Ubenimex
| |
| |
| Names | |
|---|---|
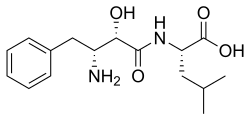
| IUPAC name
(2S)-2-[[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutanoyl]amino]-4-methylpentanoic acid
| |
| Other names
Bestatin; N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.055.917 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H24N2O4 | |
| Molar mass | 308.378 g·mol−1 |
| Melting point | 245 °C (473 °F; 518 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B),[2] leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities),[3] alanyl aminopeptidase (aminopeptidase M/N),[4] leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase),[5][6] and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia.[7] It is derived from Streptomyces olivoreticuli.[8] Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.[citation needed]

See also
References
- ^ N-((2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl)-L-leucine at Sigma-Aldrich
- ^ Umezawa,H.; Aoyagi,T.; Suda,H.; Hamada,M.; Takeuchi,T. (1976). "Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes" (29): 97–99.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Muskardin,D.T.; Voelkel,N.F.; Fitzpatrick,F.A. (1994). "Modulation of pulmonary leukotriene formation and perfusion pressure by Bestatin, an inhibitor of leukotriene A4 hydrolase" (48): 131–137.
{{cite journal}}: Cite journal requires|journal=(help) - ^ K Sekine; H Fujii; F Abe (1999). "Induction of apoptosis by Bestatin (ubenimex) in human leukemic cell lines". 13 (5): 729–734.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Nakanishi Y, Nomura S, Okada M, Ito T, Katsumata Y, Kikkawa F, Hattori A, Tsujimoto M, Mizutani S (2000). "Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta". Placenta. 21 (7): 628–34. doi:10.1053/plac.2000.0564. PMID 10985965.
- ^ Naruki M, Mizutani S, Goto K, Tsujimoto M, Nakazato H, Itakura A, Mizuno K, Kurauchi O, Kikkawa F, Tomoda Y (1996). "Oxytocin is hydrolyzed by an enzyme in human placenta that is identical to the oxytocinase of pregnancy serum". Peptides. 17 (2): 257–61. doi:10.1016/0196-9781(95)02124-8. PMID 8801531.
- ^ Hirayama, Y; Sakamaki, S; Takayanagi, N; Tsuji, Y; Sagawa, T; Chiba, H; Matsunaga, T; Niitsu, Y (2003). "Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients--effects, complications and long-term survival". Gan to kagaku ryoho. Cancer & chemotherapy. 30 (8): 1113–8. PMID 12938265.
- ^ Bauvois, B; Dauzonne, D (January 2006). "Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects". Medicinal Research Reviews. 26 (1): 88–130. doi:10.1002/med.20044. PMID 16216010.
External links
Template:Peptide receptor modulators
