GABRA3
Gamma-aminobutyric acid receptor subunit alpha-3 is a protein that in humans is encoded by the GABRA3 gene.[5]
Function
GABA is the major inhibitory neurotransmitter in the mammalian brain where it acts at GABAA receptors, which are ligand-gated chloride channels. Chloride conductance of these channels can be modulated by agents such as benzodiazepines that bind to the GABAA receptor. At least 16 distinct subunits of GABA-A receptors have been identified.[5] GABA receptors are composed of 5 subunits with an extracellular ligand binding domains and ion channel domains that are integral to the membrane.Ligand binding to these receptors activates the channel.[6]
Subunit selective ligands
Recent research has produced several ligands that are moderately selective for GABAA receptors containing the α3 subunit. Subtype-selective agonists for α3 produce anxiolytic and mild sedative effects, but without causing amnesia or ataxia, which could make them superior to currently marketed drugs.
Agonists
Inverse agonists
- α3IA
RNA editing
| Editing element of GABA-3 exon 9 | |
|---|---|
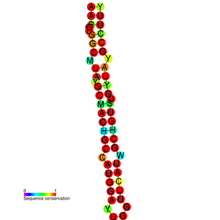 Conserved secondary structure and sequence conservation of GABA3 | |
| Identifiers | |
| Symbol | GABA3 |
| Rfam | RF01803 |
| Other data | |
| RNA type | Cis-reg; |
| Domain(s) | Eukaryota; |
| SO | SO:0005836 |
| PDB structures | PDBe |
The GABRA3 transcript undergoes pre-mRNA editing by the ADAR family of enzymes.[7] A-to-I editing changes an isoleucine codon to code for a methionine residue. This editing is thought to be important for brain development, as the level of editing is low at birth and becomes almost 100% in an adult brain.[7]
The editing occurs in an RNA stem-loop found in exon 9.[7] The structured loci was identified using a specialised bioinformatics screen[8] of the human genome. The proposed function of the edit is to alter chloride permeability of the GABA receptor.[7]
At the time of discovery, Kv1.1 mRNA was the only previously known mammalian coding site containing both the edit sequence and the editing complementary sequence.[9]
Type
A to I RNA editing is catalyzed by a family of adenosine deaminases acting on RNA (ADARs) that specifically recognize adenosines within double-stranded regions of pre-mRNAs and deaminate them to inosine. Inosines are recognised as guanosine by the cells translational machinery. There are three members of the ADAR family ADARs 1-3, with ADAR1 and ADAR2 being the only enzymatically active members. ADAR3 is thought to have a regulatory role in the brain. ADAR1 and ADAR 2 are widely expressed in tissues, while ADAR3 is restricted to the brain. The double-stranded regions of RNA are formed by base-pairing between residues in the close to region of the editing site, with residues usually in a neighboring intron but can be an exonic sequence. The region that base pairs with the editing region is known as an Editing Complementary Sequence (ECS).
Location
The editing site was previously believed to be a single nucleotide polymorphism.[10] The editing site is found at amino acid 5 of transmembrane domain 3 of exon 9. The predicted double-stranded RNA structure is interrupted by three bulges and a mismatch at the editing site. The double-stranded region is 22 base pairs in length. As with editing of the KCNA1 gene product,[9] the editing region and the editing complementary sequence are both found in exonic regions. In the pre=mRNA of GABRA3, both are found within exon 9.[7] The other subunits of the receptor are thought not to be edited, as their predicted secondary structure is less likely to be edited. Also, alpha subunits 1 and 6 have a uridine instead of an adenosine at the site corresponding to the editing site in alpha subunit 3.[7] Point mutation experiments determined that a Cytidine 15 nucleotides from the editing site is the base opposite the edited base.[7] Using a GABRA3 mini-gene that encodes for exon 9 cotransfected to HEK293 cells with either ADAR1 or -2 or none, it was determined that both active ADARs can efficiently edited the site in exon 9.[7]
Regulation
The mRNA expression of the alpha 3 subunit is developmentally regulated. It is the dominant subunit in the forebrain tissue at birth, gradually decreasing in prominence as alpha subunit 1 takes over. Also experiments with mice have demonstrated that editing of pre-mRNA alpha 3 subunit increases from 50% at birth to nearly 100% in adult.[7] Editing levels are lower in the hippocampus[11]
Conservation
At the location corresponding to the I/M site of GABRA3 in frog and pufferfish there is a genomically encoded methionine. In all other species, there is an isoleucine at the position.[12]
Consequences
Structure
Editing results in a codon change from (AUA)I to (AUG)M at the editing site. This results in translation of a methionine instead of an isoleucine at the I/M site. The amino acid change occurs in the transmembrane domain 3. The 4 transmembrane domains of each of the 5 subunits that make up the receptor interact to form the receptor channel. It is likely that the change of amino acids disturbs the structure, effecting gating and inactivation of the channel.[13] This is because methionine has a larger side chain.[7]
Function
While the effect of editing on protein function is unknown, the developmental increase in editing does correspond to changes in function of the GAGAA receptor. GABA binding leads to chloride channel activation, resulting in rapid increase in concentration of the ion. Initially, the receptor is an excitatory receptor, mediating depolarisation (efflux of Cl− ions) in immature neurons before changing to an inhibitory receptor, mediating hyperpolarisation(influx of Cl− ions) later on.[14] GABAA converts to an inhibitory receptor from an excitatory receptor by the upregulation of KCC2 cotransporter. This decreases the concentration of Cl− ion within cells. Therefore, the GAGAA subunits are involved in determining the nature of the receptor in response to GABA ligand.[15] These changes suggest that editing of the subunit is important in the developing brain by regulating the Cl− permeability of the channel during development. The unedited receptor is activated faster and deactivates slower than the edited receptor.[7]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000011677 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031343 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Entrez Gene: GABRA3 gamma-aminobutyric acid (GABA) A receptor, alpha 3".
- ^ Cromer BA, Morton CJ, Parker MW (June 2002). "Anxiety over GABA(A) receptor structure relieved by AChBP". Trends Biochem. Sci. 27 (6): 280–7. doi:10.1016/S0968-0004(02)02092-3. PMID 12069787.
- ^ a b c d e f g h i j k Ohlson J, Pedersen JS, Haussler D, Ohman M (May 2007). "Editing modifies the GABA(A) receptor subunit alpha3". RNA. 13 (5): 698–703. doi:10.1261/rna.349107. PMC 1852825. PMID 17369310.
- ^ Ohlson J, Ensterö M, Sjöberg BM, Ohman M (2005). "A method to find tissue-specific novel sites of selective adenosine deamination". Nucleic Acids Res. 33 (19): e167. doi:10.1093/nar/gni169. PMC 1275595. PMID 16257978.
- ^ a b Bhalla T, Rosenthal JJ, Holmgren M, Reenan R (October 2004). "Control of human potassium channel inactivation by editing of a small mRNA hairpin". Nat. Struct. Mol. Biol. 11 (10): 950–6. doi:10.1038/nsmb825. PMID 15361858.
- ^ Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K (February 2004). "Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene". J. Biol. Chem. 279 (6): 4952–61. doi:10.1074/jbc.M310162200. PMID 14613934.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rula EY, Lagrange AH, Jacobs MM, Hu N, Macdonald RL, Emeson RB (June 2008). "Developmental modulation of GABA(A) receptor function by RNA editing". J. Neurosci. 28 (24): 6196–201. doi:10.1523/JNEUROSCI.0443-08.2008. PMC 2746000. PMID 18550761.
- ^ Hinrichs AS, Karolchik D, Baertsch R, Barber GP, Bejerano G, Clawson H, Diekhans M, Furey TS, Harte RA, Hsu F, Hillman-Jackson J, Kuhn RM, Pedersen JS, Pohl A, Raney BJ, Rosenbloom KR, Siepel A, Smith KE, Sugnet CW, Sultan-Qurraie A, Thomas DJ, Trumbower H, Weber RJ, Weirauch M, Zweig AS, Haussler D, Kent WJ (January 2006). "The UCSC Genome Browser Database: update 2006". Nucleic Acids Res. 34 (Database issue): D590–8. doi:10.1093/nar/gkj144. PMC 1347506. PMID 16381938.
- ^ Fisher JL (April 2004). "A mutation in the GABAA receptor alpha 1 subunit linked to human epilepsy affects channel gating properties". Neuropharmacology. 46 (5): 629–37. doi:10.1016/j.neuropharm.2003.11.015. PMID 14996540.
- ^ Ben-Ari Y (September 2002). "Excitatory actions of gaba during development: the nature of the nurture". Nat. Rev. Neurosci. 3 (9): 728–39. doi:10.1038/nrn920. PMID 12209121.
- ^ Böhme I, Rabe H, Lüddens H (August 2004). "Four amino acids in the alpha subunits determine the gamma-aminobutyric acid sensitivities of GABAA receptor subtypes". J. Biol. Chem. 279 (34): 35193–200. doi:10.1074/jbc.M405653200. PMID 15199051.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
Further reading
- Buckle VJ, Fujita N, Ryder-Cook AS, et al. (1990). "Chromosomal localization of GABAA receptor subunit genes: relationship to human genetic disease". Neuron. 3 (5): 647–54. doi:10.1016/0896-6273(89)90275-4. PMID 2561974.
- Bell MV, Bloomfield J, McKinley M, et al. (1990). "Physical linkage of a GABAA receptor subunit gene to the DXS374 locus in human Xq28". Am. J. Hum. Genet. 45 (6): 883–8. PMC 1683479. PMID 2574000.
- Tögel M, Mossier B, Fuchs K, Sieghart W (1994). "gamma-Aminobutyric acidA receptors displaying association of gamma 3-subunits with beta 2/3 and different alpha-subunits exhibit unique pharmacological properties". J. Biol. Chem. 269 (17): 12993–8. PMID 8175718.
- Hadingham KL, Wingrove P, Le Bourdelles B, et al. (1993). "Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors". Mol. Pharmacol. 43 (6): 970–5. PMID 8391122.
- Belelli D, Lambert JJ, Peters JA, et al. (1997). "The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid". Proc. Natl. Acad. Sci. U.S.A. 94 (20): 11031–6. doi:10.1073/pnas.94.20.11031. PMC 23576. PMID 9380754.
- Huang RQ, Dillon GH (1998). "Maintenance of recombinant type A gamma-aminobutyric acid receptor function: role of protein tyrosine phosphorylation and calcineurin". J. Pharmacol. Exp. Ther. 286 (1): 243–55. PMID 9655866.
- Amir R, Dahle EJ, Toriolo D, Zoghbi HY (2000). "Candidate gene analysis in Rett syndrome and the identification of 21 SNPs in Xq". Am. J. Med. Genet. 90 (1): 69–71. doi:10.1002/(SICI)1096-8628(20000103)90:1<69::AID-AJMG12>3.0.CO;2-W. PMID 10602120.
- Bedford FK, Kittler JT, Muller E, et al. (2001). "GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1". Nat. Neurosci. 4 (9): 908–16. doi:10.1038/nn0901-908. PMID 11528422.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Chou KC (2004). "Modelling extracellular domains of GABA-A receptors: subtypes 1, 2, 3, and 5". Biochem. Biophys. Res. Commun. 316 (3): 636–42. doi:10.1016/j.bbrc.2004.02.098. PMID 15033447.
- Henkel V, Baghai TC, Eser D, et al. (2004). "The gamma amino butyric acid (GABA) receptor alpha-3 subunit gene polymorphism in unipolar depressive disorder: a genetic association study". Am. J. Med. Genet. B Neuropsychiatr. Genet. 126 (1): 82–7. doi:10.1002/ajmg.b.20137. PMID 15048654.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Kimura K, Wakamatsu A, Suzuki Y, et al. (2006). "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.
- Pedersen JS, Bejerano G, Siepel A, et al. (April 2006). "Identification and classification of conserved RNA secondary structures in the human genome". PLoS Comput. Biol. 2 (4): e33. doi:10.1371/journal.pcbi.0020033. PMC 1440920. PMID 16628248. Retrieved 2010-07-19.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- GABRA3+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- [1]
- Page for Editing element of GABA-3 exon 9 at Rfam
This article incorporates text from the United States National Library of Medicine, which is in the public domain.