Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
September 12
libya
Moved to Wikipedia:Reference_desk/Miscellaneous#libya - StuRat (talk) 01:14, 12 September 2012 (UTC)
Toothbrush disinfection
I tried bleach on my toothbrush to kill the germs, but, unfortunately, the bristles fell out. I'm considering trying either alcohol or hydrogen peroxide on my new toothbrush. So, will those also destroy my toothbrush ? StuRat (talk) 01:20, 12 September 2012 (UTC)
- I've used H2O2 (three percent) and do not recall it having any negative effects on the bristles, at least in a shorter time frame than you're supposed to replace it anyway. Of course I haven't actually tested whether it kills the germs. Also, I'd rinse it well before putting it in your mouth. --Trovatore (talk) 01:28, 12 September 2012 (UTC)
- Hydrogen peroxide is not very good as a disinfectant, as I understand it. Alcohol should do the trick, and I doubt it will cause much damage to the bristles, as opposed to bleach, which depending on the concentration is going to be a great deal more corrosive than alcohol. Evanh2008 (talk|contribs) 01:30, 12 September 2012 (UTC)
- Um, why are you worried about germs on your toothbrush? Unless you are putting it to unconventional use, the only place it is likely to get germs from is your mouth, and all you will be doing is putting them back (quite possibly dead) the next time you use it. Still, if you are that worried, according to WikiHow, who cite the American Dental Association, washing it under the tap both before and after use is sufficient most of the time, and you can place it inside a dishwasher occasionally for a deep cleanse: [1] I'd be wary of using a solvent like alcohol, as again the bristles might fall out, and neither bleach nor hydrogen peroxide (in germ-killing concentrations) sound particularly sensible substances to use on anything you intend to put in your mouth, even if they don't damage the toothbrush. AndyTheGrump (talk) 01:37, 12 September 2012 (UTC)
- Actually, it's a serious concern, for sort of disgusting reasons, if you keep your toothbrush where most people do (in the bathroom). I'm sure someone will be along to explain any minute now. --Trovatore (talk) 01:40, 12 September 2012 (UTC)
- One would suspect that if casual contamination or harm from germs in the bathroom was anywhere near a common occurrence to be a "serious concern" that it would 1. be something the ADA would mention it, 2. someone would sell a product for it, and 3. we'd all know people who routinely were injured in this fashion, probably including ourselves. I'm going to file this under "more things that StuRat worries about that nobody else does" unless there is some real scientific evidence of probability of harm. --Mr.98 (talk) 01:53, 12 September 2012 (UTC)
- Harm is not so clear, but contamination, yes. I'm not going to search for a cite from work, but this has been studied. Basically, flushing aerosolizes some of the contents of the toilet. Whether that's a problem depends, I suppose, on who shares the bathroom with you, and what they put in the toilet. --Trovatore (talk) 01:56, 12 September 2012 (UTC)
- Closing the lid before you flush should greatly diminish this problem. Lova Falk talk 08:23, 12 September 2012 (UTC)
- ... or keep your toothbrush in a closed container or small bag, or store it outside of the bathroom altogether. Seems to me there are much simpler and safer solutions to this problem than dipping your toothbrush in bleach etc. Gandalf61 (talk) 14:31, 12 September 2012 (UTC)
- On the other hand, the ADA suggests storing it in a closed container may not be good idea [2]. But see below.... Nil Einne (talk) 18:44, 12 September 2012 (UTC)
You can also put your tootbrush in the microwave. Count Iblis (talk) 01:41, 12 September 2012 (UTC)
- I'm not sure that would be a good idea. Apparently in some toothbrushes at least, the bristles are held in with tiny metal staples. Then again, I'm not sure that microwaving germs will necessarily kill them anyway - are you proposing to immerse the brush in water as you microwave it? AndyTheGrump (talk) 01:48, 12 September 2012 (UTC)
- (ec)Erm, technically this is a medical device, and suggesting methods of modifying it is medical advice. It's conceivably possible that you could leach out the plasticizer and make the bristles harder than the dentist would recommend, or that hydrogen peroxide could react with it to form some carcinogen, etc. Therefore I will say do not rely on any suggestions here for what you do with it. Wnt (talk) 01:51, 12 September 2012 (UTC)
- Humm, maybe you're right - though 'wash it with tapwater' is hardly medical advice I'd have thought... AndyTheGrump (talk) 01:54, 12 September 2012 (UTC)
- Well, personally I think that answering a medical question with a link to a professional medical organization's statement on the topic should always be permitted, but as we know there's a range of opinion on that around here... Wnt (talk) 02:02, 12 September 2012 (UTC)
- And in the best tradition of science reference desk original research, I can confirm that in some toothbrushes at least, a metal doo-dah is indeed used to retain the bristles. I've just sawn part of the head off one (used, needless to say - I'm not made of money ;-) ) and found a tiny sliver of metal, about 2mm x 1.2mm x 0.2mm (roughly - I've not got the tools to measure accurately) at the bottom of the brush-holes. AndyTheGrump (talk) 02:09, 12 September 2012 (UTC)
- This is all ridiculous. The mouth naturally contains a massive contingent of bacteria; a toothbrush can't possibly make a meaningful difference. Looie496 (talk) 02:19, 12 September 2012 (UTC)
- Obviously the toothbrush could make a difference. If you were living in Haiti and your toothbrush was five feet from the toilet somebody with cholera just used, you'd be reaching for the bleach! (Nay, come to think of it, in that situation who needs to brush...) Counting total numbers of bacteria is a common fallacy. Wnt (talk) 02:39, 12 September 2012 (UTC)
- Right, it's not just how many bacteria, it's which bacteria. Inside the mouth, the bacteria will normally be kept in check, but outside, you have a wet toothbrush with mouth bacteria, bits of food, and various toilet flushings sitting around like a petri dish. God only knows what will grow there. The ADA does suggest replacing brushes constantly, but I'm looking for a cheaper alternative. StuRat (talk) 03:40, 12 September 2012 (UTC)
- There appears to be a fair amount of dispute of whether you really need to replace your toothbrush due to bacterial contamination. There appears to be little dispute you probably should do it due to physical degradation. See [3] which while a forum discussion with a fair amount of typical forum nonsense does include some sourced information, include that the CDC say there is no evidence reusing toothbrushes can lead to (re)-infection. Nil Einne (talk) 08:15, 12 September 2012 (UTC)
- It's true that there are common soil microbes that can cause disease, like botulism or blastomycosis. But by and large, there is no evolutionary incentive for environmental bacteria to be harmful. So far as I know, most of the common food poisoning bacteria (like Salmonella or EPEC) come from humans or related organisms. And it seems hard to argue with the empirical observation that (unless someone ill is present in the environment) people don't generally seem to get sick from their toothbrushes, not even counting the demonstrable particulates from the toilet. It is possible to argue that theoretically there is some unnoticed rate of illness, but balance that against the hygiene hypothesis of asthma, i.e. that lack of exposure to microbes might be harmful. In the end we live in a world where there's a certain amount of turd in everything, whether it's from dust mites or the Old Faithful toilet. Wnt (talk) 15:14, 12 September 2012 (UTC)
- I don't think there could be a cheaper alternative to replacing toothbrushes constantly because dental care costs money too. The bristles also splay out after some use, making them less effective. But shaking out water after use and allowing to dry in circulating air seems like a good idea. There are two-half plastic holders for toothbrushes which have holes in them for air circulation. Sunlight would seem to be a good disinfectant but I haven't tried displaying my toothbrush on the windowsill yet. Apparently ultraviolet light is used for this purpose too. Vinegar as well as "3-percent hydrogen peroxide" are mentioned as possibilities here as well. Bus stop (talk) 15:37, 12 September 2012 (UTC)
- BTW I came across this earlier, it didn't seem that useful being a paid Colgate advert, but I now notice it mentions a study suggesting how much the effectiveness of a toothbrush can change over time [4]. Also looking at the ADA's current statement [5], even they don't seem to suggest contamination is a big reason for replacement, they too concentrate on physical degradation. (The replace due to contamination primarily comes in to play after you recover from certain diseases.) A number of suggested methods for cleaning a toothbrush are mentioned in the forum post I linked above including a dishwasher and mouth wash, but personally I'm with the person in this source [6]. Doing anything other then clean with room temperature water runs the risk you're going to damage the toothbrush more. (This includes sunlight/UV light.) And considering we have little real evidence of the risks of contamination, but evidence of the negative effects of physical degradation, there's a good chance you're causing more harm then you're preventing. Nil Einne (talk) 18:44, 12 September 2012 (UTC)
- Buy a new toothbrush? 203.112.82.128 (talk) 17:08, 12 September 2012 (UTC)
- Soak the bristles of the toothbrush in a small amount of mouthwash. — Preceding unsigned comment added by 148.177.1.210 (talk) 17:19, 12 September 2012 (UTC)
- Mouthwash is a good idea, especially since it's designed to kill oral bacteria. I'll try that. StuRat (talk) 20:19, 12 September 2012 (UTC)
I should probably mention the reason I first used the bleach. My toothbrush smelled bad, and I didn't want that smelly thing in my mouth. To me, this was most likely the result of bacterial growth. StuRat (talk) 20:19, 12 September 2012 (UTC)
- Medical advice or not, it's safe to say that if your toothbrush smells bad, it's high time to get a new one. ←Baseball Bugs What's up, Doc? carrots→ 03:59, 13 September 2012 (UTC)
- Or disinfect it, hence this Q. StuRat (talk) 04:16, 13 September 2012 (UTC)
- Or stop cleaning your teeth, if you are really concerned that the apparently-minor risk of something nasty sneaking in on your toothbrush out-weighs the more obvious risks of not cleaning them - including quite possibly bad breath, which has the advantage that you are less likely to come into close contact with other germ-carrying individuals. Your choice... AndyTheGrump (talk) 04:26, 13 September 2012 (UTC)
- Did you read the referenced discussion above? It's not clear how disinfecting it is going to help the fact that by the time it starts to smell bad, it's likely sufficiently degraded to require replacement. If you only brush your teeth because someone will nag you otherwise, then I guess disinfecting it might help since there's a chance after several years there might actually be some risk from contamination. But if you're like most people and brush your teeth to actually clean your teeth and reduce the risk of dental disease, then the merits of disinfecting remain unclear. There's also BusStop's point. Perhaps you have gold plated dental insurance which you don't pay for or something, or you live in some odd corner of the US where toothbrushes cost a few hundred dollars. If not, the cost of a new toothbrush could easily be 2 orders magnitude less then a single visit to the density or extra surgery required. So the cost savings from not replacing your toothbrush every few months are fairly unclear as avoiding even a single extra surgery or visit in 10 years could easily pay for replacing your toothbrush every 2 months in the same timeframe. Nil Einne (talk) 04:36, 13 September 2012 (UTC)
- If I wait 2 months, my toothbrush stinks. I will take the advice of soaking it in mouthwash each day. This should cost a penny a day and keep it smelling fresh. I will mark this Q resolved. StuRat (talk) 04:59, 13 September 2012 (UTC)
- Where I live, I can get toothbrushes 4 for a dollar at the local dollar store. Certainly, I can pay more, but with the cheapest toothbrushes I can buy, I could replace once a month at a cost of less than a penny per day. At that level, the cost of replacing a stinky toothbrush shouldn't be an issue. --Jayron32 05:02, 13 September 2012 (UTC)
- It would still stink by the end of that month, while it won't if I soak it in mouthwash. I also question the quality of a 25 cent toothbrush. StuRat (talk) 05:31, 13 September 2012 (UTC)
- Fair enough. But even the fancy high-end toothbrushes with the allleatherinterior and the power moon roof still cost like, what, $5.00? At some point the penny pinches back... --Jayron32 05:36, 13 September 2012 (UTC)
- If the toothbrush becomes rancid quickly, the problem might not be the toothbrush. It's time for StuRat to pay a visit to his dentist. ←Baseball Bugs What's up, Doc? carrots→ 11:26, 13 September 2012 (UTC)
Strange new observation: I dumped out the bleach and replaced it with mouthwash. The next day, the mouthwash had changed from green to blue. Could it have reacted with some remaining bleach ? StuRat (talk) 06:51, 15 September 2012 (UTC)
- Sounds likely. Bleach is highly alkaline[7], mouthwash is slightly acidic, and some of the color additives of the mouthwash may have pH indicator properties (although I could not identify any such substances, after googling the color additives of various brands of mouthwash). Another possibility: bleach is known for degrading colored substances. The green color have been produced by a mixture of blue and yellow dyes. Maybe the bleach degraded the yellow dye, but not the blue one. --NorwegianBlue talk 21:17, 15 September 2012 (UTC)
- Interesting. So I've just invented a test for residual bleach. We can use it to determine if an apparently clean suspected murder weapons was bleached clean. :-) StuRat (talk) 17:49, 16 September 2012 (UTC)
Global Ischemia and Death
I'm wondering about this--are people with global ischemia (who get revived) completely dead for several minutes or just don't have a functioning brain (brain activity) for those several minutes while the rest of their body works properly? Also, people with global ischemia can have their whole "mind" (personality, likes, hobbies, etc.) restored if they had global ischemia for several minutes or less, right? Futurist110 (talk) 02:18, 12 September 2012 (UTC)
- It helps if you provide a link to what you are talking about. μηδείς (talk) 02:34, 12 September 2012 (UTC)
- (ec)Hmmm... brain ischemia#global brain ischemia speaks of restoration after loss of heartbeat. Clearly neurons don't die the instant blood stops pumping - it takes time for the oxygen to run out, among other problems. The death is not a physical law - it can be altered by methods such as treatment with sodium valproate. [8] Deciding when a cell is dead is an example of something that is very, very difficult to define philosophically but all too easy to measure by experiment... Wnt (talk) 02:36, 12 September 2012 (UTC)
- If they come back, their brains did not entirely cease to function. If electrical activity in the brain completely disappears (sometimes known as "flatlining"), the patient is dead, dead, dead, never to return, end of story, dead. Looie496 (talk) 05:29, 12 September 2012 (UTC)
- Actually our article on brain death gives the impression that this is not absolutely certain, though with the caveat that the activity might simply not be detectable with the equipment used. It's unsourced there - ought to come up with better references... Anyway, in theory there's no reason why neurons couldn't stop firing entirely for some reason (a drug that blocks the action potential) and yet, if life support can be maintained, return later to normal function. Wnt (talk) 15:35, 12 September 2012 (UTC)
I was under the impression that electrical activity in the brain stopped completely with global ischemia? Am I wrong? Futurist110 (talk) 23:04, 12 September 2012 (UTC)
Late summer sky color
I have often noticed that in the Eastern and Midwest U.S., the sky takes on a hauntingly beautiful shade of blue in the late summer and early fall (this year, starting August 13). The sun takes on a similarly more attractive shade of golden, and together, the impact of the blue and golden on the dark vegetation of summer can be just breathtaking. At night, especially later in the season, the sky can take on a uniquely "electric" indigo shade. What's odd is that it seems so specific to this season, yet it can occur in 90-degree weather or 50-degree weather, in cloudless sky, sky half filled with cumulus clouds, sky marked with the strange swirls of cirrus that came with this week's unusually cold weather. I still do not even know for certain that it is not some purely psychological phenomenon on my part. Is this effect something known? And if so, is it explainable? Wnt (talk) 02:55, 12 September 2012 (UTC)
- I don't have a specific answer, but in general the specific color of the sky is highly dependent on the exact angle which the sun's rays are striking the atmosphere, and this angle will be highly dependent on both the time of day and the time of the year. The coloration of the sky is largely due to processes called Rayleigh scattering and Diffuse sky radiation, and accounts for the blue sky at midday and also the red sky which appears just before sunset. So, I don't know exactly what combination of effects causes the specific shade of blue you are noting (though your vivid description brought it to my mind as well!), except to direct you to the general processes that create the sky's color; it is likely just a specific combination of the normal variables that affect the color of the sky in general. --Jayron32 03:47, 12 September 2012 (UTC)
- Just a thought: Do you happen to go out at the same time each night, for a walk, say ? If so, then this might be the time of year when your walk time corresponds with sunset. There should be a second time in spring, but maybe it tends to be rainier then. StuRat (talk) 04:03, 12 September 2012 (UTC)
- No - the color varies by time of day, but it can reach this unique intensity at any of them - from the deep blue of a bright noonday to the deep indigo of late twilight. Wnt (talk) 05:01, 12 September 2012 (UTC)
- Funny, I remarked the exact same thing to myself tonight, and assumed it was a combination of the humidity and the angle of the sun. μηδείς (talk) 04:07, 12 September 2012 (UTC)
- Particulates high in the atmosphere can cause pretty sunsets. These are caused by volcanoes, forest fires, and perhaps sandstorms. Volcanoes shouldn't vary with the weather, but fires might, and sandstorms in Africa do sometimes carry particles over to the US. StuRat (talk) 04:22, 12 September 2012 (UTC)
- The sky I saw tonight went from a brilliant cyan with magenta clouds just before sunset to a rich emerald and then a deep teal after. I don't think there's anything like a volcanic eruption going on in NJ. I though particulates usually cause red sunsets. Is there some current eruption or fire in the Northern Hemisphere that has been reported as causing brilliant sunsets? My suspicion is what I saw was more due to the clarity of the sky and the angle of the sun, but I have never seen it greener. I also remember the sky on 9/11 being one of the clearest and most brilliant I have ever seen. μηδείς (talk) 05:00, 12 September 2012 (UTC)
"Strike as non-responsive."
|
|---|
|
- It's fair humor - I flew out of Newark a couple of times in the 80s and the city deserved the gibes then; maybe they helped prod it to clean up its act (which as I understand, it has done significantly) and if so, by virtue of saving many human lives, these jokes' ethics are beyond reproach. And for our question, artificial sources of particulates certainly should be considered seriously. That said... I'm not sure any industrial pollutant would be worse this particular time of year, though the great decrease in rainfall should reduce the amount that is purged from the atmosphere. My gut feeling, of course, is that pollution couldn't possibly be so pretty, but that's not a scientific argument. :) Wnt (talk) 14:54, 12 September 2012 (UTC)
- I think of this as a regular yearly phenomenon (subjectively to me, this effect together with the singing of cicadas and katydids define a season of "latesummer"). So I would discard thoughts of an eruption out of hand. Sahara dust is my leading candidate, but quick searching points to it creating haziness in Florida [9] and being limited to the South [10]. Even so, I suppose that a super fine subpopulation of the dust might make it further north and have these effects? But without seeing proof, I don't really know. Wnt (talk) 05:16, 12 September 2012 (UTC)
- Yes, it needs to be a very light layer of particulates, or you just get a dark sky. It's like the difference between light mist causing a rainbow and torrential rains just making the sky dark. StuRat (talk) 06:58, 12 September 2012 (UTC)
- Yes, it needs to be a very light layer of particulates, or you just get a dark sky. It's like the difference between light mist causing a rainbow and torrential rains just making the sky dark. StuRat (talk) 06:58, 12 September 2012 (UTC)
- Medeis: do you mean the Green flash? Ssscienccce (talk) 16:15, 12 September 2012 (UTC)
- No, I couldn't actually see the sun itself when it set, the entire sky was a distinctly greenish shade near sunset, the likes of which I have seen on maybe two or thee other occasions, somewhat like the teal green in our article, but luminous, of course. And I am not sure how true the colors are on my computer, so don't 'quote' me on that. It will be interesting to see if the weather is the same today. μηδείς (talk) 17:16, 12 September 2012 (UTC)
- Medeis: do you mean the Green flash? Ssscienccce (talk) 16:15, 12 September 2012 (UTC)
The sky was very similar again last night, with green, orange and grape-soda purple at the horizon about an hour before sunset, depending on which direction you were looking in, although I was too busy to see the color overhead at sunset. Not a single cloud was visible, which makes me think the humidity and sun angle are relevant. μηδείς (talk) 17:53, 13 September 2012 (UTC)
Janet Armstrong
What happened to the first wife of Neil Armstrong after her divorce? I've looked on the internet but she's not been mentioned except in one New York Times article about events in Neil's life where it briefly mentions that she lives in Utah. I'm wondering if she ever re-married and when she ended up moving. If this is in the wrong place, please just move it to the correct area and write where you put it on my talk page; I wasn't sure if this counted as humanities (because it was biographically related to Neil Armstrong's life) or here (because Neil Armstrong was the first man on the moon) so I chose here. --Thebirdlover (talk) 03:30, 12 September 2012 (UTC)
- Doing a people search on Janet Armstrong and her maiden name, Janet Shearon, I only found one that seems old enough, a 75 year old Janet Ann Armstrong, who has lived in Pahrump, Henderson, and Las Vegas, NV, as well as Apache Junction, AZ and Stockton, MO. No guarantee this is her, but it's possible. Does anyone know if her middle name was Ann ? StuRat (talk) 03:59, 12 September 2012 (UTC)
- Not the right one. His first wife was Janet Elizabeth Shearon, you might need to scroll down a bit. CambridgeBayWeather (talk) 23:58, 12 September 2012 (UTC)
Use of question mark and exclamation mark above equals signs
I have seen the notation and used before, and I have forgotten where. (It may have been in a highschool math textbook, maybe)
: "we would like to show that X equals Y", or "we wonder if X eqals Y"
: "Yes, X does indeed equal Y, as is now obvious"
These notations are not entirely unknown on the internet, I have found several stack overflow threads in which people ask how to produce them in LaTeX, and other threads in which people ask what they mean. However, it appears they are less well-known than I thought. So, I am looking for actual mathematics/physics papers in which these symbols are used. 81.11.174.45 (talk) 06:19, 12 September 2012 (UTC)
- I think that must be very well-known. I use it in my scratch notes when I'm trying to prove something. However, a search on Wikipedia fails to find any articles here that use it. But I'm sure it's out there somewhere.
- As for , I don't recall seeing that before, though its meaning seems obvious. Duoduoduo (talk) 21:22, 12 September 2012 (UTC)
- My feeling is that they are primarily used in notes, blackboards, and textbooks; not research papers. Really, starting with the first symbol and terminating with the second is like starting with an ansatz and ending with a Q.E.D.. My point being, researchers are likely to handle these issues with words rather than special symbols whose meanings would have to be carefully defined. SemanticMantis (talk) 21:30, 12 September 2012 (UTC)
- This German language mathchat site uses it. I don't read German so I don't know if it might help you find some published papers on the chat topic that use it. Also this one. Duoduoduo (talk) 21:46, 12 September 2012 (UTC)
- Wikipedia does have the disambiguation page =?, which mentions a unicode symbol with the question mark on top. Duoduoduo (talk) 22:17, 12 September 2012 (UTC)
- It's hard to search for this, but coincidentally a few hours after seeing your question I spotted in Exploratory Experimentation and Computation by Bailey and Borwein. I think I've never seen , and I would probably guess that it meant "is, surprisingly, equal to", not "has now been shown to equal". -- BenRG (talk) 00:50, 13 September 2012 (UTC)
- Can I just say in passing what a joy it is to learn new things on the reference desks. Had I not seen this thread, I could only have interpreted as an enquiry as to whether I have a predilection for liquorice allsorts. Now I know. AndyTheGrump (talk) 04:07, 13 September 2012 (UTC)
Gravitational pull vs. push
I saw a documentary about exoplanets the other day. They were talking about the way exoplanets make their stars wobble slightly, and that that wobbling can be detected by telescopes, and thus the exoplanets can be detected although they don't reflect enough light to show up themselves.
One of the animations was way off, though. The planet was moving around the star in a circle, and the star was moving more slowly. But the star was always moving in the same direction. Ascii art:
* .
. *
* .
- etc.
As far as I can tell, that kind of orbit means that the planet is attracted by the star, but actually repelling it. I think of this motion that it's plain impossible, however I tried to look at the math and find a solution to prove that it's impossible. My best result so far was that, to make that motion plausible, the center of gravity must stay at the same place during the orbit, or move at a constant speed and in a constant direction. If I call the masses involved M and m, and the locations of the bodies X and x, I get an equation like
- M X(t) + m x(t) = c, c being independent of t and of dimension (kg m).
thus if both locations have the same sign, the m asses must have opposite signs, not exactly a common property among celestial bodies.
Which would result in m repelling M, but also in M repelling m.
So, while the negative masses seem to prove the animation wrong, I proved at the same time that there is no stable orbit at all. As sure as I am that the animation is wrong, my calculations look wrong too, but I cannot seem to nail the error.
Is it in assuming M and m independent of t? They should be if they were moving in perfect circles? - ¡Ouch! (hurt me / more pain) 07:54, 12 September 2012 (UTC)
- In a 2-body system, both objects should orbit about the barycenter (center of mass) of the system. The barycenter will be much closer to the star, perhaps even inside it, but not at it's center. So, yes, the animation looks off (was this the Discover Channel, by any chance ?). In your equation, it isn't one of the masses which is negative, but rather one of the displacements, where negative means it is in the opposite direction from the barycenter as the other displacement. StuRat (talk) 09:49, 12 September 2012 (UTC)
I know that X(t) and x(t) are the pair which have opposite signs in real life. The point was that I wanted to explore what configuration could exhibit the behavior the animation showed, i.e. the data they fed into the computer in the first place, to produce such a degenerate case of orbit. A sign error looks quite possible, as the bloody comp will process any parameters you feed it no matter how whack-off they are.
If M and m have opposite signs, the barycenter is no longer between the two bodies, though. Effectively, if we put the barycenter into the origin, the equation simplifies to
- M X(t) = - m x(t),
which does not explain either what repels the star from the planet.
Sorry, can't say if it was on DC, I was so waisted it could have been on Complete Nonsense Network and I wouldn't remember. Now I'm sober and my brain is still doing barrel rolls trying to find the parameters for that kind of orbit. - ¡Ouch! (hurt me / more pain) 10:51, 12 September 2012 (UTC)
- Looking at the real world case, if we start with your equation:
M X(t) + m x(t) = c
- But let's just use 0 for the constant:
M X(t) + m x(t) = 0
- Now make one of the displacements negative:
M X(t) + m (-x(t)) = 0
- We get:
M X(t) - m x(t) = c
- Moving it to the other side, we get:
M X(t) = m x(t)
- If you're trying to figure out an equation to produce that nonsense animation, that seems like a pointless exercise. I suspect they just entered circular orbits for both into their animation software, around a point not between the two. So, they defined two independent circular orbits, not taking gravitational attraction into account at all. Maybe you can imagine both orbiting about a black hole, to make it all make sense (although the orbital speeds would be different, in this case). They probably weren't using astronomy software at all, but just some general animation program, which is happy to let you define circular motion however you want, with no consideration made for gravity. StuRat (talk) 11:05, 12 September 2012 (UTC)
I think I found the answer myself. Classifying forces as "attracting" and "repelling" is misleading if masses can be negative. Newton's F = m a shows that acceleration changes sign if the mass does. What this means is that a planet will orbit a star in the same way regardless of its mass (assuming it is much less than the star).
Say, the planet is Earth, m = mE.
Then, gravity is F = G M mE / (X(t) - x(t))2, and a = G M / (X(t) - x(t))2.
If the planet's mass is v times the mass of Earth, we get
F = G M v mE / (X(t) - x(t))2, and a = G M / (X(t) - x(t))2.
v cancels out, including the case v < 0. Negative inertia is a bit counterintuitive.
However, the impact the planet has on the star does depend on m. And the original solution was correct. I was wrong in thinking that there wouldn't be a stable orbit. The negative inertia fooled me.
The more important point is, however, (as you pointed out, Sturat) that they probly didn't feed any simulation data into the animation, and that their "scientific advisors" were too dazed (even more so than I was???) to catch their mistake.
And thanks for not picking up on "waisted", ;) - ¡Ouch! (hurt me / more pain) 07:01, 13 September 2012 (UTC)
/ \/ Resolved. </barrel roll>
- You're welcome. I'm amazed at how fake many documentaries and science shows are. I once saw one featuring Hero's engine which showed it rotating the wrong way. I can only conclude that they rigged an electric motor to it, to get it to spin. Then I saw a show where they demonstrated "the proper way to build a campfire". They insisted the large logs go on the bottom, then medium sized on top of those, then small twigs, with kindling on the very top. They said this is needed to keep it all stable. Only problem, is, of course, you could never light a fire this way. They were having no luck, until they went to commercial and came back, and it was blazing away like it had just been doused in gasoline. :-)
- And here's a proper resolved tag, although yours was a valiant effort. :-) StuRat (talk) 07:20, 13 September 2012 (UTC)
sound to electrical energy conversion
sound is a form of energy. each day we speak a lot i.e we waste a lot of energy. i want to use this energy. as well as there is a lot of noise in surrounding. how can we utilize this? atleast we can charge our cell phone. so, how can i convert this energy into electrical energy? — Preceding unsigned comment added by 110.172.159.134 (talk) 12:44, 12 September 2012 (UTC)
- Sound isn't very much energy. The Io of Sound intensity level is 10-12 w/m2. (Compare with the roughly one kilowatt per meter squared of sunlight. I.e. if we could "hear" sunlight it would be at 150 dB, which is well above the threshold of pain. And I don't see very many directly solar powered cars on the road. (Instead they use liquified fossilized sunshine.)) Hcobb (talk) 14:09, 14 September 2012 (UTC)
- To elaborate on that, according to the source our article cites at [11], the sound intensity level of 60 dB SIL "conversational speech" is 0.000 001 W/m2 Assuming you're in a big cubical room of, oh, 10 meters on a side, a speaker being heard anywhere in the room would need to achieve this over 600 square meters, which would take 0.0006 watts of power. Now note that someone with a basal metabolic rate of 1500 kcal/day = 6300 joules/day = 0.08 joules/second (watts) is expending 1300 times more energy lying in bed than is actually needed for you to hear him in conversation, which tends to sap the drive for improved efficiency. (Hope I didn't foul up the math again...) It would be interesting to make the same calculation for a cicada which puts a higher priority on being heard. Wnt (talk) 15:27, 12 September 2012 (UTC)
- There are instruments that convert sound to electrical energy. They are called microphones and your cell phone has one. --Wrongfilter (talk) 15:44, 12 September 2012 (UTC)
- The only practical use I know of is a sound-powered telephone using a balanced armature design for efficiency. Ssscienccce (talk) 16:11, 12 September 2012 (UTC)
- A seismograph also uses the energy in vibrations, including sound, to drive the needle. StuRat (talk) 20:12, 12 September 2012 (UTC)
Laser Light as Propulsion?
Would it be possible, theoretically, to use the 'pressure' of laser light to move a solar sail through space? Or would Newton's Third Law, (i.e., "every action produces an equal and opposite reaction") prevent this?Honeyman2010 (talk) 17:37, 12 September 2012 (UTC)
- We have a Laser propulsion article, which talks mostly about ground-based lasers hitting spacebourne vehicles. -- Finlay McWalterჷTalk 17:40, 12 September 2012 (UTC)
- Right. You couldn't use laser light emitted by the vehicle itself to move the vehicle, but you could use laser light emitted by an external source, conceivably very far away. Looie496 (talk) 17:57, 12 September 2012 (UTC)
- In terms of the physics of photons and momentum, there's no reason the laser can't be on the craft. "Couldn't" only enters in once you're talking about engineering tradeoffs. — Lomn 18:19, 12 September 2012 (UTC)
- I may have been unclear. If the laser is on the craft and you fire it at a sail attached to the craft, you aren't accomplishing anything. You could however fire the laser into empty space, using it as a sort of rocket. That would be a terrible strategy, though, since you would be using a maximum of energy to get a minimum of momentum. Looie496 (talk) 18:56, 12 September 2012 (UTC)
- With current technology, that's quite true. However, if you had a nearly infinite source of energy, let's say we get on-board fusion reactors working, and didn't need much thrust, let's just say "station keeping" (maintaining an orbit), then it might be more of a reasonable choice to launch particles at the speed of light. However, there's no need for the particles to be collimated, unless you need the beam to stay together to also drive another vehicle (say when two spacecraft separate). Since very little mass is ejected when you launch particles at the speed of light, you don't need to be worried about running out of material. StuRat (talk) 20:08, 12 September 2012 (UTC)
- Regarding a laser-firing-at-its-own-craft's-sail—how (and how well) it works depends on what the sail is made from. If it is a reflective material, then the sail will reflect the beam and give you the same amount of thrust as pointing the beam straight into space, albeit in a different direction. If the sail is nonreflective, then it will absorb the laser light and heat up; eventually it will emit blackbody radiation from its face and produce thrust in that way. It only fails to produce thrust if all of the reflected/emitted light is recaptured by other parts of the spacecraft, or if the sail is both nonreflective and sufficiently thin and thermally conductive that blackbody radiation is emitted equally from both of its faces.
- I can see the potential value of a small reflective sail on a photon rocket; the sail would be used to vector thrust without requiring reorientation of the entire laser. TenOfAllTrades(talk) 21:47, 12 September 2012 (UTC)
- Lasers are used because they allow a distant sail projectile to be targeted. If you don't need to do this, and you're carrying the light source on-board the vessel, then you don't need to use a laser and you don't need to suffer the laser's inefficiencies. You can use a simpler and more efficient light source. Andy Dingley (talk) 20:28, 12 September 2012 (UTC)
- Lasers of sufficient strength are big and heavy and require big and heavy power sources. They also don't let out a huge amount of energy for all of that weight and trouble. Their one good thing is that they can transmit their energy over long distances. So this is why most laser propulsion schemes are about putting the big, heavy, energy-hungry part — the laser — on the ground, and using it to project a high-energy beam of light to something that is lightweight, pushing it along. If you aren't going to do that, then you don't get anything beneficial out of laser propulsion. --Mr.98 (talk) 18:50, 12 September 2012 (UTC)
See also problem 7.5 on page 13 of this set of test problems. Count Iblis (talk) 19:48, 12 September 2012 (UTC)
Also, we have the article photon rocket. Count Iblis (talk) 19:54, 12 September 2012 (UTC)
- Also, the Pioneer 10 and 11 spacecraft served as an unintentional tech demonstration of this effect. See: Pioneer anomaly. Though the photon source was a 150W RTG, and the thrust produced "not very much". 8.74±1.33×10−10 m/s2 or so. After 40 years running its "photon engine", its now about 1mph slower (because the RTG is pointing outward- in the direction its moving).--Robert Keiden (talk) 00:28, 13 September 2012 (UTC)
- Exponents repaired for clarity. - ouch
- Laser beams can ping-pong between two mirrors to get more propulsion out of them, too (OR). The beam will red-shift if the moving mirror is moving away or blue-shift if it's moving towards the fixed mirror.
- Fusion is not even close to a near-infinite power source BTW. It would be of greater benefit to tap the magnetic bottle for a burst of high-v particles, rather than tapping that kinetic energy to power a light source. The average v of fusion particles is around 0.12c = 36000km/s.
- Kinetic energy of 1kg of photons: E = m c² = 9x1016Ws = 2.5x1010kWh,
- Momentum of 1kg of photons: m c = 3x108kgm/s.
- Kinetic energy of 1kg of fusion particles: E = (m/2) v² = 6.5x1014Ws = 1.8x108kWh,
- Momentum of 1kg of fusion particles: m v = 3.6x107kgm/s.
- That is, using the kinetic energy of 1kg of fusion particles, you can emit ~7g of light for a momentum of ~2x106kgm/s, or use the particles directly for roughly 18 times that momentum. The savings will only be offset if the power source is fixed (which is feasible only with a laser since you cannot project a focused particle beam into space).
- OtoH, the Pioneer anomaly does look like a workable photon propulsion, and it doesn't need a full-scale reactor core either. Once you escape Earth's gravity, it's literally smooth sailing. A parabolic mirror, a lump of radioisotopes at its focus, and a set of attitude control thrusters, and there you go, slowly spiraling outward.
- p.s. If the mirror reflects (or merely catches) neutrons, it's even better, because their momentum-to-energy ratio is superior to photons, either. - ¡Ouch! (hurt me / more pain) 07:57, 13 September 2012 (UTC)
- Interesting calcs, but what are your assumptions ? Are we talking about fusing normal hydrogen into normal helium ? How about if the reactor continues until you get iron ? You should be able to get more energy out of fusion that way. Also, you can eject the reaction products at high speed, too.
- Or, if that's still not enough, we can go with the old favorite sci-fi matter-antimatter reactor, with the anti-matter being generated prior to launch, and the matter just being waste products generated by the ship and crew (broken machinery, etc.). StuRat (talk) 08:28, 13 September 2012 (UTC)
- If you leave the laser here on earth (or preferably, on the moon or in orbit someplace), there is even a way to have the spacecraft decelerate and eventually fly back towards the laser for the 'return trip'. Have the craft carry two sails - deploy one in the conventional manner on the outward journey - then when you need to decelerate, you cut the lines holding it and deploy a second sail pointing in the opposite direction - laser light reflects off of the detached sail (which continues to accelerate away) and onto the newly deployed sail and the craft will be able to decelerate, do whatever needs to be done - and eventually return home. The real problem is the power of the laser required to do this. Even if it's left back here on earth, keeping the beam sufficiently well aimed and focussed that enough of it's light is 'caught' by the sail is a tough problem that gets worse and worse the further the craft gets - the increasing time delay for the craft to report back with laser realignment instructions would also get seriously problematic as distances increase. SteveBaker (talk) 13:29, 14 September 2012 (UTC)
Seeing Our Own Creation
Telescopes are making huge leaps in their ability to look further and further back in time, and to see greater detail than ever before. I believe astronomers have even captured images of protostars and proto solar systems, and imaging actual new planets is not too far in the future. Therefore, since we can see the creation of other solar systems, isn't it accurate to say that every event since the creation of our solar system is currently being carried by photons created at the time and now a maximum of 4.7 billion light years away from us? The creation of the moon, the extinction of the dinosaurs, etc., all luminous events could be entering some distant civilization's telescopes at this moment.
If this is the case, and if there is really no 'center' to the universe but rather we are all on this ever expanding balloon of space-time, is there any scenario where we could even capture those photons which would show us our own creation?Honeyman2010 (talk) 22:44, 12 September 2012 (UTC)
- It would seem unlikely. They're speeding away from us at the speed of light. We'd have to go faster than the speed of light to be able to interact with them so as to gather their information payload. Faster than light is, I think, forbidden in this universe. --Tagishsimon (talk) 22:49, 12 September 2012 (UTC)
- We could certainly never catch up with those photons, but they may be returned to us by a couple of means. Theoretically, a very strong gravitational lens might return light from the early solar system back to us, although it may be dim and distorted beyond all recognition. Furthermore, if the universe is finite in extent but sill centerless (as in a [hyper]spherical universe), that light will eventually return to us regardless, but that may be a very long time in the future, which would mean it's very dim, and we'd need a freakin' huge telescope to view it. Someguy1221 (talk) 22:51, 12 September 2012 (UTC)
- We've detected extrasolar planets up to 50,000 light years away, which is an impressive half of our galaxy away. In contrast, Andromeda galaxy is one of the closest galaxies to us and it is 2.5 MILLION light years away. You are asking about planetary sized objects 4.5 BILLION light years away? I don't know if I'd go so far as to say it's "impossible", but I won't be holding my breath.. Vespine (talk) 23:36, 12 September 2012 (UTC)

- At 4.5 Gly, the Milky Way, Andromeda and Triangulum galaxies would all blur together. This is what a cluster of 100 galaxies looks like, from about the same distance: --Robert Keiden (talk) 00:56, 13 September 2012 (UTC)
- It's really just a technical limitation. A sufficiently large telescope could view the pimples on your face from 4.5Gly away, although that telescope may itself be light years across. I can't remember how to calculate the limits. Someguy1221 (talk) 01:23, 13 September 2012 (UTC)
- No, it is more than a technical limitation, it is a physical limitation as well. In order to view your pimple, the telescope would have to capture some number of photons from that pimple, and at certain distances, you just can't capture enough photons. See Shot noise for a discussion of some of the problems. --Jayron32 01:31, 13 September 2012 (UTC)
- Nevertheless, Hubble Ultra-Deep Field managing to image objects as they were ~13 billion years ago is pretty cool. Sean.hoyland - talk 03:40, 13 September 2012 (UTC)
- Sure, but the objects it is seeing are considerably larger than the pimples on my face. --Jayron32 03:43, 13 September 2012 (UTC)
- So here's a factual question, assuming we have unlimited resources to build arrays of interfering telescopes, at what distance do certain things become unobservable? Individual stars, Earth-like planets, cities, people on those planets, etc. My claim came from the recollection of an astronomy lecture years ago that discussed the theoretical construction of a telescope array that could resolve to one pixel a person's head on a planet 50 light years away. It hadn't occurred to me that noise would put a limit on how far something could be seen even with an arbitrary telescope. So, back to my question, assuming a sun-like star and an Earth-like planet in orbit, typical interference from intergalactic/interstellar gas, at what distance does noise preclude any observation? Someguy1221 (talk) 06:58, 13 September 2012 (UTC)
- There's possibly no limit to the math involved, I suppose, but you start to get rediculous when it comes to actually constructing a telescope. For example, if you build a lens so large that it conatains all the mass of the known universe, or you build one so large that light could not travel from one end of the lens to the other fast enough to get there before the heat death of the universe, or the telescope is so large that its own mass causes it to collapse into a black hole, or any of a number of other ludicrous propositions, well, I don't think we're dealing with mere "technical limitations" anymore. That is, if you narrow yourself to just those equations that relate the size of a lens to its resolution, you could build a telescope of any size and have nearly infinite resolution. But that is only if you ignore all of the other laws of physics that come into play, and if you're going to ignore some set of the laws of physics, you're doing no better than invoking "magic". The article, by the way, that will answer the main question about how "far" we can see is Optical resolution, and sure, using just those equations, you could construct a telescope of any size which could resolve to any arbitrary accuracy at any arbitrary distance. But there are other considerations when constructing such a telescope, and there are limits to the size a telescope can get before other laws of physics start to make infinite resolution at any distance impossible. --Jayron32 13:10, 13 September 2012 (UTC)
- So here's a factual question, assuming we have unlimited resources to build arrays of interfering telescopes, at what distance do certain things become unobservable? Individual stars, Earth-like planets, cities, people on those planets, etc. My claim came from the recollection of an astronomy lecture years ago that discussed the theoretical construction of a telescope array that could resolve to one pixel a person's head on a planet 50 light years away. It hadn't occurred to me that noise would put a limit on how far something could be seen even with an arbitrary telescope. So, back to my question, assuming a sun-like star and an Earth-like planet in orbit, typical interference from intergalactic/interstellar gas, at what distance does noise preclude any observation? Someguy1221 (talk) 06:58, 13 September 2012 (UTC)
- Sure, but the objects it is seeing are considerably larger than the pimples on my face. --Jayron32 03:43, 13 September 2012 (UTC)
- Nevertheless, Hubble Ultra-Deep Field managing to image objects as they were ~13 billion years ago is pretty cool. Sean.hoyland - talk 03:40, 13 September 2012 (UTC)
- No, it is more than a technical limitation, it is a physical limitation as well. In order to view your pimple, the telescope would have to capture some number of photons from that pimple, and at certain distances, you just can't capture enough photons. See Shot noise for a discussion of some of the problems. --Jayron32 01:31, 13 September 2012 (UTC)
- It's really just a technical limitation. A sufficiently large telescope could view the pimples on your face from 4.5Gly away, although that telescope may itself be light years across. I can't remember how to calculate the limits. Someguy1221 (talk) 01:23, 13 September 2012 (UTC)
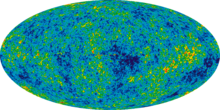
- This question has a rather unambiguous answer. The furthest back in time we can see is the cosmic microwave background radiation, before that the universe was "opaque"; i.e., a glowing cloud we can't see through. Here is an image. μηδείς (talk) 17:47, 13 September 2012 (UTC)
September 13
Storm chasing from the sky
Here in the Oklahoma City television market the local news stations will in times of tornadic thunderstorms send up their news helicopters to chase the storms and any tornadoes they produce. Strangely, despite flying in close proximity to large supercell thunderstorms, there seems to be very little turbulence (or at least the cameras on the helicopters holds a very steady picture). Why is this? Also, what distance might the helicopters have to stay away to avoid hazards from the thunderstorm, such as the tornado, hail, etc? Ks0stm (T•C•G•E) 03:10, 13 September 2012 (UTC)
- I know you asked specifically about supercells and tornados, but you may find 53d Weather Reconnaissance Squadron and Hurricane Hunters interesting, as it has some information on arial reconnaissance of major storms. --Jayron32 03:25, 13 September 2012 (UTC)
- Cells that produce tornadoes can be highly localized, so the weather may be fine where the chopper is filming. This is in contrast, say, with a hurricane. StuRat (talk) 04:14, 13 September 2012 (UTC)
- Also, most the cameras in most television station helicopters are mounted with gyroscopic stabilizers. → Michael J Ⓣ Ⓒ Ⓜ 08:59, 13 September 2012 (UTC)
- Gyro stabilizers prevent the camera from being rotated by the motion of the aircraft - but they can't prevent positional motion from producing problems. It may be that they also record a larger image than is displayed and use motion tracking (eg Digital image correlation) to crop each frame to eliminate the motion. That is the basis of the Image stabilization provided by many modern digital cameras. In this case, since the camera and the subject are many hundreds of feet apart, a foot or more of helicopter motion would only produce a few pixels of image motion between frames - and that can easily be compensated for in this way. SteveBaker (talk) 13:31, 13 September 2012 (UTC)
- I guess they would keep enough distance to be able to get away if it came towards them... Thunderstorms are steered by the winds in the area, maybe they preferably film it from the side, outside of it's predicted path. The FAA advices to stay at least 20 miles away from bad weather, but that's general advice for all aviation. Looking for info I came across a law firm page that from the looks of it specialised in "news chopper related injuries to employees" cases. Not sure if that's an indication of the risks they take, or if the firm has similar pages for every potentially lucrative law suit they could think of.
- Keep in mind that those crews have quality equipment with powerful zoom lenses, so it may look closer than it really is. Ssscienccce (talk) 19:21, 13 September 2012 (UTC)
How accurate are bathroom scales?
Seems like a simple question. How accurate are digital bathroom scales? My weight has been being shown as either 222.2lbs or 220.0lbs all through this week...never any other number. I suspect that it's internally measuring to the nearest kilogram - but displaying in pounds with a readout that's showing tenths of a pound. Just to check, this morning I was able to weigh myself then pick up two soda cans (12oz each...so 1.5lbs) and see no change whatever in the readout - so clearly there is an internal error that's at least ten to twenty times the displayed precision...and I also kinda suspect that the machine is remembering the last weight it displayed and showing the exact same number again if you weigh yourself again and the results are similar enough.
But Wikipedia has no specific article about these machines - and the general Weighing scale article doesn't mention them beyond a mention in the intro that basically says that they exist! Surfing around the web for a while, reviewers who claimed to have done careful testing gave only vague results ("Very accurate", "Accurate", "Not very accurate") with no actual numbers. Manufacturers and retailers said things like "Display is accurate to 0.2lbs"...which anyone with an ounce of critical thinking skills can parse to mean "We put a ridiculously accurate LCD display on a crappy weighing machine and we're trying to pursuade you that this is an OK thing to do"...which is evidently what happens here.
I'm trying to diet - but any reasonably sustainable diet only drops my weight by a pound or two per week...this horrifying inaccuracy in weighing myself means that I can't track my progress at any kind of reasonable rate...even if I'm careful to weigh myself right after peeing & pooping in an effort to eliminate that source of noise in the measurements - and I average my readings over a week to track progress.
Obviously I don't care much about my absolute weight so much as the rate of change - so I don't care if the machine has my weight off by a few percent, so long as it's consistent from one day to the next and able to show variations of better than 1%.
SteveBaker (talk) 13:23, 13 September 2012 (UTC)
- Interesting I had noticed what seemed to be a memory effect on scales. However please clarify the obvious: that the scales are on a hard surface in the same place, with your feet in the same place and your centre of mass similar in all cases? The old scales with counterweights seem able to spot a few grams but modern digital ones I am less convinced about. Good luck with the diet. --BozMo talk 13:33, 13 September 2012 (UTC)
- Yeah - I'm very careful to place them in the same spot on my hard bathroom floor - and to stand reasonably consistently...although shifting my position by an inch or two or leaning my weight onto one foot or the other doesn't change the readout. The "memory effect" could well be a simple software hack to make the scales SEEM more reliable by simply repeating the last readout if the measured weight is within the internal error tolerance...but I've tried weighing myself, then putting one foot on the scale and pushing down until it reads some highish number...then weighing myself again...and the results don't change. So either they are remembering the last N weighings in order to defeat my test - or the weighing mechanism is indeed heavily quantized. The problem here is that not knowing how it works makes experiments to determine it's accuracy quite challenging! SteveBaker (talk) 14:10, 13 September 2012 (UTC)
- Is your weight consistant to within 2 pounds over the course of any arbitrary length of time? It may be that the scale is accurate enough, but your weight isn't consistent, given the amount you have eaten and drunk, whether you just took your morning dump or not, how much sweating you've done, etc. Your weight is basically fluctuating +/- 1 percent and I don't know that that is outside of the normal range for what a person will do on a given day or week. The way to test this would be to get an object whose weight you know isn't changing, and then weigh it at various times. Your body is far too dynamic and unpredictable a thing to test the reliability of a bathroom scale. --Jayron32 13:47, 13 September 2012 (UTC)
- Sure, there is indeed no way that my weight could be that consistent...if I drink a can of (diet!) soda - that's 12 ounces...add a light lunch and I could easily gain a pound or more - and lose it again when I next pee/pooh. So of course it's necessary to weigh oneself after a morning trip to the toilet - and to average over a week or so...all of which I'm careful to do. But I'd hope that this would get the error bars down to within a pound or two...which is what I need to know in order to figure out whether my diet is still keeping on track. Should I walk further next week? Can I let up on the semi-starvation a bit? I know that dieting too fast is a bad idea because it can put your body into crisis/starvation mode and drop your metabolic rate to the point where your diet hits a plateau. The best advice seems to be to try to stick between one and two pounds per week of loss. That's 1% for chrissakes...I really ought to be able to measure that as an average over a week and see if I actually did lose somewhere between one and two pounds over that time.
- Bottom line is that if this machine I have is really quantizing in ~1kg increments internally - and maybe isn't even accurate to 1kg - I could easily be getting a 2% error in my measurements - and that's equivalent to an entire month of dieting - and I can't track whether my dieting behavior is excessive or inadequate. If that's the case then I need to invest in a better machine...if such a thing exists for the bathroom at a reasonable price. It's frustrating that I can't find out how accurate these things actually are in order to make an intelligent choice over whether to upgrade my bathroom scales!
- SteveBaker (talk) 14:04, 13 September 2012 (UTC)
- As a seasoned dieter, I can only repeat the advice every diet programme I've ever seen over the years gives: Only weigh yourself once a week. Resist the temptation to weigh yourself every day. Obviously this advice has the raison d'etre that (a) your weight will fluctuate naturally from day to day, and (b) the scales may not be as well calibrated as you'd like. So same time, same place every week to weigh yourself. Regardless of whether your scales are that accurate, you will at least get an idea of the weekly result and be able to compare it over time. --TammyMoet (talk) 14:55, 13 September 2012 (UTC)
- The problem with the weigh-once-a-week and the weigh-daily-and-compare-with-yesterday approaches is that both give too much 'weight' to a single measurement. One's measured weight can swing through two or three pounds based purely on minor changes in the amount of water in one's body and the fullness of one's bowels and GI tract. Daily measurements prompt both the "Sweet - I lost two pounds yesterday!" delusion as well as the "Oh my God - I was fasting and still gained half a pound!" panic. Individual weekly measurements can give similarly misleading impressions about the overall success of a diet. (If I lost two pounds in week one, and no pounds in week two, was there really a thousand-calorie-per day difference in my eating between the two weeks, or am I just seeing noisy data?)
- Far better to accept that one's measured weight varies naturally from day to day and hour to hour, and apply some sort of smoothing or averaging to one's measurement history. The Hacker's Diet provides instructions and spreadsheets for doing this; it plots a trendline based on weighted averages of preceding days measurements, to give a plausible impression of one's 'real' weight without the superimposed day-to-day noise. Even a straight three-, five-, or seven-day moving average is going to be more resistant to irrelevant fluctuations than pure daily or weekly measuring. TenOfAllTrades(talk) 15:32, 13 September 2012 (UTC)
- Yeah - I think the advice to not weigh yourself every day is for the benefit of stupid people. Intelligent, math-aware, scientific thinkers (which I aspire to be!) can only gain from having more information - providing they are smart about using it. So I gather data by averaging over a week - and look at the trend of averages to give myself some idea of whether I'm doing OK or not. However, not having a decent measurement device to collect that data - one that induces error comparable to several weeks of expected weight loss isn't making life any easier! So the question remains...how accurate are bathroom scales? SteveBaker (talk) 16:58, 13 September 2012 (UTC)
- As someone once said, I resemble that remark... I guess there's no accounting for those who would obsess over their weight. If you're not losing weight, don't blame the scales! --TammyMoet (talk) 17:39, 13 September 2012 (UTC)
- Yeah - I think the advice to not weigh yourself every day is for the benefit of stupid people. Intelligent, math-aware, scientific thinkers (which I aspire to be!) can only gain from having more information - providing they are smart about using it. So I gather data by averaging over a week - and look at the trend of averages to give myself some idea of whether I'm doing OK or not. However, not having a decent measurement device to collect that data - one that induces error comparable to several weeks of expected weight loss isn't making life any easier! So the question remains...how accurate are bathroom scales? SteveBaker (talk) 16:58, 13 September 2012 (UTC)
- OK, it's time we stopped guessing. If you have access to Consumer Reports magazine, they have done the testing. You can't read the results of the test online unless you have a subscription, but here is their methodology for studying the accuracy of bathroom scales. For any sort of consumer product testing, that's usually where I go first; they have a reputation for being thorough and impartial. If you don't have or don't want to pay for a subscription online just to view this one article, the magazine is widely availible in many libraries, so perhaps you can get it that way. Good luck! --Jayron32 17:09, 13 September 2012 (UTC)
- Well, I don't have a subscription, so I'm going to guess some more. Steve, I think you should dump the digital scales. I have done some of the same style of testing as you, and I hypothesize that they try to make themselves look more consistent than they are, by having logic that attempts to figure out if the person on them is the same one as a few minutes ago, and if so, give the same reading.
- Analog scales have limitations — the spring moves more when you step on it than the pressure sensor in a digital scale, so you might think it would soften faster. I don't know if that's true or not. But at least they're too stupid to be trying to fool you.
- Best of all should be a real doctors'-office-style balance with the moving counterweights. A little more money but might be worth it if you're looking at this level of detail. --Trovatore (talk) 17:30, 13 September 2012 (UTC)
- This would likely depend significantly on both scales. My last 2 analog scales would change about 5-10 kg depending on which way you were leaning and precisely where you were standing on the scale, even when on a flat hard surface. Nil Einne (talk) 09:44, 14 September 2012 (UTC)
- That's a .... pretty bad scale. But — at least you could tell how bad they were. I assume neither was of the doctors'-office sliding-counterweight variety? --Trovatore (talk) 09:59, 14 September 2012 (UTC)
- This would likely depend significantly on both scales. My last 2 analog scales would change about 5-10 kg depending on which way you were leaning and precisely where you were standing on the scale, even when on a flat hard surface. Nil Einne (talk) 09:44, 14 September 2012 (UTC)
- My small digital scales (500 gr and 10 gr max) weigh continuously, i.e. the readout changes when the applied weight changes. My bathroom scale gives only one reading, for a second one it has to turn off and on again. The difference is most likely because people move, so it has to average the weight over a longer timeframe. If your's is like that, picking up two soda cans will have no effect. The minimum difference of 2.2 lbs you get may be because they use a non-linear strain gauge to provide the same relative accuracy over the whole range. So a weight of 20lbs would be within 0.2lbs, but 300lbs would only be accurate within 3lbs. It would allow them to use a cheaper (lower resolution) A/D converter. But that's pure speculation on my part. Ssscienccce (talk) 21:30, 13 September 2012 (UTC)
- My girlfriend has been weighing herself every morning for about 14 weeks, while losing 0.95 lbs / week. The data actually plot pretty smoothly on a line. At 1-sigma, the typical daily difference from her measurements and the line is +/- 0.7 lbs. So, I would infer that our scale is at least that reproducible, and probably more so since her daily values should also fluctuate based on variations in the daily routine from day to day. Of course your mileage might vary, but the digital scale we have seems to do a pretty good job of tracking relative changes. Dragons flight (talk) 21:37, 13 September 2012 (UTC)
- We had a discussion of this very topic maybe a month ago here. Yes, cheap digital scales do have logic in them to provide false accuracy by not changing the reading if it's similar to a recent one. How recent and how much change it will mask may vary by model. To overcome this:
- A) Use a known weight which is over the range your scale masks. 10 pounds should do it. So, get on the scale holding the ten pound weight, and you should get your actual weight plus ten pounds. Just subtract. The next day, weigh yourself without the weight, and you should get your true weight. Just alternate days with and without the weight.
- B) Pull the battery out between uses. I bet the previous reading is in volatile memory, and will go bye-bye.
- As I mentioned in the previous discussion on this topic, the most annoying thing is that they don't tell you prior to purchase that this is what it's doing, you only figure it out later. We need some consumer protection laws here. StuRat (talk) 04:06, 14 September 2012 (UTC)
- Ideally, digital bathroom scales should behave like Ssscienccce's 500g scale — the reading stays on all the time you stand on it, and changes to reflect the weight it senses at that moment. Then you could stand there statue-like and wait for it to stop changing, and then you'd know all the transients had damped out. If they did that, I would withdraw my objection in favor of analog scales.
- Analog balances, with the movable weights, would still be better, of course. --Trovatore (talk) 04:16, 14 September 2012 (UTC)
- (Although, those actually measure your mass...not your weight...but that's probably what you ought to care about anyway! Gravity can vary by as much as half a percent depending on where you are on the earth's surface. Fortunately, I'm only planning on weighing myself (er "massing myself") in my bathroom...so it's OK.) SteveBaker (talk) 12:59, 14 September 2012 (UTC)
- Yes, as you say, mass is the important thing; weight-in-the-sense-of-gravitational-force is just a proxy for it. I'll take the opportunity to mention that it's perfectly respectable, historically, to use weight to mean "quantity of matter" rather than "gravitational force" — this is the sense in which bulk goods are sold by weight, the Bureau of Weights and Measures, and so on. The retrospective linguistic prescription imposed in high-school physics seems to be more for the convenience of physics teachers than anything else. Similar remarks could be made for velocity versus speed — if you're teaching a physics class, you need some way to explain how a body can be accelerating even though it never goes any faster or slower, but etymologically they mean the same thing, and you can find velocity as a scalar quantity attested all through the technical literature. --Trovatore (talk) 19:14, 14 September 2012 (UTC)
- (Although, those actually measure your mass...not your weight...but that's probably what you ought to care about anyway! Gravity can vary by as much as half a percent depending on where you are on the earth's surface. Fortunately, I'm only planning on weighing myself (er "massing myself") in my bathroom...so it's OK.) SteveBaker (talk) 12:59, 14 September 2012 (UTC)
- Maybe digital scale manufacturers figure (or focus groups have shown) that a dancing digital number (like many a digital multimeter measuring a jittery voltage source) that never stops due to the user's shifting his/her weight, which frequently tops 0.1lb on the sensor, frustrates the user to the point where they return the scale and the manufacturer doesn't make money. 20.137.18.53 (talk) 14:45, 14 September 2012 (UTC)
- They could eliminate that without reported your previous weight instead of your current weight. It could ignore any readings the first second you are on the scale, then average out the readings over the next second, then give you a reading of your current weight, after 2 seconds. Some type of display to tell you it was "working" would also be in order.
- The reason they do it the way they do is to make inaccurate scales appear to be accurate, since weighing yourself immediately again always gives the same result. This is deceptive marketing, and should be illegal (at least unless the customer is notified that it works this way). StuRat (talk) 18:18, 14 September 2012 (UTC)
- They could do what you say in your first paragraph, but why? I would prefer to see the numbers change, until they don't. Then I know directly when the transients have damped out and don't have to rely on the scale's firmware. I'm not going to get into the political question of whether it should be illegal, but like you I definitely don't like this practice. --Trovatore (talk) 19:51, 14 September 2012 (UTC)
- I believe the average consumer just wants to jump on the scale and have it tell them their exact weight. They don't want to have to watch for it to settle down. Also, they seem to put absurdly small watch batteries in them (mine is powered by a single CR2032, when there's room for a 9 volt battery, or maybe 4 of them). Therefore, you want to display as little as possible, so the battery might last a little longer. StuRat (talk) 21:26, 14 September 2012 (UTC)
- WP:OR of course, but mine is the same observation as StuRat's three posts above. When weighing myself within a short time frame (minutes), the first time it takes a while to settle, but then when I weigh myself again, I immediately get exactly the same readout. No matter if I shift the position of my feet, take a pee, drink a glass of water or whatever. So the scales clearly have some sort of memory. The next morning, I get a different readout. So upon reading this thread, I weighed myself several times, each time getting the readout 80.0 kg. I then weighed myself carrying a load of approximately 20 kg. Readout: 99.8 kg. And then, again without the load: 79.9 kg. Repeated measurement: 79.9 kg. Irritating... --NorwegianBlue talk 01:10, 15 September 2012 (UTC)
- Did you trying pulling the battery out ? StuRat (talk) 01:31, 15 September 2012 (UTC)
- I didn't, but decided to try it now after reading your question. First: five consecutive measurements, 78.0 kg (the 2 kg difference from last night accounted for by not having eaten breakfast and wearing less clothes). Then, turned the scales upside down to remove batteries, found I needed my glasses, spent about two minutes looking for them, turned the scales the right way up and repeated the measurement before proceeding. Five consecutive measurements of 77.8 kg. Then another two minute interruption (phone rang). Five consecutive measurements of 78.0 kg. So, first conclusion: this particular device remembers your weight for a minute or two, no more. I then got curious about what difference in weight that was necessary to get a new, real measurement. I found two 1/2 liter bottles of water. Holding one: first readout: 78.0 kg. Second readout: about to settle on 78.0 kg, then changes its mind, settles on 78.7 kg. Five consecutive readouts: 78.7 kg. Two bottles of water: First and second readout: 78.7 kg. Third readout: about to settle on 78.7 kg, then changes its mind, 79.1 kg. Five consecutive measurements: 79.1 kg. When I finally got around to removing the batteries, I found that that would involve bending some parts that looked rather brittle. So I didn't proceed. My curiosity about this particular device was satisfied. --NorwegianBlue talk 09:59, 15 September 2012 (UTC)
- Unfortunately, you may soon need to replace the battery after all that testing. :-) Your model is better than mine, which seems to remember my weight from yesterday. StuRat (talk) 20:48, 15 September 2012 (UTC)
- That doesn't sound right, Stu. It'd be off the scale with those sorts of stratospheric numbers. It's probably just recalculating your IQ. :) -- ♬ Jack of Oz ♬ [your turn] 09:06, 16 September 2012 (UTC)
- Unfortunately, you may soon need to replace the battery after all that testing. :-) Your model is better than mine, which seems to remember my weight from yesterday. StuRat (talk) 20:48, 15 September 2012 (UTC)
Merck Manual/s
Is/are Merck Manual/s worth it/are the buy and etc? Or if not what is/are worth and etc?
Basically, I was looking at it earlier and it seems its worth the buy and etc. In the end didn't get it because I'm still totally unsure about it and etc. — Preceding unsigned comment added by Mybodymyself (talk • contribs) 22:32, 13 September 2012 (UTC)
- I couldn't say, but keep in mind that a number of Merck's products have had to be yanked from the market, so use your best judgment. ←Baseball Bugs What's up, Doc? carrots→ 22:58, 13 September 2012 (UTC)
- I don't see any direct connection between a Merck drug being pulled, and the quality of The Merck Manuals. They have a long and well reputed history. And they're worth it if you like that sort of thing. It's not a question, OP, we can answer without knowing more about what it is that you're looking for in a medical handbook. --Tagishsimon (talk) 23:05, 13 September 2012 (UTC)
- I second that. Ssscienccce (talk) 23:12, 13 September 2012 (UTC)
- I don't see any direct connection between a Merck drug being pulled, and the quality of The Merck Manuals. They have a long and well reputed history. And they're worth it if you like that sort of thing. It's not a question, OP, we can answer without knowing more about what it is that you're looking for in a medical handbook. --Tagishsimon (talk) 23:05, 13 September 2012 (UTC)
- The manual is aimed at health care professionals and medical students. Still makes a good read imo, if you like that sort of thing. Don't expect layman terms, it mostly stuff like: "Immature WBCs and RBC precursors are found in the peripheral blood, and marked anisocytosis and poikilocytosis , with microcytes, elliptocytes and teardrop-shaped cells develop." (first random page I picked) If you can find the older centennial version, you get a copy of the 1899 first edition as well. Ssscienccce (talk) 23:12, 13 September 2012 (UTC)
- I own a "Merck manual of medical information, Home edition" and it seems to be a reliable source for medical information. The paperback is over 1600 pages, and is not in "medacalese" terminology requiring medical training to interpret. I recommend against buying an obsolete edition, if any reliance is to be placed on the contents for the well-being of self or family, since medical practice and recommendations are constantly changing. Any good local public library is likely to have a recent edition. Your physician should be your primary source of medical information with respect to your health, but he is unlikely to sit and give you as much information as the relevant chapter in the Merck book. Edison (talk) 18:18, 14 September 2012 (UTC)
- Also, not sure which Merck Manual the OP is interested in, but all Chemists sleep with two books on their nightstand: the Merck Index and the CRC Handbook of Chemistry and Physics, both of which are fantastic and useful reference books. From a chemists perspective, the Merck Index is worth it. Again, though, not sure which Merck publication you are interested it. --Jayron32 00:59, 14 September 2012 (UTC)
You can buy old editions rather cheap, I have bought or seen them at library sales, and you can get them at online used book sellers like Amazon and, in the US, abebooks.com μηδείς (talk) 03:14, 14 September 2012 (UTC)
Thank you for all of your wonderful responses to my section here. All of them were insightful for sure.--Jessica A Bruno (talk) 20:53, 14 September 2012 (UTC)
September 14
Removing color from/clarifying orange juice
I know it doesn't make much sense, but I am trying to make a clear green drink that contains a decent amount of orange juice. Using yellow 5 and blue 1 I can get the right color of green, but it is darker than I would like, presumably because of the orange color in the base. Diluting the juice to 10% also does nothing to eliminate the opacity - I would prefer it to be clear. Even if it stays orange-colored, I think I can fix it with food coloring as long as it is clear. Pulp-free orange juice just clogs up a coffee filter, and I'm not really sure what else to try to clear it up. Any ideas? 108.194.140.240 (talk) 02:00, 14 September 2012 (UTC)
- Have you tried to use orange extract instead of juice? --Jayron32 02:05, 14 September 2012 (UTC)
- I've found a few answers to my own question, after realizing I hadn't thought to use "clarifying" as a keyword until I wrote here. I'll have to look into extracts, and see what I think of them. I just found some suggestions to mix in a small amount of gelatin powder, freeze, and let it thaw through a strainer. The other option I've seen that is apparently used by some chefs is to use a centrifuge - but I don't have access to anything like that. I won't mark this resolved just yet, maybe someone else will have some ideas or experience with this. 108.194.140.240 (talk) 02:15, 14 September 2012 (UTC)
- Both of the techniques you note sound a lot like Molecular gastronomy which has been all the rage among the avant garde fringe of the culinary world for the past few years. --Jayron32 02:23, 14 September 2012 (UTC)
- I think this may boil down (sorry:) to what you mean by "contains orange juice", or more specifically what your intended result (rather than method to getting it) is. For example, taking orange juice and trying to clarify it (remove the suspended and emulsified...whatevers) is one of many ways of getting to a clear liquid that tastes like orange. DMacks (talk) 02:42, 14 September 2012 (UTC)
- I just found this [[12]], and also someone in the comments mentions that pectinase works on OJ. Pectinase means I can just stir and refrigerate, which is easier than any other method out there. I think I've seen enough ideas to be happy with them. Sorry I bothered the reference desk - I just was doing a bad job searching until after I posted! 108.194.140.240 (talk) 03:06, 14 September 2012 (UTC)
Pointless referencing in science articles
Whilst at University, the first thing we were told was not to reference wikipedia, but to use the journals. And wikipedia editors are somewhat insistent on journal references.
I wonder how many realise how much those journal entries cost a member of the public to view and who is taking the cash.
And I wonder how many editors are aware that some of the big name distributors now don't even offer public accounts. I believe Science Direct is one such group. What possible use could a list of $25 to $35 a view entries, inaccessible to the public, be to the public? To access the evidence, one must give up a week or twos worth of wages to check the references. — Preceding unsigned comment added by 86.150.236.65 (talk) 02:30, 14 September 2012 (UTC)
- If you wish to check the references to Journals which cost a lot of money, Wikipedia offers a service where someone with a subscription will check them for you, for free, and provide you with a copy or with relevent info as you need. See WP:REX. --Jayron32 02:39, 14 September 2012 (UTC)
- The abstracts are usually free, so you can at least get a sense of what the article is about and some of the the main conclusions. That might be enough to see whether the cited statement is already supported (or sounds likely enough that you don't need to check the nitty-gritty details) without having to spend money. Or at least help you decide which of the many cited refs you actually do want to check in detail. DMacks (talk) 02:45, 14 September 2012 (UTC)
- The reason we require references is to validate the material in the articles. It is nice if the references are easily accessible, but that isn't the essential thing. We actually don't require that references be available online at all -- many book references are not. Looie496 (talk) 03:44, 14 September 2012 (UTC)
- In addition, the full text of papers accepted for publication since April 2008 and supported by funds from the U.S. National Institutes of Health are, as a rule, freely accessible to the public under the NIH Public Access Policy. -- Scray (talk) 04:17, 14 September 2012 (UTC)
- In Australia, many university libraries allow visitors to access their online journals if they log on in person at the library. The Western Australian library service provides online access from home to thousands of textbooks via eBook Library. --Anthonyhcole (talk) 04:18, 14 September 2012 (UTC)
- I guess I'm not really adding much, but an inaccessible reference is better than no reference. The situation with availability online is changing, because most of the research is supported by universities, and they know they are getting gipped if they have to pay again for access to knowledge they probably funded in the first place. Even for my PhD, I much prefer refs that are available full text online, as a courtesy to others, and because I hate having to bother logging in. I think most people do the same, but not all knowledge is freely available. IBE (talk) 05:07, 14 September 2012 (UTC)
- I agree. Whenever two sources are equally reliable but one is free, I always cite the free one. --Anthonyhcole (talk) 05:15, 14 September 2012 (UTC)
- Unless the free one is a direct, verbatim copy of the other, the better action may be to cite them both. More source material is always more better. --Jayron32 05:17, 14 September 2012 (UTC)
- I agree. Whenever two sources are equally reliable but one is free, I always cite the free one. --Anthonyhcole (talk) 05:15, 14 September 2012 (UTC)
- There is really no problem getting access to articles, and no reason not to cite almost any orginal source as a reference. In Western Australia, if you are a student, you can get almost anything journal-wise for free, though I assume if you abuse the privilige by say requesting lots of papers on the sexual perversions of eskimos when you are an engineering student, you may be spoken to. As Anthonyhcole has said, Universities allow free access to all printed journals held, and most online journals they subscribe to, to any member of the public who visits in person. If you are a member of your local (shire) library, you can ask them to email you a scan of anything held by any public or university library in Australia. That includes just about anything - I have succesfully obtained articles that appeared in Russian journals. The only downside is that the service is very slow, typically taking several weeks. But the service is completely free! Anyone in Australia can request almost anything from the Australian National Libray - they can, via the US Library of Congress and the British Library access anything, and the service is very fast - 2 days or so. But they charge about the same as commercial services. Ratbone120.145.29.139 (talk) 11:05, 14 September 2012 (UTC)
- The thing is, Wikipedia could be set up to have articles that are no more than a list of references - no text whatever. But we don't - and the reason is that most people can't/don't-want-to get a hold of all of those referenced documents and read them all. The entire point of an encyclopedia is to provide a useful distillation of all of those core documents. You shouldn't reference Wikipedia because a better reference will always be to the things that Wikipedia references...but that doesn't make Wikipedia useless - very often you only need a summary and a reasonable presumption that the summary really does match the references it points to. But if it's a critical matter - then you're just going to have to get those source documents - no matter the cost or difficulty.
- You ask whether Wikipedia editors are aware of this - and since they presumably had to read the referenced articles first before they could choose them to back up facts in the article, I'd have to say "Yes". Your underlying question is presumably something like: "Why don't we simply shun references that are costly/difficult to read?" - but if we didn't do that, then you wouldn't even be able to find our "summary" information for a large fraction of human knowledge...and you wouldn't know when (or where) to go with large piles of cash to find out whatever is missing. So it's important that we reference those documents - especially when they are hard to find or expensive to read. SteveBaker (talk) 13:12, 14 September 2012 (UTC)
- The researchers who produce the journal articles are aware of it, and they're unhappy, too. Paul (Stansifer) 14:55, 14 September 2012 (UTC)
Dihydrogen vs. dihydrido
Can dihydrogen and dihydrido complexes be considered true tautomers? Plasmic Physics (talk) 06:31, 14 September 2012 (UTC)
- Probably not, except by extension of the definition in some way. A true tautomer is almost always an organic compound that has two stable forms that are seperated by a low energy barrier, almost always involving the migration of a hydrogen atom and the shifting of a pi-bond. The kind of isomerism you note 1) doesn't involve organic compounds 2) doesn't involve the same sort of hydrogen atom shifting and 3) doesn't involve the movement of a pi bond. Rather, I think the better term for this may be Resonance in the sense that there aren't two forms of the complex (which is what tautomerism would imply: that there were two distinct forms actually found), but rather the difference between drawing the "Dihydrogen" model and the "dihydro" model doesn't represent a real difference, rather more a shortcoming in the lewis diagram model. --Jayron32 12:50, 14 September 2012 (UTC)
- From what I gather, the article on dihydrogen complexes indicate that they are distinct with a low energy barrier. Plasmic Physics (talk) 13:28, 14 September 2012 (UTC)
- Fine then, they are, but it still isn't tautomerism, which describes a specific type of single proton transfer within a specific set of molecules. Metal-ligand coordination complexes are not the right sort of molecules, and the way in which hydrogen "moves" is not the right sort of movement. Other than "involves hydrogen in some way", there's nothing about the reaction you describe which qualifies it as "tautomerism". --Jayron32 14:10, 14 September 2012 (UTC)
- Then what kind of isomers are they? Plasmic Physics (talk) 06:18, 15 September 2012 (UTC)
- The best term may be Linkage isomerism, since the hydrogen is bonded to the metal differently in each case. It's a odd type of linkage isomerism, to be sure, because dihydrogen is a bit of an odd ligand, being that the only electrons availible for bonding are those of the H-H sigma bond; most ligands don't bond via their own sigma bonds. But generally, if the only difference is in how the ligand is bonded, its a form of linkage isomerism. --Jayron32 04:32, 16 September 2012 (UTC)
- Then what kind of isomers are they? Plasmic Physics (talk) 06:18, 15 September 2012 (UTC)
- Fine then, they are, but it still isn't tautomerism, which describes a specific type of single proton transfer within a specific set of molecules. Metal-ligand coordination complexes are not the right sort of molecules, and the way in which hydrogen "moves" is not the right sort of movement. Other than "involves hydrogen in some way", there's nothing about the reaction you describe which qualifies it as "tautomerism". --Jayron32 14:10, 14 September 2012 (UTC)
- From what I gather, the article on dihydrogen complexes indicate that they are distinct with a low energy barrier. Plasmic Physics (talk) 13:28, 14 September 2012 (UTC)
Entropy of a fan
Let's say we have a fan connected to an electric motor. The motor is switched on, and work is performed on the fan. Does the entropy of just the fan increase or decrease as a result of the motor being activated? Why?
I'm curious because, as I understand it, entropy is defined as heat flow divided by absolute temperature. Here, though, we have energy transferred from the motor to the fan in the form of work, not heat. I think.
(You may be able to tell that I've never studied physics formally.) 75.60.184.181 (talk) 14:03, 14 September 2012 (UTC)
- Friction converts work to heat, though. --Jayron32 14:04, 14 September 2012 (UTC)
- Thanks. So heat is transferred from the fan to the surrounding air? I thought that might be the answer, but then I wondered what would happen if the temperature of the air were greater than the temperature of the fan. How could heat then flow from the fan to the air? 75.60.184.181 (talk) 14:26, 14 September 2012 (UTC)
- Friction always increases the ambient temperature of what is being, well, frictioned (is that even a word? I don't know, but go with me on this). That is, whatever tempertaure it was before, the temperature will always increase as a result of friction because friction adds heat to the surroundings. So, the ambient temperature of the air and the fan is unimportant to the role of friction: friction warms the fan up, regardless of what its prior temperature was, and it will continue to do so until the relative temperature of the fan is greater than the air, at which point the heat will begin to transfer to the air. --Jayron32 14:30, 14 September 2012 (UTC)
- The word is "fricked". μηδείς (talk) 16:43, 14 September 2012 (UTC)
- I see. Am I correct, then, that the entropy of the fan will begin to decrease once it begins to transfer heat to its surroundings? Also, during the time between the activation of the motor and the point at which the fan's temperature overtakes the air's temperature, is there any entropy change? Thanks for your help. 75.60.184.181 (talk) 14:46, 14 September 2012 (UTC)
- Entropy is a property of the system, not of its components, per se, unless they themselves can be isolated as a system, which a fan in an atmosphere cannot. Most of the increased entropy will be seen as an increase in air temperature, but there will also be some small wear to the fan's mechanism. If the outside air is simply hotter than the fan, the fan itself will eventually reach the same temperature, at which time its friction (internal and with the air on the blades) will start heating the air around it. μηδείς (talk) 16:48, 14 September 2012 (UTC)
- Thanks Medeis. Well, I still don't quite understand, and I think I should hit the books again because I don't seem to understand the math well enough. But Medeis, do I understand you correctly as saying that entropy is only defined for isolated systems, that is, systems with essentially no exchange of matter or energy with the surroundings? I'm confused, because entropy change is defined in classical thermodynamics as , which I thought related entropy change to the heat absorbed by a (necessarily non-isolated) system. 75.49.2.198 (talk) 19:43, 14 September 2012 (UTC)
- Obviously there is no such thing as a truly isolated system except for the universe. But the fan and the local air body (in a room, let's say) can be treated as an isolated system, while the functioning fan cannot be. The issue with the fan is, how would you naturally treat its entropy separately from the air in which it functions? Heat will be radiated into the air from the motor casing, conducted away due to the air passing over the body, and caused by the friction of the blades on the air itself. The wind from the blades will start as coherent motion, but degenerate quickly into environmental heat. There is no way to treat of this as regards the fan itself without taking the properties of the atmosphere around it into consideration. The entropy will go into the environment, not "stay" in the fan. But a fan in outer space will behave quite differently, heating up significantly itself, and radiating away but not conducting away heat (if one can say "conducting away"). As for the math, I am biologer by science training, so you may do better asking for a physicist. I did quite well in physics and chemistry, but by intuition, not hard math. I won't be one to explain or lead you to the right equations. μηδείς (talk) 21:12, 14 September 2012 (UTC)
- Thanks Medeis. Well, I still don't quite understand, and I think I should hit the books again because I don't seem to understand the math well enough. But Medeis, do I understand you correctly as saying that entropy is only defined for isolated systems, that is, systems with essentially no exchange of matter or energy with the surroundings? I'm confused, because entropy change is defined in classical thermodynamics as , which I thought related entropy change to the heat absorbed by a (necessarily non-isolated) system. 75.49.2.198 (talk) 19:43, 14 September 2012 (UTC)
- Entropy is a property of the system, not of its components, per se, unless they themselves can be isolated as a system, which a fan in an atmosphere cannot. Most of the increased entropy will be seen as an increase in air temperature, but there will also be some small wear to the fan's mechanism. If the outside air is simply hotter than the fan, the fan itself will eventually reach the same temperature, at which time its friction (internal and with the air on the blades) will start heating the air around it. μηδείς (talk) 16:48, 14 September 2012 (UTC)
- Friction always increases the ambient temperature of what is being, well, frictioned (is that even a word? I don't know, but go with me on this). That is, whatever tempertaure it was before, the temperature will always increase as a result of friction because friction adds heat to the surroundings. So, the ambient temperature of the air and the fan is unimportant to the role of friction: friction warms the fan up, regardless of what its prior temperature was, and it will continue to do so until the relative temperature of the fan is greater than the air, at which point the heat will begin to transfer to the air. --Jayron32 14:30, 14 September 2012 (UTC)
Octopus reproductive process
I was reading our article on the Octopus and it described how the reproductive process was generally fatal to the octopus, but that researchers had managed to subvert it by removing the optic glands. I had a couple quick questions about this: Is this process invariably fatal, whether for the male or the female, and how would removing the optic glands subvert this process? Kansan (talk) 15:33, 14 September 2012 (UTC)
- As far as I can tell, yes, reproduction invariably leads to death soon after, for both males and females. This is the life-history strategy of (all?) octopods, known as semelparity (sometimes called monocarpy). All annual plants are monocarpic, and many perennials as well. In animals, more famous examples of semelparity are salmon. Basically, the selective pressures on organisms can shape their reproductive strategies in many different ways, and this is the one that worked out for octopods. You may also enjoy this paper on octopus senescence here [13]. I didn't read the paper about removal of optic glands, but the abstract indicates that removal also resulted in "cessation of broodiness," which I think means that, though they returned to eating and surviving after spawning, they did not necessarily successfully reproduce. SemanticMantis (talk) 15:55, 14 September 2012 (UTC)
- The fatality of reproduction in semelparous organisms is related to the fact that they have a breeding season, and that the likelihood of them living long enough to breed again within that second season is too low to be worth saving energy from the first breeding system to make the attempt. If octopuses could breed at any time, it might make sense for them to have smaller broods and live longer lives. But they have only a breeding season within which to reproduce. Hence, if they forgo reproduction now, they have to be sure to live until the next season, or have watsed their reproductive potential. Since they are softbodied organisms with high mortality rates, taking that risk makes little sense. It is better for them to put everything into the reproductive chance they do have now, rather than risking it on a later season that may never come. See the tradeoff section of semelparity. μηδείς (talk) 21:28, 14 September 2012 (UTC)
- Thank you (both of you); I had also been wondering how that could possibly be an evolutionary advantage and that also answers that question. Kansan (talk) 22:27, 14 September 2012 (UTC)
- The fatality of reproduction in semelparous organisms is related to the fact that they have a breeding season, and that the likelihood of them living long enough to breed again within that second season is too low to be worth saving energy from the first breeding system to make the attempt. If octopuses could breed at any time, it might make sense for them to have smaller broods and live longer lives. But they have only a breeding season within which to reproduce. Hence, if they forgo reproduction now, they have to be sure to live until the next season, or have watsed their reproductive potential. Since they are softbodied organisms with high mortality rates, taking that risk makes little sense. It is better for them to put everything into the reproductive chance they do have now, rather than risking it on a later season that may never come. See the tradeoff section of semelparity. μηδείς (talk) 21:28, 14 September 2012 (UTC)
- What are these "optic glands", and what kinds of animals have them? (Wikipedia currently has no article for optic gland.) Does removing the optic glands blind the octopus? (That's rather depressing... imagine telling an octopus, "after you have sex, to live, you must be blinded".) Does removing the optical glands also physically remove the eyes, or do their eyes remain afterward, but just sit uselessly in their heads? —SeekingAnswers (reply) 22:36, 14 September 2012 (UTC)
- The optic gland is mentioned in both the text and the references for the octopus article. Seasonality in living organisms is influenced in various ways by light sensitivity. See pineal gland for a homologous organ in vertebrates. μηδείς (talk) 23:09, 14 September 2012 (UTC)
How do they determine how many figures are "correct" or "good" for a given computation of a mathematical constant, like pi?
I'm doing a significant figures problem from a chemistry textbook that goes like: carry out the calculation, making sure your answer has the correct number of significant figures, , where r = 6.23 cm and h = 4.630 cm. Please don't get hung up on that particular thing; I know how to do the problem. And I can safely assume that I know pi to more than two correct figures. But how to they really know, when they do a given computation of one of the many mathematical definitions of pi, "OK, this time the computation is correct to 10,478,352 figures. How do they get the number of correct figures, or figures that they say are correct? 20.137.18.53 (talk) 15:40, 14 September 2012 (UTC)
- If your read the "Infinite series" section of our "Pi" article, you will see that pi is calculated with an infinite series. The terms that are being added or subtracted keep getting smaller and smaller as the series is computed. So you can estimate the uncertainty by the size of the last term that was added or subtracted. Jc3s5h (talk) 15:56, 14 September 2012 (UTC)
- (after ec) Our article Pi covers this in reasonable detail, but I can give a short version here: the value of pi (or e, or phi, or any other constant) is calculated using infinite series. These are known to sum to the correct values - generally, the description of the series is related in some way to the definition of the constant. So to get the appropriate number of decimal places, you calculate and sum terms of the series until the sum stops changing at the level of accuracy you need. So if I want to show that (say) the sum of the reciprocals of powers of 2 is 2, correct to 2sf, I'd work out terms like this:
- 1/(2^0) = 1
- 1 + 1/(2^1) = 1.5
- 1 + 1/(2^1) + 1/(2^2) = 1.75
- 1 + 1/(2^1) + 1/(2^2) + 1/(2^3) = 1.875
- 1 + 1/(2^1) + 1/(2^2) + 1/(2^3) + 1/(2^4) = 1.9375
- 1 + 1/(2^1) + 1/(2^2) + 1/(2^3) + 1/(2^4) + 1/(2^5) = 1.96875
- 1 + 1/(2^1) + 1/(2^2) + 1/(2^3) + 1/(2^4) + 1/(2^5) + 1/(2^6) = 1.984375
- And at that point, the last two terms are both 2.0, correct to 2sf. (Of course, there's more to it than that; be careful of examples like the Harmonic series.) For engineering purposes, only a few digits of pi is enough. You could probably take pi = 22/7 a lot of the time and get away with it. Mathematicians have known how to calculate pi to more places than could be needed for any real-world application for centuries. AlexTiefling (talk) 16:01, 14 September 2012 (UTC)
- (another EC) See also Approximation_of_pi#Modern_algorithms and rate of convergence. Basically, fancy math can tell us that the (numbers made up for sake of example:) 100th term of a sequence is less than 0.000001 from pi. So that tells us that at least the first four decimal places are correct. SemanticMantis (talk) 16:03, 14 September 2012 (UTC)
- Sorry, are we answering the OPs question properly? Or is the OP answering the textbook's question properly? Isn't the issue about how many significant digits to include in the answer to the problem? I thought the rule was that the answer should not be less significant, but need not be more significant than the least significant input variable. I.e., 564.56 cm**3, rather than 564.556 cm**3 (because r is only as precise as 6.23cm. The infinite precision of pi is redundant. Your Username 16:14, 14 September 2012 (UTC) — Preceding unsigned comment added by Hayttom (talk • contribs)
- No, my question was about how it is determined how many figures of a computation of a mathematical constant such as pi are correct. I only brought up the significant figures problem as background to what made me think about how it is determined how many figures of a mathematical constant are determined to be correct when computing said mathematical constant. 20.137.18.53 (talk) 16:18, 14 September 2012 (UTC)
- Sorry, are we answering the OPs question properly? Or is the OP answering the textbook's question properly? Isn't the issue about how many significant digits to include in the answer to the problem? I thought the rule was that the answer should not be less significant, but need not be more significant than the least significant input variable. I.e., 564.56 cm**3, rather than 564.556 cm**3 (because r is only as precise as 6.23cm. The infinite precision of pi is redundant. Your Username 16:14, 14 September 2012 (UTC) — Preceding unsigned comment added by Hayttom (talk • contribs)
- pi and e are transcendental; the digits go on forever, without repeating. So there's no perfectly correct way of writing out their decimal forms (without an infinite piece of paper). A textbook author might decide they only care about the first few digits, but that's just their definition. For physical things, like a temperature measurement, the equipment is only so accurate, so you might only quote a temperature with one digit after the point, because that's how accurate your thermometer is - if you had a super-accurate digital thermometer you could reasonably use more digits. -- Finlay McWalterჷTalk 17:12, 14 September 2012 (UTC)
- My question was just how people determine that a given calculation is correct to n digits. I think I get the infinite series each-time-a-smaller-amount-is-added reasoning given above. 20.137.18.53 (talk) 17:32, 14 September 2012 (UTC)
- It is also fairly easy to get a series of approximations which you can prove alternate between being above and below pi. Having a lower and upper bound makes the accuracy easy. When you are 14 or 15 at school you derive one like that based on the series expansion of arctan (1/5) or you used to when they still did maths at school. --BozMo talk 17:38, 14 September 2012 (UTC)
- My question was just how people determine that a given calculation is correct to n digits. I think I get the infinite series each-time-a-smaller-amount-is-added reasoning given above. 20.137.18.53 (talk) 17:32, 14 September 2012 (UTC)
- pi and e are transcendental; the digits go on forever, without repeating. So there's no perfectly correct way of writing out their decimal forms (without an infinite piece of paper). A textbook author might decide they only care about the first few digits, but that's just their definition. For physical things, like a temperature measurement, the equipment is only so accurate, so you might only quote a temperature with one digit after the point, because that's how accurate your thermometer is - if you had a super-accurate digital thermometer you could reasonably use more digits. -- Finlay McWalterჷTalk 17:12, 14 September 2012 (UTC)
- Interesting article: Bailey–Borwein–Plouffe formula. In which you can get a digit of pi without figuring out the whole thing! Weirdest part? "Certain combinations of specific p, q and b result in well-known constants, but there is no systematic algorithm for finding the appropriate combinations and known formulas are discovered through experimental mathematics." Now is that a red cape to the bull or what? Wnt (talk) 18:49, 14 September 2012 (UTC)
- You basically have to methologically go through all the possible sources of error and determine their size. The sum of these possible errors together determine how many accurate figures you have. In the pi example, you have two sources of error: a) you are only summing a finite number of terms of an infinite sequence: so estimate what the total size of the missing terms is and b) round-off errors if you are doing this on a computer: roughly number-of-terms * machine precision (usually 1e-16). In your volume of cylinder example above that contains measurements: how did you obtain those measurements? How many digits do you trust. To get more than a mm accuracy you will need special equipment, so likely already the last digit of 6.23cm is bogus. This becomes especially apparent if you see statistical claims like "14.28% of people like X". By what method could they have possibly determined that to 4sf? The possible sources of errors in these statements are many and are very rarely all accounted for.86.139.178.200 (talk) 22:04, 14 September 2012 (UTC)
Human viruses
Please can someone confirm that HPV is not the same as herpes? This is not a request for medical advice. It's to answer a pub discussion! --TammyMoet (talk) 18:33, 14 September 2012 (UTC)
- See Human papillomavirus and Herpes simplex virus. Totally different families of viruses. Wnt (talk) 18:40, 14 September 2012 (UTC)
- I suspect that the losing party was confusing HPV with HSV, due to similar abbreviations and similar means of transmission. StuRat (talk) 21:20, 14 September 2012 (UTC)
Yes thank you both. 1-0! --TammyMoet (talk) 09:43, 15 September 2012 (UTC)
- You're welcome. I take it we can mark this resolved while you collect your bet ? StuRat (talk) 14:44, 15 September 2012 (UTC)
does all the velocity increase come from the energy stored in air pressure when air is forced out of the lungs?
Or do the walls "accelerate" some of the air particles? I am thinking this is possible, because the average speed of air molecules (at 28 g/mol) at 300K is about 422 m/s (this accounts for 1 atm) -- so I find it conceivable that the lungs in forcing out air in a strong breath (1.5L/s) would also maybe provide kinetic energy equivalent to maybe 5% of the original PV = nRT -- maybe making the air move about 20m/s faster? That would be on the same order of magnitude as the pressure drop predicted by the Bernoulli equation, no? — Preceding unsigned comment added by 137.54.16.83 (talk) 19:55, 14 September 2012 (UTC)
- Our thoracic diaphragm clearly moves as we exhale, not prior to exhaling, so it's not just releasing pent-up pressure. StuRat (talk) 21:08, 14 September 2012 (UTC)
- Well I was thinking the Bernoulli equation would say conservation of energy means the pressure drops below atmospheric; but wouldn't the movement of the diaphragm contribute to the kinetic energy? 137.54.16.83 (talk) 22:48, 14 September 2012 (UTC)
- Yes, so a bit of both. StuRat (talk) 20:43, 15 September 2012 (UTC)
five woody clades
In The Island of the Colorblind, part of which is about cycads, Oliver Sacks mentions in passing that nature invented wood five times. I guess cycads, conifers and palms are three of these. Are broadleaf trees one clade or two? If one, what is the fifth woody clade? Bamboo? —Tamfang (talk) 20:02, 14 September 2012 (UTC)
- The so-called wood of monocots (icluding palms and bamboo) is not true wood because, although hard and "woody", it does not grow laterally from secondary xylem but apically from primary growth. The article on xylem lists five clades with more or less true wood, cycads, ginkoes, gnetophytes, conifers, and angiosperms. Note that these five clades entirely comprise the living Spermatophyta, or higher seed plants. Whether wood arose separately in each is debatable and dubious. The most primitive angiosperms include Amborella and Nymphaeales, such as Nelumbo, neither of which is truly woody. But each may have lost a primitive woody state. All other most primitive angiosperms such as the Magnolia are woody. μηδείς (talk) 20:45, 14 September 2012 (UTC)
- Let me comment that "clade" is the wrong word here. A clade consists of any species together with all of its evolutionary descendants, so clades can range from a single existing species to the entire animal kingdom. Broadleaf trees do not form a clade, but that doesn't have any bearing on this question. Regarding the question, I don't know the answer, but I'll add that tree ferns are another pseudo-woody group that are distinct from the ones that have been mentioned so far. Looie496 (talk) 21:43, 14 September 2012 (UTC)
- The five groups I mentioned (and which I assume are the ones Sacks meant) are all considered valid clades. There is no objective definition of "broadleafed". μηδείς (talk) 23:02, 14 September 2012 (UTC)
- Thank you. —Tamfang (talk) 00:24, 16 September 2012 (UTC)
- Why is clade a wrong word? The question is about sets of species descended from a common woody ancestor whose own ancestors were not woody. —Tamfang (talk) 00:24, 16 September 2012 (UTC)
- If you equate broadleaf and angiosperm, then since angiosperms are indeed a valid clade, broadleafed plants would, of course be a clade. I and Looie (I presume) were taking it to be a phenetic characteristic, like deciduous, and in my case was including Welwitschia and Ginko which have broad leaves, not needles or scales. I see someone has pointed broadleaf to angiosperm only now, so see why the question would arise. I think that piping is problematic. μηδείς (talk) 00:31, 16 September 2012 (UTC)
- Why is clade a wrong word? The question is about sets of species descended from a common woody ancestor whose own ancestors were not woody. —Tamfang (talk) 00:24, 16 September 2012 (UTC)
Purple carrots
My V8 V-Fusion drink contains purple carrot juice, yet I've never seen purple carrots sold directly in that store. Is there a reason why purple carrots aren't sold directly to consumers, or is my store just slow in changing ? StuRat (talk) 20:55, 14 September 2012 (UTC)
- I buy them at the St Lawrence Market in Toronto, and have for several years. They also come in red and yellow (and the usual orange, of course). Bielle (talk)
- Picture? Source? μηδείς (talk) 21:02, 14 September 2012 (UTC)
- I don't need a source, I trust Bielle not to lie to me. StuRat (talk) 21:03, 14 September 2012 (UTC)
- Thank you, StuRat. Nonetheless, according to this site, carrots were purple before they were orange. And there are black carrots, too. The site has photos. Bielle (talk) 21:06, 14 September 2012 (UTC)
- I think Medeis was challenging your statement that they are available at that particular store. This is a case where sources are unnecessary, since we have no reason to doubt your word. StuRat (talk) 21:10, 14 September 2012 (UTC)
- This 2000 article say there's no consumer demand for them, but I wonder how they determined that: [14]. StuRat (talk) 21:03, 14 September 2012 (UTC)
- See also Carrot for this photo among a number of others. A small restaurant in my only-slightly-larger small town serves several dishes with a bouquet of coloured carrots for "seasonal veg". I wonder if Murry (yes, that is how he spells it), the root-vegetable seller at the market might be prepared to bring a sample of his many-coloured carrots out to the street where the market sign identifies the location. I might have more success photographing a dish in the local restaurant; Costa and Annnika love any kind of publicity. But then I would have to learn how to upload photos. No, not going there; sorry, Medeis. Bielle (talk) 21:21, 14 September 2012 (UTC)
- I wasn't challenging the veracity. I simply wanted to see and read about these carrots. I love carrots and love purple, so what's not to like? μηδείς (talk) 21:30, 14 September 2012 (UTC)
- As a Ref Desk regular, Medeis, I am surprised you didn't immediately think of Carrot. :>) Bielle (talk) 21:48, 14 September 2012 (UTC)
- As a ref desk regular I am surprised (actually, not surprised at all) that you didn't provide a link with your first response. μηδείς (talk) 22:55, 14 September 2012 (UTC)
- Yes, I linked to our article in the title. If the carrot doesn't work to get Medeis to go to the article, perhaps we need to employ the stick ? :-) StuRat (talk) 22:03, 14 September 2012 (UTC)
- If not the carrot, then the trout, perhaps. I didn't see that link either.
The dark blue doesn't show up well beside the black. Wasn't linking a lighter blue at one time?Ignore that: I worked it out. I just didn't look at the title, obviously.Bielle (talk) 22:13, 14 September 2012 (UTC)
- If not the carrot, then the trout, perhaps. I didn't see that link either.
- As a Ref Desk regular, Medeis, I am surprised you didn't immediately think of Carrot. :>) Bielle (talk) 21:48, 14 September 2012 (UTC)
- Alack, our article on wild carrot - and associated Commons material - contain no roots. We need pictures like [15] and [16]. Our article actually claims the orange carrots are the result of the House of Orange, which would be a very odd twist of fate indeed. Wnt (talk) 22:36, 14 September 2012 (UTC)
- It doesn't seem all that outrageous that orange carrots were bred to honor the House of Orange. This was the Dutch, remember. They caused an economic collapse over Tulips. They take their horticulture seriously. --Jayron32 03:37, 15 September 2012 (UTC)
- In my neck of the woods, you can take your horticulture but you can't make her appreciate it. -- ♬ Jack of Oz ♬ [your turn] 23:07, 15 September 2012 (UTC)
 One of Medeis stars for you JackofOz. That's the answer of the week. --Jayron32 03:17, 16 September 2012 (UTC)
One of Medeis stars for you JackofOz. That's the answer of the week. --Jayron32 03:17, 16 September 2012 (UTC)
- To earn the star, I think Jack should have given a tip of his hat at least to Dorothy Parker. Bielle (talk) 03:32, 16 September 2012 (UTC)
- Oh, pooh on you, Bielle. What is this, some boring place where we're supposed to produce references for our statements? :) :) -- ♬ Jack of Oz ♬ [your turn] 03:40, 16 September 2012 (UTC)
- To earn the star, I think Jack should have given a tip of his hat at least to Dorothy Parker. Bielle (talk) 03:32, 16 September 2012 (UTC)
- In my neck of the woods, you can take your horticulture but you can't make her appreciate it. -- ♬ Jack of Oz ♬ [your turn] 23:07, 15 September 2012 (UTC)
- It doesn't seem all that outrageous that orange carrots were bred to honor the House of Orange. This was the Dutch, remember. They caused an economic collapse over Tulips. They take their horticulture seriously. --Jayron32 03:37, 15 September 2012 (UTC)
"Nutty" taste in cheese
What gives cheese a "nutty" flavor? Except for instances where nuts are actually inserted into cheese, I don't see where nuts would come into play in the cheesemaking process. Is this a result of the animal's diet? Also, can nutty cheeses provoke allergic reactions in people who are allergic to nuts—either a legitimate reaction or a sort of false positive based on the body's perception of nut consumption? I've looked over a few sources such as this study from North Carolina State University, but if the answer is in there, I think I'll need someone to translate it into layman's terms for me. --BDD (talk) 21:23, 14 September 2012 (UTC)
- If you come up with the answer you could patent an artificial nuttiness process and make millions scrimping on aging time.
- Hcobb (talk) 21:49, 14 September 2012 (UTC)
- Thanks—that's definitely the sort of source I'm looking for. If I'm reading that right, we don't really know what gives cheeses a nutty flavor, although that study identified compounds that could do so? --BDD (talk) 21:59, 14 September 2012 (UTC)
- They seem to be making the hypothesis that A+B(+C...?) is responsible for the nutty flavor, and they believe they have found A. This is evidenced by the fact that when added to young cheese, their compound does not significantly alter the flavor, but added to older cheeses, they see significant increases in nuttiness. Someguy1221 (talk) 23:08, 14 September 2012 (UTC)
- Thanks—that's definitely the sort of source I'm looking for. If I'm reading that right, we don't really know what gives cheeses a nutty flavor, although that study identified compounds that could do so? --BDD (talk) 21:59, 14 September 2012 (UTC)
Eggs, water, and temperature
I was steaming some eggs when the following question occurred to me: which of these two has a higher boiling point (edit:) reaches the higher temperature during steaming?
- cracked-open (liquid) chicken eggs alone
- cracked-open (liquid) chicken eggs diluted with water
—SeekingAnswers (reply) 21:24, 14 September 2012 (UTC)
- I am a seasoned cook, but I am not sure I understand the question. Do you mean boiling eggs without water in the first place? They will denature then burn, but not really boil other than giving off steam. μηδείς (talk) 21:32, 14 September 2012 (UTC)
- As the question states, I am steaming the eggs, so in the first case, the eggs are placed in a steamer above liquid water. In the second case, the eggs are diluted with water before being placed in the steamer above liquid water. —SeekingAnswers (reply) 22:04, 14 September 2012 (UTC)
- Well, what's the boiling point of liquid egg? I couldn't find that with a quick search, but if we know that, we can deduce the answer to your question. If the boiling point of a liquid egg (x, for convenience) is higher than the boiling point of water, we can expect the dilution to have a lower boiling point; if x is lower than the boiling point of water, we can expect the dilution to have a higher boiling point. But you may have already figured out that much. --BDD (talk) 21:48, 14 September 2012 (UTC)
- Egg liquid can never boil. It congeals (turns solid) at a temperature of about 150 degrees Farenheit. If you continue heating it, it gets steadily more rubbery, then turns brown, then black, then starts to smoke. Looie496 (talk) 22:01, 14 September 2012 (UTC)
- Let me rephrase (and I've edited my original question accordingly). The question I really wanted to ask was which of the two cases I gave (without water dilution and with water dilution) reaches the higher temperature during the steaming process. (I only mentioned the boiling point because liquid water reaches its maximum temperature during cooking at the boiling point, after which it vaporizes into steam, so I assumed peak temperature for liquid eggs was also their boiling point.) —SeekingAnswers (reply) 22:08, 14 September 2012 (UTC)
- It is not correct to think that once water turns to steam it is past its highest heat conductivity. Steam burns are actually worse than those from splashed boiling water. Steam conveys much more heat than boiling water. See steam and heat of evaporation. So long as the steam is not under pressure, it will not cook the egg to a much higher temperature than boiling water. But it does convey that heat much more quickly, which is why it is used as a cooking method. Adding some water to the mix will slow down the cooking, since the water will absorb some of the ambient heat. Added water will slow the cooking and help prevent overcooking, to a degree. μηδείς (talk) 22:50, 14 September 2012 (UTC)
- Let me rephrase (and I've edited my original question accordingly). The question I really wanted to ask was which of the two cases I gave (without water dilution and with water dilution) reaches the higher temperature during the steaming process. (I only mentioned the boiling point because liquid water reaches its maximum temperature during cooking at the boiling point, after which it vaporizes into steam, so I assumed peak temperature for liquid eggs was also their boiling point.) —SeekingAnswers (reply) 22:08, 14 September 2012 (UTC)
- (edit conflict) Boiling point of a mixture of components is not the average of the components' individual boiling points. See Distillation for the extremely common situation, where each behaves approximately independently, azeotrope, where "high bp + higher bp = lower-bp mix", and boiling-point elevation, where substances with extremely high bp only create a comparably small increase of bp. Back to the original question, BDD does lead in an interesting direction: does steaming really involve the egg itself boiling, or just being exposed to boiling water? And if "the egg" is boiling, is it maybe just the water of the egg boiling out of the [solid and other gooey parts]? Egg (food)#Cooking issues gives a coagulation temperature well below the boiling point of water. DMacks (talk) 22:11, 14 September 2012 (UTC)
Can anyone find out how long a tablet of Zopiclone is good for? Best guesses, and even "usually", are not useful; I can make those estimates myself, thanks. The WP article doesn't say. I'd really like a solid source. Bielle (talk) 22:35, 14 September 2012 (UTC)
- I hate to disappoint you, but the only place you're going to get an answer is the manufacturer for that pill, whatever brand it is, whenever it was made, and even that is a guess. (Manufacturers have little incentive to say just how long something will last; saying "good for a year" is often good enough for them. However, it had better last for at least as long as they say.) But a different preparation might have different pH, different excipients; the lifespan of a pill is based on the entire pill (and the bottle it comes in!), not just the active ingredient. Wnt (talk) 22:52, 14 September 2012 (UTC)
- From a practical standpoint, the lifetime of a particular pill will depend on the conditions under which it is stored. (Light, heat, and high humidity tend to be detrimental; the cabinet in the bathroom right next to the shower – you know, where everyone keeps their pill bottles – is probably one of the worst places to store drugs. [17]) If you want to know if your older prescription medications are still good, then your best bet is to ask your pharmacist. Not only will they know the shelf life, but they may also be able to advise you on how to tell if a particular medication has gone off. It wouldn't be appropriate for Wikipedia editors to advise you further. TenOfAllTrades(talk) 23:13, 14 September 2012 (UTC)
- I'm hesitant to contribute after TenOfAllTrades' final sentence, but am a bit confused about your question: doesn't the box have an expiry date? I happen to have a box of Zopiclone bought in Norway in July, 2012, that has an expiry date of January 2015. This is consistent with the proposal from the UK Medicines and Healthcare products Regulatory Agency, [18]: "A shelf life of 36 months at 25°C is proposed." --NorwegianBlue talk 00:39, 15 September 2012 (UTC)
- Our drugs are frequently, but not always, decanted. It has something to do with the Canadian government being the controller of the purchase and sale prices. Thank you for the reference. That's useful information, not advice. Bielle (talk) 03:40, 15 September 2012 (UTC)
Fungus growing without an apparent food source
I often find fungus growing in my bottles of phosphate buffer, which have been autoclaved on top of not containing anything that should support microbial growth. Seriously, water, salts (sodium, potassium, chloride, magnesium, phosphate), glass bottle. What the hell is the fungus growing on? It's always the same thing, too - a boring filamentous white fungus (sorry, no pictures). Could it be eating some trace sugars/bacteria in the bottle? Is it fixing carbon dioxide out of the air? Curiously, it doesn't spontaneously appear in other buffers in the lab. Just the one I described. Someguy1221 (talk) 23:01, 14 September 2012 (UTC)
- Something got contaminated somewhere. Fungi can't grow without a source of carbon and nitrogen, and they can't fix either from the atmosphere. Nor can they survive autoclaving. First step in this case is to replace your reagents. Try the ones from the lab next door. If the problem persists, try reagents from another producer, or of a higher grade if you're not using p.a. The water and glass seem not to be the problem as your other buffers would be probably be affected, but it would be worthwhile to check your distiller, washing and autoclaving procedures as well. Another possibility is the seal on the cap you use. Try using a groud glass stopped bottle. Dominus Vobisdu (talk) 23:15, 14 September 2012 (UTC)
- And are you certain it's a fungus ? I can imagine salts forming threadlike crystals under the proper circumstances. StuRat (talk) 23:27, 14 September 2012 (UTC)
- If put the threads on an agar dish they grow, so, yeah. Someguy1221 (talk) 23:29, 14 September 2012 (UTC)
- Fungal hyphae are rather distinct from plants and bacteria. Next time take pictures and post them. μηδείς (talk) 00:23, 15 September 2012 (UTC)
- If put the threads on an agar dish they grow, so, yeah. Someguy1221 (talk) 23:29, 14 September 2012 (UTC)
Privacy among other animals
This is sort of a sequel to my previous question about social taboos in other animals. Humans are known for this concept called "privacy", but are we the only animals with it? I'm sure at least other mammals would be familiar with the concept of "privacy", especially when it comes to raising their young, but I can't seem to find anything about it online. Are there any other animals other than humans who have the concept of "privacy" and take it very seriously? I asked this because it seems no other animal is squeamish when it comes to being naked or mating. Narutolovehinata5 tccsdnew 23:25, 14 September 2012 (UTC)
- Some cats seem to want privacy when they defecate. This might have developed since they are vulnerable then, therefore it's best to be alone, so nothing can attack them. StuRat (talk) 23:29, 14 September 2012 (UTC)
- Many animals also like privacy when they eat, presumably so nobody can steal their meal. StuRat (talk) 23:30, 14 September 2012 (UTC)
- Once again you are looking at the issue of conceptualization. Human children will happily defecate and play with themselves in public, until they are taught the idea of privacy. Animals may become aggressive or shy (which is chemically mediated) when eating, defecating, or copulating, while all evidence points to humans needing to learn this. My five year old nephew is only just mastering this, since he will insist on privacy rather hilariously when he goes to the bathroom (he doesn't even want you to realize why he is in the bathroom) then take out his penis and show you his foreskin without any shame whatsoever. Jean Piaget normally covers the development of such concepts, but I haven't found anything relevant. The notion of privacy itself is very culture-relative. Apparently, the Russian language (according to privacy) has no such word, the closest being "secrecy". I would suggest that you consider that notions that need to be taught to children using words are unlikely to exist among animals except metaphorically. μηδείς (talk) 23:52, 14 September 2012 (UTC)
- The absence of a single word for a concept in a certain language doesn't mean that the notion doesn't exist in that culture. Russians are no different from anyone else in desiring privacy when they excrete or shower or copulate or masturbate, for example. English has to resort to wordy collocations to refer to concepts for which some other languages have a single word. But you're right, privacy and secrecy are intimately entwined, and for the longest time I had the greatest difficulty in keeping them separate in my private life. (I also had a strong affinity with all things Russian from an early age, with absolutely zero family heritage to account for it. Weird, that. I suspect I was Jack the Terrible in a previous life. Now, I'm just Jack the Terribly Impressive.) -- ♬ Jack of Oz ♬ [your turn] 23:03, 15 September 2012 (UTC)
- If you find that you have a pet parrot with an intense urge to squeeze itself into dark corners of your home (e.g. beneath furniture, cupboards, drawers) and lurk there for hours at a time - just you try dislodging him/her without getting bitten or bickered loudly at. An urge for privacy? Perhaps... --Kurt Shaped Box (talk) 23:45, 14 September 2012 (UTC)
- Privacy is not normally defined as an urge to be in the dark, but to be alone. Have you found a little stash of birdie porn mags and sex toys? μηδείς (talk) 00:20, 15 September 2012 (UTC)
- An urge to be alone - but in the dark? With toys? (seriously - they do take their favourite objects in there with them sometimes). --Kurt Shaped Box (talk) 00:36, 15 September 2012 (UTC)
- Privacy is not normally defined as an urge to be in the dark, but to be alone. Have you found a little stash of birdie porn mags and sex toys? μηδείς (talk) 00:20, 15 September 2012 (UTC)
- It's more the urge to be unobserved, I think, rather than to be alone. A microphone, camera or telescope can violate someone's privacy even while he is alone, while a person behind a thick wall does not. - Lindert (talk) 00:35, 15 September 2012 (UTC)
Cannabinol in NJ?
How could one find sources determining what the protocols are for prescribing marinol pills for a (chemotherapy patient) in New Jersey? Does its use even vary by state in the US? I am not interested in "medical marijuana", which was recently legalized, but the old-fashioned cannabinol pills supposedly meant to minimize abuse. Thanks. μηδείς (talk) 23:40, 14 September 2012 (UTC)
- Note that Marinol is delta-9-tetrahydrocannabinol, the purified psychoactive component of marijuana. In healthy wild-type cannabis plants (i.e. "ditchweed") this is mostly converted to the closely related (and even more illegal) cannabidiol, a far less inebriating substance which has been credited with a variety of beneficial effects which, among other things, tend to offset some of the less desirable activities of THC. Wnt (talk) 00:31, 15 September 2012 (UTC)
- Our article says that Marinol is Schedule III in the United States, so apparently any physician can prescribe it, and patients can even obtain refills on prescriptions. Wnt (talk) 00:33, 15 September 2012 (UTC)
- We should ask governor Chris Christie. It's apparent that he's had the munchies on more than one occasion. :-) StuRat (talk) 14:37, 15 September 2012 (UTC)
- I think I may have misled with the focus of my question. I am not really concerned so much with medical marijuana or marinol itself as I am with the jurisdiction of regulation in prescribing controlled drugs. The notion arose because I know people in NY who have rather routine access to marinol, and am curious if it is regulated the same in other states. I know that how prescriptions for controlled substances are filled differs slightly in paperwork between NY and NJ (although this may be a perception of mine based on a change in federal law during between times when I had controlled prescriptions filled in both states). So, ignoring medical marijuana, since it is still illegal at the federal level, should one expect the way doctors are regulated in prescribing marinol is the same in all states, or would it vary? Thanks. μηδείς (talk) 18:13, 15 September 2012 (UTC)
September 15
continuity equation -- why partial, but not total derivative with respect to time ?
Mathematical proofs aside, I would like to intuitively grasp the meaning of the differential form. I can see flux lines going in and flux lines going out, and quantities building up, but I can't imagine how the partial differential of say charge density with respect to time relates better to charge balance than a total derivative of charge density. In fact, I have a hard time thinking about partial derivatives with respect to time versus total derivatives wrt time, whereas I can easily conceive the difference for the x,y,z counterparts. 128.143.102.189 (talk) 04:51, 15 September 2012 (UTC)
- In the article I'm not convinced about the proof [19], it moves back and forth between partial and total derivatives. For instance it uses but since q(t) is only a function of time it is the same as the total derivative, it then sticks with the partial when moving from charge to charge density but fails to discuss this. I'm not sure what the right course is though, sorry. Perhaps try math desk? 94.72.221.29 (talk) 08:03, 15 September 2012 (UTC)
- How about saying , then take time derivative:
- .
- Now shrink the volume V to a very small blob centred on , we only want at the point so we fix it with a partial deriv, hold constant and just diff wrt to time:
- .
- This is obviously very lazy, non-rigorous and probably wrong - you really need someone who knows what they are talking about. 94.72.221.29 (talk) 08:13, 15 September 2012 (UTC)
- I tried to clarify continuity equation. Ruslik_Zero 13:12, 15 September 2012 (UTC)
- Okay, what happens if you take the volume integral of the total time derivative of density? What would this generate and what would its physical meaning be? What would change about the integral and what could I add to the continuity equation to make it balance? 71.207.151.227 (talk) 22:28, 15 September 2012 (UTC)
- Hmm, from the above explanation I kind of get it, but not completely. 71.207.151.227 (talk) 22:41, 15 September 2012 (UTC)
Question about QM
I read this
Quantum mechanics is a result of human thought which is a mental analog to the microscope or telescope. It allows us to see external reality in a certain way due to the details of its construction but that very construction introduces distortions and filters that mean we are not seeing actual reality. It is a useful tool which produces "pictures" of the external world but again the pictures are not the world but a description of it.
Is it right? Bubba73 You talkin' to me? 05:05, 15 September 2012 (UTC)
- When I was in the classroom teaching various high school science classes, I used to start every year in all of my classes with This painting by René Magritte. The lesson being that of course that isn't an apple. It is a picture of an apple. Scientific theories aren't apples. They are pictures of apples. Some are really nice pictures of apples. But at no point does a picture of an apple actually become an apple. I had an organic chemistry professor who said once "Atoms do what atoms do. If our models don't accurately portray what they do, it's because our models are not atoms." In a sense, all of the knowledge in your head consists of theories and models. You don't experience all of the world in reality, what you experience is the world filtered through your brain and made into models. It is all models. When I say "tree" you have a picture in your head of some sort of tree, and you easily recognize trees as trees, and not as, say, cats. That's because you have a working model of what a tree is, a "theory of a tree" in your head. When you see a never before seen object, you are able to instantly compare it to all of the models you have constructed for yourself, and decide "this is a tree" "this is a book of poetry" "this is a rock song I am hearing", because you atomatically create theories and models all the time to make your world comprehensible. Much of science is about extending our ability to make models beyond our own senses. You aren't equipped to experience an "atom" with your own senses. So what science does is develop those theories and models that we can't do automatically, because we can't use our own senses to probe nature to that level. That's all QM is: just as you have developed an internal "theory of tree" that is an idealized tree that allows you to identify an object as a tree, QM is an idealized portrait of physics that allows us to better elucidate exactly what is going on in, say, the atom. It is a tool for understanding the inner workings of matter. But just as Magritte's painting of the apple is not an apple, QM is not reality, but a picture of reality. All pictures are, by necessity, approximations of reality, and QM is an approximation. A really good approximation, but an approximation nonetheless. If you want to explore these ideas further, philosophy of science and philosophy of mind are good places to look for insight. --Jayron32 05:31, 15 September 2012 (UTC)
- So QM is really no different from other scientific theories in that regard, right? Bubba73 You talkin' to me? 05:44, 15 September 2012 (UTC)
- No, that's what a theory is. A theory is a tool for studying a subject. It is an explanatory framework, a way of elucidating truth from some aspect of reality. Not all theories are equal, of course. QM is a particularly productive and useful theory, and it is very accurate, especially compared to the theories it supplanted, but it is a theory. It also isn't a complete picture of reality, for example it doesn't really deal with gravity in a satisfactory way. But what it does do well, it does very well, such as explaining the structures and interactions within and between atoms. --Jayron32 05:48, 15 September 2012 (UTC)
- So QM is really no different from other scientific theories in that regard, right? Bubba73 You talkin' to me? 05:44, 15 September 2012 (UTC)
- So the paragraph I quoted really applies to any scientific theory, not just QM, right? Bubba73 You talkin' to me? 16:00, 15 September 2012 (UTC)
- Not to confuse you on this point: Yes. --Jayron32 03:13, 16 September 2012 (UTC)
- I'd change that to "any model". A model, by definition, is a simplification of the real thing, such as a globe being a simplification of the Earth. StuRat (talk) 20:39, 15 September 2012 (UTC)
- George Box is often quoted on this point: "All models are wrong, but some are useful." -- 205.175.124.30 (talk) 21:38, 15 September 2012 (UTC)
- He was actually speaking of Kardashians. ←Baseball Bugs What's up, Doc? carrots→ 02:57, 16 September 2012 (UTC)
- That'd be the some that aren't useful. --Jayron32 03:14, 16 September 2012 (UTC)
- Depends on the use. ←Baseball Bugs What's up, Doc? carrots→ 05:01, 16 September 2012 (UTC)
- That'd be the some that aren't useful. --Jayron32 03:14, 16 September 2012 (UTC)
- He was actually speaking of Kardashians. ←Baseball Bugs What's up, Doc? carrots→ 02:57, 16 September 2012 (UTC)
- George Box is often quoted on this point: "All models are wrong, but some are useful." -- 205.175.124.30 (talk) 21:38, 15 September 2012 (UTC)
The return of the aether
So, just reading through some physics stuff, and let me state up front that I know next to nothing about real physics. But I was reading about the Superfluid vacuum theory, and it got me thinking, didn't I see this stuff before? Oh, yeah, Luminiferous aether. Near as I can tell, they are both ways of modeling a vacuum as a physical substance for explaining quantum-level behavior. So, any of the physics people out there. Using small words, can anyone explain how the Superfluid Vacuum Theory isn't just Luminiferous Aether wearing a new coat? --Jayron32 06:04, 15 September 2012 (UTC)
- Not knowing much about the Superfluid Vacuum theory (SFV), I'll venture that the crucial difference is that the aether establishes an absolute referential frame while the SFV doesn't. Dauto (talk) 15:40, 15 September 2012 (UTC)
- It appears that it does have an absolute rest state. More to the point, googling the phrase "superfluid vacuum theory" doesn't turn up any hits except copies of the Wikipedia article and a couple of random message board posts. I think the article should be deleted, or at least renamed to "superfluid models of the vacuum", edited to make it clear that hardly anyone believes in this stuff, and delinked from the many other articles that the page's creator added it to. -- BenRG (talk) 17:52, 15 September 2012 (UTC)
- In otherwords, it is likely mostly bullshit. Thanks for that. I was skeptical, given that most mainstream physics texts don't light on this at all, which is why I asked the question. If BenRG hasn't heard of it, I take that as a good sign that it's hokum. Thanks Ben. --Jayron32 03:11, 16 September 2012 (UTC)
- It appears that it does have an absolute rest state. More to the point, googling the phrase "superfluid vacuum theory" doesn't turn up any hits except copies of the Wikipedia article and a couple of random message board posts. I think the article should be deleted, or at least renamed to "superfluid models of the vacuum", edited to make it clear that hardly anyone believes in this stuff, and delinked from the many other articles that the page's creator added it to. -- BenRG (talk) 17:52, 15 September 2012 (UTC)
deer
can you get lyme disease from hunting and skinning deer? How big is the risk? Have there been any documented cases of this happening?--Wrk678 (talk) 11:13, 15 September 2012 (UTC)
- I'll definitely say that yes. Deer ticks are directly related to Lyme disease, and you'll be hunting in the habitat of deers. Dying might also be the perfect occasion for ticks to leave it's host and search for a new, warmer host, like a hunter. OsmanRF34 (talk) 12:59, 15 September 2012 (UTC)
- Some links for you: deer tick, Lyme disease. StuRat (talk) 14:33, 15 September 2012 (UTC)
- Given that the geographic range of the deer tick is only the eastern USA, can one say that the answer to the OP's question is "No" if outside that area? HiLo48 (talk) 16:57, 15 September 2012 (UTC)
- To the hunting part, yes, but a deer could conceivable be taken outside that region before being skinned. I'm not sure how long the ticks remain after the deer's death. StuRat (talk) 20:33, 15 September 2012 (UTC)
- The insect hangs around the dead deer for only about 4 seconds. Hence the old saying, "... 5, 6, pick up ticks." ←Baseball Bugs What's up, Doc? carrots→ 05:00, 16 September 2012 (UTC)
- Not to contradict the other answers, but perhaps to clarify them, the only recognized mode of transmission is by the bite of a tick, or (much less likely) some other biting insect. So the only way it could happen is if your activities led you to pick up ticks. Looie496 (talk) 17:26, 15 September 2012 (UTC)
- Keep also in mind that even if you never see a deer while hunting, running around in deer habitats will likely expose you to deer ticks. Whether you get bitten or not depends on whether you've taken proper precautions in your attire and etc. (I once brushed against some low-hanging tree branches in the northeast and managed to pick up no fewer than 10 ticks. Psychologically disturbing to keep finding them on me, but none of them bit me.) --Mr.98 (talk) 23:19, 15 September 2012 (UTC)
- Not only that, but as the article Lyme disease linked to above shows, one can get it anywhere in the Northern Hemisphere, not just the Eastern US from eastern deer ticks. See http://www.lymedisease.org/california/california_map.html To answer the question as worded, you cannot get the disease simply from hunting or skinning a deer per se, there needs to be blood or bodily fluid transfer, the chance of that from a dead deer is miniscule, but this being biology, anything is imaginable. μηδείς (talk) 01:09, 16 September 2012 (UTC)
- PS, the link "deer tick" Hilo linked to above was a misleading redirect to Ixodes scapularis when he posted it. I have changed it to a disambiguation page. In California the deer tick is Ixodes pacificus. μηδείς (talk) 04:31, 16 September 2012 (UTC)
- Are there no ticks in Australia? In Europe, Ixodes ricinus is sometimes called the "deer tick" or "sheep tick". I'll add this to the disambiguation page. Dbfirs 07:02, 16 September 2012 (UTC)
Conduction of heat and electricity.
Is there any material that does not conduct electricity but conducts heat and is easily available? — Preceding unsigned comment added by Daivatb (talk • contribs) 11:29, 15 September 2012 (UTC)
- Pure water (must be really pure, though, as even a tiny contamination makes it electrically conductive). Being a fluid, water also has the ability to transfer heat by convection, which can be even more effective than conduction. StuRat (talk) 14:31, 15 September 2012 (UTC)
- Glass (moderate conductor of heat). StuRat (talk) 15:03, 15 September 2012 (UTC)
- The material of choice with such properties is sapphire, which is much less exotic than diamond. Ruslik_Zero 16:36, 15 September 2012 (UTC)
- Diamond is not "exotic"! Whoop whoop pull up Bitching Betty | Averted crashes 17:08, 15 September 2012 (UTC)
- Unless you only need tiny sizes, diamond isn't likely to be "easily available". StuRat (talk) 20:27, 15 September 2012 (UTC)
- By those standards, neither is sapphire or ultrapure water. And glass is a mediocre conductor of heat. Whoop whoop pull up Bitching Betty | Averted crashes
- I already stated that about glass. Both large sapphires and pure water are easier to obtain than large diamonds. StuRat (talk) 23:29, 15 September 2012 (UTC)
- Without knowing the context or the application, it is impossible to properly answer this question. In many applications, the actuall degree or heat conduction is unimportant. An example: In electronics, power transistors need cooling - this is most often done by bolting them on to a heatsink (an aluminium piece with a large surface area), with a mica washer between the transistor and the sink. The washer conducts the heat from the transistor, but electrically insulates it. Mica is NOT a good conductor of heat, but it is a VERY good electrical insulator. In this application it works very well heat-wise, because it is very thin, typically about 0.2 mm over an area of 400 mm2. Distilled water has been used as a coolant in very high power radio transmitters, exposed to high voltages. As said above, the electrical properties with commonly available distilled water are let's say rather poor. But when you are shifting 100 kW of heat, a few watts lost due to water electrical conduction doesn't matter. Keit120.145.29.139 (talk) 03:06, 16 September 2012 (UTC)
Height of the nail

A nail is inserted in the trunk of a tree at a height of 1 meter from the groung level. After three years the nail will remain at the same position or it will rise above. Why? Sunny Singh (DAV) (talk) 16:29, 15 September 2012 (UTC)
- I believe it depends on the type and age of the tree. Many mature trees grow mainly at the tips of branches, while younger trees can also grow elsewhere. StuRat (talk) 16:34, 15 September 2012 (UTC)

- In almost all cases (I can't think of an exception, unless you are dealing with the tree's first season of growth) it will remain at the same height from the ground, but will appear to have sunk into the tree. This is because of the difference between primary and secondary growth. Primary growth is the (normally ) yearly extension of the growth of the tips of the trunk and then branches. Only this yearly growth at the tips contributes to an increase in height and length. Secondary growth in rings adds girth to the previous years' primary and secondary growth. This secondary growth will cause the growth of three new rings external to the point to which the nail had originally been sunk. Eventually the nail will be engulfed by new rings and disappear, which is why the terrorist practice of "spiking" trees is so dangerous. See also meristem on the growth of plants and especially intercalary meristem which is found in grasses and very few other plants and is the only mechanism by which a stem could grow lengthwise in its middle. μηδείς (talk) 20:53, 15 September 2012 (UTC)
- Why would driving spikes into trees (that's what I assume it means) be at all dangerous? Objects driven into a tree, unless they completely surround the trunk and girdle the tree, are harmless to the tree, unless they disrupt the sap flow immediately below a branch, in which case the only thing that dies is that particular branch. Eventually, the spike or nail or whatever will be completely engulfed by the tree, and it will become an inclusion in the tree, doing no more harm to the tree than a lithopedion does to a human. Whoop whoop pull up Bitching Betty | Averted crashes 22:22, 15 September 2012 (UTC)
- Environmental activists put spikes in trees so they will destroy the chainsaws of anyone trying to cut them down. Rather than risk this, it is presumed that the lumberjacks will leave those trees alone. However, if the lumberjack doesn't know they are there, it could also cause the chainsaw to "buck", hit the lumberjack, and injure or kill him. Not likely, but possible. StuRat (talk) 22:30, 15 September 2012 (UTC)
- (After EC, but with references) The danger is not to the tree, but to loggers. The idea is to make logging more dangerous than it already is, and thereby potentially stop logging operations due to safety risks. When a large chainsaw hits a hidden spike in a tree, the chainsaw reacts violently, and can harm the operator. I think this practice came to national attention in the USA during controversy surrounding protecting spotted owl habitat from timber harvesting. See Tree_spiking, eco-terrorism, Spotted_owl#Conservation, or google similar terms. SemanticMantis (talk) 22:32, 15 September 2012 (UTC)
- Oh. I thought Medeis meant dangerous to the tree. Apparently not. Whoop whoop pull up Bitching Betty | Averted crashes 22:34, 15 September 2012 (UTC)
- They always told us it was harmful to a tree to put nails into it, peel bark off it, etc. I don't know about that. But it's safe to say that applying a chainsaw to a tree is liable to endanger it. ←Baseball Bugs What's up, Doc? carrots→ 03:00, 16 September 2012 (UTC)
- Oh. I thought Medeis meant dangerous to the tree. Apparently not. Whoop whoop pull up Bitching Betty | Averted crashes 22:34, 15 September 2012 (UTC)
- Odd, there doesn't seem to be any good schematic drawing of this at all on the web. Much of the imagery of secondary growth seems to be related to pot plants, which don't really exhibit it in the sense of wood. But see figure three here for a hint at how a tree grows: http://www.cmg.colostate.edu/gardennotes/771.html μηδείς (talk) 21:12, 15 September 2012 (UTC)
- The caption to the image raises an interesting question: can a growing tree engulf a living part of another plant, such as a branch from another tree that was pressed against the trunk of the tree in question? Whoop whoop pull up Bitching Betty | Averted crashes 22:39, 15 September 2012 (UTC)
- Yes, and some vines/trees intentionally engulf others, even going so far as to smother them. StuRat (talk) 23:35, 15 September 2012 (UTC)
- For which see strangler fig and husband and wife trees. μηδείς (talk) 23:38, 15 September 2012 (UTC)
- Thanks for the spiking reference, had dinner not intervened I meant to come back and find the WHAAOE link. Interestingly there is an herbicide called Spike 80DF that has famously been used to murder trees, but I was talking about the saw-sabotaging practice mentioned above. μηδείς (talk) 23:38, 15 September 2012 (UTC)
- One can kill a tree, but one cannot "murder" a tree. Murder is the unlawful taking of human life. ←Baseball Bugs What's up, Doc? carrots→ 02:53, 16 September 2012 (UTC)
- Remind me not to let you water my plants while I'm away. μηδείς (talk) 02:57, 16 September 2012 (UTC)
- It's deja vu all over again. In any case, I try to keep my plants well watered. ←Baseball Bugs What's up, Doc? carrots→ 03:00, 16 September 2012 (UTC)
- Yes, I can easily imagine you murdering my Euphorbias with your careless oversaturation. Or at least negligently manslaughtering them. μηδείς (talk) 03:08, 16 September 2012 (UTC)
- That would be plantslaughtering them. -- ♬ Jack of Oz ♬ [your turn] 03:34, 16 September 2012 (UTC)
- Even if they end up in a persistent vegetative state ? StuRat (talk) 04:01, 16 September 2012 (UTC)
- Best...comeback...ever. (at least in this thread) μηδείς (talk) 04:24, 16 September 2012 (UTC)
- Keep in on life support, as with the famous Boston I.V. ←Baseball Bugs What's up, Doc? carrots→
- Best...comeback...ever. (at least in this thread) μηδείς (talk) 04:24, 16 September 2012 (UTC)
- Even if they end up in a persistent vegetative state ? StuRat (talk) 04:01, 16 September 2012 (UTC)
Speed on one point
To measure speed, do you really need to measure where you are in two points? I mean, on point p you also have speed, so could you measure this speed that you have just with data from this point? OsmanRF34 (talk) 21:36, 15 September 2012 (UTC)
- Speed is by definition distance travelled over time so if you just have one point you have no way of knowing your speed; in other words, if you only know you are at exactly 20 m from your front door at exactly noon, what is your speed? Who knows? You could be in the middle of just standing there, or running away, or driving away, or walking in circles, or any other action. However, if you know that at ten to noon you were at your front door, then you could calculate your average speed to be 20 m / 10 min or 2 m/min or 0.12 kph - there is still a problem with this, as in the intervening time you could have walked the 20 m directly, if you are a very elderly person or some kind of small animal, but you could just as well have gone around the house, or gone down the block, or gone halfway to the sun and come back if you happen to be a photon under the influence of some weird gravitational field - but at least you will have some idea of your "speed".
- Mathematically speaking, given a well-defined, differentiable position function p(t) of time t it is possible to find instantaneous speed exactly at any time, by taking the derivative p'(t) with respect to t. This is because in such a case you have or can calculate an infinite number of data points, so calculus "works". If you can model a physical phenomenon with some such function, such as the falling of an apple from a tree, then indeed you can find the speed with just one data point, the time after it has fallen. However, you will probably be slightly off due to imprecision in determining air resistance and other variables.
- In real life, you don't have a magical formula that can tell you exactly where you are at any arbitrarily precise given time, so you must use an average, hence you need more than one data point. 24.92.74.238 (talk) 21:47, 15 September 2012 (UTC)
- I don't buy that. I can theoretically measure your speed by (for example) placing an immovable obstacle in your path. When you splatter into the object, I can (in principle) calculate the rise in temperature, and knowing your mass and the mass of the object you struck, deduce the amount of kinetic energy that was involved and use that to deduce your speed at the instant of impact. This is a somewhat destructive approach - but it points the way to doing the measurement at a single point. In practical terms, a police "speed gun" does a measurement at a single point by measuring the doppler shift of reflected radio or light waves from a moving object. SteveBaker (talk) 22:06, 15 September 2012 (UTC)
- The measure doppler shift you need to measure the signal over a period of time in order to determine its frequency. Indirectly measuring the kinetic energy of an object will also take some time. To measure the speed at a single point, you need to measure it in a single instant, which you can't do. --Tango (talk) 23:18, 15 September 2012 (UTC)
- IP 24 and Tango are correct, point in space equates with instant in time. Doppler shift and splatter both imply change over time. I am not quite sure how to reference this other than suggesting a basic course in physics. μηδείς (talk) 23:27, 15 September 2012 (UTC)
- The measure doppler shift you need to measure the signal over a period of time in order to determine its frequency. Indirectly measuring the kinetic energy of an object will also take some time. To measure the speed at a single point, you need to measure it in a single instant, which you can't do. --Tango (talk) 23:18, 15 September 2012 (UTC)
- I don't buy that. I can theoretically measure your speed by (for example) placing an immovable obstacle in your path. When you splatter into the object, I can (in principle) calculate the rise in temperature, and knowing your mass and the mass of the object you struck, deduce the amount of kinetic energy that was involved and use that to deduce your speed at the instant of impact. This is a somewhat destructive approach - but it points the way to doing the measurement at a single point. In practical terms, a police "speed gun" does a measurement at a single point by measuring the doppler shift of reflected radio or light waves from a moving object. SteveBaker (talk) 22:06, 15 September 2012 (UTC)
Uh—Speed#Instantaneous speed? Isn't it easy to find the speed of an object at one instant in time by using calculus? Whoop whoop pull up Bitching Betty | Averted crashes 23:46, 15 September 2012 (UTC)
- Yes, but the calculation is still based on a measurement over time/distance. μηδείς (talk) 00:04, 16 September 2012 (UTC)
- Medies seems right on this. If you think about the practical method of finding "speed" of a moving object, even instantaneous speed, involves making a large number of discrete time-and-distance measurments to give you points on a graph, and then you fill in the "gaps" via interpolation. You can then take the derivative of that curve, but to build the curve you still have to connect the dots at some point. Or you could just use a camera and a speedometer, which measures speed continuously. --Jayron32 03:06, 16 September 2012 (UTC)
September 16
So-called "stealth" aircraft
Why has the military been foolish enough to swallow aircraft marketers' claims that their aircraft are invisible to radar? Even if the aircraft is made totally invisible to active radar, there is no way to make it invisible to passive radar. Even if the aircraft has no radar equipment on board, it can still be detected by passive radar due to the radio waves generated by its onboard electronics. And, in any case, no manufacturer in his or her right mind would design an aircraft with no onboard radar, as such an aircraft would be totally blind. Whoop whoop pull up Bitching Betty | Averted crashes 00:35, 16 September 2012 (UTC)
- Have you read our policy on not asking for opinions or starting debates? Do you have a source that backs up your claims that marketers claim "that their aircraft are invisible to radar?" Do we need to ask you formally not to contribute such nonsense? Have you even read Stealth Bomber#Stealth? Is there any reason this should not be marked closed? μηδείς (talk) 00:58, 16 September 2012 (UTC)
- The military has never simply swallowed such claims. In the case of the F-117 for instance, the military required each participant in the competition that lead to its creation to submit a model for radar testing, described briefly in Lockheed Have Blue#Experimental Survival Testbed. Our articles on the Northrop Grumman B-2 Spirit and the F-22 don't describe the selection process, but I assume radar testing was involved. The military also required flight tests of aircraft at various stages of the pre-production process, and following the aircraft with radar would have been a natural part of that process. And you're wrong on your last point. The F-117 stealth fighter has no active radar, described at Lockheed F-117 Nighthawk#Design. But it seems to work nonetheless. Six F-117s and two prototypes were lost in accidents, which is kind of pitiful when only 64 were ever built, but only one was ever lost in combat. Someguy1221 (talk) 01:07, 16 September 2012 (UTC)
- As far as alternatives to using active radar, they can get info broadcast to them from ship or ground radar, AWACs, satellites, other (non-stealth planes), UAVs, etc. StuRat (talk) 01:15, 16 September 2012 (UTC)
- As a methodological note, when you find yourself thinking that you're smarter than the people who build actual stealth bombers, you may wish to question your hubris, or at leasts do a little more research. Either you're wrong or they're wrong. I suspect you're wrong. --Mr.98 (talk) 02:36, 16 September 2012 (UTC)
- However, the US military acquisition process is susceptible to influence peddling, sometimes resulting in rather unwise multi-billion dollar defense contracts. StuRat (talk) 02:43, 16 September 2012 (UTC)
- The greatest influence peddling over military acquisitions that I'm aware of is actually coming from congressional representatives seeking pork for their state, rather than manufacturers trying to get their products purchased. This is the case, for instance, with the Boeing C-17 Globemaster III (see the "orders and deliveries" section), where congress consistently orders more planes even though the Air Force has stated they don't need any. It's a wonderful plane, however. As far as compromising actual quality, there was also a New York Times article years back, can't remember the exact link, that was criticizing the process by which the Federal Government chose where to build the Navy's ships. Rather than having manufacturers compete for contracts based on price and quality, congress was simply handing the contracts out to benefit the home states of whoever was on the relevant committees. There have certainly been some boneheaded decisions by the military in the past, but I don't think corruption had anything to do with it. The problems documented in the comical but allegedly accurate The Pentagon Wars can be blamed entirely on Generals' being unrealistic (at least that's the accusation). Going back to WWII, the Mark 14 torpedo didn't work as advertised, and in fact the Navy never tested the final product, but ordered it for the entire fleet nonetheless. More examples of simply boneheaded ideas can be found, largely centered on skepticism over repeating rifle technology, if you go back to the eras of the Spanish American war and the American Civil War. But my point is, the military does not need corruption to help them make bad decisions. Someguy1221 (talk) 03:11, 16 September 2012 (UTC)
- I would classify pork-barrel projects as a form of corruption. StuRat (talk) 03:58, 16 September 2012 (UTC)
- So the modern-day U.S. military is a hive of corrupt boneheads? Whoop whoop pull up Bitching Betty | Averted crashes 05:14, 16 September 2012 (UTC)
- I don't think so. You will find stupidity wherever you look, and shouldn't take a few examples as evidence of a larger problem. Congress is a hive of corrupt boneheads. Someguy1221 (talk) 05:18, 16 September 2012 (UTC)
- So the modern-day U.S. military is a hive of corrupt boneheads? Whoop whoop pull up Bitching Betty | Averted crashes 05:14, 16 September 2012 (UTC)
- I would classify pork-barrel projects as a form of corruption. StuRat (talk) 03:58, 16 September 2012 (UTC)
Chirality in spiders
Recently I observed a spider spinning a vertical orb-type web. I noticed that while it was laying the concentric strands, it moved in a clockwise direction (from its perspective relative to the plane of the web) and consistently used its right leg to hook the silk onto the long radial strands. Do spiders in general display a preference one way or another for clockwise or widdershins movement, or for one leg over another? If so, which way? 71.248.115.187 (talk) 01:32, 16 September 2012 (UTC)
- Is Chirality the Chirality you're talking about? ←Baseball Bugs What's up, Doc? carrots→ 02:48, 16 September 2012 (UTC)
- Yeah, he just means handedness; that is directionality: clockwise and counterclockwise are chiral directions. I did some searching, and this Google Scholar search turns up lots of results. There doesn't appear to be any general trends among orb-weaver spiders, from what I can see from those searches many spiders build their web in alternating directions: first clockwise and then return counterclockise; though others work in spirals, either clockwise or counterclockwise. Scanning several of the abstracts, I can't find any general trends, but if you're more interested, perhaps that search could aid you in finding more information. --Jayron32 03:03, 16 September 2012 (UTC)
- This is really what biologists would call laterality. This paper from 2002 describes a lateralized behavior in a species of spider. The Introduction states that the authors were not aware of any other report of lateralization in arachnids. If anything had been published in the meantime, I expect that it would have cited this paper, but Google Scholar shows nothing that does. In short, it is plausible but apparently nobody has looked into it. Looie496 (talk) 03:02, 16 September 2012 (UTC)
Cavitating bubbles party trick
Re: http://www.youtube.com/watch?v=cOeNxkksruo&feature=related
I'm pretty sure I understand the physics behind this. But at the beginning of the video, they mention that there has to be a fair bit of air at the top of the bottle, and I'm wondering why this is. As far as I can tell it shouldn't matter, but I've seen another video saying the same thing. 65.92.7.148 (talk) 02:09, 16 September 2012 (UTC)
- Here's a description of what's happening at Discovery.com. The way I would describe it myself is that the water is in effect stretched away from the bottom (and hence it needs the compressible space of the air above to move into) and then violently bouncing back against the bottom. Without the space above, the liquid would have no where to move into, away from the bottom. μηδείς (talk) 02:36, 16 September 2012 (UTC)
- The air gap is needed to give the water someplace to go. Since air is far more compressible than water, the air becomes compressed at the top while the water moves upward (relative to the bottle), and cavitation bubbles form at the bottom. StuRat (talk) 02:41, 16 September 2012 (UTC)
- sondern lasst uns angenehmere anstimmen, und zitatlosere? μηδείς (talk) 03:53, 16 September 2012 (UTC)
- No, Fawlty! It's: Alle Menschen werden Brüder, wo dein sanfter Flügel weilt. [JackofOz]
- That's a quote from a famous Sci-Fi film: Plan Ninth from Outer Space. ←Baseball Bugs What's up, Doc? carrots→ 04:52, 16 September 2012 (UTC)
- Later used as the theme music for the TV show Gunfight at the OK Choral. -- ♬ Jack of Oz ♬ [your turn] 05:45, 16 September 2012 (UTC)
- We're probably sickening the readers with all this trivia. Some of them might even Earp. Ya know, Old Ludwig's sanity was always fragile, but after he finished the first movement of the Ninth he went scherzoid. ←Baseball Bugs What's up, Doc? carrots→ 06:01, 16 September 2012 (UTC)
- Well, blame the writer of the first bit, whatever it means. Oh, I see they've signed their name now. Are you gonna let us into the secret, Medeis? -- ♬ Jack of Oz ♬ [your turn] 07:25, 16 September 2012 (UTC)
- We're probably sickening the readers with all this trivia. Some of them might even Earp. Ya know, Old Ludwig's sanity was always fragile, but after he finished the first movement of the Ninth he went scherzoid. ←Baseball Bugs What's up, Doc? carrots→ 06:01, 16 September 2012 (UTC)
- Later used as the theme music for the TV show Gunfight at the OK Choral. -- ♬ Jack of Oz ♬ [your turn] 05:45, 16 September 2012 (UTC)
- That's a quote from a famous Sci-Fi film: Plan Ninth from Outer Space. ←Baseball Bugs What's up, Doc? carrots→ 04:52, 16 September 2012 (UTC)
- No, Fawlty! It's: Alle Menschen werden Brüder, wo dein sanfter Flügel weilt. [JackofOz]
- sondern lasst uns angenehmere anstimmen, und zitatlosere? μηδείς (talk) 03:53, 16 September 2012 (UTC)
- The air gap is needed to give the water someplace to go. Since air is far more compressible than water, the air becomes compressed at the top while the water moves upward (relative to the bottle), and cavitation bubbles form at the bottom. StuRat (talk) 02:41, 16 September 2012 (UTC)
- I assume the idea is that the air space means that first you move the bottle, while the liquid stays more or less still; then the liquid hits the top of the bottle and is also jolted into motion; then the bottle stops (because you're pulling back on it) and as a result the liquid has a chance to be fast moving and slam into and break the bottom of the bottle. Wnt (talk) 04:08, 16 September 2012 (UTC)
- If the top of the bottle were hitting the liquid that hard, the top would break. What's really happening is that the water never loses contact with the bottom. Rather, in effect, it "stretches" so much that it cavitates, giving a very strong vacuum at the bottom and a much larger compressed area at the top. The net change in pressure per volume is necessarily equal above and below. But the air volume above is greater so the change in pressure it experiences is less. The cavitated volume is much, much less, so the relevant pressure is much greater, causing the "stretched" water to slam back down into it with much greater force. Effectively almost all the force of the blow is focused downward, while the air above acts like a shock absorber. μηδείς (talk) 04:20, 16 September 2012 (UTC)
- I'm thinking that when the hand strikes the top of the bottle, it starts a pressure wave that travels down through bottle and then is "reflected" off the bottom into the liquid. When the pressure transfers from the bottom of the bottle to the liquid, it causes some of the liquid to separate from the glass, resulting in discrete cavities. When these cavities refill with liquid, the force that had been distributed over the bottom of the glass is focused into a few small bubbles, creating sufficient pressure to break the glass.
- I'm not sure if the air space at the top is to allow the liquid to travel upwards off the bottom - the hand is probably compressible enough to allow this. I think the air space is to ensure that the pressure wave travels down to the bottom through the glass and not the liquid. It might also be to prevent the same cavitation breakage from happening at the top, which would lead to a cut hand.--Wikimedes (talk) 04:55, 16 September 2012 (UTC)
- If the top of the bottle were hitting the liquid that hard, the top would break. What's really happening is that the water never loses contact with the bottom. Rather, in effect, it "stretches" so much that it cavitates, giving a very strong vacuum at the bottom and a much larger compressed area at the top. The net change in pressure per volume is necessarily equal above and below. But the air volume above is greater so the change in pressure it experiences is less. The cavitated volume is much, much less, so the relevant pressure is much greater, causing the "stretched" water to slam back down into it with much greater force. Effectively almost all the force of the blow is focused downward, while the air above acts like a shock absorber. μηδείς (talk) 04:20, 16 September 2012 (UTC)
Intelligence and reproduction
Why do smart people reproduce less than other people? --128.42.223.7 (talk) 05:07, 16 September 2012 (UTC)
- Because of the KCM principle - Kids Cost Money. Ratbone124.182.32.79 (talk) 05:10, 16 September 2012 (UTC)
- Isn't it evolutionarily harmful? --168.7.232.115 (talk) 05:15, 16 September 2012 (UTC)
- [Note that both IP's are from Houston] Are you asking whether the percentage of morons in the world is continually increasing? ←Baseball Bugs What's up, Doc? carrots→ 05:21, 16 September 2012 (UTC)
- Isn't it evolutionarily harmful? --168.7.232.115 (talk) 05:15, 16 September 2012 (UTC)
- Wikipedia has an article on fertility and intelligence. I can't vouch for its quality, but it has a lot of references... -- BenRG (talk) 05:22, 16 September 2012 (UTC)
- It really has nothing to do with fertility - it has to do with personal choices... which, I can virtually guarantee you, have nothing whatsoever to do with concerns about evolutionary harm. ←Baseball Bugs What's up, Doc? carrots→ 05:24, 16 September 2012 (UTC)
Three Sisters
We unexpectedly acquired 4 cats back in June. A very pregnant mother made her way into our garden shed and delivered 3 kittens, all females. We fed them, they decided to stay, and that was that. The mother's since been run over or returned whence she came or been eaten by wandering behemoths (or beastorns), but the three kids are still with us.
One has always been noticeably larger than the other two. She's got sandy/lion colouring, while the smaller ones are grey/dappled. My partner is convinced she was the first born. He says the first born comes out about half an hour before the others, has no competition for milk, and has a better headstart in life. He knows a lot more about animals than I do, but my gut tells me that the relative sizes of animals in a litter has nothing to do with birth order. I mean, on Day 1, she was already bigger. That surely must have been determined way back at conception, and can't be attributed to the transitory benefits of being born first.
We'll never know for absolute certain, but is there a reasonable likelihood he's right about this? Thank you.
Btw, I thought you'd like to know that I see myself in the Uncle Vanya role, so I've named them collectively the Three Sisters, and individually Tolstoy, Pushkin, and Frankie (because she has beautiful blue eyes). All male names for female creatures. Purrfect. -- ♬ Jack of Oz ♬ [your turn] 06:56, 16 September 2012 (UTC)
- The birth order can't determine size, but perhaps size could determine birth order. The runt of the litter is renowned for coming out last, so perhaps the Goliath also can come out first ? What was the coloration of the mother ? And did you see a male lynx creeping around ? :-) StuRat (talk) 07:11, 16 September 2012 (UTC)
- Googling for runt litter born last finds a lot of people saying there is no relation between order of birth and being the smallest of the litter. 88.112.47.131 (talk) 07:42, 16 September 2012 (UTC)












