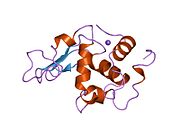
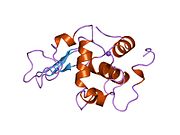
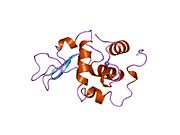
Lysozyme
| Lysozyme | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Lysozyme crystals stained with methylene blue. | |||||||||
| Identifiers | |||||||||
| EC no. | 3.2.1.17 | ||||||||
| CAS no. | 9001-63-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Template:PBB Lysozymes, also known as muramidase or N-acetylmuramide glycanhydrolase, are glycoside hydrolases. These are enzymes (EC 3.2.1.17) that damage bacterial cell walls by catalyzing hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between N-acetyl-D-glucosamine residues in chitodextrins. Lysozyme is abundant in a number of secretions, such as tears, saliva, human milk, and mucus. It is also present in cytoplasmic granules of the macrophages and the polymorphonuclear neutrophils (PMNs). Large amounts of lysozyme can be found in egg white. C-type lysozymes are closely related to alpha-lactalbumin in sequence and structure, making them part of the same family. In humans, the lysozyme enzyme is encoded by the LYZ gene.[1][2]
Function
The enzyme functions by attacking peptidoglycans (found in the cell walls of bacteria, especially Gram-positive bacteria) and hydrolyzing the glycosidic bond that connects N-acetylmuramic acid with the fourth carbon atom of N-acetylglucosamine. It does this by binding to the peptidoglycan molecule in the binding site within the prominent cleft between its two domains. This causes the substrate molecule to adopt a strained conformation similar to that of the transition state.[3] According to Phillips-Mechanism, the lysozyme binds to a hexasaccharide. The lysozyme then distorts the fourth sugar in hexasaccharide (the D ring) into a half-chair conformation. In this stressed state, the glycosidic bond is easily broken.

The amino acid side-chains glutamic acid 35 (Glu35) and aspartate 52 (Asp52) have been found to be critical to the activity of this enzyme. Glu35 acts as a proton donor to the glycosidic bond, cleaving the C-O bond in the substrate, whereas Asp52 acts as a nucleophile to generate a glycosyl enzyme intermediate. The glycosyl enzyme intermediate then reacts with a water molecule, to give the product of hydrolysis and leaving the enzyme unchanged.[4]

Role in disease
Lysozyme is part of the innate immune system. Reduced lysozyme levels have been associated with bronchopulmonary dysplasia in newborns.[5] Children fed infant formula lacking lysozyme in their diet have three times the rate of diarrheal disease.[6][failed verification] Since lysozyme is a natural form of protection from Gram-positive pathogens like Bacillus and Streptococcus,[7] a deficiency due to infant formula feeding can lead to increased incidence of disease[citation needed]. Whereas the skin is a protective barrier due to its dryness and acidity, the conjunctiva (membrane covering the eye) is, instead, protected by secreted enzymes, mainly lysozyme and defensin. However, when these protective barriers fail, conjunctivitis results.
In certain cancers (especially myelomonocytic leukemia) excessive production of lysozyme by cancer cells can lead to toxic levels of lysozyme in the blood. High lysozyme blood levels can lead to kidney failure and low blood potassium, conditions that may improve or resolve with treatment of the primary malignancy.
Serum lysozyme is much less specific for diagnosis of sarcoidosis than serum Angiotensin Converting Enzyme, since it is more sensitive, it is used as a marker of sarcoidosis disease activity and suitable for disease monitoring in proven cases [8]
History
The antibacterial property of hen egg white, due to the lysozyme it contains, was first observed by Laschtschenko in 1909,[9] although it was not until 1922 that the name 'lysozyme' was coined, by Alexander Fleming (1881–1955), the discoverer of penicillin.[10] Fleming first observed the antibacterial action of lysozyme when he treated bacterial cultures with nasal mucus from a patient suffering from a head cold.[10]
The three-dimensional structure of hen egg white lysozyme was described by David Chilton Phillips (1924–1999) in 1965, when he obtained the first 2-ångström (200 pm) resolution model via X-ray crystallography.[11][12] The structure was publicly presented at a Royal Institution lecture in 1965.[13] Lysozyme was the second protein structure and the first enzyme structure to be solved via X-ray diffraction methods, and the first enzyme to be fully sequenced that contains all twenty common amino acids.[14] As a result of Phillips' elucidation of the structure of lysozyme, it was also the first enzyme to have a detailed, specific mechanism suggested for its method of catalytic action.[15][16][17] This work led Phillips to provide an explanation for how enzymes speed up a chemical reaction in terms of its physical structures. The original mechanism proposed by Phillips was more recently revised.[18]
Chemical synthesis
The first chemical synthesis of a lysozyme protein was attempted by Prof. George W. Kenner and his group at the University of Liverpool in England.[19] This was finally achieved in 2007 by Steve Kent at the University of Chicago who made synthetic functional lysozyme molecule.[20]
See also
References
- ^ Yoshimura K, Toibana A, Nakahama K (January 1988). "Human lysozyme: sequencing of a cDNA, and expression and secretion by Saccharomyces cerevisiae". Biochem. Biophys. Res. Commun. 150 (2): 794–801. doi:10.1016/0006-291X(88)90461-5. PMID 2829884.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peters CW, Kruse U, Pollwein R, Grzeschik KH, Sippel AE (July 1989). "The human lysozyme gene. Sequence organization and chromosomal localization". Eur. J. Biochem. 182 (3): 507–16. doi:10.1111/j.1432-1033.1989.tb14857.x. PMID 2546758.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McKenzie HA, White FH (1991). "Lysozyme and alpha-lactalbumin: structure, function, and interrelationships". Adv. Protein Chem. 41: 173–315. doi:10.1016/s0065-3233(08)60198-9. PMID 2069076.
- ^ Grisham CM, Garrett RH (2007). "Chapter 14: Mechanism of enzyme action". Biochemistry. Australia: Thomson Brooks/Cole. pp. 467–9. ISBN 0-495-11912-1.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Revenis ME, Kaliner MA (August 1992). "Lactoferrin and lysozyme deficiency in airway secretions: association with the development of bronchopulmonary dysplasia". J. Pediatr. 121 (2): 262–70. doi:10.1016/S0022-3476(05)81201-6. PMID 1640295.
- ^ Lönnerdal B (June 2003). "Nutritional and physiologic significance of human milk proteins". Am. J. Clin. Nutr. 77 (6): 1537S–1543S. PMID 12812151.
- ^ Microbiology: A human perspective. Nester, Anderson, Roberts, Nester. 5th Ed. 2007
- ^ Tomita H, Sato S, Matsuda R, Sugiura Y, Kawaguchi H, Niimi T, Yoshida S, Morishita M. (1999). "Serum lysozyme levels and clinical features of sarcoidosis". Lung. 177 (3): 161–7. PMID 10192763.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Laschtschenko P (1909). "Über die keimtötende und entwicklungshemmende Wirkung Hühnereiweiß". Z. Hyg. InfektKrankh. (in German). 64: 419–427. doi:10.1007/BF02216170.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ a b Fleming A (1 May 1922). "On a remarkable bacteriolytic element found in tissues and secretions". Proceedings of the Royal Society B. 93 (653): 306–317. doi:10.1098/rspb.1922.0023.
- ^ Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR. (1965). "Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution". Nature. 206 (4986): 757–61. doi:10.1038/206757a0. PMID 5891407.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Johnson LN, Phillips DC (1965). "Structure of some crystalline lysozyme-inhibitor complexes determined by X-ray analysis at 6 Angstrom resolution". Nature. 206 (986): 761–3. doi:10.1038/206761a0. PMID 5840126.
- ^ Johnson LN (1998). "The early history of lysozme". Nat Struct Mol Biol. 5 (11): 942–944. doi:10.1038/2917. PMID 9808036.
- ^ Canfield RE (1963). "The Amino Acid Sequence of Egg White Lysozyme". J Biol Chem. 238 (8): 2698–2707. PMID 14063294.
- ^ Vernon CA (April 1967). "The mechanisms of hydrolysis of glycosides and their relevance to enzyme-catalysed reactions". Proceedings of the Royal Society B. 167 (1009): 389–401. doi:10.1098/rspb.1967.0036. PMID 4382802.
- ^ Rupley JA (April 1967). "The Binding and Cleavage by Lysozyme of N-acetylglucosamine Oligosaccharides". Proceedings of the Royal Society B. 167 (1009): 416–428. doi:10.1098/rspb.1967.0038. PMID 4382804.
- ^ Sharon N (April 1967). "The Chemical Structure of Lysozyme Substrates and Their Cleavage by the Enzyme". Proceedings of the Royal Society B. 167 (1009): 402–415. doi:10.1098/rspb.1967.0037. PMID 4382803.
- ^ Vocadlo DJ, Davies GJ, Laine R, Withers SG. (2001). "Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate". Nature. 412 (6849): 835–8. doi:10.1038/35090602. PMID 11518970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kenner GW (1977). "The Bakerian lecture. Towards synthesis of proteins". Proc. R. Soc. Lond., B, Biol. Sci. 197 (1128): 237–53. PMID 19745.
- ^ Durek T, Torbeev VY, Kent SB (2007). "Convergent chemical synthesis and high-resolution x-ray structure of human lysozyme". Proc. Natl. Acad. Sci. U.S.A. 104 (12): 4846–51. doi:10.1073/pnas.0610630104. PMC 1829227. PMID 17360367.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Muramidase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Proteopedia.org HEW Lysozyme