasks, but not all related tasks. --Modocc (talk) 20:26, 23 May 2012 (UTC)
- I guess we can only wait for #3, I feel bad for reposting this question, but even though the original discussion had a few high-quality answers, it has gone way off track. . Anyway bonobos seem to have few taboos. Joepnl (talk) 21:19, 23 May 2012 (UTC)
Diversity of sexuality (sexual behaviour) #3
Please list ways in which animals have sex which appear weird to humans (take as your baseline for "human" an Australian wikipedian in his 30s who wants the IP to get their weird animal sexual behaviour list; or if other definitions of "human" make for interesting weirdness, feel free to go with those). Citations to papers preferred! Also pictures of slugs having sex from trees. Fifelfoo (talk) 23:44, 23 May 2012 (UTC)
- Well the article linked above, Animal sexual behaviour, and I bet you can find the slug pictures for yourself if you look at the Commons, they are even in a tree and on a wall. But all in all these are one of the weirdest around. CambridgeBayWeather (talk) 07:42, 24 May 2012 (UTC)
- Dogs hump legs. HiLo48 (talk) 08:07, 24 May 2012 (UTC)
- You're going to have to confine yourself to mammals to keep the list manageable. If you're going by weird arthropod mating habits, you could write an entire book on it. In fact, that book has been written more than once. Google "sex on six legs" for I think the most recent one. Someguy1221 (talk) 09:20, 24 May 2012 (UTC)
- One of my personal favourites - in seahorses the male becomes pregnant and carries the developing eggs through gestation until birth. Gandalf61 (talk) 09:48, 24 May 2012 (UTC)
Could plants extract metabolic energy from wind?
In present day societies it seems like wind plants are being built in preference to solar plants as a source of energy. Yet thinking about plants... we think of solar power, never wind power. Of course there is wind dispersal for seed dispersal and anemophily; I'm thinking more though of plants extracting metabolic (chemical) energy from the wind.
It occurs to me that if I were to design plants to capture wind power, I would construct many elements that would flap constantly in the breeze, i.e. leaves, connected to bundles of closed fluid tubes such as xylem or maybe phloem. As the bundles would periodically be contracted and expanded by the wind, pressure differentials should exist at cell membranes. If fluid were permitted to leak through freely under pressure in one direction, but forced through a semipermeable membrane when passing in the other, a solute could build up on one side of the membrane, creating a chemical potential from which energy could be extracted.
I suppose a simpler but still useful mechanism would be simply if the flapping leaf helped pump fluid to the top of the plant, a purely mechanical process that nonetheless would spare some sort of osmotic or evaporative pump at the root or leaf (depending on the vessel type).
Is there any known method for rooted plants to harvest wind energy? Wnt (talk) 12:40, 23 May 2012 (UTC)
- Wind causes damage to the plant tissue similar to the way we damage our muscles when we workout. This damage in turn causes the plant to repair the area and it becomes stronger, thus enabling it to withstand a greater load in the future. So it does gain potential energy.165.212.189.187 (talk) 16:30, 23 May 2012 (UTC)
- But that energy to repair the leaves the plant derives not from wind, but from photosynthesis (and is thus solar power). - Lindert (talk) 16:36, 23 May 2012 (UTC)
- But the catalyst is the wind, otherwise the plant would make itself stronger initially, which it doesn't.165.212.189.187 (talk) 20:23, 23 May 2012 (UTC)
- Lindert is right. that's not what potential energy means 165.212.189.187. LukeSurl t c 21:07, 23 May 2012 (UTC)
- then what is the plants ability to resist stronger winds due to it's stronger stem, Or support a greater load?68.83.98.40 (talk) 04:40, 24 May 2012 (UTC)
- The point is that a catalyst does not supply any of the required energy. It's not the wind that makes the stem stronger, but the plants metabolism reacting to the wind, just like floods do not build dykes, but people build them in response to floods. Also, as LukeSurl pointed out, the ability to resist something is not potential energy. For example, a diamond has a high resistance to breaking, because of its strong chemical bonds. However, it has very little potential energy, i.e. very little energy could be extracted from the diamond, because it is in a very low energy state. - Lindert (talk) 09:36, 24 May 2012 (UTC)
- then what is the plants ability to resist stronger winds due to it's stronger stem, Or support a greater load?68.83.98.40 (talk) 04:40, 24 May 2012 (UTC)
- Lindert is right. that's not what potential energy means 165.212.189.187. LukeSurl t c 21:07, 23 May 2012 (UTC)
- Wind causes damage to the plant tissue similar to the way we damage our muscles when we workout. This damage in turn causes the plant to repair the area and it becomes stronger, thus enabling it to withstand a greater load in the future. So it does gain potential energy.165.212.189.187 (talk) 16:30, 23 May 2012 (UTC)
Plants metabolise gasses from the air with water, light, and minerals from the soil. Rotating movement is only possible in free moving organisms like a tumbleweed or a manis. The closest thing to a true wheel in organisms is the flagellum, and that could not power a toy battery. You couldn't power an LED light off it. ~ R.T.G 20:57, 23 May 2012 (UTC)
- Aye. While I suppose a plant generating energy from wind is theoretically possible, it seems unlikely that the efficiencies involved would make the evolving of the ability to harness it viable. LukeSurl t c 21:07, 23 May 2012 (UTC)
Science Project
I am doing a science project and I already browsed all around the web for ideas. Here's the criteria:
- It has to be something applicable in the modern household
- You need to have a investigative queation eg. What will happen...?
- We would prefer a exciting experiment such as a chemical reaction.
The main problem I have is to get something exciting and extraordinary which is also applicable in the household. Can someone please help me? I would appreciate if you could tell me of a project you did/see in the past or perhaps give me a internet link.41.125.84.204 (talk) 15:27, 23 May 2012 (UTC)
- A few thoughts: Firstly, I'm sure you've read the top of the page, but we won't do your homework for you. A large part of the objective of this exercise is likely to be getting you to be creative and understand the process behind how scientists go about setting themselves research projects, and so on. Therefore, just asking us to provide you with a topic for your project is defeating the object. A better idea might be if you could tell us some of the ideas you've had so far - don't worry how bad you think they are, or how unachievable they might be, we can work on that - and we can then help you to find ways in which to put them into practice. Secondly, if you could tell us what level this project would be at (e.g.
K-12Twelfth grade, GCSE, degree level) then we will have a better feeling for what sort of ideas we can pitch you. Hope this helps. - Cucumber Mike (talk) 15:42, 23 May 2012 (UTC)
- How about an experiment on electricity usages of various household devices. Most people have a visible electrical meter outside of your house. DO things like measure the amount of electrical energy your house uses in the aveage day, and then see how that changes when you, say, don't run the air conditioning all day, or unplug every electric device (to see the effect of Vampire power) and things like that. --Jayron32 16:50, 23 May 2012 (UTC)
- How about a comparison study between a number of different brands of a particular type of household product? Setting up a fair test to see which does the job best would demonstrate a lot about the methods of scientific investigation. LukeSurl t c 15:54, 23 May 2012 (UTC)
- Somebody just asked the question here: "Which is most efficient, using a stove or microwave to boil water for coffee ?" [14]. You could do the experiment yourself, reading the home electricity meter to determine the difference between having each appliance on and off, and then multiply that electricity usage rate by the time it takes to heat the water to boiling from a fixed starting temp. Do the experiment several times, and average out the results. You can then multiply by your electricity cost to determine the cost difference. StuRat (talk) 17:14, 23 May 2012 (UTC)
- You could compare a cleaning fluid you buy in the shop (we have Flash in the UK) with a more natural product such as lemon juice, vinegar, bicarbonate of soda, or a mixture of them, to see which is more effective at removing dirt. --TammyMoet (talk) 18:18, 23 May 2012 (UTC)
Where's the rule about how high model rockets can go in the US
I know you all are hyper-vigilant about saying yourselves what laws do and do not say. I just want to be pointed to the people who do say what the altitude limit is for model rockets in the US before you have to go get some paperwork from the FAA or whatever other agencies would be offended by a high-flying tube. I can't find that nugget of knowledge on their site. 20.137.18.53 (talk) 16:46, 23 May 2012 (UTC)
- I can give you an upper limit in that in the US everything about flight level 180 is controlled airspace. You might also find it interesting to read Airspace class (United States). I do not know what the regs about model rockets in particular are though. Egg Centric 16:51, 23 May 2012 (UTC)
- I found this page on the National Association of Rocketry website. Roger (talk) 16:58, 23 May 2012 (UTC)
- In short, for small rockets (I see they've raised the weight limit from 16 ounces to 53 ounces), there's no height limit; the only limitation is "how high can you send it on 125 grams of propellant?". --Carnildo (talk) 01:44, 24 May 2012 (UTC)
Trebuchet design.

I know that Trebuchets from medieval times had a simple pin hinge - and a beam with a gigantic weight on one end and a sling for the missile on the other. I also know that modern trebuchet enthusiasts (eg at Pumpkin chunking events) have improved the design by replacing the pin hinge with an axle with wheels on the end that run along a horizontal track (a "floating arm trebuchet") - which is better because the fulcrum can now move such that the weight can fall in a straight, vertical line without absorbing energy by accelerating horizontally as it does in the classical design where it falls through a roughly 90 degree arc. Also, this large and dangerous weight doesn't end up swinging violently back and forth.
However, it seems to me that a simpler design would be to have two pin hinges and an additional linkage to do the same basic thing (the red component in the image at right). Is there any obvious (or non-obvious) reason why the floating arm version is better than mine? Is there any precedent for trebuchets built this way? I don't see any examples in our Trebuchet article that work like this. SteveBaker (talk) 16:53, 23 May 2012 (UTC)
- There is another option. I saw a documentary where they tried to recreate a medieval trebuchet, and they concluded that the entire device was likely built on wheels, both to be able to move it into position, but also to allow the weight to fall more vertically (as the device moved under it) and to absorb the "recoil", which otherwise might destroy the device. StuRat (talk) 17:05, 23 May 2012 (UTC)
- (ec)Sorry if this is off-topic, but I'm slightly confused after looking at our 'Trebuchet' article. I thought the simple, efficient, doesn't-shake-to-pieces standard design for a large trebuchet was to mount the whole thing on wheels, such that it moves backwards and forwards as the weight swings. I don't see any trebuchets like that in our article, but I thought recreations that omitted the wheels tended to shake to pieces when used with realistic loads. 109.155.32.126 (talk) 17:08, 23 May 2012 (UTC)
- This one: File:Punkin-Chunkin-2008-Trebuchet.JPG has wheels - presumably for that reason. SteveBaker (talk) 19:35, 23 May 2012 (UTC)
- The reason it will not work as well is because the design does not in fact allow the weight to fall in a straight line. In the situation where the weight has begun to fall, you can imagine that the red component is at an angle compared to a vertical line. The force the red linkage exerts on the fixed chassis of the trebuchet must necessarily be parallel to this linkage, which means there is a horizontal component to that force. Newtons third law dictates that, because the moving components exert a horizontal force on the fixed trebuchet, an equal and opposite force is exerted by the fixed trebuchet on the moving components. Therefore, the weight will accelerate to the right (i.e. in the first part of the falling motion), though not as much as it would in the simpler design. I hope this makes sense. - Lindert (talk) 18:27, 23 May 2012 (UTC)
- Yes, I understand that - the issue is essentially the same for the floating arm trebuchets. The actual implementation of both this version and the floating arm trebuchets have some kind of a fixed vertical guide rail to force the weight to fall vertically. I didn't want to clutter the diagram too much so I left that out - I guess it's kinda important! SteveBaker (talk) 19:35, 23 May 2012 (UTC)
- What would be the problem with a more conventional crank arrangement? Position the weight directly below the main pivot, and connect it to the short end of the arm using a rod with a hinge in the middle. Tevildo (talk) 20:14, 23 May 2012 (UTC)
- Yes, I understand that - the issue is essentially the same for the floating arm trebuchets. The actual implementation of both this version and the floating arm trebuchets have some kind of a fixed vertical guide rail to force the weight to fall vertically. I didn't want to clutter the diagram too much so I left that out - I guess it's kinda important! SteveBaker (talk) 19:35, 23 May 2012 (UTC)
- Ok, thanks for clarifying. In that case I see no reason why your version could not work just as well as the floating arm design. To be sure, you might try to do a physics simulation. There may be implementational difficulties, but I wouldn't know about that. As for a precedent, not that I am aware of, but considering how many people have experimented with altered designs, I'd say there's a pretty high chance someone has tried it. - Lindert (talk) 20:29, 23 May 2012 (UTC)
Odd smell after a hailstorm
OK. Question here for the meteorologists/atmospheric scientists/amateur bullshitters out there. After a recent hailstorm, after most of the hail had melted, there was a very distinct smell in the air. It smelled like pine, maybe terpentine or pinetar. That kind of smell. It wasn't local to my house; I ran an errand a mile from my house, and the distinctive smell was there too. What about the hailstorm could have caused this? --Jayron32 21:02, 23 May 2012 (UTC)
- Are you sure it wasn't ozone ? This is common during thunderstorms. StuRat (talk) 21:09, 23 May 2012 (UTC)
- No, I recognize ozone, this was definately not that. I have smelled ozone after thunderstorms and in other contexts. This was definately piney, like terpentine. --Jayron32 21:16, 23 May 2012 (UTC)
- Question: Before the hail was the weather dry. If so you may be referring to Petrichor.--Aspro (talk) 21:17, 23 May 2012 (UTC)
- Another possibility is that there was an updraft (frequently associated with hail), which sucked up some kind of oil off the ground. For example, some roads are treated by spraying oil on them. StuRat (talk) 21:30, 23 May 2012 (UTC)
- Stu may be onto something. It wasn't petrichor: I've smelled rain before, believe it or not. This wasn't it. But I like Stu's idea: Perhaps something like a distant forest fire introduced an aerosol of piney smelling stuff high into the clouds, which provided nucleation for the hailstones, which then returned to earth and released that smell. Anyone out there know if this has been demosntrated to have happened before. --Jayron32 21:35, 23 May 2012 (UTC)
- Here's the science part ... so consecrate: Petrichor contains Terpenoids thus can smell like terpentine re: [15].The exact small depends on local soil conditions. And as you stated 'it wasn't rain' but hail so the soil may never have got wet enough to release the 'rain smell' . We get this phenomena in Europe sometimes. --Aspro (talk) 21:41, 23 May 2012 (UTC)
- Hailstones form by continuous freezing, but can form so fast that air bubbles are trapped within the structure (that's when you get "cloudy" ice); as it melts, it's not inconceivable that a large amount of organic aerosols were caught up in the updraft, frozen in the hail, then released by the melting. However, I find Aspro's explanation to be much more likely.-RunningOnBrains(talk) 22:57, 23 May 2012 (UTC)
- Actually, I am more inclined to think that it was in the hailstones themselves. A friend of a friend (someone who I don't know) told her, independent of my talking about it, about the strange piney smell following the same hailstorm, some 20 miles from where I live. It seems very likely that there was something in the ice. The soil never smells like terpentine when it rains at other times, and it rains often enough you'd think I'd notice. --Jayron32 01:00, 24 May 2012 (UTC)
- Hailstones form by continuous freezing, but can form so fast that air bubbles are trapped within the structure (that's when you get "cloudy" ice); as it melts, it's not inconceivable that a large amount of organic aerosols were caught up in the updraft, frozen in the hail, then released by the melting. However, I find Aspro's explanation to be much more likely.-RunningOnBrains(talk) 22:57, 23 May 2012 (UTC)
- My guess is that the hail banged up a lot of tender, new growth on evergreen trees, and then the variable winds of the thunderstorm (either the up- or downdraft or straight-lines) mixed the pine scent throughout the air. If I had to guess, I'd say the hail was pretty small, not enough to bring down limbs, but fairly uniform in coverage. Much less inspiringly, it could have simply been the storm blowing the smell of a logging operation or something downwind, and the hail had nothing to do with it. Juliancolton (talk) 01:41, 24 May 2012 (UTC)
- FTR, this has been documented before, which confirms my belief that the hail simply dinged up a lot of pine trees, either by stripping bark or crushing new needles.
6/9/2011 1.00 6 W SALISBURY ROWAN NC A FEW WAVES OF BRIEF HAIL FOR ABOUT 5 MINUTES...BUT LARGEST AROUND QUARTER SIZED. ENDED AROUND 525 PM.
STRONG SMELL OF PINE IN THE AIR AFTER STORM. (GSP)" Juliancolton (talk) 01:44, 24 May 2012 (UTC)
- So I guess the remaining question is, Jayron, do you live in the vicinity of trees? Because to me this seems like the most likely explanation (and I am kicking myself for not thinking of it; I'm just so used to hailstorms in the Great Plains). -RunningOnBrains(talk) 03:21, 24 May 2012 (UTC)
- That could be it. Interestingly, I live in North Carolina as the above report is from as well. The apex tree is the Carolina Pine, which is the tallest tree around, so it makes a lot of sense that it would get the brunt of the damage from the hailstorm. As you note, the stones were small (pebbles sized, probably slightly larger than a Cheerio) and uniform in their distribution. I think we have our answer. Thanks to everyone for some excellent answers, I think I'm going to go with "slight damage to lots of pine trees" as the answer, especially given that an earlier nearby storm was reported to do exactly that last year. --Jayron32 19:26, 24 May 2012 (UTC)
- So I guess the remaining question is, Jayron, do you live in the vicinity of trees? Because to me this seems like the most likely explanation (and I am kicking myself for not thinking of it; I'm just so used to hailstorms in the Great Plains). -RunningOnBrains(talk) 03:21, 24 May 2012 (UTC)
SELF CHARGING ELECTRICAL CAR
And I have a bunch of lakefront property in north Ontario for sale. CambridgeBayWeather (talk) 07:19, 24 May 2012 (UTC)
|
|---|
|
I invent the SELF CHARGING ELECTRICAL CAR and I have the same the Wright broter had no one belived it could be done,but is alrady finish. We would be ENERGY INDEPENDENT yes or no? (Please see You tube papas invention,pulling the plug- Home town News) Thank you and god bles America....great cuontry — Preceding unsigned comment added by 74.243.15.232 (talk) 21:32, 23 May 2012 (UTC)
Please, there's not even a question in here. The OP seems to be either a troll or trying to get hits for his youtube video, and you are all obliging. Vespine (talk) 04:25, 24 May 2012 (UTC) |
Oestrogen injections for sociopaths
I understand that it's believed that sociopaths lack a part of the brain to do with empathy. Personally I suspect it's unlikely that it's a binary thing, and at least with the majority of sociopaths there'll be a bit of that part lying around. Is it plausible that oestrogen injections could make that part more effective, and make the sociopath more empathetic? Egg Centric 22:41, 23 May 2012 (UTC)
- The premise is wrong: there is no serious theory saying that sociopaths lack any brain part. Some people believe that sociopathy is often caused by insensitivity to punishment, which involves a variety of brain systems. I don't see any clear reason why estrogen injections would make a difference, but really the whole topic is a bit of a boggle. Looie496 (talk) 22:51, 23 May 2012 (UTC)
- You'd be more likely to succeed with oxytocin, but pull that stunt and I might side with the sociopaths. Wnt (talk) 02:35, 24 May 2012 (UTC)
- Maybe that was chief ingredient of Love Potion Number 9. ←Baseball Bugs What's up, Doc? carrots→ 11:32, 24 May 2012 (UTC)
- You'd be more likely to succeed with oxytocin, but pull that stunt and I might side with the sociopaths. Wnt (talk) 02:35, 24 May 2012 (UTC)
Is the psi function real?
Have there been any followups to this: http://www.nature.com/news/quantum-theorem-shakes-foundations-1.9392 Bubba73 You talkin' to me? 23:58, 23 May 2012 (UTC)
- Did you see the "Related Links" section, and the comments section? Those are the followups. I can't find citations for this paper; and to be perfectly frank, it looks like a mathematical formalization of grammatical ambiguity. It's not surprising that it's on ArXiv instead of a peer-reviewed physics journal. (I see that it did make it into Nature Physics, just a few days ago). Nimur (talk) 00:59, 24 May 2012 (UTC)
- I did not read those because they could say anything. Bubba73 You talkin' to me? 01:57, 24 May 2012 (UTC)
- I don't follow it completely, but the result appears to be quite similar to Bell's theorem, which is very well known. Looie496 (talk) 02:01, 24 May 2012 (UTC)
- I agree with Nimur that this seems pretty weak, and not likely to convince anyone who wasn't already predisposed to believe in the reality of the wave function. You said that the replies "could say anything"; I'm not sure what you mean by that, but it's more or less true of the paper itself. The barrier to getting published isn't all that high. -- BenRG (talk) 02:21, 24 May 2012 (UTC)
- The barrier to getting a positive (or at least interested) mention in Nature, though, one would expect to be higher. --Trovatore (talk) 02:23, 24 May 2012 (UTC)
- I agree with Nimur that this seems pretty weak, and not likely to convince anyone who wasn't already predisposed to believe in the reality of the wave function. You said that the replies "could say anything"; I'm not sure what you mean by that, but it's more or less true of the paper itself. The barrier to getting published isn't all that high. -- BenRG (talk) 02:21, 24 May 2012 (UTC)
- When I said "people could say anything" it is because the comments seemed like a blog. Papers are subject to peer review, which is a much higher standard than simple blog comments. Bubba73 You talkin' to me? 02:30, 24 May 2012 (UTC)
- Note that ArXiv is not peer-reviewed. Anyone can author a paper there; although it is moderated, that is not necessarily an endorsement of the quality of the material. As far as I can tell this paper has not (yet) been published in a peer-reviewed journal.-RunningOnBrains(talk) 03:17, 24 May 2012 (UTC)
- Bubba, did you read the paper, though? What you linked to is a puff piece about the paper, paid for by the publisher and posted on the publisher's blog. It's not peer reviewed and I doubt the author is bound to journalistic standards of fact checking and unbiased reporting.
- Trovatore, the journal is Nature Physics, not Nature. -- BenRG (talk) 04:26, 24 May 2012 (UTC)
- The original link is to Nature. That's the comment I was referring to. I haven't looked at any of the "follow-up" links. --Trovatore (talk) 04:32, 24 May 2012 (UTC)
- Trovatore, the journal is Nature Physics, not Nature. -- BenRG (talk) 04:26, 24 May 2012 (UTC)
- No, I did not read the paper but I did read the abstract. Bubba73 You talkin' to me? 05:01, 24 May 2012 (UTC)
- Bubba, why don't you read the paper and decide for yourself? To the others: in fairness, this paper did get published in a peer-reviewed journal, Nature Physics, which is fairly prestigious. Though it is connected to the "more prestigious" journal Nature, its author information page states that it is "editorially independent, and its editors make their own decisions, independent of the other Nature journals." I have regularly read Nature and its various subject area journals, and in my mind, there's a clear difference in the significance between the journals. Nature publishes "the world's best science," and though the claim is made that "Nature Physics publishes papers of the highest quality and significance in all areas of physics," it is my opinion that the general importance of such papers is magnitudes lower than, say, the current issue of Nature's feature on Gran Sasso - a feature that is arguably not "academic" at all. So: take it or leave it: Bubba, you linked to a paper that explores subtleties of physics. It has at least passed the bar of quality set by the Ph.D-level physicists at Nature Physics, but at least in the opinion of myself and a few other reference desk regulars, is not very enlightening or novel. This is critical: I don't accuse the paper of factual inaccuracy or error; just that it's not very interesting. If you read it, and understand it, you may make up your own mind. If you read it and do not understand it, consider whether it is worth spending several years of intellectual effort to bring yourself up to speed on the physics background. If so, the paper is interesting to you and you will be able to decide for yourself whether it is "correct" or "accurate." If you don't feel that the paper is important enough to spend time to understand, then you're in agreement with a lot of people. That's how modern science works: merit is secondary to relevance. And if I may try to reclaim a shred of modesty here: who am I to decide what's important or relevant in physics? I'm not professionally employed to work in quantum physics research; this paper is out of my domain area, and I've never been published in that journal... so my opinion is moot. Nonetheless, I have formed an opinion based on wide reading, as a physicist-turned-some-type-of-engineer, I still follow the scientific literature in a variety of topics that interest me.Nimur (talk) 05:42, 24 May 2012 (UTC)
- No, I did not read the paper but I did read the abstract. Bubba73 You talkin' to me? 05:01, 24 May 2012 (UTC)
- I didn't read it because I didn't feel qualified to evaluate it. Which is why I asked here. Bubba73 You talkin' to me? 05:48, 24 May 2012 (UTC)
- I'm not qualified to evaluate the paper, either, but I will say this: theoretical interpretations, especially ones that are trying to address big "philosophical" interpretations of the ontology (that is, the reality) of quantum mechanical interpretations, are hard to evaluate. It will take some time before one can say whether this has been accepted or rejected, and whether they are accepted or rejected for "good" reasons or not may or may not be apparent. There is little urgency to it, in any case. So I doubt anyone on here is really in a position to say whether this is right or wrong or not at this point, or whether it will or won't catch on. Plumbing the depths of quantum mechanical interpretation is not, at the moment, high on too many physicists' lists for research projects. If the different ontological interpretations of the wave function do not lead to interesting physical results, then the "who cares?" response will probably dominate except in popularizations. This is essentially what Nimur is getting at when he says it is not "interesting," I believe. I am sure he would agree that on a deep level the ontological nature of the wave function is an interesting question, but this paper doesn't change the physics too much one way or another. --Mr.98 (talk) 13:10, 24 May 2012 (UTC)
I think the paper is interesting, it's a sort of no-go theorem against the idea that the wavefunction is not real. One can take issue with some details in the paper, but then it should be the people say that the wavefunction is not real, who should come up with a theory in which this is so. So, it is actually very similar to Bell's theorem as pointed out by Looie above. There are loopholes there too, but then if you are going to say that there may be a local deterministic theory that explains quantum mechanics, the ball is in your court to demonstrate this. Count Iblis (talk) 15:40, 24 May 2012 (UTC)
And about the paper's publication issues etc., one could argue that the result is trivial and could have been shown in a much simpler way, and therefore does not merit publication. I would have to carefully re-read the paper (I read it quickly some time ago), but it isn't clear to me why an argument along the following lines could not be set up. If the wavefunction is not real then it seems to me that this would imply that in at least some cases, a pure state could be replaced by a suitably chosen mixed state without that difference being detectable. But we know that this is false, you can always (in principle) tell the difference. Count Iblis (talk) 16:17, 24 May 2012 (UTC)
- (ec) I can summarize the formal part of the paper, at least. They consider the possibility that quantum states (wave functions) represent classical probabilistic distributions over some underlying kind of state, which determines the experiment outcome. Unlike the usual hidden variable setup, they are interested in the case where different quantum states sometimes (with some nonzero probability) map to the same underlying state. They rule out this case by a simple exercise in undergraduate QM.
- So much for the math. Obviously no model of the sort they rule out has ever existed (it wouldn't have worked), but moreover I can't remember anyone ever suggesting, in so many words, that they thought that quantum states could have this interpretation. The authors try to read it into passages by Jaynes and Einstein, but it's suggestive that they couldn't find anything better. I think the reaction of almost anyone to the idea of such a probabilistic interpretation would be "no, that obviously won't work", even before this paper. The authors argue that when people say that the state vector mixes objective and subjective properties of the system that they must mean this. But the state vector does mix objective and subjective properties. For starters there's the relative state (many-worlds) versus orthodox version of the state vector—different quantum states, same experiment, same predicted outcomes. Their theorem doesn't address this because theoretically there's an experiment that can distinguish these cases. It's an experiment that's impossible to actually do because it violates both locality and the second law of thermodynamics, but it technically exists as an experiment in the standard formulation of quantum mechanics. Another example is that you can pick a different inertial reference frame and get completely different time-dependent state vectors, but the same experimental predictions. There's the Hilbert picture where the wave function doesn't evolve with time. There's the fact that it's not clear how to define a wave function for the whole universe, or what that would mean. None of these problems is solved by the paper. They say they've proved the reality of something, but they can't say what.
- It all feels to me like a "Gödel's theorem, therefore God" kind of argument. It will get rave reviews from a certain contingent of people who already believed in God, and the real meaning of those reviews is "finally, something that's sure to convince the nonbelievers". And each of them has their own private conception of God which they will think is vindicated by the argument. And it probably will convince some of the more impressionable nonbelievers. And we'll never be rid of it. We're still stuck with Wigner's friend, and that was a worse paper than this one. -- BenRG (talk) 16:30, 24 May 2012 (UTC)
May 24
Calories in coffee
Does a cup of coffee have any calories in it? Or is it really simply water (i.e., no calories)? Assume that the cup of coffee that I drink in the morning has no sugar or milk (or anything else) added to it. Am I drinking a calorie-free drink (like water) ... or am I actually consuming some calories? If the latter, how many calories are there in a cup of coffee? Thank you! Joseph A. Spadaro (talk) 00:59, 24 May 2012 (UTC)
- Type "calories in black coffee" into google and you'll get your answer about 100 different ways. They all roughly agree. I won't insult you by linking any of the dozens of of answers you get. --Jayron32 01:03, 24 May 2012 (UTC)
- Yeah, but the first five pages I got of that included nothing close to a Wikipedia-grade reliable source (a reasonably low bar already). But searching coffee calories did get me to [16] from the Mayo Clinic, which says "A plain cup of brewed coffee has only two calories (and no fat)" while listing off the calories of various additives. Wnt (talk) 02:32, 24 May 2012 (UTC)
- From a reliable secondary source, instant coffee powder has 424 kJ per 100 grams, 101 kcal/100g 13.6% protein, 10% carbohydrate, 0.6% fat. ISBN 9780644138710. Graeme Bartlett (talk) 11:54, 24 May 2012 (UTC)
- Yeah, but the question is about coffee (not that other abomination to which you refer). -- ♬ Jack of Oz ♬ [your turn] 20:38, 24 May 2012 (UTC)
- Mcdonalds says 0. Shadowjams (talk) 21:01, 24 May 2012 (UTC)
- I guess they round down because they can. There are calories in brewed/espresso coffee but compared to total daily energy intake it should be negligible and dwarfed by accounting errors in the rest of the energy intake total. Diet coke territory. SkyMachine (++) 22:53, 24 May 2012 (UTC)
- Mcdonalds says 0. Shadowjams (talk) 21:01, 24 May 2012 (UTC)
- Yeah, but the question is about coffee (not that other abomination to which you refer). -- ♬ Jack of Oz ♬ [your turn] 20:38, 24 May 2012 (UTC)
- From a reliable secondary source, instant coffee powder has 424 kJ per 100 grams, 101 kcal/100g 13.6% protein, 10% carbohydrate, 0.6% fat. ISBN 9780644138710. Graeme Bartlett (talk) 11:54, 24 May 2012 (UTC)
- Yeah, but the first five pages I got of that included nothing close to a Wikipedia-grade reliable source (a reasonably low bar already). But searching coffee calories did get me to [16] from the Mayo Clinic, which says "A plain cup of brewed coffee has only two calories (and no fat)" while listing off the calories of various additives. Wnt (talk) 02:32, 24 May 2012 (UTC)
Super Heros and capes
I know that super heros are just entertainment and thus not really "bound" to our laws of physics. But, would a super hero with a cape (Superman, Thor, Batman, etc.) not have a lot more difficulty flying with that thing strapped to their neck (not to mention being strangled!). Any idea as to just how much drag such an item would create? 99.250.103.117 (talk) 07:39, 24 May 2012 (UTC)99.250.103.117 (talk) 07:44, 24 May 2012 (UTC)
- This is the kind of question that flagpole manufacturers would know how to answer, as the strength of the pole will determine the maximum size of flag it can support at a particular wind speed. Roger (talk) 07:53, 24 May 2012 (UTC)
- Anyone capable of flying on their own would, I expect, have a sufficiently strong neck to handle the strain. Last time I looked, Batman doesn't fly under his own power, just swoop around a bit, though I would venture to guess his costume would be sufficiently rigid to prevent neck injury from the darned cape getting snagged on something. Clarityfiend (talk) 09:56, 24 May 2012 (UTC)
- I think this topic was properly covered in the movie "The Incredibles"! Zzubnik (talk) 12:45, 24 May 2012 (UTC)
- Thinking about it, when humans are doing the closest thing to flying (i.e. falling out of a plane) we use sheets of fabric to create drag (i.e. a parachute or a wingsuit). Some incarnations of Batman use the cape a bit like a glider - he's not really flying, but "falling with style". Other supers have no excuse however. LukeSurl t c 16:40, 24 May 2012 (UTC)
- ...which was, in turn, inspired by a similar discussion in Watchmen. 109.155.32.126 (talk) 20:10, 24 May 2012 (UTC)
Geology of DRC
What is the origin of the giant striations, running in an East-West direction, across Nord-Kivu province? Located at approximately -0.85 decimal degrees latitude. Plasmic Physics (talk) 10:32, 24 May 2012 (UTC)
- They're a set of ridges, presumably related to different dipping rock layers, like cuestas, with variable resistance to erosion. I'll try to find out some more. Mikenorton (talk) 19:29, 24 May 2012 (UTC)
- The ridges are located at the northern edge of the mesoproterozoic metamorphic belt known as the Kilbaran Belt, but I still don't know what the specific rock types are. Mikenorton (talk) 20:05, 24 May 2012 (UTC)
- Which way do they dip? Plasmic Physics (talk) 23:13, 24 May 2012 (UTC)
Taxonomical names
This might be better at the Languages desk, but I figured the biologists here would know... how do you pronounce the Latin name for a species if it's based on a name in a non-latin language? For example: Hernandez-Camacho's night monkey is named after Jorge Hernandez-Camacho as Aotus jorgehernandezi. Would I say "Aotus Jaw-Gay-Her-Nan-Dez-Ee" or "Aotus Yor-Gay-Her-Nan-Day-Ee"?
This is the sort of thing that keeps me awake at night. Yunshui 雲水 10:42, 24 May 2012 (UTC)
- Note that neither of those pronunciations equates to the Spanish pronunciation. ←Baseball Bugs What's up, Doc? carrots→ 11:30, 24 May 2012 (UTC)
- I could also use some advice on the pronounciation of names in Spanish... Yunshui 雲水 11:57, 24 May 2012 (UTC)
- "Jorge" is pronounced "Hor-Hay" in Spanish. --Mr.98 (talk) 12:57, 24 May 2012 (UTC)
- Thanks. So: "Jaw-Gay-Her-Nan-Dez-Ee" or "Hor-Hay-Her-Nan-Day-Ee"? Yunshui 雲水 13:32, 24 May 2012 (UTC)
- "Jorge" is pronounced "Hor-Hay" in Spanish. --Mr.98 (talk) 12:57, 24 May 2012 (UTC)
- I could also use some advice on the pronounciation of names in Spanish... Yunshui 雲水 11:57, 24 May 2012 (UTC)
- I'm told that in Mexico at least, Hernandez, (or more correctly Hernández), is pronounced 'air-NAN-dez'. (As a Spanish name, it would not have "I" on the end.) Wanderer57 (talk) 16:35, 24 May 2012 (UTC)
- Yes, the "H" is silent, or effectively so. The trailing "i" is of course a Latinization, not a Spanish construction. ←Baseball Bugs What's up, Doc? carrots→ 23:28, 24 May 2012 (UTC)
- I'm told that in Mexico at least, Hernandez, (or more correctly Hernández), is pronounced 'air-NAN-dez'. (As a Spanish name, it would not have "I" on the end.) Wanderer57 (talk) 16:35, 24 May 2012 (UTC)
- IPA /ˈxorxe erˈnandes/; I guess the similar vowels would elide, so /ˌxorxerˈnandes/. — The rules of Spanish spelling are pretty easy, or at least seem that way to me because I learned them at age ~7. It always amazed me to meet educated people who had lived in California for twenty years without learning to pronounce Spanish names. —Tamfang (talk) 18:14, 24 May 2012 (UTC)
What class is a fox?
Apparently this was on an exam paper today in the UK: "A fox is a vertebrate. What class is it?" It stumped everyone, teachers included. Anyone? --TammyMoet (talk) 15:14, 24 May 2012 (UTC)
- Mammalia of course! That such a simple, easy question can stump even teachers is a pretty serious indictment of the standard of education in the UK. Roger (talk) 15:27, 24 May 2012 (UTC)
- Well the consensus was Mammals, but a quick Google got the answer "Chordata". What's the difference? --TammyMoet (talk) 15:36, 24 May 2012 (UTC)
- Chordata is the Phylum. It is a far bigger and more diverse grouping than Mammalia, which is the Class. For example, Chordata includes fish. Wanderer57 (talk) 15:53, 24 May 2012 (UTC)
- BTW In what grade (age) was this question asked? Roger (talk) 16:03, 24 May 2012 (UTC)
Thanks to LukeSur for the comprehensive answer. I believe it was a GCSE question but will check. --TammyMoet (talk) 17:35, 24 May 2012 (UTC)
- If it really is a GCSE question and even the teachers!!! were stumped it's rather conclusive proof that British education standards have "gone to the canidae"! Sad if one considers that this is the nation that just a few generations ago ruled almost half the world! Roger (talk) 18:20, 24 May 2012 (UTC)
- Maybe part of what is going on here is that Linnaeus's levels are increasingly seen as not very meaningful. Every level between phylum and genus is basically arbitrary, and modern biologists tend not to find them useful. Looie496 (talk) 19:11, 24 May 2012 (UTC)
- Indeed, modern taxonomists seem to prefer the clade system, which highlights actual decent from actual ancestors, rather than sometimes arbitrary and superficial physical characteristics present in the old Linnaean system. --Jayron32 19:17, 24 May 2012 (UTC)
- (ec) Clearly their problem was that they spent their time memorizing answers to Trivial Pursuit questions, and thereby lost their empire to smug upstarts who are unable to restrain their gratuitous use of exclamation points.
- Speaking as someone who makes a living in the field of biochemistry and who works with people who spend their days making phylogenetic trees, my response to this anecdote would have to be...'so what?' Faced with the same question, I'm not sure that I would have been able to snap off the correct answer either. What I could do is demonstrate a grasp of the more important underlying concepts— foxes are carnivorous canine mammals; foxes are vertebrates; and foxes are animals; all mammals are vertebrates (with all that implies), but not all vertebrates are mammals. Knowing which sets are proper subsets and supersets of one another is, practically speaking, more useful and meaningful than memorizing the somewhat-arbitrarily-defined Linnaean taxonomic levels and labels. (Any modern evolutionary biologist or geneticist is going to ask, why is Aves (birds) its own class, even though birds are descended evolutionarily from Reptilia—also its own class? Is it constructive to enshrine and glorify historical accidents like these as part of a strictly-taught hierarchical model?) TenOfAllTrades(talk) 19:32, 24 May 2012 (UTC)
- Indeed, Saurischia and Ornithischia were orders. Now we list Saurischia as a "clade". ;) Still, such historical terms are not entirely useless, as they give some sense of the overall degree of 'difference' people perceived among various taxonomic units. Such imprecise and arbitrary notions are nonetheless difficult to replace. Wnt (talk) 20:28, 24 May 2012 (UTC)
- The very fact that an exam question asked about the class of some creature suggests that knowledge of the Linnaean system is still required and is still taught. But is it really true that not a single teacher in the entire country knew the answer, Tammy? Who's the "everyone" you refer to? -- ♬ Jack of Oz ♬ [your turn] 20:29, 24 May 2012 (UTC)
- If we're having a rant about the UK education system, then my 1.3 pence is that this sort of rote learning is largely pointless in the current period. If you need to know a specific fact such as this there's ample places one can look it up. Hell, I even hear that there's this free encyclopaedia on the internet these days. What we should be teaching children is how to find such information, and the methods of thinking required to understand the information once it's found. LukeSurl t c 21:48, 24 May 2012 (UTC)
- The very fact that an exam question asked about the class of some creature suggests that knowledge of the Linnaean system is still required and is still taught. But is it really true that not a single teacher in the entire country knew the answer, Tammy? Who's the "everyone" you refer to? -- ♬ Jack of Oz ♬ [your turn] 20:29, 24 May 2012 (UTC)
- I'd be very surprised to find such a question on a GCSE paper, unless it had already provided context that would allow to you remember that 'mammal' was the sort of answer it wanted (or, horrors, it was multiple choice). Do we know what exam board this was, and whether it was from a Dual Science Biology paper, or from a separates sciences Biology paper? I'm curious to know who includes this knowledge on the current curriculum. 109.155.32.126 (talk) 22:15, 24 May 2012 (UTC)
Orange segments
I am curious about the segments (slices) in an orange fruit. What do they correspond to, relative to the structures in the flower? --İnfoCan (talk) 15:16, 24 May 2012 (UTC)
- The segmentation already exists in the flower's ovary. Roger (talk) 15:52, 24 May 2012 (UTC)
- You may be interested in the mutation navel orange that contains an additional set of smaller segments of a conjoined twin. See the article Orange (fruit). 84.209.89.214 (talk) 18:26, 24 May 2012 (UTC)
Household uses for reactions
I would like to know where the following reactions:
- Is found in the household/everyday life
- And what is positive an negative about each of the reactions.
- Acid ± metal→ salt ± hydrogen
- Acid ± metal oxide → salt ± water
- Acid ± metal carbonate → salt ± water ± carbon dioxide
- Acid ± alkali → salt ± water
Thanks. — Preceding unsigned comment added by 41.123.76.163 (talk) 15:39, 24 May 2012 (UTC)
- I have two questions for you, which may help others (or even me) to answer your questions. First, why would you like to know that? Second, what other research or investigation have you done other than asking here? (Even if the research, e.g. web searches, didn't produce any useful answers - what did it produce?) Third, what ideas of your own do you have on this so far? --Demiurge1000 (talk) 18:09, 24 May 2012 (UTC)
- The signs "±" in the question should all be straightforward "+". 84.209.89.214 (talk) 18:18, 24 May 2012 (UTC)
- Not trying to do your homework for you. But, I take to acid to mean a Bronsted acid, which loses a proton in solution to leave the conjugate base behind, e.g. the metal salt of carbonic acid is a metal carbonate after the protons are reduced by the metal. (And of course the proton is not naked, but rather in the form of hydronium and higher-order species.) Two protons can be reduced by a pair of electrons to form dihydrogen (H2).
- In your first case, the metal reduces (gives up a pair of electrons) the acid and thus becomes positive. The conjugate base is the acid that has lost a proton, so since a neutral species that has loses positive charge must be negative (conservation of charge), the conjugate base is negative. The salt is the combination of the positive metal cation plus the negative conjugate base, which must balance to give neutral species, if the metal and the acid both started out positive (this is not always the case, i.e. sodium dihydrogen phosphate is a negative ion that is still acidic and can lose a proton to become more negative).
- In your other cases, (metal oxides, metal carbonates, alkalis), are combinations of metal cations plus some sort of negative ion (oxide, carbonate or hydroxide). The negative ions are in general, basic, and will receive a proton to become their neutral form. Oxides receive protons to become water or hydroxide, carbonates receive protons to become carbonic acid, which are unstable and decompose rapidly to carbon dioxide and water, hydroxides receive protons to become water. Hydroxides are quite basic. Water-soluble oxides (a charge of 2-) are very basic. If you dissolve sodium oxide in water, an explosion will happen.
- In some cases, the metal and oxides form stronger bonds with each other than the solution. Acid sometimse brings the insoluble oxide into solution in the form of water, which forces the remaining metal cation into solution as well, which will be balanced by what remains of the acid after it loses a proton: the conjugate base. Remember that the combination metal cation + conjugate base is a salt. This is why mild acid can clean rust -- the insoluble iron oxide is dissolved leaving the clean metal behind (the acid is too weak to oxidise the metal). Nothing gold can stay (talk) 18:28, 24 May 2012 (UTC)
- An "everyday household" example of #3 occurs in baking when baking powder reacts to release CO2. Roger (talk) 18:33, 24 May 2012 (UTC)
- #1 is used in etching. #2 is used in dissolving rust. And you can etch silicon dioxide (glass) with acid too -- silicon is a semimetal. #3 can be found in ocean acidification -- acid breaking down the calcium carbonate shells of lifeforms (close to insoluble in neutral water, but gets increasingly soluble in acid). #4 happens when you want to clean up either acid or base by dissolving it with the other. Nothing gold can stay (talk) 20:08, 24 May 2012 (UTC)
Why does LASIK work?
The article LASIK fails to cover any basic optical theory, i.e. why ablating the cornea or creating a flap would result in higher refractive power. If you don't change the curvature of the entire lens as opposed to a small section, wouldn't it just increase optical aberrations? Can someone enlighten? 76.104.28.221 (talk) 17:37, 24 May 2012 (UTC)
- The cornea usually is thinner after LASIK, because of the removal of part of the stroma. This should reduce the optical power of the cornea, which accounts for about 2/3 of the eye's total optical power, and is the change needed to correct myopia. 84.209.89.214 (talk) 18:14, 24 May 2012 (UTC)
- But wouldn't you need to thin the cornea everywhere? Otherwise you would get optical aberrations (some of the rays are focused but some aren't). Nothing gold can stay (talk) 18:42, 24 May 2012 (UTC)
- My wife had Photorefractive keratectomy, which is a closely related procedure. IIRC, the idea is that there is enough focusing power in the cornea to make corrections such that the lens itself doesn't need to be reshaped. In other words, the effect is like turning the cornea into a permanent contact lens; the cornea is reshaped so that it refocuses the light in such a way as to correct for the abberations in the lens, much as a contact lens would. At least, that's what I remember from what my wife was told. --Jayron32 19:14, 24 May 2012 (UTC)
- I think the cornea is reshaped so that focal properties of the entire area of over the pupil are changed. It's not the whole cornea, but the whole part that transmits light to the retina. SemanticMantis (talk) 21:53, 24 May 2012 (UTC)
- Also, what are the physics behind LASIK? How do doctors know how much to correct for? How does the Lensmaker's equation apply to LASIK surgery? Nothing gold can stay (talk) 19:07, 24 May 2012 (UTC)
Solar flares and radioactive decay
According to this 2010 report, http://news.stanford.edu/news/2010/august/sun-082310.html, there is a connection between solar flares and a change in the rate of radioactive decay measured on Earth. Has this connection been confirmed or debunked since then? At Radioactive_decay#Changing_decay_rates there is discussion of the phenomena, which concludes that there is "no evidence for such correlations", but all the references in that article pre-date the 2010 news report. Happy editing! (talk) 19:42, 24 May 2012 (UTC)
- Neutrinos are definitively not the culprit, and the Purdue team is attempting to use the effect to predict solar flares. So it looks like there is still more science to come on this topic; indeed, I believe a lot more experiments are needed to find out what is happening, even if it's just experimental error (still the only culprit that makes physical sense according to current theories). -RunningOnBrains(talk) 20:20, 24 May 2012 (UTC)
Where cometh the meme about heorin being less adictive than tobacco?
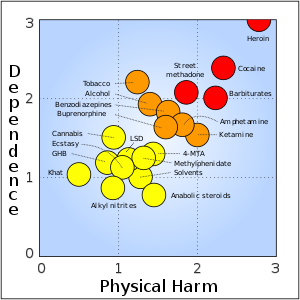
It's total rubbish that nicotine even TOUCHES the addictiveness of opioids, once one has a decent habit going. Where did this rubbish come from? Is there the slightest peace of "scientific justification" for it? Egg Centric 22:23, 24 May 2012 (UTC)
- It depends how you measure addition, and whether you are talking about nicotine in isolation or in the presence of tobacco (which complicates the chemistry). Our article on it (Nicotine#In_the_central_nervous_system, Nicotine#Psychoactive_effects) covers this with citations. It is not "rubbish"; the source seems to come from FDA studies done in the 1980s and 1990s. It is true, of course, that you don't see people robbing stores (too often) to feed their nicotine habits — but that is as much a social constraint as any pharmacological one (the legality of nicotine makes it pretty easy to obtain, and the effects are not as profound as heroin). --Mr.98 (talk) 22:34, 24 May 2012 (UTC)
- Well that lack of profundity tis the key - while heroin can be hard to get ahold of (and is more expensive) there is no comparison between acute withdrawl from the two. And even LESS comparison between the PAWS from the two. So by what means can they be called more addictive? Egg Centric 22:43, 24 May 2012 (UTC)
- I found this 1987 NYT article: NICOTINE: HARDER TO KICK...THAN HEROIN. The sensationalist headline seems to be based on not much more than anecdotal evidence really. The general public's, and public policy's, thoughts on drugs are often massively bereft of evidence, instead being based on fear-mongering and tabloid headlines (c.f. the tale of David Nutt, who basically got fired for presenting the evidence in the diagram right) --LukeSurl t c 22:45, 24 May 2012 (UTC)
- There is an irony in that were opioids legalised I am convinced they would do sod all damage. Basically you're talking about constiptation as the worst side effect (not the case for some like dextroproxyphene and pethidine but addicts can easily be moved off them to better 'uns) , and that can be combated by orally taken naloxone, biovaliable in ones gut. Nevertheless, while these wonderful chemicals stay illegal addiction to them is hellish at least some of the time (speaking from experience - I have done the occasional opioid treatment thingy and am about to start again, I currently take the equivilant of 280mg of morphine a day, I can afford this being a trader but even with a job as stupidly well paid as mine wasting 75k a year isn't ideal) and to compare it to nicoteine is honestly laughable; I have no idea where these reports are coming from but can only assume they're self serving... Egg Centric 22:49, 24 May 2012 (UTC)
- Because heroin is illegal, very little serious research can be conducted into its effects, meaning that bigots can claim whatever they like. HiLo48 (talk) 23:26, 24 May 2012 (UTC)
- My uncle was euthaniased with heroin [-]last week[/-] (checked it was a fortnight ago) (using doctrine of double effect as a legal excuse - I'm not exaggerating here btw, it was agreed with family and doctor - he had a funeral this tuesday) there's no reason in principle heroin couldnt' be experimented with in the UK that I'm aware with, I would think the political pressures ain't that strong... Egg Centric 23:35, 24 May 2012 (UTC)
- Euthanasia using heroin is completely new to me. I hope you're doing well.
- My uncle was euthaniased with heroin [-]last week[/-] (checked it was a fortnight ago) (using doctrine of double effect as a legal excuse - I'm not exaggerating here btw, it was agreed with family and doctor - he had a funeral this tuesday) there's no reason in principle heroin couldnt' be experimented with in the UK that I'm aware with, I would think the political pressures ain't that strong... Egg Centric 23:35, 24 May 2012 (UTC)
- The obvious study would be looking at heroin and smoking addicts in jail and offering them the choice between an unlimited supply of cigarettes and an unlimited supply of heroin (or possibly methadone) for the next 4 weeks. I cannot find such a study (and I don't think ethical reasons would forbid such an experiment in for example The Netherlands). My gut feeling is that this meme is invented by anti-smoking lobbyists. Joepnl (talk) 23:44, 24 May 2012 (UTC)
- Erm, an unlimited supply of heroin for jail inmates of anything other than the shortest sentences is only going to lead to one thing. BTW letting folk do as much methadone as they like would be even worse in that it would be far more likely to lead to lulzy deaths, for reasons not terribly interesting Egg Centric 00:51, 25 May 2012 (UTC)
http://www.bbc.co.uk/news/uk-11660210 Count Iblis (talk) 01:05, 25 May 2012 (UTC)
- What's got alcohol to do with this? Joepnl (talk) 01:08, 25 May 2012 (UTC)
- I've no idea why he mentioned that, but I would agree with the user. Alcohol is not as addictive as heroin (or even nicotine) but it is a MASSIVELY hard drug. It always amuses me to see anti-drug types who've taken it to huge amounts. How silly they look... But they'll never understand...
- Memes can make jumps. So, when you have a news report about research showing that alcohol is more of a problem than heroin, and people also read about nicotine in relation to drugs, then these things can get mixed up, producing the meme about nicotine being less addictive than heroin. Count Iblis (talk) 01:26, 25 May 2012 (UTC)
- I've no idea why he mentioned that, but I would agree with the user. Alcohol is not as addictive as heroin (or even nicotine) but it is a MASSIVELY hard drug. It always amuses me to see anti-drug types who've taken it to huge amounts. How silly they look... But they'll never understand...
- What's got alcohol to do with this? Joepnl (talk) 01:08, 25 May 2012 (UTC)
- Some years ago I assisted (as a volunteer) in a not-for-profit organisation providing rehabilitation to drug adicts. We were taught that there are three levels of adiction: 1) physical dependence, 2) pyschological dependence, and 3) habituation. Heroin is an example of physical dependence - it mimics a natural brain chemical, and the brain adapts by cutting back natural production (hence withdrawal symptoms). Psychological dependence is somewhat complex, but alchohol is the classic example, where alcholics get drunk to avoid their problems. Habituation is where environmental cues remind you of your craving. An example of habituation is craving tobaco when you see a friend light up, of getting a craving after dinner, if that's when you usually smoke.
- Generally, physical adiction is the strongest, though if the drug does not closely match the natural analog, it may not be. Psychological addiction is the next strongest. Habituation is the weakest. But paradoxicaly, some people can break the strongest physical addiction by making their mind up, and others cannot break habituation.
- From the above, some general principles emerge:
- A drug that operates at all three adiction levels is the hardest to break and is thereby the most damaging. Heroin is the classic example. Tobacco is an example that works mainly be habituation, and very weakly in psychological adiction.
- To get someone off drugs, you, and the addict, need to understand how the drug works in this context. If it works by physcial addition, you need to break that - cold turkey and replacement (as in methodone) are the (very imperfect) usual methods. If the addiction is psychological, you need to adress what in their life is bad - solve the problem or show that it is not so important. If the addiction is habituation, change their environment to get rid of the cues.
- It should never be forgoten that with drugs such as heroin, that while the addict has a good supply, he/she thinks life is really good and their perception of themself and their life is distorted. Thinks like Western Govt "Just say no" programms sound about as sensible as saying the moon is made of cheese.
- Opiates are disease masking. When heroin addicts came to us expressing a wish to get off it (usually when their supply has become a little unreliable), the fisrt thing we did was take then to a doctor for a really go checkout. Occaisonally they had broken bones and/or serious kidney or liver disease - and they didn't know.
- I hope that, if you read through all this, you have some understanding that the graph the OP posted is roughly about right. I've simplified the story somewhat. I would move some drugs about on the graph to a certain extent. Barbituate addiction is pretty nasty. I smoked as a teenager but found the habit easy to break. :Wickwack124.182.14.180 (talk) 01:20, 25 May 2012 (UTC)
May 25
Scientist said that Black hole pull everything even light. As I know light is made of photons which are massless, they say. How is than posible that gravity affect them? Second question. Scientist said that electromagnetic force do not affect neutrino particles. I ask how than do the experiments with neutrinos in CERN? Which force they use to accelerate them and keep them inside? They said that neutrino pass through any mater. How than keep them inside? I will be very appreciative for answer. If anybody know answer, please tell me. — Preceding unsigned comment added by Parket7 (talk • contribs) 00:11, 25 May 2012 (UTC)
What accounts for temperature increase at night in a modern home?
I live in the UK. Hot day today. But hotter night, at least in this room. No air-con.
Is it heat beating down on the bricks outside during the day, the bricks absorb it, and are now releasing that heat into the room? I've also heard that cloud cover can trap heat, could that account for it? What if we assume no clouds? --bodnotbod (talk) 00:40, 25 May 2012 (UTC)
- Are you describing real, measured temperatures, or how hot you feel? HiLo48 (talk) 00:46, 25 May 2012 (UTC)
- He's describing how hot I feel.
- To throw some numbers around, today in the UK the air temperature (outside) reached about 26C at about 5pm where I live. An hour or two later, the temperature inside my living room (not especially well ventilated) reached 23C.
- Right now at about 2am, the air temperature outside is about 15C (an unusually warm night, for here). The temperature inside my living room is *still* (probably unsurprisingly) around 23C. So it's not more, but nor has it gone down appreciably. Humans tend to perceive this as "hotter" because it's during the night, maybe? --Demiurge1000 (talk) 01:00, 25 May 2012 (UTC)
- Yeah, the heat stored in the bricks (and other parts of the building) would be a big part of the problem. Better ventilation would be a great solution, but maybe not practical. (If you have ever contemplated moving to Australia, please check our climate carefully. You may not like it.) HiLo48 (talk) 01:12, 25 May 2012 (UTC)
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17382831, please use {{cite journal}} with
|pmid=17382831instead. - ^ Cite error: The named reference
bbcprofilewas invoked but never defined (see the help page).









