Wikipedia:Reference desk/Science: Difference between revisions
AmitDeshwar (talk | contribs) |
|||
| Line 1,116: | Line 1,116: | ||
::What did it feel like?--[[User:Light current|Light current]] 03:09, 3 October 2006 (UTC) |
::What did it feel like?--[[User:Light current|Light current]] 03:09, 3 October 2006 (UTC) |
||
:::From myself, not like you'd really imagine death. Usually something interrupts the dream, or it shifts to another dream that may be partially related (but without any real explanation of how I got from the one dream to the other). No tunnels with light at the end, just dream nuttyness and such fun things. Try falling asleep in a dream, that's a trip ;) -- [[User:Consumed Crustacean|Consumed Crustacean]] ([[User talk:Consumed Crustacean|talk]]) 03:17, 3 October 2006 (UTC) |
:::From myself, not like you'd really imagine death. Usually something interrupts the dream, or it shifts to another dream that may be partially related (but without any real explanation of how I got from the one dream to the other). No tunnels with light at the end, just dream nuttyness and such fun things. Try falling asleep in a dream, that's a trip ;) -- [[User:Consumed Crustacean|Consumed Crustacean]] ([[User talk:Consumed Crustacean|talk]]) 03:17, 3 October 2006 (UTC) |
||
Well I have a junior psychology text book claiming that there is no evidence of what you are describing. I've also "died" in my sleep and am still here on Wikipedia, so it couldn't be that dangerous. [[User:AmitDeshwar|AmitDeshwar]] 06:12, 3 October 2006 (UTC) |
|||
== Big Bang / Black Hole ...? == |
== Big Bang / Black Hole ...? == |
||
Revision as of 06:12, 3 October 2006
| |||||||||
How to ask a question
| |||||||||
|
| ||||||||
After reading the above, you may
. Your question will be added at the bottom of the page. | |||||||||
How to answer a question
|
| ||||||||
September 27
I AM BORED!
Tony was here
Pseudoscience
I read that chiropractic practice is a pseudoscience. are there any explanations to why this would be based on false science or logic? Thanks
perhaps you should start with chiropractic? Xcomradex 02:15, 27 September 2006 (UTC)
- So let me first point out that many people claim to receive enormous benefit from chiropractic, and for all I know, they really do. Still, one of my favorite smirking pleasures is H. L. Mencken's spirited defense of chiropractic, which includes the line
- If a man, being ill of a pus appendix, resorts to a shaved and fumigated longshoreman to have it disposed of, and submits willingly to a treatment involving balancing him on McBurney's spot and playing on his vertebrae as on a concertina, then I am willing, for one, to believe that he is badly wanted in Heaven.
- You can see the whole essay at [1]. --Trovatore
- There is not much good published research on chiropractic methods; here is one study: A Randomized Trial of Chiropractic Manipulation and Mobilization for Patients With Neck Pain: Clinical Outcomes From the UCLA Neck-Pain Study. --JWSchmidt 02:45, 27 September 2006 (UTC)
- Quackwatch has a nice section on chiropracty ont their front page! http://www.quackwatch.org/] — X [Mac Davis] (SUPERDESK|Help me improve)06:50, 27 September 2006 (UTC)
In addition to the section regarding your question on the Chiropractic article, you should also take a look at Pseudoscience. Notice how the definition of psudoscience itself is not a clear-cut black-and-white matter.
Further more, the article with a list of pseudoscientific theories has an entry for chiropractic practice under the medicine section --Yaksha 07:32, 27 September 2006 (UTC)
- Many people claim benefit, but then lots of people claim benefit from placebos, and they also benefit from getting the practitioner's attention.--Shantavira 07:46, 27 September 2006 (UTC)
In the province of Ontario, Canada, chiropractic treatment is recognized by the healthcare system (partially). There are parts of chiropractic practice (e.g. treatment that relaxes the muscles in your back) that can be helpful to a patient with back pain, but a regular doctor will similarly suggest some sort of physical therapy that will be more or less as effective. However, the actual "back cracking" thing that they do is quite debatable. There have been (very rare) cases where doing so has unblocked some passage in the body, or something like that, but for the most part that part of what they do doesn't really seem to do anything, and it may actually be dangerous (your chiropractor will ask you to sign a waiver). - Rainwarrior 16:22, 28 September 2006 (UTC)
- Even a broken watch is right twice a day. Clarityfiend 22:53, 28 September 2006 (UTC)
- Also, there's this: http://www.chirobase.org/01General/chirosub.html , which lends much support to my opinon, derived both personally and professionally, that chiropractors are, by and large, just a bunch of sharlatans out to fleece the unsuspecting public. Mattopaedia 05:24, 30 September 2006 (UTC)
How many digits of pi for the known universe?
If I wanted to measure the circumference of the known universe to the accuracy of a planck length, how many digits of pi should I use?
PS. I'm assuming that the universe can be modelled by a very large circle.
202.168.50.40 02:47, 27 September 2006 (UTC)
- It's impossible to make such an precise measurement, and even if you could, it would take a sophisticated experimental setup, not any number of digits of some mathematical constant. Melchoir 02:54, 27 September 2006 (UTC)
- That's a pretty important assumption- see Shape of the universe. At very small and very large distances, we tend to have trouble modelling shapes and distances, and the universe almost certainly can't be modelled by a simple circle. --frothT C 02:56, 27 September 2006 (UTC)
- all of them. anything less simply won't do. Xcomradex 03:18, 27 September 2006 (UTC)
- The original question seems pretty reasonable to me. According to Observable universe#size, our universe, modeled as a sphere, is between 14 and 78 billion light-years in radius. I'm not going to try to figure out the answer, at least not tonight, but it should be doable. --Allen 03:38, 27 September 2006 (UTC)
- I'm assuming, though, that the questioner is really talking about calculating the circumference of the universe, not measuring it... so that it's a math problem, not a physics problem. --Allen 03:43, 27 September 2006 (UTC)
- With that data, it's easy: you have at most one significant digit to work with, so "pi = 3." suffices. It won't get you Planck-level accuracy, but neither will any other method without better data. Melchoir 04:21, 27 September 2006 (UTC)
- The original question seems pretty reasonable to me. According to Observable universe#size, our universe, modeled as a sphere, is between 14 and 78 billion light-years in radius. I'm not going to try to figure out the answer, at least not tonight, but it should be doable. --Allen 03:38, 27 September 2006 (UTC)
- Please see Pi#Numerical value for a start to answering the question. — Knowledge Seeker দ 04:13, 27 September 2006 (UTC)
- Your error is going to be far larger than one Planck length, unless you make the assumption from the start that the measurements you're using are perfectly accurate. -- Consumed Crustacean (talk) 04:16, 27 September 2006 (UTC)
- Radius = 78 billion light years = 78E9 * 9.461E15 = 7.38E26 metres
- Diameter = D = 2 * 7.38E26 metres = 1.476E27 metres
- Let C be the circumference
- thus
So I say you need about 63 or 64 digits of PI.
- That's a pretty precise 78 billion light years you've got there. Can you be sure of that figure? freshofftheufoΓΛĿЌ 04:24, 27 September 2006 (UTC)
- Sorry, but you missed the most important part of the error. It should read:
- And since ΔD is literally astronomical, for small values of ΔC there is no solution for Δπ. Melchoir 04:24, 27 September 2006 (UTC)
- I still agree with the anon calculator, because I think the original poster meant to ask about the number of digits of pi given a known radius of the universe. And as long as the universe's radius is in that tens-of-billions range, the answer is going to be 63 or 64 digits. And it's an interesting question, too... insofar as using pi to calculate circumferences is concerned, we will never ever need more than 64 digits for any practical problem. I bet that's what led the questioner to ask the question. --Allen 04:30, 27 September 2006 (UTC)
- If I remember correctly, some versions of inflation theory produce sizes of the universe in the range of (ref. The Whole Shebang), which throws everything out the window (try getting 10200 digits of pi...). —AySz88\^-^ 04:44, 27 September 2006 (UTC)
- The point of the question is that we long ago calculated pi to far more digits than would be needed to calculate the diameter/circumference/radius of the observable universe to subatomic uncertainties. The had it to 100,000 decimals by 1968, if I recall correctly. I believe Isaac Asimov pointed this out several decades ago. So going from 100 digits to 100,000, to 100,000,000 perhaps does not have any practical purpose. Edison 05:26, 27 September 2006 (UTC)
- pi's used for a lot more things than calculating circumferences. In which one of his 300 books did asimov say this?
- Perhaps "Asimov on Numbers" but I read it decades ago.Edison 14:48, 27 September 2006 (UTC)
- pi's used for a lot more things than calculating circumferences. In which one of his 300 books did asimov say this?
- The point of the question is that we long ago calculated pi to far more digits than would be needed to calculate the diameter/circumference/radius of the observable universe to subatomic uncertainties. The had it to 100,000 decimals by 1968, if I recall correctly. I believe Isaac Asimov pointed this out several decades ago. So going from 100 digits to 100,000, to 100,000,000 perhaps does not have any practical purpose. Edison 05:26, 27 September 2006 (UTC)
- While this is true, you need about three times as many digits to compute the volume to the same precision. So, there could feasibly be a "practical" need for a bit over 200 digits. Of course, we don't have good data on the global curvature of the universe... -- Fuzzyeric 04:46, 28 September 2006 (UTC)
- In curved space of course, the circumference of a circle is NOT equal to pi D. And depending on the curvature, you could have any value for the ratio of circumference to diameter ( as long as its greater than pi. ) What do we call this new 'constant' which is variable?--Light current 15:14, 28 September 2006 (UTC)
- Excellent point, especially in the actual universe, which is indeed curved. See http://www.uwec.edu/math/StudentFacultyResearch/Pi3_RNA.pdf as discussed in my more detailed version of the question being discussed here: http://www.piday.org/discussions/comments.php?DiscussionID=4&page=1#Comment_135 But note that in general, you can get smaller, and even negative(!) values for "PI" (the ratio of a circumference to a diameter in a non-Euclidean space). --NealMcB 03:49, 16 March 2007 (UTC)
While we have no practical use for all of these digits, the science of calculating them has been quite beneficial to computer science. The principles learned from attacking the pi problem have applications in many other places. - Rainwarrior 16:33, 28 September 2006 (UTC)
What pressure is Hydrogen stored at?
Answered
I need to know a rough pressure H2 is stored at (or what pressure it's unsafe at in a pipe). If you know any sources, please cite them, sorry I'm not too good with google. Thanks.
- i use H2 up to 100 psi without incident in an appropriate setup. and the hydrogen we buy is sent out at at least 1000 psi (just read the gauge, may be higher when new). Xcomradex 05:08, 27 September 2006 (UTC)
- ok good. I was worried about 5 atm (73.5 psi) flowing in a pipe but I guess this is quite small. THanks.
- bear in mind my "appropriate setup" is a reactor made of ~1 cm thick stainless steel Xcomradex 12:04, 27 September 2006 (UTC)
- What pressure is safe for a pipe is a property of the pipe, not the gas. --LambiamTalk 10:35, 27 September 2006 (UTC)
- Storing gas at 1000psi is one thing, but in a typical lab set up, there would be a regulator attached directly to the tank with no transitional piping. This is just a hunch, but if you need pure H2 you probably don't have a direct feed, but a feed with heated copper to scrub the O2. If that heated copper mesh is in a glass tube, then 5atm may be too high. The glass tube may be able to handle it, and it would be relatively safe (because a pipe dissection with a non-flammable gas such as air or CO2 would not be all that big of a deal) with another gas, but 74psi is probably not a good idea with an explosive gas. In general, you really should know the maximum capabilities of your piping and stay well below them with flammable/explosive gasses. Also, what's with the "anwered" thing at the top, never seen that before...Tuckerekcut 13:38, 27 September 2006 (UTC)
- That's something new we are trying, to mark questions that no longer require answers, so we don't have to keep reading thru them to figure it out. StuRat 14:18, 27 September 2006 (UTC)
- Great idea Stu. I think I'll start doing that. Should we put it in bold or as a sec header? — X [Mac Davis] (SUPERDESK|Help me improve)16:21, 27 September 2006 (UTC)
- Just like the one at the top of this question. We've been debating this on the Ref Desk Talk Page, so may end up changing things, but I'm trying it this way for now. StuRat 16:40, 27 September 2006 (UTC)
I AM BORED!!
earths motions
What are the four main motions of the earth?
June
- can you be a bit more specific? motions as in the earth as a planet moving, or motions as in things like wind and tides which are on earth and are always in 'motion'?
- Also, please take a look at Planetary motion, Earth rotation and Polar motion first to see whether they have your answer. The List of basic earth science topics may also be worth browsing through. --Yaksha 07:21, 27 September 2006 (UTC)
- Also, motions relative to what? There are components that are relative to the galaxy and to the local group of galaxies. Or you could surprise your teacher with observations about the Chandler wobble.--Shantavira 07:55, 27 September 2006 (UTC)
- "Or you could surprise your teacher" lol...just assume it's a homework question. --Yaksha 08:39, 27 September 2006 (UTC)
- Easy: Up,down, left, right--Light current 20:21, 27 September 2006 (UTC)
- Here on Wikipedia, most of us don't view existance in only two dimensions. freshofftheufoΓΛĿЌ 01:23, 28 September 2006 (UTC)
- Sounds like original research to me. :--) JackofOz 02:51, 28 September 2006 (UTC)
- "Sounds like" sounds like POV to me! freshofftheufoΓΛĿЌ 05:39, 29 September 2006 (UTC)
- " "Sounds like" sounds like" sounds like POV to me. DirkvdM 09:46, 29 September 2006 (UTC)
- Don't forget internal motions. The tidal effect of the Sun and the Moon causes the Earth to be kneaded, which I believe is part of the reason the Earth has a molten centre. I don't know the name for the phenomenon, though. DirkvdM 09:46, 29 September 2006 (UTC)
Tarsola
Has anyone ever heard of a bird or animal called a "forest tarsola"? Such an animal is mentioned in the fictional novel Journey to the River Sea by Eva Ibbotson, where a stuffed specimen is part of a museum display in Manaus. Whatever it is, if it exists at all, should be indigenous to the Amazon Basin. I am looking for a Latin name or a synonym for use in an authorised translation of the book.
Another animal, mentioned in the same book, about which I have not been able to find an independent source of information is an "Amazonion river slug". Is there such a thing, or is it just a fanciful invention of the author? Here's another one: a "tabernid fly". Ring any bells?
Thanks, --woggly 11:07, 27 September 2006 (UTC)
- I found nothing, except that there are slugs living in the Amazon river; most of these have very specific names though, and I couldn't locate an 'Amazonian river slug' as a species name. According to WP's article on Eva Ibbotson, she often features 'magical' creatures in her works. Is it possible that she just made up these names and species? Both she and her husband seem to have studied natural sciences, so I would assume she chose her names carefully. Tarsola reminded me of the (Asian) Tarsiers, or of the tarsus (insect foot or vertebrate bone cluster). Tabernid only reminded me of the silly Pseudolatin phrase 'situs vilate inisse tabernit' (which, when rearranged, is German for 'looks like Latin, but it isn't' - since she has Austrian roots this allusion is possible, but unlikely I guess). My suggestion for translation would be to keep the animal names as close to the original as possible while maintaining the 'scientific ring' they seem to suggest.---Sluzzelin 08:21, 28 September 2006 (UTC)
- Thanks for looking into it. I wouldn't put it beyond her to have invented the names, but on the other hand she makes plenty of references in this book that seem to be quite authentic, so I feel I need to be careful. For instance, she also mentioned a plant named "mashohara" which only had about ten independent Google hits but what few hits there were seemed to confirm this is Justicia Pectoralis, a medicinal plant used by native tribes in Amazonia - which is how it is described in the book. So I can't help thinking she knows lots of stuff that I don't. "Tarsola" is also similar to "Tarsila", which is the name of a famous Brazilian painter, I doubt that's a coincidence. Your suggestion is a reasonable, if not ideal solution, which we may have no choice but to adopt.
- That German/Latin sentence is pretty funny. Reminds me of a whole set of Hebrew/Japanese jokes: How do you say 'driver' in Japanese? - Ishimoto (looks Japanese, but is also Hebrew for "man with car"); How do you say 'diaper' in Japanese? - Sakimkaki (ditto for "sack of poop") etc. --woggly 10:17, 28 September 2006 (UTC)
- The Tarsila reference sounds very likely to me - you obviously already did your research. I read that the author dedicated Journey to the River Sea to her late husband. Maybe knowing more about him could help you further. Btw, I like your Japabrew examples, and, on a sidenote, I wonder whether WP has an article/collection of this type of wordplay.---Sluzzelin 13:24, 29 September 2006 (UTC)
Well, yes, I try to do my own homework - posting a question to the reference desk was a way of covering all bases, not of evading my work! As to wordplay: I wouldn't know under what name such an article would be filed. I know there is a form of poetry that follows this same idea, wherein the same sequence of sounds creates two different poems in two different languages, but I don't know that this form of poetry has a name. Here is an example, the first line of such a poem (a dirge) written by Rabbi Yehuda Aryeh of Modena in 1584.
In Italian: Chi nasce muor: Oime, che pass' acerbo! ("All that is born will die; alas! How bitter this move!")
And in Hebrew, the same pattern of sounds: קינה שמור, אוי מֶה כי פס אוצר בו ("Save your lament, alas how is it that no more is that which once encompassed")
Maybe someone at the language desk will know a name for this form of poetry. --woggly 21:44, 30 September 2006 (UTC)
- In computer science, there is such a thing as a program that works in multiple programming languages. Such a program is called a polyglot program, so I suggest the term "polyglot poetry". --WhiteDragon 19:36, 5 October 2006 (UTC)
Medical
What you mean by Health&Safety in working place
- Try reading Wikipedia's article on Occupational safety and health. --Shantavira 13:32, 27 September 2006 (UTC)
- The (British) government agency, The Health and Safety Executive has a decent website ([2]). --Dweller 14:47, 27 September 2006 (UTC)
Solar wind/Gamma rays
My question is if earth's atmosphere & magnetic field reflect(& protect us) all the gamma rays,x-rays & solar wind how come it allows visible light ,which has much lower energy, to reach us? I guess my question is a simple one but i couldn t find an answer on the net. Thank you Abhijit
- The magnetic field will only deflect ionized particles, like the solar wind. Some frequencies of radiation are reflected or absorbed by the atmosphere, while others (like infrared and visible light) pass thru. I believe that which ones are reflected, absorbed, or transmitted is related to the electron configuration of the atoms and energy quanta, but will let a physicist comment on that. StuRat 16:34, 27 September 2006 (UTC)
- All elecromagnetic radiation is made of the same stuff (photons). EM radiation above a threshold frequency will get through - this is somewhere in the radio region. Gamma rays and xrays do get through. StuRat is correct about the solar wind. See atmospheric seeing
do you metabolize trans fats?
Hi I was wondering if you are able to metabolize all fats or do the trans fats stay within your body hardening your arteries. So the question is do you breakdown trans fats? thanks so much this will help
- The article on Trans Fat may be of use to you. Benbread 20:26, 27 September 2006 (UTC)
- The article on Trans fat may be even more useful to you. David D. (Talk) 20:31, 27 September 2006 (UTC)
- There have been some studies suggesting that trans fatty acids can alter desaturase activity (example) resulting in altered levels of some fatty acids that might be important for normal health. I'm not sure that good studies have been done on humans. I think most studies have been concerned with effects of dietary trans fatty acid on levels of HDL-cholesterol and LDL-cholesterol, and other forms of circulating lipids and lipoproteins. Dietary trans fatty acids can accumulate in cell membranes of the body and it has been suggested that certain membrane-associated enzymes and receptors might have altered activity that contributes to disease (example: Dietary intake of trans fatty acids and systemic inflammation in women).
Relativity-: Theory, hypothesis ,conjecture or philosophy?
When Einstein formulated his Theory of special relativity, I believe he took as his only experimental evidence as that of the constancy of the speed of light. Einstein himself performed no experiments. In the light of this, was his 'Theory' (in modern scientific terms):
- just philosophical musings
- a scientific theory
- a hypothesis or
- a conjecture
THe underlying question is, of course, ' Can science be done without experiment'?--Light current 16:44, 27 September 2006 (UTC)
- That depends on your definition of science of course, and we could go into the philosophy of science as well... --HappyCamper 17:06, 27 September 2006 (UTC)
- MY defn of science is 'Knowledge of how the universe operates'--Light current 20:14, 27 September 2006 (UTC)
- I'd say it was a hypothesis at the time of its formulation. There's a complicating issue with moderm scientific terms: lots of things in physics are called "X theory" even if they're just a class of mathematical tools (quantum field theory), conjectures without experimental support (string theory), or largely rejected (SU(5) grand unified theory). If relativity were invented today, perhaps it would be called "relativity theory" just for looking good! Melchoir 17:29, 27 September 2006 (UTC)
- I feel that "postulate" is the best description of Einstein's work - unless I'm sorely mistaken on what his work was. As I understand it, scientists since Galileo were bashing their heads trying to find an absolute state of rest to use as a universal reference for relative motion. Einstein postulated that there is no absolute state of rest. Instead, there is an absolute state of motion - the speed of light. Everything is equally relative to that. The problem with his postulation is that it didn't fit with the already well known physical measurements of space and time. So, Einstein got around that with a second postulate that space and time can be bent, allowing the speed of light to remain constant. If both of those postulates are assumed true (which they must be or they wouldn't be postulates) the rest of his theories are just reflections on the ramifications of the absolute speed of light. --Kainaw (talk) 17:40, 27 September 2006 (UTC)
- As for the underlying question, yes, science can be done without experiment. It cannot be done without observation, and experiment is one particular type of observation which is particularly valuable to science, which is why experiment is generally used when it's feasible to do so. With experiment, you can set up two apparatuses which are identical in every respect except for one. When experiment is not feasible--for example, we cannot do experiments in astrophysics, since we don't have the technology to do experiments with stars--we can still observe the subjects of the science; it's just that it can be harder to draw conclusions since you're not going to find two stars that are the same in every respect except one, so you have to take many possible variables into account in analyzing the data. But even though astrophysics is based only on observation, not on experiment, it's still a science. Chuck 18:01, 27 September 2006 (UTC)
Of course science can be done without the experiment, but it isn't science until the experiment works. It is only a protoscience. Today, special relativity is a theory. Then, a believe it would be a postulate or a hypothesis. The real scientific method (not the crap taught in high schools and science fair projects) of theory, experiment, theory, experiment, is not fufilled by only theory. Therefore, it is a protoscience, like string theories and sociobiology.
- "The supreme goal of all theory is to make the irreducible basic elements as simple and as few as possible without having to surrender the adequate representation of a single datum of experience." -- Albert Einstein (1933)
— X [Mac Davis] (SUPERDESK|Help me improve)18:06, 27 September 2006 (UTC)
I'm afraid that I don't think your categories are appropriate. First, let me complain about the use of "just philosophical musings." I'm sure what you meant to say was something like, "ideas which are unconstrained by fact," or something like that. But, please, don't call that philosophy. Bad philosophy, like bad science, is unconstrained by fact, but it not a feature of philosophy as a whole to be widely unconstrained. And certainly, I don't think Einstein imagined he was doing that. Second, I don't understand the distinction between a scientific theory, hypothesis, and conjecture. Do you mean, to what degree did Einstein think his theory confirmed by the evidence? If this is what you mean, its not so much a question of three categories, but a matter of degree. In such a case, I think the historical record is unclear. Although his theory explains the Michaelson-Morley experiment, Einstein says contradictory things regarding his prior knowledge of the experiment. I don't think it was determined that Einstein's theory could account for the advance of the parahelion of mercury, one if its standard triumphs. Experiments about the bending of light around the sun weren't done until sometime after. On the other hand, his theory fit well with other facts known at the time, perhaps better than Newtonian mechanics. Because it had other virtues, like simplicity, Einstein may have thought it more confirmed by available evidence. --best, kevin [kzollman][talk] 23:06, 27 September 2006 (UTC)
Einstein postulates the constancy of the speed of light, indistinguishability between acceleration and force, Galilean invariance, and a few other pieces. He then constructs the (falsifiable) theory that the speed of light is an invariant with respect to intertial reference frames and that the Fiztgerald contraction is physically real. He then hypothesizes several things: clocks appearing to move appear to run slow, clocks apparently accelerating appear to run slow, fast moving meter sticks can pass through 1/2 m doors, et al. Experiments to test these hypotheses did not refute the theory (yet). However, until the theory -> hypothesis -> experiment loop is closed, there isn't Science. Ideally, every scientific theory (1) fits all prior data, (2) produces hypotheses (predictions) that discriminate it from other theories of the same phenomena, and (3) survives experiments that test the predictions. Without both the production of theories and the mechanism to reject wrong ones, it isn't science. Relativity was a theory that made a wide range of predicitions that disagreed with the prevalent prior theory (-ies) and Relativity has survived every critical test of its predictions. (So much so that it's more likely from an a priori position that dark matter or dark energy causes a deviation from the predictions of relativity than that relativity is incorrect, although this is technically muddled reasoning in the scientific framework.) -- Fuzzyeric 05:01, 28 September 2006 (UTC)
Thanks to all respondents. Esp Eric who has clarified the 'scientific' position excellently. I didnt know that he postulated the constancy of the speed of light: I though he was aware of the Michelson-Morely results. So is it fair to say that Einstien's Theories are rapidly becoming 'science' even if they are not science at the moment.--Light current 16:54, 28 September 2006 (UTC)
- If your interested in the distinction between science and non-science, see the demarcation problem. Almost all philosophers of science agree that something which is unconfirmed, but capable of being confirmed, counts as science (so long as people are actively seeking confirmation). Under most criteria SR is, and has always been, science. --best, kevin [kzollman][talk] 17:38, 28 September 2006 (UTC)
- The Michelson-Morley experiment measured no variation in the speed of light. This is different from claiming the axiom "the speed of light is constant in all reference frames". One measures a property of an experiment, the other makes a grand fundamental statement about how the universe works. Michelson and Morley make the experiment and derive no further conclusions from it. Fizgerald explains the length contraction implied by the Maxwell equations "as if" it were real, but since he doesn't take it to be a fundamental feature of the universe, doesn't derive subsequent relativistic hypotheses. Einstein says it is real and fundamental, so get used to it, then derives consequences of the claim.
- Einstein's theories were science when they were formulated. Newton's laws were not correctly predicting the precession of the orbit of Mercury, which had been measured and puzzled over. Relativity fit the Newtonian data and the Mercury data, made testable predictions (e.g. about the bending of light around the sun, clocks at different altitudes running at different rates, and so on) that differed from Newtonian predictions, and (unlike Newtonian theory) survived the experiments which tested the differences. -- 66.103.113.62 02:36, 29 September 2006 (UTC)
spirulina
I want to ask you that from where I get spirulina culture? I am residing at Anand,State-Gujarat,India.my e-mail ID is (removed for privacy) Please send me the regarding information as soon as possible to my e-mail ID.
Please send me the phone NO. of your office and contact person's name so I can contact & get all the necessary informations. I expect good ,positive reply from you.
- The Wikipedia Reference Desk is not an organization and it doesn't have a contact person. It's an online bulletin board "staffed" by volunteers. If anyone has an answer (I regret I don't), it will be posted here. --Ginkgo100 talk · e@ 19:16, 27 September 2006 (UTC)
- Also, have you heard of Google? From a simple Google [3]. Of course, you could probably find someone in India fairly easily if you search a bit harder. Nil Einne 00:01, 28 September 2006 (UTC)
velocity
if i squeeze a spot what is the maximum velocity with which the spot goo exists the spot?
FAQ!--Light current 19:18, 27 September 2006 (UTC)
geez if I had a nickel for every time that was asked! --Deglr6328 22:53, 27 September 2006 (UTC)
- Like for all matter, the velocity cannot reach the speed of light. -- --LambiamTalk 23:38, 27 September 2006 (UTC)
Speed of light
If nothing can go faster then the speed of light, how come the force of gravity or lack of it is felt sooner? It takes the light from the Sun 8.5 minutes to get to earth, but if the Sun where removed the affects would be felt that very moment and the Earth would loose it orbit well before it would get dark.
is it possibale to travel faster than the speed of light, and if so, this means that travelling faster than the speed of light will make time move backwards according to the principle of relativity which states that time slows down as you travel closer to the speed of light, and since travelling at the speed of light is impossibale then time can never absolutley come to a halt, also if time comes to a stop or moves backwards then this must mean that objects moving at the speed of light or faster than it must have a length of zero or a negative value: Length of an object = Speed of light x time diffrence.
I hope someone could clear things up for me..
- It is not currently believed to be possible for a particle with real mass to travel faster than the speed of light. See also Faster-than-light, Tachyon. JBKramer 19:33, 27 September 2006 (UTC)
- For example, if an astronaut falls towards a black hole, (which spins faster than the speed of light) time to the astronaut would be normal. But to a distant observer, their time will slow and then stop altogether as he enter the black hole. To clarify this, pretend the asrtonaut had a watch that could send time signals to Earth. If you could pick up the time signals from the astronaut falling towards a black hole, the time from the signals would go slower and slower until, as he/she entered the black hole, it would stop altogether. However, like I mentioned before, to the astronauts, time would be normal. My friend think that someone travelling faster than light would see people moving slowly. I used to have trouble understanding it, but now I do understand it! And I'm only in grade 6! WARNING: Do not try this at home! Himanyo 19:46, 27 September 2006 (UTC)
- It is possible to travel faster than the speed of light, however it is impossible to accelerate (or decelerate for anyone not accustomed to the physics meaning of acceleration) from below to above the speed of light, or from above to below. As that would violate causality, not to mention, as we know, something that is travelling at the speed of light would have infinite mass, and therefore recquiring an infinite amount of energy to move. So yeh, it is impossible to travel at the speed of light, above and below (theoretically) your fine, but we can never get above it, as that would recquire traveeling at it. Philc TECI 19:49, 27 September 2006 (UTC)
- Um, no. The current theory is that one may take two observers some distance away from "the" Kerr black hole, the "rest" observer will watch the rest of the experiment and the "moving" observer will putter around. The moving observer accelerates into the ergosphere. At no time will the moving observer accelerate to a speed faster than the speed of light relative to test particles near himself. However, to the rest observer, the moving observer can appear to exceed the speed of light. I.e., the net effect of the acceleration and the frame dragging1 in the ergosphere will produce the observation of superluminal velocity. It isn't yet decided if this is correct or not. I recall experiments planned for earlier this year using photons as the drag-inducing medium. I wonder whatever happened with that. -- Fuzzyeric 05:26, 28 September 2006 (UTC)
- I agree with Philc_0780. And sorry, black holes don't spin (?what word can I use?!) faster than light, but have gravity strong enough to suck light in. A little mistake. And gravity can also affect time. Don't forget wormholes. Also you can get teleported into the past or future if travelling faster than light according to Einstien, I think. I forgot.Himanyo 19:58, 27 September 2006 (UTC)
- Black holes do not spin faster than the speed of light. Nor is time travel through wormholes anything but science fiction at this point --frothT C 20:02, 27 September 2006 (UTC)
- My computer is running slow today. And sorry for the edit conflict Philc_0780. Himanyo 20:00, 27 September 2006 (UTC)
- I know wormholes are s.f. but some scientists think they exist. Also I wrote that the GRAVITY of the black hole affects time.
- Nah, the edconflict thing is cool! It was just seeming less and less funny as it happened again, and again, hehe!! I dont see how black holes can be wormholes, as anything that went into a black hole would be well and truly wrecked by the time it got to the centre (urr, lots of gravity) so all the wormhole would be spitting out at the other end would be a chunk of neutronium at best. Philc TECI 20:14, 27 September 2006 (UTC)
- I know that the astronaut would become like a spaghetti as he got closer to the black hole and would be toast when he's in the center, but it's just an example. Wormholes connect different parts of space-time. Scientists think they can be used as time machines and I wrote before that... Himanyo 20:42, 27 September 2006 (UTC)
- Another edit conflict! Ahh! *rips hair off head* Himanyo 20:42, 27 September 2006 (UTC)
- Actually time doesnt slow down if u travel faster than the speed of light, because travelling at speed of light would mean that time reaches zero and completley stop at 300,000,000 meters per second (speed of light), therfore moving faster than the speed of light would mean moving back in time at a very slow rate say 400,000,000 meters per second, thus i guess that traveling at double the speed of light would be like normal time but backwards.
P.S:- it would be really kewl if your parents went on a trip to a nearby blackhole for 2 weeks then you will be 20 years older than your parents when they return --RedStaR 20:55, 27 September 2006 (UTC)
- Why do you think that is likely and why would it be 'kewl'?--Light current 21:05, 27 September 2006 (UTC)
- He didn't say it was likely, but all space travel involving black holes is inherently kewl. Hyenaste (tell) 00:30, 29 September 2006 (UTC)
- The principle of relativity says that there is no preferred reference frame for constant motion. If one were to travel faster than light the maths does not say time would flow backwards - the passage of time would be measured as imaginary (some multiple of i).
- The Lorentz transformation was only designed to work up to velocites of 'c'--Light current 00:08, 30 September 2006 (UTC)
Negative Kelvin Temperatures?
How can such temperatures be obtained? Its not like you can slow atoms that are already stationary... 152.163.100.74 20:21, 27 September 2006 (UTC)
- Negative Kelvin temperatures cannot be obtained. 0K is the lowest theoretically possible. —Daniel (‽) 20:26, 27 September 2006 (UTC)
Negative Kelvin? Where did you get that from? Himanyo 20:27, 27 September 2006 (UTC)
- You might want to read negative temperature. Absolute zero is in fact an impassable discontinuity in temperature space, and infinity (in the projective reals sense) a lesser one. It is still a trick to surpass (or even reach) infinite temperature, but with certain systems it is possible. Something with a very large negative temperature is only "slightly hotter" than something with a very large positive temperature. In a sense, temperatures range from 0K to -0K (or ε to -ε). --Tardis 20:50, 27 September 2006 (UTC)
- Yup. And to clarify for the original question: you're right, the thermal motion of atoms cannot achieve a negative temperature. The only systems that can achieve negative temperatures have bounded energy spectra, such as spin ensembles. Now, if a system X at a negative temperature is brought into thermal contact with a gas, the gas will absorb heat from X until finally the temperature of X drops below infinity to a positive number; at equilibrium the two systems will have the same positive temperature. So a gas thermometer, no matter how small or unobtrusive, is incapable of measuring negative temperatures. Same goes for any other kind of material thermometer that relies on achieving equilibrium with its target. Melchoir 23:24, 27 September 2006 (UTC)
- A classical example of a negative temperature system is a lasing medium in an inverted state. There is a great deal of potential energy in the system, causing the system to be disordered, and we can extract that energy and send the system to an ordered (grounded) state by kicking off the laser emission. At thermodynamic temperature we find the common equation 1/T = dS/dE. Since the entropy of the inverted state (mix of excited and grounded atoms) is greater than the entropy of the ground state (all atoms grounded) and we can induce a decrease in entropy by adding energy, we find that the temperature is dS/dE < 0, so T < 0. -- Fuzzyeric 05:34, 28 September 2006 (UTC)
belostomatidae or giant water bug (or beetle)
I found one and I am raising it. It is two inches long. I need to know what it eats.
- Read our page please!
- They are fierce predators which stalk, capture and feed on aquatic crustaceans, fish and amphibians
- --Light current 21:10, 27 September 2006 (UTC)
- Small fish and tadpoles, try tuna or sardin see if that works --RedStaR 21:15, 27 September 2006 (UTC)
- And don't put your toe in the water. They ain't called toe-biters for nothing. Their bite is ... widely considered the most painful that can be inflicted by any insect. --LambiamTalk 23:46, 27 September 2006 (UTC)
Wetness
Is the human skin capable of detecting 'wetness'? Or is the sensation imagined from a combination of temperature etc.--Light current 21:19, 27 September 2006 (UTC)
Actually it doesnt on condition that the water you put your hands in are the same as your body temperature eg: when your in a swimming pool for a consedirable amount of time untill your body adapts to the temperature of the pool water, you cant tell the diffrence if your wet or dry --RedStaR 21:29, 27 September 2006 (UTC)
- No, no such thing as a wetness receptor.Tuckerekcut 22:16, 27 September 2006 (UTC)
- Actually, not even a proper temperature receptor. You may know the following experiment: Put one hand in cold water, the other in hot water, and the both in the same pan with medium-warm water. The temperature feels different. Hence, your skin measures not absolute temperature, but temperature change: If warmth is drawn out of your skin, you feel cold. And this is why cold water feels so much colder than cold air of the same temperature. Cold water, due to its higher heat capacity per volume can draw the heat from your body much faster. If the heat is then also dissipated (carried away from the point into the bulk of the stuff) quickly in the material, so that the temperature difference between hand and touched material (and hence the heat flow) is kept steady, it feels especially cold. This is why metals, with their very high heat conductivity, feel even colder. Simon A. 09:42, 28 September 2006 (UTC)
- I think its the thermal conductivity primarily that makes things feel hot or cold ( assuming the heat capacity is sufficiently large for you body not to affect the obejcts temperature substantially). For instance, marble or iron generally feels cool if its lower than your body temp, but hot if its above.--Light current 15:23, 28 September 2006 (UTC)
When the sun goes red giant...
...and presuming that the earth wasn't consumed, would it be possible for any of the lifeforms that exist on earth at present to survive? I'd guess that some bacteria, viruses and the obligatory 'stuff that lives near the hydrothermal vents' might be okay - but what about the plants and animals? What would the progress of the transformation of the sun be like anyway? Does it take place over hundreds/thousands of years or would it be like 'Wooosh! Suddenly the sun changes colour and fills half the sky now'? --Kurt Shaped Box 21:57, 27 September 2006 (UTC)
- What your saying wont take course before a few billions of years when humans wont probably exist or will be in another solar system--RedStaR 22:27, 27 September 2006 (UTC)
- I'm sure he knows this. - R_Lee_E
 (talk, contribs) 22:11, 27 September 2006 (UTC)
(talk, contribs) 22:11, 27 September 2006 (UTC)
- I'm sure he knows this. - R_Lee_E
- The parrots, the gulls, the magpies and quite possibly the ants may all have upped and left in their own spacecraft by that point too and have scattered across the galaxy (I would *love* to be around long enough to see what sort of spacecraft the ants would build - I don't know but I can guess that it would be a pretty cool sight)... ;) --Kurt Shaped Box 22:23, 27 September 2006 (UTC)
- Well, for one, the Earth would be completely consumed. But if we are only to consider the temperatures involved, I believe the oceans, along with any water trapped in fissures beneath the surface, would be evaporated. The atmosphere, as well, would be blown away. Hence, I'd be confident in saying that that would be the official end of life on Earth. - R_Lee_E
 (talk, contribs) 22:25, 27 September 2006 (UTC)
(talk, contribs) 22:25, 27 September 2006 (UTC)
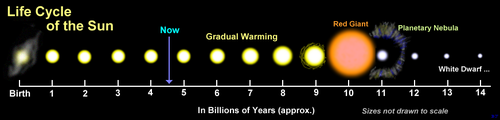
- The red giant article itself suggests that this is not nescessarily the case. The gravitational pull of the sun will have weakened by then due to its loss of mass, and it is possible that Earth may escape to a wider orbit [9]. The fate of the Earth with regard to the size of the expanding Sun is still hotly debated in the scientific community. --Kurt Shaped Box 22:26, 27 September 2006 (UTC)
- ---wow, an edit conflict with two other posters...---
- I can't answer your question, but I can tell you that should the plants die, even the bacteria living near hydrothermal vents will perish. These bacteria (actually they are mostly Archaea) use iron in their respiration. This iron, after it is used by the bacteria, becomes soluble in water and diffused into the ocean. When this diffuse (reduced) iron meets the oxegenated water near the surface, it becomes oxidized and falls back down to the bacteria, and the cycle starts over. Thus if the plants aren't around to oxegenate the air and water, even the bacteria living on the hydrothermal vents will die. Also, and don't take my word for this part, I remember hearing that when the sun becomes a red giant, it will be so large that the Earth itself will be engulfed, because its central mass will no longer be massive enough to hold the outside in ( that is, the outward explosive and centripetal forces will no longer be tiny compared to the gravitational force).Tuckerekcut 22:14, 27 September 2006 (UTC)
- uhhhhm where did you get the wild idea about archaea at thermal vents depending on FeO influx from plants above. honestly, I don't think that's even wrong. the amount of iron compounts spewed from the vent itself is immense! --Deglr6328 00:19, 28 September 2006 (UTC)
The back of my envelope suggests a surface temperature of like 1100 C. So I'd say no life on the surface or in what once were the oceans. Some bacteria that live deep underground might still be happy, but I'd say that life as we know it would pretty much need to find a new place to live. Dragons flight 01:32, 28 September 2006 (UTC)
- So (assuming the human race, or one of our successors from another evolutionary line was still around and paying attenion), how much notice would we/they have in advance of the impending 'end of (nearly) everything'? Would it be observable for a long time beforehand that the sun was about to balloon and incinerate the planet's surface? How long would it actually take for the sun to become a red giant once the process was irreversibly initiated? Minutes? Hours? Days? Weeks? Years? --Kurt Shaped Box 01:47, 28 September 2006 (UTC)
- Refer to the image above. It would be about a two-billion year process. - R_Lee_E
 (talk, contribs) 02:03, 28 September 2006 (UTC)
(talk, contribs) 02:03, 28 September 2006 (UTC)
- Refer to the image above. It would be about a two-billion year process. - R_Lee_E
- Sorry, I was trying to find out how long it would take to go from sun-sized to super-size once the thing 'blew'. Are you saying it would take 2 billion years to expand, or two billion years to reach 'critical mass' and then 'blow'? Sorry if I'm being thick - most of my knowledge in this area comes from bad sci-fi... --Kurt Shaped Box 02:11, 28 September 2006 (UTC)
- I think most of the transition might be accomplished in only a few 100 million years, but basically the transition time scale is going to be absurdly long compared to the life time of any organism that might be around to worry about it. Which I suppose could push evolution towards hyper-hyperthermophiles, but I'd still have trouble imagining anyway to survive that transition and be carbon based. Dragons flight 02:35, 28 September 2006 (UTC)
Since Deglr seems skeptical, let me explain my rationale furthur. Although reduced iron is copious at the vent surface, it must be in its oxidized form to be coupled to respiration. Fe(III) acts as a terminal e- acceptor with acetate (as acetate is one of few organic acids which has been shown to form abiotically in hyperthermic environments from the organic matter that floats down (yet another reason this life could not survive without terrestrial flora: it needs a carbon source)). Since the iron coming out of the vents is completely reduced, it makes a very poor e- acceptor, and would not be able to contribute to vent life as such. The bottom of the ocean is mostly anaerobic, so it is O2 at the surface that oxidizes it. Check out Tor, JM, Amend, JP, and Lovley, DR. 2003. Metabolism of organic compounds in anaerobic, hydrothermal sulfate-reducing sediments. Environmental Microbiology 5:583-591.. And try not to be such an assface in the future.Tuckerekcut 02:27, 28 September 2006 (UTC)
- pfft hah. real mature, what are you, like 12? that paper does not at all support your idea.--Deglr6328 03:42, 28 September 2006 (UTC)
- With all due respect, I am quite confident that vent communities existed quite happily before the invention of oxygen based metabolism. Whatever minimal impact terrestrial flora might have on them, I am certain that life can persist nearly indefinitely even without a terrestrial biosphere. Dragons flight 02:48, 28 September 2006 (UTC)
- I believe the consensus is that, in the presence of such extreme temperatures, much, if not all, of the water on Earth would be evaporated. Thus, a terrestrial biosphere is a moot point. - R_Lee_E
 (talk, contribs) 03:25, 28 September 2006 (UTC)
(talk, contribs) 03:25, 28 September 2006 (UTC)
- I believe the consensus is that, in the presence of such extreme temperatures, much, if not all, of the water on Earth would be evaporated. Thus, a terrestrial biosphere is a moot point. - R_Lee_E
- Every once in a while, someone comes around and says that they have discovered an organism that is completely uncoupled from the sun's energy. Look up SLiME (Subsurface Lithoautotrophic Microbial Ecosystem) for a well publisized example. The deep-sea hydrothermal vents are another example. It seems at first glance that they get all of their energy (that is electron donor, electron acceptor, and carbon source) from the vents, but everything is so well reduced that they must rely on oxidized materials from above. The iron coming out of the vents is no more useful to these bacteria as an electron donor than CO2 or HS is for us. So even though these organisms live far from the sun's rays, they still require the sun in an indirect way. If the terrestrial plants on the surface, which oxegenate the air with the sun's energy, were not around, these deep sea archaea would not be able to survive. There is also new talk of a photosynthesizing organism living on the sea floor which uses the dim illuminescence produced abiotically in the sea (I don't know how) to live. We will sea if this one pans out to be truly decoupled from the sun, but I'm betting it won't be either.Tuckerekcut 12:00, 28 September 2006 (UTC)
- I read years ago that when this process starts, the sun would bloom to a red giant in a matter of hours. But it would be a matter of millions of years before that process started.
Brown noise
Brown noise is a pitch with a certian frequency that makes people lose bal control, in other words it makes you crap your pants, the french experimented with it in World War II, comment on this is appreciated --RedStaR 23:11, 27 September 2006 (UTC)
- Try brown note Nil Einne 23:29, 27 September 2006 (UTC)
- A blue note makes me lose ball control. --LambiamTalk 23:59, 27 September 2006 (UTC)
- What you're talking about doesn't really exist. (And yes, there were experiments done trying to find it, possibly by the French, but they weren't successful.) There is another thing called brown noise which is a kind of sound, but has nothing to do with your bowels (it sounds kind of like distant cars on a freeway maybe). And of course the brown sound so loved by Van Halen. - Rainwarrior 16:09, 28 September 2006 (UTC)
Mythbusters tried it. No such thing. Deltacom1515 22:06, 30 September 2006 (UTC)
- Brown noise isn't pitched; although the sliding scale between a definite pitch and white noise is continuous and therefore subjective, one look at the frequency plot is enough to agree it's noise. It certainly sounds like noise - put through a very soft low-pass filter. Perhaps the brown note you're looking for does exist at extreme volumes, but personally I'd go with a frequency of about 13KHz at a ridiculous SPL if I wanted to fuck someone up. Might not work on really old people, mind. Magic Window 15:08, 1 October 2006 (UTC)
How many humans have ever lived?
What is the total population of humans that has ever lived?
I defined the function B(year) to be the number of humans born in that year.
B(2006) = number of humans born in year 2006
Next I define a new function
TotalPop(xxxx,yyyy) as B(yyyy) + B(yyyy-1) + B(yyyy-2) + ... + B(xxxx)
For example:
TotalPop( 2006 , 2006 ) = B(2006)
TotalPop( 2004 , 2006 ) = B(2006) + B(2005) + B(2004)
So my question becomes what is the value of TotalPop( -INFINITY , 2006)
If I assume that B(2006) = k P(2006) that is birth is a proportion of the actual population. And that P(2006) = r P(2005) where r > 1.
B(n) = r^1 * B(n-1) = r^2 * B(n-2)
B(n) + B(n - 1) + B(n - 2) = B(n) * ( 1/r^0 + 1/r^1 + 1/r^2 )
So
TotalPop( -INFINITY , 2006) = B(2006) * ( SUM( t=0 , t=INF, 1/r^t ) )
So what is the value of B(2006) and the value of r ?
202.168.50.40 23:20, 27 September 2006 (UTC)
WORLD POPULATION
Year Population 1700 600,000,000 1800 900,000,000 ~~> 50% increase rate/100 yrs 1900 1,500,000,000 ~~>66% increase rate/100 yrs 2000 6,000,000,000 ~~>300% increase rate/100 yrs
I'm not sure of this but if u can figure out a fixed ratio or equation with the rate of increase of the population relative to 1000yr unit multiplied by estimated human existance would get a pretty approximated number of actual history of human population, (This does not include war casulties, epidemics, plagues, or other life manipulating factors) --RedStaR 23:51, 27 September 2006 (UTC)
- As a model, it's probably not very accurate - since I wouldn't think there'd be a constant ratio difference between the total populations in 2 years now and in prehistory. The Black Death reduced Europe's population massively in the 14th century for example. But using the assumptions we have here;
- P(n) = r P(n-1) where r > 1.
- Let's rewrite that as P(n-1) = s P(n), where s < 1
- B(n) = k P(n), i.e. B(n-1) = sB(n)
- ok, so what we now have is just the sum of an infinite geometric progression, which sums to . I'm sure there are figures for P(2006) and P(2005) to calculate s, there might be figures about B(2006), so estimate . Then put it in the sum above. As I said, probably a very crude estimate Richard B 00:07, 28 September 2006 (UTC)
- The answer to your title question depends considerably on how you chose to define "human", and whether or you want to count, for example, millions of years of walking apes. For some reasonable definitions (such as saying modern humans arise only near the start of agriculture or cities), it is not a stretch to argue that a substantial fraction of all humans that have ever been born are alive today. Dragons flight 01:11, 28 September 2006 (UTC)
- I got lots of hits for "How many people have ever lived". The Straight Dope said in 1987 that demographers estimate between 69 and 110 billion. Another article dated 1999 said 96.1 billion (love that precision). The latter provides equations used to arrive at that estimate [4]. Clarityfiend 01:23, 28 September 2006 (UTC)
- Wow, that would mean that 1 out of every 15 or so humans ever to live is alive today. — Knowledge Seeker দ 01:46, 28 September 2006 (UTC)
- This is a Frequently Asked Question: Wikipedia:Reference desk archive/Humanities/2006 August 19#How to calculate the total human population, Wikipedia:Reference desk archive/Humanities/2006 August 22#Total World Population, Wikipedia:Reference desk archive/Humanities/2006 September 4#Number of Humans Since the Beginning, and Wikipedia:Reference desk archive/Humanities/2006 September 7#Number of humans that have ever lived. See also How Many People Have Ever Lived on Earth? with an estimate of 106 billion. --LambiamTalk 04:04, 28 September 2006 (UTC)
#!/usr/bin/python
from __future__ import division
def br(year):
if year >= 2000:
return 0.030
if year >= 1990 and year < 2000:
return 0.032
if year >= 1980 and year < 1990:
return 0.035
if year >= 1970 and year < 1980:
return 0.038
if year >= 1960 and year < 1970:
return 0.040
if year >= 1950 and year < 1960:
return 0.042
if year >= 1925 and year < 1950:
return 0.040
if year >= 1900 and year < 1925:
return 0.040
if year >= 1875 and year < 1900:
return 0.040
return 0.040
def dr(year):
if year >= 2000:
return 0.014
if year >= 1990 and year < 2000:
return 0.014
if year >= 1980 and year < 1990:
return 0.016
if year >= 1970 and year < 1980:
return 0.017
if year >= 1960 and year < 1970:
return 0.020
if year >= 1950 and year < 1960:
return 0.024
if year >= 1925 and year < 1950:
return 0.031
if year >= 1900 and year < 1925:
return 0.036
if year >= 1875 and year < 1900:
return 0.039
if year >= 1750 and year < 1875:
return (0.040 - 0.0057)
return (0.040 - 0.0004)
def gr(year):
return (1.0 + br(year) - dr(year))
# Main program
P = {}
P[2006] = 6.5E9
B = {}
year = 2006
total = 0
while P[year] >= 10:
B[year] = P[year] * br(year)
total = total + B[year]
print "year %d pop %9.7g birth %9.7g total %12.7g" % (year,P[year],B[year],total)
P[year - 1] = P[year] / gr(year - 1)
year = year - 1
According the the python program above. The total number is around 99 billion.
- Sweet. I want my kid to be human number 100000000000. freshofftheufoΓΛĿЌ 05:36, 29 September 2006 (UTC)
- Reality check. The above program says there were between 10 and 11 people in 43727 BC, way low. Those birth rates and death rates are just guesses.
year -43724 pop 10.01154 birth 0.4004617 total 9.852964e+10 year -43725 pop 10.00754 birth 0.4003016 total 9.852964e+10 year -43726 pop 10.00354 birth 0.4001415 total 9.852964e+10
- Also, the line "return (0.040 - 0.0057)" did you intend 'return (0.040 - 0.00057)' ? --GangofOne 10:13, 29 September 2006 (UTC)
- In defence of the routine provided, you can't expect realistic results for every year, especially far in the past where the figures become largely insignificant. In reality, there would be one year in the past where zero humans were born (because they didn't exist yet), and the following year (imagine a year where homos became sapien) would show the birth of probably a few thousand. Since we can't really guess at the exact year, it makes more sense to write a function that approaches zero the further back in time you go. I agree, though, that getting a result of 10 only 40,000 years back will skew the results quite a bit, and I have given up on giving birth to the worlds 100000000000th child. freshofftheufoΓΛĿЌ 11:56, 29 September 2006 (UTC)
- The program does illustrate the exponential takeoff; it's just not numerically realistic. If you give birth, why not try for something doable, like 'the most beautiful baby in the history of the world.' You are very likely to achieve that. --GangofOne 23:00, 1 October 2006 (UTC)
- In defence of the routine provided, you can't expect realistic results for every year, especially far in the past where the figures become largely insignificant. In reality, there would be one year in the past where zero humans were born (because they didn't exist yet), and the following year (imagine a year where homos became sapien) would show the birth of probably a few thousand. Since we can't really guess at the exact year, it makes more sense to write a function that approaches zero the further back in time you go. I agree, though, that getting a result of 10 only 40,000 years back will skew the results quite a bit, and I have given up on giving birth to the worlds 100000000000th child. freshofftheufoΓΛĿЌ 11:56, 29 September 2006 (UTC)
- Also, the line "return (0.040 - 0.0057)" did you intend 'return (0.040 - 0.00057)' ? --GangofOne 10:13, 29 September 2006 (UTC)
Where are the "Fulcher bands"?

I have been trying to validate my annotations to the discharge spectrum of a deuterium lamp for quite some time (see pic for my marked up version) and I can not for the life of me find a concise definition or spectrum showing the entire range of the so called "Fulcher bands". I am confident of my labelling of the balmer lines in the spectrum and pretty confident of the continuum radiation range note but I just don't know where the fulcher bands (are conventionally accepted to) begin. I have searched for hours through many many papers written through the last century on "fulcher bands" "fulcher system" and "secondary hydrogen emission/lines" and all I have found are refrences to the so called "q-branch" of the band, only above 600nm which is used along with the balmer lines to determine the ratio of molecular to atomic hydrogen in a plasma (only molecular H emits continuum and fulcher radiation). Can anyone here help? --Deglr6328 23:31, 27 September 2006 (UTC)
- Not even a single comment?? :( --Deglr6328 13:51, 29 September 2006 (UTC)
September 28
Corynebacterium and sweat
I know corynebacterium feed off of some types of sweat causing bad odor. My question is, are corynebacterium killed in the wash or as some suggest, do they live on and start smelling up your armpits as soon as you put your shirt back on?
- Have you heard of "killbo.com", an older defunct website? I personally tested their suggestions. The results are spectacular, at least they are with my own crop of armpit bacteria. If I take a thorough shower, use Michum antiperspirant, and then wear a shirt which was sterilized with a tiny bit of chlorox, I essentially have no armpit odor for about 100 hours. If I do the same sequence with an old shirt which hasn't been sterilized, I'll develop strong armpit odor after roughly five hours. Out of curiousity I repeated these tests every time I did laundry, while changing things slightly each time. I found that I need at least 1/3 cup of chlorox in a load of laundry. But if instead I first soak my shirts for a few minutes in a partially full washing machine, then I can use about 1/8 cup of chlorox. Less than that and the stench returns much earlier. I also found that the originally suggested bactericidal ointment didn't work very well. (It was a fungicide intended for athlete's foot.) But the bactericide in Michum deodorant works very well. Others suggest using the "phisohex" type of hexachlorophene-based medical soap, but I haven't seen it in drug stores. (Note that your clothes will develop bleach-stains unless you thoroughly mix the bleach into the wash water before adding any clothes.) --Wjbeaty 01:15, 28 September 2006 (UTC)
- Phisohex is only available via prescription, and is fairly expensive. You might be able to get it in Mexico cheap without a prescription and either do the experiment there, or risk legal trouble and take it out of the country to do the experiment.
- I once read that putting old shoes in the freezer for a length of time was the only practical way to kill off the bacteria producing the odorous substances. I never tried it though, because I don't want sneakers next to my ice cream. ---Sluzzelin 09:23, 28 September 2006 (UTC)
Edible glowing things
According to the article, honey fungus is both edible and bioluminescent. Can you eat anything else that glows? —Keenan Pepper 02:05, 28 September 2006 (UTC)
- Well, there's bacon, and all kinds of other glowing things to eat! —The preceding unsigned comment was added by 71.113.117.169 (talk • contribs) .
- Hmmm... doesn't the protein the pigs use need ATP or something to work? Also I'm pretty sure cooking the bacon would render it inactive, so that's no fun. I'm looking for things you can actually eat as they're glowing. —Keenan Pepper 02:34, 28 September 2006 (UTC)
- I have seen luciferase-expressing (genetically modified) human cells (I think they were epithelial cells, they were grown in culture), in fact, I think it's pretty common to clone luciferase into various cell-lines for various reasons (in the lab). This at least suggests that the protein itself isn't cytotoxic, as the cells continue to thrive,but it's (digestive) metabolites may be. So you probably could eat a firefly, unless there is some other toxin they make. That's not to say that you would want to though...Tuckerekcut 02:37, 28 September 2006 (UTC)
- How about fluorescent foods? I recall that Tonic Water will fluoresce with a sky-blue color, as you'll find if you order it with Gin while hanging out in a retro Disco bar with black light tubes all over the ceiling. --Wjbeaty 00:54, 30 September 2006 (UTC)
- Only marginally related, but according to Richard Feynmann, crunching wintergreen lifesavers (I think that was the flavor) releases a burst of light. He recommended doing it in a dark room to see it. Clarityfiend 03:22, 28 September 2006 (UTC)
- Yeah, I've done that before. It's a tiny, dim flash, but it's exciting when you finally see it. —Keenan Pepper 03:31, 28 September 2006 (UTC)
- The Wint-O-Green Life Savers sparking was even used in an episode of Cheers. The sparking is mentioned on the Life Savers page. Dismas|(talk) 03:35, 28 September 2006 (UTC)
- Triboluminescence would be the cause of that. Same with neco and tape. — X [Mac Davis] (SUPERDESK|Help me improve)04:50, 28 September 2006 (UTC)
- Triboluminescence can also be observed when crunching polo mints between your teeth. Green flashes. --MerKaBa 00:34, 2 October 2006 (UTC)
- Radium! — X [Mac Davis] (SUPERDESK|Help me improve)04:54, 28 September 2006 (UTC)
- You could eat a cyalume glowstick. I hear they're pretty yummy [5] (turn volume down-annoying music). Some glowing vibrio species spontaneously grow on too old seafood...you could eat that. You could also eat the genetically engineered deep sea bioluminescent food additive "prolume" which is supposedly safe and awaits fda approval [6].--Deglr6328 05:00, 28 September 2006 (UTC)
Dancers glow. You could always eat one of those. --Dweller 13:55, 28 September 2006 (UTC)
- Anything white under a black light would appear to glow too. --Fastfission 22:22, 28 September 2006 (UTC)
Gender??
When is gender determinable?
- Nine weeks it is.[7] — X [Mac Davis] (SUPERDESK|Help me improve)04:32, 28 September 2006 (UTC)
- External sexual differentiation starts in week 8 according to our article on the matter (although it isn't specifically sourced). Genetically, sex can be determined as soon as the egg is fertilized. Fetal screening is usually performed sometime between weeks 11 and 15, although I don't know why. -- Plutortalkcontribs 10:58, 28 September 2006 (UTC)
- For in vitro fertilization, it can be done by day 5, see Preimplantation genetic diagnosis. --JWSchmidt 11:15, 28 September 2006 (UTC)
- Note though that gender is not exactly the same thing as sex. --Fastfission 22:20, 28 September 2006 (UTC)
- Note also that the definitions have been so thoroughly confused that one cannot infer that the terms are being used as anything other than synonyms unless their definitions have been explicitly stated. - Nunh-huh 22:20, 29 September 2006 (UTC)
Wrist
My wrist is so thin compared to some people even some girls! Is there anyway to make it look thicker?
- You didn't tell us your age (from the question, I'm assuming you're male). It could be simply your physical makeup. When I was young, my wrists were tiny too, but it changed over time. Healthy eating (no hunger diets) and plenty of exercize should help you along. - Mgm|(talk) 08:16, 28 September 2006 (UTC)
- Don't worry about how your wrists look silly. The only people that really notice are you. If anybody makes fun of you for it be glad it is your wrists, and not your face, or your intellegence. — X [Mac Davis] (SUPERDESK|Help me improve)14:40, 28 September 2006 (UTC)
- Wrist falsies? --jpgordon∇∆∇∆ 15:19, 28 September 2006 (UTC)
- This may come in handy if you ever have the cuffs put on!--Light current 15:28, 28 September 2006 (UTC)
You might want to wear something to cover them, like a watch or long sleeve shirts. They will likely get thicker as you get older. StuRat 17:34, 28 September 2006 (UTC)
- 'Dancing with yourself' a few times a day might help. Well, it couldn't do any harm... ;) --Kurt Shaped Box 17:39, 28 September 2006 (UTC)
- Wouldn that only build up one wrist? OR, you could try playing with a gull in each hand! 8-)--Light current 01:15, 29 September 2006 (UTC)
- I do wrist curls with a 10 kg weight in each hand. If you keep your arms dead straight (pointed down) it starts to really burn after about 30 reps. This will not do much to increase the size of your wrists (there's not much muscle close to the wrist bones), but it will make your forearms bigger and make the size of your wrists less noticeable, and more natural. It easy to strengthen your wrists when you're bored too, just by pushing on your opposing hand. freshofftheufoΓΛĿЌ 05:30, 29 September 2006 (UTC)
I'm 15 years old MacGyverMagic.
- Nice to hear that. You could still do less than... 30 reps. Yikes. — X [Mac Davis] (SUPERDESK|Help me improve)08:08, 1 October 2006 (UTC)
Largest Dinosaurs
The above title mostly more or less explains my question, which is, "Which was the largest dinosaur?" I've been searching for the answer across many sites, but I haven't got a definite answer. Most of the best sites, like www.enchantedlearning.com, or www.miketaylor.com normally give you a list of Italic textlarge dinosaurs. Although some sites do say Argentinosaurus is the largest, the same sites give much bigger figures for others, like Bruhathkayosaurus or Amphicoelias fragillmus. I'm pretty sure that the answer is one of the above two, but hey, I'm no expert. I was hoping someone could answer my above question. I'd really appreciate it.
- See Dinosaur#Size for a discussion of this. Batmanand | Talk 10:28, 28 September 2006 (UTC)
- Try taking a look at the history of our List of dinosaur. Whoever did the most work or expansion on it will probably be able to give you a good source. - Mgm|(talk) 09:51, 29 September 2006 (UTC)
- S/he'll have even better luck with List of dinosaurs. :-) Anchoress 10:10, 29 September 2006 (UTC)
- Be careful, since the rarest dinosaurs are known by just a few bones. This means, of course, some of them may not actually exist, as was the case with Supersaurus. What is almost for sure is that the largest is a Brachiosaurid, my bet goes for Argentinosaurus, relatively well-known and damn huge ;)
Null-G Manufacturing?
Hey. What are the potential benefits of manufacturing in zero-g? I can't think of any besides having a high-quality vacuum and drastically reduced friction. The downsides seem to outweigh the ups. Am I missing something here? Thanks. --Demonesque 12:14, 28 September 2006 (UTC)
- Also, the sun doesn't go down. --Wjbeaty 00:59, 30 September 2006 (UTC)
- See Microgravity environment. Our article could use some fleshing out, but there are a few applications listed. TenOfAllTrades(talk) 12:21, 28 September 2006 (UTC)
- I always think of the balls and rollers of bearings as the prime example of what you'd want to manufacture without gravity. :-) —Bromskloss 12:53, 28 September 2006 (UTC)
- One thing they always said they'd make in orbit was metal foam - they'd melt aluminium, blow gas through it until it formed a foam, and then chill it so that it solidified in the foamed form. This would, the theory went, produce very strong lightweight components or aerospace and the like (the bones of birds are structure similarly, with a far greater proportion of voids than human bones, for example). It looks like material scientists have figured out how to make metal foam on Earth anyway, but there's still research that proposes doing it in orbit (presumably the orbital variety is better) - [8]. Middenface 13:59, 28 September 2006 (UTC)
- Well first of all: there are no ups or downs in space so 'the downsides cant outweigh the ups'. And secondly, in the case of ball bearings you can run them as fast as you like because in space, 'no one can hear them scream' 8-)--Light current 15:08, 28 September 2006 (UTC)
- And second of all? Or was that just 'first of one'. ?
- We dont use the 'of all' for anything after first in UK. Did you not notice the word 'secondly' I ve bolded it for you.--Light current 12:45, 29 September 2006 (UTC)
- One reason materials constructed in zero gravity might be stronger is that cristals can grow completely free and to any size you wish. Thus, you can create huge monoliths. DirkvdM 10:04, 29 September 2006 (UTC)
- And the use of such large monoliths would be to make more realistic versions of 2001/2010? --Light current 01:15, 30 September 2006 (UTC)
- All that said, at the present time nobody has come up with a serious business plan where products can be manufactured that are of sufficient value to pay for the very, very substantial costs of launching the raw materials, the factory to transform them into finished goods, and possibly the staff needed to run the factory.
- If you want a decent discussion of this, you could try Entering Space by Robert Zubrin. His conclusion is that space (that is, the empty bits between natural bodies like the Earth, Moon, Mars and asteroids) is like the terrestrial oceans, where the profits gained by harvesting it directly will be far outweighed by the value of the cargoes transported across it. --Robert Merkel 00:45, 30 September 2006 (UTC)
- I always liked the idea of making new real estate. (Invest in real estate, they've started making it again?) Just take a largish asteroid, surround it with mylar mirrors to focus sunlight on its surface, then when nice and melty you'll inflate it into a bubble using a large explosion, or perhaps just stick a few comets in the center before you start. This gives you a large, hollow shell made of glassy rock/iron. A thousand and one uses! Add some engines, steal Earth's water, then ferry it back to your own solar system. (Or steal earth's women... same thing.) Or just fill it with air and set up housing the inside surface. (A bit hard to build one of these on Earth!) --Wjbeaty 01:08, 30 September 2006 (UTC)
pathology
what does refrence range mean
- Do you mean reference range? Jack Daw 13:34, 28 September 2006 (UTC)
Lactose and blood glucose
Stupid question, but, in lactase persistent, is lactose digested into glucose and consequently raises blood glucose levels? Jack Daw 13:32, 28 September 2006 (UTC)
What do you mean by lactase persistent? In normal humans, lactose is digested in the gut to glucose and galactose in roughly equal amounts. The glucose and galactose are rapidly absorbed by the mucosa of the small intestine into the portal vein, mostly ending up in liver cells. The simple answer then is that half of lactose is rapidly digested to glucose and would certainly raise the blood glucose in a short time. A more complete answer is, as usual slightly more complicated. High galactose levels in liver cells can inhibit gluconeogenesis and actually impair liver glucose production in people who cannot efficiently metabolize galactose (e.g., galactosemia) or conduct gluconeogenesis (e.g., glycogen storage disease type I). alteripse 15:01, 28 September 2006 (UTC)
- From a quick Google, it appears lactase persistent is used to desribe adults who are not lactose intolerant or more specifically, adults who continue to produce lactase, as needed, in adulthood and are therefore able to consume products containing lactose such as cow's milk without issue Nil Einne 15:07, 28 September 2006 (UTC)
- Ok, then the answer is still applicable. If gut digestion is incomplete, less sugar will enter the circulation and metabolism but (except for the lesser amount) these processes are still the same regardless of the efficiency of digestion. alteripse 15:10, 28 September 2006 (UTC)
Cold and Flu viruses
I have been fighting a cold and/or flu bug for several weeks using OTC medicines such as nyquil.
What are the current viruses and do they respond to the OTC stuff?
Thank you
O'Dell from South Dakota
- Nothing you can get over the counter will have any effect whatever on a cold or flu virus. Products like nyquil just ease the symptoms a bit while your own immune system fights the virus. If you're still sick after several weeks, it's definately time to see a doctor. Middenface 16:08, 28 September 2006 (UTC)
- Agreed. It also could be something else, like a mold allergy. StuRat 17:21, 28 September 2006 (UTC)
- Is easing the symptoms ok for your body? The symptoms are usually there to try and make you better! i.e. fever, runny nose, coughing. Would it take longer to get better if you took away the "symptoms?" — X [Mac Davis] (SUPERDESK|Help me improve)17:33, 28 September 2006 (UTC)
- Well, some of the symptoms are there for the benefit of the virus. Coughing and sneezing, for instance, are very useful strategies for spreading lots of virus-laden droplets around. On the other hand, a moderate fever tends to be helpful because it stimulates certain classes of immune cells. On the third hand, suppressing symptoms can be problematic because it encourages some people to remain active and stress themselves further when they ought to be resting. That, in turn, leads to the fourth hand, on which one might note that you will be better able to fight infection if you're well-rested—which won't happen if you're up all night coughing and sneezing and sniffling. TenOfAllTrades(talk) 23:14, 28 September 2006 (UTC)
- Cure pathogens for hijacking the body's systems. STDs, poxes, and retroviruses! I am skeptical of the "get well better with sleep" idea. I need to see references argueing both sides before I'll believe that. It seems like sleep is a nice way to "get away" from your illness. Your immune system can battle while you don't have to feel it happening. — X [Mac Davis] (SUPERDESK|Help me improve)07:24, 29 September 2006 (UTC)
- Well I think it really depends on what you have. If it's a cold, I agree that rest doesn't make a difference. If I have a cold, I take Sudafed during the day, Neo Citran at night, and who knows how long the thing lasts lol; it's business as usual. If it's the flu, I definitely have to rest. If I continue as usual, with OTC remedies or without, I get sicker, ending up with bronchitis or something worse. And if it's something bacterial like strep throat, tonsillitis or a sinus infection, whether or not I rest depends on how I feel. I can fight the infection OK while working unless I'm really sick; if I feel bad enough that I need to rest, not resting will definitely make me sicker, longer. I agree with other posters, though; if it's been weeks without improvement, a doctor is the next order of business. Anchoress 07:37, 29 September 2006 (UTC)
- Cure pathogens for hijacking the body's systems. STDs, poxes, and retroviruses! I am skeptical of the "get well better with sleep" idea. I need to see references argueing both sides before I'll believe that. It seems like sleep is a nice way to "get away" from your illness. Your immune system can battle while you don't have to feel it happening. — X [Mac Davis] (SUPERDESK|Help me improve)07:24, 29 September 2006 (UTC)
- Well, some of the symptoms are there for the benefit of the virus. Coughing and sneezing, for instance, are very useful strategies for spreading lots of virus-laden droplets around. On the other hand, a moderate fever tends to be helpful because it stimulates certain classes of immune cells. On the third hand, suppressing symptoms can be problematic because it encourages some people to remain active and stress themselves further when they ought to be resting. That, in turn, leads to the fourth hand, on which one might note that you will be better able to fight infection if you're well-rested—which won't happen if you're up all night coughing and sneezing and sniffling. TenOfAllTrades(talk) 23:14, 28 September 2006 (UTC)
While there are a lot of people who would agree with Middenface, there is controversy. See Cold_virus#Zinc_Preparations. --Allen 18:45, 28 September 2006 (UTC)
- Several weeks, eh? If you went to a physician and got tested, you might find that you don't have the flu at all but some other viral disease which can have similar symptoms. Or, as StuRat suggested, not a viral caused illness at all. Sticking to the viral hypothesis, there are some scary sounding viral illnesses which are thought to be grossly underreported because everyone assumes they have the flu (unless they get so sick they wind up in the hospital). (There oughta be a list... no, probably not!) You probably didn't want to hear that, huh? If you're worried, I'd see that physician. If you're not worried, I wouldn't be surprised if you get better on your own, eventually.---CH 06:16, 29 September 2006 (UTC)
- Human Growth Hormone production increases during sleep. Apart from making you grow, it has important reparitive functions both in the structural sense and in stimulation of cellular immune functions. The article has a reasonable discussion of these. I also read somewhere that HGH pulses are larger during slow-wave (deep) sleep. If you're sleep deprived you have proportionally less slow wave sleep. It seems snoozing through the sniffles might be more then just an avoidance tactic. Mattopaedia 07:43, 30 September 2006 (UTC)
glutamine
I am interested in whether there's a relationship between the amino acid supplement glutamine and the neurotransmitter recently associated with depression called glutamate. Is there a way to email directly to any of the contributors to the glutamine article or to get a reference to someone who might know anything about this question?
Thanks,
Florence
Interestingly enough, both are amino acids. Glutamine has an amide functional group (in addition to the amine and carboxyl groups inherent to all AAs) whereas Glutamate has a carboxylic acid functional group. Glutamate is used to makke proteins, and is itself, as you mentioned, involved in neurotransmission. Glutamine is also incorporated into proteins, and is also used extensively in the body for storage and transfer of nitrogen. Check out the articles glutamic acid (same thing as glutamate, just at different pH) and glutamine for more information. They are "related" in that they have similar structures, with one (very important) difference in side chain functional groups, and they are readily interconverted in the body.Tuckerekcut 19:59, 28 September 2006 (UTC)
Florence, to contact any of the editors of an article, go to the article's history page (such as the one for Glutamine) and you will see a list of contributors. Click on the editor's name and you will go to their User page. On that page, you will see an "email this user" link. Click on it to send them an email. Not all users have this capability enabled, however. User:Zoe|(talk) 20:36, 28 September 2006 (UTC)
white oak tree
Two questions really. I wish to add about 18 inches of gravel and sand over 1/4 of the drip line of my very large oak, two feet out from the trunk. No lime, and not a driveway or heavy traffic area. Will this harm my oak? And I'm wondering if it is characteristic for a white oak to have many small shoots off the main branches(not the trunk)? Thank you so much in advance for your considerations.
- I don't see any harm in the gravel, per se, but, if it leads to people parking cars there that leak oil and other fluids, that could have a detrimental effect on the oak. StuRat 00:41, 29 September 2006 (UTC)
Speed of molecules
At the same STP, a block of wood would has its molecules moving slower than a bucket of water. Apart from differences in state, what exactly does that depend on? Is it the masses, densities, and pressures?
- a whole bunch of things. the amount of interaction each molecule has with its neighbours is one of the most important factors, which will decide the state the compound is in at a given temperature. eg. water has a strong network of hydrogen bonds, which helps slow the vibrations of the water, forming a liquid, boiling point 100 deg C. but weaken those bonds by changing H2O to H2S, and you get a boiling point of -60 deg C. in wood's case, the cellulose that its made of has multiple hydrogen bonds to neighbouring chains of cellulose, resulting in a very strong (and solid) material. Xcomradex 22:02, 28 September 2006 (UTC)
Increasing the volume of my ejaculations?
Is it true that regularly eating asparagus increases the volume of semen produced? --81.79.28.234 23:02, 28 September 2006 (UTC)
- No, but try eating a high protein diet, that should do it. StuRat 00:21, 29 September 2006 (UTC)
- Why should it?--Light current 01:06, 29 September 2006 (UTC)
- Sperm are largely composed of protein. Men's bodies therefore produce sperm when they have excess protein. Enough seminal fluid is also produced to accompany the sperm. StuRat 13:08, 29 September 2006 (UTC)
- There is an interesting story behind this. William Kellogg, famous as the founder of the breakfast cereal company, came up with the idea of cereal as a low protein alternative to the traditional bacon and egg breakfast, specifically to keep men from being "horny bastards", due to what he considered to be "excess sperm production". It does work; try a low protein diet for a week and see what it does to your sex drive, then try a high protein diet for a week. StuRat 17:38, 29 September 2006 (UTC)
Wouldn't that make your semen smelly? Maybe just urine. — X [Mac Davis] (SUPERDESK|Help me improve)01:15, 29 September 2006 (UTC)
- It does tend to make your urine smell like fried bacon, which is really the smell of protein broken down to amino acids. StuRat 17:45, 29 September 2006 (UTC)
I've heard that eating Lecithin does this. (Haven't tried it though.) Or zinc and lecithin? - Rainwarrior 04:24, 29 September 2006 (UTC)
- I don't see why zinc (dose?) would do anything more than negligible. Don't worry about volume of ejaculate, that's silly. Quanity doesn't matter anyway—there's enough sperm in there to impregnate a signficant portion of the women in the world. — X [Mac Davis] (SUPERDESK|Help me improve)07:19, 29 September 2006 (UTC)
- The more 'spunk' that comes out in one shot, the better it feels. Trust me - my spunk levels vary. --Kurt Shaped Box 20:47, 29 September 2006 (UTC)
- So, you are not the kinda guy that could wait two days. — X [Mac Davis] (SUPERDESK|Help me improve)08:10, 1 October 2006 (UTC)
- So, would you rather have one almighty 'shot', or about 25 slightly less powerful ones? I remember a comedy programme once where... no better not! 8-)--Light current 20:52, 29 September 2006 (UTC)
- The more 'spunk' that comes out in one shot, the better it feels. Trust me - my spunk levels vary. --Kurt Shaped Box 20:47, 29 September 2006 (UTC)
- 25 that could blow the shower door off its hinges would be nice. --Kurt Shaped Box 21:16, 29 September 2006 (UTC)
- Of course it would! But you have to be realistic here and realise that the human penis is NOT a cannon! --Light current 21:32, 29 September 2006 (UTC)
- Of course not! It's more like an ICBM - in my pants. --Kurt Shaped Box 21:47, 29 September 2006 (UTC)
- Well, as your brain gets bigger, your libido will get smaller. Thats for sure! 8-)--Light current 21:55, 29 September 2006 (UTC)
- Of course not! It's more like an ICBM - in my pants. --Kurt Shaped Box 21:47, 29 September 2006 (UTC)
- Increasing the volume of some fluids could have a detrimental effect on oaks|oats. -- DLL .. T 20:43, 29 September 2006 (UTC)
- Reading these answers made coffee spew out my nose!
- Was that an ejaculATION from an unusual orifice, or just you choking on your biscuit? 8-)--Light current 22:23, 30 September 2006 (UTC)
NMR Spectroscopy
I'm not going to lie. This is part of a homework problem. But I can't figure it out on my own (notes, textbook, and wikipedia aside), so i'm going to ask conceptually. If you would like to respond conceptually, I would be more than happy with that.
Given an NMR with a specific field strength (H0), an isotope with a given magneto-gyric ratio (I've calculated the Lamar frequency (vo) from these values if I need it), how does one calculate the number of Hz in 1 ppm for the given nucleus?
Thanks --Michael 23:45, 28 September 2006 (UTC)
There's a big "theory" section in the Nuclear magnetic resonance page, and the intro to the NMR spectroscopy page has some info about unit conversion. DMacks 01:35, 29 September 2006 (UTC)
how about this:
- ν = γB0/2π
and fyi the symbol for field strength that i am used to is B0, in T. γ is the magnetogyric ratio, in radians T-1 s-1. ν the frequency for that nuclei at that field strength, in Hz. getting to 1 ppm from there should be cake. Xcomradex 04:12, 29 September 2006 (UTC)
- lolz. Lamar[9] want's to know how you calculated his frequency and he says that perhaps you meant the Larmor frequency.--Deglr6328 13:49, 29 September 2006 (UTC)
Ringtones
How to send ringtones online.i.e from net to mobile using a sms sending site. plz can any one can tell me the format because i have got he ringtone notes with me?
- My information is a few years old but I'm pretty sure there's no free way to do this. There's probably a few websites that host some sort of "ring tone maker" online program, that in turn will send it to your (if supported) cellphone in a format that it will understand, for a fee. If you have a ringtone composer on your phone, search for a website that can convert to and from ringtone notes for different types of phones. Those sites often instruct you how to easily input the ringtone directly into your phone as well. freshofftheufoΓΛĿЌ 05:23, 29 September 2006 (UTC)
September 29
magnetic north
Please explain in a concise sentence the diffrence between magnetic north and true north. Thank you! :-) — Preceding unsigned comment added by 24.241.22.219 (talk • contribs)
- Magnetic north is where compasses point; true north is on the axis of the Earth's rotation. --Allen 00:51, 29 September 2006 (UTC)
- See this for a more detailed explaination. And don't forget to sign your comments with four tildes: ~~~~ Dar-Ape (talk) 00:57, 29 September 2006 (UTC)
leptin???
can i buy this anywhere??? my doctor thinks this would be a good thing for me to take but how to buy??? thanks bruce woolley phone phone number deleted
email address deleted
- You know what would be cool - a website that is sort of a wiki and sort of an encyclopedia. I know, we could call it something like Wikipedia. And then, there could be articles on it about things like leptin and people can describe what it is and include links in the article to people who hawk garbage like leptin as a miracle weight loss pill. --Kainaw (talk) 02:10, 29 September 2006 (UTC)
- Ah, but if someone started up this crazy "Wikipedia" you speak of, then it would compete with Wikipedia and we'd be forced to destroy it like all the others. Melchoir 02:24, 29 September 2006 (UTC)
- what about this one [10]? Xcomradex 04:14, 29 September 2006 (UTC)
- Black Ops isn't interested in eliminating Uncyclopedia; we have our reasons. Melchoir 04:34, 29 September 2006 (UTC)
- i for one find its coverage far more accurate (eg. [11], its definetely this first place i look for actual info. Xcomradex 04:49, 29 September 2006 (UTC)
- Ah, but we've got a much more comprehensive catalog of Pokemon. Melchoir 04:59, 29 September 2006 (UTC)
- C'mon guys, try to make an effort to be at least pretend to be nice to the new folks. – ClockworkSoul 06:15, 29 September 2006 (UTC)
- We even have a nice template I created for this use now! Template:Refq If not for us, for the newbies. — X [Mac Davis] (SUPERDESK|Help me improve)07:14, 29 September 2006 (UTC)
- C'mon guys, try to make an effort to be at least pretend to be nice to the new folks. – ClockworkSoul 06:15, 29 September 2006 (UTC)
If you have a weight problem, the chances are at least 99.99% that you already have more leptin than most of us and do not need more. Leptin is produced by fat tissue and serves as a signal to the brain that you have plenty of fat (a different signal than looking in the mirror). In an ideal world, this signal would result in less eating. However, the leptin signal, like looking in the mirror, often does not make a large change in eating behaviors. Injecting more leptin might be less effective than posting a picture of yourself on your refrigerator as a signal to your brain that you have more fat than you need and should eat less. When leptin was first discovered in the early 1990s it was found that mice who were genetically deficient were obese and if they were given leptin injections they lost weight; as you can imagine, pharmaceutical companies invested enormous amounts of money into leptin research until it was clear that leptin deficiency is such a rare cause of human obesity that less than 10 cases have been found and that leptin injections do nothing for people who already have plenty of leptin (the rest of us). alteripse 07:18, 29 September 2006 (UTC)
Rib Removal: 11th and 12th: Why?
Is removal of ribs 11 and 12 possible and why would one do so? What is this procedure called?70.242.47.227 03:22, 29 September 2006 (UTC)Dominique
- I can't find the name of the procedure, but it may help you accomplish a frontbend. freshofftheufoΓΛĿЌ 05:16, 29 September 2006 (UTC)
I believe it helps women or gay men get the "wasp waist" shape, which is considered by many to be beautiful. StuRat 08:49, 29 September 2006 (UTC)
- One hopes that this is a (bad) joke.--Deglr6328 13:41, 29 September 2006 (UTC)
- I wish it was. StuRat 17:22, 29 September 2006 (UTC)
Prince (musician) was meant to have had some ribs removed so that he could bend down and suck his own penis more easily. --84.65.99.86 11:35, 29 September 2006 (UTC)
- People say the same thing about Marilyn Manson, but he says it's bullshit. freshofftheufoΓΛĿЌ 11:42, 29 September 2006 (UTC)
Read the Snopes page. It's all just urban legend. – ClockworkSoul 16:40, 29 September 2006 (UTC)
- A rib resection of the 11th and/or 12th ribs is sometimes carried out to allow surgical access for a nephrectomy, either due to renal cancer or because the patient is donating it. (The first rib may be resected in thoracic outlet syndrome.)Mmoneypenny 18:28, 30 September 2006 (UTC)
Albratrosses and gulls
How closely related are they?
- They are both of class aves. See albatross and gulls. freshofftheufoΓΛĿЌ 11:43, 29 September 2006 (UTC)
- The Sibley-Ahlquist taxonomy lumps them together in a new, greatly enlarged order Ciconiiformes. "New" here apparently means that the old term has been given a new meaning. --LambiamTalk 13:30, 29 September 2006 (UTC)
- Interesting! Note to anyone still reading: The new definition is not uncontested. freshofftheufoΓΛĿЌ 13:32, 30 September 2006 (UTC)
Daleks vs Magpies vs Gulls
3 vintage RD topics, all fighting it out, who wins?--172.163.55.148 12:34, 29 September 2006 (UTC)
- the bagel whips them all.Xcomradex 13:15, 29 September 2006 (UTC)
- Moles are kings of the undergorund 8-)--Light current 16:32, 29 September 2006 (UTC)
- Daleks, really? Must be before my time. Melchoir 23:05, 29 September 2006 (UTC)
- Your time doesn't stretch back more than a few days I guess? Daleks even flying ones have appeared on television pretty recently and we have some great articles on those episodes and a featured article called Dalek. - Mgm|(talk) 23:19, 30 September 2006 (UTC)
- Yes yes, but on the reference desk? Melchoir 18:01, 1 October 2006 (UTC)
- Your time doesn't stretch back more than a few days I guess? Daleks even flying ones have appeared on television pretty recently and we have some great articles on those episodes and a featured article called Dalek. - Mgm|(talk) 23:19, 30 September 2006 (UTC)
- Well, they all fly... Ah, magpies can pick things up, even gulls, and Daleks can't, so... -- Fuzzyeric 00:26, 30 September 2006 (UTC)
- Who wins? Easy. The first one to travel faster than light wins! Mattopaedia 09:06, 30 September 2006 (UTC)
- if you want the gull to win, just drop your sandwich Mattopaedia 09:09, 30 September 2006 (UTC)
- By my perception, Daleks can't fly. Daleks have trouble with stairs. However, there's something to be said for death rays. --Amanaplanacanalpanama 22:49, 30 September 2006 (UTC)
- Daleks most certainly can fly. Watch any of the Dalek storylines in the two most recent series of Dr Who for confirmation. We also see one flying up a flight of stairs in the Sylvester McCoy era Dalek episodes... --Kurt Shaped Box 22:54, 30 September 2006 (UTC)
Whoever wins - we lose. --Kurt Shaped Box 10:15, 30 September 2006 (UTC)
- Kurt Shaped Box is right on the money. They can all fly, but unless the doctor is around magpies and gulls would be exterminated. Daleks win, we lose. - Mgm|(talk) 23:19, 30 September 2006 (UTC)
Nuclear density
Is it true that basically all (atomic) nuclei have roughly the same density? I heard it once from a fellow undergrad physicist, and it sounded strange but plausible. I could go off an calculate the densities of every element's nucleus, but to be frank I cannot be bothered if someone can give me an answer here quicker. Thanks in advance Batmanand | Talk 12:50, 29 September 2006 (UTC)
- Since nuleii are only composed of neutrons and protons then if the densities of neutrons and protons are te same, then the idea is plausible. Looks like they are the same density from our pages on them. --Light current 16:42, 29 September 2006 (UTC)
- If you use the formula provided in Atomic nucleus#Nucleus size for calculating the densities, you will come up with almost exactly the same value for each element, simply because, according to that formula, the size is proportional to the mass number, and the mass of a nucleus is an almost constant factor times the mass number. I have no idea how the size of a nucleus would actually be measured, and how good an approximation that formula is. --LambiamTalk 16:58, 29 September 2006 (UTC)
- Yes, as far as I remeber from my own undergrad time, that's correct: the density of nuclear matter is roughly idepend of the atomic species. I'm not sure about the reason but I'd venture the guess that it has to do with asymptotic freedom, which makes the strong force increase with distance and hence keeps the quarks in the nucleons together, but not too tightly as on these minute distances, the force becomes weak. Also, the Yukawa coupling keeps the nucleons quite tighly together. On the other hand, however: The nucleons are not in close-packing, but organized as described by the nuclear shell model. It is also quite difficult to define the radius of a nucleus (or even of a nucleon) and hence to give an exact meaning to the term "nuclear denisty". If you define the diameter of, say, a proton, via the Fourier transform of the result of a scattering experiment in a collider, you get differet results whether you scatter off accelerated electrons or protons. Hence, there is an "electromagnetic" and a "strong force" radius. Also, I now remeber that some nucleii do deviate from the constant density rule quite noticeably, and rather recent research associates this to the idea of nuclear halos, i.e. hollow shells of nuclear matter surrounding the core part and leaving some empty space between inner nuclear core and halo. Now, as I pulled all I remember from the back of my head, your "homework": Research the details of this stuff and expand it to our nuclear halo article which has only two lines su far. Simon A. 21:57, 29 September 2006 (UTC)
Nuclear Fusion
If one of the goals of nuclear fusion is to create more energy than is consumed, then how is it possible for fusion to ever be a reality? Doesn't that violate some laws of thermodynamics or something? A Clown in the Dark 15:35, 29 September 2006 (UTC)
- No, the idea is to convert energy from one type to another, not to create it. There is an energy barrier though, which may be what you mean.In that case, if the energy in is less than the energy out, all it means is that the fusion reaction is exothermic, with more of the energy in the nuclei being converted to heat than the amount of energy required to allow the reaction to take place. No enegry is created, just some converted. 80.169.64.22 15:53, 29 September 2006 (UTC)
- In nuclear fusion mass is converted to energy according to Einstein's famous equation E = mc². The law of thermodynamics commonly called "conservation of energy" might more appropriately be called "conservation of mass-energy" or even just "conservation of mass" (since energy E has mass m = E/c²). --LambiamTalk 16:44, 29 September 2006 (UTC)
- Have you read the article on fusion power? It should answer most of your questions about how nuclear fusion would work as an energy source. — QuantumEleven 17:15, 29 September 2006 (UTC)
- Hah! I knew stars were just lumps of burning coal. Clarityfiend 17:26, 29 September 2006 (UTC)
- Seriously though, a team at UCLA has initiated fusion using a tabletop machine, though it still takes more energy than it produces ([12]). Clarityfiend 01:02, 30 September 2006 (UTC)
- Size matters here. The bigger it gets the easier it will be to maintain the necessary temperatures to keep the fusion going. The present setups are just for experimentation. Building the first productive one will cost loads, so it makes sense to first make sure you build something that will work. Alas we don't quite have the time to wait until we have the knowledge to build the 'perfect' reactor at the first attempt. Also alas, the money spent on this is a fraction of the money spent on fossil fuels (largely finding them, I suppose). DirkvdM 11:42, 30 September 2006 (UTC)
Case of the Missing Printhead
From where might I get a Canon S500 printhead? I bought a printer off eBay that said the printhead wasn't included - I thought printheads where part of the cartridge but I guess it depends on the printer. I can use the printer until I locate the printhead. Any idea why someone is selling printers in which the printhead has been removed? --Username132 (talk) 15:54, 29 September 2006 (UTC)
- To have a joke on unsuspecting people? 8-)--Light current 16:39, 29 September 2006 (UTC)
- As others have stated you can have buy printheads new (if they're still available) although it would make little sense given the cost. However it's easily possible people would have some around for whatever reason (e.g. printer died print heads okay). Since the person clearly specified it didn't have a printhead, I would suggest it's username's fault for not properly researching before buying. Nil Einne 04:01, 30 September 2006 (UTC)
It was probably damaged or lost. Then, they found they couldn't buy one anywhere, so they decided to sell the printer. StuRat 17:08, 29 September 2006 (UTC)
I just did a Google search on "canon s500 print head" and found many sites that will sell you one, so you're in luck ! StuRat 17:13, 29 September 2006 (UTC)
I had the print head die on a Canon S300 a while back (at least with the 300 the head was a separate unit, into which you'd plug the ink carts). The cost for a new print head was such a large proportion of the cost of the entire printer (I think a new head was about £50 and the printer had only cost about £80) that the only smart thing to do was dump the entire printer and buy a cheap laserprinter (it was a pretty duff printer to begin with, with slow, pretty crummy prints and a tendency for the heads to dry out). -- Finlay McWalter | Talk 22:47, 29 September 2006 (UTC)
Bathing after meals
Ppl use to say not to bath right after meals, why? is there any scientific support?
- Swimming is an extremly physical sport, recquiring a lot of blood supply to alomost all muscles, which diverts it from the gut, which as a result recieves minimal blood supply, so if it has more food that it can cope with given the energy provided (the oxygen in blood is recquired for energy release) then it will jettison some of the food. I.e. you'll throw up. Philc TECI 18:45, 29 September 2006 (UTC)
- I never thought of that... now it all makes sense! --Russoc4 19:04, 29 September 2006 (UTC)
- It makes sense, but it doesn't actually happen... I've never seen someone throw up just because they ate dinner and then went swimming, or even swam in a raceXcfrommars 19:10, 29 September 2006 (UTC)
- I've thrown up beofre for over exerting myself over a meal, and I know other people have (though not specifically swimming, but the principles the same), you will feel a lot of butterflies prior to it happening, as this is caused by lack of blood supply to a full stomach aswell. Philc TECI 20:48, 29 September 2006 (UTC)
- Before taking martial arts classes we were told not to eat a large meal, as your digestive system will take energy to digest it, and there would be a possibility that you would pass out, since there isn't enough oxygen/energy to go around. Of course, we were also told to eat SOMETHING (like an apple) to provide us with some energy for practice. --Bennybp 21:29, 29 September 2006 (UTC)
- It makes sense, but it doesn't actually happen... I've never seen someone throw up just because they ate dinner and then went swimming, or even swam in a raceXcfrommars 19:10, 29 September 2006 (UTC)
- I never thought of that... now it all makes sense! --Russoc4 19:04, 29 September 2006 (UTC)
- The reason they suggest it is that you are likely to experience painful cramps when exerting yourself shortly after eating a meal (for the blood supply issue stated above) and if you panic as a result, and are in deep water, you could potentially drown. --Jmeden2000 19:55, 29 September 2006 (UTC)
- Swimming was sometimes thought risky after a meal, but bathing was ok, especially if the person was a messy eater.Feed the baby, then bathe the baby. Then bathe the parent. Edison 20:26, 29 September 2006 (UTC)
- Hmm, maybe that tryptophan and energy required for digestion combined with the warm, soothing water would cause undesirable effects. Hyenaste (tell) 21:36, 29 September 2006 (UTC)
- Swimming just after eating is likely to cause side stitch. —Keenan Pepper 21:52, 29 September 2006 (UTC)
- Try reading what Snopes has to say about this urban legend: [[13]] Rmhermen 00:38, 30 September 2006 (UTC)
- Do or do not. There is no try. freshofftheufoΓΛĿЌ 13:22, 30 September 2006 (UTC)
- Snopes is right, it's not dangerous, but it does hurt when it happens. —Keenan Pepper 17:01, 30 September 2006 (UTC)
How much pot is too much?
Is a 20-spliff-a-day habit bad for you in the long term? --84.66.42.217 22:13, 29 September 2006 (UTC)
- See our article on Cannabis, but note our content disclaimers. JBKramer 22:14, 29 September 2006 (UTC)
I think everybody knows what it does. Smoking it by itself does not cause any physiological harm, however it affects your thinking and judgement, as well as making you a lazy slob. — X [Mac Davis] (SUPERDESK|Help me improve)23:16, 29 September 2006 (UTC)
- Will your buddies really want to hang out with a lazy, fat, permanently-stoned hippy? I dunno - maybe all your buddies *are* lazy, fat, permanently-stoned hippies? ;) --Kurt Shaped Box 23:37, 29 September 2006 (UTC)
You might get lung cancer one day? Though if you smoke 20 spliffs a day, you probably don't care about that. :) --Kurt Shaped Box 23:33, 29 September 2006 (UTC)
- Yes, smoking pretty much anything will cause lung cancer, since lungs aren't made to handle much inhaled smoke. StuRat 00:12, 30 September 2006 (UTC)
- I'd say that smoking pretty much anything can cause lung cancer. Not to mention mouth cancer and throat cancer. It won't necessarily happen to you, but then again it just might. It's a huge risk, not worth taking. JackofOz 00:17, 30 September 2006 (UTC)
- I have never personally researched it. I bet there is a strong correlation but does that imply causation? — X [Mac Davis] (SUPERDESK|Help me improve)02:32, 30 September 2006 (UTC)
- In this case, yes. One obvious requirement is that the "cause" occurs prior to the "effect", which is true here. Another requirement is that there be a mechanism for the cause to produce the effect. The presence of numerous carcinogen chemicals in tobacco smoke is a known mechanism for causing cancer. There is also a lack of alternative explanations for the correlation. StuRat 05:08, 30 September 2006 (UTC)
- How do the carcinogens work than? I would guess we don't know that well, since we don't know where cancer comes from. I am pretty much skeptical because we seem to be missing a vital link in the chain of events. It isn't just smoke>cancer. — X [Mac Davis] (SUPERDESK|Help me improve)08:14, 1 October 2006 (UTC)
- I think people put way too much weight on the few people who never get cancer as a result of their smoking, for the simple reason that it's in their genes. Actually, this comment leads into loads of instigations involving big tobacco companies and greedy politicians, so I'll revoke it until I'm ready to push my opinions about all that other stuff too. freshofftheufoΓΛĿЌ 13:15, 30 September 2006 (UTC)
- In this case, yes. One obvious requirement is that the "cause" occurs prior to the "effect", which is true here. Another requirement is that there be a mechanism for the cause to produce the effect. The presence of numerous carcinogen chemicals in tobacco smoke is a known mechanism for causing cancer. There is also a lack of alternative explanations for the correlation. StuRat 05:08, 30 September 2006 (UTC)
Yeah kids - don't smoke. It's a bad habit and it's not big, hard or clever (says I, with the 30th cigarette of the day danging from my mouth as I type). --Kurt Shaped Box 00:24, 30 September 2006 (UTC)
By spliff do you mean a joint that is a mix of cannabis and tobacco? If so then the known health risks of tobacco use make it bad. If it is just weed, from personal experience it would prob make you slow in the head for awhile. Depends on the potency of the weed. I say go ahead. just dont try and go anywhere. Sosobra 03:32, 30 September 2006 (UTC)
- Cannabis smoke has been found to be worse for you than tobacco, cancer-wise. This might be because cannabis smokers tend to inhale deeper. —Pengo talk · contribs 11:10, 30 September 2006 (UTC)
- But nobody "chain smokes" cannabis like people do with tobacco, so the total effect on the lungs is far less. StuRat 12:14, 30 September 2006 (UTC)
- There are a number of obvious reasons, and inhalation depth is the least easy to prove. Cannabis is often in a much more potent form than any tobacco you can buy in the western world, which can mean a few things, mainly additional (unhealthy) chemicals. There is also no filter on (most) people's spliffs, so they're getting the full blast from the smoke. freshofftheufoΓΛĿЌ 13:19, 30 September 2006 (UTC)
- But tobacco contains a host of chemicals added by the cig companies to make them more addictive, as well as all the unintended chemicals, like pesticides. Since tobacco additives are completely unregulated and unreported, the public doesn't really know what's in them. StuRat 23:53, 30 September 2006 (UTC)
- Something that I wish I had known before know. What a pethetic thing this is we call "democracy". freshofftheufoΓΛĿЌ 06:19, 1 October 2006 (UTC)
- It is incredible how many people have missed the essential part of the question. Everyone jumps on the bandwagon of indoctrination saying that "smoking is bad for you". Mac Davis even makes the classic mistake of saying that "everybody knows that". But the question was how bad it is to smoke 20 spliffs. That is not only an essential aspect that is usually missed, but it was even actually the question here. And still everyone misses it (except Kurt). That said, I can't answer the question either, in part because I don't know what kind of weed it is (there is a huge variation), how big the joints are and if yo mix with tobacco. Buit if you smoke big joints fille dpurely with Dutch quality weed, never mind your lungs. You're messing up your brain. Bad lungs will make you live shorter. Bad brains will make you miss what life you have, which is much more important 9another important aspect that is often missed). DirkvdM 18:59, 2 October 2006 (UTC)
Largest sensor size in compact digital cameras
Are there any compact digital cameras available which use (physically) large sensors? In point and shoot camera it says that "The sensor used in these types of cameras tends to be smaller than their SLR counterparts", but does not mention any exceptions. Sony Cyber-shot DSC-R1 mentions the use of an APS-C sensor in this 2005 camera, and says it's the first time it has been used in a non-DSLR camera. Have any other non-SLR cameras used this sensor since? I'm interested in any information on digital cameras which could legitimately be called compact (ie. a couple of inches thick when closed, not a giant like the Sony above; I'm not counting the Leica M8 as a compact either!) which use a larger than normal sensor. Digital photography lists a number of sensor sizes between 1/2" and 4/3", but doesn't give any examples of where they are used. Thanks. CarelessHair 23:45, 29 September 2006 (UTC)
- See this blog post for a good discussion about why this isn't done. --203.214.55.189 02:04, 30 September 2006 (UTC)
top of the world?
Answered
Hello there ,
my question is so simple ? where is the top of the world is it the north pole or the souh or nither of them ?
Ahmed salah
thanks of your time
- Neither. StuRat 00:07, 30 September 2006 (UTC)
- In space, no-one has the right to tell you which way is 'up'. --Kurt Shaped Box 00:10, 30 September 2006 (UTC)
- You could say Mount Everest, though. If up is away from the center of the earth, the peak of Mount Everest would be the most up, and perhaps you could call that the "top". --Allen 00:18, 30 September 2006 (UTC)
Except that Everest isn't the farthest point from the center of the Earth. The equatorial bulge means that Chimborazo (volcano) is the farthest from the center, according to Extreme points of the world. Rmhermen 00:30, 30 September 2006 (UTC)
- Thanks, Rmhermen; that's both a good fact and a good article to know about. It's cool to see that the land and sea poles of inaccessibility are almost exactly the same distance away from their respective land/sea borders. --Allen 02:10, 30 September 2006 (UTC)
- What Kurt is trying to tell you is that "up" or "down", in the context of maps of the world, is arbitrary. There's no reason why you couldn't draw perfectly accurate maps which have the south pole at the top, or any other point on the earth (though for navigation purposes it makes good sense to put the poles at the top).
- Consider that you're floating in space on the edge of the solar system. You get out your telescope and look at the Earth. Relative to your current orientation, everything looks normal, with the north pole at the top of your view.
- Then you fire the retrorockets in your space ship a little, so you spin 180 degrees. Now the south pole is at the top. Then you spin yourself another 90 degrees, and a point on the equator looks like it's at the top from your point of view!
- Why put the northern hemisphere at tht top of maps? It's probably got a lot to do with the fact that the societies responsible for the first "conventional" maps (as distinct from the string maps of the Polynesians) were all based in the northern hemisphere. --Robert Merkel 00:56, 30 September 2006 (UTC)
- In fact, see the article Reversed map for world maps with south (or something else than north) on top. The very term "reversed" implies a non-neutral point of view, of course. --LambiamTalk 01:03, 30 September 2006 (UTC)
- Actually one thing that the article fails to mention is that the Greenwich meridian is of course completely. arbitary. Others such as the Paris meridian were used historically. While it makes sense to have the international dateline dissecting primarily in the ocean, it could of course be the Atlantic rather then the Pacific. One advantage with having north as up is that east is left. Together with the (completely arbitary) cartesian coordinate system this means east is positive. It makes sense that east is positive due to the rotation of the earth (think why east timezones are positive)... Nil Einne 03:31, 30 September 2006 (UTC)
- One advantage with having north as up is that east is right. Peter Grey 15:27, 30 September 2006 (UTC)
- Note that the North\South direction we use in maps is used because that's the axis which the Earth spins around itself. ☢ Ҡi∊ff⌇↯ 04:03, 30 September 2006 (UTC)
I suppose the "top" could be the point on Earth furthest from the Earth's orbital plane about the Sun, as well. This would put it on the Arctic circle or Antarctic Circle, at a different point on the circle depending on the time of day. StuRat 04:59, 30 September 2006 (UTC)
Magnetic North and South Poles could also be used. StuRat 04:59, 30 September 2006 (UTC)
- If you were to place the Earth on a large enough table, it might have an "up". —Pengo talk · contribs 11:05, 30 September 2006 (UTC)
- Or, alternatively, if you put the Earth through a vertical wind-tunnel test, it would presumably orientate itself into some sort of "upwards" position. freshofftheufoΓΛĿЌ 13:08, 30 September 2006 (UTC)
- How do you define "vertical" and "up" for your wind tunnel? --LambiamTalk 14:57, 30 September 2006 (UTC)
- Or, alternatively, if you put the Earth through a vertical wind-tunnel test, it would presumably orientate itself into some sort of "upwards" position. freshofftheufoΓΛĿЌ 13:08, 30 September 2006 (UTC)
- If you were to place the Earth on a large enough table, it might have an "up". —Pengo talk · contribs 11:05, 30 September 2006 (UTC)
- If the guy has the technology and power to build a planetary wind tunnel, I wouldn't really argue with him, heh ☢ Ҡi∊ff⌇↯ 18:18, 30 September 2006 (UTC)
- True! But for the record, since we always head into the wind, and cut through the wind head-first, I would choose the head as the top of my planet. freshofftheufoΓΛĿЌ 06:18, 1 October 2006 (UTC)
- When I'm in a really good mood, the top of the world is wherever I am. DirkvdM 19:30, 1 October 2006 (UTC)
September 30
Singing parrots?
Is the budgerigar the only member of the parrot family that (to human ears) can be described as having a 'song'? All other parrot species I've ever encountered tend to either 'tweet', 'screech' or 'squawk' - nothing melodic there at all. Anyone know of any other 'singing parrots'? --Kurt Shaped Box 00:36, 30 September 2006 (UTC)
- The genus name Melopsittacus, or "singing parrot" (melo- as in "melody"), suggests that this is indeed something particular to budgies. --LambiamTalk 01:15, 30 September 2006 (UTC)
- Answering my own question here - but now I come to think of it, I seem to remember reading that the lineolated parakeet has quite a melodic song too. Quite strange little birds, they are - they rarely fly (preferring to walk, climb and swing), hang upside down from their perches like a bat when sleeping and have been described as the 'most even-tempered of all parrots'. Been thiking of getting a linnie for a while now. --Kurt Shaped Box 02:11, 30 September 2006 (UTC)
- I guess it also depends on what you call a song. I personally feel the kakapo's sound is melodic enough [14] to be called a song but some people might not agree... Nil Einne 03:45, 30 September 2006 (UTC)
- The basic rule is, the smaller the bird, the greater need to sing to indicate its presence. I can't think of anything bigger than a currawong that could be said to have a song. A few ground dwelling tropical birds possibly, but not parrots. Still, they're good mimics, so you could teach them.--Shantavira 06:19, 30 September 2006 (UTC)
- Parrots tend to pick up swear words when around me. I used to have an african grey parrot that would loudly exclaim "OH FOR FUCK'S SAKE!" and "JESUS H. FUCK!" (my catch-all curses for when something goes wrong and I'm letting off steam in private). He was full of expletives when I got him too - my parrot would call me a cunt just about every day... ;) --Kurt Shaped Box 10:57, 30 September 2006 (UTC)
- I've heard Amazon parrots sing -- but they were mimicking human songs. And very off-key, I might add. Has anyone heard a parrot mimick a song with good pitch? --Ginkgo100 talk · e@ 17:31, 30 September 2006 (UTC)
- Ooh. That sounds quite nice. --Kurt Shaped Box 10:19, 30 September 2006 (UTC)
- But humming birds don't sing. They hum, which can get annoying after some time. Shoo shoo, little bird. --LambiamTalk 10:35, 30 September 2006 (UTC)
- Completely untrue that parrots cannot sing. I was in a petshop where a very clever parrot was a longtime resident. The lights went off due to a momentary power failure, and the bird sang "Rockabye baby, in the treetop" all the way through. No shit. I expect someone had trained him to do it, but it was quite understandable and tuneful. So this myth is BUSTED.Edison 17:48, 30 September 2006 (UTC)
- But that's not "singing" as in birdsong; that's mimicking humans. --jpgordon∇∆∇∆ 19:52, 30 September 2006 (UTC)
- It did answer my question about parrots mimicking song in tune, though. --Ginkgo100 talk · e@ 23:41, 30 September 2006 (UTC)
- But that's not "singing" as in birdsong; that's mimicking humans. --jpgordon∇∆∇∆ 19:52, 30 September 2006 (UTC)
- Completely untrue that parrots cannot sing. I was in a petshop where a very clever parrot was a longtime resident. The lights went off due to a momentary power failure, and the bird sang "Rockabye baby, in the treetop" all the way through. No shit. I expect someone had trained him to do it, but it was quite understandable and tuneful. So this myth is BUSTED.Edison 17:48, 30 September 2006 (UTC)
STAFFING A POLITICAL LEADERS OFFICE
I am forming a new Political Party in my country.
Please advise me of the staff I will need to run my political officeie Personal Assisstant, Researchers, Press Secretary.
The basic responsibilities of the functions will be greatly appreciated
Regards.
- I suggest you try the humanities section instead. But bear in mind the kind of question your asking is extremely complicated and I suggest you do a large amount of research before you ask for help. It will also likely vary a lot depending on the laws and political system of your country so I would also recommend you let people know where you from. Personally, I would suggest you get involved in another political party first. The experience you gain will likely be more valuable then all the time you spend researching. Choose one that is most compatible with your values and beliefs. Oh and finally, please don't use all caps, even for the subject line. 03:23, 30 September 2006 (UTC)
- for personal assistant i recommend Monica Lewinsky; for researcher i recommend Al Gore, and for press secretary you can't go past Mohammed Saeed al-Sahaf. Xcomradex 03:57, 30 September 2006 (UTC)
- You will need an Instructions Interpreter: someone who can interpret simple instructions to you, such as the one at the top of the page that states: Sign your question. Then you will need a Science Adviser: someone who can explain to you that this question does not belong on a Science reference desk. Further an official Party Historian to document the glorious deeds of the Leader, a Tax Consultant to make sure the money you honestly deserve as a Leader is not greedily taken away, and a Lawyer for explaining why the Tax Consultant's constructions are legal. Then a Ghost Writer for writing your rousing speeches, answering your fan mail, and penning down your autobiography, and ... you're all set! Simple, isn't it? Congratulations on your new career. --LambiamTalk 14:55, 30 September 2006 (UTC)
Farrukh (nursing and heart transplants)
Answered
When was the nursing system start? when was the first heart trasnplant?
- For nursing, while there have always been "assistants" during various medical endeavors, I would say one of the first modern nurses, concerned with maintaining hygiene in the operating room, was Florence Nightingale. (However, the very first nurses, if you include midwives, may even predate modern humans.) StuRat 04:35, 30 September 2006 (UTC)
- The first heart transplant was performed on December 3, 1967 (South African surgeon Christiaan Barnard conducted the first heart transplant on 53-year-old Louis Washkansky). StuRat 04:44, 30 September 2006 (UTC)
Electrical power
For an educational book on water conservation that I am helping to produce, I have stated that a desalination plant of a particular size will use approximately 25MW/day of electricity. I want to compare this to something meaningful. The booklet is for high school students in south-east Queensland, so something like, "This is comparable to powering the city of ______ [place in Queensland or Australia] for one day" or "powering a town of ______ homes for one day" would be a helpful comparison. BenC7 06:35, 30 September 2006 (UTC)
- Well first of all, since a megawatt is a unit of power not energy, I would drop the '/day' and say it uses 25 MW continuously. If you think of electric 1kW fires this is equivalent to 25,000 of them running together.--Light current 06:47, 30 September 2006 (UTC)
- As Light current mentions, definitely recheck your power statistic, since a watt is one joule per second, it itself is a rate of energy use; 25 MW/d (or 25 000 000 joules / sec / day) would be a rate of increase (or decrease) of energy use (~acceleration)
- One needs to be careful here; as the editors above have noted, the units provided aren't aren't meaningful, and you'll have to go back to check your original references to determine what they should be. The rate of power consumption may be a flat 25 MW, or it may have been meant to be 25 MW·h/day. (Energy usage is often measured in watt-hours and its multiples.)
- Once you've got the units figured out, I'll note that this site gives the average quarterly household electricity consumption as 1625 kWh per quarter (6500 kWh per year, or a shade less than 18 kWh per day). That, in turn, would be a continuous average power draw per household of about 750 watts. TenOfAllTrades(talk) 13:16, 30 September 2006 (UTC)
- OK, I've double-checked and it appears to be 25MW, not 25MW/d. Given the figure from TenOfAllTrades above (750W per household, on average), I calculate that it would be the equivalent of powering over 33,000 homes! BenC7 01:48, 1 October 2006 (UTC)
- And to make this really meaningfull, you'd then have to add in info on how may households can be serviced by that water plant and, consequently, how big a part of a household's power consumption would be for water production if this was all done with desalination. But why use households? Why not doing it per person? Something like "providing the water for one person through desalination would cost the equivalent amount of power of so many 50W light bulbs" (constantly on, of course). Of course, that's just the production, not the transportation and different production methods will have differnt locations and transportation costs. Also, are you talking about people in the US, Europe or where? The water consumption will be roughly similar, but the power consumption will differ greatly. DirkvdM 07:38, 2 October 2006 (UTC)
illness
I have heared about this neurological illness in which some of the cappilaries of a persons brain stop working thus making his/her brain weak. Can any one please tell me the Name of that illness.
- Mattopaedia hit the nail, but your questions sounds a bit that you were not imagining a sudden event but rather a chronic problem. So, also have a look at vascular dementia to see whether this is what you mean. Simon A. 08:53, 30 September 2006 (UTC)
- vascular dementia used to be called multi-infarct dementia, reason being, multple small cerebral infarcts over time knock off the grey matter and tend to preserve the long tracts, whereas, what we typically consider to be a stroke is really an infract on a larger scale, long enough to cause long tract signs, plus or minus cognitive impairment. You could also have a look at transient ischaemic attack. A TIA is a cerebrovascular occlusive event like stroke, or vascular dementia, but it doesn't last long enoght to infarct brain. Mattopaedia 06:57, 2 October 2006 (UTC)
Nutritionist
Answered
Any resources for learning a lot about properties of different kinds of food? Thanks.
- Try this site for it's food search capability (upper right corner): [15]. StuRat 11:54, 30 September 2006 (UTC)
Thank you very much.
- You're quite welcome. StuRat 23:46, 30 September 2006 (UTC)
Aircraft Turbulence
If aircrafts produce turbulence, then how do fighter planes fly in formation?
- All of the turbulance created by a plane occurs behind it, just like the wake of a boat (this is not technically true, but it is sufficient to think that for the purposes of this explanation). The faster the plane moves, the smaller the cone of turbulance behind it. As long as the planes aren't flying single file (which I doubt you'll ever see anyone try, though don't quote me on that), they won't affect each other's heading. freshofftheufoΓΛĿЌ 12:51, 30 September 2006 (UTC)
- Check out the article on Wake turbulence, and here's an external site with additional pictures: [Tip Vortices]. 192.168.1.1 9:16, 30 September 2006 (my Birthday!) (PST)
- Also, if they fly in v-shaped formation or diagonal formation, the turbulence is much less because the tip vortices of the neighboring wings get cancelled, and the planes act like one larger wing. At low speeds, if speed and aircraft mass it constant, then the larger the wing, the less fuel is needed. That's why migrating geese fly in V-formation. --Wjbeaty 04:33, 1 October 2006 (UTC)
Chemistry Help
Hey guys! Our chemistry teacher gave us a scare by announcing that there is going to be a problem-solving test in Stoichiometry and Gaseous State chapters. He also said that it contains some very tough problems....not exactly tough but very logical...heard that my friends from other batch were not able to solve more than 3-4 questions out of a total of 25! Even though i am pretty confident on Stoichiometry, and since we are preparing for IIT-JEE, the questions are bound to be surprising. So, could you please tell me some really good sites that contains some tough questions WITH answers and also some suggestions for problem-solving. Thanks! (PS: This is my chance to impress my teacher :) )
`Milind
- Here's a site for you. [16]. There are a couple of links to quizzes and such, with answers. If you want a hand with something specific, I'm sure people here would be happy to help you. BenC7 01:55, 1 October 2006 (UTC)
Trouble Identifying Cactus

Hello. I was wondering if any of you botanists out there could tell me what Cactus this picture is portraying? I think it is quite encyclopedic and would add it to the appropriate article, but I don't know which cactus it is in the first place. So far, I think it might be a very large type of Houseleek or Jovibarba. Thanks in advance. NauticaShades(talk) 13:47, 30 September 2006 (UTC)
- It's actually not a cactus. Cacti are in the family Cactaceae, while agaves are in Agavaceae. I'm not sure if it is Agave parryi though. The picture could be added to Agavaceae, since that article is lacking in pictures of agaves that show more than the flowers. Gary 15:42, 30 September 2006 (UTC)
- I agree that it looks like A. parryi, probably var. truncata. --jpgordon∇∆∇∆ 19:47, 30 September 2006 (UTC)
a plant can not be identified correctly without viewing its flowers, this could be a euphorbia, an aeonium, or one or many different succulents.Hejlotta 02:32, 1 October 2006 (UTC)
Simple AC circuit?
What's the simplest way to use a DC cell to generate AC current? Preferably answers that don't involve "pick up a battery with your hand, and spin it at 3 hZ" or something silly like that--162.84.213.178 14:26, 30 September 2006 (UTC)
- Inverter (electrical). -- Finlay McWalter | Talk 14:35, 30 September 2006 (UTC)
- I don't suppose you could just reverse the setup on a rectifier?--162.84.213.178 12:29, 1 October 2006 (UTC)
- You could use an old type doorbell powered by a cell. THese have a coil and an interrupter contact that will give a square wave voltage across the coil. Or did you want a sinewave?--Light current 20:53, 30 September 2006 (UTC)
- Electricity can be dangerous, and even small currents can cause the heart to stop. Experiments with electricity which might create high voltage should be done only by those trained in the safety precautions for dealing with high voltage. That said, a transformer with a winding in series with the doorbell and the battery will have changing current in that winding. This will produced an alternating current in the other winding, although since the doorbell current is an ugly squarish wave, the output current will be a highly distorted ac wave. The turns ratio will determine how high the induced voltage is, so extreme caution is urged to prevent a dangerous shock from the induced voltage. The turns ratio will determine whether the voltage is stepped up, stepped down, or the same as in the primary (the winding in series with the doorbell). It is hard to predict the peak voltage in the output, since the input will be distorted. The resistance and reactance of the transformer windings and doorbell, as well as the turns ratio will effect the efficiency, output voltage, and output current. An interrupter more efficient than the doorbell would convert dc to pulsating dc more efficiently. Vibrators devices were used in former times to chop the dc of a car's 12 volt electrical system so it could be stepped up to high voltage ac then rectified to provide the plate supply for tube type car radios. A motor generator was another approach: a dc motor powered an ac generator. Utilities used single armature rotary convertors for changine megawatts of dc to ac, ac to dc, dc to higher/lower voltage dc, or ac to ac at a different frequency, or single phase to multiphase ac.Edison 18:09, 30 September 2006 (UTC)
- If you only wanted low power ac, you could make a simple square wave (relaxation oscillator) or sinewave oscillator--Light current 22:01, 30 September 2006 (UTC)
- If you don't care about efficiency or noise, an electric motor turning an AC generator is pretty simple, and produces a pure AC sinewave. --Serie 22:56, 2 October 2006 (UTC)
Kinetic energy's dissipation in vacuum
In true vacuum - with one object in a space where there are no atoms (perhaps neutrinos or something going about, but that's it) - will any kinetic energy be lost? Thanks in advance, again. 81.93.102.3 19:55, 30 September 2006 (UTC)
No. After all, according to Newtons first law, an object that feels no force will keep its velocity. And without background matter to cause friction, the object has no force acting upon it, so it won't change. Actually, according to the principle of relativity, it would not even be clear what losing kinetic energy meant. As kinetic energy is defined by relating to a velocity, we need a frame of reference relative to wich the velocity is measured. In case of braking due to friction, this is the inertial frame in which the background gas is at rest. Simon A. 21:15, 30 September 2006 (UTC)
- It would seem not unless the opbject were to encounter some curved space. This would act like a hill or valley in 3d space thus adding to or subtracting from the KE of the object. But this is unlikely, since you specify a true vacuum--Light current 12:19, 1 October 2006 (UTC)
Moving charge
To give a particle (with non-zero mass) a push, so that it starts to move, takes some energy which turns into kinetic energy. But if the particle is electrically charged, its movement generates a magnetic field (due to Ampère's law) which is also carries some energy. Does it take more energy to move the charged particle because of this, and how can that be explained? Is it a relativistic effect (the particle is moving, albeit slowly, with respect to the pusher)?
I figure this is the same phenomenon that manifests itself as radiation resistance of an antenna. That is, viewed from the feed point, the antenna looks like a resistance (or at least an impedance with a resistive component) even if it is made of a perfectly conducting material. —Bromskloss 21:19, 30 September 2006 (UTC)
I think that a subtle point here is to distinguish between energy of a field which stays with the particle and a energy that is carried away as electromagnetic radiation. Now, the magnetic field due to Ampere's law arises because of the costant-velocity movement. It satys with the particle, and I wonder whether it's energy can be considered as part of the kinetic energy of the particle. After all, if one taps the magnetic field energy, the particle is breaked (see Lenz's law). On the other hand, in order to accelerate (push) the particle, one needs more energy than to push a uncharged particle of the same mass -- precisely because during acceleration, energy is radiated off, as Bromskloss has pointed out. Note, by the way, also, that the magnetic field of the movig particle vanishes in its own frame of reference, as there, it is resting. Hence, special relativity is involved here, because Ampere's law is, in a way, a genuine relativistic effect, probably even the most important one as it is noticable at low speed. Simon A. 21:43, 30 September 2006 (UTC)
- But radiation resistance would be more like friction, since you can't get the radiated energy back again. Electromagnetic mass is odd because it's not associated with the particle, it's associated with the system (i.e. the electrons in a wire would have a different EM Mass if the wire was coiled versus straight, or if the wire was near a chunk of iron.)--Wjbeaty 04:29, 1 October 2006 (UTC)
- Thanks for the article you pointed to. Unfortunately, it's not very elaborate. Any other suggestions of where I could look (I already read the only reference in the article)? I was hoping that a lively discussion would break out here, but that doesn't seem to have happened. —Bromskloss 20:12, 1 October 2006 (UTC)
- Yes, a charged particle has kinetic energy and magnetic field energy when moving, and so it takes "more" energy to accelerate it. Moreover, a moving charge carries an additional electric field due to the changing magnetic field (Faraday), and that has energy too. But is it really that mysterious? If we push a perfectly conductive metal box that, unbeknownst to us, contains a free electron, then after a while we expect interactions with the box to have accelerated the electron, and so there's this "extra" energy taken up to generate the field. But from the outside, all that we can see is that we applied so much energy/force and got yea much velocity. If the velocity is smaller (or the energy larger) than we expected, what do we conclude? Merely that the box is more massive than we thought. In other words, the fact that a moving charge has more than energy can be treated as a different m: extra inertia that, in this case, is added to the system by the very act of pushing it. This is hardly surprising: all objects gain inertia when you push on them from special-relativistic concerns. Of course, with a charge we have the added concerns of radiation emitted because of the acceleration (which implies that yet more energy is required to get it moving); at this point your best bet is to go read up on such things as electromagnetic mass, radiation reaction, and the electromagnetic self-force, none of which (lamentably) have very useful articles here. Does this help? --Tardis 18:35, 2 October 2006 (UTC)
Steering trams
How do you steer a tram?
I know they go on rails but sometimes the number 3 goes left and the number 5 right at the same place, if you haven't got a fancy pants gps computer points thing (like they didn't have in the 40s, how do you make it go they right way)
- Do I get points for answering this one? --Light current 22:17, 30 September 2006 (UTC)
- In St Petersburg, I saw a tram stop at a points, the driver got out, used a rod to change them, got back in, and drove on. When I remarked that I felt sorry for the driver having to get out to do that (this was in early Feb at -15C), the American friend I was with noted that typically even in more technically advanced systems, the driver still gets out to change them in order to ensure they don't get blood clots from sitting down for too long. --Mnemeson 22:23, 30 September 2006 (UTC)
- They also used have a second set of points, I think called a frog, which is attached to the wires. These force the trolley to travel along the correct path (there's nothing worse than the tram going left and the trolley going right!), but now that pantographs have taken over from trolleys, they don't normally exist anymore. Laïka 22:58, 30 September 2006 (UTC)
- Railroad switch is the article you want. Amazingly, switching seems not to be mentioned in the tram article, and trams are barely mentioned in the railroad switch article. Switches on modern tram systems are usually computer controlled of course.--Shantavira 08:19, 1 October 2006 (UTC)
How much sleep
I usually sleep for 9-10 hours and then wake up on my own (i.e. without alarm clocks), but I'm usually unable to get out of bed for another 2 hours or so, unless I really have to get up. Anyway, I was wondering if, the time I sleep before I wake up on my own, is that the amount of sleep I personally need (as requirements differ from person to person), or is my excessive tiredness following my waking up indicating that I should sleep longer? Jack Daw 22:37, 30 September 2006 (UTC)
- My experience is that the more I sleep the more tired (and sick) I become. The more I excercise, the more awake I am. --Kainaw (talk) 22:54, 30 September 2006 (UTC)
- They were saying on QI that 4-7 hours sleep is best; those who slept longer had a short life expectancy. However, always remember with this sort of data that correlation DOES NOT imply causation; maybe people who sleep longer tend to be the people who exercise less, for example. Laïka 23:09, 30 September 2006 (UTC)
- I don't know how old you are, but I heard (at least 15 years ago), that adults need around 8 hours of sleep. Personally, when I stay in too long, I tend to stretch it out longer and stay tired for the rest of the day. With the comments so far, it may pay to get an alarm clock and try to cut down an hour or two. - Mgm|(talk) 23:12, 30 September 2006 (UTC)
- If you sleep more than you need to, its bad for you!--Light current 23:22, 30 September 2006 (UTC)
- Not being able to get out of bed after you wake up sounds strange to me. You might want to mention this to your doctor. --Ginkgo100 talk · e@ 23:39, 30 September 2006 (UTC)
- I always feel/felt like that. Its not unusual-- is it ?--Light current 00:13, 1 October 2006 (UTC)
- I have researched sleep a little and found that we don't really have any clue about recommended hours of sleep per night. You really need to sleep however you want, your body will tell you. Taking it easy getting out of bed is normal, as far as I know, the majority of people do it if they don't have an appointment, or don't like to wake up extra early. I do it so that I can fall in and out of sleep frequently to increase my memorable lucid dream count. Everytime I get out of bed without the two buffer hours I am lethargic for half and hour or so unless I do physical activity to wake myself up. If you are a marathon runner you might want and need to sleep a bit more than if you are a computer programmer, so that needs to be taken into consideration too. I casually only do four or six hours of sleep per night because I have finally gotten sedentary (woo hoo!). Back to requirements, what is interesting, is that the more segments you break your sleep up into, the less sleep you actually need to stay cognitively functional—polyphasic sleep is more efficient than monophasic. As a disclaimer, long term health effects are unknown, and uncharted. — X [Mac Davis] (SUPERDESK|Help me improve)08:29, 1 October 2006 (UTC)
- I absolutely agree with Mac Davis, it seems that we don't have much actual scientific evidence about recommended hours of sleep. I hate it when people show hypothesis as if they were theories. I have never been able to sleep more than eight continuous hours a night, but anyway, I feel much better now that I sleep 6 hours or less per night and take some power naps. Probably it also depends on the person. --GTubio 09:18, 2 October 2006 (UTC)
Valium + caffeine: Why does it make me feel like Jesus' son?
Prescribed valium, 5mg every four hours. It makes me feel dopey, so I've started drinking loads of very strong coffee to keep me awake. For some reason, this combination makes me feel supercharged - euphoric, happy, like I could do anything that I put my mind to, like all's right with with the world, like I love everyone I talk to. Not tired/sick at all - just, well *great*.
Anyone know what's going on here? --84.66.184.76 23:31, 30 September 2006 (UTC)
- Your doctor probably could tell you. I recommend contacting him or her as soon as possible. --Ginkgo100 talk · e@ 23:38, 30 September 2006 (UTC)
- How does Jesus's sun feel? I hope that stuff doesn't make you feel nonexistant. Or worse, debatably nonexistant. — X [Mac Davis] (SUPERDESK|Help me improve)08:30, 1 October 2006 (UTC)
- I think it's a Velvet Underground lyric. My unprofessional opinion is that if you feel weird it's because you're full of drugs - don't drink so much coffee and tell your doctor you think the dose is making you dopey. Rentwa 12:36, 1 October 2006 (UTC)
- Nice feeling. --Proficient 02:41, 2 October 2006 (UTC)
- I Am Not A Doctor, and this is not medical advice, but, essentially, Diazepam (Valium) is a sedative and anti-anxiety medication (amongst other effects like relaxing muscles), and caffiene is a stimulant. Put those two effects together and you've got, well, a combination of effects not unlike what you describe.
- I once read a book by a former GP in which he explained that the original 'Mother's Little Helpers' used to treat people who were 'down' were actually a combination of benzodiazepine and amphetamine. The benzos relax/calm you, the speed peps you up and allows you to go about your business without feeling doped - I guess that lots and lots of coffee might have a similar effect (limitless energy and no worries). Supposedly works really well for a time - until some sort of 'brick wall' is hit by the patient. Anyone able to elaborate on this further? It's literally been years since I read about it... --Kurt Shaped Box 13:32, 2 October 2006 (UTC)
- Like Rentwa said, self-medication is a bad idea, and if the side effects of your medication are bothering you, consult your doctor. --Robert Merkel 13:12, 2 October 2006 (UTC)
- Caffiene is also a seritonin stimulator.
October 1
Food becoming stale
What makes crunchy foods like crackers and popcorn become stale when exposed to air? Is it a physical or chemical change? I think (correct me if I'm wrong) that bread becomes stale because the moisture evaporates and it looses its sponginess, but it seems like drying out crackers would make them more crunchy, not less. —Keenan Pepper 00:16, 1 October 2006 (UTC)
- Bread 'dries out' and crackers absorb moisture?--Light current 00:25, 1 October 2006 (UTC)
- But if that was true, then we could restore the bread by putting it in extremely high humidity for awhile. I recall an article about this: the bits of liquid starch in bread will crystallize, so bread hardens without drying. IIRC, the article said that refridgeration won't stop this, but freezing the bread will. --Wjbeaty 04:23, 1 October 2006 (UTC)
- I believe microwaving stale bread will help to soften it a bit. Frost-free freezers can ruin bread, by driving all the moisture to one side. When thawed, one side is stale and the other is soggy. I believe the high salt content in most crackers also causes them to absorb more moisture from the air. StuRat 12:30, 1 October 2006 (UTC)
- Interesting. Maybe bread starts out with more moisture than the atmosphere, but crackers start out with less. That leads to another question: do crackers ever get stale in very dry places, say Arizona or Arica, Chile? —Keenan Pepper 00:33, 1 October 2006 (UTC)
- Crackers are stale to start with. Are you crackers or something? 8-)--Light current 00:39, 1 October 2006 (UTC)
- I always thought it had to do with reactions with gases in the air. The less air you have exposed to the food, the longer it takes to get stale. Temperature and humidity matter, but are secondary factors I thought. — X [Mac Davis] (SUPERDESK|Help me improve)08:32, 1 October 2006 (UTC)
- No, only the humidity matters. StuRat 12:34, 1 October 2006 (UTC)
So nobody has googled it yet? We could actually get an answer. Google Scholar is godlier than Google. Almost.[17][18][19][20][21] "Bread staling fall into 2 categories: crust staling and crumb staling. Crust staling is generally caused by moisture transfer from the crumb to the crust (Lin and Lineback 1990), resulting in a soft, leathery texture and is generally less objectionable than is crumb staling (Newbold 1976)." The crumb staling is the result of a bunch of chemical reactions. I haven't read them all yet.
Then there is also something interesting I came across: studies on microwave induced staling. You can never put bread in the microwave for a few seconds at a time. For those with time, check 'em out.[22][23] — X [Mac Davis] (SUPERDESK|Help me improve)22:39, 1 October 2006 (UTC)
- I've personally microwaved stale bread (usually with cheese or something on it) and had it get softer, so I know it works. StuRat 10:27, 2 October 2006 (UTC)
Back pain across bra line
For 15years I have suffered terrible burning pain across the bra line on my back if I stand or work for 45 min utes. No doctor has ever helped except to offer pain pills. Lying down for an hour helps, but then the cycle repeats when I stand leeb60207.200.116.204 01:20, 1 October 2006 (UTC)
- You might want to see a musculoskeletal specialist. A herniated disc, which can be aggrivated by vertical pressure on it (i.e., by standing), can cause symptoms similar to what you are talking about. If you have seen a GP already, I am assuming that heart conditions etc have been ruled out. The treatments for back problems can be pretty drastic (surgery), other times it is just pain management. I have had a slightly prolapsed disc in my lower back; doing the stretches recommended to me by a specialist have helped greatly. But obviously, I am not a doctor. I can only recommend that you see a specialist. BenC7 02:01, 1 October 2006 (UTC)
The obvious answer is that the bra may be the problem. If you have particularly large breasts you may even want to consider breast reduction surgery. Perhaps all you need is a better designed bra, that puts more of the weight on the shoulders and less on the back. For comparison, consider belts versus suspenders. Belts are fine for people with small bellies, but suspenders are needed for those with large bellies. StuRat 11:43, 1 October 2006 (UTC)
baby nose color
As a mother of a 1 yr old healthy boy, his little nose tip is quite orange/yellow. I have not introduced carrots,squash or similar colored foods, so what is doing this? Thanks very much.
Contrary to popular belief, feeding your baby carrots will not make him turn orange. Discoloration of skin is often an indication of some sort of chemical imbalance, which can be caused by diet, but implying that would require me to ask you to take your child to see a doctor and ask him for his educated opinion. (Ignore this comment) freshofftheufoΓΛĿЌ 06:07, 1 October 2006 (UTC)- Jaundice? I had jaundice when I was that age, younger actually. I can't rememeber the experience though... must have been the Men in Black. — X [Mac Davis] (SUPERDESK|Help me improve)08:34, 1 October 2006 (UTC)
- Nah, it was neonatal jaundice. I remember (based on what my parents told me) that they just took me in the sun for a while for a few days, as the doctor recommended. Haha, look at that loser baby with a tanning bed corset. :P— X [Mac Davis] (SUPERDESK|Help me improve)08:37, 1 October 2006 (UTC)
- Loser? He's looking pretty pimpin to me! --frothT C 16:31, 1 October 2006 (UTC)
- Nah, it was neonatal jaundice. I remember (based on what my parents told me) that they just took me in the sun for a while for a few days, as the doctor recommended. Haha, look at that loser baby with a tanning bed corset. :P— X [Mac Davis] (SUPERDESK|Help me improve)08:37, 1 October 2006 (UTC)
It is very common for healthy babies between 9 and 18 months of age to have a distinctly yellow-orange hue to the skin from dietary carotene from pureed vegetables: squash is the usual source, but carrots and other yellow vegetables as well may do it, as can some vitamin supplements. This is so much more common at this age than every pathologic cause that I suspect she is simply not recognizing the source. Pathologic causes of bad color are much rarer and likely to be accompanied by obvious failure to thrive or other problems. Jaundice at this age indicates severe liver disease, but it produces a greenish rather than orange tint to the yellow and it is most obvious in the whites of the eyes whereas carotenemia does not affect the whites of the eyes. PS: Mac, may I respectfully suggest you include the fact that you are a high school student with your medical answers? Neonatal jaundice is long gone by age 1 and is not relevant to this question. alteripse 10:11, 1 October 2006 (UTC)
- I don't see the relevance of your final statement. He gave correct information, and wasn't misleading in the least with his light-humoured comments. freshofftheufoΓΛĿЌ 15:24, 1 October 2006 (UTC)
- Nonsense. Feeding carrots is one of the most common reasons for a baby to have an orange tint. A yellow-orange tint at this age is not a sign of a "chemical imbalance", but a common finding in healthy infants. Jaundice is a very unlikely possibility in a well child and neonatal jaundice is indeed completely irrelevant to the coloring of a 1 year old. The only thing he said that wasn't either misleading or irrelevant was "see your doctor for an educated opinion." alteripse 17:50, 1 October 2006 (UTC)
- I owe an apology to Mac. I missed your signature after the first answer and thought it was all his. Sorry. alteripse 19:01, 1 October 2006 (UTC)
- Hehe; All is well. — X [Mac Davis] (SUPERDESK|Help me improve)22:16, 1 October 2006 (UTC)
- Yes, I realized my error as well after realizing the active chemical in jauntice was actually carotene, so I guess I'm the one who's sorry. I was a little bit to quick up on that response. I don't see the error in my statement about chemical balance though, for is carotene not a chemical, and carotenemia not a condition signifying a higher-than-average amount of carotene in the body? I also said "is often" and "can be" to protect myself, for there are many conditions resulting in changes of skin color that are caused by chemical imbalance. Regardless, my initial comment was improper, and I'll remember to give the questioner more credit next time. freshofftheufoΓΛĿЌ 05:34, 2 October 2006 (UTC)
- The pigment in jaundice is bilirubin, not carotene. While every substance is a chemical in one sense, we usually do not refer to healthy conditions as a "chemical imbalance". That phrase has acquired a clear pathologic connotation in both popular and medical American English of the last 3 decades. The color of carotenemia is classically in the tip of the nose, especially obvious if you touch it with a fingertip. The color of jaundice is usually first obvious in the whites of the eyes and if you saw it in your child you would not mistake it for anything healthy and would not be asking about it here. You can hide behind "often" and "can be", but that was as clear and succinct a description of carotenemia as you will get in a sentence. alteripse 10:14, 2 October 2006 (UTC)
- Yes, I realized my error as well after realizing the active chemical in jauntice was actually carotene, so I guess I'm the one who's sorry. I was a little bit to quick up on that response. I don't see the error in my statement about chemical balance though, for is carotene not a chemical, and carotenemia not a condition signifying a higher-than-average amount of carotene in the body? I also said "is often" and "can be" to protect myself, for there are many conditions resulting in changes of skin color that are caused by chemical imbalance. Regardless, my initial comment was improper, and I'll remember to give the questioner more credit next time. freshofftheufoΓΛĿЌ 05:34, 2 October 2006 (UTC)
- Hehe; All is well. — X [Mac Davis] (SUPERDESK|Help me improve)22:16, 1 October 2006 (UTC)
- I owe an apology to Mac. I missed your signature after the first answer and thought it was all his. Sorry. alteripse 19:01, 1 October 2006 (UTC)
- Nonsense. Feeding carrots is one of the most common reasons for a baby to have an orange tint. A yellow-orange tint at this age is not a sign of a "chemical imbalance", but a common finding in healthy infants. Jaundice is a very unlikely possibility in a well child and neonatal jaundice is indeed completely irrelevant to the coloring of a 1 year old. The only thing he said that wasn't either misleading or irrelevant was "see your doctor for an educated opinion." alteripse 17:50, 1 October 2006 (UTC)
Missing posts
Has anyone else noticed posts going missing on these pages? Like you click on an item in your watch list by UserXXXX, and it aint there? Im posting this msg on all ref desks.--Light current 11:26, 1 October 2006 (UTC)
- It is perhaps an unintended effect of an experimental way of archiving – which also has its advantages. --LambiamTalk 11:38, 1 October 2006 (UTC) · See also Wikipedia talk:Reference desk#Archive dump. --LambiamTalk 11:41, 1 October 2006 (UTC)
Contributors for Pharmacogenomics Wiki wanted
Hi, I am fully not sure if here is the right place for looking for contributors or helpers for http://www.wiki.pcg-portal.com. If it is wrong, please displace this post to the right section in the wikipedia. Fact is, that it would be great, if my team and me could find here some guys who could help us in the english version of the mentioned wiki link http://wikien.pcg-portal.com/index.php/Main_Page. Helpers have the possibility, to become admins at this wiki then. This project is supported by a german university and two german companies.If somebody is now interested, please don not hesitate to contact me. Contact possibility can be found on the page itself. In principle we need someone, who is familiar in dealing with the wiki software itself and optimising the general structure und the administration, It is not necessary, that you bring in specific knowledge for the topic PCG. Thanks a lot for your help, and again sorry for the topic here if it should be on the wrong place ... Waiting anxious for answers, regards MoritzE 12:07, 1 October 2006 (UTC)
- Is that site in German ? If so, I would post any request to the German Wikipedia, or at least to the Language Ref Desk. StuRat 14:39, 1 October 2006 (UTC)
- So. there is actually a german and an english version. As teh german version is from the structure and function almost complete, i am looking for people helping for the english section.
MoritzE 16:36, 1 October 2006 (UTC)
Hairy back
Why do the hairs on my back cuase such itching? Or is it the hairs at all?--Light current 12:22, 1 October 2006 (UTC)
- Ingrown hairs can cause lots of itching. Those can happen especially if you shave your back. I've also notice that stretching my skin tight makes it itch. StuRat 12:37, 1 October 2006 (UTC)
- How the hell do you shave your back, may I ask? With a remote controlled razor? Or do you have double jointed elbows/shoulders and a head that can rotate 180 deg? 8-)--Light current 01:18, 3 October 2006 (UTC)
- That makes three of us. I'm quite hairless though so I always blame dry skin. It's difficult to clean the back properly when showering, and I also tend to sweat quite a lot on my back during the summer months. Some kind of lotion really helps. A cool side-effect of frequent scratching: I can almost grab my elbows behind my back. freshofftheufoΓΛĿЌ 15:39, 1 October 2006 (UTC)
- What do you mean almost grab your elbows? You mean like... grab your elbow? I'm guessing not everybody is that flexible? — X [Mac Davis] (SUPERDESK|Help me improve)02:53, 2 October 2006 (UTC)~
- Yes! I can't grab my elbows though, only about 3/4 the way up my forearms. I can also spin my arms in different directions, and make a clover-leaf with my tongue! freshofftheufoΓΛĿЌ 07:31, 2 October 2006 (UTC)
- What do you mean almost grab your elbows? You mean like... grab your elbow? I'm guessing not everybody is that flexible? — X [Mac Davis] (SUPERDESK|Help me improve)02:53, 2 October 2006 (UTC)~
- That makes three of us. I'm quite hairless though so I always blame dry skin. It's difficult to clean the back properly when showering, and I also tend to sweat quite a lot on my back during the summer months. Some kind of lotion really helps. A cool side-effect of frequent scratching: I can almost grab my elbows behind my back. freshofftheufoΓΛĿЌ 15:39, 1 October 2006 (UTC)
Sorry but as men are notorious for lack of hygene and sskin care knowledge, let me give you a better answer. Exfoliate vigourously and often and the itching will stop. Removing the layer of dead skin cells is important to keep the pores unclogged and prevent itching. Doing as most men do by swiping a bar of soap across your body is not enough. Get a loofah or a backscrubber of some type so you put some friction on the skin. Of course this works all over, but the back is most often neglected since it is harder to reach. pschemp | talk 19:17, 2 October 2006 (UTC)
- So you reccommend a scrub with the loofah daily? Ill try anything once. Like the chap who went to the doctor with a steering wheel slipped over his penis. He said 'Doctor you gotta help me. This thing is driving me nuts!'--Light current 21:04, 2 October 2006 (UTC)
- A vigourous scrub. Need to do it everytime you shower to make a difference. pschemp | talk 21:07, 2 October 2006 (UTC)
In tides, why is the eighth wave always the largest?
When waves strike on shore, the eighth wave is always larger than those previous to it. Why is it always the eighth one? Thanks! Robinoke 14:20, 1 October 2006 (UTC)
- It sounds like you have two different frequencies of waves, with one being 8 times as long as the other, and reinforcing it. I'm not sure what causes this, however. StuRat 14:36, 1 October 2006 (UTC)
- Is this a question about tides or waves? I don't know where you got this idea about the eighth wave, but I can find no support for it. I suppose it could conceivably be a local phenomenon somewhere? Or you may be thinking of spring tides and the lunation cycle?--Shantavira 17:43, 1 October 2006 (UTC)
- Indeed. Why would the 8th be the largest? It is probably a local event, as suggested. --Proficient 02:41, 2 October 2006 (UTC)
- See Sneaker wave which says the common belief is that the seventh wave is the large one. (Love is the Seventh Wave from The Dream of the Blue Turtles by Sting, etc.) Rmhermen 03:49, 2 October 2006 (UTC)
- But surely I'm not the only person to have seen this? I really dont think it's localised -- I've seen it all over Britain, but then again, not in Florida. Could this be because Florida waves are constructive (low, I think) and British are destructive (where they rise up and crash, dragging the beach back with them)? User:Rmhermen might be right, maybe it is the seventh one. Regardless, the article on sneaker waves claims it is an unscientific claim, but i'm seeing it happen whenever I walk down the shore. What's going on? Robinoke 14:15, 2 October 2006 (UTC)
- I can picture two bodies of water coming together, each with different frequency and magnitude waves (caused by different winds), to produce this effect. For example, large, long frequency waves may come in off the Atlantic Ocean, and combine with small, short frequency waves off the English Channel. StuRat 17:09, 2 October 2006 (UTC)
- Yeah, that explains things a bit more for me. Thanks guys. Robinoke 21:11, 2 October 2006 (UTC)
PAL I vs. PAL BG
I wonder if a camcorder purchased in Hong Kong, where the PAL I system is used, can be utilized without any adaptation in countries where the the PAL B/G system is in use.
--132.66.7.213 14:20, 1 October 2006 (UTC)
Sperm in Facial Treatments?
Does anyone have any information on the practise of using sperm not necessarily human in skin care remedies, face creams etc?--14:26, 1 October 2006 (UTC)
- Never heard of it. They do, however, sometimes contain urea, a component of urine. StuRat 14:31, 1 October 2006 (UTC)
- You're not confused with this, are you? DirkvdM 19:34, 1 October 2006 (UTC)
- From Lewis Black's Wikiquote page: "You ever read the ingedients in sunblock? I've never seen those words anywhere. You don't even know what you're putting on your face, do you? You go, 'Oh no, the sun's out!' It could be zebra cum; you don't know. You may not like that joke, but you don't know." --jh51681 21:20, 1 October 2006 (UTC)
- There's a myth that male ejaculate is good for the skin. I recall a website where someone put semen on the same spot of their face for a couple of weeks and on the opposite side left blank or applied with some professional skin treatment (I can't remember which) and they found that the semen made the skin drier. Boys will tell their girlfriends anything. AEuSoes1 23:42, 1 October 2006 (UTC)
- Drier is good? Didn't you get that the wrong way around? I've heard of moisturisers, not drierisers. DirkvdM 07:59, 2 October 2006 (UTC)
- Er – well that's why it's a myth, i.e. wrong. Does this need adding to the old wives' tale article?--Shantavira 08:29, 2 October 2006 (UTC)
- I don't remember the last time old wives discussed this subject seriously. — X [Mac Davis] (SUPERDESK|Help me improve)11:35, 2 October 2006 (UTC)
- Er – well that's why it's a myth, i.e. wrong. Does this need adding to the old wives' tale article?--Shantavira 08:29, 2 October 2006 (UTC)
primary colours
80.47.9.47 15:51, 1 October 2006 (UTC)please can you tell me which of the three primary colours is likely to fade fast in daylight or flourescent lighting, and which is the strongest pigment→ : blue , red, or yellow. (I need this for my homework and can`t find an answer anywhere.) Thank you, pamela
- First, this obviousley depends on the type of ink used. Some ink resists fading due to light well and some less well, and if you paint something outside, you better take a good ink or paint. Nevertheless, there is an obvious answer. Have you ever looked at old posters in windows e.g. of stores closed long ago? Or just as any poster that has been hanging outside for many years? It is always the same colour which is missing. Now, as this is a homeowrk question, I stop here, forcing you to a walk through your neighborhood to look for an old poster. Simon A. 16:06, 1 October 2006 (UTC)
- Oh, and one more thing: It's true that painters tend to consider red, yellow and blue the primary colors, event though they use more different pigments than just these three colors (see pigment). Printers however (at least for posters (as in my example), magazines and so on, not for high-quality artwork), mix (or rather: dither) there colors from magenta, yellow, cyan and black (see CMYK). Simon A. 16:09, 1 October 2006 (UTC)
- Or the ones in between, the famous rgb. See also colour spectrum. DirkvdM 08:14, 2 October 2006 (UTC)
Cannabis
I was reading a previous post on here and I don't understand why weed seems to be so bad for us. Is it really all that harmful? I've read the Cannabis page... but it doesn't really say. Cathycat790 19:18, 1 October 2006 (UTC)
- First of all it is usually smoked, which has roughly the same bad effect that tobacco has on the lungs. Worse even, per quantity, but then one doesn't normally smoke over 10 grams per day (hell, even one gram would be too much). And that indicates an important thing about how bad something is for you. It depends on quantity. There's lots of stuff that is good for you in the right dosage, but bad if you get too much of it. Such as vitamin a. Or too little, like vitamins in general. In the Netherlands, over the last few decades, nederwiet (Dutch marijuana) has been turned into what is now considered a hard drug because of its high THC content. Which brings me to a more useful distinctyion, between hard use and soft use. I suppose one could use opiates without really suffering much adverse effects. It's just that drugs usually come in too concentrated a form and thus make overuse too easy and tempting. Which is at least in part caused by the widespread illegality of many drugs. I assume that during prohibition in the US spirits became more popular (anyone know?).
- And now I'm going to light a joint (not kidding). DirkvdM 19:43, 1 October 2006 (UTC)
- It's a bit of a minefield at the moment. On the one hand there are lots of people who sincerely (if ignorantly) believe it's dangerous, and lots of politicians who know better but also know it's political suicide to say so. On the other hand there are thousands of young people who believe it's entirely harmless even though there's very good evidence that it causes psychosis in a significant minority. Rentwa 20:47, 1 October 2006 (UTC)
- Pardon me, but that seems to be a bit of a one-sided view. Aside from doing really stupid things while high (eg trying to drive), it's well known that inhaling smoke damages your lungs and greatly increases the risks of lung cancer. So, yes, cannabis use is 'dangerous' in that it increases the chances that you will become ill and die. However, the risk increase is often outweighed in the user's mind by the good feelings they get from using it, and that's their choice - we live in a free society, after all. It works the same way as, say, a skier who accepts the increased risk of injury as a tradeoff for the enjoyment of skiing. But saying that "cannabis isn't dangerous" is incorrect, it's not a "consequence-free high", you have to understand its effects and make a decision to use it (and how much), or not, based on that. — QuantumEleven 12:54, 2 October 2006 (UTC)
- It's a bit of a minefield at the moment. On the one hand there are lots of people who sincerely (if ignorantly) believe it's dangerous, and lots of politicians who know better but also know it's political suicide to say so. On the other hand there are thousands of young people who believe it's entirely harmless even though there's very good evidence that it causes psychosis in a significant minority. Rentwa 20:47, 1 October 2006 (UTC)
- Marijuana is extremely cheap when it is not illegal, and the price is a lot more when it is illegal. Might this have something to do with the legality? — X [Mac Davis] (SUPERDESK|Help me improve)02:43, 2 October 2006 (UTC)
- If the government were to legalize it, they could tax it. ;) --Kurt Shaped Box 11:08, 2 October 2006 (UTC)
- There's pretty good support for the claim that its use is linked to depression and other mental illnesses in later life. Aaadddaaammm 06:34, 2 October 2006 (UTC)
- I'll say it again, just shorter. Anything can be good or bad, depending on the quantity. DirkvdM 08:02, 2 October 2006 (UTC)
Giant Redwoods and their cells
What kind's of cells compose a Giant Redwood?
– — … ° ≈ ± − × ÷ ← → · § Allie: 71.252.82.143 21:04, 1 October 2006 (UTC)
- At the top of the page it says to do your own homework you know. ;) Based on the Coast Redwood article, Sequoias are conifiers, which are softwood. You can take a look at leaves and roots, pith, periderm, phloem, secondary phloem, xylem, secondary xylem, vascular rays, vascular cambium, heart wood, sap wood, bark, early wood, and parenchyma. It would probably be easier to read the page or two in your book or just look at the diagram, but check the links if you are interested. — X [Mac Davis] (SUPERDESK|Help me improve)22:26, 1 October 2006 (UTC)
conifers? Lukas 01:23, 2 October 2006 (UTC)
Indoor Plants
Now that fall is upon us, and winter is approaching. I am thinking about growing some typically spring/summer plants inside. Ive done some research for mimicing the sun cycles and the amount of time the sun is up beginning in the spring (8-10hours) and then to summer (10-12) and then back to late summer/fall (8-10). What I am wondering is what if I just kept some artificial light on the plants 24 hours from the beginning? Would that have an adverse or positive effect?
Thanks!!!
Are some plants immortal?
I've been thinking about some of the really old trees in the world - some of them are thousands of years old, right? Is there any reason why these trees could not live 'forever', provided no external factor (e.g. fire, chainsaw) results in their destruction? Are they, in effect, immortal? --Kurt Shaped Box 22:37, 1 October 2006 (UTC)
- Don't forget disease! The oldest trees are around 2000 years old, but they pale in comparison to the oldest bushes. The King Clone has been dated as over 11500 years old, and the King's Lomatia in Tasmania is dated for at least 43000 years. However trees come back in again and The Pando's roots are believed to be over 80000 years old. These clonal colonies are respected as organisms, but I don't see how you couldn't count each tree/bush as a single living organism, and give the title to Coast Redwoods. The only thing that could really get them is disease. They survived thousands of years of shit, and they are pretty tough. — X [Mac Davis] (SUPERDESK|Help me improve)22:44, 1 October 2006 (UTC)
- Trees get to at least 5,000 (not 2,000) - see Prometheus (tree), a bristlecone pine. Rmhermen 03:41, 2 October 2006 (UTC)
- I was talking about currently living trees. — X [Mac Davis] (SUPERDESK|Help me improve)07:14, 2 October 2006 (UTC)
- Life forms with no set life span can certainly live for a very long time, but not forever, for two reasons:
- Probability dictates that, so long as there is any possibility they will die in any given year, and there are a finite number of life forms, then they will all die eventually. In practice, this seems to work out to be thousands of years, but not millions or billions.
- Eventually some cataclysmic event is likely to kill all life forms on Earth. This could be the Sun going red giant, a "big crunch", proton decay, etc.
- I think the Sun going red giant will make the big crinch irrelevant here. :) Also, if all plants live in the same place then a meteor will probably hit that spot or somewhere close enough before that. Or a volcanic eruption. DirkvdM 08:32, 2 October 2006 (UTC)
- I was allowing for the possibility that some trees would survive, perhaps by being relocated to a safe location by us. StuRat 10:09, 2 October 2006 (UTC)
- That is pretty cool though. --Proficient 05:06, 2 October 2006 (UTC)
- I've seen the term "functionally immortal" used. "Immortal" like Tolkien's elves are immortal. Guettarda 03:47, 2 October 2006 (UTC)
- I could be immortal too if I just kept on cloning myself. Though those are some pretty bad-ass bushes for figuring out how to do that naturally. freshofftheufoΓΛĿЌ 06:14, 2 October 2006 (UTC)
- Fascinating. Even though the examples given so far dwarve the Bodhi Tree it's worth adding it as a very well-known example to the list. The Bodhi Tree is said to be the tree under which the Buddha was sitting when he was enlightened. Even though the tree tied, clones still exist, and even thoigh one cannot prove that Buddha indeed sat under their clonal parent, they are clearly quite old. And have been cloned artificially. Simon A. 07:09, 2 October 2006 (UTC)
- Relevant aricles should be Maximum life span and Longevity, but they seem to discriminate against plants. Shouldn't we have an article on oldest life forms? DirkvdM 08:32, 2 October 2006 (UTC)
- Ah, but we do. List of long-living organisms. — X [Mac Davis] (SUPERDESK|Help me improve)11:34, 2 October 2006 (UTC)
- I bet there are some bacteria or fungi that don't change genetically and should therefore count as a single life form. Or what about this one? I once heard about bacteria (probably) that had been captured in the salt of a dried up lake, were kept 'safe' there for hundreds of thousands of years (or 'thereabouts') and were revived. They had just been 'asleep' or something. The standard terminology (like 'sleep' and 'single life form') seems to break down here. As usual, when you explore the extremes of something, you just find yourself redefining what you thought was clear. Which is one of the most educative things one can do. DirkvdM 08:41, 2 October 2006 (UTC)
- Well, they're "dormant." Kind of like hibernation, or how frogs can freeze during the winter. — X [Mac Davis] (SUPERDESK|Help me improve)11:34, 2 October 2006 (UTC)
I don't count clones as the same life form. After all, if you could clone yourself, would you consider your clone to be you ? I don't think identical twins ever consider themselves to be the same person, either. StuRat 09:54, 2 October 2006 (UTC)
- It is a clonal colony. They are part of the same "organism." I don't get it either. Maybe they're just wrong. — X [Mac Davis] (SUPERDESK|Help me improve)11:34, 2 October 2006 (UTC)
- I would definitely require that, to be considered one life form, the organism must be contiguous. So, clones are not one organism, under that definition. StuRat 11:46, 2 October 2006 (UTC)
- I'd agree. However, it seems that the example given for a record-holder in age, namely the quaking aspen known as Pando (tree) shares a root system and hence may be considered as one organism. Simon A. 17:59, 2 October 2006 (UTC)
- I think the missing info here is that a clonal colony has a single root system - which may be cut artificially, but not by the plant itself. I read about it in a study on a bush in Yucca Valley (in the Mojave). The roots of one bush were connected to another one, which was connected to another one, and so on. After mapping out a lot of the bush connections, it became apparent that there was only one root system spiraling out from the center where assumably the first bush began. --Kainaw (talk) 18:07, 2 October 2006 (UTC)
- Don't fungi also consist largely of 'roots' that can extend over huge areas? how old do fungi get? The article doesn't say. DirkvdM 19:19, 2 October 2006 (UTC)
October 2
Taxonomy for Extraterrestrial Life
There's something I've been wondering about for years: If/when we do find extraterrestrial life, how would we go about classifying it? Considering the ideal of having taxonomy reflect genetic lineage, would you start an entirely new tree for them - maybe put all life on Earth in "Superdomain Terrania", and put all life on Mars in "Superdomain Martiana"? Does anyone know of any proposals that have been made for extraterrestrial taxa?
Taking the question a bit further, if we were to make a new superdomain for another planet, then would taxonomy tolerate "duplications" of basic taxa used for life on Earth? I mean, could you have "another" Domain Eukaryota for another planet, if a group of extraterrestrial organisms fit our commonly accepted standards of what a eukaryote is? Could you have "another" Kingdom Animalia for another planet, if a group of organisms unambiguously met our commonly accepted standards of what an animal is?
Many thanks to whomever responds, --The Lazar 00:43, 2 October 2006 (UTC)
- See Xenobiology, for starters. — X [Mac Davis] (SUPERDESK|Help me improve)01:15, 2 October 2006 (UTC)
- Well, I've looked there, but it doesn't really seem to touch on this specific issue of "xenotaxonomy". Google searches haven't yielded much either. I asked over at Talk:Extraterrestrial life, but they recommended that I ask here. --The Lazar 02:39, 2 October 2006 (UTC)
- A very interesting question. Since our classification scheme tends to represent evolutionary relationships (cladistics), I would assume as you say that a new tree would have to be developed. Of course we don't know what extraterrestrial life might be like, organic, cellular, and so on. I tend to think that using duplicate names would be a bad idea in general. Especially with today's taxonomic thinking, the "commonly accepted standards" are more of a surrogate for genetic lineage; that is, the true definition of some division is something like "all descendants of the most recent common ancestor of x and y", and if they all just happen to be give birth to live young or something due to their shared ancestry, that just makes it easier to classify. Of course, xenobiology might reveal either that life can come in enormous variety or perhaps there aren't that many ways for life to be successful; so if we were to find extraterrestrial life it may end up that we find similar characteristics as on our own world. — Knowledge Seeker দ 02:42, 2 October 2006 (UTC)
- I had thought about xenotaxonomy for a while too and I came up to pretty much the same idea that you have, Lazar. A new planet and new genetic lineages would mean you could start over with the names. There might be some confusion but when clarifying one could simply insert the superdomain somewhere like "Apis florea Martiana" and "Apis florea Terrania".
- I really hope there aren't already bees on Mars. Ƶ§œš¹ [aɪm ˈfɻɛ̃ⁿdˡi] 03:24, 2 October 2006 (UTC)
- I doubt that the nomenclatural bodies would allow the reuse of names, assuming that Linnean taxonomy still stands. If something like PhyloCode is etablished, then that problem doesn't exist.
- As for the original question, it should form a new clade, distinct from all other known life. Guettarda 03:54, 2 October 2006 (UTC)
Its possible however that we find extraterrestial life that is related to life on Earth, in which case those species could be integrated into the existing classification schemes. AmitDeshwar 06:07, 3 October 2006 (UTC)
Codon inversion
Hi
I'm doing a third year bioinformatics course at varsity, and I'm researching for a presentation. My topic is codon inversions/reversal in DNA. Eg ABC DEF GHI -> GHI DEF ABC. I've come across them from time to time when sequence gazing. Does anyone know of any work done on these? Any cases in the literature? I've had quite a hard look, but I'm finding search terms hard to pin point, and information even harder. The only paper I've found is Santos et al 2004 J. Biol. Chem. pp17596-17606. Thanks for your help!
Aaadddaaammm 03:05, 2 October 2006 (UTC)
- third year bioinformatics? you must know already the NCBI is your friend B-). go to pubmed, and get searching. a keyword to get you started is pericentric inversion, a rather special case of gene inversion. good luck! Xcomradex 07:33, 2 October 2006 (UTC)
air pressure cuts
I read somewhere that there is a phenomena where air pressure can cut people or things, it has something to do with a vacuum or something. apparently in japan they call it "kamaitachi" is this a real phenomena or just a legend? if it is real how does it work?
curious 17:00 01 october 2006
- A high pressure air source certainly could cut like a torch, particularly on soft material like human flesh. Of course, the air velocity would quickly dissipate, so the person would need to be very close to the source to be cut by it. StuRat 16:45, 2 October 2006 (UTC)
High-pressure water can cut you, since it can cut through metal. Here's a funny article on whether high-pressure steam can cut a person in half (probably a magician has done it on doctored video!) [24] But nothing on air. --Zeizmic 17:18, 2 October 2006 (UTC)
- Note: we do have an article on kamaitachi, though it refers to a mythological phenomenon. freshofftheufoΓΛĿЌ 03:30, 3 October 2006 (UTC)
- High pressure steam will cut to the bone as well as scald. High pressure hydraulic fluid from a small pinhole leak can penetrate the flesh and accumulate in the tissues, requiring surgery.Edison 04:41, 3 October 2006 (UTC)
Glucosamine and Diabetes
Dear Sir/Madam: I am looking specifically for any information or sources you can offer to reference regarding the effects of orally taking Glucosamine for someone with Diabetes. This person is not insulin dependent, the diabetes is primarily controlled by a combination or diet and pills(not sure what kind). Any help you can offer would be greatly appreciated.
Thank You, 69.130.36.6 16:02, 2 October 2006 (UTC)
- As far as I know, the two are completely unrelated, glucosamine is for joint health, and diabetes is a pancreas/blood sugar issue. 16:42, 2 October 2006 (UTC)
Since millions of older adults have both conditions the question is a good one. The theoretical concern is some animal evidence based on large doses (larger per weight than humans would take) showed moderate reduction of insulin sensitivity. This might be a concern in someone with partial or early type 2 diabetes, because it could hasten progression of the diabetes. On the other hand there have been remarkably few reports of this in people. Here is a concise and reassuring summary of the problem. At least with diabetes (as opposed to, for example, cancer) you have in your hands the tool to see if a treatment is helping or worsening the diabetes. For an excellent brief overview of glucosamine and chondroitin for arthritis, see Stephen Barrett's synopsis of the evidence. Diet supplement company sites should be considered advertising, not balanced information. Good luck. alteripse 17:38, 2 October 2006 (UTC)
Bird Eggs
Why do birds lay unfertilized eggs? For such small animals, wouldn't it be a tremendous disadvantage to be losing so many nutrients every day? (In the case of chickens.) --Demonesque 16:21, 2 October 2006 (UTC)
- It's similar to menstruation in certain mammals. Unfortunately, no elegant method has ever developed to allow the animal to know whether an egg is fertilized, so it can make a decision about what to do. Most animals just make full preparations as if the egg were fertilized. One exception seems to be cats, where the male penis has a barb that injures the female, and this somehow triggers the preparation for kittens. Not a very elegant method, but better than none, I suppose. StuRat 16:32, 2 October 2006 (UTC)
- Actually it doesn't trigger preparation for kittens at all. Cats are induced ovulators which means if they aren't mated with by a male with barbs (neutered males lose the barbs) that they don't ovulate at all and no eggs are released. Great mechanism to ensure conception, but somewhat unrealted to chickens. pschemp | talk 19:07, 2 October 2006 (UTC)
- And you don't consider cat ovulation to be "preparation for kittens" ? There would be very few kittens without ovulating cats. StuRat 20:37, 2 October 2006 (UTC)
I suppose there could be an evolutionary advantage to unfertilized eggs, if they provide decoys for predators who might otherwise get the fertilized eggs. StuRat 16:37, 2 October 2006 (UTC)
- In rare cases the unfertilized birds eggs develop into embryos. See also parthogenesis for comparison; it exists among many non-avian species.---Sluzzelin 16:42, 2 October 2006 (UTC)
Don't forget that chickens have been selectively bred for the egg laying trait, so its not the same as if their genes were not subjected to artificial selection. pschemp | talk 19:07, 2 October 2006 (UTC)
Wow, thanks. --Demonesque 17:42, 2 October 2006 (UTC)
Birds are morelikely to lay infertile eggs when they are in unnatural circumstances. I used to have a single lovebird which laid eggs regularly. In the wild, she would have been around other lovebirds and had a mate to fertilize those eggs. --Ginkgo100 talk · e@ 21:43, 2 October 2006 (UTC)
Strobe
Is there a way I could produce strobe flashes, using only batteries as the power source? All bright strobes seem to use mains power, but if I need only a few flashes, would batteries do? I need a strobe for a school science fair, where we are not allowed to use mains power. We want to try and stop the motion of various things. Many thanks! --86.142.195.245 18:10, 2 October 2006 (UTC)
- Since you used the term "mains power" I'll guess that you are in the UK. However, a simple Google search returns a lot of hits. You'll just have to narrow it down to local suppliers. --LarryMac 18:19, 2 October 2006 (UTC)
Sure, just shine a flashlight ("electric torch") through a battery-powered fan (they sell those for stadiums, etc.). (I'm afraid I don't know what you call an electric fan in the UK, perhaps a "flugglethump" ?) :-) StuRat 20:05, 2 October 2006 (UTC)
You can use a battery to charge up a capacitor, and then press a switch to discharge it through a lightbulb, or an LED (make sure you don't put too much current through it though). The hard part might be getting a suitably short pulse of light. A quick finger on the switch might be able to do it (or even just the capacitor's discharge time, if resistance isn't too strong), but you can also build a circuit that produces a certain pulse length with a 555 timer IC (rated for a suitable voltage). - Rainwarrior 20:24, 2 October 2006 (UTC)
- It wasnt my first thought, but StuRats idea is probably the simplest a nd safest way to do this. Instead of a fan though, you could make your own strobe wheel- just a carboard wheel with some narrowish slots cut around the periphery. A small dc electric motor to drive it and a bright flash lamp would probably do the trick all without mains power 8-)
Many thanks for your time and effort people, I think I like the idea of the fan, that'll be the simplest to implement. Last year we got a bit over-ambitious, and I'd like to keep it simple, so we'll try the strobe wheel. Thanks! This reference desk is very useful!--86.142.195.245 21:01, 2 October 2006 (UTC)
- Battery + Inverter + mains-powered-stroboscope. Or if you're electrically creative and competent to work with ~300 volts, I think you'll find that you could take the circuitry from a battery-powered camera strobe (as found in disposable cameras), exchange the bulk storage capacitor cor a much lower value (so it recharges much faster), rig up a repetitive triggering circuit, and Bob's your uncle.
- Yes thats how we'ed do it, but I didnt want to encourage schoolkids to mess around with dangerous voltage! 8-(--Light current 00:52, 3 October 2006 (UTC)
I'm searching for information on the workability and physical characteristics of pure Tungsten metal
Monday, 10-2-06 Portland, OR 12:57pm West Coast, Pacific Time
Who on the Reference desk/Science page would be an authority/expert on pure Tungsten metal? I'm searching for a cost effective retail source of this metal; considering my extremely low income level, what is the best, lowest price of retail pure Tungsten? There are plenty of websites that sell either scrap or new tungsten, but unfortunately the purity of either of these forms can vary from website to website. The purist form that I found on one particular website is 99.95% in foil form at $146.0 for a specified size(in metric units). Does anyone in the Reference desk/Science page heve any physical work experience with pure Tungsten metal? Is the metal compatible with common workshop tools such as powertools, tinsnips, handsaws, etc.? With these common workshop tools, I wish to fabricate my own storage/holding container for "Dry Ice": This is for a private, experimental science project that I'm cogitating; Is Tungsten tough and strong enough to tolerate physical contact with "Dry Ice" for an indefinite duration of time, or does pure Tungsten undergo any adverse chemical reaction with "Dry Ice"?
From: MyPresentCPUisTooSlow, new registered user since 9/06
- Google should give you all the suppliers. [25]--Light current 20:59, 2 October 2006 (UTC)
a few thoughts:
- pure tungsten is rather expensive. at some stage you need decide an allowable level of impurites, because you will never get pure 100% tungsten. i'm not entirely sure of the specifics of your application, but from the description i'm guessing you want the tungsten for its mechanical properties, so i'm guessing you can get away with relatively impure (<99%) tungsten. this will bring down the cost.
- there are much, much cheaper ways to store dry ice if this is your intention, everything from glass dewars down to waxed cardboard and polystyrene (better) store it well.
- tungsten can be cut with a hacksaw apparently, and i imagine if you are using tungsten carbide drills etc you shouldn't have too much trouble. but apparently crappy tungsten can be quite brittle.
- from a (overpriced) supplier, >99.9% foil is availible in thicknesses of 0.05 up to 1 mm (but a 10cm x 10cm x 0.5mm piece is NZ$500)
hope there is something of use here. Xcomradex 21:30, 2 October 2006 (UTC)
Collect a large number of lightbulb filaments, straighten them out a bit, and knit yourself a tungsten sweater for your dry ice.Edison 04:47, 3 October 2006 (UTC)
Nokia phones
I noticed that on most nokia mobile (cell) phones I have ever owned that when I press the * button, the second time a + appears, then a p on the third and a w on the fourth. I know what the + does but what about the p and w, what are they for?
- When you are typing in the code to unlock the phone from a specific network and stuff, I think for some reason p's and w's are involved. Philc TECI 22:37, 2 October 2006 (UTC)
- Is this when entering phone numbers into speed dial/contacts/etc.? I have an LG phone, and at the moment I don't remember exactly how to enter them, but when entering phone numbers into memory, I can include a "p" (representing a brief pause, two seconds I think, before proceeding to the next digit), or a "w" (representing a wait, i.e., when dialing the number, when the phone gets to the w, it waits for the user to press a key before continuing with the rest of the number). Chuck 23:15, 2 October 2006 (UTC)
- I think it is only unique to Nokia. Press star button quickly. Just like you do to get a,b from 2 button.nids(♂) 23:16, 2 October 2006 (UTC)
- Considering that Nokia is a Finnish company, it might be a Finnish abbreviation, but I doubt it. The Fins are not the French. :) DirkvdM 06:09, 3 October 2006 (UTC)
What chemical is this?
What do you get if you bond phosphorus with tungsten and nitrogen? --216.164.249.49 22:30, 2 October 2006 (UTC)
- Some sort of tungsten nitro phosphide?--Light current 22:38, 2 October 2006 (UTC)
- Bets a wikidollar on this being a wordjoke with PWN. - Dammit 22:47, 2 October 2006 (UTC)
- Yes! I wanted to see how long it would take for somebody to get it. --216.164.249.49 22:50, 2 October 2006 (UTC)
- Except wouldn't it be WPN, using the IUPAC nomenclature of inorganic chemistry? Hyenaste (tell) 00:11, 3 October 2006 (UTC)
- 216.164.249.49, you must be the life of your "chemistry for ten year olds" class. Xcomradex 00:28, 3 October 2006 (UTC)
- Don't be such a bismuth technetium hydride. :( Hyenaste (tell) 00:53, 3 October 2006 (UTC)
- no need to be an arsole ;-) Xcomradex 04:27, 3 October 2006 (UTC)
- and you are owned by the clearly superior alloy of phosphorus, tungsten and neodymium. Xcomradex 04:33, 3 October 2006 (UTC)
- I like the quote from the page that arsole is only mildly aromatic. I bet the editors has a good laugh writing that--Light current 06:01, 3 October 2006 (UTC)
Valuable substances
What is the most valuable substance in existance?
P.S. Don't say a substance if the only reason it is valued is because some collector wants it for no other reason than to have it. P.S.S. By substance I mean either a chemical element or a compound(not a mixture). --216.164.249.49 22:46, 2 October 2006 (UTC)
- According to this (www.collegebowl.com/games/05nct01.pdf), it's antimatter at 1.75 trillion dollars per ounce. Clarityfiend 23:16, 2 October 2006 (UTC)
- Maybe anti-platinum? freshofftheufoΓΛĿЌ 03:21, 3 October 2006 (UTC)
I just read this article by random chance, and noticed that it existeed, question, it talks about the environmental impact of lithium ion watch batteries on nature. question, how much "nature" can there be surrounding a suitably large ferrometallic surface? Doesn't a large metal building with lots of people walking in and out of it have more of an environmental impact that a dozen Li Ion cells and LED parts?
- It doesn't actually mention the word "nature", though I understand what you're getting at. The overall environmental impact may be small relative to the amount of pollution that the building they are stuck to causes, but that's not exactly an excuse to just throw these things out. freshofftheufoΓΛĿЌ 03:09, 3 October 2006 (UTC)
October 3
Dying in sleep
Hey there everyone! I got another curious question! I've heard for most my life from teachers, peers, and sometimes colleagues about death from sleep because you die in your dreams. Is it possible that if you do die in your dream you really do die in real life? Just curious if anyone has any articles, people or any stories about this --Agester 00:02, 3 October 2006 (UTC)
- There's an easy answer to that - if it were true, how the hell would we know? Confusing Manifestation 00:12, 3 October 2006 (UTC)
- I've heard of dreams (including my own) where the person involved would have died if the situation was in real life. There's no reason to think that dieing in a dream would kill you in reality, except that it makes for an interesting novel / paranormal kook book / movie / etc. Heck, since one doesn't even know what dieing really is like until arrival, there's no reason to think you even can experience "death" in a dream.
- Not doubt you'll find articles, stories, and probably even books on this if you look hard enough, but they'll all be oogy-boogy nonsense, if anything. -- Consumed Crustacean (talk) 00:24, 3 October 2006 (UTC)
- Sounds like a FAQ to me (or it will be)--Light current 00:27, 3 October 2006 (UTC)
- You know, this would make a mediocre Twilight Zone episode. Clarityfiend 01:00, 3 October 2006 (UTC)
- No. I've died tons of times (and killed myself) in my dreams, and here I am. Friday the 13th (film series) is not real! No harm from a dream will be there when you wake up. Unless you have a heartattack or a stroke or something. Yeesh. — X [Mac Davis] (SUPERDESK|Help me improve)02:15, 3 October 2006 (UTC)
- There's actually a movie called Friday the 13th? When I saw the commercial, I thought they put "Friday the 13th" in there because it's going to be released on Friday, October 13, 2006. --Bowlhover 03:10, 3 October 2006 (UTC)
- Yeah, there are 11 of them. :P -- Consumed Crustacean (talk) 03:17, 3 October 2006 (UTC)
- What did it feel like?--Light current 03:09, 3 October 2006 (UTC)
- From myself, not like you'd really imagine death. Usually something interrupts the dream, or it shifts to another dream that may be partially related (but without any real explanation of how I got from the one dream to the other). No tunnels with light at the end, just dream nuttyness and such fun things. Try falling asleep in a dream, that's a trip ;) -- Consumed Crustacean (talk) 03:17, 3 October 2006 (UTC)
- There's actually a movie called Friday the 13th? When I saw the commercial, I thought they put "Friday the 13th" in there because it's going to be released on Friday, October 13, 2006. --Bowlhover 03:10, 3 October 2006 (UTC)
Well I have a junior psychology text book claiming that there is no evidence of what you are describing. I've also "died" in my sleep and am still here on Wikipedia, so it couldn't be that dangerous. AmitDeshwar 06:12, 3 October 2006 (UTC)
Big Bang / Black Hole ...?
If the early universe was contained within a small volume of space, wouldn't it be inside it's own Schwarzschild radius? If so, how did it get out? Or did it? -- 84.65.71.50 00:18, 3 October 2006 (UTC)
- A heck of a lot of interesting links at Google. Not trying to be rude by saying that, I'm just pointing out that there's some really cool shtuff there. -- Consumed Crustacean (talk) 00:24, 3 October 2006 (UTC)
- It came out the white hole the other end!--Light current 00:28, 3 October 2006 (UTC)
- Actually, we can't know. The laws of physics as we know, including the ones we use to determine the Schwarzschild radius, break up at that level of energy density in the earlier Universe. ☢ Ҡi∊ff⌇↯ 01:47, 3 October 2006 (UTC)
- We could still be inside the primordeal black hole!--Light current 03:10, 3 October 2006 (UTC)
- I agree. See [26]. Pfalstad 03:32, 3 October 2006 (UTC)
- No, no, this is based upon a common misunderstanding: see this entry from the sci.physics FAQ. More generally, the Cosmology FAQ] maintained by Ned Wright (a working cosmologist) is well worth bookmarking. ---CH 04:31, 3 October 2006 (UTC)
- And Pfalstad please note: the page you cited actually is an attempt to explain why the Universe is not, in fact, a black hole, so consistent with the two sources I just cited. HTH---CH 04:33, 3 October 2006 (UTC)
Terrifying, recurring nightmare I used to have...
From being a small child, all the way up to early adulthood, I used to experience a really, really bad dream, perhaps once a month or so. It was always the same - I was lying in my bed, looking up towards the ceiling and there would be a large, shadowy male figure leaning over me, with a balaclava over his face, only his eyes and mouth visible. His hands would be around my neck, choking the life out of me, pressing down on my windpipe with all his weight (I could feel his fingers digging into my throat). I'd try to struggle but I couldn't move my body at all to fight back. Then I'd wake up screaming and screaming and screaming, clawing at the air, gasping for breath. It was always exactly the same - I'd also tend to lash out violently at anyone that tried to approach me when I awoke. It'd often take me half an hour or so of being curled up in the foetal position in a corner to realize that 'it was only a dream' (no matter what anyone told me). One time, I picked up a hunting knife (that I kept near my bed 'for my own protection', heh) and started slashing madly at the air, not even realizing that I'd picked it up by the blade and had sliced my own hand open...
I haven't had that dream for nearly five years now but I still occasionally think about it and it still scares me, slightly. Anyone have any explanations as to what would cause such a violent, vivid dream like that, the same every single time I had it? I don't recall any traumatic event from my childhood that might've brought it on. --Kurt Shaped Box 00:43, 3 October 2006 (UTC)
- Your muscles are paralysed whlst dreaming- so how dod you pick up the knife? Do you do sleepwalking?--Light current 00:49, 3 October 2006 (UTC)
- I think I sleepwalked a couple of times when I was really young. I think the knife thing happened in the 'just awoken in terror - not sure if my assassin is still there/don't really know what the hell I'm doing' phase of sheer panicked frenzy. In the past, when it happened, when one of my parents ran into my room because I was screaming, I'd sometimes bite them, punch, kick or try to scratch their eyes out before I fully regained my senses. --Kurt Shaped Box 00:58, 3 October 2006 (UTC)
- Sounds like a night terror. I remember watching a video about them, and a common occurence was for people to sense a man in black or a large dark woman who was strangling them. Hyenaste (tell) 00:56, 3 October 2006 (UTC)
- How do you get the 'large (sexy) dark woman' dream please? 8-))--Light current 00:59, 3 October 2006 (UTC)
- Have you tried summoning a succubus? ;) --Kurt Shaped Box 01:03, 3 October 2006 (UTC)
- The large woman is not sexy. Hyenaste (tell) 01:23, 3 October 2006 (UTC)
- Who says so? 8-)--Light current 01:31, 3 October 2006 (UTC)
- The large woman is not sexy. Hyenaste (tell) 01:23, 3 October 2006 (UTC)
- It also usually happened within an hour or so of me getting to sleep, if that's any help. --Kurt Shaped Box 01:05, 3 October 2006 (UTC)
- Is an hour enough time to get to stage 4 sleep? The article doesn't say! Hyenaste (tell) 01:23, 3 October 2006 (UTC)
- Actually, this sounds more like me... --Kurt Shaped Box 01:49, 3 October 2006 (UTC)
- Nope, hypnagougia is a lot different. Your parasomnia is a night terror. They are not uncommon. — X [Mac Davis] (SUPERDESK|Help me improve)02:12, 3 October 2006 (UTC)
- Actually, this sounds more like me... --Kurt Shaped Box 01:49, 3 October 2006 (UTC)
If requesting medical advice, please consider asking a doctor instead of Wikipedia. That said, there are sleep disorders in which the paralysis normally present during dreaming is absent, and a dreamer may jump up and "fight" with the boogeymen attacking him, injuring himself or a bedpartner. This is a related to sleepwalking. Some medications may make it more likely. A sleep study can be done for a few thousand dollars at a sleep center of a hospital. The dreaming about someone choking you suggests obstructive sleep apnea.Edison 04:57, 3 October 2006 (UTC)
Dreaming of dying
Has anyone here dreamed of life after death? If so, what is it like? I've always wondered this since I dreamed of falling from a high place. However, in that dream, I woke up before I hit the ground. --Bowlhover 03:15, 3 October 2006 (UTC)
- Falling is quite a common dream I believe. When I used to have that one, I would slowly decelerate and end up on a very soft pile of pillows-- end of dream!--Light current 03:18, 3 October 2006 (UTC)
- Like an episode of ReBoot, combined with purple dogs. Really, dreams are incredibly variable from person to person and night to night (and I am serious about that dream). -- Consumed Crustacean (talk) 03:30, 3 October 2006 (UTC)
- I am either an intangible entity, or there is just another of me. — X [Mac Davis] (SUPERDESK|Help me improve)03:43, 3 October 2006 (UTC)
- See various scriptures for other dreams of life after death. --GangofOne 04:38, 3 October 2006 (UTC)
Measuring Mass/Volume
I need to know how to measure the mass and volume of an object like a rock or a nail. Can someone please help me?
-Thanks, anon
- Mass: buy a scale, put the object on the scale, and take a reading.
- Volume: Buy a graduated cylinder, fill it partially with water, and take a reading. Then throw your object in (making sure it's completely submerged) and take another reading. Subtract the first reading from the second, and that's the volume. --Bowlhover 04:11, 3 October 2006 (UTC)
That stuff between your toes
Wher does it actually come from & what is it?--Light current 05:06, 3 October 2006 (UTC)
- It's the ichor of the Sons of the Bird. It was put there to make them fearful. And no, I'm not going to tell you what your profession is; you should have figured it out by now. --Trovatore 05:32, 3 October 2006 (UTC)
- Everybody's a critic. Oh, wait... Clarityfiend 05:48, 3 October 2006 (UTC)
- I'm guessing that it's similiar to Navel lint. Mostly material from your socks. -- Consumed Crustacean (talk) 05:41, 3 October 2006 (UTC)













