Wikipedia:Reference desk/Science: Difference between revisions
→Storks: new section |
|||
| Line 579: | Line 579: | ||
: [[User:SteveBaker|SteveBaker]] ([[User talk:SteveBaker|talk]]) 23:54, 3 December 2009 (UTC) |
: [[User:SteveBaker|SteveBaker]] ([[User talk:SteveBaker|talk]]) 23:54, 3 December 2009 (UTC) |
||
:I understand that an interesting question is why the night sky is not bright white rather than black, as an infinite number of stars would lead to the former. [[Special:Contributions/89.242.105.246|89.242.105.246]] ([[User talk:89.242.105.246|talk]]) 01:13, 4 December 2009 (UTC) |
|||
== The most useless particle == |
== The most useless particle == |
||
Revision as of 01:13, 4 December 2009
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
November 29
Solutes
When I dash salt into a bowl of water, it seems as though the salt falls and 'drags water with it down into the water' upon hitting the surface, but when I dash paprika, it seems as though the paprika falls and 'pulls the water' along its own surface, causing the paprika to rapidly spread along the surface. Does this happen because the salt is immediately ionized and falls into the water, while paprika doesn't ionize and isn't nearly as readily brought into solution, and perhaps because of surface tension, fails to be incorporated? DRosenbach (Talk | Contribs) 01:10, 29 November 2009 (UTC)
- Paprika will not likely dissolve appreciably at all. Most of the compounds in paprika are hydrophobic and/or insoluble in water. If you shake the mixture, you can generate a Suspension, but that is not at all the same thing as a solution. As far as the visual effect of the mixing of the two, well, I can't make much comment on that as I really don't understand what you are getting at (the difference between "drag" and "pull".) But for sure, the way in which salt mixes with water (which is a true solution, see solvation for more details) and the way in which paprika mixes with water are very different. --Jayron32 02:03, 29 November 2009 (UTC)
- Salt being dropped into water "falls into the water," while paprika dropped into water does not "fall in," but spreads out onto the surface. Try it in a white vessel and you'll see the effect. DRosenbach (Talk | Contribs) 02:28, 29 November 2009 (UTC)
- What you're probably seeing is most likely a surface tension effect. Individual grains of salt are larger and more massive than individual specks of paprika powder; table salt is also a denser material than paprika. Consequently, each grain of salt exerts a substantially greater force per unit area on the water's surface and is more likely to fall through. The other factor is that I expect paprika (being dried plant matter) is probably appreciably more hydrophobic than salt; the salt is much more readily wetted by water. TenOfAllTrades(talk) 02:56, 29 November 2009 (UTC)
- May be some component of the Paprika has a surfactant effect? That would explain the phenomenos described. Dauto (talk) 03:44, 29 November 2009 (UTC)
- Most herbs and spices are used for their volatile oils, which explains the hydrophobia you're seeing. --Sean 15:19, 30 November 2009 (UTC)
Coffee problem
I was given the following problem: if you have a cup of coffee that's too warm to drink, and you want to cool it as quickly as possible, should you add cream immediately or after?
My first thought was that the cream should be added later, because the coffee will cool down faster when it is warmer (owing to the greater difference in temperature between the coffee and the air). But then I thought, the cream will do a better job of decreasing the temperature of the coffee if it is added immediately, because that is when the difference in temperature between the coffee and cream is greatest. So which is: immediately or later? —Preceding unsigned comment added by 24.200.1.37 (talk) 03:30, 29 November 2009 (UTC)
- Haha, my first concern would be more of preventing precipitation due to solubility issues (and keeping everything supersaturated), but I think adding it first would be more rapid, if your target temperature is above ambient temperature. Of course, adding creamer later will help to achieve lower cooling temperatures, but I don't think that's your goal. (You could also perform an empirical experiment...) John Riemann Soong (talk) 03:45, 29 November 2009 (UTC)
This question is a classic. It's such a classic in fact, that I think it's a homework question. But you could always google for the answer, there are probably hundreds of sites that explain it, and would do a better job than a few paragraphs here. Ariel. (talk) 03:47, 29 November 2009 (UTC)
- The question isn't a homework one, but yes I did try to google it. The problem is, everyone seems to ignore the fact that that the cream will be a more effective cooler if it is added immediately, thereby making the correct answer ambiguous.
- Well, what do you mean by "cool down faster". Do you mean the total time between the starting temperature and the final temperature OR do you mean the instantaneous rate of change of the temperature at any given point along the way. Depending on which definition of "faster" you mean, will result in a different approach to finding the answer. --Jayron32 03:49, 29 November 2009 (UTC)
- Total time.
- I think adding it later works better exactly for the reasons you provided. Dauto (talk) 03:51, 29 November 2009 (UTC)
- But the reasons I gave work against each other...
- No, think about it in terms of energy. Mixing the cream and the coffee doesn't change the total heat energy in the cream and coffee system. The only way the heat of the system changes is by the coffee getting rid of it to the environment as it cools. Rckrone (talk) 07:37, 29 November 2009 (UTC)
- But the reasons I gave work against each other...
- Add the cream immediately to the hot coffee because that increases the volume of liquid in contact with the cup hence reducing the thermal resistance between liquid and atmosphere. But add that cream gently without stirring to avoid heating by Turbulence kinetic energy. OTOH if you have a very cold spoon you know where to stick it. Cuddlyable3 (talk) 11:28, 29 November 2009 (UTC)
- The simplest way to find out is to try it out ourselves. All you need is a thermometer, a clock and some coffee. With two cups you don't even need to measure time. Too bad I don't have all of these right now, else I would do the experiment myself.--131.188.3.20 (talk) 14:32, 29 November 2009 (UTC)
- The heat loss will be in proportion to the temperature difference between the coffee and its surroundings. If the 'cream' is at room temperature rather than chilled, then the temperature difference will be greater if you do not add it to the coffee. If the 'cream' (do Americans really use cream rather than milk - all that saturated fat and calories - somehow I'm not surprised) is chilled, then leave it in the fridge until the last moment. Case solved. 89.240.199.137 (talk) 15:23, 29 November 2009 (UTC)
- This is indeed a classic. There are probably a dozen conflicting possibilities...these are the ones I immediately recall:
- The rate at which energy is lost from the coffee is proportional to the difference in temperature with the environment. (See Newton's law of cooling) So hot coffee loses heat energy faster than cooler coffee. Adding the cream at the end should be the right thing to do if you want cooler coffee.
- Cream lightens the color of the surface of the coffee - that changes it's thermal emissivity - so it radiates heat differently.
- Adding cream increases the volume of the liquid - which increases the surface area - which improves heat loss.
- When you add the cream, you induce turbulance in the coffee which allows the hotter coffee in the center of the volume to swirl out to the edges where it'll cool more effectively.
- If you take the cream out of the fridge when you first make the coffee - it's warming up towards air temperature while you're thinking about adding it. That heat ends up in the coffee when you mix it. If you added the cream immediately, that extra heat would not be accumulated.
- If you are using a cup with a narrow base and a wide rim - then as you add more cream, the exposed surface area of the coffee to the air increases. If you use a cylindrical mug - then that's not a factor. If you use one of those cups that has a narrower rim than base (I have one of those) - then the reverse is the case.
- I'm sure there were others too. Short answer: It's crazy complicated - which is why people like to argue about it - it's like the ridiculous "Airplane on a treadmill" - except this one is actually hard to answer. I believe that for "normal" shapes of coffee cups and normal coffee/cream ratios and normal room temperatures - the nice Mr Newton was right and you should add the cream at the last moment if you want the coolest coffee possible in the time available. SteveBaker (talk) 16:41, 29 November 2009 (UTC)
- That's right, and stir the coffee vigorously (and blow on it at the same time) whilst you are waiting to add the cream. Dbfirs 17:50, 29 November 2009 (UTC)
- I don't understand why there is any question. Cool it down means to reach a certain lower temperature point. Why would you delay adding the cooler liquid? You are trying to reach a target temperature in the shortest possible time. And no other factors are supplied in the question as posed. Given the wide range of possible factors that are not specified, it is possible that that target temperature could be reached almost instantaneously. Bus stop (talk) 17:58, 29 November 2009 (UTC)
- If that was the case we would be solving a stupid problem. I would rather solve a more interesting problem and assume that you cannot reach the target temperature simply by pouring the cream and therefore waiting to the last minute is the right answer. Dauto (talk) 18:30, 29 November 2009 (UTC)
- I experience this problem every single day and my solution is this: add more milk than usual. Take it right to the temperature you want it at, right away. If we are taking a scientific view then Steve Baker's first point is the most pertinent. Vranak (talk) 18:33, 29 November 2009 (UTC)
- Obviously if you have enough (and can tolerate enough) cream to drop the temperature to the desired amount immediately - then you should - but that's just a rather uninteresting special case of a more general problem. If Newton's law of cooling is the only issue then the best algorithm is:
- Calculate the coffee temperature at which adding the maximum allowed amount of cream will bring the temperature to the ideal value more or less instantly. Call this 'taddcream'.
- Assume that the ambient air temperature is 'tambient'.
- Grab the hot (black) coffee: call it's temperature 'tcoffee'.
- While the tcoffee is greater than taddcream - wait.
- Add the cream.
- The case that Bus stop and Vernak describe are just trivial subsets of this more general solution.
- There are all sorts of corner cases where more extreme initial conditions would favor adding the cream at the outset and then waiting - but Bus stop is basically incorrect in 'typical' cases where tcoffee is greater than taddcream - because Newton's law of cooling says that the coffee will lose heat energy much faster if tcoffee is a lot bigger than tambient than if it's closer to tambient. Cooling the coffee down by adding the cream before taddcream will cause the coffee to remain higher than ttarget for much longer than if you waited for the black coffee to cool to taddcream naturally. SteveBaker (talk) 19:12, 29 November 2009 (UTC)
- Obviously if you have enough (and can tolerate enough) cream to drop the temperature to the desired amount immediately - then you should - but that's just a rather uninteresting special case of a more general problem. If Newton's law of cooling is the only issue then the best algorithm is:
- This sounds like it would be a good problem for Mythbusters. Of course it lacks the excitement of blowing something up or inflicting pain. I suppose the pain could come from one of the cast having to drink the hot or not so hot coffee. As long as the volumes and temperatures of coffee (as poured and at the highest allowed drinking temperature) and cream are unspecified, the problem may have multiple solutions. The standard answer [1] is that adding the cream immediately leads to the coffee being warmer, say 15 minutes later, because of Newton's cooling law leading to greater heat loss during the long period of high delta temp if the coffee goes uncreamed until it is to be consumed. Conversely, coffee goes from brewing temp to a safe drinking temp quicker if you wait to add the cream [2] If I recall correctly, some science popularizer like [Isaac Asimov]] or Mr. Wizard said this many years ago (no citation readily available). Edison (talk) 22:15, 29 November 2009 (UTC)
- Some original research with hot chocolate - mmmm! Can't tell the differenc6, gonna have to try again :) hydnjo (talk) 01:30, 30 November 2009 (UTC)
Guys! We've had this question before - so often that I wrote the official Wikipedia Reference Desk Coffee And Milk Simulator some time back (in response to our December 29, 2008 query). Our best responses can be found by searching the archives. The decisive conclusion is that adding milk later always causes the coffee to end colder, but it takes a lot of work (or a lot of milk) to have a very significant effect. Nimur (talk) 03:17, 30 November 2009 (UTC)
- That is a wonderous thing of great beauty Nimur!
- But it misses a bunch of second order effects that can dramatically overturn the decision in some cases. As the graph shows, the amount of gain you get by dumping the cream in late isn't all that great and it could be overwhelmed by effects such as the increase in the volume of the liquid.
- I'm playing Devil's advocate here - because I believe that your simulation is 'good enough' in common cases. But suppose the "cup" is a tall, thin cylinder such that the area of the base of the cup and the liquid exposed to the air at the top is negligable compared to the area of the sides of the cup. If you increase the volume if liquid by 10% by adding the cream - then you also increase the surface area by close to 10%. Newton's law of cooling says that the rate of heat loss is proportional to the temperature difference multiplied by the surface area - so if adding the cream drops the temperature difference by less than it increases the surface area - then adding the cream early results in cooler coffee than adding it later.
- This is complicated though - if the cup itself is made of a material like steel that's a good conductor of heat then the "surface area" exposed to the ambient air is the area of steel in contact with the air - plus the surface area of the coffee. In this situation, then as the surface area of the liquid increases the area of hot steel that's exposed to the cooler air decreases and we're back with adding the cream as late as possible - but for a new and interesting reason. But if the material that the cup is made of is a good insulator then the reverse might be the case. For more conical coffee cups - the amount of surface area increase gained when you add the cream is disproportionately in the region where the liquid meets the ambient air rather than the porcelain of the cup - which alters the rate of heat flow across the interface. Just this one complicating factor (and there are MANY more) means that there isn't a clean answer in every possible case.
- Bottom line is that the simulation is missing some important details that can definitely change the outcome.
- As a long-time veteran of this particular debate - I can tell you that the large number of sophisticated arguments makes any kind of simplistic answer invalid for all but the simplest of cases - and that's why this debate dragged on for so long back in the early days of the Internet.
- Think about this one, for example: If you add cream slowly (like if you're making Irish coffee) and it floats on top of the coffee instead of mixing properly - then it forms an insulating layer (and as a highly fatty liquid, it's a better insulator than black coffee) - that reduces the amount of heat lost to the air from the hot coffee beneath.
- It's really complicated.
- That's freakin' awesome. :) --Sean 14:49, 30 November 2009 (UTC)
- I totally agree with Steve's nitpicks - I assume many things; most notably, uniform mixing, which is probably the worst assumption. Steve brings up weird-shaped vessels, which could also throw things out of whack. Fortunately the simulation is open-source:
clear;close all;
room_ambient_temp = 25;
tau_nlc = 60; % Time constant (Seconds) for the cooling
r_nlc = 1/tau_nlc; % constant for Newton's Law of Cooling in the form dT/dt = -r(T-Tamb)
% Initial coffee and milk parameters
coffee_volume = 300; % mL
coffee_temp0 = 100; % degrees C
milk_volume = 30; % mL
milk_temp0 = 5; % degrees C
% Initialize the coffee and milk parameters
coffee1_temp(1) = (coffee_temp0*coffee_volume + milk_temp0*milk_volume)/(coffee_volume+milk_volume)
coffee2_temp(1) = coffee_temp0;
%%Loop over time, measured after 2:00 PM, in seconds
for t = [1:60*30]
coffee1_temp(t+1) = coffee1_temp(t) - r_nlc*(coffee1_temp(t)-room_ambient_temp);
coffee2_temp(t+1) = coffee2_temp(t) - r_nlc*(coffee2_temp(t)-room_ambient_temp);
% At time 2:10, add milk to Coffee #2
if (t == 60*10)
coffee2_temp(t+1) = (coffee2_temp(t)*coffee_volume + milk_temp0*milk_volume)/(coffee_volume+milk_volume)
end
end
%% Plotting, including time-stamped x-axis
time_labels = datenum(2008, 12, 29, 14, 0, 0) + 1/(24*60*60)*[0:60*30];
plot(time_labels,coffee1_temp, 'r'); hold on;
plot(time_labels,coffee2_temp, 'b');
datetick('x', 16);
xlim([datenum(2008, 12, 29, 14, 0, 0), datenum(2008, 12, 29, 14, 20, 0)]);
legend('Coffee 1 - Milk Added at 2:00', 'Coffee 2 - Milk Added at 2:10');
xlabel('Time');
ylabel('Temperature (Celsius)');
title(['Hot Coffee Simulation with \tau = ' , num2str(tau_nlc) , ' sec']);
- So fellow milk/coffee enthusiasts can feel free to write plugins for various additional parameters and I'll throw those into my web interface. Nimur (talk) 15:03, 30 November 2009 (UTC)
- I was (gnomically) waiting for those already engaged in this conversation to say so, but WOW! Nimur, posting open-source code for GNU Octave so that anyone can run and tweak this model is just so cool. Wikipedia at its finest! -- Scray (talk) 04:30, 1 December 2009 (UTC)
- So fellow milk/coffee enthusiasts can feel free to write plugins for various additional parameters and I'll throw those into my web interface. Nimur (talk) 15:03, 30 November 2009 (UTC)
Cope elimination
Can you do it with a six-membered transition state? (I'm guessing not, because of the electron-pushing?) John Riemann Soong (talk) 03:48, 29 November 2009 (UTC)
Molecular structure
Does anyone know what the wiggly line means in the molecular structure diagram in the top right-hand corner of Amphetamine? —Preceding unsigned comment added by 86.138.105.112 (talk) 04:00, 29 November 2009 (UTC)
It means that both enantiomers prolly have similar properties on the body -- e.g. chirality doesn't matter. John Riemann Soong (talk) 04:13, 29 November 2009 (UTC)
- Thanks, do you mean that there are "left-handed" and "right-handed" versions of the molecule, but the structure diagram is not distinguishing between them (it could represent either)? 86.138.105.112 (talk) 04:29, 29 November 2009 (UTC)
- That's exactly right. There are two non-superimposible mirror image versions (i.e. "left" and "right" handed), the wavy line indicates that the actual version doesn't matter to the discussion at hand, or that there is a racemic mixture of the two versions under discussion. --Jayron32 04:38, 29 November 2009 (UTC)
- Got it, thanks! 86.138.105.112 (talk) 04:46, 29 November 2009 (UTC)
- That's exactly right. There are two non-superimposible mirror image versions (i.e. "left" and "right" handed), the wavy line indicates that the actual version doesn't matter to the discussion at hand, or that there is a racemic mixture of the two versions under discussion. --Jayron32 04:38, 29 November 2009 (UTC)
Earth's season
Hi! my question is how the season change occur in earth? and a season is same all around the world? i.e same season occur in all countries? —Preceding unsigned comment added by Nirmal kumaran (talk • contribs) 07:36, 29 November 2009 (UTC)
- No, at any given time, the entire world does not experience the same season. What the current season is depends on where you are. See Season. Red Act (talk) 08:12, 29 November 2009 (UTC)
- Seasons are reversed in the Northern hemisphere vs the Southern hemisphere (winter in one is summer in the other, spring is autumn, and vice versa). The nearer to one or other pole you are, the more extreme the seasons are. There basically aren't any seasons in the tropics (although you do get wet season and dry season in some places). The seasons are caused by the axial tilt of the Earth, which means sunlight hits a particular place on the Earth at different angles at different times of year (or, put another way, the sun gets higher in the sky during summer than winter). Sunlight shining directly on the ground heats it up more than sunlight hitting the ground at a glancing angle. --Tango (talk) 11:41, 29 November 2009 (UTC)
- And in addition, when the sun is higher in the sky, you also get more hours of daylight, so the sun has more time to heat things up. --Anonymous, 07:53 UTC, November 30, 2009.
- The greatest effect by far is the angle at which the sunlight hits the ground, as Tango said. --121.127.200.51 (talk) 09:07, 30 November 2009 (UTC)
- Wrong -- that's true if you're relatively near the equator, but the length of the day becomes a more important factor as you get nearer to the poles. Especially once you pass the Arctic or Antarctic Circle and you start getting continuous daylight in one part of the year and continuous night in another part! --Anonymous, 21:40 UTC, November 30, 2009.
- The greatest effect by far is the angle at which the sunlight hits the ground, as Tango said. --121.127.200.51 (talk) 09:07, 30 November 2009 (UTC)
Evaporating water
When evaporating water , are you creating new oxygen?
- Evaporating water doesn't create oxygen or anything else. The water molecules (H2O) stay exactly the same. 86.134.90.230 (talk) 12:12, 29 November 2009 (UTC).
- Agreed. Evaporated water becomes water vapor, not free oxygen. StuRat (talk) 13:18, 29 November 2009 (UTC)
- For more details see liquid and gas. In water, the H2O molecules are all stuck to each other, when it evaporates all that happens is the H2O molecules seperate from each other, but they remain intact H2O molecules. There are means of seperating the H's from the O's to give you H2 gas and O2 gas, but this usually involves much more energy than is typical of evaporation. --Jayron32 20:34, 29 November 2009 (UTC)
- However, water has strange properties. It does not have a set condensation nor an evaporation point, because there is supercooled water and mist can from from a puddle under sunlight below freezing, or from one's breath (which is condensation that can occur either above or below freezing). The presence of air also seems to have an effect on this. ~AH1(TCU) 01:46, 2 December 2009 (UTC)
How do I prevent wrinkles around my eyes
I'm 27 and just starting to see wrinkles. Oh no! How do I prevent wrinkles as I get into my thirties? —Preceding unsigned comment added by 92.224.206.187 (talk) 12:25, 29 November 2009 (UTC)
- There must be hundreds of different anti-wrinkle products - lotions, eyepads, Botox, etc.. We can't really recommend one. The problem with judging these products (except Botox, I suppose, but that is only a temporary solution and has plenty of side effects) is that it is several years, at least, before you can really assess their effectiveness, so you aren't likely to get useful advice by just asking your friends what they would recommend. I've had a quick look for academic papers doing proper comparisons of treatments, but I haven't found anything useful... --Tango (talk) 13:03, 29 November 2009 (UTC)
- Wouldn't drinking adequate amounts of water, getting adequate sleep, and not smoking, all be a good idea if wrinkles around the eyes are trying to be avoided? Bus stop (talk) 13:08, 29 November 2009 (UTC)
- Also avoid sunlight and/or wear hats and sunscreen to prevent damage from UV light. StuRat (talk) 13:12, 29 November 2009 (UTC)
- I got my first wrinkle round my eyes aged 48. I attribute this to: 1. Good bone structure on my face. 2. Not having children. 3. Never smoking. 4. I only wash my face once a day. 5. I try and sleep 8 hours a night. 6. I've used moisturising cream daily since I was aged 14. --TammyMoet (talk) 13:21, 29 November 2009 (UTC)
- And 7. You got lucky. One datapoint really isn't useful. --Tango (talk) 13:23, 29 November 2009 (UTC)
- Thanks Tammy. That washing once per day: is it with ice cold, cold, lukewarm, warm, hot, or steaming hot water? How long do you shower? 92.230.65.123 (talk) 14:41, 29 November 2009 (UTC)
- I wash with hot water, and I have a bath once a day. And yes I agree: I got lucky. --TammyMoet (talk) 16:17, 29 November 2009 (UTC) And I forgot something quite important: I'm about 4 stone overweight. Thin people don't have the layer of fat which makes their face fill out, and are more prone to wrinkles. --TammyMoet (talk) 16:18, 29 November 2009 (UTC)
- Avoid squinting, smiling, frowning, scowling or laughing. And don't smoke.[3] Fences&Windows 13:38, 29 November 2009 (UTC)
- One more piece of advice. Stop worrying about wrinkles. Worrying gives you wrinkles... Dauto (talk) 14:59, 29 November 2009 (UTC)
- Have a healthy balanced diet. Take exercise. Stay slim. Don't smoke, avoid alcohol. My pet theory is that drinkers (and smokers) look older than their years. 89.240.199.137 (talk) 15:27, 29 November 2009 (UTC)
- You will get more and more wrinkles around your eyes. Laugh and smile as much as you can. Nothing is more attractive than smile wrinkles. DVdm (talk) 17:20, 29 November 2009 (UTC)
- Get new parents. A large proportion of your wrinkles are a product of genes; some is likely environement, but it may be difficult to seperate the wrinkles you have just because of your genetic make up versus the wrinkles you have because you abused your skin. --Jayron32 20:37, 29 November 2009 (UTC)
- Forgot to add - stay out of the sun. If you live in a sunny area, the wrinkles may be sun-damage, and you can look forward to other skin problems from sun-damage in the future. 78.147.183.186 (talk) 22:34, 29 November 2009 (UTC)
- Screw it! Just keep trying to look like Clint ;) hydnjo (talk) 01:21, 30 November 2009 (UTC)
What happens after a Pulmonary embolism?
Our article doesn't explain what happens to the blood from the lungs, assuming the patient survives and recovers without need for surgery. Where does the blood go? --Dweller (talk) 17:03, 29 November 2009 (UTC)
- I'm not sure what blood you're referring to, but if you mean the initial blood clot (assuming it's a thrombotic P.E.), it is either broken down by fibrinolysis, or the thrombus is organized and recanalized so that a new channel forms through the clot. See this section of our thrombus article (only #3, #4, and maybe #2 apply if the patient "survives and recovers" as you say). Blood flow is restored most rapidly in the first day or two, then improvement gradually slows [4]. It's often impaired permanently, but may be recovered to some degree. - Draeco (talk) 18:06, 29 November 2009 (UTC)
- Perhaps you were referring to "blood in the cavity surrounding the lungs" instead of "blood in the lungs." DRosenbach (Talk | Contribs) 23:16, 29 November 2009 (UTC)
- Note that the cavity around both lungs would be pleural. :-) StuRat (talk) 03:34, 30 November 2009 (UTC)
- I'm going to use that one! DRosenbach (Talk | Contribs) 13:03, 1 December 2009 (UTC)
- Excellent point (and pun); it's also interesting to note singular pleural human cases PMID 16714521, PMID 14602881. The cases described there highlight what I think is a great "did you know" fact - bison have a communication between the right and left pleural cavities that may help explain the ease with which Native Americans hunted them, and their near-extinction by white hunters - any injury causing pneumothorax (such as an arrow or bullet into the lung) would be rapidly fatal. I see plenty of human medical sources for this information, and lots of Google hits, but I don't have a veterinary reference at hand that I would consider definitive. -- Scray (talk) 14:39, 1 December 2009 (UTC)
- Note that the cavity around both lungs would be pleural. :-) StuRat (talk) 03:34, 30 November 2009 (UTC)
Ah. I thought blood actually entered the lungs (coughing blood is cited as a symptom). I just wondered how it would drain from there, given that blood isn't supposed to go there! If blood doesn't enter the lung, how does the PE cause people to cough blood? --Dweller (talk) 10:30, 30 November 2009 (UTC)
- Hemoptysis may result from pulmonary infarction, i.e. death of lung tissue due to inadequate perfusion. The lung has a dual blood supply from pulmonary and bronchial arterioles, and there are anastomoses between these circulations. With small PEs (in the pulmonary artery), the bronchial circulation provides adequate perfusion to sustain the lung tissue; however, in certain vulnerable distributions, or with a large enough PE, the bronchial circulation can be overwhelmed (or even stagnate due to the anastomoses) leading to infarction. When that happens, lung tissue (including vascular walls) dies, and blood does leak into the airways (alveoli, then bronchi, etc) resulting in hemoptysis. The dead tissue eventually gets remodeled through processes like organization by agents that include alveolar macrophages. The center of that dead area generally remains a scar (or, less commonly, a cavity), whereas the peripheral portions may recover some function. The blood itself can be removed completely by the mucociliary escalator then through the digestive tract or simply by coughing, though some will have to be cleared locally, e.g. by macrophages that may process the iron for re-use, though some will remain for a time as characteristic hemosiderin-laden macrophages. BTW, I'm reminded of my comment in this thread, in which I suggested that the connection between the airway and the esophagus might not be totally detrimental. -- Scray (talk) 12:33, 30 November 2009 (UTC)
- Thanks Scray. I understood some of that. You're saying (I think) that there are mechanisms (including, but not limited to coughing) that will get rid of blood from the lungs, it won't just sit there. --Dweller (talk) 13:50, 30 November 2009 (UTC)
- In any case, it won't remain as blood - some will be coughed up or cleared by the "escalator" to which I referred, but the blood that remains will be broken down with components cleared in various ways. The plasma (serum, really, since the fibrinogen and clotting factors will be consumed in a fibrin-based clot) will be cleared by lymphatics, the platelets will become part of the local clot, the white cells will most likely die there, and along with the red blood cells will be trapped in the clot. Organization of that clot will result in entry and activation of phagocytic cells, primarily macrophages, that will ingest the debris. Most components of blood cells (proteins, lipids, sugars, nucleic acids) are readily broken down; however, the Heme is difficult for the body to metabolize, and the iron therein requires special handling. Phagocytosis of red blood cells by macrophages often results in accumulation of hemosiderin that may remain for a long time in the tissue (described in that article) and draining lymph nodes, but iron can be recycled through pathways involving ferritin and transferrin. If some of this jargon is not clarified by the linked articles, please ask. -- Scray (talk) 15:52, 30 November 2009 (UTC)
- Thanks Scray. I understood some of that. You're saying (I think) that there are mechanisms (including, but not limited to coughing) that will get rid of blood from the lungs, it won't just sit there. --Dweller (talk) 13:50, 30 November 2009 (UTC)
Citing this CDC webpage
Hi, how would I cite this webpage (I use ref-works)? [5]
Many thanks 188.220.144.215 (talk) 22:23, 29 November 2009 (UTC)
- Do you mean for use on Wikipedia? If so, you can use the {{Cite web}} template which will correctly format it for you. Otherwise, it depends which of the many citation styles you are required to use in your document. SpinningSpark 00:24, 30 November 2009 (UTC)
- Since you say you use RefWorks, it sounds like you're not looking for a Wikipedia template. Ref-works does not represent a citation format, in fact it supports many of them. I think what you want is a breakdown of MMWR into conventional fields. I Google'd "how to cite mmwr" and got a variety of useful resources, including this one which gives the APA format for a MMWR citation. MMWR is a little odd among journals (which are what RefWorks is used for most), but I would map it this way:
- Authors: Centers for Disease Control and Prevention
- Title: Update: Measles --- United States, January--July 2008
- Journal: Morbidity and Mortality Weekly Report
- Date of publication: 22 Aug 2008
- Volume: 57
- Issue: 33
- Pages: 893 - 896
- Hope this helps. -- Scray (talk) 00:45, 30 November 2009 (UTC)
hydride donation to amides
When a hydride attacks an amide, does it attack the carbonyl carbon or the amide nitrogen? The nitrogen would be more positive, wouldn't it? John Riemann Soong (talk) 22:27, 29 November 2009 (UTC)
Recognising, just with a glance, that a squeleton is the one of an americain
Hello. I'm French. Recently, watching an episode of the serial "Bones", we saw something difficult to explain. I asked the question on the French Reference desk (called l'Oracle) and got good informations but no good answers. That's why I try the same thing here.
The situation took place in Norway. A squeleton is discovered, after some seconds of observation, the specialist says that it's the squeleton of an American. We think it's mean white, caucasien from the USA.
The squeleton is sent to the USA where the Dr Brennan, at the same speed, gets to the same conclusion.
We must give more informations. The squeleton is not old enought to be from World War 1 or 2.
Considering that all the ancestors of the white americans came from Europe, how a single glance to a squeleton can lead to such a conclusive conclusion ?-Reims (Champagne area)-France---Joël DESHAIES (talk) 22:47, 29 November 2009 (UTC)
- This is not necessarily possible in reality. Oftentimes, in television series (such as Bones) facts are embellished or "bent" (I believe in French, this would be "ont dénaturé") to further the plot line. Intelligentsium 23:49, 29 November 2009 (UTC)
- Perhaps from a dental filling? A lot of different kinds of dental restorative materials have been used, so perhaps the skeleton had a dental filling made of a material that was only used in the US at the time of the filling? Or depending on the skeleton's age, perhaps from a relative lack of cavities? Water fluoridation was first used in the US. Colorado brown stain on the teeth would be an indication, too. Or perhaps the skeleton had an intramedullary rod, or surgical screw or plate or wire of a type that was only used in the US at the time of a bone fracture? It does seem like a well-written script would explain anything along those lines, though, if it's used as an important plot device. Or maybe the script did give a plausible explanation, but that part got edited out due to the episode running over time. Red Act (talk) 01:08, 30 November 2009 (UTC)
- I googled and found that this episode is called "Mayhem on a Cross". Several links around the web indicate that the identification as American was due to the visible orthodonture. Perhaps if you watched this episode dubbed it was lost in translation? P.S. it's spelled "skeleton" in English. --Sean 15:05, 30 November 2009 (UTC)
The speed of the reaction Ca(OH)2+CO2
What is the approximate speed of the reaction between calcium hydroxide in suspension and carbon dioxide in air at standart conditions? Renaldas Kanarskas (talk) 22:48, 29 November 2009 (UTC)
- How much of each? Without a quantity, the only answer I can provide is: slowly. Intelligentsium 23:53, 29 November 2009 (UTC)
- Imagine 1 gr of Ca(OH)2 mixed with 1 gr of water. This suspension is lying on the glass in our room, concentration of CO2 in air is 0,04%. As water evaporates, new quantities are being added immediatly. Renaldas Kanarskas (talk) 00:21, 30 November 2009 (UTC)
- I don't actually know the answer, but you may be rate limited by how fast CO2 dissolved in the water. So a thin layer with high surface area will react quicker than a lump. You could reduce this variation by saying dry Ca(OH)2. Graeme Bartlett (talk) 20:57, 2 December 2009 (UTC)
- Imagine 1 gr of Ca(OH)2 mixed with 1 gr of water. This suspension is lying on the glass in our room, concentration of CO2 in air is 0,04%. As water evaporates, new quantities are being added immediatly. Renaldas Kanarskas (talk) 00:21, 30 November 2009 (UTC)
November 30
Do microwaves disrupt Wi-Fi signals?
Do microwaves disrupt Wi-Fi signals? The other day I was connected to a weak signal on my laptop. I was sitting about fifteen feet away from a microwave oven, and the instant it was turned on, I lost my signal and couldn't regain it until the microwave finished cooking. Is it possible for the microwaves to disrupt the signals, or was this just an odd coincidence? Also, if a closed microwave oven can disrupt Wi-Fi signals from fifteen feet away, can those escaped microwaves be harmful to people? Livedtype (talk) 01:08, 30 November 2009 (UTC)
- Microwave ovens use frequencies around 2.45GHz. The 802.11 variety of WiFi uses frequencies around 2.4GHz - so, yep - any leakage from your microwave will interfere with your WiFi. So, no surprises there. Now - is your WiFi going to cook you? No - it produces at most 100 milliWatts - and even the smallest microwave oven produces 500 Watts - 5,000 times more energy. US Federal standard limits the amount of microwaves that can leak from a microwave oven to 5 milliwatts per square centimeter - but there is a lot more than 100 square centimeters on the surface of your oven - so it can easily overwhelm your puny WiFi connections. Try repositioning your oven - or your WiFi gizmo's - or try switching them to a different frequency band (you'll need to consult the manual for your WiFi router to figure out how to do that). SteveBaker (talk) 01:25, 30 November 2009 (UTC)
- Insert obligatory xkcd reference here. Zunaid●for your great great grand-daughter 08:43, 30 November 2009 (UTC)
Artificial black hole
Would it be (theoretically) possible for humans to create an artificial micro black hole by encapsulating a piece of non-fissile matter (say, a small lead sphere) with extremely powerful, shaped nuclear charges, which are then detonated in order to compress the sphere beyond its Schwarzschild radius and trigger implosion? --Kurt Shaped Box (talk) 01:36, 30 November 2009 (UTC)
- The following may help: Micro black hole#Can we produce micro black holes? Red Act (talk) 02:08, 30 November 2009 (UTC)
- Is this about the Large Hadron Collider, which started collisions on the day of posting? ~AH1(TCU) 01:43, 2 December 2009 (UTC)
- Nope. Didn't even realize that it had. --Kurt Shaped Box (talk) 21:11, 2 December 2009 (UTC)
Question
Suggest possible diagnoses given the following symptoms; if there is a symptom that does not fit, note this:
- Diarrhea
- Coughing
- Low fever/chills
- Fatigue
- Slight headache
- "Stuffed up" ears
Use your own words. Do NOT copy out of the textbook or any other source. This is PLAGIARISM and will result in a lowered/FAILING grade. 76.228.195.148 (talk) 02:29, 30 November 2009 (UTC)Tristan (at my mom's compy)
 Please do your own homework.
Please do your own homework.- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our aim here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. --Tagishsimon (talk) 02:30, 30 November 2009 (UTC)
- OK, OK, I admit I was tryin to put one over on the man there :). I've so far considered influenza, but that doesnt fit low fever, 'cause influenza has high fever. Common cold doesnt fit because I thought that didn't cuase diarrhea. Stuffed up ears has me stumped, because I don't even know the name for that symptom. TOnsilitis doesn't fit the symptoms in the rear, and app'itis doesn't fit anything in the top Help? P.S.:The professor said something about no zebra, and he gave a hint that coughing was wet This is due tomorrow help! 76.228.195.148 (talk) 02:50, 30 November 2009 (UTC)Tristan
- Keep in mind that people with a condition can have varying degrees of a symptom, which may also vary over time (i.e. don't be too dogmatic). Stuffed up ears sounds like Eustachian tube dysfunction. You seem to be thinking about many reasonable possibilities - you might expand on things that are similar to your strongest suspicion, whatever you decide that is. -- Scray (talk) 03:06, 30 November 2009 (UTC)
- Alrgiht, I have a new thought: Could it be Swine flu?? Swine flu explains most symtoms, exept the ears (and I think the prof meant like acute stuffed up, not like from birth, or recurring). OK, Im almost done with this paper (Thank God; it's past 9pm where I am at, and I need to get up before 5!). All I need now is prognoses. If it's swine flu, assuming the subject is not immuno-compromised, I think prog is something along the lines of "good chance of full recovery", no? 76.228.195.148 (talk) 03:22, 30 November 2009 (UTC)tristan
- Wait, I got a email from the prof. It's copied below
- Keep in mind that people with a condition can have varying degrees of a symptom, which may also vary over time (i.e. don't be too dogmatic). Stuffed up ears sounds like Eustachian tube dysfunction. You seem to be thinking about many reasonable possibilities - you might expand on things that are similar to your strongest suspicion, whatever you decide that is. -- Scray (talk) 03:06, 30 November 2009 (UTC)
- OK, OK, I admit I was tryin to put one over on the man there :). I've so far considered influenza, but that doesnt fit low fever, 'cause influenza has high fever. Common cold doesnt fit because I thought that didn't cuase diarrhea. Stuffed up ears has me stumped, because I don't even know the name for that symptom. TOnsilitis doesn't fit the symptoms in the rear, and app'itis doesn't fit anything in the top Help? P.S.:The professor said something about no zebra, and he gave a hint that coughing was wet This is due tomorrow help! 76.228.195.148 (talk) 02:50, 30 November 2009 (UTC)Tristan
Hey, Med-I students. Regarding the assignment (I'm assuming most of you are done with it since you've had all Thanksgiving to do it), I want to give it a little...twist. For extra credit, talk about what the prognosis would be if our patient has been sick for a while (more than a week or two, but not as long as...let's say two months). I am offering 20! extra credit points for this—in other words, about a fourth of the assignment. But the real twist is, you don't get an extension! It's due tomorrow, ec or not.
I really need this credit. Help again? —Preceding unsigned comment added by 76.228.195.148 (talk) 03:30, 30 November 2009 (UTC)
- Could be lupus? 74.105.223.182 (talk) 03:48, 30 November 2009 (UTC)
- Which part of "We are not allowed to do your homework" do you not understand? No, no, NO! SteveBaker (talk) 04:08, 30 November 2009 (UTC)
- Backed up by "who wants to be treated by a doctor who got his qualifications by cheating?" AndrewWTaylor (talk) 11:17, 30 November 2009 (UTC)
- In case repetition helps - we provide nudges when people get stuck and ask for a little assistance, but you're past that now. -- Scray (talk) 05:11, 30 November 2009 (UTC)
- I diagnose it as "laziness and procrastination", waiting until the last minute and hoping someone will tell you the answer rather than actually reading your available class and reference materials to learn about the topic. Here's a freebie: the goal of this assignment is the information contained in the answer itself and also how to figure it out. DMacks (talk) 14:35, 30 November 2009 (UTC)
- Q: What do you call the guy who cheated his way through medical school? A: "ɹoʇɔop". --Sean 15:16, 30 November 2009 (UTC)
- Have you considered that a person can have more then 1 disease at the same time? Googlemeister (talk) 16:28, 30 November 2009 (UTC)
- www.webmd.com has a symptom checker that you can put in your list of symptoms and it will spit back a list of possible diseases. So your list runs the gammut of the common cold to Cryptococcosis, a fungus that grows on pidgeon droppings! Your professor's comment about a zebra was probably the old doctors adage: "When you hear hoofbeats, think horses not zebras." Which means if you see this list of symptoms, don't jump on the most complicated because you are probably wrong (we have an article on it [6]. Livewireo (talk) 16:51, 30 November 2009 (UTC)
- Have you considered that a person can have more then 1 disease at the same time? Googlemeister (talk) 16:28, 30 November 2009 (UTC)
- Sounds like an episode of House to me. Obviously you need to put the patient in an MRI scanner to suck the iron out of their tattoo ink while your coworkers break into their house and locate their stash. Meanwhile your prof can practice his yoyo or spin a plate on the end of his walking stick. And remember - (a) it's never lupus and (b) the patient's prognosis is favourable unless it's the end of series finale ! Gandalf61 (talk) 17:05, 30 November 2009 (UTC)
Di-t-boc
It seems kind of like bad atom economy to make it symmetrical -- why not use a halide leaving group in the presence of some base? John Riemann Soong (talk) 02:32, 30 November 2009 (UTC)
- If you think of Di-tert-butyl dicarbonate the problem is that the substitution of one t-Bu-O by one Cl leads to a unstable molecule. The substitution of the t-Bu-O by CH3 makes the molecule inreactive. The diester of carbonic acid just in the midle. It is not especially green, but this word is of no specially interest in the science lab and the industry would avoid a that expensive protection group.--Stone (talk) 10:56, 30 November 2009 (UTC)
- Wait it's an anhydride-type species, right? Putting a halide has a stronger activating effect than anhydride, but don't we usually use non-aqueous solvents when using t-boc? (I mean, aqueous solvents will hydrolyse t-boc too...). Or is it just cheaper to produce the monomer and dimerise it with some P2O5? John Riemann Soong (talk) 17:31, 30 November 2009 (UTC)
1,3 carbon overlap in carbon backbones
I was thinking about space-filling models when I realised what's been bothering me (in terms of counterintuition) though I didn't realise it till now -- in the space-filling model there appears to be possible overlap between atoms that aren't directly bonded to each other! Is this related to inductive effects or hyperconjugation? What about polarisability or if you have a big fat iodide atom on your chain?
Take the space-filling model for cyclohexane
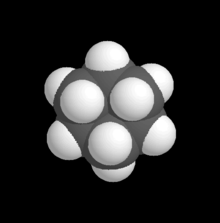
-- it appears that the six carbon atoms have some degree of overlap with each other, even if they aren't directly bonded to each other! And I've always wondered about carbon-halide bonds on rings -- with skeletal diagrams the issue seems well-concealed but sterics appears to be quite an issue! John Riemann Soong (talk) 04:13, 30 November 2009 (UTC)
- Space-filling models are just that: models. The probability density functions for the electrons do not have sharp borders, rather they have nonzero probability that extends outward indefinitely. Proximity will result in complex interactions that simple space-filling models won't capture. So, yes there is overlap in probability density among all of the atoms in a molecule. Maybe I miss your point. -- Scray (talk) 05:21, 30 November 2009 (UTC)
- (EC with below) Furthermore, space filling models are based on the covalent radius of atoms; that is the average bonding distance between atoms in a same atom bond, i.e. a C-C bond or a H-H bond. This distance isn't really related to the actual size or distribution of molecular orbitals or atomic orbitals in terms of their wave functions/probability distributions. That is, space filling models don't really represent what the molecular electron cloud will look like; if the C atoms appear to meet in the middle of the cyclohexane space-filling model, it does NOT mean that the actual electron distribution will cause "orbital overlap" (or if you prefer "molecular orbitals") between those atoms. As noted by Scray, space-filling models are models, useful for judging the overall size and shape of a molecule, but not for making any greater statements about bonding in the molecule. --Jayron32 05:30, 30 November 2009 (UTC)
- Yes, but what would you call overlap between C-R-C bonds? (95% density cutoff) ... induction? Hyperconjugation? John Riemann Soong (talk) 05:26, 30 November 2009 (UTC)
- Nothing. "A bond is at this place I draw the line or cylinder" is totally a fiction we use to explain certain things about molecules. There are not two real entities that exist and interact the way two physical objects do, because they are just probability regions. And they are regions that are part of the same molecule, so they are derived from the same functions. For example, even simple MO explanations say that there is one orbital that includes the whole A–B–C (the whole σ plane) and a second that includes "both σ bonds but not the central atom" rather than just two distinct A–B and B–C bonds. A and C certainly interact, both via sterics and electronics, depending on (as usual) their sizes and electron densities. DMacks (talk) 17:12, 30 November 2009 (UTC)
- Ok other than some elementary photochemistry and three-membered molecules I haven't really done "MO from scratch" since they pretty much abandoned that approach in my education in favour of hybridisation ... say you have a straight-chain hydrocarbon like hexane. Is there really a sigma-symmetry orbital that overlaps all six carbons (and maybe hydrogens)? So now let's put a halogen on it and let an alcohol attack by SN2... how would a nucleophile distort that orbital? Would it? John Riemann Soong (talk) 17:28, 30 November 2009 (UTC)
- Also, when you're doing something like retro-aldol cleavage ... what are you doing to those "delocalised" sigma bonds? My first intuition is that they would be some of the lowest orbitals in the molecule, but I know C-C bonds immediately formed by aldol addition (before condensation) are relatively weak. (Since there are EDG and EWG effects, are there pi interactions too?) John Riemann Soong (talk) 17:38, 30 November 2009 (UTC)
- To answer the first question, even MO theory and hybridization theory are models. As Dmacks noted, the reality is that electrons are fully mobile and non-localizable; that means that while, on average, a bond may be able to be modeled as two electrons shared between two atoms, in reality all 48 electrons in the molecule pretty much move wherever they want, randomly. Any "molecular orbitals" or "overlap between hybridized orbitals" is a model used to explain the probability distribution of the electrons in the molecule as a whole. Some models more accurately represent certain aspects of molecular shape, electron distribution, size, relative angles, etc. etc. than other models. The best models to describe the location of all electrons in a molecule would be a quantum mechanical based mathematical model, likely a Schrödinger equation. Of course, with models like this, the more accurate they become, the less intuitive and useful they are for the average user. --Jayron32 18:15, 30 November 2009 (UTC)
- Well yes, but when a radical say, extracts electrons from a bond -- those delocalised electrons must all of a sudden "localise" so they can leave? Do electrons routinely exchange between orbitals kinda like protons get exchanged in hydrogen bonding? John Riemann Soong (talk) 18:48, 30 November 2009 (UTC)
- See activated complex. When electrons "tranfer" between two molecules, they do so via a transition state called an "activated complex", which is basically a short-lived single molecule consisting of a merged complex of the two (formerly seperate) molecules. The rules for electron localizability in this activated complex are no different than for any other molecule. No single electron is transfered in a single act. The two molecules come together to form one molecule, and then seperate again; or if they stay together (such as in a Lewis acid-base reaction or in the radical bond formation you note), it doesn't matter. Also, remember that real atomic orbitals only exist in lone atoms. Once you make a molecule, you end up with molecular orbitals. There still is no need for "localization" in any of these processes. Do two drops of water "localize" their molecules when they combine to form one drop? --Jayron32 20:40, 30 November 2009 (UTC)
- Well yes, but when a radical say, extracts electrons from a bond -- those delocalised electrons must all of a sudden "localise" so they can leave? Do electrons routinely exchange between orbitals kinda like protons get exchanged in hydrogen bonding? John Riemann Soong (talk) 18:48, 30 November 2009 (UTC)
- To answer the first question, even MO theory and hybridization theory are models. As Dmacks noted, the reality is that electrons are fully mobile and non-localizable; that means that while, on average, a bond may be able to be modeled as two electrons shared between two atoms, in reality all 48 electrons in the molecule pretty much move wherever they want, randomly. Any "molecular orbitals" or "overlap between hybridized orbitals" is a model used to explain the probability distribution of the electrons in the molecule as a whole. Some models more accurately represent certain aspects of molecular shape, electron distribution, size, relative angles, etc. etc. than other models. The best models to describe the location of all electrons in a molecule would be a quantum mechanical based mathematical model, likely a Schrödinger equation. Of course, with models like this, the more accurate they become, the less intuitive and useful they are for the average user. --Jayron32 18:15, 30 November 2009 (UTC)
- Nothing. "A bond is at this place I draw the line or cylinder" is totally a fiction we use to explain certain things about molecules. There are not two real entities that exist and interact the way two physical objects do, because they are just probability regions. And they are regions that are part of the same molecule, so they are derived from the same functions. For example, even simple MO explanations say that there is one orbital that includes the whole A–B–C (the whole σ plane) and a second that includes "both σ bonds but not the central atom" rather than just two distinct A–B and B–C bonds. A and C certainly interact, both via sterics and electronics, depending on (as usual) their sizes and electron densities. DMacks (talk) 17:12, 30 November 2009 (UTC)
- Yes, but what would you call overlap between C-R-C bonds? (95% density cutoff) ... induction? Hyperconjugation? John Riemann Soong (talk) 05:26, 30 November 2009 (UTC)
- Elaborating a bit on what Jaryon said: Every set of possible nuclear coordinates has a different electronic ground state, so you have a potential energy surface for your electronic ground state in terms of nuclear coordinates. The local minima on that surface define stable compounds and the saddle-points between those valleys are the (optimal) transition-states. The motion of the nuclei is so slow compared to that of the electrons that you can regard them as instantly adjusting their energy/motion to any change of nuclear coordinates (Born-Oppenheimer approximation). You have a smooth transition from one electronic wave function to another. Transition states are tricky beasts that can't really be modeled in a simple way. The transition-state between two compounds is not typically at the halfway point in terms of coordinates, nor at the halfway point in terms of where the electrons are. E.g. I recently looked at one where the electrons were about 70% of where they'd be in the 'product' although the nuclei had only moved 20% of the way. So that's the fundamental outlook you need to have: Abandon the idea of discrete numbers of electrons in definite locations. A radical with an unpaired electron 'on' a certain atom only means it's mostly on that atom. Oxidation states are never actually integers. And so on) --Pykk (talk) 07:52, 1 December 2009 (UTC)
- Minor quibble: The "consider electrons moving nearly instantaneously on a framework of fixed nuclear positions" is more the Franck–Condon principle than Born-Oppenheimer. DMacks (talk) 08:24, 1 December 2009 (UTC)
- Elaborating a bit on what Jaryon said: Every set of possible nuclear coordinates has a different electronic ground state, so you have a potential energy surface for your electronic ground state in terms of nuclear coordinates. The local minima on that surface define stable compounds and the saddle-points between those valleys are the (optimal) transition-states. The motion of the nuclei is so slow compared to that of the electrons that you can regard them as instantly adjusting their energy/motion to any change of nuclear coordinates (Born-Oppenheimer approximation). You have a smooth transition from one electronic wave function to another. Transition states are tricky beasts that can't really be modeled in a simple way. The transition-state between two compounds is not typically at the halfway point in terms of coordinates, nor at the halfway point in terms of where the electrons are. E.g. I recently looked at one where the electrons were about 70% of where they'd be in the 'product' although the nuclei had only moved 20% of the way. So that's the fundamental outlook you need to have: Abandon the idea of discrete numbers of electrons in definite locations. A radical with an unpaired electron 'on' a certain atom only means it's mostly on that atom. Oxidation states are never actually integers. And so on) --Pykk (talk) 07:52, 1 December 2009 (UTC)
Map of the Moon
If we have satellites to map accurately every point of the surface of the Earth, so that you can even see individual lampposts on Google Earth, how come we haven't got that kind of resolution on lunar maps? —Preceding unsigned comment added by 213.229.148.222 (talk) 08:33, 30 November 2009 (UTC)
- Because we don't have a cloud of equally sophisticated satellites orbiting the moon (or any satellites for that matter). 218.25.32.210 (talk) 09:45, 30 November 2009 (UTC)
Google Earth is most likely Aerial photography at the resolutions where you can see lamp posts and cars and similar, and satalite data is only used for high level low resolution photos. Non Military satellite imagery from companies in the US are only allowed to have a resolution of 1 pixel to 0.5m (See Satellite imagery) Gunrun (talk) 10:27, 30 November 2009 (UTC)
The Mars Reconnaissance Orbiter has the camera you need and takes pictures of mars for three years now and you are far from a coverage you whant to have. The moon was uninteresting from 1972 till late 1990s and neither Clementine nor the following missions had a camera of that quality. For the maned landing they will run the camera of the Lunar Reconnaissance Orbiter for several years and they will only get coverage of the most important places.--Stone (talk) 11:02, 30 November 2009 (UTC)
- Indeed. MRO has captured images of the Phoenix lander on the surface of Mars, as well as the smaller Opportunity rover and Spirit rover, which are only about 2m across. LRO has imaged the descent stages of several of the Apollo Lunar Modules on the surface of the Moon (4m wide) and can resolve features that are 3m wide. Gandalf61 (talk) 15:13, 30 November 2009 (UTC)
Blooming of flowers.
Which chemical compound makes the flowers bloom vigourously?Is there any way to make flowers bloom in such a way? —Preceding unsigned comment added by 113.199.164.42 (talk) 11:56, 30 November 2009 (UTC)
- For some, ethylene gas might work. DRosenbach (Talk | Contribs) 13:03, 30 November 2009 (UTC)
- However, ethylene terminates the blooming of orchids (flowers wilt and drop off). On the other hand, 6-Benzylaminopurine (BAP) induces flowering in orchids [7] and possibly in other plants. See also Gibberellin --Dr Dima (talk) 03:13, 1 December 2009 (UTC)
- Florigen may be of interest, although it's not a great article. However it does cover the gist although perhaps a bit out of date i.e. we still aren't sure what substance/s induce flowering and it's one of the mysterious that interests quite a few plant biologists and there have been some recent major breakthroughs. The article also does mention that this is something that's been going back and forth, e.g. first people got all excited about the idea of a flowering hormone then after fruitless searches and some research people began to think that perhaps there wasn't a flowering hormone (i.e. florigen) after all but just a specific combination of hormones that induced flowering but that had problems too and recent evidence suggests we may have finally found the florigen. Also the name is fairly unique so you can find lots of likely useful articles by searching e.g. [8] [9] [10] Nil Einne (talk) 11:45, 1 December 2009 (UTC)
- However, ethylene terminates the blooming of orchids (flowers wilt and drop off). On the other hand, 6-Benzylaminopurine (BAP) induces flowering in orchids [7] and possibly in other plants. See also Gibberellin --Dr Dima (talk) 03:13, 1 December 2009 (UTC)
Antidepressants losing effectiveness over a decade.
If a person took the antidepressant bupropion for over a decade or two, would this lose its effectiveness of the subject becomes too used to it? --Reticuli88 (talk) 15:01, 30 November 2009 (UTC)
- In the abstract (i.e. in general) this phenomenon apparently does occur PMID 9671339, though some dispute its frequency PMID 17854252. If you're asking about an individual case (you're question is worded generally, so I'm assuming that you are not), that person should ask their doctor. -- Scray (talk) 15:31, 30 November 2009 (UTC)
- Most antidepressants are actually placebos, and only work if you believe in them [11]. So over time if you occasionally felt depressed anyway, you might start to loose faith in the drug, and then it won't work anymore. I hope that knowing this does not cause you medical problems :( I hope you are posting this on behalf of someone else, and do not tell them. Also, I should clarify: the drug does not have zero effect, it has a small one, but the majority of the effect is the placebo. Read the actual studies on it (and others) if you want the details. Ariel. (talk) 19:05, 30 November 2009 (UTC)
- Your statement is wrong. No approved antidepressant is a placebo, as they are all biochemically active compounds. The article is saying that some antidepressants appear to be no more effective than the placebo effect in the treatment of depression, which is not the same thing as saying that the drug is a placebo. Just because a compound is (or becomes) ineffective at treating its intended ailment does not mean it is inert. It could still have other effects (both positive and negative). Dragons flight (talk) 19:19, 30 November 2009 (UTC)
- Not that what is used as placebo in medical research is always inert[12]. Unomi (talk) 19:49, 30 November 2009 (UTC)
- Your statement is wrong. No approved antidepressant is a placebo, as they are all biochemically active compounds. The article is saying that some antidepressants appear to be no more effective than the placebo effect in the treatment of depression, which is not the same thing as saying that the drug is a placebo. Just because a compound is (or becomes) ineffective at treating its intended ailment does not mean it is inert. It could still have other effects (both positive and negative). Dragons flight (talk) 19:19, 30 November 2009 (UTC)
Looking for planets visibility
I would like to know what planets are visible to the unaided eye, and when they would be visible at 42 deg N for the night of April 17-18, 2010 At timezone -6 GMT. Googlemeister (talk) 19:52, 30 November 2009 (UTC)
- You can use the sky chart at Heavens Above to generate a view for any arbitrary time. Note that time zone doesn't matter for stellar/planetary observations, and the observer's latitude isn't a major concern for viewing the planets. It appears that Mercury and Venus will both be in the western sky near sunset, with a crescent moon slightly behind. Saturn and Mars should be overhead for much of the night. Jupiter may be visible pre-dawn. — Lomn 20:10, 30 November 2009 (UTC)
- And those are the 5 naked eye planets - Mercury, Venus, Mars, Jupiter and Saturn. Uranus is sometimes visible under excellent conditions if you know just where to look, but generally speaking you need binoculars for it. --Tango (talk) 21:53, 30 November 2009 (UTC)
- I have never had much luck with Mercury, but then it is only visible for a very short time right? Googlemeister (talk) 22:00, 30 November 2009 (UTC)
- Yep - it's orbitting so close to the sun that it rises and sets quite close to sunrise/sunset. That gives you only a small window of time - and since it's so close to the horizon, you're looking through a lot of atmosphere - which may still be scattering sunlight from the sun that's only just below the horizon. SteveBaker (talk) 03:39, 1 December 2009 (UTC)
- Is Mars the easiest to see with the naked eye? I seem to think I'm seeing Mars pretty often. Bus stop (talk) 03:44, 1 December 2009 (UTC)
- At peak brightness, Venus and Jupiter are the two brightest planets. "Easiest" may be subjective, though. — Lomn 03:50, 1 December 2009 (UTC)
- OK. Thanks. Bus stop (talk) 04:12, 1 December 2009 (UTC)
- Venus and Jupiter are very bright, but Mars is quite bright too at its brightest, and certainly stands out in the sky. Also, it really is red and therefore easy to identify.--Rallette (talk) 07:54, 1 December 2009 (UTC)
- OK. Thanks. Bus stop (talk) 04:12, 1 December 2009 (UTC)
- I would say that Venus and Jupiter are easier to see than Mars, the problem is that they look rather like stars to the untrained eye. Mars is clearly Mars because it is red. Venus and Jupiter can only really be distinguished from stars by the fact that they are brighter than any star (they also twinkle less due to having a larger angular diameter and are always found on the ecliptic). --Tango (talk) 12:28, 1 December 2009 (UTC)
- I would imagine that at certain times, Mars is brighter then both Jupiter and Venus depending on the positions of the planets, but it is rather rare for that to occur. Googlemeister (talk) 14:55, 1 December 2009 (UTC)
- Well, yes - because Venus (and Mercury) are closer to the Sun than the Earth, they show 'phases' rather like the moon. When Venus is directly between the earth and the sun, we're looking at the dark side and it will be essentially invisible - when Venus is on the far side of the sun, it's a lot further away - but now we're looking at the bright, sunny side - so it's pretty bright. The trouble is that Venus is at it's brightest when it's furthest away and when it's so close to the sun that we can't see it in daylight and sets too soon to be visible at night. The best time to see it is when it's about half-lit and off to the side of the sun. Mars is a different matter though. Being further out than the Earth, it's brightest when it's on the opposite side of the Earth from the Sun - and that's also when it's closest and when you have the darkest sky to see it against. So there are certainly times when Mars is brighter than Venus. SteveBaker (talk) 16:06, 1 December 2009 (UTC)
- I would imagine that at certain times, Mars is brighter then both Jupiter and Venus depending on the positions of the planets, but it is rather rare for that to occur. Googlemeister (talk) 14:55, 1 December 2009 (UTC)
- At peak brightness, Venus and Jupiter are the two brightest planets. "Easiest" may be subjective, though. — Lomn 03:50, 1 December 2009 (UTC)
- Is Mars the easiest to see with the naked eye? I seem to think I'm seeing Mars pretty often. Bus stop (talk) 03:44, 1 December 2009 (UTC)
- Yep - it's orbitting so close to the sun that it rises and sets quite close to sunrise/sunset. That gives you only a small window of time - and since it's so close to the horizon, you're looking through a lot of atmosphere - which may still be scattering sunlight from the sun that's only just below the horizon. SteveBaker (talk) 03:39, 1 December 2009 (UTC)
- I have never had much luck with Mercury, but then it is only visible for a very short time right? Googlemeister (talk) 22:00, 30 November 2009 (UTC)
- Also try YourSky, and enter your latitude, longitude, and the date and time in UTC. ~AH1(TCU) 01:26, 2 December 2009 (UTC)
- Currently, Jupiter is visible on evenings, Mars is visible later at night and appears reddish, and Venus and Saturn are visible late night to morning. On December 3, the disc of Mars will exceed ten arcseconds. The Moon will be directly above Mercury on December 18, which looks like a relatively faint object, but should rise for long enough to see; I've been successful at viewing Mercury through a telescope with its cresent. On December 20, between 7:33 and 9:08 pm, Jupiter will have a double shadow transit of two of its moons, while on that night Jupiter will be closest to Neptune in the field of view of a telescope. Uranus is located close to Jupiter, in Pices. ~AH1(TCU) 01:33, 2 December 2009 (UTC)
- And those are the 5 naked eye planets - Mercury, Venus, Mars, Jupiter and Saturn. Uranus is sometimes visible under excellent conditions if you know just where to look, but generally speaking you need binoculars for it. --Tango (talk) 21:53, 30 November 2009 (UTC)
Ray-tracing for sound not light
Would the principles of and algorithmns for ray-tracing work with sound as well as with light? Rather than creating an image, instead I would like to make a contour map of the sound intensity. 89.242.99.245 (talk) 22:35, 30 November 2009 (UTC)
- Sure. That's how Medical ultrasonography works. 169.139.217.77 (talk) 23:19, 30 November 2009 (UTC)
- Sort of. But medical ultrasonography works with wavelengths that are roughly 1000 times smaller than typical audible wavelengths, so it can deal with objects that are about 1000 times smaller before interference and diffraction become a consideration. Red Act (talk) 00:10, 1 December 2009 (UTC)
- Yes and no. They do use ray tracing for acoustics sometimes; see Ray tracing (physics)#Ocean acoustics. However, what might be a problem is that depending on the sounds and the environment involved, the wavelengths involved might not be much smaller than the sizes of the objects involved, in which case interference and diffraction would no longer be negligible effects. That's problematic because normal ray tracing algorithms don't model interference and diffraction. But that might not be an important issue if you're only dealing with high enough sound frequencies and/or large enough objects. Red Act (talk) 00:10, 1 December 2009 (UTC)
- We use acoustic ray tracing all the time in seismic imaging. Here is an overview to the method as adapted for reflection seismology. Nimur (talk) 01:12, 1 December 2009 (UTC)
- There are some tricky problems - for example, our eyes only see three frequencies of light (Red, Green, Blue) - so you can do a very good approximation for almost any frequency-dependent effect by simply tracing monochromatic red, green and blue rays separately. But for audio, our ears are very sensitive to frequency and we can distinguish very complex mixtures of them...that trick isn't going to work for sound. Also, it takes an insanely small gap to cause diffraction in light - to the extent that we can basically just ignore it. But audio diffracts as it passes through a doorway! On the plus side - the resolution of audio is so much lower than light that we don't need to trace even a tiny fraction of the number of 'rays' - if you want the same kind of calculation times - you can lavish much more CPU time on your audio rays. SteveBaker (talk) 03:36, 1 December 2009 (UTC)
- Wait, wait, wait. "our eyes only see three frequencies of light (Red, Green, Blue)"? That isn't true. Our eyes perceive different frequencies as combinations of Red, Green and Blue, but it isn't true that we only see those frequencies. We can see yellow light: we see it by perceiving it as a combination of red and green, but we still see it. I know you know loads about this, so I assume you're making some other point Steve. Could you explain a bit more? 86.166.148.95 (talk) 19:39, 1 December 2009 (UTC)
- Yes, I think Steve has made a mistake there. As astounding as it may sound, it does happen! --Tango (talk) 20:21, 1 December 2009 (UTC)
- Well, yes and no. In the context of Ray Tracing - we are going to display the resulting image on a display that very literally only displays red, green and blue light. Our eyes can't tell the difference between that and a full spectrum of frequencies. But yes - I spoke a little loosely. We have only three colors of 'sensor' in our eyes - we can't tell the difference between pure yellow light (such as you get from a sodium vapor lamp) and an appropriate mixture of pure red and pure green light - they look completely identical to our poor eyes. On the other hand - if we take the audio analogy if you play a 'D' as a pure sine-wave - it sounds very different from a chord containing a 'C' plus an 'E'. The only case where we can tell the difference between a pure color and an "optical chord" is with magenta (red+blue) - which looks a lot different from green. SteveBaker (talk) 00:45, 2 December 2009 (UTC)
- Yes, I think Steve has made a mistake there. As astounding as it may sound, it does happen! --Tango (talk) 20:21, 1 December 2009 (UTC)
- Wait, wait, wait. "our eyes only see three frequencies of light (Red, Green, Blue)"? That isn't true. Our eyes perceive different frequencies as combinations of Red, Green and Blue, but it isn't true that we only see those frequencies. We can see yellow light: we see it by perceiving it as a combination of red and green, but we still see it. I know you know loads about this, so I assume you're making some other point Steve. Could you explain a bit more? 86.166.148.95 (talk) 19:39, 1 December 2009 (UTC)
premolut n
Plz provide detail about drug premolut n —Preceding unsigned comment added by 117.254.28.61 (talk) 23:16, 30 November 2009 (UTC)
- This is a drug involved with the timing of menstruation. On wikipedia, we don't provide medical advice. You can get more information by contacting a physician or other medical provider or simply searching it on the internet. Tyrol5 [Talk] 23:21, 30 November 2009 (UTC)
- Premolut N is a brand name for norethisterone, on which we do at least have a stub article. I just now created a redirect. Red Act (talk) 00:21, 1 December 2009 (UTC)
December 1
Zygotes implanted in the wombs of other species
Has this ever been done in controlled experiments? I wonder how close the species have to be (if anything less than exact species matches can) for the fetus and mother to be able to create a compatible placental link without the mother's body seeing it as a foreign substance and killing it. 71.161.61.41 (talk) 01:06, 1 December 2009 (UTC)
- I don't think the mothers body would see it as foreign. The problems are more likely in other areas. For example, a newborn has a special kind of hemoglobin which has a higher affinity for oxygen than the mother (so it can pull oxygen out of the mothers hemoglobin. So that has to match in a different species. The nutrition requirements must match, and the physical size of course. Possibly the most difficult is getting the placenta (which has the dna of the child) to embed in the mother. I have a vague memory of hearing about an extinct (or close to it) species being cloned, then embedded in a different, but similar, species (but I don't know if they did it, or just planned to). All that said, this is a very interesting question, and I don't know the answer. But I figured I'd post what I knew, since you didn't have any other replies. Ariel. (talk) 02:07, 1 December 2009 (UTC)
- That would be the woolly mammoth in an elephant, I believe. 90.195.179.130 (talk) 06:37, 1 December 2009 (UTC)
- Doesn't the blood get exchanged? Won't the blood cells be identified as foreign? 66.65.141.221 (talk) 03:47, 1 December 2009 (UTC)
- No, unless there is a fetomaternal hemorrhage, the blood of the fetus and the mother do not mix. See Placenta#Fetoplacental_circulation. --NorwegianBlue talk 22:54, 1 December 2009 (UTC)
- I'm pretty sure it would get rejected. Just look at how difficult it is to transplant organs between different humans. Or the fact that humans can form antigens even against their own fetuses. (e.g. Rhesus factor) Add to that different gestation periods and easily thousands of different factors that have to match up, most of which are probably unknown to us. I don't believe it's been attempted and I think it would be outright unethical to even attempt such a bizarre thing, given that there's zero probability of success. You're talking about literally millions of foreign proteins and compounds, every single one of which could possibly trigger an immune system response in the host/mother. --Pykk (talk) 06:45, 1 December 2009 (UTC)
- As our article on Cloning#Cloning extinct and endangered species briefly mentions, it has been attempted before, obviously only in related species; with the zygote species usually being endangered. It obviously often comes up when discussing cloning extinct mammal species (for obvious reasons) and cloning or IVF reproduction of endangered mammal species (using mothers of the endangered species may be a bit risky and limiting). For example, this [13] shows some research in 1988 i.e. long before Dolly; this shows a horse mare and it's zebra surrogate child [14] [15]; and in fact this ref says the first case was in the 1970s [16]. Obviously for some of this stuff e.g. zebra/horse it's not that surprising it works since zebra/horse hybrids are possible; in the surrogate case the offspring does not have any genetic complement from the parent species unlike hybrids which have half, but on the other hand it doesn't have to cope with any problems arising from a mismatch (perhaps not the best word but can't think of anything else) of genes. I believe most research/interest now has been for cloning, probably because IVF likely still has some significant risk for the mother and because cloning is the hot thing nowadays. Or perhaps there's a fair amount of research ongoing with cases of non-cloned animals it's just harder to find refs since it isn't so 'hot'. [17] discusses the case of the Pyrenean Ibex (also mentioned there) which has so far been unsuccessful. There's also been discussion about panda cloning [18] and as that mentions and discussed here [19] there was some success with implanting panda embroyos in rabbits, obviously not with the intention of the rabbits carrying the pandas to term but to show implantation is possible (I believe using rabbits as temporary hosts is common so using them to attempt implantion would follow on). Obviously a better surrogate species would be necessary, what I'm not sure but presemuably some kind of bear. This perhaps raises one of the other issues. If you're using a cow, horse, sheep, goat, pig, etc as a surrogate that's easy since they're domestic animals and you can get large numbers. On the other hand if you're using a lion as surrogate for a tiger you may not be making things much easier then just using tiger surrogates while creating additional problems. Anyway although the last ref on pandas is from 2002, there doesn't seem to have been any significant new news I can find perhaps it was abandonded due to opposition [20] or was simply unsuccessful. There's also [21] which could be of interest altho I'm not sure if it discusses anything not in the other refs/our article and this [22] for a more scientific viewpoint. Interspecies surrogacy appears to be the common term but we have no article and while a useful search term you also get stuff like mother cats looking after puppies. Nil Einne (talk) 10:16, 1 December 2009 (UTC)
Gene Therapy and Hormones
In theory, could gene therapy ever be used to replace or increase the estrogen and progesterone hormones, even if a woman's ovaries have been removed? —Preceding unsigned comment added by 71.156.167.121 (talk) 02:28, 1 December 2009 (UTC)
- Gene therapy is in its very early infancy, so what all will ultimately be possible with it is really quite speculative at this point. So I think the sentence at the top of this page may apply here: "The reference desk does not answer requests for opinions or predictions about future events." Red Act (talk) 10:20, 1 December 2009 (UTC)
- Perhaps your understanding of gene therapy is a bit misguided. All cells of the body have the theoretical potential to express all genes and produce all proteins. That being said, certain limitations exist for the vast, vast majority of cells in an organism such that they differentiate and lose totipotency/pleuropotency. Because all cells still retain the DNA to make all proteins, though, gene therapy, which works to supplant missing DNA (as in the case of a hereditary, genetic disorder) won't really accomplish what you are asking it to do. What your case would need is a de-differentiation/redifferentiation process by which another cell type would be able to produce ovarian hormones -- something that might work with a cloning-like procedure but is likely not happening anytime soon. DRosenbach (Talk | Contribs) 13:19, 1 December 2009 (UTC)
- I have to disagree. There's no reason to think that, "in theory", gene therapy could not accomplish the production of estrogen and progesterone from a non-ovarian tissue. Gene therapy isn't about de-differentiating a cell type but rather adding back a normal copy of a mutant gene or adding some exogenous gene that will help to correct a disease (in this case, lack of ovaries). Each application of gene therapy is going to have a different target cell type and different approach. There may be some cases in which a small patch of muscle tissue (or other tissue) could produce enough of a certain gene product to reverse the symptoms of a disease. However, other disorders would require replacing the defective gene in a large proportion of the body's cells (which we currently can't do). The problem with the OP's question is that the gene therapy we have now would be like using a sledgehammer to try to cut the facets of a gemstone. It requires some really fancy tricks to mimic the endogenous switching between estrogen production and progesterone production in a fashion similar to the female menstrual cycle. Ideally, you would want your transgene to be under the control of the endogenous gonadotropins released by the pituitary gland, FSH and LH, which would be even more complicated. The answer to the question is that yes, it could probably be achieved using gene therapy, but in reality it seems unlikely that this type of therapy would ever be developed given the already existing (and relatively inexpensive) hormone replacement therapy or combined oral contraceptive pills. --- Medical geneticist (talk) 14:19, 1 December 2009 (UTC)
- Perhaps your understanding of gene therapy is a bit misguided. All cells of the body have the theoretical potential to express all genes and produce all proteins. That being said, certain limitations exist for the vast, vast majority of cells in an organism such that they differentiate and lose totipotency/pleuropotency. Because all cells still retain the DNA to make all proteins, though, gene therapy, which works to supplant missing DNA (as in the case of a hereditary, genetic disorder) won't really accomplish what you are asking it to do. What your case would need is a de-differentiation/redifferentiation process by which another cell type would be able to produce ovarian hormones -- something that might work with a cloning-like procedure but is likely not happening anytime soon. DRosenbach (Talk | Contribs) 13:19, 1 December 2009 (UTC)
Air bubles in tap water
In modern tap water we see a lot of air bubbles, from where it comes and when we put our hands the splashing is very less compared to old taps?203.199.205.25 (talk) 09:31, 1 December 2009 (UTC)
- The bubbles are there due to the faucet aerator, which not only reduces splashing, but also saves water. Unfortunately, the little stub article explains that the aerator breaks the stream into little droplets, but doesn't explain why that would help reduce splashing compared to a laminar flow of water. Red Act (talk) 09:50, 1 December 2009 (UTC)
Is everything energy?
As the subject line says. Is all matter made ultimately of energy? (Please excuse me for posting this. I should know the answer but I'm full of cold and my head is made of cotton wool.) --TammyMoet (talk) 10:16, 1 December 2009 (UTC)
- That's basically correct. The mass of a body of matter is a measure of its energy content. See Mass–energy equivalence. Red Act (talk) 10:28, 1 December 2009 (UTC)
- Thanks Red Act, I knew we had an article on it somewhere! --TammyMoet (talk) 11:45, 1 December 2009 (UTC)
- Red Act is correct to point to mass-energy equivalence, but that does not mean that "all matter is made ultimately of energy". Energy is just our label for the quantity that is conserved in the conservation of energy law. Mass-energy equivalence establishes an "exchange rate" for converting between mass and energy. It means that conservation of energy and conservation of mass, which had origimated as two separate conservation laws, are now merged into a single conservation law. But the conversion does not have a preferred direction - we could just as easily say that the energy content of a body is a measure of its mass. From this point of view, we could, for example, assign a relativistic mass to photons, and add up the relativistic masses of colliding particles instead of adding their energies. But this is just a change of viewpoint - it does not mean that all energy is made ultimately of matter. There are several other conservation laws, and each of them gives us some information about which physical processes are possible and which ones are impossible (although when you introduce quantum mechanics, things are not as black and white as this, and possible/impossible becomes probable/improbable). But none of these conservation laws tell us anything conclusive about the ultimate constituents of matter. Gandalf61 (talk) 13:00, 1 December 2009 (UTC)
- Mass-energy equivalence is one of the fundemental threads that actually shows up, in one form or another, in all of modern theoretical physics. It isn't that matter is a form of energy, or that energy is a form of matter. Its that the two are the same exact thing, but being observed under different conditions or in different environments. The same basic principle is at work in wave-particle duality, that is all of the universe can be said to behave in a wave-like (energy-like) manner, or as particles (matter-like) manner. That is, everything is both an object (particle) and a wave (energy), its not so much that anything "chooses" one form or another, its that the method of observation of something determines which properties of that thing you actually see; some methods of observation lead to wave-like properties being highlighted, while others lead to particle-like properties (see Double-slit experiment for a discussion of the principle at work in electrons and photons). For really big things, we only have observation methods that highlight the object-like nature of them, but when you get smaller and smaller, you can arrive at more and more wave-like properties comeing out. See also DeBroglie wavelength for the theoretical wave-particle duality in large objects, Schrödinger equation for the complex mathematics of wave functions of matter (there is even a possible Schrödinger equation which describes the entire universe as a single wave function), and Copenhagen interpretation for some of the philosophical implications of mass-energy equivalence. --Jayron32 20:57, 1 December 2009 (UTC)
- Actually no. What it means is that mass and energy are the same thing! They are two words that mean the same thing. There is no test that can distinguish them, and no definition that discriminates between them. They are not interconvertable, they are the same. Note that "matter" and energy are not the same. But "mass" and energy are. Matter is energy plus quantum numbers, like charge or boson, spin, etc. And don't get confused by concepts like a photon being massless - it's actually that it has no rest mass, not that it's massless. All that said, I think the convention is that energy that is tightly bound in matter is called "mass". So the binding energy of an atom is called mass, but the heat, and velocity of an atom, it called energy, even though both contribute to it's gravity, and weight, etc. Heat is easy to remove from an atom, the binding energy is not. And to answer the question in the title, everything is not energy, because there are quantum numbers that are conserved, which also make up the universe. Ariel. (talk) 21:14, 1 December 2009 (UTC)
- Regarding your last sentence, it depends on what the meaning of the word 'is' is. I would take the question "is everything energy?" to mean something like "within a closed region of flat spacetime, does what is contained within that region differ from a vacuum if and only if the total energy within that region differs from the total energy of a vacuum?". The answer to that question is "yes". You're taking the question "is everything energy" to mean something like "within a closed region of flat spacetime, is total energy the only conserved property of what's within that region?". The answer to that question is indeed "no", but I don't think that was the intent of the original question. Red Act (talk) 23:34, 1 December 2009 (UTC)
- Regarding quantum numbers; don't quantum numbers all sum to zero when added across the whole universe anyways (i.e., the universe is neutral?). So there is nothing "extra" there that needs to be conserved once you have taken account of the energy/mass. Yes, they are conserved, but only to maintain the neutrality of the universe; there's nothing "left over" once you factor out the energy/mass! If so, then the singularity at the big bang would have had a net charge, and that doesn't make much sense... --Jayron32 00:41, 2 December 2009 (UTC)
- Jayron32: That's the whole question of baryogenesis. Red Act: Your first question is also not what the op asked. You are basically saying there can be no quantum numbers unless there is also energy. A fine statement (is that really what you meant to say though?), but not what the op asked. The op asked is there anything else besides energy in the universe. And the answer is no, there are also other conserved quantities (as Gandalf61 said). In truth we are arguing over words, because the op's question is not well defined. But hopefully reading this will give him the answer anyway. And BTW, your answer to him was correct, I was arguing with Gandalf61 who implied that mass and energy were different, but they are not. They are not simply interchangeable - they are the same. Ariel. (talk) 01:36, 2 December 2009 (UTC)
- Yes, loosely speaking, I was basically saying that there can be no quantum numbers unless there is also energy. Something physically exists if and only if it has energy. (I'm here ignoring vacuum energy and the complexity of the quantum vacuum.) So it works as a reasonable perspective to say that everything consists of energy, and quantum numbers are a way of describing some of the properties of that energy. I'm not saying that that's the only correct perspective, merely that it's a reasonable one. If you have a cubical granite rock, most people would say that it clearly consists of granite, and has a property of being cubical, rather than arguing that it consists of granite and cubicality. But the distinction between "consists of" and "has a property of" is a lot more arbitrary at small enough scales. That's ultimately not well-defined AFAIK. Red Act (talk) 07:29, 2 December 2009 (UTC)
- I agree that energy and mass are indistinguishable and interchangeable - energy has mass, and mass has energy. And therefore we cannot say "everything consists of energy" without also saying "everything consists of mass". But the latter formulation sounds just wrong because it begs the questions "where does the mass come from ?" and "why do different bits of mass (i.e. fundamental particles) have different properties ?". You might as well say "everything consists of blue - but some of that blue appears to be green or red". Gandalf61 (talk) 12:01, 2 December 2009 (UTC)
- Yes, loosely speaking, I was basically saying that there can be no quantum numbers unless there is also energy. Something physically exists if and only if it has energy. (I'm here ignoring vacuum energy and the complexity of the quantum vacuum.) So it works as a reasonable perspective to say that everything consists of energy, and quantum numbers are a way of describing some of the properties of that energy. I'm not saying that that's the only correct perspective, merely that it's a reasonable one. If you have a cubical granite rock, most people would say that it clearly consists of granite, and has a property of being cubical, rather than arguing that it consists of granite and cubicality. But the distinction between "consists of" and "has a property of" is a lot more arbitrary at small enough scales. That's ultimately not well-defined AFAIK. Red Act (talk) 07:29, 2 December 2009 (UTC)
- Jayron32: That's the whole question of baryogenesis. Red Act: Your first question is also not what the op asked. You are basically saying there can be no quantum numbers unless there is also energy. A fine statement (is that really what you meant to say though?), but not what the op asked. The op asked is there anything else besides energy in the universe. And the answer is no, there are also other conserved quantities (as Gandalf61 said). In truth we are arguing over words, because the op's question is not well defined. But hopefully reading this will give him the answer anyway. And BTW, your answer to him was correct, I was arguing with Gandalf61 who implied that mass and energy were different, but they are not. They are not simply interchangeable - they are the same. Ariel. (talk) 01:36, 2 December 2009 (UTC)
- Regarding quantum numbers; don't quantum numbers all sum to zero when added across the whole universe anyways (i.e., the universe is neutral?). So there is nothing "extra" there that needs to be conserved once you have taken account of the energy/mass. Yes, they are conserved, but only to maintain the neutrality of the universe; there's nothing "left over" once you factor out the energy/mass! If so, then the singularity at the big bang would have had a net charge, and that doesn't make much sense... --Jayron32 00:41, 2 December 2009 (UTC)
- Regarding your last sentence, it depends on what the meaning of the word 'is' is. I would take the question "is everything energy?" to mean something like "within a closed region of flat spacetime, does what is contained within that region differ from a vacuum if and only if the total energy within that region differs from the total energy of a vacuum?". The answer to that question is "yes". You're taking the question "is everything energy" to mean something like "within a closed region of flat spacetime, is total energy the only conserved property of what's within that region?". The answer to that question is indeed "no", but I don't think that was the intent of the original question. Red Act (talk) 23:34, 1 December 2009 (UTC)
- Actually no. What it means is that mass and energy are the same thing! They are two words that mean the same thing. There is no test that can distinguish them, and no definition that discriminates between them. They are not interconvertable, they are the same. Note that "matter" and energy are not the same. But "mass" and energy are. Matter is energy plus quantum numbers, like charge or boson, spin, etc. And don't get confused by concepts like a photon being massless - it's actually that it has no rest mass, not that it's massless. All that said, I think the convention is that energy that is tightly bound in matter is called "mass". So the binding energy of an atom is called mass, but the heat, and velocity of an atom, it called energy, even though both contribute to it's gravity, and weight, etc. Heat is easy to remove from an atom, the binding energy is not. And to answer the question in the title, everything is not energy, because there are quantum numbers that are conserved, which also make up the universe. Ariel. (talk) 21:14, 1 December 2009 (UTC)
- Mass-energy equivalence is one of the fundemental threads that actually shows up, in one form or another, in all of modern theoretical physics. It isn't that matter is a form of energy, or that energy is a form of matter. Its that the two are the same exact thing, but being observed under different conditions or in different environments. The same basic principle is at work in wave-particle duality, that is all of the universe can be said to behave in a wave-like (energy-like) manner, or as particles (matter-like) manner. That is, everything is both an object (particle) and a wave (energy), its not so much that anything "chooses" one form or another, its that the method of observation of something determines which properties of that thing you actually see; some methods of observation lead to wave-like properties being highlighted, while others lead to particle-like properties (see Double-slit experiment for a discussion of the principle at work in electrons and photons). For really big things, we only have observation methods that highlight the object-like nature of them, but when you get smaller and smaller, you can arrive at more and more wave-like properties comeing out. See also DeBroglie wavelength for the theoretical wave-particle duality in large objects, Schrödinger equation for the complex mathematics of wave functions of matter (there is even a possible Schrödinger equation which describes the entire universe as a single wave function), and Copenhagen interpretation for some of the philosophical implications of mass-energy equivalence. --Jayron32 20:57, 1 December 2009 (UTC)
Sell copyrights of my research work
Hi folks,
I have done a research work and project has come really well. My research paper has been chosen for publication in a Internatinal Journal in USA. I am basically from India. I was thinking like it would be better if I could give the copy rights for any of the research company so that my work (which I believe will cater the need of many doctor's world over) will get improved more. To be more specific My project is an Ophthalmology related one. Its not about drugs, its about diagnosing a device. So it will be a great help for me if any of you folks coul give me some information with regards to this (I don't have much knowledge in this field thats why asking).I am really sorry if I have posted in the wrong section.Thanks in Advance.
Thanks in Regards. —Preceding unsigned comment added by 202.54.176.51 (talk) 10:46, 1 December 2009 (UTC)
- Does copyright help? Also, if your research pertains to an ophthalmological diagnostic device, it seems like patent may also be a helpful article. However, we can't give you legal advice about copyrights and patents as they may apply to your specific case. Red Act (talk) 10:59, 1 December 2009 (UTC)
- (ec)You don't have copyright in the scientific results of your work - these could, if at all, only be protected by a patent. Copyright only protects the expression, not the ideas or the data. You do hold copyright to the actual text you wrote, but if you publish it in a journal, you almost certainly have to transfer copyright to the publisher - at best, you give him a fairly unrestricted license, but more typically you transfer it and receive some rights back. Sorry, that's how science works, and that's why scientists are rarely rich ;-). If you only want the results to be used (fame for you, better life for humanity), make sure you publish in a good journal and bring the paper to the attention of people who might be interested. If you want to make extra money, you need to apply for a patent, but a) it could be too late and b) its a fairly involved and none too cheap process, and there is no guarantee that you will recoup the money. --Stephan Schulz (talk) 11:07, 1 December 2009 (UTC)
- One nitpick. In the US system you can't copyright scientific data, but in the UK and some other places one can claim a copyright over data. (Personally I think such copyrights are dumb, but I don't write the laws.) Dragons flight (talk) 01:47, 2 December 2009 (UTC)
- If you publish your article in a traditional subscription journal you will be asked to transfer copyright to the journal as part of the process - and they won't pay you for it. See Scientific journal#Copyright. If you publish in an open access journal, i.e. a journal that is free to read and reuse, you will generally retain copyright, but you will usually have agree to release your work under a free license like the Creative Commons license. You need to pay to publish in many but not all open access journals,[23] and many open access journals will give full or partial waivers of the publication fees for researchers from countries like India. Fences&Windows 15:47, 1 December 2009 (UTC)
Size of processor dies
Does anyone know how large a typical processor die (I believe they're also referred to as wafers prior to assembly into a processor) would be? This [24] is exactly what I'm referring to - but I couldn't see a scale. 157.203.42.175 (talk) 13:19, 1 December 2009 (UTC)
- First google hit has some numbers for recent chips: [25]. --Sean 13:32, 1 December 2009 (UTC)
- FYI: I work in a fab where things such as that are manufactured. We refer to them as 'chips'. A 'wafer' is a round piece of silicon on which several to several hundred chips are built. See wafer (electronics). Dismas|(talk) 14:14, 1 December 2009 (UTC)
- Sean, thanks. Not sure how I missed that one. 157.203.42.175 (talk) 15:04, 1 December 2009 (UTC)
- You can also look for pictures of naked cores, i.e. with the IHS removed. Extreme overclockers particularly those using water cooling sometimes do it (it's probably less common now then it was before because they are usually soldered) to improve cooling. The solder may confuse things a bit but if it's sufficiently removed you can usually see the size of the actual core. E.g. [26] [27] [28] [29] [30] [31] [32]. While these don't carry measurements (well one of them does and actually tells you the official size so you can see it's fairly close), you can work it out if you know the size of the entire processor (i.e. including circuit board) which probably isn't that hard to find out given that these are consistent within a processor socket for obvious reasons. Also [33] of a GPU (harder to find the dimensions but it helpfully includes a Singaporean coin). I'm not sure if all modern GPUs include an IHS anyway. I also came across [34] of a poor removal (of an already dead processor) which may be of interest because you can actually see some of the layers Nil Einne (talk) 23:46, 1 December 2009 (UTC)

- Back before we had flash memory - lots of chips would have on-board UV-erasable, Programmable, ROM (UV-EPROM). In order to get the UV light into the chip to erase it - they had quartz windows over them - which handily lets you see the naked die inside. The processor in the photo to the right here is an ancient (and very simple) 8048. For scale, the pins on the side of the package are 1/10th of an inch apart. SteveBaker (talk) 00:36, 2 December 2009 (UTC)
Red apples inside and out
I have some apples that we bought at the grocery store last week. Unfortunately, I can't tell you their country/state of origin since we've thrown out the bag that they came in. When I bite into them, the flesh of the apple is stained red in parts. Mostly nearest the skin. The entirety isn't reddish, just parts. And if I cut into the apple, it doesn't get reddish inside. It's only when I bite into it. What's causing this?
Note: This is not a request for medical advice. I've been eating these apples for going on a week now and have not noticed any change in my health. Nor any changes in my wife's health. There have been no recalls of apples from my local grocer and there hasn't been anything in the local papers about poisoned apples. Furthermore, I am not dead and trying to reason out just what killed me. Dismas|(talk) 14:08, 1 December 2009 (UTC)
- Some apples do this naturally: Discovery's one such. --TammyMoet (talk) 15:00, 1 December 2009 (UTC)
- The list of apple cultivars provides a compendium of apple varieties (I had no idea how many there are). Among these, a quick search for "flesh" turns up Pink Pearl (apple) as having naturally bright pink flesh. Not sure that it fits with the color of the skin, though. A google search for "apple pink flesh" turns up some other possibilities. --- Medical geneticist (talk) 16:14, 1 December 2009 (UTC)
- It could be paint, most red apples are artificially colored. Is your apple uniformly red? With no variations, where it's slightly darker or greener in spots? If so it was painted, and some of the color seeped inside. Ariel. (talk) 21:18, 1 December 2009 (UTC)
- What? Ariel's entry should be tagged [citation needed]. Nimur (talk) 21:31, 1 December 2009 (UTC)
- I can't find one for apples, but here's one for oranges: [35] Does putting food color on the outside of a fruit really surprise you so much? A natural fruit is not a solid color, but consumers expect it to be. Ariel. (talk) 21:57, 1 December 2009 (UTC)
- No, the color is not uniform. Dismas|(talk) 21:57, 1 December 2009 (UTC)
- And now that I've had a chance to look, no, they are not Pink Pearls. Dismas|(talk) 01:30, 2 December 2009 (UTC)
- That reference is from 1955, I expect a lot has changed since then. While you may have heard of apples being artificially coloured, I doubt they mean with dyes. They mean by shining light on it in the right way while it is ripening and maybe spraying some plant hormones on it. --Tango (talk) 01:46, 2 December 2009 (UTC)
- No, the color is not uniform. Dismas|(talk) 21:57, 1 December 2009 (UTC)
- I can't find one for apples, but here's one for oranges: [35] Does putting food color on the outside of a fruit really surprise you so much? A natural fruit is not a solid color, but consumers expect it to be. Ariel. (talk) 21:57, 1 December 2009 (UTC)
- What? Ariel's entry should be tagged [citation needed]. Nimur (talk) 21:31, 1 December 2009 (UTC)
- Food in industrial food production is often dyed,[36] but I doubt whole apples are dyed. Here's an article about apple slices being dyed:[37] Apples can take up dye, but they do it mostly through the blossom end and only a little through the skin.[38] Fences&Windows 23:52, 3 December 2009 (UTC)
- It could be paint, most red apples are artificially colored. Is your apple uniformly red? With no variations, where it's slightly darker or greener in spots? If so it was painted, and some of the color seeped inside. Ariel. (talk) 21:18, 1 December 2009 (UTC)
the acidic group in aspartate/glutamate is COOH. what do you call the basic group in arginine?
That R-NH-(C=N)-NH2 motif. Why isn't this motif more common among organic compounds as carboxylic acids? It's kinda like the basic equivalent of COOH, isn't it? John Riemann Soong (talk) 15:48, 1 December 2009 (UTC)
- It's a Guanidine group. I have to ask...did you actually look in the arginine article before posting the question about what its sidechain motif is called? It's stated in the first sentence of arginine#Structure. DMacks (talk) 17:00, 1 December 2009 (UTC)
- I took a quick glance. I have to say, the phrase "the distal end of which is capped by a complex guanidinium group" isn't the most eye-catching sentence ever. Well anyway, why isn't the C+ carbocation resonance contributor shown? I would think it would be a major resonance structure, since with three electronegative nitrogens I imagine that that carbon is very positive. (In fact some 3-21 calculations show it to have over full positive charge!) Is a guanidine group much more reactive to nucleophiles than carboxylic acids, especially when protonated? Or does the delocalised positive charge, etc. make it react like amides? Basically why isn't this group hydrolysable under mild conditions (physiological conditions even) -- I imagine that hydrolysing a guanidine group on a protein (maybe converting it into an amide) would be potentially bad. John Riemann Soong (talk) 18:16, 1 December 2009 (UTC)
- When you protonate the imine to make the guanidinium ion, this puts a + charge on a nitrogen. Basic rules of resonance will show that such charges are shared among alternate atoms; if you do the "electron pushing" to shift between resonance forms, there is no possible resonance form which puts a + charge on the carbon. Thus, there is no carbocation contribution to the resonance structure of guanidinium. The formal charge on the carbon is 0, and on each nitrogen is +1/3. Yeah, the electronegativity would indicate that having nitrogen at a higher formal charge would seem to be an intuitively backwards situation; but its just that there is no mechanism to have any charge on that carbon in guanidinium. --Jayron32 21:06, 1 December 2009 (UTC)
- If the π electrons of C=N+H2 are pulled into being a lone-pair on that N without another N lone-pair pushing it, you get C+(NH2)3 (for the parent structure). All this arrow-pushing would mean each N is sp2 and the whole guanidinium is planar and highly resonance-stabilized, which is a common explanation for why guanidine is such a strong base. Of course, Nature is more clever than we are (once we actually study Her): turns out the NH2 are actually rotated (propeller-like geometry around the C) and therefore there is not nearly as much resonance as simple arrow-pushing might predict. And that the high basicity is due to H-bonding of the added proton, not really enhanced electronic stability of the structure. See doi:10.1021/ja00059a035 for info. DMacks (talk) 22:51, 1 December 2009 (UTC)
- In other words, it depends on whether the imine-N lone pair OR the imine C=N double bond is a better lewis base as to whether you end up with the carbon with a +1 formal charge OR you end up with the three nitrogens with a +1/3 formal charge. I'm pretty sure that in most cases, lone pairs are better lewis bases than π-bonding electrons are, which gives us the + charge distributed among the N's and not the C. --Jayron32 00:34, 2 December 2009 (UTC)
- If the π electrons of C=N+H2 are pulled into being a lone-pair on that N without another N lone-pair pushing it, you get C+(NH2)3 (for the parent structure). All this arrow-pushing would mean each N is sp2 and the whole guanidinium is planar and highly resonance-stabilized, which is a common explanation for why guanidine is such a strong base. Of course, Nature is more clever than we are (once we actually study Her): turns out the NH2 are actually rotated (propeller-like geometry around the C) and therefore there is not nearly as much resonance as simple arrow-pushing might predict. And that the high basicity is due to H-bonding of the added proton, not really enhanced electronic stability of the structure. See doi:10.1021/ja00059a035 for info. DMacks (talk) 22:51, 1 December 2009 (UTC)
- When you protonate the imine to make the guanidinium ion, this puts a + charge on a nitrogen. Basic rules of resonance will show that such charges are shared among alternate atoms; if you do the "electron pushing" to shift between resonance forms, there is no possible resonance form which puts a + charge on the carbon. Thus, there is no carbocation contribution to the resonance structure of guanidinium. The formal charge on the carbon is 0, and on each nitrogen is +1/3. Yeah, the electronegativity would indicate that having nitrogen at a higher formal charge would seem to be an intuitively backwards situation; but its just that there is no mechanism to have any charge on that carbon in guanidinium. --Jayron32 21:06, 1 December 2009 (UTC)
Putting cancer cells to sleep
I was reading about Kodiak bears' reproduction, specifically the part that says:
As soon as the egg is fertilized and divides a few times, it enters a state of suspended animation until autumn when it finally implants on the uterine wall and begins to grow again.
this made me wonder if any researchers are seeing if it would be possible to find out how to do that to other cells than Kodiak bear embryo cells by finding out how exactly it's done there (if that suspended animation functionality is a property of the Kodiak embryo cells themselves, an interaction in that specific kind of womb, or something else). The first application that came to my mind for stopping cell division of targeted cell types was cancer treatment. 20.137.18.50 (talk) 18:52, 1 December 2009 (UTC)
- As I understand it - the difficulty isn't with killing or preventing subdivision of cancer cells - the problem is with finding ways to attack ONLY the cancer cells. If you have some way to direct a drug or other treatment to JUST the cancer cells - then there are any number of nasty things you can do to them to wipe them out. SteveBaker (talk) 00:30, 2 December 2009 (UTC)
Is there a medical term for this?
I think this might be closed-eye hallucination but I'm not sure...when I have my eyes closed when I'm about to go to sleep I sometimes see wallpaper-like repetitive patterns of leaves and flowers, or sometimes just ambiguous droplet-like dots. What is this called, if it isn't just CEH?--Editor510 drop us a line, mate 19:26, 1 December 2009 (UTC)
- This seems to be a stage between consciousness and dreaming, akin to hypnogogic imagery. The mind may be perceiving the random sensory data as patterns. A higher level of dreaming would be perceiving the patterns as scenes rife with meaning, in vivid detail. If one attends to the incipient dream stage, the increasing level of consciousness causes the percept to revert to the original random patterns. See also the controversial Lucid dreaming. Edison (talk) 06:47, 2 December 2009 (UTC)
- You may also find Prisoner's cinema interesting. cheers, 10draftsdeep (talk) 18:52, 2 December 2009 (UTC)
aspartame
I think saccharin is considered safe because it is broken down into non-toxic components in the stomach. What about aspartame? Is it broken down into non-toxic components during digestion or does it break down into toxic components during digestion which can be absorbed into the blood stream? 71.100.160.161 (talk) 21:38, 1 December 2009 (UTC)
- Aspartame has some information pertaining to this. Unomi (talk) 21:42, 1 December 2009 (UTC)
Gums Bleeding
Before this question is dismissed as medical advice, please read carefully.
Why do one's gums bleed when there are a lot of bacteria proliferating on them? For instance, when someone avoids bruching their teeth for a while, and then brushes them, a lot of bleeding is observed. Why?130.127.52.67 (talk) 21:56, 1 December 2009 (UTC)
My understanding is this has nothing to do with bacteria at all. Gum tissue is simply sensitive, and if brushed regularly "toughens" against the assault of the bristles. The same can be said for flossing. If you don't floss for ages and then start, you'll look like you got on the wrong side of Mike Tyson.61.189.63.183 (talk) 22:30, 1 December 2009 (UTC)- That would be a misunderstanding. DRosenbach (Talk | Contribs) 03:33, 2 December 2009 (UTC)
- It is bacteria - see the link below. --Tango (talk) 22:38, 1 December 2009 (UTC)
- See the two articles linked to from Gum disease. --Tango (talk) 22:38, 1 December 2009 (UTC)
- There is a space between the gum and the tooth called the sulcus or gingival crevice. In health, the depth of the sulcus occurs where the junctional epithelial cells attach to the tooth. When the gum tissue is inflamed due to plaque, the depth of the sulcus no longer consists of fit, rigid junctional epithelial cells, but rather of flimsy, somewhat irregular epithelial cells. The epithelial barrier exists in an ulcerated state during gingival inflammation (otherwise known as gingivitis) and thus, bleeding from the underlying connective tissue occurs more easily, either when stimulated, or in cases of worse inflammation, even unstimulated. Plaque causes gingivitis and junctional epithelial ulceration because it is essentially minute food particles colonized by bacteria. Bacteria secrete all sorts of noxious and nasty chemicals, such as endotoxin and collagenase that stimulate a host response that induces inflammation via histamine and various interleukins and degrades colalgen, respectively. Let me know if there's anything else you'd like to know. DRosenbach (Talk | Contribs) 01:46, 2 December 2009 (UTC)
the economics of enantioselective chemistry
Are enantiotopically pure substances typically:
- Less than twice as expensive as a racemic mixture
- About as twice as expensive as a racemic mixture, with a little more to compensate for reagent consumption/workup
- More than 2x expensive as the racemic mixture?
Basically I'm wondering how price economics might convince companies whether to adopt an asymmetric process versus a chiral resolution process (where you discard half your yield). I imagine it might be variable for the substance involved (like if one enantiomer of a drug was particularly poisonous, for instance). John Riemann Soong (talk) 23:56, 1 December 2009 (UTC)
- Depends on the source. Biologically derived molecules are usually provided in their enantiomerically pure state, and with the "unnatural" isomer being anywhere to slightly to ungodly more expensive (Because they have to be synthesized chemically). For example, L-phenylalanine from Sigma is about $390/kg, whereas D-phenylalanine is $2000/kg, and DL-phenylalanine is $430/kg (all for 98-99% purity, no extra testing/certification). For compounds which are both chemically synthesized, it depends on the starting materials. If chirally pure starting materials are available for reasonable prices and the chemical reactions to synthesize doesn't introduce/scramble any positions, entantiomerically pure compounds won't be any more expensive than the racemic mixture. The final case is where the stating compounds aren't chiral. If the synthesis incorporates entantioselective reactions/catalysts, the entantomerically pure compounds will be slightly more expensive than racemic, as entantioselective catalysts tend to be more expensive than the ones which give racemic products. If none of the previous points hold, the only practical way you can get entantiomerically pure compounds is by chiral column chromatography or by making a diastereomeric derivative. This makes the entantiomerically pure compounds ungodly expensive, as chiral chromatography columns are seriously expensive, and diastereomeric derivitization is a p.i.t.a. -- 128.104.112.95 (talk) 16:11, 3 December 2009 (UTC)
December 2
several questions
Here are two questions I that keep popping into mind and I have not had the chance to ask.
If you release a 100 pound mass from a geostationary orbit will it still be attracted to the Earth at the speed of 32 feet per second per second and if not what speed?
If you vibrate a magnet will it send out a magnetic wave at the frequency of vibration and if so will this magnetic wave in turn produce an electrical wave?
71.100.160.161 (talk) 00:13, 2 December 2009 (UTC)
- For question 1, you need to specify at what height you are dropping from. The acceleration due to gravity should be (roughly) uniform at any given height, but will decrease with distance from the earth. However, if you drop such a weight from a geostationary orbit, it will not sink towards the ground; rather it will remain exactly where you let go of it, since it is in free fall with you when you release it, so unless you push it, it will remain in free fall right next to you. If you push it, it will move at whatever speed and whatever direction it was going when it left contact with your hand.
- For question 2, electromagnetic waves are not seperable, they basically move together, orthogonal to each other. See this picture:

- --Jayron32 00:29, 2 December 2009 (UTC)
- (ec) If you release something while in orbit that something will also be in orbit and won't fall at all. If something started out at the altitude of geostationary orbit but with no horizontal momentum, then it would fall at a little under 1 foot per second per second (at first - it would increase as the object got closer to the Earth). Geostationary orbit is at about 36,000km above the centre of the Earth and the surface is about 6,000km above the centre. That's a factor of 6 different and, since gravity follows an inverse square law, that results in a factor of 36 difference between the gravitational acceleration. --Tango (talk) 00:29, 2 December 2009 (UTC)
- For question 2, Jayron32, are you implying that if you move a magnet you make light (radio)? I guess it makes sense, since if you move a charge (electron in a wire) it makes light. Can you shake a magnet at kilohertz speeds? Ariel. (talk) 02:32, 2 December 2009 (UTC)
- The OP implied that. I said nothing of the sort. I was dodging the question by merely noting that "magnetic" and "electric" waves cannot exist in isolation from each other; they are two sides to the same coin. His question is unanswerable as written because it presupposes something which is not true (the seperate nature of "electric" and "magnetic" waves). --Jayron32 05:02, 2 December 2009 (UTC)
- "Fields and waves" was far from my strong point in school, but altering the current flow in a set of coils can create a rotating magnetic field, as was established by Nikola Tesla. Similarly, vibrating a magnet would cause induced voltage in a nearby coil, similar to varying current in a parallel coil. At least so far as "near field" effects are concerned, a moving magnet or a varying current in a wire or coil could have the same effect. Not so clear on antenna effects and radiated field. Electromagnetic waves have no lower frequency, and a charged particle or a magnet vibrating at 1 Hz or 100 Hz or 1000 Hz or 10 KHz should create an electromagnetic field, though at a lower frequency than what we usually consider "radio." Clearly a little horseshoe or bar magnet from the dime store, or a ceramic refrigerator magnet would fly apart or come loose from the moving thing if you attempted to rotate or vibrate it at megahertz radio frequencies, or microwave frequencies, or light frequencies. That is a material science problem and unrelated to the underlying physics. It should be possible to use a sound transducer to move a little neodymium magnet back and forth at 20 KHz, which is toward the upper end of the Very low frequency radio band (3Hz to 30KHz). I have doubts about the moving magnet acting as an efficient antenna or the actual emitted power. Edison (talk) 17:57, 2 December 2009 (UTC)
Evolution
http://www.jesus-is-savior.com/Evolution%20Hoax/devilution.htm
Is this a joke, or is that person serious? --70.250.212.43 (talk) 00:35, 2 December 2009 (UTC)
- Sadly, he's probably serious. It doesn't make him correct, but I would fathom that the author of that bullshit actually believes it. --Jayron32 00:43, 2 December 2009 (UTC)
- On the internet, if it looks crazy, it probably is. Hooray for democratization of communication channels—for every nut, a megaphone! --Mr.98 (talk) 01:48, 2 December 2009 (UTC)
- It can sometimes be difficult to tell if a site or person is being serious or intended in as satire or parody. (Many people think Edward Current's work on Youtube is genuine/serious for example.) However that site appears to be genuine, I can find numerous discussions of it an evidentally it has had hosting problems Nil Einne (talk) 02:13, 2 December 2009 (UTC)
- I'm beginning to be able to tell that a site is junk just by looking at the fonts, colors and centering of text on the page! It's truly incredible. Look at almost any site on perpetual motion, crystal healing, moon-landing conspiracies or anything like that - they all have the same basic look - primary colors, too many font sizes, centered text and pages that scroll on for dozens of screenfulls. It gets to the point where you don't even need to read the words anymore! SteveBaker (talk) 02:20, 2 December 2009 (UTC)
- It's true that really amateurish/crazy sites stand out (e.g. Time Cube) in their embrace of vernacular web design... but looking good doesn't mean the content is any more sensible. Answers in Genesis has a wonderfully professional-looking site... but it's still full of junk. Looking bad doesn't necessarily mean that you're a crackpot, but I think most non-crackpots recognize that looking like a crackpot does not get their message across and change it. I think people who are 100% crackpots probably can't tell the difference. --Mr.98 (talk) 02:46, 2 December 2009 (UTC)
- Mr.98, you only say that because you've been educated stupid by evil academia. :) A Quest For Knowledge (talk) 03:59, 3 December 2009 (UTC)
- "primary colors, too many font sizes, centered text and pages that scroll on for dozens of screenfulls". Just like Daniel Brandt... Fences&Windows 03:31, 2 December 2009 (UTC)
Check out http://www.islamdenouncesterrorism.com/darwinism_materialism.html; it's even more ridiculous! They blame evolution for every societal problem since Darwin. Even things before Darwin are blamed on "Darwinism's" "materialistic roots". ----J4\/4 <talk> 17:58, 2 December 2009 (UTC)
- The general principle of "If you sling enough mud - some of it will stick." definitely applies here! SteveBaker (talk) 19:28, 2 December 2009 (UTC)
- I love a good comedy ;-). Falconusp t c 21:15, 2 December 2009 (UTC)
- There are lots of crazy sites out, and yes, some people really believe this crap. What gets me is that they think we're the naive ones. Add WP:FTN to your watchlist to get a sampling of the craziness. A Quest For Knowledge (talk) 05:01, 3 December 2009 (UTC)
- BTW, we really could use some watchful eyes on the Fringe theory noticeboard, especially from editors knowledgeable about science. The fringe theorests were having a field day with the Climatic Research Unit e-mail hacking incident until we had to lock down the page. A lot of the time, it's hard to keep up with them because there are so many. A Quest For Knowledge (talk) 05:25, 3 December 2009 (UTC)
- *wonders if anyone else thought of this Fringe when they first saw that board mentioned, and wondered how it managed to get so bad that we needed an entire noticeboard for one tv show...* :P J.delanoygabsadds 05:42, 3 December 2009 (UTC)
- BTW, we really could use some watchful eyes on the Fringe theory noticeboard, especially from editors knowledgeable about science. The fringe theorests were having a field day with the Climatic Research Unit e-mail hacking incident until we had to lock down the page. A lot of the time, it's hard to keep up with them because there are so many. A Quest For Knowledge (talk) 05:25, 3 December 2009 (UTC)
greater permittivity than a vacuum?
Is it possible that there is something, perhaps an effected area of space such as in a pre-particle, (an area of space after the Big Band but before any particle has formed) where permittivity is greater than in a vacuum, allowing a particle to form? 71.100.160.161 (talk) 01:41, 2 December 2009 (UTC)
- By Big Band, I assume you mean the big bang? Quantum mechanics states that even a vaccum has energy, so an absence of energy may indicate lower mass or permeability, but how the universe formed in the first place is probably beyond our scope of knowledge. ~AH1(TCU) 02:35, 2 December 2009 (UTC)
- By "vaccum," I assume you mean Vacuum. Edison (talk) 06:36, 2 December 2009 (UTC)
- I assume that maximum permittivity is for a vacuum is determined by use of a perfect vacuum which would offer greater permittivity than a partial vacuum, So yes, is there the possibility of greater permittivity than a perfect vacuum such as an area in space so small it is too small to contain anything? 71.100.160.161 (talk) 15:39, 2 December 2009 (UTC)
- I don't think this is an appropriate forum. There are several physics forums on the internet where you can discuss new theories and questions that are beyond the current scientific knowledge, but here our answear can merely be that no, there are no evidence nor generally accepted theories describing something like what you propose. (Which of course can be something exciting from one point of view :-) EverGreg (talk) 12:09, 2 December 2009 (UTC)
- Such a variation has been proposed (published version requires payment) as an explanation for the Pioneer anomaly, but is far from being accepted. SpinningSpark 12:52, 2 December 2009 (UTC)
- What's the permittivity of the space between two Casimir plates? --Tango (talk) 15:15, 2 December 2009 (UTC)
- The same as a vacuum, the plates attract each other with an electromagnetic force. The difference is due to the plates. Graeme Bartlett (talk) 20:21, 2 December 2009 (UTC)
2,3-Dihydropyran as a protecting group
How do you take it off? I know you take it off with acid, but how do you guarantee that you get your actual alcohol back, with high yield? I would imagine the protected group might cleave in acid several ways ... John Riemann Soong (talk) 01:42, 2 December 2009 (UTC)
- There are no guarantees in chemistry. You have to do the actual experiment using the actual reactants (and often screen several different times, temperatures, acids, reactant ratios, solvents, etc.) to find "what works best for intended reaction with minimum of unintended reactions". One reason there are so many different protocols for doing reactions is that every substrate is different, and (unlike in textbooks) every little thing may matter and may matter in totally unexpected ways. And the reason there are so many different protecting groups is because different substrates may have different sensitivies to various specific chemicals. Again, Nature is cleverer than the total of every journal article ACS has ever published.It's called research because you have to search over and over and over to find how to actually perform what seemed like an obvious and easy proposed idea:) DMacks (talk) 02:14, 2 December 2009 (UTC)
- Well "guarantee" is a relative term. My prof mentioned it as a protecting group and now I am following up on it. According to design, is the ring oxygen supposed to be protonated sometimes? Or is the ring geometry making the ring oxygen act like an ethoxy oxygen on an anomeric carbon? Basically I'm worried the protected group will hydrolyse the wrong way. John Riemann Soong (talk) 02:29, 2 December 2009 (UTC)
- In the real world, it doesn't matter which of the O is protonated first during acid-catalyzed hydrolysis deprotection. The acetal will fall apart either way, and eventually the ROH (deprotected alcohol) will detach—the product of acid-catalyzed hydrolysis of an acetal is ROH, R'OH, RCHO and you just happen to have "R' and R attached to the same molecular chain). On paper (i.e, not tied to the real world) you can do any and every possibility until you get to the one your prof wants:) But seriously, the reactions are reversible, and conditions are chosen to push towards the desired product by kinetics, thermodynamics, or concentration effects, etc. DMacks (talk) 21:14, 2 December 2009 (UTC)
- Well "guarantee" is a relative term. My prof mentioned it as a protecting group and now I am following up on it. According to design, is the ring oxygen supposed to be protonated sometimes? Or is the ring geometry making the ring oxygen act like an ethoxy oxygen on an anomeric carbon? Basically I'm worried the protected group will hydrolyse the wrong way. John Riemann Soong (talk) 02:29, 2 December 2009 (UTC)
Also I have a question regarding the synthesis posted in that article. Do you dehydrate with alumina in neutral conditions? Is there a carbocation rearrangement involved? John Riemann Soong (talk) 01:50, 2 December 2009 (UTC)
Typhoon Nida
Hi. Please refer to the discussion topic I posted on Talk:2009 Pacific typhoon season. I am not forum shopping, just trying to incite quicker discussion. This seems a little disturbing, and the weather for the past few days has been unpredictable (just one example is that we've had black ice for the past two mornings). Thanks. ~AH1(TCU) 02:43, 2 December 2009 (UTC)
- If you wish to avoid accusations of "forum shopping" - then you need to ask us a specific question that you're unable to answer for yourself with the resources you have at hand. SteveBaker (talk) 12:46, 2 December 2009 (UTC)
- Is the super typhoon responsible for all these ocean temperature changes, will this enhance El Nino, and could the pool of warm water actually cut of the Humboldt Current? ~AH1(TCU) 00:32, 4 December 2009 (UTC)
ring opening/closure, reaction rates and entropy
I've been trying to organise the overload of reactions depending on some special entropy-reducing quality of rings or where two substituents are held in a conformed position next to each other ... can I get this straight:
Assuming enthalpy of the reaction is near zero (e.g. esterification <___---> hydrolysis), ring-closure reactions are slightly disfavoured (e.g. K will be a little less than 1) because of reduction in entropy. (Unless there is some weird effect where closure might actually relieve strain and increase the rotational degrees of freedom?) However, being in a ring will catalyse the reaction e.g. make both forward and reverse reactions faster.
Take the phthalic anhydride and pthalic acid. The enthalpy of forming the anhydride is positive (and the reduction in entropy hurts it further), but however kinetically, the rates of hydrolysis and anhydride-formation are both increased cuz of the ring effect. Distillation of water and heat will define the equilibrium point, but the ring proximity thing will make both reverse and forward reactions faster? I'm trying to frame this in terms of thermodynamics. John Riemann Soong (talk) 02:45, 2 December 2009 (UTC)
Speed at which levels of melanin in humans adapt to UV light
As I understand it, the varying levels of melanin in humans (and thus varying skin tones, and so forth) are believed to have evolved at least partly in response to differing levels of ultraviolet light — the high levels of UV light in equatorial areas prompt retention of melanin, while in places closer to the poles, there's less UV light and thus not so much melanin is kept. (I'm setting aside other contributing factors like diet, for now.) My question is: how quickly would changes in UV light be reflected in melanin? That is, if a group migrated from one place to another, how long would it take before there were noticeable changes in melanin levels (setting aside other factors) as a result? I don't expect this is the sort of thing that can be determined with any precision, but even rough figures would help. Ten thousand years? A hundred thousand? -- 203.97.105.173 (talk) 03:02, 2 December 2009 (UTC)
- I recall being told that it would take ~10,000 years for black to go to white or vice versa. Dragons flight (talk) 03:05, 2 December 2009 (UTC)
- To slightly side-step the question, some research suggests that light skin in Europe may have evolved as recently as 5,500 years ago:[39] Fences&Windows 03:28, 2 December 2009 (UTC)
- In modern times, it may be never. Since everyone pretty much lives indoors now, theres little reproductive pressure to favor one skin tone over another, so there is little reason to suppose any such changes will occur in the future. Furthermore, there is little genetic isolation anymore, so there is little reason to believe that an isolated population would develop such uniform traits among themselves. --Jayron32 04:59, 2 December 2009 (UTC)
- Jayron, you should come out sometime and enjoy the outdoors. Everyone does not pretty much live indoors. Your second point stands. Dauto (talk) 06:07, 2 December 2009 (UTC)
- Do you work outside? Most don't anymore. --Tardis (talk) 15:59, 2 December 2009 (UTC)
- Jayron, you should come out sometime and enjoy the outdoors. Everyone does not pretty much live indoors. Your second point stands. Dauto (talk) 06:07, 2 December 2009 (UTC)

- Who said anything about work? Dauto (talk) 01:42, 3 December 2009 (UTC)
- Evolution can run pretty quickly when the genetic basis for the change is already in the population and the environmental change makes a significant dent in the ability of less useful genes to be passed on. It's likely that the genes for different skin colors are present in the general population - so if the UV levels changed suddenly and started to cause a dramatic change in birth rates and survivability then evolution might happen very rapidly. But that second point is the issue here. The disadvantage to light skinned people in sunny places or dark skinned people in less sunny places just isn't that dramatic. If there is only (let's say) a 1% chance of someone being so seriously disadvantaged by inappropriate skin tone that they failed to reproduce - then the rate of evolutionary change will be fairly slow. But if 90% of people with the wrong skin tone died before reaching child-bearing age - then we'd evolve in not too many generations. I don't know what the case was as mankind migrated around the world - or what it is today - but my gut feel is that this would be on the slower end of the scale in the past and essentially non-existant today in a technological world of sunblock, indoor living, clothing and
vitamin Evitamin D(sorry!) supplements that allow anyone to live successfully in any climatic conditions. SteveBaker (talk) 12:44, 2 December 2009 (UTC)
- That concept that skin tone evolution has stoped because we all live indoors, use cloth and sunblock and vitamin E (That last one is probabily irrelevant anyways) doesn't hold water. Those factors will make us evolve towards a lighter skin tone instead of a darker one. evolution still happens. 169.139.217.79 (talk) 15:54, 2 December 2009 (UTC)
- Why would they make us evolve towards a lighter skin tone at all? What is the reproductive pressure to do so? Do darker-skinned people reproduce less effectively when they wear sunscreen and clothes and work in offices? The idea that people's skintone will change just because their environment changes smacks of Lamarckism, a thoroughly disproven idea. --Jayron32 18:56, 2 December 2009 (UTC)
- People with dark skin tones at high latitudes do have an increased risk of rickets, but it is fairly minimal. --Tango (talk) 19:01, 2 December 2009 (UTC)
- It's minimal in a modern world with good nutrition, vitamin-D supplements in milk and decent medical care - but when darker skinned people started to migrate into Northern Eurasia - none of those benefits were present - and that would have been enough of a problem to reduce reproductive success - and hence evolutionary benefit for people with lighter skin tones. The need to wear clothing as protection from the colder climate (thereby covering up yet more skin and making vitamin D production still less effective) probably added to the problem. Within enough generations - voila - lighter skin color. The benefit must have been substantial enough to offset the costs in terms of skin cancers and such caused by having a lighter skin on sunny days in the summer...which also isn't such a big deal in a modern world with people staying indoors much more and having clothing and sunblock. SteveBaker (talk) 19:23, 2 December 2009 (UTC)
- Sorry, yes, I meant in the modern world. --Tango (talk) 19:43, 2 December 2009 (UTC)
- It's minimal in a modern world with good nutrition, vitamin-D supplements in milk and decent medical care - but when darker skinned people started to migrate into Northern Eurasia - none of those benefits were present - and that would have been enough of a problem to reduce reproductive success - and hence evolutionary benefit for people with lighter skin tones. The need to wear clothing as protection from the colder climate (thereby covering up yet more skin and making vitamin D production still less effective) probably added to the problem. Within enough generations - voila - lighter skin color. The benefit must have been substantial enough to offset the costs in terms of skin cancers and such caused by having a lighter skin on sunny days in the summer...which also isn't such a big deal in a modern world with people staying indoors much more and having clothing and sunblock. SteveBaker (talk) 19:23, 2 December 2009 (UTC)
- People with dark skin tones at high latitudes do have an increased risk of rickets, but it is fairly minimal. --Tango (talk) 19:01, 2 December 2009 (UTC)
- Why would they make us evolve towards a lighter skin tone at all? What is the reproductive pressure to do so? Do darker-skinned people reproduce less effectively when they wear sunscreen and clothes and work in offices? The idea that people's skintone will change just because their environment changes smacks of Lamarckism, a thoroughly disproven idea. --Jayron32 18:56, 2 December 2009 (UTC)
- Surely you mean vitamin D? --Tardis (talk) 15:59, 2 December 2009 (UTC)
- Yes - I do, sorry! Vitamin D is produced in the skin when exposed to sunlight. Light skinned people need very little exposure to the sun each day to make all the Vitamin D they need. But dark skinned people who live in the extreme North or South of the planet - or in places where there is dense cloud cover for extended periods - don't absorb enough sunlight to make sufficient quantities of this vitamin - and that (presumably) is the main reason for the evolution of lighter skin colours as humans migrated away from the tropics. However, in our modern civilisation - this problem is easily overcome by eating an appropriate diet or (in extreme cases) taking vitamin supplements. Hence, there is unlikely to be any evolutionary pressure for lighter skin to continue to evolve in modern times. SteveBaker (talk) 19:15, 2 December 2009 (UTC)
- Actually, there is a considerable body of opinion that vitamin-D deficiency (less serious than that needed to cause rickets) is a widespread problem even among light-skinned people. I remember this being in the news when I lived in Canada, where obviously it would be more of an issue than at my current California latitude.
- The experts can't seem to agree on whether to recommend oral supplementation, partly because D, as a fat-soluble vitamin, is possible to overdose on. (Experts don't trust people much, as a general rule.) In any case I took D supplements during the Canadian winter. --Trovatore (talk) 02:02, 3 December 2009 (UTC)
Since going outdoors might involve disconnection from the internet and Wikipedia, could this be fatal?Trevor Loughlin (talk) 12:49, 2 December 2009 (UTC)
- Wikipedia does not offer medical advice. Of course, if disconnecting yourself from Wikipedia could be fatal, we can hardly suggest that you go see a doctor either. Do you have an iPhone? --Tardis (talk) 15:59, 2 December 2009 (UTC)
- The article Human skin color has a bit about all this. One gene which only arose between 5 and 12 thousand years ago accounts for about a third of the difference between black Africans and white Europeans. The previous ones in all likelihood spread even faster as they would have had a greater effect. So about 20 thousand years for most of the change would probably be quite reasonable. As to the amount of difference needed for genetic advantage to take effect I've read that a real advantage as little as one in a thousand will eventually spread throughout a population and not get swamped by random factors. This is why it always interesting to ask about possible advantages in some harmful genetic disease or predisposition to it to account for its existence. Dmcq (talk) 09:20, 3 December 2009 (UTC)
vitamin c chart at bottom of vitamin c article.
Is it possible to include kale in the vitamin c chart at the bottom of the vitamin c article? —Preceding unsigned comment added by 97.96.127.130 (talk) 10:13, 2 December 2009 (UTC)
- Yes, it is possible. Be bold! Red Act (talk) 10:21, 2 December 2009 (UTC)
- Remember to include your source for the information. SpinningSpark 10:25, 2 December 2009 (UTC)
- You can get the vitamin c content for kale from the first of the three references cited for the table. If you get a value from that source, there's no need to cite a reference specifically for the new kale line that you add to the chart. All you need to do is add a new line in the table for kale, using the value you found in the reference. Red Act (talk) 10:36, 2 December 2009 (UTC)
- Remember to include your source for the information. SpinningSpark 10:25, 2 December 2009 (UTC)
GIS + ray-tracing algorithms = traffic noise estimation
I've put this here rather than in the Computing section as I think it is more likely to catch the eye of the appropriate people. I'd like to write a freeware program to predict the amount of traffic noise at various distances from busy roads, and display it in noise-intensity contours. Are there any freely available relevant algorithms or programming code that I could use rather than starting from scratch? Or does a free program already exist? The use of ray-tracing for sound rather than light was discussed in an earlier question on this page. Thanks. 89.242.106.49 (talk) 15:30, 2 December 2009 (UTC)
- I see an immediate problem with this. First, assume that the entire world is perfectly flat - no trees, houses, fences, hills, etc... Noise is easily measured by the distance to traffic source(s) -- some places may get noise from more than one road. You can use something like Google Traffic to measure traffic density and simply reduce the noise the further from the road you get. Now, what if there is a large solid fence next to the road to block the noise? No online mapping service will map the fence. What if there is a large hill? You will need to overlay an elevation map to reduce sound quicker when going uphill. So, it seems to me that the first step is to create a map that has proper elevation based on land (hills/trees) and manmade objects (fences/buildings). Then, you can work on getting traffic data. -- kainaw™ 18:40, 2 December 2009 (UTC)
Please! I do have at least a double digit IQ and I'm well aware of all that already thanks. 89.242.106.49 (talk) 20:00, 2 December 2009 (UTC)
- I think you may be making a mistake in fixating on ray tracing. That's an appropriate way to handle optical rendering - but it may not be the best way for audio. I would expect some kind of wave-front model to be better at handling your problems. As I explained in answer to your previous question on the computing desk - ray tracers (for light) presume light travels in straight lines without diffraction. If you employ that technique for audio then if the simulated microphone doesn't have "line of sight" to the cars on your virtual road - then it'll hear silence. But we know that in reality, a small rise in the ground does very little to cut out the noise of a nearby road (although it easily blocks 100% of the light coming from it!) - so you know, without doing very much deep thinking, that the problem you're trying to solve isn't a good fit for a classic raytracing solution. I typed "acoustics" into the search box at http://www.sourceforge.net (probably the largest repository of OpenSourced software in the world) and came up with several possible hits. One is FOAC - which claims to be: "software for calculating acoustic field by finite difference time domain method( FDTD )". It appears to be a plugin for Matlab. In the FOAC forums, there was a list of other opensourced acoustic packages: HERE. The language they use is completely foreign to a graphics guy like me - so I guess you just hit the limit of my expertise. Good luck! SteveBaker (talk) 18:53, 2 December 2009 (UTC)
I repeat my comment given to the previous poster. While a very simple program would suit settings in the countryside in a flat landscape - and that would be useful to many people including myself - a program suitable for an urban area would need to take into account reflecting off surfaces such as walls and being absorbed by other things such as vegetation as well. For an urban area I would need something which is capable of dealing with the equivalent of large scale maps that include buildings, and ideally being able to add ray-tracing capabilities to it. Doing it as a flat 2d plan would be the simplest case, but in some instances the differing elevetations would matter also. 89.242.106.49 (talk) 20:00, 2 December 2009 (UTC)
- If you are going to start from scratch you will need something like Uniform theory of diffraction. I studied Geometric theory of diffraction before, and the formula's were huge, each filling up one complete page. This will account for waves of sound diffracting around obstacles. It will vary with frequency. Graeme Bartlett (talk) 20:10, 2 December 2009 (UTC)
A complex forumula may not be a problem, particularly if you can put it in a subroutine. 92.29.36.113 (talk) 23:37, 2 December 2009 (UTC)
El nino
Will it bring more snow to North Texas than usual?Accdude92 (talk to me!) (sign) 17:19, 2 December 2009 (UTC)
- Take a look at NOAA's weekly El Niño status report -- the last few slides give an overview of the effects of El Niño on precipitation across the USA. Looie496 (talk) 17:53, 2 December 2009 (UTC)
- I don't know whether El Nino caused it - but North Texas had some snow last night - and that's "more than usual" for early December. SteveBaker (talk) 18:22, 2 December 2009 (UTC)
- There is a giant Gulf low currently in the Midwest, formed from a combination of at least five different low pressure systems, and it is travelling extremely fast. It looks to be worse than the 1993 Storm of the Century for those on the East Coast of the US and Southern Ontario, because the storm is drawing air and moisture from the Gulf of Mexico, the East Pacific, and the Caribbean, while the cold Arctic air extends to Hudson Bay and the northern reaches of Nunavut. Yesterday by this time, the low was still three separate systems, one in the Gulf later to acquire a classic tornado signature, another near New Mexico that resembled a hurricane on land, and yet another that was a cold front near the coast of Texas. Meanwhile in the western Pacific, Typhoon Nida recently weakened from a category five to a category one, but when it weakened to a category four it was perhaps briefly larger than Tip. Now a second system is starting to form behind Nida in the gap in the Subtropical High that the storm created. The warmest pool of water is now in the Central Pacific, and the Humboldt current is becoming choked by this warm pool as it moves east. Currently all the warmest water in the West Pacific is south of the equator. This could bring an early and extra boost to El Nino. Already, the warmer water in the south Eastern Pacific is affecting temperatures in the Gulf and Caribbean, and this was likely enhanced by the warmer water temperatures. Off Brazil, a zone of 26C+ water extends almost to Uruguay, and this is what happened in March of 2004 when Cyclone Catarina formed. ~AH1(TCU) 23:42, 2 December 2009 (UTC)
- Ok but what does that mean for North texas? More snow?Accdude92 (talk to me!) (sign) 14:02, 3 December 2009 (UTC)
- There is a giant Gulf low currently in the Midwest, formed from a combination of at least five different low pressure systems, and it is travelling extremely fast. It looks to be worse than the 1993 Storm of the Century for those on the East Coast of the US and Southern Ontario, because the storm is drawing air and moisture from the Gulf of Mexico, the East Pacific, and the Caribbean, while the cold Arctic air extends to Hudson Bay and the northern reaches of Nunavut. Yesterday by this time, the low was still three separate systems, one in the Gulf later to acquire a classic tornado signature, another near New Mexico that resembled a hurricane on land, and yet another that was a cold front near the coast of Texas. Meanwhile in the western Pacific, Typhoon Nida recently weakened from a category five to a category one, but when it weakened to a category four it was perhaps briefly larger than Tip. Now a second system is starting to form behind Nida in the gap in the Subtropical High that the storm created. The warmest pool of water is now in the Central Pacific, and the Humboldt current is becoming choked by this warm pool as it moves east. Currently all the warmest water in the West Pacific is south of the equator. This could bring an early and extra boost to El Nino. Already, the warmer water in the south Eastern Pacific is affecting temperatures in the Gulf and Caribbean, and this was likely enhanced by the warmer water temperatures. Off Brazil, a zone of 26C+ water extends almost to Uruguay, and this is what happened in March of 2004 when Cyclone Catarina formed. ~AH1(TCU) 23:42, 2 December 2009 (UTC)
- I don't know whether El Nino caused it - but North Texas had some snow last night - and that's "more than usual" for early December. SteveBaker (talk) 18:22, 2 December 2009 (UTC)
Children on Wikipedia
I have a six year old daughter who I want to start using Wikipedia as a reference. However I'm afraid she'll click a few links and suddenly be staring at pictures I wouldn't want her to see for several more years. I believe this is a legitimate concern, for example, it only takes 3 clicks to go from Man to Human sexuality to Bondage (BDSM) where there's quite pornographic material. There must be near infinite similar examples. Is there a children's version she could use (not the Simple English wiki, I would like her to develop her vocabulary)? TheFutureAwaits (talk) 18:39, 2 December 2009 (UTC)
- There are third-party ports of Wikipedia which have stripped out all of the "naughty bits". These are not actively edited versions of Wikipedia, so often they are not entirely as up-to-date as Wikipedia itself is, but if you are more concerned with protecting your children from pornographic material (a VERY legitimate concern) than it may be worth it to be dealing with a slightly out-of-date version of Wikipedia which has been cleaned up for the kids. Wikipedia for Schools is the one I recommend for others. Good luck! --Jayron32 18:52, 2 December 2009 (UTC)
- A child of six shouldn't be left alone with an Internet connected computer - regardless of which site she uses. The way to have kids get the best out of the Internet (and Wikipedia) is to keep the computer in whatever room your family hangs out in - and for parent and child to surf together - just like you'd read a book together or (hopefully) watch TV together. Any of those things could potentially expose your kid to stuff you don't think is appropriate - so these activities need to be a "family time" kind of thing. That being the case - you can use Wikipedia with complete safety - with YOU deciding what's appropriate and not some anonymous (and fallible) Wikipedia-clone maker. If something difficult needs explaining - you are there to explain it. The computer is not a child-minder and shouldn't be treated as such. More importantly - you can also direct your child towards interesting/useful/appropriate things - not just away from inappropriate material - no amount of censorship of the content will help you there! A 6 year old is not able to make the connections that you can make - to find answers in an online encyclopedia that you can find. SteveBaker (talk) 19:39, 2 December 2009 (UTC)
- I find it rather offensive that you're telling me how to raise my child. I simply asked if there was a children's version which Jayron was helpful in providing. It's a lot to assume I'm letting her on the internet unsupervised. It's good to know other people treat the issue with concern but please don't assume I neglect my children. TheFutureAwaits (talk) 19:57, 2 December 2009 (UTC)
- I'm actually more concerned with truth than whether you might find it offensive or not. If you are supervising her then what do you have to worry about? Just use regular Wikipedia. What am I to assume from the nature of your question? The best way to increase her vocabulary is to read to her. When a kid doesn't understand a word - they just skip over it and guess the meaning (often incorrectly). SteveBaker (talk)
- The suggestion to surf together is a pretty common answer to questions like yours. I don't think you should get offended, it's a good suggestion. It's not that anyone thinks you are negligent or that they're trying to tell you how to raise your child, it's just the fact that the internet, by it's very nature, is only a couple links away from content you might not want your kids to see. -- JSBillings 20:23, 2 December 2009 (UTC)
- I had this problem a while back with a sequence which went Tudors>>Anne Boleyn>>Incest (which then was the wikilinked crime on her death warrant). If you do want to avoid being online at all the Schools Wikipedia also comes as a free download which we will mail you without charge if needed (yes, me). It is less popular compared to Wikipedia but a lot of schools intranets load it. As said it is currently mainly March 2008 content and Obama is still only a senator (there will be a big update in Q1 2010). --BozMo talk 20:38, 2 December 2009 (UTC)
- It doesn't help the original poster, but I've experimented with creating filtered subsets of Wikipedia using categories and keywords to choose what material to exclude. My impression is that I can actually do a pretty good job at this. Not as good as hand-checking for obvious reasons, but the result I'd hope for would be a much larger collection of "good" articles while excluding most of what people find objectionable. I've thought about trying to finish this early next year. Dragons flight (talk) 20:48, 2 December 2009 (UTC)
- Unfortunately, Wikipedia culture is such that we demand that our readers accept our "anything goes" policy as a price of using Wikipedia. You'll never get any prior warning of what the next click will bring you to; that's just not the way we roll here. However, Openmoko's Wikireader is a portable device for reading a static version of Wikipedia offline, and advertises itself as having parental controls. It might be the solution to the original questioner's problem (since Wikipedia resolutely refuses to provide a solution). - Nunh-huh 21:22, 2 December 2009 (UTC)
- The wikireader parental controls are just a keyword based filter as I understand it. Dragons flight (talk) 22:29, 2 December 2009 (UTC)
- We did make a request yonks ago for a hidden tag of "unsuitable for young children" be included in content so that a reader could switch it on or off but the community is for some reason extremely hostile to any concept of censorship. --BozMo talk 22:34, 2 December 2009 (UTC)
- One problem is defining what is and is not "unsuitable for young children" on an international site. E.g. Europeans are pretty relaxed about children seeing non-sexualized female toplessness, but get an accidental 2-second "wardrobe malfunction" on US television, and you won't hear the end of it for months. -- 128.104.112.95 (talk) 23:09, 2 December 2009 (UTC)
- Parental supervision while small children read Wikipedia is advisable, since a vandal can introduce any content into all but the few protected articles at any time, or can substitute an extremely obscene image for another image, with it typically taking several minutes or several days for articles few are watching to get reverted to the good version. Wikipedia is not censored, and content you might think inappropriate for a small child is only a couple of clicks away. Children doing unsupervised websurfing are also likely to be stalked by pedophiles. A School-appropriate version of Wikipedia as mentioned above would be useful, with staleness being the main problem. Edison (talk) 23:29, 2 December 2009 (UTC)
- Lets not be silly children unsupervised websurfing are not 'likely' to be stalked by pedophiles. That is a ridiculous comment. They are clearly at more risk than when supervised with an adult, but the risk itself is still extremely low. 194.221.133.226 (talk) 10:13, 3 December 2009 (UTC)
- You can get a huge reduction in simple vandalism by not serving any page unless it is at least a few hours old without further editing. That's another kind of automated filter one can use when collecting good versions. Dragons flight (talk) 23:41, 2 December 2009 (UTC)
- The main trouble is that it's difficult for anyone else to anticipate exactly which images you would find unsuitable for your child, without knowing you or your child. One option is Internet filtering software, such as CyberSitter or NetNanny, which you can configure to suit your particular concerns. A conservative option is to turn off images altogether and browse text only. Dcoetzee 23:51, 2 December 2009 (UTC)
- Exactly - there are widely differing opinions between parents about what is or is not appropriate at a whole range of ages. It's quite unreasonable to expect one filtered source to be appropriate for all parent's requirements, for all children (they are all different you know?!) over a wide range of ages. Then, we have three MILLION articles - nobody, no organisation, can possibly have checked them all. So we're left with some flaky keyword-based thing - which can't figure out what's in photos anyway. That will exclude some appropriate articles and fail to exclude some noxious ones. I simply don't believe this can work. But in any case - leaving a 6 year old alone with an internet-connected computer is just not reasonable. I'm sorry our OP objects - but it's true. Work with your kid - both you and she will be better off as result. SteveBaker (talk) 00:39, 3 December 2009 (UTC)
- With keyword-based filters you also have the problem of the unmentionable British town. -- 128.104.112.95 (talk) 22:44, 3 December 2009 (UTC)
- Buying the latest edition of Worldbook or just taking your child to the closest public library to read about things in that or similar encyclopedias is another great idea. Edison (talk) 00:24, 3 December 2009 (UTC)
Another question about visual phenomena...
When I close my eye and push hard on it, I see weird psychedelic greyness, like a swirly tunnel with RGB dots. What's that called?--Editor510 drop us a line, mate 18:52, 2 December 2009 (UTC)
- Not sure that is a good thing to do, but I am no eye doctor... Googlemeister (talk) 19:47, 2 December 2009 (UTC)
- Phosphene#Mechanical stimulation -- Finlay McWalter • Talk 19:48, 2 December 2009 (UTC)
- Thank you, Finlay. Googlemeister, I asked for an answer, not medical advice. Keep that in mind for next time.--Editor510 drop us a line, mate 20:08, 2 December 2009 (UTC)
- I guess this is a situation exemplifying the statement "There are no stupid questions... " Well, I am sure you can fill in the rest. Googlemeister (talk) 21:04, 2 December 2009 (UTC)
- "...There are only stupid people who quote trite clichès and leave off the end for others to fill in themselves."--Jayron32 21:18, 3 December 2009 (UTC)
- I guess this is a situation exemplifying the statement "There are no stupid questions... " Well, I am sure you can fill in the rest. Googlemeister (talk) 21:04, 2 December 2009 (UTC)
- Thank you, Finlay. Googlemeister, I asked for an answer, not medical advice. Keep that in mind for next time.--Editor510 drop us a line, mate 20:08, 2 December 2009 (UTC)
why do i feel hotter after a bath?
It gets cold where I live and I have no central heating. The house feels cold and the fire seems to not do much, however after a hot bath in the evening the house seems to be 50% hotter than it was. Is it my core temperature or something? —Preceding unsigned comment added by 89.242.173.139 (talk) 19:14, 2 December 2009 (UTC)
- There's probably a couple of things at work here. First, to point out the obvious, after a hot shower or bath you are warmer. People typically have an internal temperature of about 37C, but it's much cooler than that at your extremities (feet, hands) and, to a lesser extent, any part of you exposed to the environment (i.e. your skin). A hot bath can raise the temperature of your extremities, making you feel warmer overall. Your core temperature changes very little except under rather uncomfortable extremes such as hyperthermia, so I doubt you've affected your core temp more than a tiny amount. The benefit to having your extremities warmed (to me) is that they stop acting like heat sinks for the rest of the body. Cold feet, by themselves, are no big deal, but on some chilly days it almost feels like they're draining the heat right out of you. When you add heat energy to your feet through hot water, rather than through your own efforts, it seems like you get paid twice - your feet are warm and you haven't had to freeze your hands (or your partner's back!) to do it. Matt Deres (talk) 21:33, 2 December 2009 (UTC)
- Your temperature-detection equipment is in your skin; a hot bath raises the temperature of your skin, so there you are. As an aside, a simple fireplace will often cause a net heat loss in your house. You want some kind of wood stove or fireplace insert to make the fire put off more heat than it sucks up the chimney. --Sean 17:27, 3 December 2009 (UTC)
Children aargh
Ok, so I cannot always answer my offspring's question, here is one: "If you exclude a primary colour such as blue from the visual experience of a baby through to childhood we have been told they will not develop the ability to see and process that colour mentally, which I understand, but what if you exclude a secondary colour whilst allowing the separated components into their world: will their brain mix the colours and develop the secondary colour (say, green) via dreams etc or will they not develop a capacity to "see" the secondary colour in cognitive terms". Oh dear. Any help? --BozMo talk 20:43, 2 December 2009 (UTC)
- Who said that excluding a primary color will prevent a child from being able to see it? I'm just curious, because I haven't heard that, and I am wondering how they managed to test it. Falconusp t c 21:11, 2 December 2009 (UTC)
- Who said it in this case was a biology teacher so it could be suspect although I suspect "see and process" should be taken together. There is lots of stuff around about lazy eyes and some eye conditions do selectively filter frequencies/colours so "testing it" I assumed was more "observing it" --BozMo talk 21:17, 2 December 2009 (UTC)
- Firstly, green is a primary colour (it's additive or psychological primary colours that are relevant here and green is in both those groups, subtractive primary colours, which don't include green, are only relevant to painters). Secondly, could you provide a reference for your initial assertion? I'm not sure what would happen if you someone doesn't see a particular colour during their childhood. Under the opponent process theory, we perceive colour along two axes red-green and blue-yellow. I don't think you could exclude red, say, without also excluding green. That would mean you reduce colour to one axis. If the brain doesn't form the ability to see the other axis (and I don't know if it would or not) then you would essentially be colour blind (red-green colour blind or blue-yellow colour blind depending on which axis you exclude). If you exclude a secondary colour, it might be similar to the situation described in Opponent process#Reddish green and yellowish blue (which is about creating new secondary colours). In that case, people described it as a new colour that they couldn't recognise, but some were able to describe it as a combination of colours they knew. It would be reasonable to assume showing someone a secondary colour which is new to them would have a similar effect. --Tango (talk) 21:13, 2 December 2009 (UTC)
- That's helpful thanks, and good enough I hope to keep my daughter thoughtful for a couple of hours. --BozMo talk 21:19, 2 December 2009 (UTC)
Christmas lights
What is it that makes new-fangled LED Christmas lights look so harsh and cold, while old-fashioned incandescent Christmas lights look more warm? Something about the narrowness of their spectrum, prior life experience of them, or something else? - Nunh-huh 21:16, 2 December 2009 (UTC)
- I expect it is the narrowness of the spectrum. --Tango (talk) 21:18, 2 December 2009 (UTC)
- I think it's because most LEDs are clear while the larger, older lights are usually frosted to an extent. If you've ever bought the wrong type of (regular) light bulb for your home, the difference should be immediately obvious. ~ Amory (u • t • c) 21:45, 2 December 2009 (UTC)
- I suspect the answer is along the lines of what User:Tango said. Incandescent Christmas lights work by emitting white light, which is composed of all visible wavelengths, and then filtering it through a colored glass so that it appears colored, but light from the whole spectrum still gets through. LED lights, on the other hand, actually produce light of only one certain color, in a very narrow spectrum. It's this narrow spectrum that you're seeing as "harsh." Mildly MadTC 21:53, 2 December 2009 (UTC)
- I think the color temperature is a good answer, as 77.22.37.20 suggested. The retina is not a good spectrum analyzer to determine what wavelengths are present. The size of the source may also be a factor, since most incandescent Christmas bulbs are much larger than their LED rivals. Edison (talk) 23:21, 2 December 2009 (UTC)
- It may also be that LEDs are typically strobed to work at maximum efficiency - i.e. they flash on and off very rapidly. This should be faster than the eye can detect, but may still cause an effect of harshness. You can check whether they flash by moving them quickly - you should be able to see the flashes that way. --Phil Holmes (talk) 09:48, 3 December 2009 (UTC)
- I thought the strobing is due to plug-in LEDs running off AC current causing flickering at 50 or 60 Hz. I've never noticed the flickering when they operate under DC power. -- Flyguy649 talk 15:45, 3 December 2009 (UTC)
- Strobing LEDs definitely makes it look unnatural. If you slide your eyes quickly across a string of such lights you get a weird 'digital looking' trail instead of a smooth blur like you would with an incandescent
- If you're thinking of white Christmas lights, then a big part of the issue is the actual color of the lights. They're not quite white. "White" incandescent tree lights are slightly yellowish which we mentally associate with warmth, candles, etc. Most "white" LED lights are actually slightly blue, maybe not enough that you notice consciously, but subconsciously you associate them with coldness, and metal, and artificial light sources. APL (talk) 16:47, 3 December 2009 (UTC)
- Since most "white" LED's are typically ultraviolet LED's used to excite a phosphor coating - they could presumably fix that by reducing the amount of blue phosphor in the coating. However, because LED Xmas lights are not a vast market for white LED manufacturers - there isn't enough volume to justify making special off-white ones. I suspect that this will happen though - it's such a joy to have a string of lights actually work reliably after they've been pulled off a tree - stored in a garden shed for 340 days and then dumped back onto the tree again...when the prices fall by just a little more, the incandescent kind will go the way of the Dodo. I made my first set of LED Xmas lights myself - back before there were any that you could buy. I used 50 bi-color red/green LED's which can show any color in the red/orange/yellow/green range - all of which seem pretty Xmassy. They are still working - but the big DC power supply it takes to run them is a pain to deal with...so now I have store-bought ones. SteveBaker (talk) 18:39, 3 December 2009 (UTC)
- But going through each bulb on a series circuit testing each one to see where the loose one is is part of Christmas tradition! --Tango (talk) 18:48, 3 December 2009 (UTC)
- Since most "white" LED's are typically ultraviolet LED's used to excite a phosphor coating - they could presumably fix that by reducing the amount of blue phosphor in the coating. However, because LED Xmas lights are not a vast market for white LED manufacturers - there isn't enough volume to justify making special off-white ones. I suspect that this will happen though - it's such a joy to have a string of lights actually work reliably after they've been pulled off a tree - stored in a garden shed for 340 days and then dumped back onto the tree again...when the prices fall by just a little more, the incandescent kind will go the way of the Dodo. I made my first set of LED Xmas lights myself - back before there were any that you could buy. I used 50 bi-color red/green LED's which can show any color in the red/orange/yellow/green range - all of which seem pretty Xmassy. They are still working - but the big DC power supply it takes to run them is a pain to deal with...so now I have store-bought ones. SteveBaker (talk) 18:39, 3 December 2009 (UTC)
which element most toxic?
Which pure element is the most toxic if ingested? Natural elements only, don't worry about stuff heavier then Uranium. Is it arsenic? Googlemeister (talk) 21:41, 2 December 2009 (UTC)
- How much and in what form are we talking about here? Chlorine gas is up there, but if you could figure out how to do it I imagine elemental Fluorine would be far worse. ~ Amory (u • t • c) 21:51, 2 December 2009 (UTC)
- Well assume the natural state of the element at STP. So whereas drinking liquid helium would probably kill you, swallowing a bit of helium gas would not be lethal, so it is not that helium is naturally toxic. Googlemeister (talk) 21:56, 2 December 2009 (UTC)
- Beryllium, Plutonium, Mercury, Arsenic, Cadmium are all pretty nasty.[40] Fences&Windows 22:27, 2 December 2009 (UTC)
- But of lot of those are not as toxic as their salts, many of the heavy metals won't dissolve much in stomach acid, and small amounts will pass right through. Therefore one should consider things like sodium, potassium, bromine - not that you would ever be able to swallow them! Ronhjones (Talk) 22:35, 2 December 2009 (UTC)
- Polonium isn't great - it was used to kill Alexander Litvinenko in the UK a couple of years ago. Brammers (talk) 23:15, 2 December 2009 (UTC)
- But it didn't kill him via chemical toxicity, but by radiation. Polonium (any isotope) is so radioactive that there is probably no experiment that can measure its chemical toxicity, which is negligible in comparison. --Trovatore (talk) 00:28, 3 December 2009 (UTC)
- Right... and if you are allowed to pick non-stable elements, you can find some reeeaaaallly nasty ones, albeit they have very short half-lives. Whether you count these as "natural elements" of course relies on different definitions of "natural". --Mr.98 (talk) 03:25, 3 December 2009 (UTC)
- My objection was to the word "toxic". Radioactives will kill you, but they're not toxic, any more than a bullet is. Toxicity is a chemical property. --Trovatore (talk) 10:36, 3 December 2009 (UTC)
- Hmm while I'm not disagreeing with you our article does toxic Nil Einne (talk) 15:30, 3 December 2009 (UTC)
- I think toxicity means a bit more than you are claiming it does ("chemical toxicity" is one sub-variant of "toxicity"). I am fairly sure that highly radioactive substances are classed as highly toxic (ergo, Polonium#Toxicity). Where things get trickier are weakly radioactive but long-living substances (like, say, plutonium), which are not very acutely toxic (you will not keel over dead if you are exposed to it) but have long-term carcinogenic risks (you will get lung cancer in some number of years if you inhale a lot of it). --Mr.98 (talk) 17:51, 3 December 2009 (UTC)
- Plutonium is toxic in addition to being radioactive, because it's a heavy metal. Not very toxic, though; supposedly about as toxic as caffeine.
- I really think it's a misuse of the term to call radioactive substances "toxic" as a function of their radioactivity. Litvinenko wasn't poisoned, he was irradiated. Outcome is the same, of course, but it's a different thing. For that matter it strikes me as a bit off to call things poisons just because they are very acid or very alkaline — those are chemical properties but shading into physical ones. --Trovatore (talk) 21:01, 3 December 2009 (UTC)
- I think toxicity means a bit more than you are claiming it does ("chemical toxicity" is one sub-variant of "toxicity"). I am fairly sure that highly radioactive substances are classed as highly toxic (ergo, Polonium#Toxicity). Where things get trickier are weakly radioactive but long-living substances (like, say, plutonium), which are not very acutely toxic (you will not keel over dead if you are exposed to it) but have long-term carcinogenic risks (you will get lung cancer in some number of years if you inhale a lot of it). --Mr.98 (talk) 17:51, 3 December 2009 (UTC)
- Hmm while I'm not disagreeing with you our article does toxic Nil Einne (talk) 15:30, 3 December 2009 (UTC)
- My objection was to the word "toxic". Radioactives will kill you, but they're not toxic, any more than a bullet is. Toxicity is a chemical property. --Trovatore (talk) 10:36, 3 December 2009 (UTC)
- Right... and if you are allowed to pick non-stable elements, you can find some reeeaaaallly nasty ones, albeit they have very short half-lives. Whether you count these as "natural elements" of course relies on different definitions of "natural". --Mr.98 (talk) 03:25, 3 December 2009 (UTC)
- But it didn't kill him via chemical toxicity, but by radiation. Polonium (any isotope) is so radioactive that there is probably no experiment that can measure its chemical toxicity, which is negligible in comparison. --Trovatore (talk) 00:28, 3 December 2009 (UTC)
- I think beryllium (by far) is the most toxic element. The term you are looking for LD50. Try googling for LD50 and the names of some potential elements. I checked those that Fences posted, and beryllium easily beat the others. Also you have to distinguish between ingestion and inhalation (and injection possibly). Different things have different toxicity depending on the route of administration. Ariel. (talk) 01:23, 3 December 2009 (UTC)
- Isaac Asimov's 1954 short story 'Sucker Bait' hinges on the realisation (by a non-specialist polymath whom Asimov terms a "mnemonic") that a higher than usual level of beryllium in the crust of a recently discovered planet is responsible for the death of its first colonists. 87.81.230.195 (talk) 14:10, 3 December 2009 (UTC)
- Are you sure? In our article on Beryllium...
- Isaac Asimov's 1954 short story 'Sucker Bait' hinges on the realisation (by a non-specialist polymath whom Asimov terms a "mnemonic") that a higher than usual level of beryllium in the crust of a recently discovered planet is responsible for the death of its first colonists. 87.81.230.195 (talk) 14:10, 3 December 2009 (UTC)
Ingestion
Swallowing beryllium has not been reported to cause effects in humans because very little beryllium is absorbed from the stomach and intestines. Harmful effects have sometimes been seen in animals ingesting beryllium.[41]
Googlemeister (talk) 16:20, 3 December 2009 (UTC)
- Did you read the rest of the long toxicity section in the article? --Mr.98 (talk) 17:46, 3 December 2009 (UTC)
- I admit, I only skimmed the rest since my question specifically was referring to ingestion and not inhalation or skin contact. Googlemeister (talk) 19:18, 3 December 2009 (UTC)
- Did you read the rest of the long toxicity section in the article? --Mr.98 (talk) 17:46, 3 December 2009 (UTC)
The most toxic element is anti-matter. Ingesting even 1/1000 gram of anti-matter is fatal. 139.130.57.34 (talk) 20:55, 3 December 2009 (UTC)
- Possibly, but I don't see how you are going to get the antimatter (we will use antihydrogen since technically antimatter is not it's own element) into your mouth to swallow it before it annihilates. Googlemeister (talk) 21:14, 3 December 2009 (UTC)
- This is not toxicity; this is vaporization. --Trovatore (talk) 21:17, 3 December 2009 (UTC)
Solar panels
If you used a big magnifying glass to focus the sun's energy on one spot of a solar panel, could you therefore have a smaller panel while producing the same amount of power? What about a concave mirror? Dismas|(talk) 22:15, 2 December 2009 (UTC)
- Yes and yes. People often use reflectors and similar but there is a geometric problem that the sun moves so you have to move them or accept limited effectiveness. I have 11m2 of solar panel (water heating) on my roof plus a similar area of reflectors. It roughly doubles the measured output when the sun is in the correct position but not all day long. --BozMo talk 22:19, 2 December 2009 (UTC)
- At some degree of concentration the solar energy would destroy the photovoltaic panel segment it was focussed on, like setting a piece of paper on fire with a magnifying lens. There is clearly a limit on the amount of concentration a panel can tolerate, and a maximum on the electricity it can generate. Edison (talk) 23:18, 2 December 2009 (UTC)
- There's also a safety issue, as concentrating too much light can start fires, blind people, or burn them. Also, the "giant lens" might very well cost more than just having more solar cells. StuRat (talk) 07:16, 3 December 2009 (UTC)
- A giant reflector is a lot easier to make than a giant lens, which is why this Australian company chose to use those instead. --antilivedT | C | G 10:31, 3 December 2009 (UTC)
- On a related note, focussed sunlight is used to run concentrating solar power stations; there the sunlight generates heat to run a turbine (rather than using a solar panel). -- Finlay McWalter • Talk 01:34, 3 December 2009 (UTC)
December 3
Medical experiment that went terribly wrong
I remember reading a news story from a few years ago about a medical experiment that went terribly wrong. My memory is foggy but I'll try as best I can to explain what I remember. The researchers were testing an experimental drug. It was the first trial on humans. The test subjects had a terrible reaction to the drug. I can't recall if any of the test subjects died, but a couple might have. I remember that part of the controversy was that the scientists administered the drug to the test subjects back to back, rather than waiting to make sure that the first person didn't have a negative reaction. They may have violated medical protocols. This happened maybe 2 or 3 years ago. It recieved some mainstream media attention. Again, my memory is foggy, but I think I read about it at BBC News. Does anyone know what I'm talking about? A Quest For Knowledge (talk) 05:16, 3 December 2009 (UTC)
- Sounds a lot like the trials of TGN1412 — Zazou 05:34, 3 December 2009 (UTC)
- I think you've got it. It happened in England in March 2006; six patients got the drug at 10-minute intervals and it only took an hour before they began suffering one after the other. Nobody died, but they were all severely affected. --Anonymous, 08:28 UTC, December 3, 2009.
- Presuming this is what you mean, and it sounds to me like it is, while the 10 minutes interval thing generated a lot of controversy amongst other things and did seem like a dumb thing to do to many, I don't believe it was a violation of protocols or particularly unusual. In fact, as this ref suggests [41] for example, giving sufficient time for a reaction to be observed is a new recommendation arising from the trial Nil Einne (talk) 10:40, 3 December 2009 (UTC)
- They gave systemic doses of a previously untested drug instead of giving it topically to begin with. It was a drug designed to boost the immune system, they gave it to healthy patients, and it resulted in a cytokine storm. This was definitely predictable and as an immunologist noted "not rocket science". Fences&Windows 14:40, 3 December 2009 (UTC)
- Maybe but that doesn't mean it violated the protocols of the time which was the point I was addressing. To put it a different way, they may have screwed up badly, but it doesn't mean they ignored established protocols, more that perhaps they didn't think properly whether the protocols were appropriate in the specific instance. On the other hand this [42] does suggest it's normal to try hazardous agents on one patient first so it may not have been uncommon as the earlier ref. However it isn't peer reviewed. There is of course still research ongoing as a result of the case. E.g. [43] [44] Nil Einne (talk) 15:44, 3 December 2009 (UTC)
- They gave systemic doses of a previously untested drug instead of giving it topically to begin with. It was a drug designed to boost the immune system, they gave it to healthy patients, and it resulted in a cytokine storm. This was definitely predictable and as an immunologist noted "not rocket science". Fences&Windows 14:40, 3 December 2009 (UTC)
- Presuming this is what you mean, and it sounds to me like it is, while the 10 minutes interval thing generated a lot of controversy amongst other things and did seem like a dumb thing to do to many, I don't believe it was a violation of protocols or particularly unusual. In fact, as this ref suggests [41] for example, giving sufficient time for a reaction to be observed is a new recommendation arising from the trial Nil Einne (talk) 10:40, 3 December 2009 (UTC)
- Perhaps the X-linked severe combined immunodeficiency gene therapy trial? Or less likely the gene therapy trial that killed Jesse Gelsinger. 75.41.110.200 (talk) 06:40, 3 December 2009 (UTC)
Yes, that's it. Thanks! A Quest For Knowledge (talk) 23:44, 3 December 2009 (UTC)
Butterfly sensation from infatuation.
There's a girl I've recently become infatuated with and I think she reciprocates my affections at least to some degree. Sometimes, I'll go many minutes without thinking of her and then suddenly, in a flash, I'll remember her-- infectious laughter, her supple contour, her stellar character, her daring wit, & her infinite, limpid, brown eyes... Accompanying these thoughts, I often experience a sinking sensation in my stomach or heart -- butterflies, I think it's sometimes called. What is the cause of this delicious sinking feeling? What are the biological and physical reasons for it? —Preceding unsigned comment added by 66.210.182.8 (talk) 05:41, 3 December 2009 (UTC)
- Wikipedia has an article on everything. Looking at that article, it seems that the main component is due to anxiety, possibly due to adrenalin. Vimescarrot (talk) 09:52, 3 December 2009 (UTC)
- and good luck! --pma (talk) 13:31, 3 December 2009 (UTC)
- Well, we really ought to have an article on the neurobiology of love; there is enough of a literature. In the absence of an article, here is a pointer to a recent paper with a lot of information, a bit technical though. Looie496 (talk) 17:22, 3 December 2009 (UTC)
- Also Esch, Tobias (2005). "The Neurobiology of Love" (PDF). Neuroendocrinology Letters. 3 (26).
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Fences&Windows 23:25, 3 December 2009 (UTC) - And a video about how the key to love is oxytocin. Fences&Windows 23:29, 3 December 2009 (UTC)
- Also Esch, Tobias (2005). "The Neurobiology of Love" (PDF). Neuroendocrinology Letters. 3 (26).
- Well, we really ought to have an article on the neurobiology of love; there is enough of a literature. In the absence of an article, here is a pointer to a recent paper with a lot of information, a bit technical though. Looie496 (talk) 17:22, 3 December 2009 (UTC)
Physical fallacies
Hi, I posted this question about speed of light calculations few months ago. Is there an article discussing such physical fallacies? If yes, can anyone volunteer to explain where the wrong use of physical laws was made in that website? I think such information should have the same interest as has been done to mathematical fallacy article.--Email4mobile (talk) 09:26, 3 December 2009 (UTC)
- It seems to me you got a good answer last time. What else do you want to know? Second the paragraph "Variable Speed of Light" is not true. Not at all. It's completely contrary to the theory of relativity. And third scientists have NOT confirmed the existence of Dark Energy. Why do you want to learn anything at all from a website that does not understand science? If you want to theorize on changes to science go for it. But don't think for a second that what they say is correct by current theories. Unlike some, I don't mind speculating on changes to current thinking (historically the accepted scientific thinking of the day has been wrong quite often, I see no reason to believe we are in a unique period today) - but it's always important to note when your speculations differ from current understanding. Ariel. (talk) 11:07, 3 December 2009 (UTC)
- I agree with Ariel, it is usually pretty pointless entering a scientific discussion with fundamentalists. The fundamentalist position starts from the premise that all truth emanates from their holy book (of whatever religion). It is intolerable to them that anyone else can obtain "truth" from another source, hence the strong desire to "prove" that their holy reference manual contains that truth, though it was previously somehow overlooked by everyone. I guarantee that no-one has previously interpreted that passage in the Koran as meaning the speed of light until long after science came up with an accurate measurement of it. Science starts from a radically different position, and mutually incompatible with the fundamentalist view. The scientific position is that truth (the laws of nature) is the simplest possible interpretation consistent with the experimetal results. This means that science will modify its laws in the light of new evidence. The fundamentalist can never do this, contradictory evidence will only cause the reasoning to become ever more contrived in order to make the holy book remain true.
- I like the postulate on that site that Angels travel at the speed of light. If that is true, it means they are inside our own light cone and exist in our universe, not in some other ethereal existence. In principle then, they are scientifically detectable - but it is strange that no experiment, so far, has found them. SpinningSpark 14:03, 3 December 2009 (UTC)
- Perhaps, but then we have never found any dark matter either. Googlemeister (talk) 14:22, 3 December 2009 (UTC)
- Angels are photons, God is a singularity, and Satan is the heat death of the universe. Fences&Windows 14:31, 3 December 2009 (UTC)
- That analogy doesn't work. In christian and jewish versions of the story, Satan is an angel who "turns to the dark side". I don't see how the heat death of the universe is also analogous to a photon. The information content of a singularity is restricted to it's mass and maybe it's spin...doesn't bode well for something that's supposed to be all-knowing and therefore containing an infinite amount of information!
- Anyway - these kinds of websites are nonsense. It's very easy to come up with similar nonsense - it doesn't prove anything - the best you can do is ignore them. You can find approximate coincidences in ratios of numbers everywhere - it doesn't prove anything. Precise relationships are more interesting - but even then may not mean much. Let's look at one "fact" from that page:
- "But 1400 years ago it was stated in the Quran (Koran, the book of Islam) that angels travel in one day the same distance that the moon travels in 1000 lunar years, that is, 12000 Lunar Orbits / Earth Day. Outside the gravitational field of the sun 12000 Lunar Orbits / Earth Day turned out to be the local speed of light!!!" - Well, how far does the moon travel in 1000 "lunar years"? What the heck is a "lunar year" anyway? If it's the time it takes the moon to orbit the sun - then that's almost exactly the same as a regular year - and the distance the moon travels over that time (relative to the earth) is 1.022km/s x 1000 x 365.25 x 24 x 60 x 60 = 32,251,000,000km - the distance light travels in a day is 1,079,000,000 km/hr x 24 = 25,896,000,000 km. So these supposed angels are travelling at about 25% faster than the speed of light. I'm not sure what the gravitational field of the sun has to do with it - the speed of light is constant and the sun's gravity can't change that, it can distort time a bit - but nothing like 25%. Now, you might consider the distance travelled by the moon relative to the sun...that's a bit tougher to calculate but it's got to be a lot more than it moves relative to the earth - so that just makes the situation worse. So this guy has an error of 25% in his calculations - that's simply not acceptable in any kind of scientific argument. The errors in our measurements of the speed of light and the speed of the moon are tiny TINY fractions of a percent. So this argument must be incorrect...period. SteveBaker (talk) 17:43, 3 December 2009 (UTC)
- Not really related, but satan in jewish thought is NOT an angel that went to the dark side. Stan is more akin to a prosecutor, who works for god, has no free will! Ariel. (talk) 20:19, 3 December 2009 (UTC)
- While I agree with Steve's overall sentiment, he is a bit overzealous with regard to numerical accuracy in astrophysics. For a lot of parameters, 25% error is acceptable in astrophysics... for example, look at some of the tolerances on the parameters of a typical exoplanet, CoRoT Exo B, as documented by the ESA. Its density is quoted with a 30% error bar. I've seen much more speculative numbers with worse uncertainty in other publications. Stellar physics publications are lucky if they can estimate some numbers to within a factor of 10. But these parameters are not the speed of light, which is well known to better than one part in a billion. In general, a "high level of accuracy" is context-specific. In any case, the above argument is making an outlandish claim, so a greater burden of proof is in order. While I can stomach a 50% uncertainty about whether an exoplanet is iron- or silicate-core, I don't have the same tolerance for the "angels are photons" argument. Because those claims are much more unbelievable, I would expect a much higher standard of accuracy before giving them even the slightest little bit of credibility. I guess my point can be summarized as follows: the above claims are false - but not simply because the numerical error is very large. Numerical error is acceptable, if the scientific claims are qualitatively correct. The above claims about "lunar years" are simply wrong, so it's useless to even bother analyzing their accuracy. Nimur (talk) 17:52, 3 December 2009 (UTC)
- No, I'm not being overzealous. Errors that big are acceptable only when the data you're working from has error bars that big. The error bar on the speed of light is a very small fraction of a percent - and so is the speed of the moon, the length of a year and all of the other things that made up that calculation. The numbers I calculated for the distance travelled by the moon over 1000 years and the distance travelled by light in a day are accurate to within perhaps one part in a thousand. The discrepancy between them is 25%!! There is no way that those numbers back up that hypothesis - and no respectable scientist would say otherwise. Since our confidence in the speed of the moon, etc is very high - the hypothesis that the Koraan is correct about the nature of angels is busted. It flat out cannot be true. (Well, technically - the number "1000 years" has unspecified precision. I suppose that if the proponents of this theory are saying "1000 years plus or minus 50%" and therefore only quoting the number to one significant digit - then perhaps we have to grant that it is possible (not plausible - but possible). But I'm pretty darned certain that the proponents of this theory would tell us that when this holy book say 1000 - it means 1000.0000000000000000000000...not 803.2 - which would be the number required to make the hypothesis look a little more credible! Hence, probably, the necessity of muddying the water by dragging the sun's gravitational field into the fray - the hope being that anyone who tries the naive calculation above can be bamboozled into accepting the result as being 100% correct once general relativity has been accounted for...but sadly, that's not the case because none of the bits of the solar system involved are moving anything like fast enough relative to each other and the sun's gravitational field simply isn't that great.) SteveBaker (talk) 18:26, 3 December 2009 (UTC)
- For the purposes of establishing the actual facts of this claim, I looked up the quoted passage and got;
- He regulates the affair from the heaven to the earth; then shall it ascend to Him in a day the measure of which is a thousand years of what you count. (The Adoration 32:5)
- I was going to post just the quote and leave it at that. However, I was intrigued by the lack of mention of the moon in the passage, or indeed, in the entire book (or chapter or whatever the Koran calls its subdivisions). Apparently we must read "the measure of what you count" as meaning a lunar year. So looking a bit further I found this;
- To Him ascend the angels and the Spirit in a day the measure of which is fifty thousand years. (The Ways of Ascent 70:4)
- Sooo, to be consistent we must interpret that the same way and now have angels travelling at 50C, and if the interpretation that angels travel at the speed of light or slower is to be maintained we must conclude that the Koran would have the speed of light to be at least 1.5x1010. I think that pretty much rules out the Koran as a potential reliable source for Wikipedia purposes. SpinningSpark 19:07, 3 December 2009 (UTC)
- For the purposes of establishing the actual facts of this claim, I looked up the quoted passage and got;
- No, I'm not being overzealous. Errors that big are acceptable only when the data you're working from has error bars that big. The error bar on the speed of light is a very small fraction of a percent - and so is the speed of the moon, the length of a year and all of the other things that made up that calculation. The numbers I calculated for the distance travelled by the moon over 1000 years and the distance travelled by light in a day are accurate to within perhaps one part in a thousand. The discrepancy between them is 25%!! There is no way that those numbers back up that hypothesis - and no respectable scientist would say otherwise. Since our confidence in the speed of the moon, etc is very high - the hypothesis that the Koraan is correct about the nature of angels is busted. It flat out cannot be true. (Well, technically - the number "1000 years" has unspecified precision. I suppose that if the proponents of this theory are saying "1000 years plus or minus 50%" and therefore only quoting the number to one significant digit - then perhaps we have to grant that it is possible (not plausible - but possible). But I'm pretty darned certain that the proponents of this theory would tell us that when this holy book say 1000 - it means 1000.0000000000000000000000...not 803.2 - which would be the number required to make the hypothesis look a little more credible! Hence, probably, the necessity of muddying the water by dragging the sun's gravitational field into the fray - the hope being that anyone who tries the naive calculation above can be bamboozled into accepting the result as being 100% correct once general relativity has been accounted for...but sadly, that's not the case because none of the bits of the solar system involved are moving anything like fast enough relative to each other and the sun's gravitational field simply isn't that great.) SteveBaker (talk) 18:26, 3 December 2009 (UTC)
- At the core of the issue, it's difficult/impossible to assess the scientific merits of an unscientific line of reasoning. This theory, and others like it, are very inconsistent, are not based on empirical observation, and do not draw logical conclusions from experimental data. Therefore any assertions that it makes are categorically unscientific. It doesn't matter what the error-bars are on its numeric results. A lot of numerology finds exact values via convoluted procedures. That "accuracy" does not mean the methods are sound or scientific. In the same way, the inaccuracy of the above numbers is irrelevant - the method is simply wrong. Nimur (talk) 19:09, 3 December 2009 (UTC)
- Also, I object to SpinningSpark's comment, "that pretty much rules out the Koran as a potential reliable source for Wikipedia purposes." The Koran is a reliable source for information about Islam'. It is a very reliable source for Wikipedia's purposes when those purposes are related to Islam. It'd be hard to find a more reliable source for our article about Islam, for example. But, the Quran is not a scientific book, and sourcing scientific claims from it would be invalid. Since this is the science desk, we should never source our references from the Quran or any other "holy book;" nor should we source scientific claims from history books, poetry books, or other non-scientific references. However, that doesn't mean that these are unreliable sources - it's just the wrong source for the Science Desk or science-related issues. Nimur (talk) 19:16, 3 December 2009 (UTC)
- Quite so, I had intended to qualify that with "...for scientific articles" or some such, but typed the more general "Wikipedia" instead. SpinningSpark 19:32, 3 December 2009 (UTC)
- The issue is not that people believe the Quran - that's entirely their own problem - it's that some people are attempting to portray what it says as somehow reliably relevant and applicable to modern science. Plainly, it's not...or at least not as that website explains it. But if he can't get his science right and he can't quote the Quran accurately then it's really no use to anyone. SteveBaker (talk) 19:44, 3 December 2009 (UTC)
- Quite so, I had intended to qualify that with "...for scientific articles" or some such, but typed the more general "Wikipedia" instead. SpinningSpark 19:32, 3 December 2009 (UTC)
- Also, I object to SpinningSpark's comment, "that pretty much rules out the Koran as a potential reliable source for Wikipedia purposes." The Koran is a reliable source for information about Islam'. It is a very reliable source for Wikipedia's purposes when those purposes are related to Islam. It'd be hard to find a more reliable source for our article about Islam, for example. But, the Quran is not a scientific book, and sourcing scientific claims from it would be invalid. Since this is the science desk, we should never source our references from the Quran or any other "holy book;" nor should we source scientific claims from history books, poetry books, or other non-scientific references. However, that doesn't mean that these are unreliable sources - it's just the wrong source for the Science Desk or science-related issues. Nimur (talk) 19:16, 3 December 2009 (UTC)
- At the core of the issue, it's difficult/impossible to assess the scientific merits of an unscientific line of reasoning. This theory, and others like it, are very inconsistent, are not based on empirical observation, and do not draw logical conclusions from experimental data. Therefore any assertions that it makes are categorically unscientific. It doesn't matter what the error-bars are on its numeric results. A lot of numerology finds exact values via convoluted procedures. That "accuracy" does not mean the methods are sound or scientific. In the same way, the inaccuracy of the above numbers is irrelevant - the method is simply wrong. Nimur (talk) 19:09, 3 December 2009 (UTC)
- A lunar year is 12 lunar months, which is about 354 days. That makes it a little closer than your calculation gave, but not by much. --Tango (talk) 22:37, 3 December 2009 (UTC)
- Well, I'm an Arab and Muslim too; though I don't believe for any reason to connect between religion and Science. Unfortunately many Muslims believe. I'm afraid to say the one who tried to prove this fallacy was originally a professor as I heard. If I were just an engineer then how could I convince so may people who are spreading such information not only in that website but in the schools and universities. How can they believe me such information are totally mess unless I can verify that from reliable sources and I believe in Wikipedia because it either gives reliable sources or proofs. On the one hand, I still believe this problem is not just in Muslim countries but almost all religions have some extremist who would like to convince others by any means. Anyhow thank you very much for this wonderful interaction.--Email4mobile (talk) 20:38, 3 December 2009 (UTC)
- I agree - it's certainly not just the Quran that makes these kinds of error. The Christian bible says that Pi is 3 and that bats are a species of bird. This is what happens when you try to take written material that's several thousands of years old and apply it to everything we've learned in the meantime. The fact is that we shouldn't expect this stuff to be halfway reasonable - the problem isn't the books - it's that people are still trying to apply it to modern situations. SteveBaker (talk) 00:53, 4 December 2009 (UTC)
- Steve, I know you're not a big fan of the Bible, fine, but don't say nonsense about it. Nowhere does it say pi is 3. It says someone made a "molten of sea" that was 10 cubits across and 30 cubits round about. From there to "pi==3" there are a couple of large logical jumps. --Trovatore (talk) 00:59, 4 December 2009 (UTC)
- I agree - it's certainly not just the Quran that makes these kinds of error. The Christian bible says that Pi is 3 and that bats are a species of bird. This is what happens when you try to take written material that's several thousands of years old and apply it to everything we've learned in the meantime. The fact is that we shouldn't expect this stuff to be halfway reasonable - the problem isn't the books - it's that people are still trying to apply it to modern situations. SteveBaker (talk) 00:53, 4 December 2009 (UTC)
- Back to Steve: A Lunar year is a year in the lunar calendar, i.e. in this case likely the Islamic calendar. It consist of 12 lunar months, i.e. 354 or 355 days, depending on how the fractions work out. That's how the original author arrives at the 12000 (12 months times 1000 years). So the error is about 3 percentage points worse than your result. --Stephan Schulz (talk) 20:50, 3 December 2009 (UTC)
- Yes, Stephan, I think I've already mentioned that in the previous discussion but not sure it SteveBaker noticed that. To me I've accepted this step of calculations but was surprised when he again used another kind of conversions to achieve cos(26.92952225o) in order to reach 0.01% error. That was the point I wanted to swallow, but couldn't understand how (See the details here).--Email4mobile (talk) 21:02, 3 December 2009 (UTC)
- Apart from that other verse talking about 50,000 years a day, let's first verify the 1000 years a day calculation, Spinningspark.--Email4mobile (talk) 21:21, 3 December 2009 (UTC)
Lines of little circles of light on camera
How come when a camera shoots something very bright like a brief shot of the sun, you often see little circles, usually as if they were strung together along a line? 20.137.18.50 (talk) 12:56, 3 December 2009 (UTC)
- It's caused by light reflecting back and forth between the surfaces of the lenses. Cameras with high quality lenses don't do it nearly so much. The dots you see in the 'flare' aren't always circles - sometimes they are pentagonal or hexagonal. In this photo they seem to be 7-sided. SteveBaker (talk) 17:16, 3 December 2009 (UTC)
- Fixed your link. APL (talk) 17:22, 3 December 2009 (UTC)
- It's caused by light reflecting back and forth between the surfaces of the lenses. Cameras with high quality lenses don't do it nearly so much. The dots you see in the 'flare' aren't always circles - sometimes they are pentagonal or hexagonal. In this photo they seem to be 7-sided. SteveBaker (talk) 17:16, 3 December 2009 (UTC)
Rainbow ham?

What causes the rainbow color that I sometimes see in ham and other cured meats? This says it's a "chemical reaction" (not telling much more), this says it's birefringence, which is a nicer word, but our article on birefringence doesn't mention this effect at all. (If it is birefringence, this is probably one of the most common effects of birefringence encountered in the typical life of citizens of the western world. Probably deserves a mention.) Staecker (talk) 17:35, 3 December 2009 (UTC)
- A lot of cured meats are soaked in a brine, saline solution, or other liquid to add volume and flavor to them. The birefringence or other optical effects are often the result of these saline liquids suspended in the interstitial spaces of the meat. Nimur (talk) 17:46, 3 December 2009 (UTC)
- There are several possibilities - one is that we're seeing an "oil on water" effect because oils from the meat are mixing with water - another is that we're seeing some kind of Dichroism effect - yet another is some kind of coherent scattering - similar to the thing that makes the colorless scales of a butterfly's wing show up in such vivid, iridescent colors. There are a lot of related effects and this could easily be any one of them - or even some complicated combination of them. Without some kind of expert study - I don't think we should speculate. SteveBaker (talk) 18:07, 3 December 2009 (UTC)
- We can, however, point to prior research, e.g. Prediction of texture and colour of dry-cured ham by visible and near infrared spectroscopy using a fiber optic probe, Journal of Meat Science, 2005. Virtually everything that can possibly be observed, and many things that can't, has already been studied and published somewhere. Nimur (talk) 18:10, 3 December 2009 (UTC)
- Darn! How did I miss that? I'm such an avid reader of the Journal of Meat Science! SteveBaker (talk) 19:38, 3 December 2009 (UTC)
- There are several possibilities - one is that we're seeing an "oil on water" effect because oils from the meat are mixing with water - another is that we're seeing some kind of Dichroism effect - yet another is some kind of coherent scattering - similar to the thing that makes the colorless scales of a butterfly's wing show up in such vivid, iridescent colors. There are a lot of related effects and this could easily be any one of them - or even some complicated combination of them. Without some kind of expert study - I don't think we should speculate. SteveBaker (talk) 18:07, 3 December 2009 (UTC)
Does such a disease exist?
Is there a disease where the neurons of the brain spontaneously form synapses with all their neighboring neurons at an accelerated rate, essentially forming one very deeply interconnected mess? 20.137.18.50 (talk) 18:27, 3 December 2009 (UTC)
- Never heard of anything like that. If there were a mutation that did that, it seems likely to me that it would be fatal at a pretty early stage of embryonic development. Looie496 (talk) 20:41, 3 December 2009 (UTC)
- Relevant articles are Synaptogenesis and Synaptic pruning. Landau–Kleffner syndrome and continuous spikes and waves during slow sleep syndrome, related to epilepsy, both involve too much synaptogenesis during childhood due to electrical activity that strengthens the synapses.[45] Fences&Windows 23:12, 3 December 2009 (UTC)
- There is a great variety of proteins that participate in axonal guidance and/or affect synaptogenesis. See, for example, FMR1, Thrombospondin, semaphorins, and Amyloid precursor protein. I am not familiar with the specific pathology you refer to, though. --Dr Dima (talk) 00:48, 4 December 2009 (UTC)
cheesewring stones
It does not say in the article, but is the Cheesewring a natural formation, or is it man made like Stonehenge? Googlemeister (talk) 20:26, 3 December 2009 (UTC)
- Looks natural to me. In southern Arizona there are hundreds of rock formations that look like that -- made of sandstone rather than granite though. Looie496 (talk) 20:37, 3 December 2009 (UTC)
- The article states "Geological formation" which implies natural rather than man made source. In Southwestern Utah there are formations called Hoodoos (you've seen them in the old Wile E. Coyote cartoons). Geology + psychology is capable of some remarkable looking formations. I remember taking some college friends to Northern New Hampshire to see the Old Man of the Mountain (RIP), and they kept asking "No really, who carved that? Was it the Indians?" I kept trying to tell them it was just a natural formation. Other fun natural formations which have been mistaken for manmade include the Giant's Causeway in Ireland, the Pingos of northern Canada, the Badlands Guardian of Alberta, the Cydonia face on Mars, etc. --Jayron32 21:07, 3 December 2009 (UTC)
- Apparently we are lucky that it still exists. Looie496 (talk) 21:22, 3 December 2009 (UTC)
- The article states "Geological formation" which implies natural rather than man made source. In Southwestern Utah there are formations called Hoodoos (you've seen them in the old Wile E. Coyote cartoons). Geology + psychology is capable of some remarkable looking formations. I remember taking some college friends to Northern New Hampshire to see the Old Man of the Mountain (RIP), and they kept asking "No really, who carved that? Was it the Indians?" I kept trying to tell them it was just a natural formation. Other fun natural formations which have been mistaken for manmade include the Giant's Causeway in Ireland, the Pingos of northern Canada, the Badlands Guardian of Alberta, the Cydonia face on Mars, etc. --Jayron32 21:07, 3 December 2009 (UTC)
North Korea's closed-circuit speaker system
In this article and at least one other at the Wall Street Journal, they say that the North Korean authorities notified the citizenry of the replacement of the North Korean won by means of "a closed-circuit system that feeds into speakers in homes and on streets, but that can't be monitored outside North Korea."
Speakers in homes? Really? Do we have a Wikipedia article on this system? Is this cable TV but without the TV? How many homes are equipped with this technology? I have a raft of questions. Tempshill (talk) 21:54, 3 December 2009 (UTC)
- Is this science? Anyhow... from the New York Times: "Every North Korean home has a speaker on the wall. This functions as a radio with just one station -- the voice of the Government -- and in rural areas speakers are hooked up outside so that peasants can toil to the top 40 propaganda slogans. Some of the speakers are hooked directly into the electrical wiring, so that residents have no way of turning them off; they get up when the broadcasts begin and go to sleep when the propaganda stops. In some homes, however, the speakers have a plug, and people pull the plug when they want some quiet."[46] Just like in 1984. Something similar but less scary in Australia: "loudspeakers are sprouting like mushrooms on Sydney streets, peering down from the tops of traffic lights. The State Government has begun to put in place a permanent public address network that will, in some unspecified emergency, tell people what to do."[47] Fences&Windows 22:47, 3 December 2009 (UTC)
- You might find this: [48] link interesting. It has a photo of a similar hard wired radio(?) in russia. Ariel. (talk) 00:03, 4 December 2009 (UTC)
- Back in the 70s and 80s -- and probably well before that -- there was a ubiquitous contraption called "radiotochka" (radio spot) in the USSR households. IIRC the radio signal was transmitted via the electric wires of the power grid and not by air. I do not know how the signal was modulated, but I am pretty sure it was separated in frequency from the 50 Hz AC current the wires were carrying. There was only one station. Yes, it was government-controlled, but so was the TV, anyway; and it could be turned off or unplugged any time you like, of course :) . I doubt it that it transmitted anything back, but in principle I guess it could double as a bug for the bolsheviks to eavesdrop on you. --Dr Dima (talk) 00:08, 4 December 2009 (UTC)
- (I haven't seen Ariel's post when I edited mine, but I didn't get the EC screen either. Weird.) anyway, Ariel, yes, that's it in the picture. It had one station only, though, not three; or maybe it had three in some places. Or maybe the other two were added after I emigrated :). --Dr Dima (talk) 00:13, 4 December 2009 (UTC)
- Would you mind creating an article on it? It's ok if you don't know everything about it, just get it started and put in what you do know. (I know nothing about it. But maybe I can ask the person who posted the photo to contribute.) Ariel. (talk) 00:34, 4 December 2009 (UTC)
- Last time I've seen a radiotochka was about 20 years ago. I do not think my memory from back then is accurate enough for me to write a Wikipedia article about it now. Sorry. --Dr Dima (talk) 01:07, 4 December 2009 (UTC)
- Would you mind creating an article on it? It's ok if you don't know everything about it, just get it started and put in what you do know. (I know nothing about it. But maybe I can ask the person who posted the photo to contribute.) Ariel. (talk) 00:34, 4 December 2009 (UTC)
- (I haven't seen Ariel's post when I edited mine, but I didn't get the EC screen either. Weird.) anyway, Ariel, yes, that's it in the picture. It had one station only, though, not three; or maybe it had three in some places. Or maybe the other two were added after I emigrated :). --Dr Dima (talk) 00:13, 4 December 2009 (UTC)
- Back in the 70s and 80s -- and probably well before that -- there was a ubiquitous contraption called "radiotochka" (radio spot) in the USSR households. IIRC the radio signal was transmitted via the electric wires of the power grid and not by air. I do not know how the signal was modulated, but I am pretty sure it was separated in frequency from the 50 Hz AC current the wires were carrying. There was only one station. Yes, it was government-controlled, but so was the TV, anyway; and it could be turned off or unplugged any time you like, of course :) . I doubt it that it transmitted anything back, but in principle I guess it could double as a bug for the bolsheviks to eavesdrop on you. --Dr Dima (talk) 00:08, 4 December 2009 (UTC)
Environmental Impact of ebooks vs paper books
I've seen some e-book distributors advertising ebooks as environmentally friendlier than the 'dead tree' version. On the face of it this seemed reasonable; no trees, no chemicals for paper and ink making, no distribution of heavy books, no bricks and mortar stores (and all the energy to run them), but then I started thinking about the computing required to deliver ebooks. So, which is more environmentally friendly? I'll leave it to you to decide how much of the production / distribution / consumption chain to include, also what constitutes 'environmentally friendly'. Scrotal3838 (talk) 22:02, 3 December 2009 (UTC)
- Hmm well this page and this page outline some perceived problems with paper. See also Pulp (paper). On the other hand Electronic waste is often portrayed as being bad fairly serious, and factories that produce Kindles or computers or whatever of course also pollute. On the balance, however, I'd say that electronic distribution is much more environmentally friendly. It could (theoretically) replace a huge amount of printed material, and I just don't think there's any way the pollution generated making a kindle could add up to the pollution generated making a piece of paper for every page a kindle electronically displays. As far as energy to run servers and the devices themselves, I really doubt you could quantify ebooks as being anything but a marginal energy use. I don't see why ebook distribution would take up any more energy than a regular website, which on an energy per unit of information basis is extremely efficient.
- However, the argument should be taken with a grain of salt, in my opinion. People were predicting similar improvements with the advent of email replacing memos. But paper use over the period when email became widespread increased, due to it being much easier to produce documents with modern printers and (ironically?) people printing out their work emails to have a paper copy. I forget where I read that last bit, I think it was in the Economist. Regardless, I think ebooks could be portrayed as better for the environment if it can be demonstrated that the user in fact uses less paper, and doesn't just use the same amount of paper and an electronic device that has an environmental impact in its creation, operation and disposal. TastyCakes (talk) 23:26, 3 December 2009 (UTC)
Stars
How are we able to see stars if they are so far away? jc iindyysgvxc (my contributions) 22:02, 3 December 2009 (UTC)
- They are bright. --Jayron32 22:09, 3 December 2009 (UTC)
- There's not a lot in the way. Light doesn't just fade away over long distances -- it has to go through plenty of interstellar dust before becoming indiscernible. Vranak (talk) 22:12, 3 December 2009 (UTC)
- It does spread out, though. The brightness of nearby stars is determined more by the inverse square law than extinction. --Tango (talk) 22:15, 3 December 2009 (UTC)
A more interesting question might be: "How are we able to look at any of the night sky and not see stars?" See Olbers' paradox. Dragons flight (talk) 23:22, 3 December 2009 (UTC)
- The previous answers are missing a critical point - and (sadly) it's a somewhat complicated explanation.
- The sun is a star - a pretty normal, boring kind of star just like many others in the sky. It's so bright that you can't look at it for more than the briefest moment without wrecking your eyesight. Most of the other stars out there are at least that bright - and space is pretty empty - interstellar gasses and dust make very little difference. So the only real effect is that of distance.
- As others have pointed out, that's driven by the "inverse square law" - when one thing is twice as far away as another similar thing - it's four times dimmer - four times further away means 16 times dimmer and so on. The sun is only 93 million miles away - that's 8 light-minutes. The nearest star is 4 light-years away. Let's consider Vega (which is one of the brightest stars in the sky) - if you were 93 million miles away from it - it would be about 37 times brighter than our sun and you'd need some pretty good sunglasses and a good dollop of SPF-50! But fortunately, it's 25 light years away. So, Vega is 25x365x24x60/8...about one and a half million times further away. Which means that even though it's 37 times brighter when you're up close, it's 1.5Mx1.5M/37 times dimmer from where we're standing (73 billion times dimmer) because of that inverse-square law thing.
- Our eyes are able to see a range of brightnesses from the maximum (which is about where the sun's brightness is) to a minimum of about 10 billion times dimmer than that. On that basis, Vega ought to be about 7 times too dim for us to see - but it's not. It's actually pretty bright. So you can tell right away that that inverse square law that everyone is going on about ISN'T the whole story.
- There is obviously something else going on - and that is that the total amount of light from the sun is spread over that large disk you see in the sky - and while Vega is 73 billion times dimmer, all of that light is collected into one tiny dot. It gets hard to calculate the effect that has - but it's actually rather significant because the apparent size of the sun compared to that of Vega is gargantuan. In fact, the apparent area of an object obeys the same inverse-square law as the brightness does - so when you double the distance to something, it looks four times smaller (in area, that is). That concentration of light from a perceptually large object into progressively smaller areas of our retina exactly counteracts the inverse-square law.
- Someone's going to complain about that - but think about it...that's why you can see something quite clearly when it's 200 feet away and it's not 40,000 times dimmer than when it's 1 foot away!
- That means that until you are so far away that the sun is just a speck that's comparable to the resolution of your retina - it's not really any dimmer to look at than it is up close. The total amount of light is much less - but the light coming hitting each cell in your retina is exactly the same - until the projected image of the sun on the back of your eye starts to get smaller than the size of a single cell. So if you were out at the orbit of (say) Pluto - where the sun casts almost no heat and very little light - staring at the sun's tiny disk would still ruin a very small patch of your eyeball.
- But still, 73 billion is a big number - Vega is still a heck of a lot dimmer - as you'd expect. However: remember that the sun is bright enough to literally blind you - and that your eyes are really sensitive - we can see things that are 10 billion times dimmer than the sun - so it's actually quite easy to see Vega even in very light-polluted cities. Much dimmer stars are also visible to the naked eye.
- I understand that an interesting question is why the night sky is not bright white rather than black, as an infinite number of stars would lead to the former. 89.242.105.246 (talk) 01:13, 4 December 2009 (UTC)
The most useless particle
Say you had to choose one type of subatomic particle to be completely rid of: every single particle of that kind would completely disappear and no process would ever produce them ever again. Which would make the least difference to the Universe? Vitriol (talk) 22:37, 3 December 2009 (UTC)
- I strongly suspect there is no answer to this - they are all absolutely 100% necessary. Take any one away (if that's even possible - string theory says "No") then the universe would be a dramatically different place - probably life as we know it wouldn't exist. But there is no "marginally less useful" particle. SteveBaker (talk) 23:04, 3 December 2009 (UTC)
- Oh, I don't know. A universe without a top quark might not differ much. Top is very hard to create and decays in ~5×10−25 s. Now there might be secondary effects on the rest of the standard model if one removed the top, and I'm not sure how to predict what modifications to the larger theory might be necessary, but the top by itself seems of little importance. Dragons flight (talk) 23:14, 3 December 2009 (UTC)
- There is no particle that could be removed from the Standard Model without either making it inconsistent or making life impossible. However, we could remove a whole group of particles, such as the third generation of the standard model (which comprises the tau, tau neutrino, top quark, and bottom quark) This is the only of the three generations with no practical applications. 74.14.108.210 (talk) 23:13, 3 December 2009 (UTC)
- Not to hijack the question but could you elaborate on that a little? Why would it be inconsistent or non-life sustaining if, for example, the top quark didn't exist? Maybe not so many pleasing symmetries would exist but where are the serious effects? 129.234.53.144 (talk) 23:56, 3 December 2009 (UTC)
- In physics the math always balances. If the top quark was missing, some physical interaction would not balance which is impossible. So some other particle or effect would, nay MUST, happen instead. Which would then have implications, etc, etc. Make any change, and everything else changes too. Ariel. (talk) 00:01, 4 December 2009 (UTC)
- Not to hijack the question but could you elaborate on that a little? Why would it be inconsistent or non-life sustaining if, for example, the top quark didn't exist? Maybe not so many pleasing symmetries would exist but where are the serious effects? 129.234.53.144 (talk) 23:56, 3 December 2009 (UTC)
- More importantly, per Murray Gell-Mann, "that which is not forbidden is mandatory" in particle physics. The existance of the top and bottom quarks is necessitated by the symmetry in the Standard Model. The entire system predictes the existance of said particles, therefore they are ALL equally vital. We have a pschological sense that particles like electrons are more vital because we tend to work with them more often, but the entire system of particles is not seperable; you must take them all, because the laws that created the top quark also created the electron; you could not create a universe with one and not the other. You can think of the Standard Model like a house of cards. If you remove any part of it, the whole system does not stand. See also anthropic principle for more on this. --Jayron32 00:16, 4 December 2009 (UTC)
- Here's an interesting article for you Weakless Universe, they imagine a universe where something is missing. But as you see they had to change various other things too to make it work. Ariel. (talk) 00:31, 4 December 2009 (UTC)
- Excuse me, but that's totally nonsense answer. If there were no top quark, the standard model would be seriously broken, I agree. But that's still just a human model of physical reality. If the universe had no top quark, then that would imply physicists need to discover a theory of particle physics that is different from the standard model, and one in particular where top quark formation is forbidden. However, because the top quark is almost never involved in interactions at human scales, more likely than not one could invent a new theory (perhaps much less elegant) that still gave the same predictions for human life as we have now. The Standard Model might be a "house of cards", but physical reality need not adhere to your sense of aesthetic beauty in determining its laws. For another example, the Higgs boson has but long sought after and not yet found. Most physicists seem to believe the Higgs will eventually be found, but one can just as well replace the Standard Model with one of several Higgsless models and our physical reality would look the same. Dragons flight (talk) 00:35, 4 December 2009 (UTC)
Storks
Why do you get storks in places like Germany and Holland but not in Britain? Germany has a more severe winter than Britain, so that cannot be the reason. 89.242.105.246 (talk) 01:08, 4 December 2009 (UTC)

