Wikipedia:Reference desk/Science: Difference between revisions
| Line 509: | Line 509: | ||
:Without seeing the leaves or flowers, I don't think you can say more than that they are Papaver. Lots of poppies have fruit (or whatever you call it) that looks like that. [[User:Looie496|Looie496]] ([[User talk:Looie496|talk]]) 15:37, 24 June 2012 (UTC) |
:Without seeing the leaves or flowers, I don't think you can say more than that they are Papaver. Lots of poppies have fruit (or whatever you call it) that looks like that. [[User:Looie496|Looie496]] ([[User talk:Looie496|talk]]) 15:37, 24 June 2012 (UTC) |
||
:: Based on OR I am about 80% certain they are papaver somniferum. The pale bluish [[glaucous]] leaves are visible at the bottom of the photo. [[User:Richard Avery|Richard Avery]] ([[User talk:Richard Avery|talk]]) 17:56, 24 June 2012 (UTC) |
:: Based on OR I am about 80% certain they are papaver somniferum. The pale bluish [[glaucous]] leaves are visible at the bottom of the photo. [[User:Richard Avery|Richard Avery]] ([[User talk:Richard Avery|talk]]) 17:56, 24 June 2012 (UTC) |
||
== Trying to make equivalent colors match equivalent return rates == |
|||
I want to alter the color scheme in [https://docs.google.com/spreadsheet/ccc?key=0AjxOp0_3zRt0dFQyYkFIQnY3cVZfNHpESHVDdHRYWXc this triangular spreadsheet graph] so that it matches the color scale on [http://www.nytimes.com/interactive/2011/01/02/business/20110102-metrics-graphic.html this ''New York Times'' chart.] Any ideas? [[Special:Contributions/71.212.226.91|71.212.226.91]] ([[User talk:71.212.226.91|talk]]) 18:21, 24 June 2012 (UTC) |
|||
Revision as of 18:21, 24 June 2012
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
June 20
Weird brown egg

Does anyone know what the bumps are at the bottom of the egg? Smallman12q (talk) 15:26, 20 June 2012 (UTC)
- It's an egg defect. More specifically, an irregular calcification. Quite common in home-grown eggs, and also far from rare in industrial eggs. For more info, see this resource [1], which would describe the defect as "pimples", or perhaps a "misshapen egg." SemanticMantis (talk) 18:02, 20 June 2012 (UTC)
- Just wait until they engineer cube-shaped eggs, to better fit in the shelf space. :-) StuRat (talk) 18:39, 20 June 2012 (UTC)
- It's just a matter of time, and not at all biologically impossible. Gallstones are often cubical, for example. In any case, there appears to be some readiness among the egg-consuming public for cubical eggs, and you can make your own in the comfort and privacy of your own humble abode with this: [[2]]. Dominus Vobisdu (talk) 18:48, 20 June 2012 (UTC)
- Just wait until they engineer cube-shaped eggs, to better fit in the shelf space. :-) StuRat (talk) 18:39, 20 June 2012 (UTC)
Do fish have tongues ?
StuRat (talk) 18:27, 20 June 2012 (UTC)
- Yes. Here's an article describing how fish tongues work a little differently from mammal tongues [3]. Unless, of course, the tongue has been replaced by this tongue-eating parasitic louse. SemanticMantis (talk) 18:31, 20 June 2012 (UTC)
Are plastic houses doable?
Plastic is known to be very durable, even if it would possibly be more expensive than mortar and bricks. OsmanRF34 (talk) 19:35, 20 June 2012 (UTC)
- Doesn't seem like a good idea, for several reasons:
- 1) Plastics outgas toxic fumes, so the house might be too toxic to live in for the first year or so.
- 2) Plastics are flammable, again giving off toxic fumes when they burn. This is true of wood, too, but to a lesser extent. Burning plastics can also stick to human flesh, like napalm.
- 3) Plastics tend to degrade under UV light. A protective coating might help here, though.
- 4) Plastics have a high coefficient of thermal expansion, and some become noticeably softer when hotter and harder when cold. Thus, they could crack in winter and sag in summer.
- 5) Plastics don't have as much compression strength as other common building materials, like bricks. StuRat (talk) 19:40, 20 June 2012 (UTC)
- It might have been revolutionary in the 1960s, but plastic houses have been around for a long time. A Google search will turn up lots of hits. Dominus Vobisdu (talk) 19:48, 20 June 2012 (UTC)
- Note that plastics are used extensively in building houses, though. Here are a few ways:
- a) Carpets made from synthetic fibers (outgassing from these can be a problem, too).
- b) Thermal insulation made of Styrofoam, etc.
- c) Plastic vapor barrier sheets in walls.
- d) PEX plumbing.
- You're conception of "house" seems to be a bit culturocentric and narrow, Stu. Dominus Vobisdu (talk) 20:07, 20 June 2012 (UTC)
- If you're talking about igloos, grass huts, etc., I don't tend to call those "houses", no, those are other types of dwellings. StuRat (talk) 20:11, 20 June 2012 (UTC)
- No, I'm not. Think about the houses that most of the planet lives in, not most of the people in your neighborhood. Dominus Vobisdu (talk) 20:16, 20 June 2012 (UTC)
- I think electric wiring with plastic insulation is pretty universal. In additon to the features StuRat listed, there's also vinyl floor covering, which in some cases goes right through the house, and the adhesives that hold the whole mess together. The extensive use of plastics in the constrution of houses, combined with the large amount of plastic in furniture, white goods, sterosystems, etc. seems to belie StuRat's claims about outgassing of plastics making homes unlivable after construction though. 203.27.72.5 (talk) 21:37, 20 June 2012 (UTC)
- Small quantities of plastic aren't a problem, but large quantities, like certain floor coverings, can cause sick building syndrome. StuRat (talk) 21:44, 20 June 2012 (UTC)
- There are already houses built that use a great deal of plastic. White/brown/etc ultraviolet resistant PVC foam insulated weatherboarding clad the outside. Double glazed UV PVC windows and doors stop the wind blowing through. Inside surfaces are clad in plastics. Plumbing often is now mostly plastic. Much of the wood furniture is made from chip wast, formed into sheets by bonding with plastic. The jacuzzi is often plastic. The carpets are made from synthetic fibre (plastic). And in hot climates they are surrounded by drought resistant astro-turf; surrounded in turn by plastic white picket fences.--Aspro (talk) 21:48, 20 June 2012 (UTC)
- Does the house come with free health insurance for cancer, asthma, birth defects, eczema and breathing mask with suit ..? :-) Electron9 (talk) 14:33, 21 June 2012 (UTC)
Smokeless powder compared to bullets
Lets say you shoot a blank cartridge. Would the escaping gasses accelerated out the muzzle have a total kinetic energy greater than a bullet accelerated by those same escaping gasses? The reason I ask is if the gasses are doing work on the bullet, shouldn't there be inefficiencies involved that make the total energy imparted to the bullet less than the total energy that the escaping gasses would normally have if there were no bullet? ScienceApe (talk) 19:52, 20 June 2012 (UTC)
- Yes, given the same amount of powder in either scenario, the bullet will get some of the kinetic energy, and the gases will get some (or all, if there is no bullet). However, that kinetic energy from the gases will quickly dissipate by moving air, while the bullet will keep most of it's kinetic energy far longer and farther. However, shooting somebody point-blank with a blank can still kill them, as the gases still have sufficient potential energy to do so at that distance. StuRat (talk) 19:56, 20 June 2012 (UTC)
- What StuRat said isn't wrong, but the line between kinetic energy, sound energy (in the pressure wave) and heat is a bit less obvious in this situation. Whether there is more energy as kinetic, heat or in the pressure wave at any given point depends on how you want to delineate each. 203.27.72.5 (talk) 20:48, 20 June 2012 (UTC)
June 21
Boiling hotter, boiling faster?
It's a commonly noted fact that salted water boils hotter than unsalted water. But my question is, does it boil faster? If I have a pot of salted water, will it boil away faster than an otherwise identical pot of unsalted water? If I synchronize the pots by when I turn the flame on? If I synchronize the pots by when they begin to boil? Thanks. Someguy1221 (talk) 00:38, 21 June 2012 (UTC)
- Possibly of interest. Short Brigade Harvester Boris (talk) 00:45, 21 June 2012 (UTC)
- Assuming both start at ambient temperature, the salted water will boil slightly slower, since it has to heat up further to reach it's boiling point. 203.27.72.5 (talk) 00:47, 21 June 2012 (UTC)
I suppose another consideration is that salt water has more entropy than non-salted, so it will resist evaporation. That will lead to it losing less heat and possibly boiling faster. That will of course depend on the concentration of salt and ambient humidity, etc.203.27.72.5 (talk) 00:50, 21 June 2012 (UTC)- Scratch that. The higher entropy of the salt water is what makes the boiling point higher to start with. The amount evaporating will still increase as the temperature approaches the boiling point. The salt water will boil ever so slightly slower, and hotter. As an aside, any food in the pot will cook faster in boiling water at 100°C than it will in not-yet-boiling-salt-water at 100°C. This is because steam at 100°C has much more internal energy than water and can transfer it to the food faster through the agitation of a rolling boil. 203.27.72.5 (talk) 01:08, 21 June 2012 (UTC)
- Keep in mind that a given volume of pure water actually contains more H2O than the same volume of salt water. Because salt water is an imperfect mixture, NaCl (or rather, its dissolved ions) represents a statistically significant portion of the "water", and salt does not boil at 100C. The specific thermic properties of NaCl notwithstanding, you're actually starting off with less water to begin with in the case of salt water. I'll let people smarter than me tell you what that means in practical terms. Evanh2008 (talk|contribs) 01:16, 21 June 2012 (UTC)
- In practical terms, nothing. Dissolving NaCl in water doesn't appreciably change its volume, which, incidentally, is why brine is much denser than water and tends to sink. This drives various climatic processes as I understand it. 203.27.72.5 (talk) 01:49, 21 June 2012 (UTC)
- Keep in mind that a given volume of pure water actually contains more H2O than the same volume of salt water. Because salt water is an imperfect mixture, NaCl (or rather, its dissolved ions) represents a statistically significant portion of the "water", and salt does not boil at 100C. The specific thermic properties of NaCl notwithstanding, you're actually starting off with less water to begin with in the case of salt water. I'll let people smarter than me tell you what that means in practical terms. Evanh2008 (talk|contribs) 01:16, 21 June 2012 (UTC)
- Scratch that. The higher entropy of the salt water is what makes the boiling point higher to start with. The amount evaporating will still increase as the temperature approaches the boiling point. The salt water will boil ever so slightly slower, and hotter. As an aside, any food in the pot will cook faster in boiling water at 100°C than it will in not-yet-boiling-salt-water at 100°C. This is because steam at 100°C has much more internal energy than water and can transfer it to the food faster through the agitation of a rolling boil. 203.27.72.5 (talk) 01:08, 21 June 2012 (UTC)
Using genetics to determine the potential for undiscovered species
I often read stories about a new species of plant/animal being discovered. My question is: Can the genetic code of a plant/animal specimen provide clues to the existence of a yet-to-be discovered species. Take tree frogs, for example. They cover a pretty wide range of variations and species, and it is likely a new species is yet to be discovered. Could a scientist interested in tree frogs look at the genes of a known species, and extrapolate the potential for other, undiscovered species of tree frogs to exist...or at least the potential to exist? Quinn ✹SUNSHINE 03:23, 21 June 2012 (UTC)
- Well, sure -- the potential, not the actuality. But I suspect if you knew more about genetics you would realize that that fact is not very interesting. People who do genetic engineering invent new species every day -- many of them have the potential to exist before they are created, in a theoretical sense. The number of viable gene combinations is unimaginably huge. Looie496 (talk) 03:45, 21 June 2012 (UTC)
- Do genetic engineers really make new species? As in, they modify the genetic material to such an extent that a population of organisms with the modified genetic material cannot mate with the general population of organisms with natural genetic material to produce viable offspring (but can produce viable offspring within their own population). I was not aware of that being possible. 203.27.72.5 (talk) 04:06, 21 June 2012 (UTC)
- It's possible. Take, for example, a very extreme case of engineered underdominance, described in theoretical capacity in this paper, section 5 if you can access it. You can also cheat and just make an organism incapable of mating for some stupid reason, like engineering a self-fertilizing nematode to have no opening through which to mate. It's self-fertilizing, so it's still viable, but now reproductively isolated. And finally, what is actually done, but may not count as "engineering", us using colchicine treatment to change the copy number of chromosomes in a plant. The resulting plant cannot yield anything but sterile offspring with the plant of origin (as the progeny will have a sterility-inducing odd number of chromosomes). So in that sense, it is at least reproductively isolated, if not a new species. You decide if that's genetic engineering. Someguy1221 (talk) 04:22, 21 June 2012 (UTC)
- Do genetic engineers really make new species? As in, they modify the genetic material to such an extent that a population of organisms with the modified genetic material cannot mate with the general population of organisms with natural genetic material to produce viable offspring (but can produce viable offspring within their own population). I was not aware of that being possible. 203.27.72.5 (talk) 04:06, 21 June 2012 (UTC)
Answering the original question, yes, it can provide clues. You can take any two species, there were almost certainly intermediate genetic steps between those two organisms and their most recent common ancestor. Any one of those intermediate organisms may have itself given rise to more than one descendant species. So when you are looking at a phylogeny and there appears to be a large gap between two closest relatives, a biologist will often suspect that some extra species might fit in there. For example, Caenorhabditis elegans has essentially no close relatives (at least by comparison to other members of Caenorhabditis), and there is currently a $5000 prize offered to whichever lucky field biologist finds a species that is more closely related to elegans than anything else. But there are a couple problems with this. It's possible that all of those intermediate forms separating two closest relatives may have not given rise to any other species, or alternatively, those other species might all be extinct. Someguy1221 (talk) 04:31, 21 June 2012 (UTC)
Solar Energy and Its Production
How many units are present in 1 KW of electricity? What is the present cost of the project for producing 1 MW of electricity with the help of solar panels, etc. without the inclusion of the cost of land? — Preceding unsigned comment added by 122.172.176.45 (talk) 04:07, 21 June 2012 (UTC)
- 1 kW is 1000 Watts of power. A Watt is 1 Joule per second. A Joule is the energy required to accelerate an object with a mass of 1 kilogram at 1 meter per second per second for 1 second. The kilowatt is therefor a derived unit, and is a combination of the base units kilograms, meters, and seconds. You can also express it in terms of these base units as Mg·m2/s3. 203.27.72.5 (talk) 04:13, 21 June 2012 (UTC)
- As for your second question, it depends on where the solar panels will be located. Different areas have very different incident sunlight. Solar panels will also cost different amounts in different places due to logistics, supply of raw materials, labour, government subsidies, etc. 203.27.72.5 (talk) 04:25, 21 June 2012 (UTC)
- Solar panels vary considerably but a solar panel that can produce 250W measures about 1m x 1.6m, you'd need 4000 of those to get 1MW under ideal conditions. Expect to pay $500 for such a panel and you get about 2 million bucks for the solar panels alone. It would also take up about 6400 square meters of space if they were installed edge to edge, which I'm sure is probably not practical. Then you have to install them and also all the infrastructure needed to collect and condition and invert and whatever else you need to do, I wouldn't be surprised if it was another 2 million. Having said that, I'm sure you could get a good deal if you were buying $4 Million dollars of solar power equipment, but this is only intended as a very rough ballpark figure. Vespine (talk) 05:08, 21 June 2012 (UTC)
- To put that in perspective, a rugby field minus the try zone is 6800m2.
- Solar panels vary considerably but a solar panel that can produce 250W measures about 1m x 1.6m, you'd need 4000 of those to get 1MW under ideal conditions. Expect to pay $500 for such a panel and you get about 2 million bucks for the solar panels alone. It would also take up about 6400 square meters of space if they were installed edge to edge, which I'm sure is probably not practical. Then you have to install them and also all the infrastructure needed to collect and condition and invert and whatever else you need to do, I wouldn't be surprised if it was another 2 million. Having said that, I'm sure you could get a good deal if you were buying $4 Million dollars of solar power equipment, but this is only intended as a very rough ballpark figure. Vespine (talk) 05:08, 21 June 2012 (UTC)
- As for your second question, it depends on where the solar panels will be located. Different areas have very different incident sunlight. Solar panels will also cost different amounts in different places due to logistics, supply of raw materials, labour, government subsidies, etc. 203.27.72.5 (talk) 04:25, 21 June 2012 (UTC)
- By "units" the OP may be refering to the terminology used by power companies/authorities when charging for the use of electricity. For example, in Australia, 1 Unit is 1kw for 1 hour - about the energy consumed in one hour by 17 60 watt incadescent light globes, or the energy consumed in one hour by a small bar radiator. As to the OP's second question, the answer given above is entirely right, however, without govt subsidies, solar power is so costly compared to fossil fuel generation, no same person would ever consider it, unless fossil fuel generation cannot be used at the location. The unsubsidised cost of panels, electronics, batteries, installation, etc, for 1 kw for 24 hours a day is of the order of $200,000, with a service life of about 10 to 15 years. By using solar power to back feed power into the grid, rather than owner-conmsumption, batteries are eliminated, however without govt subsidies and a value per unit (kw-hr) of the order of 6 to 10 times the normal electricity charge, it is not cost effective. Ratbone121.215.67.177 (talk) 05:23, 21 June 2012 (UTC)
- So using that definition of unit, 1kW is 1/3600 units. 203.27.72.5 (talk) 05:27, 21 June 2012 (UTC)
- No. Kilowatts (kw) are the units of power. Units, as used by power companies, are the units of energy. Energy is power multiplied by time. 1 kw for 1 hour (or 2 kw for 1/2 hour, etc) is 1 Unit, as I previously stated. By your use of 1/3600, you possibly were thinking of 1 kw.second. Ratbone121.215.67.177 (talk) 06:44, 21 June 2012 (UTC)
- Yeah, so
- 1kW is the energy per 1 second, and over the hour that sums to 3600kJ. I didn't express myself very clearly though. What I should have said was, "you can't ask for a conversion factor between power and energy. That's like asking for a converstion factor between distance and speed." 203.27.72.5 (talk) 08:20, 21 June 2012 (UTC)
- Yes, and now that we have the diffrence between power and energy sorted out, you can see that Vespine's answer is really nonsense. I could use solar panels of arbitarily small area to charge a storage device (battery or capacitor), and then discharge it thru a very low resitance. The power upon discharge could be a megawatt, if only for a microsecond. Ratbone58.167.254.173 (talk) 16:53, 21 June 2012 (UTC)
- I wouldn't say it's complete nonsense. It calculates the minimum size required to generate 1MW continuously, assuming continuous sunlight. Of course in reality, we don't have continuous sunlight, so to replace a 1MW gas turbine it would need to be far larger than his estimate and it would need to store most of its energy for later consumption (peak energy requirements for most areas are in the early evening). Since the OP is asking for a cost of all of the components excluding land, perhaps his idea is to stick solar panels on a large number of houses? If this is the case you'd also have to account for the slope of the roof and how it affects the amount of sunlight incident on the panel. 203.27.72.5 (talk) 20:44, 21 June 2012 (UTC)
- Evidently, wikipedia has an article on this topic. 203.27.72.5 (talk) 08:23, 21 June 2012 (UTC)
- There is also an article on the Kilowatt hour. Jørgen (talk) 08:44, 21 June 2012 (UTC)
- Yes, and now that we have the diffrence between power and energy sorted out, you can see that Vespine's answer is really nonsense. I could use solar panels of arbitarily small area to charge a storage device (battery or capacitor), and then discharge it thru a very low resitance. The power upon discharge could be a megawatt, if only for a microsecond. Ratbone58.167.254.173 (talk) 16:53, 21 June 2012 (UTC)
- Yeah, so
- No. Kilowatts (kw) are the units of power. Units, as used by power companies, are the units of energy. Energy is power multiplied by time. 1 kw for 1 hour (or 2 kw for 1/2 hour, etc) is 1 Unit, as I previously stated. By your use of 1/3600, you possibly were thinking of 1 kw.second. Ratbone121.215.67.177 (talk) 06:44, 21 June 2012 (UTC)
- So using that definition of unit, 1kW is 1/3600 units. 203.27.72.5 (talk) 05:27, 21 June 2012 (UTC)
What is RNA ‘Ribonucleic acid’ and what is DNA 'Deoxyribonucleic Acid'
What is RNA ‘Ribonucleic acid’ and what is DNA 'Deoxyribonucleic Acid' — Preceding unsigned comment added by 175.140.179.46 (talk) 06:41, 21 June 2012 (UTC)
- Have you checked the articles DNA or RNA? I imagine they would have something to say on the subject. Evanh2008 (talk|contribs) 06:43, 21 June 2012 (UTC)
Cells~~(*for cells questions)
What are the three main categories of organelles within the cytoplasm? — Preceding unsigned comment added by 175.140.179.46 (talk) 06:48, 21 June 2012 (UTC)
- 1) Those which do their own homework.
- 2) Those which call other cells on their cell phone to get homework answers.
- 3) Those which try to get people on Wikipedia to do their homework for them. StuRat (talk) 06:57, 21 June 2012 (UTC)
- Looking at organelle, I was disappointed to discover that they aren't female organs, but perhaps that article will have the answers you seek. However, at first glance, I only see two categories listed, but definitions may vary, so I suggest you consult your textbook. StuRat (talk) 07:01, 21 June 2012 (UTC)
- Now you made me look up "female organs"... 92.80.52.54 (talk) 08:26, 21 June 2012 (UTC)
after classifying plants
In the novel La tournée de Dios by Enrique Jardiel Poncela God comes down to Earth and, well, stuff happens. Anyway, in one of the chapters God is invited to a botanical garden and ask to the staff "and when you have classified all plants and animals, then what?"
Anybody has a good answer to God?--85.55.200.82 (talk) 10:58, 21 June 2012 (UTC)
- Then we start to make our own animals and plants by genetic engineering! But I expect that God would point out that humans have not looked every where yet for life forms, especially at other planets. Graeme Bartlett (talk) 12:06, 21 June 2012 (UTC)
- There's a lot to untangle here. First: taxonomy is a distinctly human concept, and our taxonomies are constantly being rearranged, due to new information and new techniques (e.g. systematics, phylogenetics and cladistics are used much more currently, compared to the morphological comparisons used in the past. Second: life form themselves are constantly speciating, and I'm not sure that the rate of classification of new species is greater than the worldwide rate of speciation. So, it is entirely possible (and IMO likely) that we will never "finish" classifying life. Finally: suppose we did. It is a very narrow view of botany and zoology to think that classification is a primary concern (surely God would know better ;). In the modern era, much more research is done in other areas. Even if we had classified all life (and it stopped speciating), we'd have plenty left to discover. How do birds migrate? How does bark form on trees? Why don't naked mole rats get cancer? How do symbioses form? How does a bee hive function collectively? These are all very simple questions that have not yet been completely answered. Actually I would be more concerned that rigorous classification is getting too little attention these days; I've heard many biologists lament that the last of the great taxonomists are dying, and the younger generation is not filling in the ranks. SemanticMantis (talk) 13:34, 21 June 2012 (UTC)
- Why? Despite many biologists wishing it weren't so? I can understand if this was something like prewar-era jazz singer or newspaper editor or something but why this? Is it funded completely by lint taxes? Is the rest of biology so compelling that no biology major/grad student has ever been able to resist the power of the dark side? They're people who're most interested in computational linguistics or train timetables for goodness sake, why not this? Are there no science types left who're also into 18th/19th century stuff? Doesn't seem like it should be the case as some young people still like Steampunk. And aren't there British biologists? And British people should be more likely to like old stuff than Americans. Sagittarian Milky Way (talk) 17:43, 22 June 2012 (UTC)
- Nice try, Semantic. God is obviously a young earth creationist, so he doesn't believe in speciation. 203.27.72.5 (talk) 20:56, 21 June 2012 (UTC)
- Ha! Well, I'm not here to discuss religious beliefs, but suffice it to say that not all who believe in a god believe in YEC. Unfortunately, it seems to be the case that the craziest religious people make the most noise :-/ SemanticMantis (talk) 21:28, 21 June 2012 (UTC)
"Be patient and get in the queue behind the insects, we'll get round to You when we're ready." DriveByWire (talk) 23:35, 24 June 2012 (UTC)
"God, you know more about evolution than we do. You know the process of speciation is endless and ongoing, and the number of small population species of smaller creatures (insects, bacteria, etc) is astronomical. We will never finish cataloging life, your grandest creation, as you well know. However, we can set a new goal of sequencing the DNA of every species, along with cataloging all the proteins that exist. Thank you for the Periodic Table and our ability to create computers based on the electrical and chemical properties of Silicon, with which we can decode the molecules of life." in the Hebrew tradition, god welcomes challenges from us to his questions.(mercurywoodrose)50.193.19.66 (talk) 17:02, 25 June 2012 (UTC)
TV with a particular type of EPG
I am in the UK. I need to buy a small television, and I have only one requirement and that is that the current live TV channel is displayed within a small window in the EPG, i.e. I can continue to watch and listen while browsing the channels. Unfortunately this information is missing from almost all the online descriptions of the many different models available, and the manufacturers' websites turn out to be useless. The only comparison chart I've been able to find is this one for video recorders, and it only lists a few obscure brands and none of the big names. However, I strongly suspect that is the manufacturer that is significant rather than the TV model, as they tend to use basically the same software for much of their range. My trusty Philips set-top box does what I want perfectly. But I have looked at friends' Sanyo, Toshiba, and JVC TVs, and they are no good, so it seems that what I want is a minority feature. Before you say ask the salesman, in my experience they are mostly clueless in the UK when it comes to buying electrical equipment, so I thought I would risk a little original research and ask whether any UK refdeskers have this feature on their TV and if so what is the make and model?--Shantavira|feed me 11:16, 21 June 2012 (UTC)
- Most retail salespeople are technically clueless. The best bet for now and long term is likely an PC with one or more DVB-T/C/S receiver cards and MythTV etc. The TV industry is quite dumbed down, computers is the game. Electron9 (talk) 14:37, 21 June 2012 (UTC)
- I have a fairly recent-ish Sony flatscreen - it doesn't have your desired feature. It overlays the EPG on top of the current picture, pretty much obscuring it. So scratch them off the list. You might be advised to wander into Currys, Dixons, John Lewis, etc., and see if you can get their salespeople to show you this function working on one of their TVs. --Tagishsimon (talk) 14:42, 21 June 2012 (UTC)
- Agreed, you can't trust salesmen, get them to show you the feature before you buy a model. I've had the same problem with cars, they either don't know how the "fancy buttons" work, just make it up as they go along, or outright lie to you. I test everything out myself before I agree to buy, and you should take your TV for a "test drive", too. StuRat (talk) 03:53, 22 June 2012 (UTC)
- My Samsung does this and so does a friends, do a google image search for 'Samsung EPG' and you can see what it looks like. Nanonic (talk) 06:39, 22 June 2012 (UTC)
- This answer doesn't apply to you because if your computer was running Linux you would know of this already. Yet, if you were running Linux, you would not have to worry about what TV to buy. You could watch TV on MythTV which provides a small live image of main channel as you require and since Linux allows you to have multiple desktops, you can quickly switch over to surfing the web (whilst still listening to the TV broadcast) and/or listen to music at the same time, and even do image processing, or what ever else you desire to do on your computer. If you like, you can have a larger image -of what ever size you so wish- and have it follow you around different desk tops. What to change channel at any point … No problem; just hit the numeric key number of the channel you now wish to watch. Come to think of it, I don't know why Microsoft hasn’t thought of coming out with a compact and cheap ($ 0.00) home-entertainment system like this. It would sell like hot pies. --Aspro (talk) 23:57, 22 June 2012 (UTC)
- It's a bread & butter company without visions, except in shareholder profit. As for Unix like BSD or Linux, getting any OS to handle video without interruption is the harder bit. Integration and being handle everything with remote control, device drivers etc.. is another thing to deal with. Electron9 (talk) 05:38, 24 June 2012 (UTC)
how much friction does water flowing through a (decent-sized) pipe undergo? if the pipe is long enough, would it not flow out, even vertically?
The question is how much friction water flowing through a (decent-sized) pipe undergoes, and whether, if the pipe is long enough, the water wouldn't even fall out the other side vertically, despite gravity and the other side being lower?
My thought process is as follows. It's very hot where I am right now. I noticed that the water that flowed out of the shower (set to cold water only) was still, after a moment, very very cold. Even standing a few feet away a draft of cool air hit me. If I had run it a very long time, it would have cooled my whole apartment off, which doesn't have an air conditioner in it. Of course, running water just for cooling is wasteful.
My next thought was: well, I'm not actually using it. Could someone hypothetically just run it, in a (clean) configuration that makes a big narrow wall of water with very large surface area, and simply (the water is still clean) feed it back into the system. Probably that would be possible. But it wouldn't be fair to the neighbors across the street (you could run it into their water intake) even if it's clean and sterile, because it's now warm. Why should they get warm water in the heat? (assuming they also want cold water.)
My next thought was: ah, but do you know who DOES want warm water: extreme latitudes where it's winter now. And vice versa. (If they cooled water, I would want it).
So here is my next thougth. Hypothetically, if we had a very long pipe between winter places that want warm water and summer places that want cold water, and we set the pipe to exactly the same altitude between the two places, then wouldn't the tiniest push (or vertical drop, like an aqeuaduct, which drops only a tiny amount over distance) of the end at either place cause the water to in the correspondeing direction? Since it doesn't actually take work (the physics concept) to keep something moving at the same momentum and altitude, less friction, the only thing that would stop such a scheme working for the next 100 years is if it were built is...water resistance in the pipe.
so, the question is: what IS that resistance? What stops my scheme from working, if anything? THe scheme is free cooling for summer climate and free heating for winter climate by running a water-based heat exchange between them with almost no work performed after building the aparatus. 84.3.160.86 (talk) 11:19, 21 June 2012 (UTC)
- by the time the water's reached the other side of the world it would have reached ambient temperature. Besides friction is considerable-try an experiment using drinking straws joined together, and blow down them. It would be easier to heat water through geothermal power or something. As for cooling, its generally reasonably cold a few meters under ground-which is why your water is cold in the first place. — Preceding unsigned comment added by 109.155.4.192 (talk) 12:17, 21 June 2012 (UTC)
- I'll answer the 2nd question first: The flow of water in a pipe is indeed determined by a form of friction - the viscosity of the water resists the movement of water against a surface. The flow of fluid (eg water) through a smooth pipe can be calculated by means of the Fanning-Darcy equation. There are two forms of Fanning-Darcy, one for simple laminar (stright line) flow, and one for turbulent flow. For laminar flow, the Fanning-Darcy equation is:-
- F = ( ΔP π D4 ) / ( 128 μ L )
- where F is the flow rate in m3/s; ΔP is the pressure difference along the length of the pipe in Pascals, π is 3.14.., D is the diameter of the pipe in meters, μ is the absolute viscosity of the fluid in mPa.s, L is the length of the pipe in meters. For turbulent flow, the formula is somewhat more complicated. To find whether you have laminar or turbulent flow, calculte the Reynolds Number:-
- Re = ( 4 ρ F ) / ( π μ D )
- If the Reynolds number is less than 2000, the flow will be laminar; if above 3000 it will be turbulent; between these 2 limits, some engineering judgement is required.
- All this means that (a) you will always get some flow no matter how long the pipe, and (b) the flow is inversley proportiona to pipe length, and proportional to the 4th power of the diameter (laminar flow; for turbulent, it is proportional to diameter raised to the power 19/7).
- As to the first question: Water walls are sometimes seen as a decorative feature in restaurants and hotel lobbies. It has some psychological effect in cooling perception, but the actual cooling effect is minimal, essentially being due to the conduction of heat from the air to the water over the water wall surfcae area. As far far more effective method of cooling is to evaporate the water, as the latent heat of vaporisation of water is very high. This is the principle being evaporative type airconditioners, often nicknamed "swampies" in the trade due to the use of wood shavings and the like to provide a very large surface area in a small volume.
- Don't forget that humans sweat - this is our built-in personal swampy, and makes you feel cool when in moving air, even if the air is not cool. You don't have to actually feel wet with sweat for this to work.
- Ratbone58.167.254.173 (talk) 12:31, 21 June 2012 (UTC)
- Also note that someone (or something, or some process ;-) has already put this system into production, and it seems to work well with only minor hickups so far! However, the tenants are playing with the thermostat and may possibly break it. --Stephan Schulz (talk) 13:57, 21 June 2012 (UTC)
- Good point, and well put too! Ratbone58.167.254.173 (talk) 15:40, 21 June 2012 (UTC)
- Also note that someone (or something, or some process ;-) has already put this system into production, and it seems to work well with only minor hickups so far! However, the tenants are playing with the thermostat and may possibly break it. --Stephan Schulz (talk) 13:57, 21 June 2012 (UTC)
- Water evaporation (usually spraying) is efficient for cooling and spreading legionella.. Electron9 (talk) 14:40, 21 June 2012 (UTC)
- Water evaporation does NOT spread legionella (bacteria can't hide in individual water molecules), however water spray or mist does spread it. However this must be seen in the proper context. When a large number "legionaires" who were at the Belevue-Stratford Hotel in 1976 got sick, the reason was investigated, and was shown to be an infection with a type of bacteria not previously known to science, sourced from the building aircon cooling towers. They named this "new" bug legionella. Soem years later, patients at an English hopital got sick and died. An investigation showed legionella was the cause, again sourced from airconditioning cooling towers. These and other incidents led to the rapid implementation of laws in various countries requiring owners of large buildings with aircon cooling towers to sample the water regularly and dose the water to keep the legionella count below arbitarily determined levels. It has since become very apparent that (a) almost invariably only people with compromised immune systems (such as hopital patients already sick and/or on prescribed drugs) are susceptable to legionella, and (b) legionella is ubiquitous in the enviroment - one of the most common forms of bacteria around. It can be argued that these laws are unnecessary - certainly they were enacted in haste without a true understanding of the issue. I worked for 6 years as a building manager responsible for several multi-storey sites with aircon cooling towers. Our aircon legionella count, due to dosing with bacteriacide per legal requirements, was lower than the count in water straight from the city water supply. Nobody legislates about legionella in domestic swampy airconditioners - it isn't necessary. Ratbone58.167.254.173 (talk) 16:09, 21 June 2012 (UTC)
- Not only do readers get impromtu tutorials here, we also have an encyclopedia with an article about Legionellosis. DriveByWire (talk) 23:25, 24 June 2012 (UTC)
- Water evaporation does NOT spread legionella (bacteria can't hide in individual water molecules), however water spray or mist does spread it. However this must be seen in the proper context. When a large number "legionaires" who were at the Belevue-Stratford Hotel in 1976 got sick, the reason was investigated, and was shown to be an infection with a type of bacteria not previously known to science, sourced from the building aircon cooling towers. They named this "new" bug legionella. Soem years later, patients at an English hopital got sick and died. An investigation showed legionella was the cause, again sourced from airconditioning cooling towers. These and other incidents led to the rapid implementation of laws in various countries requiring owners of large buildings with aircon cooling towers to sample the water regularly and dose the water to keep the legionella count below arbitarily determined levels. It has since become very apparent that (a) almost invariably only people with compromised immune systems (such as hopital patients already sick and/or on prescribed drugs) are susceptable to legionella, and (b) legionella is ubiquitous in the enviroment - one of the most common forms of bacteria around. It can be argued that these laws are unnecessary - certainly they were enacted in haste without a true understanding of the issue. I worked for 6 years as a building manager responsible for several multi-storey sites with aircon cooling towers. Our aircon legionella count, due to dosing with bacteriacide per legal requirements, was lower than the count in water straight from the city water supply. Nobody legislates about legionella in domestic swampy airconditioners - it isn't necessary. Ratbone58.167.254.173 (talk) 16:09, 21 June 2012 (UTC)
- Water evaporation (usually spraying) is efficient for cooling and spreading legionella.. Electron9 (talk) 14:40, 21 June 2012 (UTC)
Science of weight loss and fitness
Can anyone direct me to some good source of science on the subject? Also I would like to ask a couple of questions, Are raw eggs any better than cooked eggs? Should you take protein just before working out or you just need to have them in your diet? Bastard Soap (talk) 14:09, 21 June 2012 (UTC)
- The science on this is still very much uncertain, you could search in some scientific journals, like here:
- Count Iblis (talk) 16:03, 21 June 2012 (UTC)
- Unless they are burnt, proteins are broken down into the same amino acids whether they are cooked or not. Cooking has the huge benefit of killing potentially dangerous bacteria. So long as you do not burn the eggs you will not lose any nutritional values. Boil or scramble them. If convenience matters, one or two eggs scrambled in a bowl and then microwaved for about a minute (until dry, you may need to stir once) will come out quite nice. Since just one egg already exceeds a normal person's daily recommended cholesterol you might look into other sources like whey protein. Consult a professional. μηδείς (talk) 16:35, 21 June 2012 (UTC)
- My understanding is that those cholesterol recommendations are out of date, because of considerable data showing that dietary cholesterol has only a weak influence on the buildup of cholesterol in arteries. But in any case none of this protein stuff really matters except to extreme bodybuilders, and you certainly don't need major protein supplementation to achieve weight loss. Looie496 (talk) 18:04, 21 June 2012 (UTC)
It's so strange to not have definite answers about this stuff, it's what most people are most interested in and the science is very lacking.Bastard Soap (talk) 22:04, 21 June 2012 (UTC)
- My own experience suggests that some of the fundamentals of nutritional science are totally flawed like the link between calorie intake and weight. On theoretical grounds, you would not expect there to be a strong link, because if you increase your calorie intake, you'll eventually reach a dynamical equilibrium where you burn the same amount of calories as you are consuming. That your weight should be linked to that dynamical equilibrium is not clear at all. The argument that if you eat more than you burn, you'll store more fat, is a red herring, as it doesn't address the issue of dynamical equilibrium.
- People who are naturaly thin have been tested in an experiment where they were given 5000 Kcal/day to eat. Their weights didn't increase by a lot at all.
- Then my own experience also contradicts the idea of there being a strong link between calorie intake and weight. I consume about 3500 Kcal/day, yet I only weigh about 60 kg. Some of my obese friends and family members eat a lot less than I do, but they don't eat healthy foods. This suggests that what matters is the vitamins and the minerals you consume. The body needs these to burn carbohydrates and fat efficiently. Count Iblis (talk) 02:04, 22 June 2012 (UTC)
- Perhaps excess calories go undigested in some, but I can't believe your metabolic rate goes up tenfold if you eat ten times as many calories. You'd burst into flames. StuRat (talk) 03:34, 22 June 2012 (UTC)
- I presume the excess calories are turned into bacteria, and then pooped out. Your gut microbiome is pretty good at getting rid of most of the nutrition you couldn't absorb yourself. Regarding the question of protein intake, even in the case of bodybuilders there is only so much protein your body can process in a day. In fact, the average american's diet contains more than enough protein for even a weightlifting regimen. All those protein supplements for weight building may as well be snake oil (along with most nutritional supplements for that matter). Someguy1221 (talk) 07:44, 22 June 2012 (UTC)
- Perhaps excess calories go undigested in some, but I can't believe your metabolic rate goes up tenfold if you eat ten times as many calories. You'd burst into flames. StuRat (talk) 03:34, 22 June 2012 (UTC)
As far as I know when they list calories that's the total ammount not the digestible ammount, they burn the shit and tell you how much power it gave them. One single experiment means very little it could easily be biased, it becomes a truth when a lot of scientists replicate the results. The dynamic equilibrium makes little sense to me and is easily disproven by eating only pizzas for a week. Sturat I think you're closer to the truth and some people are less efficient.Bastard Soap (talk) 07:35, 22 June 2012 (UTC)
- Just eat a bit less every day. You don't starve to death if you eat 100 Kcal/day less and keep your activity level the same (or increase it a bit to make sure that during exercise you still burn the same amount of calories or more than you used to, despite having lost some weight). Yet, the simplistic models used for weight gain would predict that after 10 years, you should have lost 45 kg body weight. Clearly, that won't happen, but that means that the body maintains the energy balance by regulating the metabolic rate. But that implies that you won't keep on gaining weight if you eat 100 Kcal per day more, day after day. Count Iblis (talk) 18:11, 22 June 2012 (UTC)
gauss and gauss meters
Could someone please explain to me in very simple terms (I am not a physicist or a scientist, but an archaeologist) what a gauss meter measures and how to interpret the results I get from it? I am not really clear on what gauss represents, either. Thank you. — Preceding unsigned comment added by 184.9.192.187 (talk) 14:15, 21 June 2012 (UTC)
- A gauss meter measures the strength of a magnetic field. See Gauss (unit). Red Act (talk) 14:39, 21 June 2012 (UTC)
- Please see Magnetic survey (archaeology). In very simple terms, buried objects distort the Earth's magnetic field at the surface. By measuring the field at a large number of points, you can create a map showing where the field varies and by how much. --Heron (talk) 18:05, 21 June 2012 (UTC)
- See also gaussmeter. Red Act (talk) 19:01, 21 June 2012 (UTC)
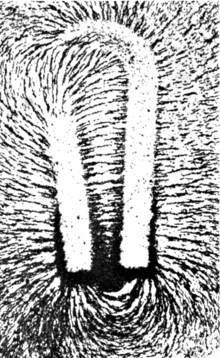
Have you ever looked at iron filings lining up like the Earth's magnetic field due to a magnet? Where the lines are more spread out the magnetic field is weaker. Where the lines are more densely packed the field is stronger. The Gauss meter is measuring the (invisible to you) density of those lines. μηδείς (talk) 03:06, 22 June 2012 (UTC)
What feels like a throat but isnt'?
I've been practising martial arts for a while now and I would like to get a good test of my kill strikes. Are there any common fruits or foods or other common objects which have a similar consistency and strength to the human throat or solar plexus?Bastard Soap (talk) 14:29, 21 June 2012 (UTC)
- If you don't get queasy easily, get a dead pig, they are somewhat close to us biologically - skin, bones, meat. The carcass is gonna get ripe on you though. Compare that feel with melons and such to find something that feels similar - or at least looks cool when you make a mess of a melon in front of your friends. A more practical way is to look in your local yellow pages for companies that sell martial arts equipment; they can sell you a practice dummy that won't break or turn rancid. 88.114.124.228 (talk) 17:50, 21 June 2012 (UTC)
- 'Tis should be a strange world if we should feel comfortable with answering "I would like to get a good test of my kill strikes."
- I've removed the question, and OP you should obviously give this some some thought thought before you get you get into any trouble. --80.99.254.208 (talk) 19:37, 21 June 2012 (UTC)
They give some advice in Human Wrecking Balls. Count Iblis (talk) 19:47, 21 June 2012 (UTC)
Wow, I did not expect this from wikipedia. All of martial arts is about making yourself a weapon and to be used only if absolutely necessary, I have no intention of using it except if I'm attacked by some rabid dog, I just want to know if I'm able to. If I wanted to kill someone I'd get a knife jesus.Bastard Soap (talk) 21:22, 21 June 2012 (UTC)
- Veiled legal threats, ad hominem and crystall ballery aside, I don't see how this question violates policy, or how removing it is appropriate. I've replaced it. 203.27.72.5 (talk) 22:11, 21 June 2012 (UTC)
- I second the meat hypothesis. Vespine (talk) 23:10, 21 June 2012 (UTC)
- Do you mean if a rabid dog attacks you, you intend to strike the throat of the nearest human just to prove you were able to kill someone by a throat strike before you potentially lose your life? Nil Einne (talk) 05:17, 22 June 2012 (UTC)
Grapefruit. μηδείς (talk) 23:28, 21 June 2012 (UTC)
I imagine dog throats are not that different.Bastard Soap (talk) 08:27, 22 June 2012 (UTC)
- I suggest WP:DNFTT. --Saddhiyama (talk) 08:34, 22 June 2012 (UTC)
- But why would you ask for human throats if what you actually want to practice on is dog throats? Nil Einne (talk) 05:35, 24 June 2012 (UTC)
You could train your dog to attack you and then claim self defense when you kill it. good luck165.212.189.187 (talk) 15:40, 22 June 2012 (UTC)
It sounds like none of you have ever taken martial arts, being able to kill and actually killing or wanting to kill are very seperate thingsBastard Soap (talk) 10:41, 23 June 2012 (UTC)
- Actually I did when I was younger, but never desired to learn how to kill. In fact I think if I'd told my instructor I wanted to learn to kill, I probably would have been kicked out of the class. I would note many martial arts concentrate on self defence, particularly the ability to disable, at least sufficiently to escape since being able to kill is often not of particular use. Nil Einne (talk) 05:35, 24 June 2012 (UTC)
Robots
Could you make a robot with real human skin? 176.250.196.132 (talk) 15:26, 21 June 2012 (UTC)
- Well you can get human skin book covers, so I don't see why that shouldn't be extended to robots. Access for maintenance would be a bit tricky, and of course the skin would be dead and not very convincing if you're trying to make an android.--Shantavira|feed me 15:59, 21 June 2012 (UTC)
- But can you make an android with living human skin. 176.250.196.132 (talk) 17:46, 21 June 2012 (UTC)
- In principle, yes. But you would have to work out a way to supply it with all the necessary nutrients and complex molecules, which means adding an artificial bloodstream and a lot of very complex biochemical fabricating machinery. I can't imagine any possible benefit that would be worth the enormous effort. Looie496 (talk) 17:53, 21 June 2012 (UTC)
- Oh, it's essential. Non-living matter can't travel backwards in time. Apparently, unless it's covered by living matter — that part never seemed to be explained in detail, but anyway it was a nice excuse to show a (fairly chaste) nude of Summer Glau on broadcast TV in prime time. --Trovatore (talk) 19:11, 21 June 2012 (UTC)
- Because that worked so well the first time! —Tamfang (talk) 03:40, 25 June 2012 (UTC)
- Uh-oh. Not only has Skynet acheived self-awareness, it's asking questions on the Wikipedia Ref Desk.FlowerpotmaN·(t) 19:16, 21 June 2012 (UTC)
- Oh, it's essential. Non-living matter can't travel backwards in time. Apparently, unless it's covered by living matter — that part never seemed to be explained in detail, but anyway it was a nice excuse to show a (fairly chaste) nude of Summer Glau on broadcast TV in prime time. --Trovatore (talk) 19:11, 21 June 2012 (UTC)
- In principle, yes. But you would have to work out a way to supply it with all the necessary nutrients and complex molecules, which means adding an artificial bloodstream and a lot of very complex biochemical fabricating machinery. I can't imagine any possible benefit that would be worth the enormous effort. Looie496 (talk) 17:53, 21 June 2012 (UTC)
- But can you make an android with living human skin. 176.250.196.132 (talk) 17:46, 21 June 2012 (UTC)
Would it be possible that in the distant future, it wouldnt be so complex, with advancing biological research? 176.250.196.132 (talk) 21:54, 21 June 2012 (UTC)
- Well, you can already remove human bone and replace it with metal. It's not such a big leap to remove all human bones and replace them with synthetic materials, though it would be much more technically difficult (you might exceed the $6million budget). Then you could look at doing a brain transplant, but instead of a brain, install some sort of computer control device. I'd say the technology will be that advanced relatively soon, but unfortunately, the we've long since past the times where that might be ethically possible. 203.27.72.5 (talk) 22:03, 21 June 2012 (UTC)
- It is uncertain whether the above poster means "we've long passed the times when" or "we're long past the times when", either of which is grammatically possible. DriveByWire (talk) 23:11, 24 June 2012 (UTC)
- Even such a limited thing as bones illustrates the difficulty. Replacing one bone is relatively minor, but bone marrow is what generates blood cells, so replacing all the bones would leave you with mere plasma for blood. Looie496 (talk) 22:26, 21 June 2012 (UTC)
- You could use perflourocarbon blood substitutes. HominidMachinae (talk) 23:59, 21 June 2012 (UTC)
- Even such a limited thing as bones illustrates the difficulty. Replacing one bone is relatively minor, but bone marrow is what generates blood cells, so replacing all the bones would leave you with mere plasma for blood. Looie496 (talk) 22:26, 21 June 2012 (UTC)
- A much lower level of technology could provide fake skin indistinguishable from the real thing. StuRat (talk) 03:06, 22 June 2012 (UTC)
- Sorry StuRat, but the laws of time travel cannot be fooled. 203.27.72.5 (talk) 03:58, 22 June 2012 (UTC)
- Currently, no. However, futurists like Ray Kurzweil have imagined our technology reaching the Picotechnology scale. If and when we can design at the atomic or subatomic level, our current distinctions of Human being, robot and android would become moot. Would you describe a dna and protein based, laboratory grown intelligent organism which looks human, and has an enhanced brain using quantum computing a human (ie has perhaps a soul), a robot (a machine designed to do work for us), or an android (a device designed to mimic the human form). for that matter, will WE still be human. Greg Bear says we wont be recognizably human in the near future. Practically speaking, the cost and effort involved to create a synthesized organism with elements of a machine, with human skin and the supporting biological systems that would keep the skin alive and functional (sweat glands, blood flow and oxygen transport, nerves connected to a brain or computer, etc), make it unlikely we will be making a terminator type "robot with skin" at any point. too many other things to do at that point of our technological evolution. What purpose would it serve? skin would make it more vulnerable to the conditions that we usually design robots to handle (working conditions not ideal for humans).(mercurywoodrose)50.193.19.66 (talk) 17:14, 25 June 2012 (UTC)
- An android would be useful for many purposes, such as a nannybot to care for the kids. If you gave them a nanny that looked like Robby the Robot, it wouldn't be very acceptable to your average toddler (and might cause a potty training failure when they first see it). One that looks human would be better. StuRat (talk) 18:43, 25 June 2012 (UTC)
- I can imagine assigning a nanobot as understudy to each cell in your body, or at least each nerve cell; when the cell dies, the understudy takes over its function, interfacing with its neighbors. Eventually you're all artificial but the difference may be hard to tell. In such a scenario, I'd want to keep my skin as long as practical, for social reasons and for the pleasures of tactility. —Tamfang (talk) 18:13, 25 June 2012 (UTC)
Refractive index
What kind of material has the highest refractive index?--Jsjsjs1111 (talk) 15:52, 21 June 2012 (UTC)
- When asking what material has the highest refractive index, it's necessary to specify what wavelength you're interested in, because the refractive index varies widely with the wavelength, in a non-uniform way. The link Zzubnik gives refers to a material that has a maximum refractive index of 38.6 at frequencies near 0.3 THz, which corresponds to a wavelength (in vacuum) of about 1 mm. However, standard refractive index measurements are taken with light with a wavelength in vacuum of 589 nm (the sodium D line), which is more than a thousand times smaller. The highest refractive index at that wavelength listed in our list of refractive indices, at least, is 4.01, for germanium. Red Act (talk) 16:59, 21 June 2012 (UTC)
- For minerals in light you can check out rutile (2.9) and hematite (3.22). Around absorption lines you can get some rapidly changing high numbers. Also for artificial substances look at Barium titanate and Lead zirconate titanate which will have very high values at longer wavelengths. Graeme Bartlett (talk) 21:35, 21 June 2012 (UTC)
I see. Thank you very much!--Jsjsjs1111 (talk) 07:15, 22 June 2012 (UTC)
Creep (deformation)
At my job, I'm often moving around big stacks of boxes (just a few at a time; I don't have any power machinery to help me) that are full of newspapers. As you can imagine, the stacks tend to lean if the boxes aren't placed properly, and improper placement of boxes toward the bottom of stacks can result in damage. In some stacks, there are different sizes of boxes, with the wider ones sitting on top of the narrower ones, and the wider ones are damaged — look at this:
__________________________ |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| | ____________________ | \/ | | \/ |__________________|
The edges of the bottom of the lowest wide box have nothing under them, so the lowest wide box is gradually squished and its edges splay out around the top of the not-so-wide box. I've never seen this happen instantly — I can put a big stack of heavy wide boxes on top of a single not-so-wide box, and they all just sit there without problems, but if I come back a few weeks later, there is substantial damage even if nobody else touch the stack. Is all of this an example of Creep (deformation)? I don't understand the article well enough to say either yes or no. Nyttend (talk) 21:30, 21 June 2012 (UTC)
- It's analygous to creep, but not exactly the same thing. Creep is essentially caused by stresses in metals having an effect on the solid state diffusion through the crystal lattice, so as to increase relaxation. That's why being close to the melting point dramatically increases creep. In this case I think the friction between the sheets of newspaper prevents instant relaxation but as it sits and goes through a few day/night cycles of heating and cooling the sheets will move a tiny amount accross each other as the aggregate structure deforms around the smaller box placed underneath. 203.27.72.5 (talk) 21:56, 21 June 2012 (UTC)
- (EC)It's an example of slow permanent deformation, so it probably can be considered an example of creep. Our article won't help you though with mechanism by which it occurs as the cardboard (I'm assuming that's the material that the boxes are made from) won't deform like a metal or ice. No doubt there are many papers written on this topic and here's the sort of apparatus that can be used to test the creep behaviour of corrugated cardboard boxes[6]. Mikenorton (talk) 22:03, 21 June 2012 (UTC)
- And here's a paper on just this sort of behaviour [7]. Mikenorton (talk) 22:09, 21 June 2012 (UTC)
- What a great question and reference/answer! SemanticMantis (talk) 00:00, 22 June 2012 (UTC)
- Thanks very much for the help! Yes, they're corrugated cardboard boxes; sorry that I forgot to mention that before. They're normally full, although for some newspaper titles we don't quite have enough to fill a box, and those ones (not surprisingly) appear to be more vulnerable to deformation — they sometimes exhibit comparable behavior in the middle of a stack, when they have equally sized boxes both above and below them. In such cases, the sides splay out and the boxes on top will appear to have sunken into the partly empty box, squishing the partly empty box's lid in the process. I think I'll read the Georgia Tech paper if I get the chance. Nyttend (talk) 14:54, 22 June 2012 (UTC)
- What a great question and reference/answer! SemanticMantis (talk) 00:00, 22 June 2012 (UTC)
- And here's a paper on just this sort of behaviour [7]. Mikenorton (talk) 22:09, 21 June 2012 (UTC)
Why exactly does water stop bullets?
Hello. Mythbusters recently showed a clip from the time they tested shooting bullets into water, and watched how they disintegrated a few feet in. Apparently they blamed this stopping power on the "incompressibility of water". Nevermind water is not exactly "incompressible" (just very hard to compress); does saying "water is (nearly) incompressible" (or something similar) really explain the bullet-stopping power? Or what exactly would you have to say about water to explain why it's so effective against bullets? Thanks in advance. --Kreachure (talk) 23:28, 21 June 2012 (UTC)
- well, nothing is exactly incompressible. --Trovatore (talk) 23:42, 21 June 2012 (UTC)
There are a few factors at play. First of all, many modern bullets for civilian use are what they call frangible, or meant to break up or mushroom on impact. This is so that they transfer their energy more quickly and more completely into the target. Virtually every hunting round falls into this category and even non-frangible civilian ammo is jacketed soft-point at best. Only the military uses fully jacketed rounds that do not deform normally in use. The water causes the round to start to mushroom or break apart (depending on the type of round) and this destroys its aerodynamics (hydrodynamics?) and causes it to lose speed more rapidly due to resistance. In addition the density of the water rapidly saps kinetic energy from the bullet. Remember most bullets are VERY fast but actually don't have all that much in terms of actual momentum because they typically weigh comparatively very little. if you take into account the fact the round is being pushed out of shape it can lose speed in as little as a foot or two of water. the rounds that perform best in water are smaller, very fast and smooth-nosed rounds like the 9x19mm Parabellum (as proven on mythbusters) but if the round is too tiny it will fracture (as mythbusters saw with the 5.56mm NATO round). There are special rounds designed for underwater use, in use by Russia notably in a modified AK-47 frame. The rounds are designed like flechettes or darts with a very low cross-section and long length and designed for hydrodynamics. I have NO basis for this but logic would indicate there may also be a hydrodynamic influence, if the bullet created a low-pressure cavitation behind it, that would further slow it. HominidMachinae (talk) 23:45, 21 June 2012 (UTC)
- To further what Hominid said, civilian ammunition is designed to break up because that causes the most damage to flesh. It might seem counter intuitive that military ammunition has a key design element that inhibits it from damaging flesh, but that's an historical artifact from the hague convention. Anyways, since flesh is mostly water, the round behaves in water much the same way as it does in flesh; its cross-section expands and it breaks up. 203.27.72.5 (talk) 01:12, 22 June 2012 (UTC)
- As to what physical processes cause it to break up, when the bullet is travelling though air, it has very little to resist its motion. When the tip of the bullet makes contact with water/flesh, the resistance is much greater and it slows very quickly (it may still be travelling quite fast, but it's rate of deceleration is very high). This sends a shockwave through the length of the bullet which causes it to break apart. The shock wave travels at the speed of sound (in the metal that the bullet is made out of) with respect to the bullet. Since the bullet itself may be going faster than the speed of sound, the shockwave may not reach the back of the bullet until it's already travelled more that it's own length into the flesh. If the bullet has a soft nose, the lead at the front will also be deformed and material will be forced down into the nose and cleave it apart leading to the mushroom effect. If it has a hollow nose, this effect can be even stronger.
- The flechette style rounds that Hominid mentioned are made of hard metals that don't break up easily (such as steel alloys) and as he said, their small cross section aids them in getting through flesh without being clogged up with flesh in the nose (like a hypodermic syringe). You could say that the fact that air is compressible is what leads to it not resisting the bullet's motion. You could also just say that its density is so much lower that there is less mass to heave out of the way. The fact that water/flesh is compressible is important in the damage caused by bullets also; a compression wave originiates at the point of impact and propagates through the body potentially causing damage to organs far away from the bullet's path. 203.27.72.5 (talk) 01:22, 22 June 2012 (UTC)
- Excellently put, I'd also like to point out that military ammo has other constraints on it other than maximum flesh damage. A full metal jacket round is kinder on barrels especially when firing fully automatic (important if you want your rounds to be compatible with your squad support weapons), it also has greater armor penetration. To top it all off the noses of hollow points (unless capped) have problems feeding in some automatic mechanisms, that was the reason the smooth-nosed 9mm Parabellum was invented to begin with. Some self-defense experts advise using revolvers for defense and concealed carry for that very reason, you can carry a mushrooming round without risking a feed jam, and if a round misfires you can just pull the trigger again and rotate the cylinder. HominidMachinae (talk) 01:37, 22 June 2012 (UTC)
Not to trivialize an interesting discussion but, could you then say in simple terms that water can stop bullets "because water is much denser than air, and a bullet encounters so much resistance when in water that it can't clear a path and breaks apart"? I hope I'm not oversimplifying too much. Apart from this, the shockwave aspect that was mentioned I find very hard to believe. Do you mean that things that are slowed very fast are destroyed by the resulting shockwave, rather than by the "being slowed" itself? If this is the case, could you provide other examples where this happens? --Kreachure (talk) 02:04, 22 June 2012 (UTC)
- I'm not sure that you're grasping the concept of a shockwave. The nose of the bullet slows first. The particles (of Pb) that comprise the nose slow down and the bonds to the particles immediately behind them are compressed. This compression continues in a wave through to the back of the bullet. At points such as lattice defects the bond compression may be enough to break the bonds and consequently break the bullet apart. You can't distinguish the shockwave caused by the deceleration from the "being slowed" itself. 203.27.72.5 (talk) 02:28, 22 June 2012 (UTC)
- I don't think you're oversimplifying much, except that the mechanism of the bullet mushrooming is vital to understanding the issue here, and why some bullets might NOT be affected that much by water. HominidMachinae (talk) 02:30, 22 June 2012 (UTC)
- A full Coke can certainly can stop relatively low speed projectiles such as air rifle pellets, or projectiles with poor aerodynamic characteristics like shotgun pellets. It might stop a .22 or .177. It would have a better chance if you shot it length ways down from the spout. It won't stop a .50cal though. Not even length ways. 203.27.72.5 (talk) 02:34, 22 June 2012 (UTC)
Speed of sound in lead is 1 km/s, while pistol rounds travel half that fast. Ergo no shockwave inside the bullet. Hcobb (talk) 02:42, 22 June 2012 (UTC)
- Your conclusion does not follow from your premise. The abrupt change in velocity is what constitues the shockwave, not the velocity itself. 203.27.72.5 (talk) 02:50, 22 June 2012 (UTC)
- Hcobb is right. Most bullets do not travel fast enough to experience internal shock waves on impact. The bullets experience an impact (mechanics) resulting in a shock (mechanics) that creates an extreme compression wave in the bullet that does tear the bullet apart. However, since the bullets are usually traveling slower than the speed of sound in lead, the resulting wave is merely a compression wave and not a shock wave. An actual shock wave requires a disturbance that propagates faster than the internal sound speed, which will not occur at typical bullet speeds. This distinction is technical, but not horribly relevant. The subsonic compression waves created by a bullet's impact are still more than enough to rip it apart. Dragons flight (talk) 05:28, 22 June 2012 (UTC)
Um, water stops bullets for the same exact reason human the human body stops bullets. It's mostly made of water. μηδείς (talk) 02:55, 22 June 2012 (UTC)
- To rephrase that slightly, water stops bullets for the same reason that bullets stop people. Hcobb (talk) 13:35, 22 June 2012 (UTC)
- I guess I've never seen a "modern bullet" which shatters when it hits water or anything else. I've fired rifle and pistol bullets in years past which were basically a streamlined piece of lead. If "modern bullets" feel "shock waves" which cause them to fall apart when they hit water, then how come ballistics testing of a pistol starts with firing it into a water tank, after which the intact bullet is examined microscopically for striations which can match it to crime scene bullets? Certainly bullets extracted from someone's body may be mushroomed or distorted, but not always shattered into fragments. I've heard of the "frangible rounds" being used by sky marshals, so they don't shoot holes in a pressurized airplane, or so a bullet does not pass through the "bad guy" and hit a "good guy," but if I were shooting a game animal for the pot, I would rather have one bullet to remove than bits of shattered bullet fragments. If I were a soldier or cop shooting at an opponent, I would not want to have fragile bullets which shattered on contact with something, while they had non-shattering bullets which could go through a door or car and still kill me. Edison (talk) 14:51, 22 June 2012 (UTC)
- Where are you shooting your game? You don't need to remove the bullet from the brain, unless you want to eat that and risk prion infection. And not too many people have a taste for heart for game animals. In forensics, the bullets are shot into water containing a surfactant to minimize surface tension and at a shallow angle. It can also take many firings to produce an intact specimen even under those circumstances. 203.27.72.5 (talk) 23:02, 22 June 2012 (UTC)
- See this eBook for an excellent explanation of what goes on as a bullet impacts flesh, water and other surfaces. 203.27.72.5 (talk) 23:13, 22 June 2012 (UTC)
- The bullets break up and they try to id the fragments apparently[8]. Or some suggest they use low-velocity loads in water tanks. Rmhermen (talk) 23:38, 22 June 2012 (UTC)
- I said SOME frangible bullets are designed to break up, typically some kinds of anti-personnel loads and training rounds, far more common is the mushrooming kind (jacketed hollowpoint, hollowpoint, wadcutter, semi-wadcutter, AET, AET composite). There are fragmenting rounds like Glaser, Spartan, Equalloy and the potentially mythical mercury). HominidMachinae (talk) 17:38, 23 June 2012 (UTC)
- Where are you shooting your game? You don't need to remove the bullet from the brain, unless you want to eat that and risk prion infection. And not too many people have a taste for heart for game animals. In forensics, the bullets are shot into water containing a surfactant to minimize surface tension and at a shallow angle. It can also take many firings to produce an intact specimen even under those circumstances. 203.27.72.5 (talk) 23:02, 22 June 2012 (UTC)
June 22
Photosynthesis, CO2, etc.
So, I have a few questions concerning photosynthesis, carbon dioxide and this whole global warming issue:
- How long does it takes green plants/trees to start photosynthesis?
- On average, how much oxygen is produced by trees through photosynthesis?
- Can plants get carbon dioxide "poisoning" (i.e., having too much?)
- I came up with a thought a little while ago. Companies that burn fossil fuels for energy just dump that carbon dioxide gas into the atmosphere. Why can't companies transfer the gas into say, a greenhouse beside the factory, with plants that could simply convert that CO2 into oxygen, as a way to reduce emissions into the atmosphere?
Thanks, 64.229.5.242 (talk) 00:16, 22 June 2012 (UTC)
- 1: Plants start photosynthesis as soon as the cotyledons break the soil surface, not long after germination. (At least most of them. Some vascular plants never photosynthesize, such as Indian pipe or broom rape).
- 2: You'll have to specify what you mean. mass per year per plant? Which plant? Mass per year per hectare? What community? This is a broad area of research. For starters, put /plant "carbon fixation" estimate/ into google scholar, or see e.g. here [9] for some methods that focus on certain forest types (O2 production will be related to CO2 fixation, which is a more common key term).
- 4: Plants do tend to grow better/more with more CO2, but sometimes their insect pests and plant pathogens also do better too. So whether your proposal would help much is unclear. SemanticMantis (talk) 00:31, 22 June 2012 (UTC)
- 5: (which you didn't ask) I don't think that most air pollution comes in nicely sorted flows. It is probably very challenging and expensive to selectively isolate CO2 from industrial sources. But, still an interesting question: I think pairing greenhouses with factories in some way could probably work out well... :) SemanticMantis (talk) 00:31, 22 June 2012 (UTC)
I'm only going to touch on the last question. Plants are not infinite CO2-->Oxygen conversion machines, and that's because the CO2 is not actually being converted into molecular oxygen. Water is broken up into hydrogen ions and oxygen, while the carbon dioxide is converted into carbohydrates (don't get on me about all the ions and electrons, I don't feel like keeping track). Some of those carbohydrates are burned for energy, and actually just get spat back out as carbon dioxide (and water vapor). The carbon dioxide that disappears is from the carbohydrate mass that is used to build the physical stuff of the tree itself. In this sense, the tree is acting as a carbon sequestration system. While a tree is growing, it is certainly removing CO2 from the atmosphere, but once it stops growing it has zero net impact. You'd have to keep planting new trees in perpetuity to continually cancel out the emissions of a factory. In fact, the article I linked to gives a lot of ideas on this. Someguy1221 (talk) 00:36, 22 June 2012 (UTC)
- 3:It is possible, although plants use CO2 for a carbon source and produce O2, plants alos need to breath in O2 just like animals do. If you placed a plant into a jar of CO2 and gave it no light, it would suffocate to death. In the atmosphere, if CO2 levels rise, so will the global temperature which hurts plants in a vast number of ways, higher respiration (anti-photosynthesis) rates, increased pest levels and spread of pests to previously cold areas, more severe weather events, aka hurricanes, floods, droughts, decreased stability in percipitation, increased rate of evaporation from soils, etc.
- 4:Greenhouses often increase CO2 levels to increase growth rates, up to 1500ppm (4x normal), over that it begins causing headaches in workers and has diminishing economic returns. I know of greenhouses that burn coal for heating and buy CO2 to pump into because, to paraphrase the owner "the emissions would severly damage the crop"...so he put up a smokestack so the neighbouring farmers can deal with his problem. Unique Ubiquitous (talk) 01:29, 22 June 2012 (UTC)
- Exhausts contain more than just CO2. They may also contain CO and NOx which are bad news for animals (including people). I don't know what they do to plants though. Is there an eqivalent of heme in plants? 203.27.72.5 (talk) 01:33, 22 June 2012 (UTC)
- Several pollutants are known to mess with a plant's stoma. Either they force the stoma shut when it should be open - reducing CO2 uptake thus stopping photosynthesis, or forcing them open when they should be closed - increasing water loss to critical levels. By heme, I expect you mean something for gas transportation? If so, then no, live plant cells are never too far from the air, I say live because cells say in the middle of the trunk of a tree are dead and don't need air. Cells, apart from the epidermis, are kinda loose allowing air to diffuse through the air spaces between them. And yes, roots also breath on their own and for plants like rice, which grow on flooded land, they have special air spaces which go from the above water plant to down below. Unique Ubiquitous (talk) 02:42, 22 June 2012 (UTC)
- Exhausts contain more than just CO2. They may also contain CO and NOx which are bad news for animals (including people). I don't know what they do to plants though. Is there an eqivalent of heme in plants? 203.27.72.5 (talk) 01:33, 22 June 2012 (UTC)
- To answer the OP's question 4: It is not practical for factories and processing sites to set up a greenhouse next door, as the land area required is huge. And its not necessary - oxygen and carbon dioxide difuse through the atmosphere with great facility. The percentage oxygen and percentage carbon dioxide in the air is negligibly different whether in rainforrest areas, deserts, or major industrial areas. Rainforrests in Indonesia can deal with carbon dioxide emitted in Australian industry for example. What we need politicians world wide to do is act more strongly to protect, nurture, and grow green plant areas. Wickwack120.145.68.66 (talk) 03:46, 22 June 2012 (UTC)
- Actually, we'd be better of in terms of CO2 sequestration if we grew green plant areas and then cut down all of the trees and stuck them somewhere where the cellulose won't break down and then grow more trees on that land and repeat. Also, massive sources of CO2 are often located far away from built up areas (i.e. coal/gas fired powerstations, smelters, etc.) so the land requirements aren't the big issue you make them out to be. Also, the comments about CO2 from Australia being dealt with by Indonesian rainforrests misses the OP's point; you can use the very high concentrations in exhaust gas to speed up the process. 203.27.72.5 (talk) 03:55, 22 June 2012 (UTC)
- Yet for some reason we do the opposite, taking the stored carbon from out the ground and throwing it into the air for all to suffer from...there is no point in starting to sequester so long as we are digging up fossil fuels. Unique Ubiquitous (talk) 04:47, 22 June 2012 (UTC)
- Sure there is; to reduce the net effect. If we did it enough we could even be carbon neutral through this process. I'm sure greenies would love that, "Save the Earth by cutting down old growth forrests, sequestering their carbon and planting fast growing weeds!" 203.27.72.5 (talk) 04:55, 22 June 2012 (UTC)
- Yet for some reason we do the opposite, taking the stored carbon from out the ground and throwing it into the air for all to suffer from...there is no point in starting to sequester so long as we are digging up fossil fuels. Unique Ubiquitous (talk) 04:47, 22 June 2012 (UTC)
- Actually, we'd be better of in terms of CO2 sequestration if we grew green plant areas and then cut down all of the trees and stuck them somewhere where the cellulose won't break down and then grow more trees on that land and repeat. Also, massive sources of CO2 are often located far away from built up areas (i.e. coal/gas fired powerstations, smelters, etc.) so the land requirements aren't the big issue you make them out to be. Also, the comments about CO2 from Australia being dealt with by Indonesian rainforrests misses the OP's point; you can use the very high concentrations in exhaust gas to speed up the process. 203.27.72.5 (talk) 03:55, 22 June 2012 (UTC)
- There's something called "Carbon capture and storage" that will reduce the released CO2 by 80-90%, but will also consume 25%-40% more fuel and thus increase the cost with 21-91%. However with successful research, development and deployment (RD&D), sequestered coal-based electricity generation in 2025 may cost less than present unsequestered coal-based electricity generation. Electron9 (talk) 10:00, 22 June 2012 (UTC)
Deflecting a lead bullet with a magnetic field
Hi, I found this discussion on repelling bullets with a magnetic field. Since most bullets are made of lead, is it indeed possible to deflect bullets with a strong enough magnetic field, either using lead's diamagnetic properties, or eddy currents, or something else? How would this work? --Kreachure (talk) 02:29, 22 June 2012 (UTC)
- If you shot a lead bullet at a magnetar, yes it would probably be deflected by the magnetic field. It would also be attracted by the extreme gravity, so catch-22. If you tried to make a massive magnet that would appreciably deflect lead bullets away from you, it would also cause damage to your tissues due to the diamagnetism of water (the effect a magnetic field has on water is about half of what it has on lead). 203.27.72.5 (talk) 02:44, 22 June 2012 (UTC)
- Ah, interesting. I suppose the user of such a magnet would require a magnetic shielding suit of some sort to be effectively protected. Or is it too optimistic to think that the material's permeability wouldn't fail under such a powerful magnetic field? --Kreachure (talk) 03:16, 22 June 2012 (UTC)
- The issue isn't magnetizing the user, but exerting magnetic force upon him. While the former is bounded as you suggest, the latter is not. Someguy1221 (talk) 03:19, 22 June 2012 (UTC)
- Hmm, I don't follow. Isn't being inside a magnetic field required for magnetic forces to be felt? Magnetic shielding not only prevents magnetization, but "isolation from external magnetic fields" as the article says. What do you mean by "bounded"? --Kreachure (talk) 03:32, 22 June 2012 (UTC)
- Sorry, I think I misread what you said. I thought you were saying that good shielding wasn't necessary because a material can only be magnetized so much. Also, see bounded function and boundedness for other uses. Someguy1221 (talk) 05:22, 22 June 2012 (UTC)
- While my understanding is that you could shield the user from the magnetic field using concentric spheres of magnetically permeable metals, that metal in and of itself would probably be sufficient to stop the bullets. Then you'd only need to turn on the magnetic field to kill your enemies once they get close enough... 203.27.72.5 (talk) 04:13, 22 June 2012 (UTC)
- Hmm, I don't follow. Isn't being inside a magnetic field required for magnetic forces to be felt? Magnetic shielding not only prevents magnetization, but "isolation from external magnetic fields" as the article says. What do you mean by "bounded"? --Kreachure (talk) 03:32, 22 June 2012 (UTC)
- The issue isn't magnetizing the user, but exerting magnetic force upon him. While the former is bounded as you suggest, the latter is not. Someguy1221 (talk) 03:19, 22 June 2012 (UTC)
- Ah, interesting. I suppose the user of such a magnet would require a magnetic shielding suit of some sort to be effectively protected. Or is it too optimistic to think that the material's permeability wouldn't fail under such a powerful magnetic field? --Kreachure (talk) 03:16, 22 June 2012 (UTC)
- In case the Mythbusters are an acceptable source to you, it may be worth noting that they played around with how powerful a magnetic field was necessary to significantly alter the path of a bullet in flight. [10] Jwrosenzweig (talk) 06:21, 22 June 2012 (UTC)
- That website seems to be saying that the bullet was attracted by the magnetic field. If the magnetism was causing that effect those magnets would be very difficult to handle; since they apparently attract diamagnetic substances (assuming the bullets were Pb) they'd stick to flesh. Maybe there's more complex physics at work to explain why that happened. Or maybe it's just bollocks. 203.27.72.5 (talk) 07:11, 22 June 2012 (UTC)
- Maybe the magnets were pulling the gun out of it's precise position and causing it to point further downward than before. 203.27.72.5 (talk) 07:15, 22 June 2012 (UTC)
- Most modern bullets have a copper jacket around the lead core. They also spin at very high rates, modeling the induced eddy currents in such a situation is not easy. Roger (talk) 14:17, 22 June 2012 (UTC)
- I just watched the Mythbusters episode. I'm pretty sure they used bullets either made of something other than lead, or made of lead jacketed with metal (copper, cupronickel, etc.). I'm gonna go with Ockham's Razor (usually a good idea :) ) and say that this is why the bullet was attracted to the magnets; and not because of "complex physics" or "experimentation flaw" (or "bollocks"). --Kreachure (talk) 15:37, 22 June 2012 (UTC)
- But copper is also diamagnetic. 203.27.72.5 (talk) 20:15, 22 June 2012 (UTC)
- Fine, not copper then. --Kreachure (talk) 23:31, 22 June 2012 (UTC)
- And I wouldn't say they spin at very high rates at all. A typical rifling twist rate in a semi-automatic pistol is 1 revolution per 30". 203.27.72.5 (talk) 20:56, 22 June 2012 (UTC)
- But copper is also diamagnetic. 203.27.72.5 (talk) 20:15, 22 June 2012 (UTC)
- I just watched the Mythbusters episode. I'm pretty sure they used bullets either made of something other than lead, or made of lead jacketed with metal (copper, cupronickel, etc.). I'm gonna go with Ockham's Razor (usually a good idea :) ) and say that this is why the bullet was attracted to the magnets; and not because of "complex physics" or "experimentation flaw" (or "bollocks"). --Kreachure (talk) 15:37, 22 June 2012 (UTC)
- Most modern bullets have a copper jacket around the lead core. They also spin at very high rates, modeling the induced eddy currents in such a situation is not easy. Roger (talk) 14:17, 22 June 2012 (UTC)
- Maybe the magnets were pulling the gun out of it's precise position and causing it to point further downward than before. 203.27.72.5 (talk) 07:15, 22 June 2012 (UTC)
- That website seems to be saying that the bullet was attracted by the magnetic field. If the magnetism was causing that effect those magnets would be very difficult to handle; since they apparently attract diamagnetic substances (assuming the bullets were Pb) they'd stick to flesh. Maybe there's more complex physics at work to explain why that happened. Or maybe it's just bollocks. 203.27.72.5 (talk) 07:11, 22 June 2012 (UTC)
- Eddy currents have easily observable effects, If the poles of a fairly strong magnet have a small gap between them, and you drop various flat pieces of material between them, non-electrically conductive substances like plastic and wood seem unaffected and drop as if there were no magnet there, but copper or lead seem to hesitate and slide v-e-r-y s-l-o-w-l-y through the gap. If a spinning disc of aluminum or other conductive material has an ordinary permanent magnet brought near it, it slows dramatically and noticeably. If a lead bullet were fired at a distant target, and early in its flight passed through a magnetic field achievable with common means (neodymium magnets near the path, or a modest electromagnet, even a small deflection would cause it to miss the aiming point far away at the end of the trajectory. The closer the magnet is to the target, the more extreme the magnetic field would have to be to modify the path meaningfully. All in all, deploying magnets to deflect bullets does not seem practical, compared to spending the same money and effort to build a barrier, or detection systems and weapons to shoot the shooter before he pulls the trigger. Edison (talk) 14:39, 22 June 2012 (UTC)
If the bullet is stopped in a bullet proof vest then the forces that make that happen are ultimately electromagnetic in origin. Count Iblis (talk) 17:57, 22 June 2012 (UTC)
Why are mammals smarter than other animals?
Not sure if this question has any meaningful answer, but I thought I would throw it out there. I was just reading Great white shark and I just thought to myself, why are they so dumb? Especially compared to Orcas. Assuming that mammals are indeed (typically) smarter than other animals, which I believe is true. But why is this? Is there a correlation with warm bloodedness and intelligence? Is it just that some mammals happened to (coincidentally) evolve intelligence, and then from there animals that evolved from them were intelligent as well? Dolphins are intelligent... Orcas are intelligent. Pigs are intelligent. Dogs are intelligent. Great apes are intelligent. Humans are intelligent. Seems that animals that eat meat are typically more intelligent that herbivores. But if I'm not mistaken we have a closer common ancestor with deer than we do with dolphins right? Not sure. In terms of lifespan, do mammals typically live longer than other animals? ScienceApe (talk) 04:21, 22 June 2012 (UTC)
- Some birds are also very intelligent, such as crows. They are also warm blooded and many eat meat. A large brain does have a high energy demand, and energy density is higher in meat than in vegetation. Octopuses are apparently quite intelligent too. Hunting also requires a high degree of intelligence, so carnivores have an advantage in the evolutionary selection there. 203.27.72.5 (talk) 04:32, 22 June 2012 (UTC)
- (ec)No, some of the longest living animals are reptiles, in the turtle family specifically. Also, there are some intelligent birds, like parrots, and even intelligent cephalopods, like cuttlefish. StuRat (talk) 04:29, 22 June 2012 (UTC)
- Whales also have a very long lifespan. Trying to work out an average for two entire classes so you can compare them is both difficult and largely meaningless though. 203.27.72.5 (talk) 04:36, 22 June 2012 (UTC)
A higher body temperature and both a greater relative and absolute brain size. μηδείς (talk) 15:10, 22 June 2012 (UTC)
Anecdotally, my vegetarian friends are also much dumber than their meat-eating counterparts.Just kidding...friends don't let friends become vegetarians.203.27.72.5 (talk) 04:41, 22 June 2012 (UTC)
- Paging Dunning–Kruger. Would Mr. Dunning & Mr. Kruger please come to the RD. --Tagishsimon (talk) 14:01, 22 June 2012 (UTC)
- I don't know what relevant commentary they could have on the matter. Their hypothesis involved inept people who judge themselves to be better than they really were and vica-versa. My comment compared two groups of other people. 203.27.72.5 (talk) 22:29, 22 June 2012 (UTC)
- Deer and dolphins are actually pretty closely related; they both belong to the clade Cetartiodactyla. Regarding the main question, I think the answer is a combination of (1) mammals have larger brains in relation to body size; (2) mammal brains use myelinated axons, which make their internal connections much more efficient; (3) only mammals have a fully developed cerebral cortex. Looie496 (talk) 06:46, 22 June 2012 (UTC)
- Also keep in mind some arthropods are very intelligent, and some mammals are very stupid. Salticids, particularly Portia labiata are said to "have hunting tactics as versatile and adaptable as a lion's" (see ref in our article). I think it is a general trend that, for a certain clade, predators tend to be more intelligent than herbivores. Omnivores may be smarter yet, such as the new caledonian crow, or the raccoon. Both of these can work simple latches to escape cages, and the former is celebrated for its tool use. SemanticMantis (talk) 13:10, 22 June 2012 (UTC)
- Some insects/arthropods may appear to be intelligent, but, due to their brain size, that can't be true intelligence, but rather all instinct. The way to test this theory is to expose them to situations not encountered in nature (like the monkey that has to stack boxes to get the banana) and see if they can design a strategy to solve those problems, or not. StuRat (talk) 19:06, 22 June 2012 (UTC)
- If you touch a cockroach often, does it have an instinct to start running farther and more furious as that's something that might happen in nature? If so, is it's instinct so good that it only displays the heightened response to things that look like what's chased it before, for example, things that look like shoes? Sagittarian Milky Way (talk) 17:33, 23 June 2012 (UTC)
- Possibly. We seem to have similar instincts. For example, if we see insects, we then have a heightened sense of awareness and sensitivity on our skin, even to the point of imagining bugs crawling on us that aren't really there. However, insects probably do have a small ability to learn, only in their case it's like 10% of their behavior versus 90% for us. StuRat (talk) 20:05, 23 June 2012 (UTC)
- I think mammals are relative latecomers, evolutionarily, thus they had to find a niche or an advantage that could allow survival, and intelligence provided that edge over the non-mammalian population that dominated the Earth. Intelligence itself is not good for anything under evolutionary pressure—except for survival. If intelligence helped mammals to survive then it would continue to accompany them in their competition for survival with one another, thus we have mammals competing with one another on the basis of intelligence, leading to greater intelligence in some species of mammals. But as humans we have to bear in mind that there is nothing intrinsically valuable about intelligence. It is merely one more factor that probably has bearing on the survival of a life-form. Bus stop (talk) 13:42, 22 June 2012 (UTC)
- It's also worth remembering that from an evolutionary point of view, very high intelligence is pretty clearly a fluke (one species in 10 billion or so over Earth's history), and humanity has yet to demonstrate that high intelligence is, in the long run, a positive evolutionary adaptation (the last 300 years have been hell-bent on a path to unsustainable self-destruction; the last 10,000 years of civilization may prove to be just a weird blip on the evolutionary timeline). --Mr.98 (talk) 14:06, 22 June 2012 (UTC)
- If you are talking about human intelligence, I agree, but a fair degree of intelligence seems to have developed independently on Earth several times. Right now we have some birds (crows, etc.) and octupi/squid/cuttlefish, in addition to mammals (some of which, like aquatic mammals, aren't very close to us). In the past, there were probably several others cases of intelligence developing independently. StuRat (talk) 04:16, 23 June 2012 (UTC)
- Have to agree with Mr.98. IQ measurements seems to centre around solving puzzles dreamt up by other people who find solving puzzles fascinating and an end to themselves. Yet Mensa members can be found among the welfare queues because they can't feed themselves. Real intelligence means being able to survive this short sojourn on earth and eating and producing offspring before getting eaten oneself. Even the humble Cockroach has been around about 350,000,000 obits of this blue and green planet and it succeed in trained the Homo sapiens to provide the cockroach kinship with nice comfy all year round habitats and regular supplies of food. However, mice have long since overtaken both the cockroach, and Alan Turing. --Aspro (talk) 14:51, 22 June 2012 (UTC)
Clindamycin
When and how was Clindamycin discovered?--Jsjsjs1111 (talk) 05:37, 22 June 2012 (UTC)
- This article from 1970 says that the drug was "newly developed" from lincomycin. 203.27.72.5 (talk) 06:32, 22 June 2012 (UTC)
- (edit conflict) 1966, modified from Lincomycin ("Chemical Modifications of Lincomycin", Antimicrobial Agents and Chemotherapy (1961-70) (1966), 1966, 727-36.), is the earliest reference I can find to it. Buddy431 (talk) 06:41, 22 June 2012 (UTC)
- Here's a 1970 paper on its synthesis. It probably has the information you're looking for, but it's behind a pay wall. 203.27.72.5 (talk) 06:42, 22 June 2012 (UTC)
- Apparently its synthesis was first announced in 1965 at the Fifth interscience conference on antimicrobial agents and chemotherapy by Robert Birkenmeyer, et al. 203.27.72.5 (talk) 06:50, 22 June 2012 (UTC)
- Thank you very much! BTW, your IP address looks oddly familiar. You're from Brisbane?--Jsjsjs1111 (talk) 07:20, 22 June 2012 (UTC)
- I'm in the NT, but my organisation is headquartered in Brisbane, so maybe that has something to do with it. 203.27.72.5 (talk) 07:23, 22 June 2012 (UTC)
- Thank you very much! BTW, your IP address looks oddly familiar. You're from Brisbane?--Jsjsjs1111 (talk) 07:20, 22 June 2012 (UTC)
- Apparently its synthesis was first announced in 1965 at the Fifth interscience conference on antimicrobial agents and chemotherapy by Robert Birkenmeyer, et al. 203.27.72.5 (talk) 06:50, 22 June 2012 (UTC)
- Here's a 1970 paper on its synthesis. It probably has the information you're looking for, but it's behind a pay wall. 203.27.72.5 (talk) 06:42, 22 June 2012 (UTC)
- (edit conflict) 1966, modified from Lincomycin ("Chemical Modifications of Lincomycin", Antimicrobial Agents and Chemotherapy (1961-70) (1966), 1966, 727-36.), is the earliest reference I can find to it. Buddy431 (talk) 06:41, 22 June 2012 (UTC)
Explosive question
In the news, whenever I hear about the number of people died and injured in a bomb explosion, the number of people injured is usually higher than those killed in it (a natural consequence of the fact that the bomb's destructive power is higher in lower distances, but the number of people is less in the area covered by that distance, but the destructive power is less in higher in further distances, while more people (area) is covered in that distance) now my question is, can people speculate what kind of a bomb used only knowing the number of the killed/injured and the <average> population area density at that time of the day in that place? Has it ever been done? How good an estimate is it?--Irrational number (talk) 09:25, 22 June 2012 (UTC)
- Depends exactly what you mean. Firstly, yes, people can, and will, always speculate on just about everything. Secondly, if there is a massive death toll over a wide area it may be safe to assume the bomb was nuclear or even thermonuclear. Since you're probably talking about people being killed in the middle east or north Africa by IEDs and suicide bombs (unless you happen to be reading a newspaper from 1945), it would be much easier just to guess based on what the most common type of terrorist explosive is in that region. 101.171.127.238 (talk) 09:58, 22 June 2012 (UTC)
- (EC)No. A small amount of a powerful explosive is presumably as destructive as a larger amount of a less powerful explosion. SO that rules out knowing exactly what sort of bomb you're dealing with. The vaguries of the position of the bomb, the environment in which is is placed, and a whole host of other uncontrolled variables would entirely frustrate the sort of analysis you;re looking for. --Tagishsimon (talk) 10:01, 22 June 2012 (UTC)
- You can't determine the type of bomb, but you can estimate the explosive yield (that's a red link... try TNT equivalent - can anyone find a better article to redirect it to?). There is a whole field of study into estimating yields based on damage and injuries and things. Once you know the yield, you may be able to make a guess about what kind of bomb is likely to have that yield, but generally Tagishsimon's point is true and you can't distinguish between a small amount of high explosive and a large amount of low explosive (except from context - if you know who made the bomb you'll know what they usually use or what they have access to). --Tango (talk) 11:30, 22 June 2012 (UTC)
- Another thing to consider, is that the detonation wave from say a 60lb charge is only about 50 feet. This wave can rupture lungs and things, bringing about eventual if not immediate death. Yet a bomb loaded with ball bearings and other such objects, can cause injures many times this distance. --Aspro (talk) 15:05, 22 June 2012 (UTC)
- The blast wave of a real bomb in an urban environment is by no means spherical; it follows the obstacles in its way. If the bomb happened to be under a car, the death toll will likely be small, especially if the only people around that car are surrounded by other cars. If it goes off in an open market square and sends shrapnel everywhere, many more people will be killed. Even putting the bomb behind a table leg can provide enough shielding to save someone on the other side of the table leg, as evidenced by the 20 July plot. --140.180.5.169 (talk) 05:06, 23 June 2012 (UTC)
Nuclear fusion in black holes
Could nuclear fusion theoretically occur in or around a black hole? If not, why not? And if so, what kind of superheavy elements might be synthesised? Evanh2008 (talk|contribs) 10:45, 22 June 2012 (UTC)
- Nothing of any meaning to human comprehension can happen "in" a black hole. Around it, yeah potentially fusion could happen, especially if it's a quasar. Neutronium might be made there. That's not really an element though since even if you take it to be a nucleus, it's atomic number is still zero. It's pretty heavy though. Strange matter could also be made there, shortly before plunging into the blackhole itself. 101.171.127.238 (talk) 11:11, 22 June 2012 (UTC)
- Since when is an atomic number greater than zero a definition requisit for an element? Plasmic Physics (talk) 11:24, 22 June 2012 (UTC)
- Well, an element is a category of atoms with the same atomic number, and atoms are a nucleus surrounded by a cloud of electrons. Neutrons cannot hold electrons, so they cannot form atoms by themselves, therefore neutronium cannot be a chemical element. That's just my logic in deriving my conclusion though. It's such an inconsequential grey area that I don't think anyone really cares whether or not neutronium is considered an element. 101.171.127.238 (talk) 11:40, 22 June 2012 (UTC)
- An atom is a nucleus, composed of x protons and y neutrons, surrounded by x electrons. In neutronium, x = 0, so there are 0 protons, y neutrons, and 0 electrons. So despite the lack of electrons, neutronium is still an element, composed of atoms. Whoop whoop pull up Bitching Betty | Averted crashes 14:33, 22 June 2012 (UTC)
- By the way, if an atomic number of zero can still be an element, can an atomic weight of zero also constitute an element? That would make the lightest isotope of neutronium nothing. I'm going to name it now (assuming I'm the first to consider this). I shall call it vacuumium. It's the most plentiful element in the universe. 101.171.127.238 (talk) 11:42, 22 June 2012 (UTC)
- Last time I checked, a simple neutron does not weigh nothing. Why are electrons required for an element, neutronium consists of nucleons. There are several arguements available from acedemic sources, supporting the classification of the neutron as element 0, under several proposed names: Neutrium, Neutronium, Nilium, Nihilon, or just Neutron. Plasmic Physics (talk) 13:31, 22 June 2012 (UTC)
- I think the point was, that if neutronium is an element, and you removed one neutron to make a lighter isotope of neutronium, you'd have nothing left. 203.27.72.5 (talk) 22:32, 22 June 2012 (UTC)
- That's true, I'm not argueing against that. What does that prove though? Plasmic Physics (talk) 01:13, 23 June 2012 (UTC)
- Nothing. It just forms the basis of a reductio ad absurdum that results in the vacuum being considered an isotope of neutronium. The debate is just over semantics anyway. Call it an element, don't call it an element, potato, potata. 203.27.72.5 (talk) 03:38, 23 June 2012 (UTC)
- Yes, that would be an extreme interpretation to the point of being absurd. It ventures into the domain of philosophy, akin to the 'nothing' paradox: any definition of 'nothing', leads to the logical conclusion that 'nothing' is 'something'. Therefore 'nothing' cannot exist. It is not absurd to say that a glass is empty, it is absurd to say: I have a glass of water, it contains 0 mL of water. Plasmic Physics (talk) 05:57, 23 June 2012 (UTC)
- Does anyone really say potata? Plasmic Physics (talk) 05:58, 23 June 2012 (UTC)
- I think so, but the pronounciation of the second a is like in tater tots. 203.27.72.5 (talk) 06:14, 23 June 2012 (UTC)
- Nothing. It just forms the basis of a reductio ad absurdum that results in the vacuum being considered an isotope of neutronium. The debate is just over semantics anyway. Call it an element, don't call it an element, potato, potata. 203.27.72.5 (talk) 03:38, 23 June 2012 (UTC)
- That's true, I'm not argueing against that. What does that prove though? Plasmic Physics (talk) 01:13, 23 June 2012 (UTC)
- I think the point was, that if neutronium is an element, and you removed one neutron to make a lighter isotope of neutronium, you'd have nothing left. 203.27.72.5 (talk) 22:32, 22 June 2012 (UTC)
- Last time I checked, a simple neutron does not weigh nothing. Why are electrons required for an element, neutronium consists of nucleons. There are several arguements available from acedemic sources, supporting the classification of the neutron as element 0, under several proposed names: Neutrium, Neutronium, Nilium, Nihilon, or just Neutron. Plasmic Physics (talk) 13:31, 22 June 2012 (UTC)
- Well, an element is a category of atoms with the same atomic number, and atoms are a nucleus surrounded by a cloud of electrons. Neutrons cannot hold electrons, so they cannot form atoms by themselves, therefore neutronium cannot be a chemical element. That's just my logic in deriving my conclusion though. It's such an inconsequential grey area that I don't think anyone really cares whether or not neutronium is considered an element. 101.171.127.238 (talk) 11:40, 22 June 2012 (UTC)
- Since when is an atomic number greater than zero a definition requisit for an element? Plasmic Physics (talk) 11:24, 22 June 2012 (UTC)
- Contrary to 101.171.127.238's answer, it's certainly possible for nuclear fusion to occur inside of a black hole. From the perspective of an object that falls into a black hole, spacetime is locally unremarkable as the object crosses the event horizon. So for example if a star undergoing fusion falls into a supermassive black hole, there's nothing immediately that would stop the fusion within the star from continuing after it crosses the event horizon. (The same can't be said after the star hits the gravitational singularity, though.)
- However, there's nothing particular about falling into a black hole that would cause nuclear fusion to occur. As you get further inside of a black hole, tidal forces become extremely strong, eventually resulting in the spaghettification of infalling objects. However, in spaghettification, the compression experienced along two spatial dimensions is counterbalanced by stretching along the third, resulting in no change in the object's volume. So the tidal forces aren't likely to cause the pressure within the object to get high enough to cause fusion. Of course, all bets are off as to what happens in the very last little bit right near the gravitational singularity. Red Act (talk) 15:17, 22 June 2012 (UTC)
- As I understand it, as you fall towards a black hole, there is always a point in front of you from which you cannot observe light returning to you i.e. a point at which the required velocity to reach your eyes is greater than c. That's why nothing remarkable happens, from your perspective you never actually hit the event horizon, it always receeds away from you. When you look out towards the rest of the universe, there will be no event horizon behind you, just a very blue shifted universe. So there is no observer who can see inside the black hole, not even one who to another far away observer has crossed the event horizon. 203.27.72.5 (talk) 22:25, 22 June 2012 (UTC)
- Nuclear fusion occurs everywhere you have hydrogen (and other light elements too). You need high temperatures and/or pressures to make the rate significant, but even at room temperature there is a nonzero chance at any given moment that two hydrogen nucleii in a bag of hydrogen will fuse. So, of course fusion occurs around a black hole. If the matter gets hot and/or dense there, then fusion will occur faster. --Srleffler (talk) 03:06, 23 June 2012 (UTC)
I should point out that around black holes is an accretion disk, which is one of the most efficient methods of turning mass into energy. Up to and perhaps exceeding 40% efficiency, which far beats nuclear fusion which is only about .7% efficient. Black_hole#Accretion_of_matter. ScienceApe (talk) 14:41, 23 June 2012 (UTC)
- From the article on accretion disks it isn't really clear to me the mechanism that results in matter being turned into energy. Obviously, any chemical bonds will be broken by the heat and pressure releasing their stored energy. The heat and pressure will also be high enough to enable fusion. But what accounts for the really efficient mass to energy converstion? 203.27.72.5 (talk) 23:13, 23 June 2012 (UTC)
- (ec)The mechanism would likely still be fusion, simply more complete (higher temperature and repeated) fusion than one finds in main-sequence stars where not all the matter is fused to iron before the star explodes or fusion otherwise stops. Excitation strong enough to create high-energy gamma rays could produce matter-antimatter pairs. However, the energy released by their annihilation would be the same as the energy needed to create the gamma-ray in the first place. The mechanism of gamma-ray bursts, which are short lived, are not understood according to http://en.wikipedia.org/wiki/Gamma-ray_burst#Energetics_and_beaming μηδείς (talk) 01:02, 24 June 2012 (UTC)
- No, the mechanism is simple friction. ScienceApe (talk) 01:00, 24 June 2012 (UTC)
- How does friction convert mass to energy? Normally friction as I know it converts kinetic energy to heat. 203.27.72.5 (talk) 01:05, 24 June 2012 (UTC)
- (EC)That doesn't really add up. If you fuse 2H+ to make 2H+ then fuse thatwith another H+ to give 3He and fuse two of those to make 2H+ and 2He, you still have ~6 atomic mass units just like you did at the start. A small fraction is now energy, but not all that much. No where near 40%. Even if you continue through to 56Fe, there's no way 40% of the mass is now released as binding energy; and after that point fusion is endothermic. 203.27.72.5 (talk) 01:05, 24 June 2012 (UTC)
- Actually, assuming the friction can make gamma rays of high enough energy, it would be the equivalent of the annihilation of matter-antimatter pairs. I was assuming that this would have to be driven by fusion, but I think ScienceApe is correct that friction alone might excite the matter enough to produce gamma rays of such high energy. I am not competent to work that out. μηδείς (talk) 01:15, 24 June 2012 (UTC)
- That's still only converting kinetic energy to heat, and then radiating it as a gamma ray, and then creating and annihilating some matter and antimater. There's not net change of matter to energy there. How does the matter become energy? 203.27.72.5 (talk) 01:43, 24 June 2012 (UTC)
- There's an unsourced claim on the article for pair production that says near a black hole, one of the created anti particles could be sucked into the hole before it gets to annihilate, so then there's a net creation of energy from matter only when one particle and not the other gets sucked into the hole and only when that particle is the electron. In the other case where you end up with an electron outside and a positon sucked in, you actually create mass from energy cancelling out the effect of the first case. 203.27.72.5 (talk) 01:59, 24 June 2012 (UTC)
- That's Hawking radiation, and it's only significant in small black holes without accretion disks. As for converting matter to energy by friction, shouldn't the kinetic energy of mass falling into a black hole be relatively easy to calculate? I'll leave it to some math or physics major. μηδείς (talk) 02:10, 24 June 2012 (UTC)
- Of course it's easy to calculate. It's also completely beyond the point. A huge amount of kinetic energy being converted into a huge amount of heat is just that. A conversion from one form a energy to another. There's no converstion of mass to energy. According to ScienceApe, >40% of the mass can be converted to energy (according to the wikipedia article on accretion discs it's 10 percent and neither of them has cited a source). How does that happen? 203.27.72.5 (talk) 03:18, 24 June 2012 (UTC)
- You seem to be missing the fact that black body radiation can be emitted at any energy, and that while it is often called heat, since we equate it in most cases with infrared radiation, it can have any energy, up to x-ray and gamma radiation. Remember, also, that E=mc^2, and hence gamma rays are the equivalent to a certain amount of mass. μηδείς (talk) 03:35, 24 June 2012 (UTC)
- I'm not missing that at all. As I said earlier "That's still only converting kinetic energy to heat, and then radiating it as a gamma ray". I know you can radiate internal energy as gamma rays. ScienceApe said "an accretion disk...is one of the most efficient methods of turning mass into energy.". The only possibile interpretation of that is "rest-mass into energy". Otherwise it just becomes "Mass energy to mass energy". Gamma rays are the equivalent of a certain amount of mass, but what mass? What mass is gone? I can see where there was some gravitational potential energy, which became kinetic energy in the motion of the accetion disc, and then friciton converts it to heat which is then radiated as gamma rays. I can't see how any mass went to being energy. 203.27.72.5 (talk) 03:46, 24 June 2012 (UTC)
- There are two questions. First is that of the balanced equation. If the kinetic energy is sufficient to create powerful enough gamma rays, they will carry away a certain amount of energy, and hence, by E=mc^2, a certain amount of mass. Surely you agree with that? Second, there is the question of the intermediate steps. There's the rub, apparently, since the sources we have in wikipedia don't seem to give the proposed mechanisms. But that doesn't mean we don't know that we start with a certain amount of friction and end up with apparently 40% of the mass being converted to radiative energy? Or do you disagree at some point? μηδείς (talk) 03:54, 24 June 2012 (UTC)
- The second part is fully and absolutely understood. The first part is what I'm having trouble with. The net change is kinetic energy to gamma rays, correct? Yes, mass is equivalent to energy, but if you talk about mass being converted to energy (as ScienceApe did), clearly, you're delineating between rest-mass (which is converted to energy in nuclear fusion) and other mass energy. Gamma rays have no rest mass and kinetic energy is by definition the non-rest-mass component of total energy. The rest mass is therefore unchanged i.e. there is no converstion of mass to energy at all. 203.27.72.5 (talk) 04:27, 24 June 2012 (UTC)
- There are two questions. First is that of the balanced equation. If the kinetic energy is sufficient to create powerful enough gamma rays, they will carry away a certain amount of energy, and hence, by E=mc^2, a certain amount of mass. Surely you agree with that? Second, there is the question of the intermediate steps. There's the rub, apparently, since the sources we have in wikipedia don't seem to give the proposed mechanisms. But that doesn't mean we don't know that we start with a certain amount of friction and end up with apparently 40% of the mass being converted to radiative energy? Or do you disagree at some point? μηδείς (talk) 03:54, 24 June 2012 (UTC)
- I'm not missing that at all. As I said earlier "That's still only converting kinetic energy to heat, and then radiating it as a gamma ray". I know you can radiate internal energy as gamma rays. ScienceApe said "an accretion disk...is one of the most efficient methods of turning mass into energy.". The only possibile interpretation of that is "rest-mass into energy". Otherwise it just becomes "Mass energy to mass energy". Gamma rays are the equivalent of a certain amount of mass, but what mass? What mass is gone? I can see where there was some gravitational potential energy, which became kinetic energy in the motion of the accetion disc, and then friciton converts it to heat which is then radiated as gamma rays. I can't see how any mass went to being energy. 203.27.72.5 (talk) 03:46, 24 June 2012 (UTC)
- You seem to be missing the fact that black body radiation can be emitted at any energy, and that while it is often called heat, since we equate it in most cases with infrared radiation, it can have any energy, up to x-ray and gamma radiation. Remember, also, that E=mc^2, and hence gamma rays are the equivalent to a certain amount of mass. μηδείς (talk) 03:35, 24 June 2012 (UTC)
- Of course it's easy to calculate. It's also completely beyond the point. A huge amount of kinetic energy being converted into a huge amount of heat is just that. A conversion from one form a energy to another. There's no converstion of mass to energy. According to ScienceApe, >40% of the mass can be converted to energy (according to the wikipedia article on accretion discs it's 10 percent and neither of them has cited a source). How does that happen? 203.27.72.5 (talk) 03:18, 24 June 2012 (UTC)
- That's Hawking radiation, and it's only significant in small black holes without accretion disks. As for converting matter to energy by friction, shouldn't the kinetic energy of mass falling into a black hole be relatively easy to calculate? I'll leave it to some math or physics major. μηδείς (talk) 02:10, 24 June 2012 (UTC)
- Actually, assuming the friction can make gamma rays of high enough energy, it would be the equivalent of the annihilation of matter-antimatter pairs. I was assuming that this would have to be driven by fusion, but I think ScienceApe is correct that friction alone might excite the matter enough to produce gamma rays of such high energy. I am not competent to work that out. μηδείς (talk) 01:15, 24 June 2012 (UTC)
- No, the mechanism is simple friction. ScienceApe (talk) 01:00, 24 June 2012 (UTC)
- (ec)The mechanism would likely still be fusion, simply more complete (higher temperature and repeated) fusion than one finds in main-sequence stars where not all the matter is fused to iron before the star explodes or fusion otherwise stops. Excitation strong enough to create high-energy gamma rays could produce matter-antimatter pairs. However, the energy released by their annihilation would be the same as the energy needed to create the gamma-ray in the first place. The mechanism of gamma-ray bursts, which are short lived, are not understood according to http://en.wikipedia.org/wiki/Gamma-ray_burst#Energetics_and_beaming μηδείς (talk) 01:02, 24 June 2012 (UTC)
- That seems easily addressed. Imagine a particle and an antiparticle next to each other. They are moving towards each other with negligible but real velocity. They touch and are annihilated. A huge amount of energy is released. Almost none of this is from the original kinetic energy of the particles. That is what is happening in the accretion disk. Friction is creating gamma rays. The gamma rays are as energetic as matter-antimatter pairs. Hence they spontaneously create matter-antimatter pairs. Whose relative velocities are negligible, but real. So the pairs annihilate each other. Converting a huge amount of mass into an even huger amount of energy. μηδείς (talk) 04:54, 24 June 2012 (UTC)
- Ok, from reading a couple of non-wiki sources on the subject, I think I get it now. The logic of it still seems a bit odd, but I think I know what they're getting at now when they say 10% of the mass is converted to energy (the 40% estimate is now considered wrong because it would require almost all known quasars to have black holes rotating at 99% of the maxiumum allowable speed, and that was based on incorrect early mass estimates of the black holes themselves). If you throw something into the blackhole, friction, and the simple act of radially accelerating charged particles to near light speed causes it to emit light, and the total amount emited is proportional to its graviational potential energy, which is proportional to its mass. The total amount emitted sums to ~10% of it's rest mass. The reason this is a bit odd, is because even after it has emited most of that energy, but before it has crossed the event horizon, it still has the same rest mass it started off with so it's mass is not really being converted to energy. But still, the amount of electromagnetic energy that is extracted is still a fraction of it's mass, and the mass certainly cannot be recovered once it has crossed the event horizon. 203.27.72.5 (talk) 05:11, 24 June 2012 (UTC)
- Yes, your saying it emits light now, rather than heat, is the important part. But I myself find it hard to believe or understand that the rest mass stays exactly the same. (Is it just that c in E=mc^2 is so large?) Can you link to your source? μηδείς (talk) 05:38, 24 June 2012 (UTC)
- I've just left work, so I don't have the links in front of me, but they didn't discuss the fact that the rest mass stays the same anyway. Whether it's light or heat doesn't matter; gravitational potential energy is converted to light through the various mechanisms, including friction which is by definition the conversion of kinetic energy to heat. The rest mass must remain the same because rest mass is invariant by definition. Imagine a single atom of H flying into the black hole. When the distance is still great, is accelerates towards the hole, as it would towards any other graviational field. At first it emits very low frequency EMF, but as its velocity increases, it goes right through the spectrum to gamma. Tidal forces will strip the electron from it and if it's path curves towards the black hole, it will emit synchrotron radiation. If it bumps into another particle, it will lose kinetic energy and impart heat energy into the thing it hit. The amount of energy it will emit in total is about 10% of its rest mass. But right up until it crosses the event horizon, it will still have ~1 atomic mass unit of rest mass. 101.173.170.147 (talk) 11:03, 24 June 2012 (UTC)
- I should also point out guys that a great deal of the mass-energy generated in the accretion disk is imparted to the polar jet aka relativistic jet if the particles are moving at extremely high velocities. The jets are essentially titanic sized particle accelerators. The mechanism for how they are formed is not well understood yet. ScienceApe (talk) 14:22, 24 June 2012 (UTC)
- "The rest mass must remain the same because rest mass is invariant by definition" doesn't make a whole lot of sense. Invariant isn't the same as conserved. The rest mass of each of the elementary particles is a constant, but rest mass isn't conserved in general.
- I should also point out guys that a great deal of the mass-energy generated in the accretion disk is imparted to the polar jet aka relativistic jet if the particles are moving at extremely high velocities. The jets are essentially titanic sized particle accelerators. The mechanism for how they are formed is not well understood yet. ScienceApe (talk) 14:22, 24 June 2012 (UTC)
- I've just left work, so I don't have the links in front of me, but they didn't discuss the fact that the rest mass stays the same anyway. Whether it's light or heat doesn't matter; gravitational potential energy is converted to light through the various mechanisms, including friction which is by definition the conversion of kinetic energy to heat. The rest mass must remain the same because rest mass is invariant by definition. Imagine a single atom of H flying into the black hole. When the distance is still great, is accelerates towards the hole, as it would towards any other graviational field. At first it emits very low frequency EMF, but as its velocity increases, it goes right through the spectrum to gamma. Tidal forces will strip the electron from it and if it's path curves towards the black hole, it will emit synchrotron radiation. If it bumps into another particle, it will lose kinetic energy and impart heat energy into the thing it hit. The amount of energy it will emit in total is about 10% of its rest mass. But right up until it crosses the event horizon, it will still have ~1 atomic mass unit of rest mass. 101.173.170.147 (talk) 11:03, 24 June 2012 (UTC)
- Yes, your saying it emits light now, rather than heat, is the important part. But I myself find it hard to believe or understand that the rest mass stays exactly the same. (Is it just that c in E=mc^2 is so large?) Can you link to your source? μηδείς (talk) 05:38, 24 June 2012 (UTC)
- Ok, from reading a couple of non-wiki sources on the subject, I think I get it now. The logic of it still seems a bit odd, but I think I know what they're getting at now when they say 10% of the mass is converted to energy (the 40% estimate is now considered wrong because it would require almost all known quasars to have black holes rotating at 99% of the maxiumum allowable speed, and that was based on incorrect early mass estimates of the black holes themselves). If you throw something into the blackhole, friction, and the simple act of radially accelerating charged particles to near light speed causes it to emit light, and the total amount emited is proportional to its graviational potential energy, which is proportional to its mass. The total amount emitted sums to ~10% of it's rest mass. The reason this is a bit odd, is because even after it has emited most of that energy, but before it has crossed the event horizon, it still has the same rest mass it started off with so it's mass is not really being converted to energy. But still, the amount of electromagnetic energy that is extracted is still a fraction of it's mass, and the mass certainly cannot be recovered once it has crossed the event horizon. 203.27.72.5 (talk) 05:11, 24 June 2012 (UTC)
- That seems easily addressed. Imagine a particle and an antiparticle next to each other. They are moving towards each other with negligible but real velocity. They touch and are annihilated. A huge amount of energy is released. Almost none of this is from the original kinetic energy of the particles. That is what is happening in the accretion disk. Friction is creating gamma rays. The gamma rays are as energetic as matter-antimatter pairs. Hence they spontaneously create matter-antimatter pairs. Whose relative velocities are negligible, but real. So the pairs annihilate each other. Converting a huge amount of mass into an even huger amount of energy. μηδείς (talk) 04:54, 24 June 2012 (UTC)
- When you pull objects apart against their mutual gravitational attraction, you're adding gravitational potential energy to the system. In general relativity that energy is mass and gravitates, so this system's rest mass and distant gravitational field are larger after this procedure than before. That's the "mass" that's being "converted into energy" in the accretion disc. But you'll never get an objective answer to a question about whether mass is being converted into energy in any given situation because the laws of physics just don't distinguish between mass and energy at all. The answer depends on which energy you've chosen to call mass. -- BenRG (talk) 18:02, 24 June 2012 (UTC)
I want to import chemical feedstocks from Chinese suppliers at alibaba.com
This is for a possible laboratory / business to make biochemical products (finished goods) to resell in the United States (to other research labs). Is it true that all you need to do at customs is for someone to "positively" certify that "all chemical substances in this shipment comply with all applicable rules or orders under TSCA and that I am not offering a chemical substance for entry in violation of TSCA or any applicable rule or order under TSCA"? i.e. I could just get a customs broker to do this for me? 72.229.155.79 (talk) 14:30, 22 June 2012 (UTC)
- Which China? Whoop whoop pull up Bitching Betty | Averted crashes 14:34, 22 June 2012 (UTC)
- We cannot give legal advice, consult a qualified professional. Roger (talk) 15:32, 22 June 2012 (UTC)
- This is not legal advice, just the obvious: Quite simply -don't try. Iodine, pseudoephedrine, phosphorous, hydrogen peroxide and a host of others are just some of the chemicals one can buy freely abroad. When it comes to biochemicals you are getting into a minefield. Unless you are already an established US business with good paper trail audits (and ways of fiddling them when there are not so good) and millions to spend lobbing those in high government office that you are really the good guys. Then you are open to letting your competitors to get you arrested for supporting terrorism, attempted insurrection and anything else they can dream up that sounds impressive on Faux News. The present laws, regulations and what-not have been angled to keep the small guy out of it.--Aspro (talk) 15:37, 22 June 2012 (UTC)
why don't swampies use non-exchanging heat exchange with air?
reading the "swampy" (evaporative cooling) article, most problems seem to be related to the humidity it generates. But why not just use a swampy in a gridlock maze of long (very large surface area) celophane compartments filled with air but with a maze structure such that the inside air is actually cut off from the swampie-cooled air, and the latter circulates out of the house at the end of the maze? Then you would have the cooing effect without the humidity, at a cost of almost 0 in materials and a very low volume of sacrificed space (representing a huge surface area of exchanged heat).
what I mean is that the exchange should be conductive through the celophane between the swampie-cooled air and the inside air. at the end, the swampie-cooled air becomes warmer and the inside air becomes colder, but the inside air isn't filled with humidity. thoughts? — Preceding unsigned comment added by 188.6.90.91 (talk) 18:28, 22 June 2012 (UTC)
- What article are you reading? Our article called "Swampy" is about a person. --Tango (talk) 18:38, 22 June 2012 (UTC)
- Evaporative cooler. StuRat (talk) 18:52, 22 June 2012 (UTC)
- Because inevitably most of the "coolth" is carried away with the water vapor. And, in the dry climates where an evaporative cooler works well, the added moisture inside is actually welcome. StuRat (talk) 18:55, 22 June 2012 (UTC)
- What do you mean? Given enough surface area in a tunnel-shape, by the time the swampie (topologically "outside") air gets to the end of it it will be in equilibrium with the inside air. i.e. both be warm. I imagine it like this:
100 s> 75 -> 76 -> 77 -> 78 -> 79 -> 80 -> 81 -> ... -> 87 -> outside -------------------------------------------------------...--------- 100 -> 99 -> 98 -> 97 -> 96 -> 95 -> 94 -> 93 -> ... -> 87 -> inside
i.e. the swampy cools the air down a lot, but it's very moist. By the end it's at equilibrium. If you don't want to "waste" the heat difference between 87 and 100, you can again use the difference between 87 and 100 to cool some of the air before it even enters the system through a second loop (again it's closed so it doesn't make the newly entering air any wetter, just colder). Then the air would enter at e.g. 90 instead of 100, if 90 is the equilibrium temperature between 87 and 100. You see what I'm getting at? You can get the two things to equilibrium if you just expose a long surface area through conductive celophane. This can be bundled up in a maze-like structure that is topologically equivalent to the above... --80.99.254.208 (talk) 19:23, 22 June 2012 (UTC)
- in other words, if my calculations are correct you can make a swampy arbitrarily close to an "perfect air conditioner" (not wet and humid output) without wasting heat, by increasing the number and length of loops to arbitrary length. Of course, if you have to add another 100 meters of heat exchange wall to keep from emiting 98 degree humid air instead of 99.2 degree humid air (when the outside is 100 degrees), then it's probably not worth it. So you would probably draw the line and say: We will capture 96% of the swampy's cooling power and let it exit slightly cooler than the outside air because we don't want to build a long enough exchange to equalize over such a small temperature differential. But the point is initially the temperature differential is quite large, and the closed exchange wouldn't have to have a prohibitively large inner surface area, would it? Link title — Preceding unsigned comment added by 80.99.254.208 (talk) 19:30, 22 June 2012 (UTC)
- ( note that this is our relevant article: http://en.wikipedia.org/wiki/Heat_exchanger ) — Preceding unsigned comment added by 80.99.254.208 (talk) 19:31, 22 June 2012 (UTC)
- I lived in Tucson for 20 years and relied on swamp coolers for most of that time, so I can answer this question from personal knowledge. First, the added moisture is definitely not a benefit, because it makes the air feel muggy in spite of being cooler. But regarding the main question, the thing you have to realize is that swamp coolers are only moderately effective at best, and anything that makes their effects less direct would make them essentially useless. I don't follow the calculations above, but the conclusions have nothing to do with reality. Looie496 (talk) 22:59, 22 June 2012 (UTC)
- If I understand the OP's idea/question correctly, he is talking about using water evaporation to cool air drawn in, as in anormal "swampy", but instead of using that now cool and high humidity air inside the building, pass it through an air-to-air heat exchanger (maze in the OP's words) to transfer the "coolth" without the humidity. This idea is quite valid, and has been tried in remote areas of Australia, where the only electricity available is local solar power - so energy consumption by aircon must be kept to an abolute minimum, due to solar power cost. It works just fine, bu the trouble with it is the huge size of the heat exchanger (termed "flat-plate counter-flow" heat exchangers) actually required - the ones I have seen have the heat exchanger occupying the same volume as the building. A number of patents have been granted for manufacturing methods to get the leargest possible surface area without requiring prohibitive power to force the air through the heat exchanger - this is the reason for the huge size. You can make the heat exchanger small, and by suitable laborithian design, get enough surface area. But then the air path will be too tortuous and require too much fan power to force the air through. Huge size means high cost as well - essentially, if you need to to build a building twice as big as what you need for its' function, it will cost twice a much as well.
- Another problem is that the heat exchnager must be kept scrupulously clean inside. Only a small amount of dust on the plates significantly reduces heat transfer and obstructs air flow. And wasps and other creepies reckon its a good place to set up residence. This means high maintenace costs.
- On the other hand, using a cooling system like this in conjunction with solar power gneration makes some good sense, because you get maxium solar power during the heat of the day on hot summer days - just when you need cooling. So if you size the solar panels for adequate power in winter, you have some excess power in summer to run the fans in the airconditioning.
- Keit58.164.229.57 (talk) 03:52, 23 June 2012 (UTC)
That's right Keit. (I'm the OP here.) I am glad to know the invention works and has been used by others. In fact I have over 3000 ideas in every branch of humanity that fall into four categories and a separate category (5): 1) things I think are "very easy" (like two lines of code, e.g. a single SQL join, but nobody has thought to do it or knows why it works), 2) moderately difficult (things that I have completed and can, for example, patent, but would take an R&D budget to refine into something you can start selling), 3) mostly finished or risky (not completely ready to write the patent or don't have complete design specs, however the idea is finished) 4) not sure I could do it myself (working on it, but I have a proof that it is physically possible), 5) not really the same category as the others, but "probably possible" (strong evidence but no proof that it would work), this means it is not done (e.g. I've devoted time to it and have reason to do so but the problem is open to me. It may be impossible or I may hear of another team's solution.)
Without including any of the fourth or fifth category, I am happy to share all 3000 ideas with someone who would finance the "best" of them. I propose the following methodology: I send you 3 thousand ideas each encrypted with a different private key, you pick a random selection of 50 of them, giving me the numbers that you have selected, and I will share the keys for those numbers. This makes it statistically impossible for me to "fluff" the number or quality of the ideas. You evaluate the fifty (much as you evaluated the present idea) and seeing the merit in them begin to fund me as a 1-man R&D firm. Straight up-front I would like funding for a great many patents, some web apps, and other practically no-cost ways to begin certain businesses. As I fbegin to accrue IP I will be able to seek outside funding and pay you a return on your initial investment. Obviously, the reason I have 3000 solid ideas to my name is that I have devoted a great deal of thought to working them out. Initially I will invest your money in filing for several patents - those most easily manufactured - and then immediately begin to shop them around to very large companies. the total cost here is about 5000-7000 buckses (based on my initial research). I believe no follow-on investment will be necessary before I can pay you a big multiple of your investment, and of course the patent may be joint if you require or until you have been repaid on your investment it can be assigned to you. all this is after you review the 3000 ideas (that is, a statistically significant sample of 50 you choose at random). Please let me know if you are interested. You may reach me at ranbir.bacchan at gmail.com. I hope to hear from you. --84.3.160.86 (talk) 15:41, 23 June 2012 (UTC)
- Um I think you've misunderstood where you are. This is the wikipedia reference desk, not a venture capital firm or the Dragons' Den internet edition. I suggest you put some thought in to finding appropriate places to ask for funding rather then spending all your time coming up with random ideas then spaming about them to random places. You may also want to look in to whether your ideas have already been tried before spending too much time on them, or asking for funding. Alternatively, perhaps rather then coming up with 3000 ideas, spend more time on one idea and then perhaps when it makes the megaloads of 'buckses' you expect, you can self fund the other 2999. Nil Einne (talk) 16:36, 23
- Hi, I was surprised that this has already been done and works. I wasn't sure, it's outside my main areas of expertise. I'm not looking for venture capitalists and I don't have a company. I'd just like to monetize a great many ideas with a partner, such as the one who evaluated this one. The monetary needs are minimal, such as the collaboration between a certain mathematician and Mr. Hardy. It wasn't really about the money. At any rate I've put a "resolved" next to this item. I do appreciate that your suggestion not to "spend all my time coming up with random ideas" is probably appropriate to most traditional businesses, and I have nothing to say on this subject. 84.3.160.86 (talk) 17:41, 23 June 2012 (UTC)June 2012 (UTC)
- Agreed. I also list possible invention ideas I have, but more like 30 than 3000. The difference is that I try to filter out the impractical ideas and those which have already been done, neither of which have any any financial value. I suggest you go through that filtering process on your own ideas, before searching for investors. StuRat (talk) 17:35, 23 June 2012 (UTC)
- The "filtering process" I have resources for is to separate great, workable ideas from ones that don't work or can't work. The question here has done just that for one of the category 4/category 5 ideas. I don't currently have the resources to see which have been independently discovered by other people and which have not. This is part of the patent-application process, which costs a few hundred buckses in attorney's fees. At any rate the offer is not to evaluate 30, but a random sampling of 3000 patentable or and marketable ideas. These are not business plans. I'm not soliciting an investment but given your helpful nature in evaluating my question - and thus resolving it - if you would like to get in touch with me I've told you how. 84.3.160.86 (talk) 17:41, 23 June 2012 (UTC)
Obviuosy none of you here are VC's, but I also don't think a VC is equipped to evaluate any mechanisms of the type that I have. They evaluate busineses. To be clear, I don't have a business. You are saying I shouldn't get involved with anyone who isn't already rich? No matter how talented and able to evaluate mechanisms and patentable processes in many disciplines, which is what I would like. 84.3.160.86 (talk) 17:55, 23 June 2012 (UTC)
And Sirs, I apologize if I have offended you. Perhaps as a separate question we can find a better forum for me, I do not think the VC plan is appropriate. 84.3.160.86 (talk) 18:00, 23 June 2012 (UTC)
- On the chance that the OP genuinely thinks he has a stock of good ideas, and is not spamming/trying to find a sucker with cash, here's some further advice:-
- People who think up literally thousands of good ideas are 10 a penny. But folks who think up good ideas that have have not been tried before are very rare.
- If you think you have a good idea, keep it to yourself. Park it in the back of your mind. Over the next 3 months, 3 years, or whatever, consult relavent trade and academic journals - 99.99% or the time you will discover your idea is not new.
- Read up appropriate textbooks and skill up on the applicable theory. This will either reveal that it has been done before, or why it's not a good idea after all.
- Try and work out what the terminology/nomenclature of the various parts would be. Then web-search on this terminology. That will a) reveal further that it has been thought of before, & b) lead you to the right terminology. True understanding of technology is 70% knowing the terminology and 30% mastering relavent text book theory. Only with true understanding can you correctly filter good ideas from bad.
- I have been a technician and succesful professional engineer for over 40 years. I too over the years have had thousands of "good ideas" for new products. But, using the above 4 principles, I have eliminated all but a dozen or so, and only a couple of things have had commercial success. Which leads me to one more principle, perhaps the most important of all:-
- 5 Commercial success depends principally on a) good marketing b) adquate finance c) excellent risk management and business management skills. Comming up with the good technical idea is just a tiny part of it. A person with a good idea but no business skills linking up with a person who has a bag of money but no technical skills or business skills generally just means a depleted bag of money and dissapointment. Succesfull venture capitalists are the way to go, but they won't just fund on an idea - they'll want a comprehensive package. If they don't, they are fools. Keit120.145.65.23 (talk) 03:56, 24 June 2012 (UTC)
Why do Neon lamps burn out?
I have several electrical devices, such as power strips, which have a Neon lamp to indicate the power is on. After a few years use, they flicker, or do not light at all, or go on and off randomly. They are cold cathode gas discharge devices, and there is no hot filament to burn out, as in an incandescent lamp, and they are just a resistor in series with the neon bulb, so there is not ballast or starter to fail, and no phosphors inside the tube to fail, as in a fluorescent lamp. So what gets "used up" in a neon lamp, when it is not operated at excessively high voltage or current? How many hours does a neon lamp such as an NE-2 operated, on average, before failure? Edison (talk) 20:30, 22 June 2012 (UTC)
- The metal that makes up the electrodes evaporates over time. They may not be hot to you but metal atoms are getting continuously knocked off. Eventually the normal voltage is not sufficient to strike an arc. Place same flickering lamp across a transformer that provides a higher voltage and the lamp will shine brightly again. --Aspro (talk) 22:08, 22 June 2012 (UTC)
- There must be more to it than that. The electrodes in a dud neon don't look much different to those in a good neon. Certainly any loss of material from the electrodes cannot significantly alter the spacing. The striking voltage depends on the spacing, the gas pressure, and the type of gas (ref http://en.wikipedia.org/wiki/Breakdown_voltage). So the gas pressure must be changing somehow, or the gas gets contaminated. As to how many hours of operation, that does seem to depend on the operating current density. Before zener diodes became available, neon tubes, both NE-2 type and larger types designed for the purpose, were used as voltage references in (tube-based) regulated power supplies. In my experience such regulator tubes in such equipment as oscilloscopes, TV cameras, etc, operated without failure all day every day for 10 years or more - but they glowed only dimly compared to NE-2 used as visual indicators. Keit121.221.1.107 (talk) 12:00, 23 June 2012 (UTC)
- Here is a pons asinorum: Neon is more or less inert. So little is going to happen the gas pressure or composition. (2) Glass in a good insulator at these temperatures. (3) The electrodes are good conductors at these temperatures. (4) There is no oxygen in the bulb – just neon. (5) The metal ions that boil off the electrodes 'condenses' on the cool inside envelop of glass -i.e., it does not form a non-conductive oxide film . (6) The inside coat of conductive metal slowly reduces the potential across the xeon gap by conduction. (7) A point will come when the potential across the gap will not reach the afore said breakdown voltage. (8) As this condition is approached, flickering of light output will be observable. (9) If these electrodes did not evaporate, the lamp would not fail -unless for other reasons. (10) The metallic film on the inside of the glass envelope has a resistance – so if you increase the electrode voltage, the arc will eventually re-strike; as this current path (between the electrodes) will again form the lowest resistance. Platinum has a lower vapour pressure than tungsten, so this as electrode element might be better.
- Unless you live in one of those countries that just trains its pupils to mechanically pass exams, you will come to learn all this, in your science lessons. Your CD's get their shinny aluminium coat by the same principle of vacuum deposition. There is likely to be many more example of this processes in your own home.--Aspro (talk) 20:16, 23 June 2012 (UTC)
- How about those contries that spend more time on subject-verb agreement? μηδείς (talk) 21:09, 23 June 2012 (UTC)
- Either the tubes break or the electrodes burn out. Or God intervenes. μηδείς (talk) 21:09, 23 June 2012 (UTC)
- How about those contries that spend more time on subject-verb agreement? μηδείς (talk) 21:09, 23 June 2012 (UTC)
- Here is a pons asinorum: Neon is more or less inert. So little is going to happen the gas pressure or composition. (2) Glass in a good insulator at these temperatures. (3) The electrodes are good conductors at these temperatures. (4) There is no oxygen in the bulb – just neon. (5) The metal ions that boil off the electrodes 'condenses' on the cool inside envelop of glass -i.e., it does not form a non-conductive oxide film . (6) The inside coat of conductive metal slowly reduces the potential across the xeon gap by conduction. (7) A point will come when the potential across the gap will not reach the afore said breakdown voltage. (8) As this condition is approached, flickering of light output will be observable. (9) If these electrodes did not evaporate, the lamp would not fail -unless for other reasons. (10) The metallic film on the inside of the glass envelope has a resistance – so if you increase the electrode voltage, the arc will eventually re-strike; as this current path (between the electrodes) will again form the lowest resistance. Platinum has a lower vapour pressure than tungsten, so this as electrode element might be better.
Skin color and temperature
If a black person is standing next to a white person in sunlight, shouldn't the black person feel hotter? Maybe African-descended persons are better adapted for high heat than say Northern Europeans but is that enough to compensate? Sagittarian Milky Way (talk) 21:57, 22 June 2012 (UTC)
- I remember my biology teacher from grade 12 talking about how pigmentation causes black people to lose heat to the environment much faster.
I don't remember why, but now it's got me interested...203.27.72.5 (talk) 22:35, 22 June 2012 (UTC)- It's because they have higher emissivity, of course. I was thinking they were standing the sun and still getting colder than a white person. So yeah, black people should get hotter in direct sunlight without going into any other adaptations some people may possibly have. 203.27.72.5 (talk) 22:41, 22 June 2012 (UTC)
- Dark surfaces reflect less solar radiation than light surfaces, so of two otherwise identical persons of different skin pigmentation in your scenario, the darker-skinned person would absorb more solar radiation. That's a purely physics explanation (it could apply to any surface, not just human skin) - heat and the perception of heat are going to depend on more factors than simply the skin colour. As to the evolutionary reasons for the variation in skin pigmentation in humans, see Skin pigmentation#Evolution of skin color. LukeSurl t c 22:47, 22 June 2012 (UTC)
- It's because they have higher emissivity, of course. I was thinking they were standing the sun and still getting colder than a white person. So yeah, black people should get hotter in direct sunlight without going into any other adaptations some people may possibly have. 203.27.72.5 (talk) 22:41, 22 June 2012 (UTC)
If an object is darker at all wavelenghts (visible light and infrared), then it won't get any hotter. It is only when an object is darker at visible wavelengths but less dark in the infrared, that it will get hotter. Count Iblis (talk) 22:53, 22 June 2012 (UTC)
- I'm not sure you are right. An object at human body temperature will want to emit radiation at much longer wavelengths than the peak of the solar spectrum. Heating of such an object in direct sunlight will be dominated by absorption in the near infrared, while cooling will be dominated by absorption (emission) in the mid- to far-infrared.
- Melanin absorbs strongly in the near ultraviolet, and not much at all in the infrared.[11] It's not clear whether dark skin will cause a person to get hotter or cooler in direct sunlight, but clearly the apparent dark color in the visible will have much less effect than one might suppose, because it is not dark in the infrared where the solar spectrum is much stronger.--Srleffler (talk) 02:49, 23 June 2012 (UTC)
- The Sun's radiation peaks around 500 nm, which is in the green band of the visible spectrum. See [12]. If it peaked in the infrared, our eyes would have evolved to see infrared, and we would name it "visible". Because of the long high-wavelength tail, there might be more total radiation in the infrared than in the visible (it's hard to tell from the graph, and I'm too lazy to do the integration), but in any case absorption in the visible is highly significant.
- I'm also not sure that the predominant source of cooling for a human body is radiation. Do you have a source for that? If the main source of cooling isn't radiation, black skin should definitely be hotter, unless it's much less absorptive in the infrared than white skin. — Preceding unsigned comment added by 140.180.5.169 (talk) 04:55, 23 June 2012 (UTC)
- Humans have three main ways to loose heat: 1) radiation from skin, 2) conduction from skin to air passing over it, 3) evaporation of sweat. (1) is very ineffective compared to (2). (3) is vastly more effective than (2), but the body automatically adjusts sweating from zero to dramatic as required. The body can also adjust the blood flow to the skin in order to control (1) + (2). For thse and the other reasons posted above (the visual colour has little to do with the radiation coefficient at infrared), it matters not a whit what your skin colour is. When I was a first year at university, they had us do a series of lab experiments measuring the heat loss from a piece of aluminium, unpainted and shiny, painted red, painted black, unpainted and roughened, various airflows from zero to a gentle breeze. These lab tests proved convicingly that radiation in all manner of practical applications, where the object temperature is only moderate (say up to 40 C above ambient), is unimportant, though measureable. Surface roughness is just as, and often more, important re radiation than is visual colour. Wickwack58.170.139.183 (talk) 06:45, 23 June 2012 (UTC)
- But the question was in sunlight does the black person feel hotter. The difference in temperature need only be very slight for a person to feel it. And the heat radiated from the sun will rapidly change the temperature of the skin's surface before mechansims (2) and (3) start to respond. 203.27.72.5 (talk) 07:09, 23 June 2012 (UTC)
- Ultimately, I suppose this cannot be answered, as there is an acute shortage of people who change colour. However, the difference is so slight that I expect that acclimatisation would render it impreceptable - much as folks in London feel warm on a 25 C day (hot for London) but folks in Perth Australia feel cool on a 25 C day (average for Perth). Wickwack58.170.139.183 (talk) 10:42, 23 June 2012 (UTC)
- But the question was in sunlight does the black person feel hotter. The difference in temperature need only be very slight for a person to feel it. And the heat radiated from the sun will rapidly change the temperature of the skin's surface before mechansims (2) and (3) start to respond. 203.27.72.5 (talk) 07:09, 23 June 2012 (UTC)

(1) Regardless of skin colour, body shape is different in long-limbed Africans and thick-trunked Northern Europeans. (2) Yes, Black people whom I have touched normally feel warmer than White people. (3) Anyone who has lived in NYC will know you will often see Black people bundled in heavy jackets or hats in weather of 70 degrees Fahrenheit, while you will rarely see the same in White people above 60 degrees Fahrenheit. μηδείς (talk) 21:05, 23 June 2012 (UTC)
Yes, a darker skinned individual will probably feel hotter, but they won't burn, or their risk of getting burned is significantly reduced. Melanin very efficiently converts ultraviolet radiation into heat which is harmless, so while you may feel a little hotter in the sun, you won't get sunburned. I know it's possible for a dark skinned person to get sunburned at a certain point, but I'm a dark skinned guy myself, and I've never been sunburned in my life. Considering the risk of sunburn, melanoma, and other skin cancers, I'll take my dark skin any day. ScienceApe (talk) 22:45, 23 June 2012 (UTC)
Sun's magnetic field doubled?
Has the Sun's magnetic field doubled over the last 100 years? Bubba73 You talkin' to me? 23:23, 22 June 2012 (UTC)
- The Solar variation#Changes in the solar wind and the Sun's magnetic flux article section says it has. However, despite a couple surprising statements in that section, there isn't a single citation in that section, and solar variation is something that people into climate change denial like to bring up (see Global warming controversy#Solar variation), so it might not be good to blindly rely on that article section as being accurate. Red Act (talk) 01:17, 23 June 2012 (UTC)
- The statement (the one that made me ask) that it had doubled most likely came from a climate change denier. Bubba73 You talkin' to me? 01:41, 23 June 2012 (UTC)
- Yes, the open magnetic flux has increased by something like a factor of two in the past century, a rough number given the uncertainty in the reconstructions. See e.g., Lockwood (2003), figure 12. Notice that the flux was about as high as now back during the late 1700s, a period that was a good bit cooler than now (see Little Ice Age). Short Brigade Harvester Boris (talk) 01:42, 23 June 2012 (UTC)
- Why would there be any connection between the global temperature and the Sun's magnetic flux? 203.27.72.5 (talk) 03:33, 23 June 2012 (UTC)
- The Sun's magnetic flux correlates with the intensity of the solar wind, which could hypothetically influence cloud formation, which itself influences global temperatures and other climate effects. But since the flux has doubled in a hundred years (allegedly) and we're not all dead, I assume the influence is small. I leave it to climate modelers to say what the influence might actually be. Someguy1221 (talk) 03:38, 23 June 2012 (UTC)
- "But since the flux has doubled in a hundred years (allegedly) and we're not all dead, I assume the influence is small." So would you say the same thing about atmospheric CO2? 203.27.72.5 (talk) 03:49, 23 June 2012 (UTC)j
- Wow, I didn't even see myself walking into that one. Someguy1221 (talk) 04:10, 23 June 2012 (UTC)
- (edit conflict)None, basically. There's the supposed effect of galactic cosmic rays on production of cloud condensation nuclei (which is what I assume you're referring to). That GCR can affect production of tiny particles is accepted, but those particles are orders of magnitude smaller than the actual particles that serve as CCN. Short Brigade Harvester Boris (talk) 03:51, 23 June 2012 (UTC)
- "But since the flux has doubled in a hundred years (allegedly) and we're not all dead, I assume the influence is small." So would you say the same thing about atmospheric CO2? 203.27.72.5 (talk) 03:49, 23 June 2012 (UTC)j
- The Sun's magnetic flux correlates with the intensity of the solar wind, which could hypothetically influence cloud formation, which itself influences global temperatures and other climate effects. But since the flux has doubled in a hundred years (allegedly) and we're not all dead, I assume the influence is small. I leave it to climate modelers to say what the influence might actually be. Someguy1221 (talk) 03:38, 23 June 2012 (UTC)
- Why would there be any connection between the global temperature and the Sun's magnetic flux? 203.27.72.5 (talk) 03:33, 23 June 2012 (UTC)
- Yes, the open magnetic flux has increased by something like a factor of two in the past century, a rough number given the uncertainty in the reconstructions. See e.g., Lockwood (2003), figure 12. Notice that the flux was about as high as now back during the late 1700s, a period that was a good bit cooler than now (see Little Ice Age). Short Brigade Harvester Boris (talk) 01:42, 23 June 2012 (UTC)
- The statement (the one that made me ask) that it had doubled most likely came from a climate change denier. Bubba73 You talkin' to me? 01:41, 23 June 2012 (UTC)
June 23
Dissolving sulfur
I have a nylon cloth that has minerals impregnated in it. I think that one of them is elemental sulfur. The rest of them should be sulfides and oxides of iron, zinc, calcium and lead, and lead sulphate may be there as well. The cloth has a red/orange colour to it that is removed if I soak it in dilute HCl, HNO3 or H2SO4, but not removed if I soak it in EDTA or water. The EDTA liquor contains a fair bit of iron, so I think the red colour is not due to iron. The article on sulfur says that it dissolves in carbon disulfide, benzene and toluene, but my ICP-OES isn't set up to run organics. Is there anything aqueous that can selectively dissolve only the elemental sulfur, if it is in fact the cause of the red colour? 203.27.72.5 (talk) 07:26, 23 June 2012 (UTC)
- Is there no emulsifier to do the job? Plasmic Physics (talk) 08:49, 23 June 2012 (UTC)
- Can you elaborate a bit on that? I don't really want an emulsion, because I want to analyse it on the ICP, but I suppose if I formed an emulsion I could separate the sulfur suspended in the liquor from the rest of the cloth and then oxidize to sulphate to solubulize and go from there. What emulsifier could I use for that? 101.172.85.63 (talk) 09:43, 23 June 2012 (UTC)
- I did a bit of googling and found that ethoxy nonylphenol is a good candidate for the emulsification of sulfur. Plasmic Physics (talk) 10:36, 23 June 2012 (UTC)
- I'm not familiar with the opperation of that particular piece of laboratory equipment, but why not just just dissolve the sulfur in benzene or tolluene, and then recover it by vacuum distillation? Plasmic Physics (talk) 10:41, 23 June 2012 (UTC)
- My lab isn't really set up for organics at all...I don't have a vacuum distillation rig or anything like that. I could just wait until the stuff evaporates in the fumecupboard though. The lab normally only does metal assays on mining samples, but they've thrown me a bit of a curve ball. I don't think I'm going to have any of the emulsifiers on hand, so I think I'm just going to soak the fabric in CS2 and see if it gets rid of the red colour. Do you think elemental sulfur could account for the red colour in a nylon fabric? I'm thinking red/orange is pretty close to yellow sulfur, but it could potentially also be another allotrope, couldn't it? 101.172.85.63 (talk) 11:00, 23 June 2012 (UTC)
- I'm really not sure, I'm not familiar enough with sulfur allotropes to give an answer to that. Plasmic Physics (talk) 11:55, 23 June 2012 (UTC)
- It could be an arsenic sulfide. Plasmic Physics (talk) 12:23, 23 June 2012 (UTC)
- That's not unreasonable, considering arsenic is often associated with heavy metals. Plasmic Physics (talk) 12:52, 23 June 2012 (UTC)
- Now that I think about it, what would free sulfur be doing in the sample anyway? It's usually associated with reducing soils, which does not seem to be the case here. Plasmic Physics (talk) 12:59, 23 June 2012 (UTC)
- It's possibly produced as a colloid in the treatment of sulfide ores. 203.27.72.5 (talk) 23:25, 23 June 2012 (UTC)
- ~15 hours of soaking in 100% CS2 did not remove the red colour. Now I'm thinking the EDTA only moved iron oxides and hydroxides, but left the pyrite behind. I'm getting desperate, so I just dumped samples of the cloth into hydrogen peroxide, calcium hypochlorite and household bleach. Hope fully something cleans it. The HCl I originally used actually cleaned it up pretty nicely, but chloride ion is a bit too corrosive to use in this application. 203.27.72.5 (talk) 00:49, 24 June 2012 (UTC)
- Have yout tried an alkaline solution yet? Plasmic Physics (talk) 05:47, 24 June 2012 (UTC)
- Try a free radical reaction with a Fenton type solution: a combination of iron(2+) sulfate and hydrogen peroxide. That should pack a punch. Plasmic Physics (talk) 06:20, 24 June 2012 (UTC)
- A strong alkaline solution will dissolve the nylon too, so best to steer clear of that. Stong acids will also damage nylon, and so will heat. Is it better to throw out the cloth and buy whatever it is new? As all thos chemicals and your time will cost. Graeme Bartlett (talk) 10:42, 24 June 2012 (UTC)
- Larox filter cloths are $20,000 each, and they're clogging up 5x faster than expected. 101.173.170.147 (talk) 11:45, 24 June 2012 (UTC)
- ~15 hours of soaking in 100% CS2 did not remove the red colour. Now I'm thinking the EDTA only moved iron oxides and hydroxides, but left the pyrite behind. I'm getting desperate, so I just dumped samples of the cloth into hydrogen peroxide, calcium hypochlorite and household bleach. Hope fully something cleans it. The HCl I originally used actually cleaned it up pretty nicely, but chloride ion is a bit too corrosive to use in this application. 203.27.72.5 (talk) 00:49, 24 June 2012 (UTC)
- It's possibly produced as a colloid in the treatment of sulfide ores. 203.27.72.5 (talk) 23:25, 23 June 2012 (UTC)
- If it is an arsenic sulfide, it should be soluble in a solution of oxalic ammine with excess ammonia. Plasmic Physics (talk) 11:57, 24 June 2012 (UTC)
- The manufacturer recommended trying oxalic acid but I don't have any. I thought that the EDTA would be a good alternative, and it did nothing, so I think the oxalic acid will be much the same. I've now tried; HCl, H2SO4, HNO3, water, EDTA, diesel, bleach, Ca(OCl)2, H2O2, acetone, THF, ethanol, methanol and carbon disulfide. HCl gave the best results, but the engineers are worried about corrosion on the filter press itself if we use that. Other than that, only H2SO4 and HNO3 did anything, and they didn't do much. Fenton's reagent sounds like it will attack the nylon, but thanks for pointing it out anyway; I realised it was just what I was looking for for something else! I doubt it's arsenic. I checked the assay numbers and arsenic is at ppm levels. 101.173.170.147 (talk) 12:02, 24 June 2012 (UTC)
- Oxalic acid is quite different from ammonium oxalate, and works differently from EDTA. Oxalic acid can be obtained from a local hardware store, it's used to treat wood before it's painted. Just neutralise some oxalic acid with excess ammonia over some heat, that should give you ammonium oxalate. In any case, have you tried running diagnostics on a sample of the residue? Take a scraping, or pick off some pieces of the contaminant and react it with concentrated hydrochloric acid, analyse the resulting solution with the IPC-OES against a hydrochloric reference. At least that way you know what you are dealing with. That way, we can be more helpful, rather than just guessing. Plasmic Physics (talk) 12:25, 24 June 2012 (UTC)
- The very first thing I did was cut of 4 samples of ~0.1g and digest them in conc. HNO2, HCl and HF and analyse on the ICP. The results showed that it contained significant Fe, Zn, Pb, Ca and S. There was a little bit of Si, but not much. Everything else that is detectable was trace. Other than S and Si, I can't see non-metals on the ICP. 101.173.170.147 (talk) 12:52, 24 June 2012 (UTC)
- No, no, I meant after you've removed the other contaminants. Treat a sample with EDTA to was out all the contaminants to leave just the unknown residue on te cloth. Then wash it thoroughly with deionised water, and treat it with the conc. HCl. Plasmic Physics (talk) 12:59, 24 June 2012 (UTC)
- The very first thing I did was cut of 4 samples of ~0.1g and digest them in conc. HNO2, HCl and HF and analyse on the ICP. The results showed that it contained significant Fe, Zn, Pb, Ca and S. There was a little bit of Si, but not much. Everything else that is detectable was trace. Other than S and Si, I can't see non-metals on the ICP. 101.173.170.147 (talk) 12:52, 24 June 2012 (UTC)
- Oxalic acid is quite different from ammonium oxalate, and works differently from EDTA. Oxalic acid can be obtained from a local hardware store, it's used to treat wood before it's painted. Just neutralise some oxalic acid with excess ammonia over some heat, that should give you ammonium oxalate. In any case, have you tried running diagnostics on a sample of the residue? Take a scraping, or pick off some pieces of the contaminant and react it with concentrated hydrochloric acid, analyse the resulting solution with the IPC-OES against a hydrochloric reference. At least that way you know what you are dealing with. That way, we can be more helpful, rather than just guessing. Plasmic Physics (talk) 12:25, 24 June 2012 (UTC)
- The manufacturer recommended trying oxalic acid but I don't have any. I thought that the EDTA would be a good alternative, and it did nothing, so I think the oxalic acid will be much the same. I've now tried; HCl, H2SO4, HNO3, water, EDTA, diesel, bleach, Ca(OCl)2, H2O2, acetone, THF, ethanol, methanol and carbon disulfide. HCl gave the best results, but the engineers are worried about corrosion on the filter press itself if we use that. Other than that, only H2SO4 and HNO3 did anything, and they didn't do much. Fenton's reagent sounds like it will attack the nylon, but thanks for pointing it out anyway; I realised it was just what I was looking for for something else! I doubt it's arsenic. I checked the assay numbers and arsenic is at ppm levels. 101.173.170.147 (talk) 12:02, 24 June 2012 (UTC)
- My lab isn't really set up for organics at all...I don't have a vacuum distillation rig or anything like that. I could just wait until the stuff evaporates in the fumecupboard though. The lab normally only does metal assays on mining samples, but they've thrown me a bit of a curve ball. I don't think I'm going to have any of the emulsifiers on hand, so I think I'm just going to soak the fabric in CS2 and see if it gets rid of the red colour. Do you think elemental sulfur could account for the red colour in a nylon fabric? I'm thinking red/orange is pretty close to yellow sulfur, but it could potentially also be another allotrope, couldn't it? 101.172.85.63 (talk) 11:00, 23 June 2012 (UTC)
- Can you elaborate a bit on that? I don't really want an emulsion, because I want to analyse it on the ICP, but I suppose if I formed an emulsion I could separate the sulfur suspended in the liquor from the rest of the cloth and then oxidize to sulphate to solubulize and go from there. What emulsifier could I use for that? 101.172.85.63 (talk) 09:43, 23 June 2012 (UTC)
Entomologist named "Nonfried" (fl. 1893-1900)
Hi all. Nonfried (fl. 1893-1900, initials A. F.) is apparently the species authority for a number of beetles. See: Eligmodermini, Lampetis, Metopocoilus giganteus, and so on. So, who was this chap? --Shirt58 (talk) 11:02, 23 June 2012 (UTC)
- This document gives a bit of information: "Anton Franz (Antonín František) Nonfried (1854–1923). Based in Rakovník (= Rakonitz), Czech Republic, Nonfried ran a business with insects. His private collection was mostly sold (e.g., the cetonids are deposited in Museum für Naturkunde Berlin, Germany; HORN et al. 1990) but a small part, including several types, was acquired by NMP" [National Museum, Prague]. Obviously a sober, responsible person. Clarityfiend (talk) 15:28, 23 June 2012 (UTC)
- Thank you! And NONFRIED Anton Franz, *16.10.1854, +16.12.1923... Oh dear, not much to start an article on... --Shirt58 (talk) 16:36, 23 June 2012 (UTC)
- I'm guessing that Nonfried had a basket full of half-baked theories ? :-) StuRat (talk) 17:38, 23 June 2012 (UTC)
- Ran a business with insects? Clarityfiend (talk) 23:15, 23 June 2012 (UTC)
- Some Chiroptera guano "differently realitied" editor has started an article about the guy.--Shirt58 (talk) 12:04, 24 June 2012 (UTC)
- Ran a business with insects? Clarityfiend (talk) 23:15, 23 June 2012 (UTC)
Status of a request for a qualified expert to create a Wikipedia article on the concept of "new water".
To whom it may concern:
I made a request for an article on the subject of "new water", and supplied enough information so such a person could be found. Now I'm unable to find the request and the message I previously wrote. The following is what I supplied:
−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−
I am not qualified to write an article about this subject, but it is of extreme importance currently. I would like to request that a qualified writer, not pre-disposed to consider what follows to be the work of a charlatan in the water prospecting business, create the article.
In the 1960's a mining engineer, named Stephen Riess, discovered a water generating process within the Earth he called "new water". It was part of his management of mining operations in silver mining at high elevations in Nevada. Some of the mines he was associated with were experiencing flooding that could not be explained by the hydrologic cycle because of the high elevations in which the flooding occurred. I was fascinated by an article appearing in the Los Angeles Times and obtained the article from a library. A book was written called "New Water For A Thirsty World" by Michael Salzman, who interviewed Riess. Based on Riess' mining experience he began a business prospecting for water, that, among others, attracted the Sparklets Spring Water Co to drill a well for them near Ramona, CA. The well Riess created successfully produced water for the company. This was one of many successful water projects. At the time Riess also approached the State of California for an alternative to the Feather River Project as a much less expensive way to supply adequate amounts of water. His proposals were rejected.
Riess was an educated person, having received his engineering degree in Switzerland. I actually met with him and his wife in Ojai, CA in the early 80's with some friends, one of whom was a scientist for Hughes Aircraft. At that time he was in his 80's. During that visit I learned that one of his professors in Switzerland was Albert Einstein. He also explained that one of his professors in the petroleum engineering field showed how crude oil could be created in the laboratory, which explains the theories of some that "abiotic oil" exists, and is not the result of dead dinosaurs, something space exploration is confirming from the exploration of planets in our Solar system. He had created a successful well on his property on Sulfur Mountain Road, which is also at an elevation well above any explanations that traditional hydrologists can provide.
The reason I believe this article would be of great current interest is because of the nuclear disaster in Fukashima, Japan. The radioactive contamination has reached as far east as Philadelphia, PA, and is showing up in sea life, like the blue fin tuna, on the west coast of the United States. If an article, such as this, was created it should generate a great deal of interest in renewing the search for uncontaminated water anywhere that radioactive contamination has become a public health problem. In the book mentioned above there is a passage in the book about above ground atomic testing by the United States. This widely distributed radioactivity that was born by air currents across the Earth. However, the wells Riess constructed never became contaminated because they were not part of the hydrologic cycle. The current implications of this should be evident, and is why I believe an article created by a qualified expert, who is unafraid of criticism within his discipline, could draw widespread interest.
I have done some cursory research provided by others to help explain this request as follows:
http://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.51.4.627-b
NEW WATER FOR A THIRSTY WORLDBy Michael Salzman. Los Angeles, Calif.: Science Foundation Press (73314 Ascot Station), 1960. 210 pp. Price, $5.95. This book projects a theory that: (1) there are everlasting supplies of new waters in little-explored, solid-rock fissures within the earth; and (2) they originate from chemical processes occurring there and are unrelated to the familiar ground water, dependent on insoak, which man conventionally taps to supplement surface supplies. These new waters, the author asserts, can be scientifically located and economically brought to the surface, even in deserts. Examples are cited. This would be a boon to our present dilemma -a rapidly closing gap between water demands and the fixed supply of the hydrologic cycle. Water pollution and its health implications are briefly discussed. Mentioned is "the constant pollution of our drinking water by hundreds of new chemical products whose effects on human health are totally unknown." The author projects a possible link between today's increasing chronic (cancer and cardiovascular- renal) disease rate and drinking water supplies. Apparently painstaking research went into the preparation of this thought provoking and somewhat convincing treatise. The effort was spurred by the work of Stephan Riess, a Californian. Riess formulated the "new water" theory and has gained attention in the press by drilling good water-producing wells where others had failed. Although Riess has been called by some "a charlatan, a witch, and a fake," the author is convinced that the Riess theory is authentic and that it can ex- plode certain established scientific and hydrologic principles if delved into with "open mind." Except for brief recitations in highly academic and scientific terms and formulas, this book is easily read by the layman. Salzman demonstrates a remarkable cross-knowledge of the earth sciences and believes that less narrowminded specialization could improve scientific achievement by chemists, physicists, metallurgists, mineralogists, and crystallographers. Certainly for each of these professions this book holds something of interest. Likewise, both professionals and students in forestry, soil, agronomy, and the water resource field will find this book readable and an excellent reference. The book documents in detail 198 references. GORDON MCCALLUM
Amazon source:
New water for a thirsty world. (Paperback) by Michael H. Salzman (Author)
Nablus, Palestine: The city stands at an elevation of around 550 meters (1,800 ft) above sea level
This is the location of Jacob's well, which has been unaffected by sparse rainfall since its creation thousands of years ago. That is one of the other characteristics of these water sources, which is another way of discounting hydrology as a water source.
If anyone who reads this knows of a person that could contribute to this request, please contact them. Please know that statistical studies are showing that the Fukushima reactor failures from the tsunami are already showing evidence of increased cancer rates in the United States.
−−−−−−−−−−−−−−−−−−−−−−−−−−−−−
As I explained, I'm not an expert in this field, as are those at the links I supplied above. So I would like to follow the status of my request. Based on what I supplied above can anyone please locate what I previously wrote and provide a link to it. If no one can be found to respond as an expert on the subject I'll do my best. In the meantime I would like to follow the status of my request. — Preceding unsigned comment added by NewWaterWorld (talk • contribs) 18:27, 23 June 2012 (UTC)
- Here is your edit: [13]. It looks like you unintentionally deleted large portions of the existing page when you posted, so were reverted some 6 hours later. Here's how it looked after you posted: [14]. Here's how it looked before you posted (which is also how it looks now): [15]. Try posting again, at that last link, but be a bit more careful not to delete anything else. StuRat (talk) 18:42, 23 June 2012 (UTC)
- I reverted your original edit for the reasons that StuRat said. Also note that requests normally only include the name of the requested article and a few words on why, often with a link to a supporting source or two, not a long essay - it completely overwhelms the page, which is a concise list of requested articles. Mikenorton (talk) 19:07, 23 June 2012 (UTC)
- That would be fine - unfortunately (in my view) the introduction at the top of the page does say "please include as much information as possible", but that's because people often just add a redlink with no other information at all. Mikenorton (talk) 20:39, 23 June 2012 (UTC)
- Is there any data from drilling that support the "New Water" theory?, any peer review of said material? Electron9 (talk) 00:18, 24 June 2012 (UTC)
- You may also be interested in juvenile water which is formed from magma. Graeme Bartlett (talk) 08:58, 24 June 2012 (UTC)
Would it be possible to dream with one eye open?
Real dreaming, with actual video, not just daydreaming. Sagittarian Milky Way (talk) 19:33, 23 June 2012 (UTC)
- Maybe in complete darkness, or if totally blind, so the light wouldn't awaken then, although having an eye open might keep them awake even then. StuRat (talk) 19:58, 23 June 2012 (UTC)
Yes, it is possible. Not everyone sleeps with their eyes closed. Some people normally sleep with their eyes open, and there are some who have a condition called Nocturnal lagophthalmos, who have a type of facial paralysis. Both are able to dream. For an interesting story about someone with nocturnal lagophthalmos, read this: [[16]]. Dominus Vobisdu (talk) 20:10, 23 June 2012 (UTC)
- "Video"? Evanh2008 (talk|contribs) 20:38, 23 June 2012 (UTC)
- That seemed a rather elegant way of putting it, actually.
- That video would not be in 3D then with only one eye open. Why not open both eyes for the full 3D hi def suround sound dream experience. SkyMachine (++) 21:59, 23 June 2012 (UTC)
- That seemed a rather elegant way of putting it, actually.
Google the lyrics to Enter Sandman. μηδείς (talk) 20:50, 23 June 2012 (UTC)
- I've been asleep and dreaming with both eyes open before. But not just one. It is a bizare feeling when you wake up because you know your eyes were open and that they must have seen things but you can't remember what they saw. 203.27.72.5 (talk) 23:33, 23 June 2012 (UTC)
- Being able to see things without being properly awake is extremely common. As is being able to carry out complex activities (e.g., finding alarm clock and then configuring it to shut up and not keep beeping) without being properly awake. --Demiurge1000 (talk) 23:44, 23 June 2012 (UTC)
If there are no pain receptors in the central nervous system, why do headaches hurt?
Topic says it all. ScienceApe (talk) 20:08, 23 June 2012 (UTC)
- The simple answer is that, the blood vessels that run through the brain have nerves sensitive to pain. The symptoms of a head ache originate from them. As to why, you best ask a neurologist but id may has something to do with the release of prostaglandin ; (or in my case too much alcohol).--Aspro (talk) 20:27, 23 June 2012 (UTC)
- Migraines are believed to be caused by internal bloodflow. But there are also the more common sinus headaches and muscle-tension headaches. μηδείς (talk) 20:47, 23 June 2012 (UTC)
I have a follow-up. Where do blood-flow headaches hurt? Is the sensation actually internal? (I only get sinus and tension headaches.) μηδείς (talk) 20:53, 23 June 2012 (UTC)
- This is close to giving medical advice, therefore the only advice I will give is to take your check-book along to your
quackdoctor and ask him. If you're mildly computer literate there is a world-wide-web search engine called Google. That has thrown up this: [17] I won't say that simple meditation has been found to work better that drugs for tension headaches, as that would be medical advice and youquack(dam, why does me spell checker doing this?) will not like to hear that.--Aspro (talk) 21:15, 23 June 2012 (UTC)
- What a rude, ill-considered, uninformative, and, frankly, hysterical response. I am not asking for medical advice. I don't suffer from blood-vessel caused headaches, so I do not know what they feel like. I am simply curious. My question, if I can make it clear, is, where does the pain seem to originate from for those people who suffer such headaches? μηδείς (talk) 21:39, 23 June 2012 (UTC)
- You stated I only get sinus and tension headaches" Wikipedia:Reference desk/Guidelines/Medical adviceHow else could I have answered it?--Aspro (talk) 21:56, 23 June 2012 (UTC)
- Yeah, I see how talking about quacks, giving me unsolicited medical advice on how to treat tension headaches, and giving a link that says nothing about where pain is actually felt is called for when I asked exactly what I meant, "where do blood-flow headaches hurt?" Please don't respond to me again unless you actually have an answer to my question. μηδείς (talk) 22:04, 23 June 2012 (UTC)
- Aspro, many of us don't need to take our cheque-books along to our doctors, since we have state-provided medical care. If you're having to bribe your medical professionals in order to get treatment, you may have a headache of a more general character than the others under discussion here. AlexTiefling (talk) 22:36, 23 June 2012 (UTC)
- Alex, please read WP:SOAP. Aspro, please read User:Kainaw/Kainaw's criterion — Medeis is adding a comment to clarify the reason for asking; you can answer the question completely without providing a diagnosis, prognosis, or treatment advice. Nyttend (talk) 03:18, 24 June 2012 (UTC)
- Aspro, many of us don't need to take our cheque-books along to our doctors, since we have state-provided medical care. If you're having to bribe your medical professionals in order to get treatment, you may have a headache of a more general character than the others under discussion here. AlexTiefling (talk) 22:36, 23 June 2012 (UTC)
- Thanks! Kainaw's criteria are actually quite brilliantly expressed. I was going to object that I was neither asking for a diagnosis nor a treatment if challenged again. I see I omitted prognosis, but am obviously not asking for that either. In any case, can anyone answer the question? μηδείς (talk) 03:46, 24 June 2012 (UTC)
- I don't know about migraines, but I (thankfully rarely these days) get cluster headaches, a type of vascular headache. The pain always originates just behind my right eye, before radiating out over my temple. It's hard to describe any pain, and I can't put it any better than our cluster headache article, which says it is like having a red-hot poker inserted into the eye. The pain is definitely more "internal" than a tension headache. 92.20.237.148 (talk) 11:29, 24 June 2012 (UTC)
- Thanks! Kainaw's criteria are actually quite brilliantly expressed. I was going to object that I was neither asking for a diagnosis nor a treatment if challenged again. I see I omitted prognosis, but am obviously not asking for that either. In any case, can anyone answer the question? μηδείς (talk) 03:46, 24 June 2012 (UTC)
The following is medical advice but such obviously crackpot advice that only scientologists who don't read Wikipedia are likely to heed it. Their cult founder advised one to throw a headache out of one's own head into someone else's head. DriveByWire (talk) 14:09, 24 June 2012 (UTC)
Pre-amp for oscilloscope?
My oscilloscope is crumby and isn't sensitive enough for my needs even with max gain. Is there some way I can pre-amplify the signals (presently 2-6 volts) which are sometimes AC, sometimes DC? I don't know much about electronics. — Preceding unsigned comment added by 94.1.194.66 (talk) 23:12, 23 June 2012 (UTC)
- So why does somebody without much knowledge of electronics use, or even own, an oscilloscope ? (Knowing how you use it might allow for better answers.) StuRat (talk) 00:18, 24 June 2012 (UTC)
- I'm learning about electronics as a hobby and have a few circuit designs I want to try for dynamo-driven bike lights. I need the oscilloscope to better understand the designs and the effects of inclusion of certain parts. It's just a rubbish old oscilloscope off eBay. --94.1.194.66 (talk) 00:56, 24 June 2012 (UTC)
- I don't understand why you would need to amplify signals in the 2-6 volt range. If you were talking millivolts or microvolts I could understand it. 2-6 volts should be ideal for an oscilloscope, if my memory serves me. Looie496 (talk) 02:08, 24 June 2012 (UTC)
- I concur with what's written above. Virtually all oscilloscopes ever made have sensitivities in the range 1mV to 10mV per division or per cm, regardless of whther they are cheapies or professional lab-grade units worth a year's salary. Sensitivity worse than 50 mV would be very unusual. For a typical screen having a height of 6 to 10 cm, that means a signal only 10 to 100 mV will fill the screen. What you might have purchased in error (yours or the seller's) is not an oscilloscope but a "modulation monitor" intended for use by radio hams. These look like a very simple osciloscope, but have no internal amplifier, as they are intended to be driven by the transmitter output - so that the ham can tell whether he is overmodulating or undermodulating. If this is the case (make contact with a local ham if you need), I recommend you scrap it or sell it again on ebay. You could build an amplifier for it, but such an amplifier requies skills and experience. Keit120.145.65.23 (talk) 03:25, 24 June 2012 (UTC)
- The oscilloscope is a Heathkit IO 102 which can be seen here http://www.youtube.com/watch?v=27s3QynquoI - I get a deflection of two small divisions for 6.5 volts. Edit: According to an old advert, this machine should be able to measure down to 30mV per cm. Furthermore, I get the exact same deflection from applying 1.3 volts as 6.5 volts (using NiCd batteries). Could the machine be damaged? --Seans Potato Business 11:38, 24 June 2012 (UTC)
- You mean this advert? I agree that your scope appears to be broken. If you tried to amplify your signals using a laboratory preamplifier, you would probably exceed the input range of your scope and fry what's left of its vertical amplifier. According to that advert, the scope has a one volt peak-to-peak test output (might be labelled "1-V"). Try feeding that into the vertical input and see if you see a square wave. --Heron (talk) 12:26, 24 June 2012 (UTC)
- Yes, the compression of signals to only two divisons indicates a fault in the vertical amplifier, and a preamplifier will definitely not help. Since you are new to electronics, and repair by a commercial business will cost far more than a new oscilloscope of this class, I recommend that you make contact with a local ham radio chap. Not all hams have sufficient skills to diagnose faults in electronic circuits, but whoever you do contact will know another ham who does. The cost of parts to fix it may well be of the order of 20 cents - a few dollars if you are unlucky. Each country has its own ham radio association (eg ARRL in the USA, RSGB in Britain, WIA in Australia) - they would be a good starting point if you don't know any hams. Most hams will try to be very helpful to anybody starting in electronics as a hobby. Good luck. Keit120.145.65.23 (talk) 13:18, 24 June 2012 (UTC)
- The first step to repairing your oscilloscope is to get a copy of the circuit diagram which you can do here. DriveByWire (talk) 13:57, 24 June 2012 (UTC)
- The oscilloscope is a Heathkit IO 102 which can be seen here http://www.youtube.com/watch?v=27s3QynquoI - I get a deflection of two small divisions for 6.5 volts. Edit: According to an old advert, this machine should be able to measure down to 30mV per cm. Furthermore, I get the exact same deflection from applying 1.3 volts as 6.5 volts (using NiCd batteries). Could the machine be damaged? --Seans Potato Business 11:38, 24 June 2012 (UTC)
June 24
yoga asanas as therapy for herniated disc
I am a yoga teacher - trained in the Iyengar method. I have a client who wants help with debilitating back pain and stress due to a herniated disc. Guidance would be welcome. Pippa — Preceding unsigned comment added by 41.221.159.83 (talk) 11:27, 24 June 2012 (UTC)
- Ummm...we're not supposed to give medical advice. I've never seen someone ask the RD for medical advice so they can treat a paying customer though. That is a new one. 101.173.170.147 (talk) 11:49, 24 June 2012 (UTC)
- (after ec) We don't give medical advice here. However, I suggest you ask a physiotherapist as some of their exercises are based on yoga asanas. --TammyMoet (talk) 11:50, 24 June 2012 (UTC)
- For those not as familiar with it as the OP, Wikipedia has an article about Iyengar Yoga. There is also an article about Spinal disc herniation and I echo strongly the urge above to seek advice from a qualified doctor, both because a proper assessment is done with imaging equipment such as MRI and because there are a number of treatments, among which spinal manipulation is controversial or contraindicated. DriveByWire (talk) 13:45, 24 June 2012 (UTC)
Papaver somniferum
Are these Papaver somniferum? 88.173.200.107 (talk) 14:46, 24 June 2012 (UTC)
- Without seeing the leaves or flowers, I don't think you can say more than that they are Papaver. Lots of poppies have fruit (or whatever you call it) that looks like that. Looie496 (talk) 15:37, 24 June 2012 (UTC)
- Based on OR I am about 80% certain they are papaver somniferum. The pale bluish glaucous leaves are visible at the bottom of the photo. Richard Avery (talk) 17:56, 24 June 2012 (UTC)
Trying to make equivalent colors match equivalent return rates
I want to alter the color scheme in this triangular spreadsheet graph so that it matches the color scale on this New York Times chart. Any ideas? 71.212.226.91 (talk) 18:21, 24 June 2012 (UTC)


