Ciprofloxacin: Difference between revisions
Davidtfull (talk | contribs) →Overprescribing and bacterial resistance: moved text from licensed use to here as suggested by another editor on the talk page. |
→Overprescribing and bacterial resistance: Adding ref. |
||
| Line 337: | Line 337: | ||
==Overprescribing and bacterial resistance== |
==Overprescribing and bacterial resistance== |
||
{{See also|Antibiotic abuse|Antibiotic resistance}} |
{{See also|Antibiotic abuse|Antibiotic resistance}} |
||
Ciprofloxacin is commonly used for urinary tract and intestinal infections (traveler's diarrhea) and |
Ciprofloxacin is commonly used for urinary tract and intestinal infections (traveler's diarrhea) and |
||
was once considered a powerful antibiotic of last resort<ref> Biosecurity requires drug reform. Jan 1, 2002 World Watch ISSN: 0896-0615 </ref><ref> Electrochemical DNA biosensor for the study of ciprofloxacin–DNA interaction Haq Nawaza, Sakandar Raufa, Kalsoom Akhtara and Ahmad M. Khalid, Bioprocess Technology Division, National Institute for Biotechnology and Genetic Engineering (NIBGE), P.O. Box 577, Jhang Road, Faisalabad, Pakistan May 2006</ref><ref> The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment Stacey L. Knobler, Stanley M. Lemon, Marian Najafi, Tom Burroughs 2003 ISBN 0-309-08854-2 |
was once considered a powerful antibiotic of last resort<ref> Biosecurity requires drug reform. Jan 1, 2002 World Watch ISSN: 0896-0615 </ref><ref> Electrochemical DNA biosensor for the study of ciprofloxacin–DNA interaction Haq Nawaza, Sakandar Raufa, Kalsoom Akhtara and Ahmad M. Khalid, Bioprocess Technology Division, National Institute for Biotechnology and Genetic Engineering (NIBGE), P.O. Box 577, Jhang Road, Faisalabad, Pakistan May 2006</ref><ref> The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment Stacey L. Knobler, Stanley M. Lemon, Marian Najafi, Tom Burroughs 2003 ISBN 0-309-08854-2 |
||
| Line 346: | Line 345: | ||
The ever increasing bacterial resistance to ciprofloxacin, (which is a major concern), may very well threaten its future viability to treat bacterial infections. Years ago the FDA had added warnings regarding the proper use of these drugs within the package inserts to combat such overprescribing. Advising physicians that ciprofloxacin: "...should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria..."(See the monographs for this class) |
The ever increasing bacterial resistance to ciprofloxacin, (which is a major concern), may very well threaten its future viability to treat bacterial infections. Years ago the FDA had added warnings regarding the proper use of these drugs within the package inserts to combat such overprescribing. Advising physicians that ciprofloxacin: "...should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria..."(See the monographs for this class) |
||
As with other fluoroquinolones their use as first line agents is not generally recommended. They are usually rreserved for use in patients who are seriously ill and may soon require immediate hospitalization.<ref>Jim Hoover, for Bayer Corporation, Alaska Pharmacy and Therapeutics Committee March 19, 2004 http://www.hss.state.ak.us/dhcs/PDL/minutes_meetings_pdl/minutes_031904_pdl.pdf</ref> The European Center for Disease Prevention and Control recommend that fluoroquinolones and the antibiotic [[clindamycin]] are avoided in clinical practice due to their high association with [[clostridium difficile]], a potentially life-threatening super-infection.<ref>{{cite web |author=Dr Ralf-Peter Vonberg |title=Clostridium difficile: a challenge for hospitals |url=http://www.ihe-online.com/feature-articles/clostridium-difficile-a-challenge-for-hospitals/trackback/1/index.html |work=European Center for Disease Prevention and Control |publisher=IHE |location=Institute for Medical Microbiology and Hospital Epidemiology |accessdate=27 July 2009}}</ref> Though considered to be a very important and necessary drug required to treat severe and life threatening bacterial infections, the associated overprescribing of ciprofloxacin remains unchecked, which has contributed to the problem of bacterial resistance.<ref>http://www.nyc.gov/html/doh/html/tb/tb4a.shtml</ref> The [[overuse of antibiotic]]s such as happens with children suffering from otitis media has given rise to a breed of super bacteria which are resistant to antibiotics entirely.<ref>Antimicrobials for acute otistis media; A review from the International Primary Care Network". British Medical Journal July 5, 1997.</ref> “''Fluoroquinolone resistance is an increasing problem not only in the U.S. but also worldwide, potentially due to the widespread misuse of this class of antimicrobials.''”<ref>http://econnect.uspharmacist.com/econnect/Default.aspx?tabid=53&page=publish/content/8_1868.htm</ref> |
As with other fluoroquinolones their use as first line agents is not generally recommended. They are usually rreserved for use in patients who are seriously ill and may soon require immediate hospitalization.<ref>Jim Hoover, for Bayer Corporation, Alaska Pharmacy and Therapeutics Committee March 19, 2004 http://www.hss.state.ak.us/dhcs/PDL/minutes_meetings_pdl/minutes_031904_pdl.pdf</ref> Fluoroquinolones such as ciprofloxacin are strongly associated with causing C Difficile infections. Fluoroquinolones are more strongly associated with C difficile infections than other antibiotics including clindamycin, 3rd generation cephalosporins beta latamase inhibitors. One study found that fluoroquinolones were responsible for 55% of C difficile infections. Fluoroquinolones are the most common cause of C Difficile infections.<ref>{{cite journal |author=Pépin J, Saheb N, Coulombe MA, ''et al.'' |title=Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec |journal=Clin. Infect. Dis. |volume=41 |issue=9 |pages=1254–60 |year=2005 |month=November |pmid=16206099 |doi=10.1086/496986 |url=http://www.journals.uchicago.edu/doi/abs/10.1086/496986}}</ref> The European Center for Disease Prevention and Control recommend that fluoroquinolones and the antibiotic [[clindamycin]] are avoided in clinical practice due to their high association with [[clostridium difficile]], a potentially life-threatening super-infection.<ref>{{cite web |author=Dr Ralf-Peter Vonberg |title=Clostridium difficile: a challenge for hospitals |url=http://www.ihe-online.com/feature-articles/clostridium-difficile-a-challenge-for-hospitals/trackback/1/index.html |work=European Center for Disease Prevention and Control |publisher=IHE |location=Institute for Medical Microbiology and Hospital Epidemiology |accessdate=27 July 2009}}</ref> Though considered to be a very important and necessary drug required to treat severe and life threatening bacterial infections, the associated overprescribing of ciprofloxacin remains unchecked, which has contributed to the problem of bacterial resistance.<ref>http://www.nyc.gov/html/doh/html/tb/tb4a.shtml</ref> The [[overuse of antibiotic]]s such as happens with children suffering from otitis media has given rise to a breed of super bacteria which are resistant to antibiotics entirely.<ref>Antimicrobials for acute otistis media; A review from the International Primary Care Network". British Medical Journal July 5, 1997.</ref> “''Fluoroquinolone resistance is an increasing problem not only in the U.S. but also worldwide, potentially due to the widespread misuse of this class of antimicrobials.''”<ref>http://econnect.uspharmacist.com/econnect/Default.aspx?tabid=53&page=publish/content/8_1868.htm</ref> |
||
For example the use of the fuoroquinolones had increased three-fold in an emergency room environment in the United States between 1995 and 2002, while the use of safer alternatives such as macrolides declined significantly.<ref> MacDougall, C., Guglielmo, B.J., Maselli, J., and Gonzales, R. (2005, March). "Antimicrobial drug prescribing for pneumonia in ambulatory care." (AHRQ grant HS13003). Emerging Infectious Diseases 11(3), pp. 380–384. |
For example the use of the fuoroquinolones had increased three-fold in an emergency room environment in the United States between 1995 and 2002, while the use of safer alternatives such as macrolides declined significantly.<ref> MacDougall, C., Guglielmo, B.J., Maselli, J., and Gonzales, R. (2005, March). "Antimicrobial drug prescribing for pneumonia in ambulatory care." (AHRQ grant HS13003). Emerging Infectious Diseases 11(3), pp. 380–384. |
||
</ref><ref> Fluoroquinolone prescribing in the United States: 1995 to 2002. Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. |
</ref><ref> Fluoroquinolone prescribing in the United States: 1995 to 2002. Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. |
||
Revision as of 00:37, 1 August 2009
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, intravenous, topical (ear drops, eye drops) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 69%[2] |
| Metabolism | Hepatic, including CYP1A2 |
| Elimination half-life | 4 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.026 |
| Chemical and physical data | |
| Formula | C17H18FN3O3 |
| Molar mass | 331.346 g·mol−1 |
| 3D model (JSmol) | |
| |
Ciprofloxacin (INN) is a drug used to treat bacterial infections. It is a second generation fluoroquinolone antibacterial. It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate.
Ciprofloxacin is marketed worldwide with over three hundred different brand names. In the United States, Canada and the UK, it is marketed as Ciloxan, Cipro, Cipro XR, Cipro XL Ciproxin and, most recently, Proquin. In Mexico it is available over the counter and marketed under the names Ciproflox or Ciprofloxacino. In Ecuador it is available and marketed under the name Cidrax. Additionally, ciprofloxacin is available as a generic drug under a variety of different brand names and is also available for limited use in veterinary medicine.
Ciprofloxacin was first patented in 1983 by Bayer A.G. and subsequently approved by the United States Food and Drug Administration (FDA) in 1987.
Ciprofloxacin has 12 F.D.A.-approved human uses and other veterinary uses, but it is often used for non-approved uses (off-label).
Ciprofloxacin interacts with other drugs, herbal and natural supplements, and thyroid medications.[3]
History
Bayer introduced the first broad-spectrum oral fluoroquinolone, ciprofloxacin, in 1987. In 1991 the intravenous formulation was introduced. Ciprofloxacin is available in more than 100 countries. Under the trade name Cipro HC, it is distributed by Alcon. Ciprofloxacin appears to have been first patented Jan 21, 1986, in Germany, and was later approved by the U.S. Food and Drug Administration on October 22, 1987 for use in the United States to specific bacterial infections. The current United States patent appears to be held by Bayer being the assignee.[4] The patent was applied for Jan., 1987 but not approved until 1996 according to its patent history, although the patent history makes reference to a 1982 European Patent (patent number 0049355), as well.
In 2004 ciprofloxacin and levofloxacin together command 65% ($3.3 billion) of the global sales of the fluoroquinolone class.[5] The first nine months of 2008 sales for Ciprofloxacin were $242 million, as compared to $324 million for Bayer Aspirin.[6]
Licensed uses

The licensed uses for ciprofloxacin in the United States are as follows:
Oral and I.V. fluoroquinolones are not licensed by the FDA for use in children due to the risk of permanent injury to the musculoskeletal system, with two exceptions as outlined below. Within the studies submitted in response to a Pediatric Written Request (Ciprofloxacin, circa 2004) the rate of atrophy was reported to be 9.3% at one month and 13.6% at one year.[7] As such the pediatric use of ciprofloxacin is restricted to proven complicated urinary tract infections and pyelonephritis due to E. coli and inhalation anthrax.[8] Although alleged to be effective, ciprofloxacin is not to be considered a first line agent for inhalation anthrax in the pediatric population.[9][10][11][12] The CDC revoked its recommendation regarding the use of ciprofloxacin as a first line agent in treating anthrax due to the unacceptable risk documented within the Antimicrobial Postexposure Prophylaxis for Anthrax study (aka Cipro 60 day study).[13] However, the fluoroquinolones are licensed to treat lower respiratory infections in children with cystic fibrosis in the UK.
In the adult population ciprofloxacin is limited to the treatment of proven bacterial infections such as:
- Urinary tract infections
- Acute uncomplicated cystitis in females
- Chronic bacterial prostatitis
- Lower respiratory tract infections
- Acute sinusitis
- Skin and skin structure infections
- Bone and joint infections
- Infectious diarrhea
- Typhoid fever (enteric fever) caused by Salmonella typhi
- Uncomplicated cervical and urethra gonorrhea (due to N. gonorrhoeae) – however, this indication is no longer effective in some areas (i.e. Asian Countries [14], United States, Canada and Hawaii)[15]) due to bacterial resistance.[16]
As well as in combination with other specific drugs:
- Complicated intra-abdominal infections (in combination with metronidazole);
- Empirical therapy for febrile neutropenic patients (in combination with piperacillin)
In the pediatric population ciprofloxacin is limited to the treatment of proven bacterial infections such as:
- Complicated urinary tract infections and pyelonephritis due to Escherichia coli
- Inhalational anthrax (post-exposure)
Ciprofloxacin is not a drug of first choice in the treatment of presumed or confirmed pneumonia secondary to Streptococcus pneumoniae. As such ciprofloxacin is not recommended for community acquired pneumonia and other such chest infections.[17] Antibiotics may not improve the long-term clinical outcome for sinusitis.[18] When prescribed for community acquired pneumonia, chronic bronchitis, and acute bacterial sinusitis, the use of the fluoroquinolone class offers no compelling advantages over established treatment.[19] Nor does antibiotic treatment help sore throats.[20] The use of antibiotics such as ciprofloxacin to treat bronchitis is to be considered unnecessary and as such exposes the patient to an unacceptable risk of suffering a severe adverse reaction.[21] Additionally ciprofloxacin and other fluoroquinolones have no effect upon viral infections such as the common head cold.
NOTE: Ciprofloxacin may be licensed for other uses, or restricted, by the various regulatory agencies worldwide.
Availability
Ciprofloxacin is available as:
- tablets (250 mg, 500 mg or 750 mg)
- intravenous solutions (5% and 10%, 100 mL)
- eye and ear drops
In most countries, all formulations require a prescription.
See the latest package insert for ciprofloxacin (Cipro) for additional details.[22]
Mode of action
Ciprofloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV[23], enzymes necessary to separate bacterial DNA, thereby inhibiting cell division.
This mechanism can also affect mammalian cell replication. In particular, some congeners of this drug family (for example those that contain the C-8 fluorine) [24], display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models. [25] Although quinolones are highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone induced DNA damage was first reported in 1986 (Hussy et al.)[26].
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.[27][28][29][30] As such some fluoroquinolones may cause injury to the chromosome of eukaryotic cells.[31][32][33][34][35][36]
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe adverse reactions experienced by some patients following fluoroquinolone therapy.[37][38][39]
Contraindications
As noted above, under licensed use, ciprofloxacin is also now considered to be contraindicated for the treatment of certain sexually transmitted diseases by some experts due to bacterial resistance. [40]
There are only four contraindications found within the 2009 package insert:[22]
- “Coadministration of ciprofloxacin with other drugs primarily metabolized by CYP1A2 results in increased plasma concentrations of these drugs and could lead to clinically significant adverse events of the coadministered drug.”
- “Concomitant administration with tizanidine is contraindicated”
- “Ciprofloxacin is contraindicated in persons with a history of hypersensitivity to ciprofloxacin, any member of the quinolone class of antimicrobial agents, or any of the product components.”
- “Local I.V. site reactions are more frequent if the infusion time is 30 minutes or less. These may appear as local skin reactions which resolve rapidly upon completion of the infusion. Subsequent intravenous administration is not contraindicated unless the reactions recur or worsen.”
Due to growing prevalence of antibiotic resistance to the fluoroquinolones in southeast Asia, the use of Ciprofloxacin in patients who have been to southeast Asia is increasingly being contraindicated.[41]
Ciprofloxacin is also considered to be contraindicated within the pediatric population (except for the indications outlined under licensed use above), pregnancy, nursing mothers, and in patients with epilepsy or other seizure disorders.
- Pregnancy
The fluoroquinolones rapidly cross the blood-placenta and blood-milk barrier, and are extensively distributed into the fetal tissues. For this reason the Fluroquinolones are contraindicated during pregnancy due to the risk of spontaneous abortions and birth defects. The Flouroquinolones have also been reported as being present in the mother’s milk and are passed on to the nursing child, which may increases the risk of the child suffering from this syndrome as well, even though the child had never been prescribed or taken any of the drugs found within this class.[42][43]
- Pediatric population
Fluoroquinolones are not licensed by the FDA for use in children due to the risk of fatalities [44] as well as permanent injury to the musculoskeletal system, with two exceptions. Ciprofloxacin is being licensed for the treatment of Complicated Urinary Tract Infections and Pyelonephritis due to Escherichia coli and Inhalational Anthrax (post-exposure) and Levofloxacin was recently licensed for the treatment of Inhalational Anthrax (post-exposure). However, the Fluoroquinolones are licensed to treat lower respiratory infections in children with cystic fibrosis in the UK.
Within one study it was stated that the pediatric patient has a 3.8% chance of experiencing a serious musculoskeletal adverse event.[45] Within the studies submitted in response to a Pediatric Written Request (Ciprofloxacin, circa 2004) the rate of athropy was reported to be 9.3%.[46]
Two recent pediatric studies involving the use of levofloxacin indicates that the pediatric patient has a greater than 50% chance of experiencing one or more adverse reactions. Which would be consistent with the studies found within the NDA (new drug application) for Levofloxacin[47] which showed and Adverse Drug Reaction (ADR) rate in excess of 40%, as well as a number of reported fatalities. Within the first study[48] it is stated that “Of the 712 subjects evaluable for safety, 275 (52%) levofloxacin-treated subjects experienced one or more adverse event... Serious adverse events were reported in 33 (6%) levofloxacin-treated subjects... Two serious adverse events in levofloxacin-treated subjects resulted in fatal outcomes.” Within the second study[49] it is stated that “Of the 204 subjects evaluable for safety, 122 experienced one or more adverse events...Twelve subjects (6%) discontinued study drug due to an adverse event...Seven subjects (3%) experienced 8 serious adverse events.” (circa 2007)
Within the BPCA Pediatric Studies Summary for ciprofloxacin[50] it was stated that the overall incidence of adverse events at six weeks was 41%. This would be consistent with the safety profile found with the other fluoroquinolones studied in the pediatric population, as noted above with levofloxacin. As such the current ban on the use of the fluoroquinolones in the pediatric population is both reasonable and supported by various clinical studies. The risk of permanent injury outweighs the potential benefits.
Adverse effects
Some of the serious adverse effects which occur more commonly with fluoroquinolones than with other antibiotic drug classes include central nervous system (CNS) side effects and tendon toxicity.[51][52] Additional serious adverse reactions include psychosis and chorea (involuntary muscle movements).[53][54][55] Phototoxicity, neurological symptoms, impaired colour vision, exanthema, abdominal pain, malaise, drug fever, peripheral neuropathy, dysaesthesia and eosinophilia have also been observed as adverse effects of ciprofloxacin.[56][57]
The serious events may occur with therapeutic or with acute overdose. At therapeutic doses they include: central nervous system toxicity, cardiovascular toxicity, tendon / articular toxicity, and rarely hepatic toxicity.[58][59][60] Events that may occur in acute overdose are rare and include: renal failure and seizure.[58] Children and the elderly are at greater risk.[52][61] Adverse reactions may manifest during, as well as after fluoroquinolone therapy.[62] Unusual but potentially serious adverse reactions occur as a result of ciprofloxacin administration bone marrow depression, interstitial nephritis and hemolytic anemia occur during ciprofloxacin use.[63][64]
Some groups refer to these adverse events as "fluoroquinolone toxicity". These groups of people claim to have suffered serious long term harm to their health from using fluoroquinolones. This has led to a class action lawsuit by people harmed by the use of fluoroquinolones as well as legal action by the consumer advocate group Public Citizen.[65][66] Partly as a result of the efforts of The State of Illinois and Public Citizen the FDA ordered a black box warnings on all fluoroquinolones advising consumers of the possible toxic effects of fluoroquinolones on tendons.[67]
Pseudotumor cerebri, commonly known as idiopathic intracranial hypertension (IIH), also referred to as increased intracranial pressure, has been reported to occur as a serious but isolated adverse reaction to ciprofloxacin.[68] An unusual case of seizures has been reported with ciprofloxacin ear drops in an elderly lady.[69]
Interactions
The toxicity of drugs that are metabolised by the cytochrome P450 system is enhanced by concomitant use of some quinolones. Coadministration may dangerously increase coumadin warfarin activity; INR should be monitored closely. They may also interact with the GABA A receptor and cause neurological symptoms; this effect is augmented by certain non-steroidal anti-inflammatory drugs.[70] Quercetin, a flavonoid occasionally used as a dietary supplement, may interact with fluoroquinolones, as quercetin competitively binds to bacterial DNA gyrase. Some foods such as garlic and apples contain high levels of quercetin; whether this inhibits or enhances the effect of fluoroquinolones is not entirely clear.[71] Ciprofloxacin can reduce phenytoin plasma levels which may in some cases result in seizures.[72] Ciprofloxacin may interfere with the levels of thyroid medications resulting in hypothyroidism.[73]
Concurrent administration of ciprofloxacin, with magnesium or aluminum antacids, sucralfate or products containing calcium, iron, or zinc (including multivitamins or other dietary supplements) may substantially decrease the absorption of ciprofloxacin, resulting in serum and urine levels considerably lower than desired.[74]
Significant drug interactions
Some drug interactions are associated with molecular structural modifications of the quinolone ring, specifically interactions involving NSAIDS and theophylline. The fluoroquinolones have also been shown to interfere with the metabolism of caffeine[75] and the absorption of levothyroxine. The interference with the metabolism of caffeine may lead to the reduced clearance of caffeine and a prolongation of its serum half-life, resulting in a caffeine overdose. Ciprofloxacin has been shown to interact with thyroid medications (levothyroxine) resulting in unexplained hypothyroidism.[76]
The use of NSAIDs (Non Steroid Anti Inflammatory Drugs) while undergoing fluoroquinolone therapy is contra-indicated due to the risk of severe CNS adverse reactions, including but not limited to seizure disorders. Fluoroquinolones with an unsubstituted piperazinyl moiety at position 7 have the potential to interact with NSAIDs and/or their metabolites, resulting in antagonism of GABA neurotransmission.[77] Whether or not such reactions occur after completion of therapy is a matter of considerable debate. Patients have reported reactions to NSAIDS long after completion of fluoroquinolone therapy, but there does not appear to be any research that would either confirm or deny this association other than these anecdotal reports.
Some quinolones exert an inhibitory effect on the cytochrome P-450 system, thereby reducing theophylline clearance and increasing theophylline blood levels. Coadministration of certain fluoroquinolones and other drugs primarily metabolized by CYP1A2 (e.g. theophylline, methylxanthines, tizanidine) results in increased plasma concentrations and could lead to clinically significant side effects of the coadministered drug. Additionally other fluoroquinolones, especially enoxacin, and to a lesser extent ciprofloxacin and pefloxacin, also inhibit the metabolic clearance of theophylline.[78]
Such drug interactions appear to be related to the structural changes of the quinolone ring and the inhibitory effect on the cytochrome P-450 system. As such, these drug interactions involving the fluoroquinolones appear to be drug specific rather than a class effect.
The use of Ciprofloxacin concomitantly has also been associated with transient elevations in serum creatinine in patients receiving cyclosporine, on rare occasions, resulted in severe hypoglycemia with sulfonylurea. Renal tubular transport of methotrexate may be inhibited by concomitant administration of ciprofloxacin, potentially leading to increased plasma levels of methotrexate. This might increase the risk of methotrexate toxic reactions. Altered serum levels of phenytoin (increased and decreased) have been reported in patients receiving concomitant ciprofloxacin. Probenecid interferes with renal tubular secretion of ciprofloxacin and produces an increase in the level of ciprofloxacin in serum. The fluroquinolones have also been reported to enhance the effects of the warfarin or its derivatives. [79]
Current or past treatment with oral corticosteroids is associated with an increased risk of Achilles tendon rupture, especially in elderly patients who are also taking the fluoroquinolones.[80] This effect seems to be restricted to people aged 60 or over, and within this group concomitant use of corticosteroids increases this risk substantially. Though technically not to be considered a drug interaction, mention of this is made here due to fact that the etiology of such ruptures remains elusive and further research may confirm such a drug interaction may play a role in this particular reaction. However, at the moment, this is to be considered speculatory in nature and additional research to confirm or deny is required.
Interactions with endogenous compounds
There are a number of the endogenous compounds that have been reported to be affected by ciprofloxacin as inhibitors, alteraters and depletors. See the latest package insert for additional details.[clarification needed]
Overdose
"In the event of acute overdosage, reversible renal toxicity has been reported in some cases. The stomach should be emptied by inducing vomiting or by gastric lavage. The patient should be carefully observed and given supportive treatment, including monitoring of renal function and administration of magnesium, aluminum, or calcium containing antacids which can reduce the absorption of ciprofloxacin. Adequate hydration must be maintained. Only a small amount of ciprofloxacin (< 10%) is removed from the body after hemodialysis or peritoneal dialysis." Quoting from the 2009 package insert for Ciprofloxacin[74]
Chemistry
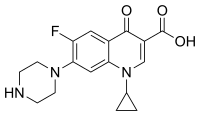
Ciprofloxacin is 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its molecular weight is 331.4 g/mol. It is a faintly yellowish to light yellow crystalline substance.[74]
Ciprofloxacin hydrochloride (USP) is the monohydrochloride monohydrate salt of ciprofloxacin. It is a faintly yellowish to light yellow crystalline substance with a molecular weight of 385.8 g/mol. Its empirical formula is C17H18FN3O3HCl•H2O.[74]
Pharmacokinetics
"The pharmacokinetics of ciprofloxacin are linear over the dose range of 200 to 400 mg administered intravenously. Comparison of the pharmacokinetic parameters following the 1st and 5th I.V. dose on a q 12 h regimen indicates no evidence of drug accumulation. The absolute bioavailability of oral ciprofloxacin is within a range of 70–80% with no substantial loss by first pass metabolism. An intravenous infusion of 400-mg ciprofloxacin given over 60 minutes every 12 hours has been shown to produce an area under the serum concentration time curve (AUC) equivalent to that produced by a 500-mg oral dose given every 12 hours. An intravenous infusion of 400 mg ciprofloxacin given over 60 minutes every 8 hours has been shown to produce an AUC at steady-state equivalent to that produced by a 750-mg oral dose given every 12 hours. A 400-mg I.V. dose results in a Cmax similar to that observed with a 750-mg oral dose. An infusion of 200 mg ciprofloxacin given every 12 hours produces an AUC equivalent to that produced by a 250-mg oral dose given every 12 hours." Quoting from the 2009 package insert for Ciprofloxacin.[74]
Biotransformation is hepatic. The half life is 4 hours.[79]
Dosing
The status of the patient’s renal function and hepatic function must also be taken into consideration to avoid an accumulation that may lead to a fatal drug overdose. Ciprofloxacin is eliminated primarily by renal excretion. However, the drug is also metabolized and partially cleared through the liver and the intestine. Modification of the dosage is recommended using the table found within the package insert for those with impaired liver or kidney function. (Particularly for patients with severe renal dysfunction.) However, since the drug is known to be substantially excreted by the kidneys, the risk of toxic reactions to this drug may be greater in patients with impaired renal function. The duration of treatment depends upon the severity of infection and the usual duration is 7 to 14 days.[81]
NOTE: The patient’s serum levels should be monitored during therapy to avoid a drug overdose. See the most current Package Insert for proper dosing guidelines and relevant warnings/precautions.
Susceptible bacteria
Aerobic gram-positive microorganisms
- Enterococcus faecalis (Many strains are only moderately susceptible.)
- Staphylococcus aureus (Methicillin-resistant strains tend to also be resistant to ciprofloxacin)
- Staphylococcus epidermidis (Methicillin-resistant strains tend to also be resistant to ciprofloxacin)
- Staphylococcus saprophyticus
- Streptococcus pneumoniae (Unreliable activity)
- Streptococcus pyogenes
Aerobic gram-negative microorganisms
- Campylobacter jejuni
- Proteus mirabilis
- Citrobacter diversus
- Proteus vulgaris
- Citrobacter freundii
- Providencia rettgeri
- Enterobacter cloacae
- Providencia stuartii
- Escherichia coli
- Pseudomonas aeruginosa
- Haemophilus influenzae
- Salmonella typhi
- Haemophilus parainfluenzae
- Serratia marcescens
- Klebsiella pneumoniae
- Shigella boydii
- Moraxella catarrhalis
- Shigella dysenteriae
- Morganella morganii
- Shigella flexneri
- Neisseria gonorrhoeae
- Shigella sonnei
Current litigation
A class action had been filed against Bayer AG on behalf of employees of the Brentwood Post Office in Washington, D.C., and workers at the U.S. Capitol, along with employees of American Media, Inc. in Florida and postal workers in general who allege that they have suffered serious side effects from taking the antibiotic ciprofloxacin (Cipro) in the aftermath of the anthrax attacks in 2001. The action alleged that Bayer failed to warn class members of the potential side effects of the drug, thereby violating the Pennsylvania Unfair Trade Practices and Consumer Protection Laws. According to the allegations within the complaint, exposed individuals were not informed of the true safety profile of Ciprofloxacin, the high rate of adverse events associated with its use or the availability of safer and equally effective alternative drugs. The complaint further alleged that, as a result of taking Cipro, many individuals suffered severe and debilitating injuries. The action sought funding for a medical monitoring program and compensatory damages for those workers who have suffered side effects. In 2004, the law firm of Goodell, DeVries, Leech & Dann, LLP were retained as national counsel in this litigation. The class action was defeated and the litigation abandoned by plaintiffs. [82] [83] [84] A similar action had been filed in New Jersey that covers New Jersey postal workers. Final disposition of that lawsuit is unknown. Following the addition of the Black Box Warning in 2008, regarding tendon damage, a significant number of product liability law firms began soliciting clients who have suffered a spontaneous tendon rupture following fluoroquinolone therapy.
Regulatory history
Ciprofloxacin was first patented in 1983 by Bayer A.G. and subsequently approved by the U.S. Food and Drug Administration (FDA) for use in the United States in 1987.
October 1987:
- FDA approval. The NDA (New Drug Application) documents are no longer available on the FDA site. There is a regulatory gap between 1987 and 1994 as well as between 1994 and 1997, resulting in ten years worth of regulatory documentation missing on the FDA site concerning the regulatory history of ciprofloxacin.
October 1996:
- Within a FDA Medical Bulletin issued October 1996, the FDA gave notice to practicing physicians within the United States regarding spontaneous tendon ruptures and chronic tendonitis being associated with the fluoroquinolones.[85][86]
September 1997:
- Addition of the CNS adverse reaction warnings.[87]
September 1998:
- Addition of taste loss as an ADR. Study added that showed 22% of the pediatric cystic fibrosis patients treated with ciprofloxacin experienced musculoskeletal adverse reactions to ciprofloxacin, some of which persisted for a length greater than eight months.[88]
August 30, 2000:
- Approval of the adult and pediatric use of ciprofloxacin for inhalational anthrax (post-exposure).[89]
April 17, 2002:
- Removal of the warning that stated “prolonged use of ciprofloxacin may result in overgrowth of nonsusceptible organisms. Repeated evaluation of the patient’s condition and microbial susceptibility testing is essential.”
- Removal of the warning that stated “There are, however, no adequate and well-controlled studies in pregnant women. Ciprofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.”
- Removal of the warning that stated “Adverse events that were considered likely to be drug related occurred in 7.3% of patients treated, possibly related in 9.2% (total of 16.5% thought to be possibly or probably related to drug therapy), and remotely related in 3.0%. Ciprofloxacin was discontinued because of an adverse event in 3.5% of patients treated." Replaced with the statement “Most of the adverse events reported were described as only mild or moderate in severity, abated soon after the drug was discontinued, and required no treatment.”[90]
In 2004 the FDA requested new warning labels to be added to all of the Fluoroquinolones regarding Peripheral Neuropathy (irreversible nerve damage), Tendon Damage, Heart Problems (prolonged QT Interval / Torsades de pointes), Pseudomembranous colitis, Rhabdomyolysis (muscle wasting), Steven Johnson Syndrome, as well as concurrent usage of NSAIDs contributing to the severity of these reactions. A review of the last 2004 label revisions for ciprofloxacin failed to indicate that any warnings concerning Heart Problems (prolonged QT Interval / Torsades de pointes), Rhabdomyolysis (muscle wasting), or Steven Johnson Syndrome were added to the label. It was not until the latest label (2008) that the warnings concerning prolonged QT Interval / Torsades de pointes appear. Warnings concerning Rhabdomyolysis (muscle wasting) and Steven Johnson Syndrome are still conspicuously absent.
March 15, 2004
- Addition of the warning concerning ruptures of the Achilles and other tendons in relation to the concomitant use of corticosteroids, especially in the elderly.[91]
- Removal of the warning that “Allergic reactions ranging from urticaria to anaphylactic reactions have been reported.” "Sweating" added as an ADR in its place.[92]
March 25, 2004
- Approval of the pediatric use to treat Complicated Urinary Tract Infections and Pyelonephritis due to Escherichia coli.[92]
July 14, 2004
- Addition of the peripheral neuropathy warning, and concomitant use of NSAIDS warning.[93]
November 9, 2005
- Addition of warnings concerning Ciprofloxacin being an inhibitor of humancytochrome P450 1A2 (CYP1A2) mediated metabolism. “Coadministration of ciprofloxacin with other drugs primarily metabolized by CYP1A2 results in increased plasma concentrations of these drugs and could lead to clinically significant adverse events of the coadministered drug.”[94]
June 25, 2007
- Addition of the warning that “Other serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including ciprofloxacin.”[95]
- Addition of the warning that Clostridium difficile associated diarrhea (CDAD) is associated with the use of ciprofloxacin.[96]
January 18, 2008
- Phototoxicy warnings reduced significantly.[97]
October 3, 2008
- Addition of the BLACK BOX WARNINGS regarding tendon ruptures.[98]
April 6, 2009
- Addition of warning that ciprofloxacin should not be administered through feeding or NG (nasogastric) tubes.[99]
April 27, 2009
- Issuance of a Medication Guide and revisions to include new safety information. The FDA has determined that Ciprofloxacin poses a serious and significant public health concern, requiring the distribution of a Medication Guide.[100][101]
June 24, 2009 Updating of the carton and container labels to include a statement to let dispensers know that a Medication Guide must be dispensed with the product.[102]
Note: Although the FDA had requested that the revised labeling (which were to include the Black Box Warnings) accompany the package inserts for any newly shipped products (effective January 2009) there are continuing reports that as of July 2009, that the products continue to contain the older labels, and not the revised labels, and that the Medication Guides (absent of the Black Box Warnings) were not made available for distribution. [98][103]
History of the black box warnings
Musculoskeletal disorders attributed to use of quinolone antibiotics were first reported in the medical literature in 1972, as an adverse reaction to nalidixic acid.[104] Rheumatic disease after use of a fluoroquinolone (norfloxacin) was first reported eleven years later.[105] In a 1995 letter published in the New England Journal of Medicine, representatives of the U.S. Food and Drug Administration (FDA) stated that the agency would "update the labeling [package insert] for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."[106]
By August 1996, the FDA had not taken action, and the consumer advocacy group Public Citizen filed a petition with the FDA prompting the agency to act.[107] Two months later, the FDA published an alert in the FDA Medical Bulletin and requested that fluoroquinolone package inserts be amended to include information on this risk.[108]
In 2005, the Illinois Attorney General filed a petition with the FDA seeking black box warnings and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter.[109] In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for a black box warning.[109][110] In January 2008, Public Citizen filed suit to compel the FDA to respond to their 2006 petition.[111][112] On July 7, the FDA ordered the makers of systemic-use fluoroquinolones to add a boxed warning regarding tendon rupture, and to develop a Medication Guide for patients.[113] The package inserts for Ciprofloxacin, Avelox (moxifloxacin), Proquin XR, Factive (gemifloxacin), Floxin (ofloxacin), Noroxin (norfloxacin) and Levaquin (levofloxacin) were amended on September 8, 2008 to include these new warnings.[114] Bayer, which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes.[115] Ortho-McNeil, the manufacturers of Levaquin, issued a similar letter in November.[116] through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals.
Review of the FDA website indicates that the generic versions of the fluoroquinolones have not been updated to include this Black Box Warning as of January 2009. And there are numerous reports that this information has not been dessiminated to the pharmacist, the products continue to contain the previous labels that are absent of this warning, and the Medication Guide has not been made available to the pharmicist or physician for distribution.
FDA warning letters
Additionally the manufacturers of ciprofloxacin (Bayer A.G.) received numerous warning letters from the United States Food and Drug Administration regarding false advertising and failure to provide adequate warnings within their promotional materials. [117] [118] [119] [120]
Overprescribing and bacterial resistance
Ciprofloxacin is commonly used for urinary tract and intestinal infections (traveler's diarrhea) and was once considered a powerful antibiotic of last resort[121][122][123], used to treat especially tenacious infections. Not all physicians agreed with this assessment, as evidenced by its wide spread use to treat minor infections as well as non-approved uses. As a result in recent years many bacteria have developed resistance to this drug, leaving it significantly less effective than it would have been otherwise.[124][125]
Resistance to ciprofloxacin and other fluoroquinolones may evolve rapidly, even during a course of treatment. Numerous pathogens, including Staphylococcus aureus, enterococci, and Streptococcus pyogenes now exhibit resistance worldwide.[126] Widespread veterinary usage of the fluoroquinolones, particularly in Europe, has been implicated.[127]
The ever increasing bacterial resistance to ciprofloxacin, (which is a major concern), may very well threaten its future viability to treat bacterial infections. Years ago the FDA had added warnings regarding the proper use of these drugs within the package inserts to combat such overprescribing. Advising physicians that ciprofloxacin: "...should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria..."(See the monographs for this class)
As with other fluoroquinolones their use as first line agents is not generally recommended. They are usually rreserved for use in patients who are seriously ill and may soon require immediate hospitalization.[128] Fluoroquinolones such as ciprofloxacin are strongly associated with causing C Difficile infections. Fluoroquinolones are more strongly associated with C difficile infections than other antibiotics including clindamycin, 3rd generation cephalosporins beta latamase inhibitors. One study found that fluoroquinolones were responsible for 55% of C difficile infections. Fluoroquinolones are the most common cause of C Difficile infections.[129] The European Center for Disease Prevention and Control recommend that fluoroquinolones and the antibiotic clindamycin are avoided in clinical practice due to their high association with clostridium difficile, a potentially life-threatening super-infection.[130] Though considered to be a very important and necessary drug required to treat severe and life threatening bacterial infections, the associated overprescribing of ciprofloxacin remains unchecked, which has contributed to the problem of bacterial resistance.[131] The overuse of antibiotics such as happens with children suffering from otitis media has given rise to a breed of super bacteria which are resistant to antibiotics entirely.[132] “Fluoroquinolone resistance is an increasing problem not only in the U.S. but also worldwide, potentially due to the widespread misuse of this class of antimicrobials.”[133] For example the use of the fuoroquinolones had increased three-fold in an emergency room environment in the United States between 1995 and 2002, while the use of safer alternatives such as macrolides declined significantly.[134][135]
Fluoroquinolones had become the most commonly prescribed class of antibiotics to adults in 2002.[136] Nearly half (42%) of these prescriptions were for conditions not approved by the FDA, such as acute bronchitis, otitis media, and acute upper respiratory tract infection, according to a study that was supported in part by the Agency for Healthcare Research and Quality.[137][138] Additionally they are commonly prescribed for medical conditions that are not even bacterial to begin, with such as viral infections, or those to which no proven benefit exist.
Social and economic impact
Ciprofloxacin has proven to be a blockbuster drug for Bayer A. G., generating billions of dollars in additional revenue. "In 1999, Cipro was the eleventh most prescribed drug in the United States based on new prescriptions and ranked twentieth in total United States sales. In 1999, Bayer's gross sales of Cipro in the United States were approximately $1.04 billion."[139] The sale of ciprofloxacin increased dramatically following the anthrax scare of 2001. On October 24, 2002 the Bush Administration (2000–2008) announced a deal between the government and Bayer Pharmaceuticals to purchase 100 million tablets of ciprofloxacin at a reduced price of $0.95 per pill. A full course of ciprofloxacin for postexposure prophylaxis (60 days) resulting from this arrangement costs the government $204 per person treated compared with $12 per person treated with doxycycline, the drug normally used to treat anthrax, a difference of $192.[140]
- Generic equivalents:
On October 24, 2001, The Prescription Access Litigation (PAL), filed suit to dissolve an agreement between Bayer, Barr Laboratories, and two other generic drug companies that it claimed was blocking access to adequate supplies and cheaper, generic versions of ciprofloxacin. The plaintiffs charged that Bayer Corporation, a unit of Bayer AG, had unlawfully paid three of its competitors—Barr Laboratories, Rugby, and Hoechst-Marion Roussel—a total of $200 million to prevent cheaper, (generic versions) of ciprofloxacin being brought to the market, as well as manipulating the price and supply of ciprofloxacin. Numerous other consumer advocacy groups joined this lawsuit. On October 15, 2008, five years after Bayer’s patent had expired, the United States District Court for the Eastern District of New York granted Bayer’s and the generic defendants’ motion for summary judgment, holding that any anti-competitive effects caused by the settlement agreements between Bayer and the generic defendants were within the exclusionary zone of the patent, and thus could not be redressed by federal antitrust law.[141] In effect upholding Bayer’s agreement to pay—Barr Laboratories, Rugby, and Hoechst-Marion Roussel—a total of $200 million to prevent the marketing a generic equivalent of ciprofloxacin.
- Patent Extensions:
Under the George W. Bush administration (2001–2008) patent extension legislation was signed into law that allowed Bayer A. G., as well as other drug companies, a six month patent extension for testing their products for safety in children. It has been estimated that Bayer A.G.'s revenue increased an extra $358 million due to ciprofloxacin's pediatric patent extension. The legislation that was signed by President Bush, granting Bayer and other drug manufacturers a six month extension on their patents (to conduct pediatric testing), was drafted after extensive lobbying of numerous members of Congress by Bayer A.G. and others. One of the four sponsors of this legislation was Chris Dodd (D-CT), who at the time, ranked as one of the top three beneficiaries of campaign contributions by drug companies. Sen. Edward Kennedy (D-Mass.), who chaired the committee with jurisdiction over the bill, refused to fight over the language that (if it had been included) would have reduced the drug company's profits due to these patent extensions. The reasons for Sen. Edward Kennedy's decision not to fight for the inclusion of this language were not made known.[142]
The results of these pediatric trials indicated that arthropathy occurred more frequently in patients who received ciprofloxacin (within these studies). The affected joints included the knees, elbows, ankles, hips, wrists, and shoulders of the pediatric patients. In one study at six weeks arthropathy was seen in 9.3% of ciprofloxacin patients. These rates increased significantly after one year to 13.7% of the ciprofloxacin patients. Such arthropathy occurred more frequently in patients treated with ciprofloxacin than any other control drug, regardless of whether they received IV ciprofloxacin or the oral version of the drug. Ciprofloxacin patients reported more than one event and on more than one occasion when compared to the control patients. The overall incidence of adverse events at six weeks was 41% in those patients being treated with ciprofloxacin. Serious adverse events were seen in 7.5% of these patients and 3% of the patients discontinued the drug due to adverse events. In spite of this unacceptable safety profile the FDA stated that “The data support updating the package insert to include safety; and treatment recommendations for pediatric patients between 1 and 17 years of age with complicated urinary tract infection or pyelonephritis.”[143]
Within a 2005 memo the FDA reviewed seventeen unique pediatric cases reported to the FDA during the thirteen month period after the pediatric exclusivity for ciprofloxacin had been granted. During this period there was one death, two reports of disability and four of hospitalization. The disabilities involved the inability to walk (in a 12-year female patient) and the inability to run (in a 12-year old male patient). The hospital admissions were for pseudomembranous colitis, pancytopenia, tendonitis and Stevens Johnson Syndrome. The female patient received 5 weeks of ciprofloxacin oral therapy at the recommended doses. Even though ciprofloxacin was discontinued, she could not stand or ambulate and required a wheelchair one month later. These seventeen unique pediatric cases showed mostly hematological, musculoskeletal, allergic/hypersensitivity, and CNS adverse events. It does not appear that this executive summary was ever released to the medical community.[144]
- Economic impact: adverse reactions:
The adverse drug reaction profile of ciprofloxacin and other fluoroquinolone drugs has spawned a grass root movement of those so effected to lobby for Black Box Warnings and Dear Doctor Letters as well as the petitioning of the FDA for the removal of some fluoroquinolone drugs from clinical practice.[145][146][147][148][149][150][151][152]
A number of class action lawsuits as well as malpractice litigation has been spawned by this safety profile. The various manufacturers have countered these allegations stating that they believe that these drugs are both safe and effective antibiotics, well tolerated with a minimum of side effects, such reactions are “rare” (contrary to the literature) and the benefits of such therapy outweigh the perceived risks.
Risk/benefit assessment
The benefits of ciprofloxacin therapy have also been widely disputed. There are major discrepancies between the promoted image and the clinically interpreted usefulness of ciprofloxacin, and what has been reported within the leading medical journals or the results of independent double blind studies, in a number of instances.
- Respiratory infections:
In a 1986 issue of the Journal of Antimicrobial Chemotherapy, a leading article on quinolones in chest infections concluded that there is little reason for optimism about the role of quinolones in chest infections mainly because of problems with resistance, recurrence, and reinfection with Pseudomonas aeruginosa and S pneumoniae.[153][154] Melinda et al., in 2003, confirmed the validity of the concerns raised within 1986 issue of the Journal of Antimicrobial Chemotherapy (referenced to previously), concerning the use of the fluoroquinolones to treat acute respiratory infections. Stating that: “Overuse of these antibiotics will eventually render them useless for treating antibiotic-resistant infections, for which broad-spectrum antibiotics are supposed to be reserved.”[155]
In spite of this caveat, the use of the fluoroquinolone to treat community acquired pnuemonia (CAP) increased by >50%, from 25% to 39% of all prescriptions. This increase was at the expense of the macrolide class of antimicrobial drugs, the use of which declined 20% during the study period.[156]
Antibiotics do not improve sinusitis symptoms a number of studies have shown. Primary care physicians (family doctors) commonly prescribe ciprofloxacin to treat acute maxillary sinusitis (inflamed membranes of the sinuses), although there is no evidence that this approach is effective. A report in the British medical Journal the Lancet found that antibiotics did nothing more than the placebos used as the control.[157]
Only about 5-10% of bronchitis cases are caused by a bacterial infection. Most cases of bronchitis are caused by a viral infection and are "self-limited" and resolve themselves in a few weeks.[158]
- Chronic bacterial prostatitis:
Prostatitis has been termed "the waste basket of clinical ignorance" by prominent Stanford University Urologist Dr. Thomas Stamey. Campbell's Urology, the urologist's most authoritative reference text, identifies only about 5% of all patients with prostatitis as having bacterial prostatitis which can be "cured" at least in the short term by antibiotics. In other words, 95% of men with prostatitis have little hope for a cure with antibiotics alone since they don't actually have any identifiable bacterial infection. Within a 2003 study involving the use of fluoroquinolones to treat Chronic Bacterial Prostatitis it was found that the level of improvement was no different from that associated with placebo.[159] Within a 2004 randomized, double-blind trial involving ciprofloxacin specifically, it was found that ciprofloxacin “did not substantially reduce symptoms in men with long-standing CP/CPPS who had at least moderate symptoms.”[160] Chronic pelvic pain (category IIIB) is often misdiagnosed as chronic prostatitis and needlessly treated with ciprofloxacin. Within a Bulgarian study, where by definition all patients had negative microbiological results, we see a 65% adverse drug reaction rate for patients treated with ciprofloxacin in comparison to a 9% rate for the placebo patients. This was combined with a higher cure rate (69% v 53%) found within the placebo group. The authors stated that “The results of our study show that antibiotics have an unacceptably high rate of adverse side effects as well as a statistically insignificant improvement over placebo...”[161]
- Infectious diarrhea:
A Clostridium difficile infection is the principal cause of ciprofloxacin-associated diarrhea and pseudomembranous colitis.[162][163] In June 2007 the FDA changed the package insert for ciprofloxacin to include the warning that that Clostridium difficile associated diarrhea (CDAD) is associated with the use of ciprofloxacin.[96] As such the efficacy of ciprofloxacin to treat infectious diarrhea would be called into question.
- Uncomplicated cervical and urethra gonorrhea:
As previously stated the use of ciprofloxacin to treat this disease has been severely compromised by bacterial resistance.
Applying a reasonable risk/benefit assessment to the use of ciprofloxacin there would be very few cases where the use of a fluoroquinolone drug, such as ciprofloxacin, should be considered by the treating physician to be a first line agent.[164][165][166] Ciprofloxacin has been associated with significant collateral system toxicity during therapy; as such the potential for benefit may not outweigh the proven risk when there is a safer alternative available to the treating physician.
See also
Package insert links
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J (1986). "Absolute oral bioavailability of ciprofloxacin". Antimicrob Agents Chemother. 30 (3): 444–6. doi:10.1128/AAC.. PMC 180577. PMID 3777908.
{{cite journal}}: Check|doi=value (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cooper JG, Harboe K, Frost SK, Skadberg Ø (2005). "Ciprofloxacin interacts with thyroid replacement therapy". BMJ. 330 (7498): 1002. doi:10.1136/bmj.330.7498.1002. PMC 557149. PMID 15860826.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://patft.uspto.gov/netacgi/nph-Parser?u=%2Fnetahtml%2Fsrchnum.htm&Sect1=PTO1&Sect2=HITOFF&p=1&r=1&l=50&f=G&d=PALL&s1=5286754.PN.&OS=PN/5286754&RS=PN/5286754
- ^ https://www.leaddiscovery.co.uk/reports/843/Commercial_Perspectives_Fluoroquinolones_Established_Products_Drive_Market_Growth
- ^ http://www.stockholders-newsletter-q3-08.bayer.com/en/bayer_stockholders_newsletter_3Q_08.pdfx
- ^ Division of Special Pathogen and Immunologic Drug Products Summary of Clinical Review of Studies Submitted in Response to a Pediatric Written Request 3/16/04 Applications: 19-537/S-049, ciprofloxacin tablets 20-780/S-013, ciprofloxacin oral suspension 19-847/S-027, ciprofloxacin IV 10 mg/mL 19-857/S-031, ciprofloxacin IV 5% dextrose Applicant: Bayer Corporation, Pharmaceutical Division Established: Ciprofloxacin Route: Oral or IV
- ^ Pediatric section of package insert http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019537s68,19847s42,19857s49,20780s26,21473s24lbl.pdf
- ^ Division of Special Pathogen and Immunologic Drug Products Summary of Clinical Review of Studies Submitted in Response to a Pediatric Written Request
- ^ Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France.Chalumeau M, Tonnelier S, D'Athis P, Treluyer JM, Gendrel D, Breart G, Pons G; Pediatric Fluoroquinolone Safety Study Investigators. Perinatal and Pediatric Pharmacology Unit, Universite Rene-Descartes, Hopital Saint-Vincent-de-Paul (AP-HP), Paris, France.
- ^ 62 Meeting of the Anti-Infective Drugs Advisory Committee
- ^ ODS POSTMARKETING SAFETY REVIEW Consult: One-Year Post Pediatric Exclusivity Postmarketing Adverse Events Review Drug: Ciprofloxacin NDA: 19-537, 19-847, 19-857, 20-780, 21-473, 21-554 Pediatric Exclusivity Approval Date: December 22, 2003
- ^ Antimicrobial Postexposure Prophylaxis for Anthrax: Adverse Events and Adherence Colin W. Shepard,* Montse Soriano-Gabarro,* Elizabeth R. Zell,* James Hayslett,* Susan Lukacs,* Susan Goldstein,* Stephanie Factor,*† Joshua Jones,* Renee Ridzon,* Ian Williams,* Nancy Rosenstein,* and the CDC Adverse Events Working Group1
- ^ (World Health Organization (WHO) Western Pacific Region Gonococcal Antimicrobial Susceptibility Programme (GASP) Report- 2000. Commun Dis Intell 2001; 25:274-277).
- ^ Gonococcal Isolate Surveillance Project (GISP) Annual Report - 2003
- ^ CDC's Morbidity and Mortality Weekly Report, April 13, 2007; vol 56: pp 332–336.
- ^ BMJ 1994;308:1437 (28 May)Letters Ciprofloxacin in general practice
- ^ van Buchem, F. L. (1997). The Lancet. 349 (9053): 683–687. doi:10.1016/S0140-6736(96)07585-X.
{{cite journal}}: Missing or empty|title=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - ^ Moxifloxacin – a new fluoroquinolone antibacterial BNF 5.1.12 DTB Vol 42 No 8 August 2004
- ^ British Medical Journal N0 7104 Volume 315, August 9, 1997
- ^ The Journal of Family Practice 1997;44(3):261-265
- ^ a b http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019537s69,19847s43,19857s50,20780s27lbl.pdf
- ^ Drlica K, Zhao X (1997). "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiol Mol Biol Rev. 61 (3): 377–92. PMC 232616. PMID 9293187.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - ^ Antimicrob Agents Chemother. 1992 April; 36(4): 751-756 Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of the C-8 fluorine group. M J Robinson, B A Martin, T D Gootz, P R McGuirk and N Osheroff Department of Biochemistry, Vanderbilt University School of Medicine, Nashville, Tennessee 37232-0146. http://www.ncbi.nlm.nih.gov/pubmed/1323952
- ^ The quinolone family: from antibacterial to anticancer agents.Sissi C, Palumbo M. Dept. of Pharmaceutical Sciences, V. Marzolo 5, 35100 Padova, Italy. claudia.sissi@unipd.it http://www.ncbi.nlm.nih.gov/pubmed/14529452
- ^ Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U (June 1986). "Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts". Antimicrob. Agents Chemother. 29 (6): 1073–8. PMID 3015015. PMC: 180502. http://aac.asm.org/cgi/reprint/29/6/1073.pdf.
- ^ Hosomi JA. Maeda Y. Oomori T. Irikura and T. Yokota (1988). "Mutagenicity of norfloxacin and AM-833 in bacteria and mammalian cells". Rev. Infect. Dis 10 (Suppl. 1): S148–S149.
- ^ Forsgren A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF (May 1987). "Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth". Antimicrob. Agents Chemother. 31 (5): 774–9. PMID 3606077. PMC: 174831. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=3606077.
- ^ Gootz TD, Barrett JF, Sutcliffe JA (January 1990). "Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems". Antimicrob. Agents Chemother. 34 (1): 8–12. PMID 2158274. PMC: 171510. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=171510&blobtype=pdf.
- ^ Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC (February 1993). "4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells". J. Cell. Biochem. 51 (2): 165–74. doi:10.1002/jcb.240510208. PMID 8440750.
- ^ Elsea SH, Osheroff N, Nitiss JL (July 1992). "Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast". J. Biol. Chem. 267 (19): 13150–3. PMID 1320012. http://www.jbc.org/cgi/reprint/267/19/13150.
- ^ Suto MJ, Domagala JM, Roland GE, Mailloux GB, Cohen MA (December 1992). "Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity". J. Med. Chem. 35 (25): 4745–50. doi:10.1021/jm00103a013. PMID 1469702
- ^ Enzmann H, Wiemann C, Ahr HJ, Schlüter G (April 1999). "Damage to mitochondrial DNA induced by the quinolone Bay y 3118 in embryonic turkey liver". Mutat. Res. 425 (2): 213–24. PMID 10216214.
- ^ Kashida Y, Sasaki YF, Ohsawa K, et al. (October 2002). "Mechanistic study on flumequine hepatocarcinogenicity focusing on DNA damage in mice". Toxicol. Sci. 69 (2): 317–21. doi:10.1093/toxsci/69.2.317. PMID 12377980. http://toxsci.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12377980.
- ^ Thomas A, Tocher J, Edwards DI (May 1990). "Electrochemical characteristics of five quinolone drugs and their effect on DNA damage and repair in Escherichia coli". J. Antimicrob. Chemother. 25 (5): 733–44. doi:10.1093/jac/25.5.733. PMID 2165050. http://jac.oxfordjournals.org/cgi/reprint/25/5/733.
- ^ "Fluoroquinolones and Quinolones". The American Academy of Optometry (British Chapter). http://www.academy.org.uk/pharmacy/fluoroq.htm. Retrieved on 29 January 2009
- ^ Yaseen A. Al-Soud; Najim A. Al-Masoudi (2003). "A new class of dihaloquinolones bearing N'-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity". J. Braz. Chem. Soc 14 (5). doi:10.1590/S0103-50532003000500014. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532003000500014&lng=es&nrm=iso&tlng=es. "Nevertheless, some quinolones cause injury to the chromosome of eukaryotic cells.21,22 These findings prompted us to optimize the substituent at C-3, by...".
- ^ Yaseen A. Al-Soud a and Najim A. Al-Masoudi (2003). "A New Class of Dihaloquinolones Bearing N’-Aldehydoglycosylhydrazides, Mercapto-1,2,4-triazole, Oxadiazoline and α-Amino Ester Precursors: Synthesis and Antimicrobial Activity". J. Braz. Chem. Soc 14 (5): 790–796. http://jbcs.sbq.org.br/jbcs/2003/v14_n5/13-048-02.pdf. "Although the current quinolones are not considered to be potent inhibitors of eucaryotic topoisomerases, some effects on these and other enzymes involved with DNA replication have been observed".
- ^ Sissi C, Palumbo M (November 2003). "The quinolone family: from antibacterial to anticancer agents". Curr Med Chem Anticancer Agents 3 (6): 439–50. doi:10.2174/1568011033482279. PMID 14529452. http://openurl.ingenta.com/content/nlm?genre=article&issn=1568-0118&volume=3&issue=6&spage=439&aulast=Sissi. "The present review focuses on the structural modifications responsible for the transformation of an antibacterial into an anticancer agent. Indeed, a distinctive feature of drugs based on the quinolone structure is their remarkable ability to target different type II topoisomerase enzymes. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models."
- ^ http://www.nycms.org/article_view.php3?view=947&part=1
- ^ "Fluoroquinolone resistance in Neisseria gonorrhoeae—Colorado and Washington, 1995". MMWR Morb Mortal Wkly Rep. 44 (41): 761–4. 1995. PMID 7565558.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Shin HC, Kim JC, Chung MK; et al. (2003). "Fetal and maternal tissue distribution of the new fluoroquinolone DW-116 in pregnant rats". Comp. Biochem. Physiol. C Toxicol. Pharmacol. 136 (1): 95–102. doi:10.1016/j.cca.2003.08.004. PMID 14522602.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dan M, Weidekamm E, Sagiv R, Portmann R, Zakut H (1993). "Penetration of fleroxacin into breast milk and pharmacokinetics in lactating women". Antimicrob. Agents Chemother. 37 (2): 293–6. PMC 187655. PMID 8452360.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Adverse drug reaction monitoring of ciprofloxacin in pediatric practice. sudden death after intravenous ciprofloxacin Indian Pediatr. 1992 Feb;29(2):181-8. Karande SC, Kshirsagar NA. Department of Pharmacology, Seth G.S. Medical College, Parel, Bombay
- ^ Noel GJ, Bradley JS, Kauffman RE; et al. (2007). "Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders". Pediatr. Infect. Dis. J. 26 (10): 879–91. PMID 17901792.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Division of Special Pathogen and Immunologic Drug Products Summary of Clinical Review of Studies Submitted in Response to a Pediatric Written Request 3/16/04 Applications: 19-537/S-049, ciprofloxacin tablets 20-780/S-013, ciprofloxacin oral suspension 19-847/S-027, ciprofloxacin IV 10 mg/mL 19-857/S-031, ciprofloxacin IV 5% dextrose Applicant: Bayer Corporation, Pharmaceutical Division Established: Ciprofloxacin Route: Oral or IV
- ^ FDA (20 December 1996). "Levaquin (Levofloxacin) NDA 20634 Approved: R.W. Johnson". USA. Retrieved 29 January 2009.
- ^ "SYNOPSIS" (PDF). USA. Retrieved 29 January 2009.
- ^ "SYNOPSIS" (PDF). USA. Retrieved 29 January 2009.
- ^ Ciprofloxacin (Cipro) - BPCA Pediatric Studies Summary: http://fqresearch.org/pdf_files/19537_Cipro_BPCA_clinical.pdf
- ^ Owens RC, Ambrose PG (2005). "Antimicrobial safety: focus on fluoroquinolones". Clin. Infect. Dis. 41 Suppl 2: S144–57. doi:10.1086/428055. PMID 15942881.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Iannini PB (2007). "The safety profile of moxifloxacin and other fluoroquinolones in special patient populations". Curr Med Res Opin. 23 (6): 1403–13. doi:10.1185/030079907X188099. PMID 17559736.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mulhall JP, Bergmann LS (1995). "Ciprofloxacin-induced acute psychosis". Urology. 46 (1): 102–3. doi:10.1016/S0090-4295(99)80171-X. PMID 7604468.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Reeves RR (1992). "Ciprofloxacin-induced psychosis". Ann Pharmacother. 26 (7–8): 930–1. PMID 1504404.
- ^ Azar S, Ramjiani A, Van Gerpen JA (2005). "Ciprofloxacin-induced chorea". Mov. Disord. 20 (4): 513–4, author reply 514. doi:10.1002/mds.20425. PMID 15739219.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Therapy of acute and chronic gram-negative osteomyelitis with ciprofloxacin. Report from a Swedish Study Group". J. Antimicrob. Chemother. 22 (2): 221–8. 1988. doi:10.1093/jac/22.2.221. PMID 3053554.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zehnder D, Hoigné R, Neftel KA, Sieber R (1995). "Painful dysaesthesia with ciprofloxacin". BMJ. 311 (7014): 1204. PMC 2551120. PMID 7488901.
One case of peripheral neuropathy has been reported. We report two cases of generalised painful dysaesthesia due to ciprofloxacin, a reaction not previously associated with this particular fluoroquinolone
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Nelson, Lewis H.; Flomenbaum, Neal; Goldfrank, Lewis R.; Hoffman, Robert Louis; Howland, Mary Deems; Neal A. Lewin (2006). Goldfrank's toxicologic emergencies. New York: McGraw-Hill, Medical Pub. Division. ISBN 0-07-143763-0. OCLC url=http://books.google.com/books?id=cvJuLqBxGUcC&pg=PA849&dq=goldfranks+Fluoroquinolone+toxicity.
{{cite book}}: Check|oclc=value (help); Missing pipe in:|oclc=(help)CS1 maint: multiple names: authors list (link) - ^ Sherman O, Beizer JL (1994). "Possible ciprofloxacin-induced acute cholestatic jaundice". Ann Pharmacother. 28 (10): 1162–4. PMID 7841570.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zimpfer A, Propst A, Mikuz G, Vogel W, Terracciano L, Stadlmann S (2004). "Ciprofloxacin-induced acute liver injury: case report and review of literature". Virchows Arch. 444 (1): 87–9. doi:10.1007/s00428-003-0917-9. PMID 14994731.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Owens RC, Ambrose PG (2005). "Antimicrobial safety: focus on fluoroquinolones". Clin. Infect. Dis. 41 Suppl 2: S144–57. doi:10.1086/428055. PMID 15942881.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Saint F, Gueguen G, Biserte J, Fontaine C, Mazeman E (2000). "[Rupture of the patellar ligament one month after treatment with fluoroquinolone]". Rev Chir Orthop Reparatrice Appar Mot (in French). 86 (5): 495–7. PMID 10970974.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dutta TK, Badhe BA (1999). "Ciprofloxacin-induced bone marrow depression" (PDF). Postgrad Med J. 75 (887): 571–3. PMC 1741342. PMID 10616701.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lim S, Alam MG (2003). "Ciprofloxacin-induced acute interstitial nephritis and autoimmune hemolytic anemia". Ren Fail. 25 (4): 647–51. PMID 12911170.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ http://www.consumeraffairs.com/news04/2006/08/pubcit_cipro.html
- ^ http://www.mnd.uscourts.gov/MDL-Levaquin/index.shtml
- ^ "FDA orders 'black box' label on some antibiotics". Retrieved 2008-07-08.
- ^ Winrow AP, Supramaniam G (1990). "Benign intracranial hypertension after ciprofloxacin administration". Arch. Dis. Child. 65 (10): 1165–6. doi:10.1136/adc.65.10.1165. PMC 1792342. PMID 2248512.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Orr CF, Rowe DB (2003). "Eardrop attacks: seizures triggered by ciprofloxacin eardrops". Med. J. Aust. 178 (7): 343. PMID 12670280.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Brouwers JR (1992). "Drug interactions with quinolone antibacterials". Drug Saf. 7 (4): 268–81. doi:10.2165/00002018-199207040-00003. PMID 1524699.
- ^ Hilliard JJ, Krause HM, Bernstein JI; et al. (1995). "A comparison of active site binding of 4-quinolones and novel flavone gyrase inhibitors to DNA gyrase". Adv Exp Med Biol. 390: 59–69. PMID 8718602.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Carol Langlois (1998). "Risk of seizures from concomitant use of ciprofloxacin and phenytoin in patients with epilepsy" (PDF). Canada: Canadian Adverse Drug Reaction Newsletter. Retrieved 30 January 2009.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Cooper JG, Harboe K, Frost SK, Skadberg Ø (2005). "Ciprofloxacin interacts with thyroid replacement therapy". BMJ. 330 (7498): 1002. doi:10.1136/bmj.330.7498.1002. PMC 557149. PMID 15860826.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019537s073,020780s030lbl.pdf
- ^ Harder S, Fuhr U, Staib AH, Wolff T (1989). "Ciprofloxacin-caffeine: a drug interaction established using in vivo and in vitro investigations". Am. J. Med. 87 (5A): 89S–91S. doi:10.1016/0002-9343(89)90031-4. PMID 2589393.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cooper JG, Harboe K, Frost SK, Skadberg Ø (2005). "Ciprofloxacin interacts with thyroid replacement therapy". BMJ. 330 (7498): 1002. doi:10.1136/bmj.330.7498.1002. PMC 557149. PMID 15860826.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Domagala JM (1994). "Structure-activity and structure-side-effect relationships for the quinolone antibacterials". J. Antimicrob. Chemother. 33 (4): 685–706. doi:10.1093/jac/33.4.685. PMID 8056688.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Janknegt R (1990). "Drug interactions with quinolones". J. Antimicrob. Chemother. 26 Suppl D: 7–29. PMID 2286594.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b http://www.drugbank.ca/drugs/DB00537
- ^ van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HM, Rowlands S, Stricker BH (2003). "Increased risk of achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids". Arch. Intern. Med. 163 (15): 1801–7. doi:10.1001/archinte.163.15.1801. PMID 12912715.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019537s68,19847s42,19857s49,20780s26,21473s24lbl.pdf See DOSAGE AND ADMINISTRATION – ADULTS
- ^ http://www.sheller.com/NewsDetails.asp?NewsID=72
- ^ http://www.lunewsviews.com/legal_briefs_archives.htm#cipro
- ^ http://www.gdldlaw.com/content/bio_goodell.htm
- ^ * October 1996 * Volume 26 Number 3 REPORTS OF ADVERSE EVENTS WITH FLUOROQUINOLONES
- ^ http://www.fqresearch.org/text_documents/FDA_Medical_Bulletin_1996.doc
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/nda/97/019537_S025_CIPRO.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/nda/98/019537_S033_CIPRO.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2000/20780S08ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/19537s41ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/19537slr048,050,051,20780slr012,014,015ltr.pdf
- ^ a b http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/19537s049,19857s031,19847s027,20780s013ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/19537s053,054,20780s017,018ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/019537s60,019847s36,019857s41,020780s20,021473s13ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2006/019537s62,020780s23,019847s37,019857s42,021473s16LTR.pdf
- ^ a b http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2007/019537s65,19847s39,19857s46,20780s24,21473s22ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/019537s067,020780s025ltr.pdf
- ^ a b http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/019537s068,019847s042ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/019537s073,020780s030ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/019537s070,019847s044ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019537s701984744198575120780282147325L.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/019537s071,019847s045,019857s052,020780s029,021473s026ltr.pdf
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/019537s070,019847s044ltr.pdf
- ^ Bailey RR, Natale R, Linton AL (1972). "Nalidixic acid arthralgia". Can Med Assoc J. 107 (7): 604 passim. PMC 1940945. PMID 4541768.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bailey RR, Kirk JA, Peddie BA (1983). "Norfloxacin-induced rheumatic disease". N Z Med J. 96 (736): 590. PMID 6223241.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Szarfman A, Chen M, Blum MD (1995). "More on fluoroquinolone antibiotics and tendon rupture". N Engl J Med. 332 (3): 193. doi:10.1056/NEJM199501193320319. PMID 7800023.
{{cite journal}}:|format=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399)". Public Citizen. August 1, 1996. Retrieved on December 27, 2008.
- ^ "Reports of adverse events with fluoroquinolones". FDA Medical Bulletin. 26 (3). October 1996. Retrieved on December 27, 2008. alternate link: http://www.fqresearch.org/text_documents/FDA_Medical_Bulletin_1996.doc
- ^ a b "Madigan, Public Citizen, petition FDA for "black box" warning regarding potential adverse effects of certain popular antibiotics" (Press release). Office of the Illinois Attorney General. August 29, 2006. Retrieved 2008-12-27. Full text of the 2005 petition and FDA response available from the Fluoroquinolone Toxicity Research Foundation, a U.S. consumer advocacy group.
- ^ "Public Citizen Petitions the FDA to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781)". Public Citizen. August 29, 2006. Retrieved 2008-12-27.
- ^ "Public Citizen v. Food and Drug Administration (FDA) (Fluoroquinolone)". Public Citizen. January 3, 2008. Retrieved 2008-12-27.
- ^ Ravn, Karen (August 18, 2008). "Behind the FDA's 'black box' warnings". Los Angeles Times. Retrieved 2008-12-27.
{{cite news}}: Italic or bold markup not allowed in:|publisher=(help) - ^ "FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs" (Press release). U.S. Food and Drug Administration. 2008-07-08. Retrieved 2008-10-11.
- ^ The complete labeling history of each drug is available from Drugs@FDA. Medication Guides are available from the FDA's MedWatch system.
- ^ MacCarthy, Paul (October 22, 2008). "Important Change in the Avelox (moxifloxacin hydrochloride) and Cipro (ciprofloxacin) Complete Prescribing Information – Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture" (PDF). Bayer HealthCare Pharmaceuticals. Retrieved 2008-12-27.
- ^ Rosenthal, Norman (November 2008). "Important Change in the LEVAQUIN (Ievofloxacin) Complete Prescribing Information -Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture" (PDF). Ortho-McNeil Janssen Scientific Affairs, LLC. Retrieved 2008-12-27.
- ^ FDA (2 April 1999). "NDA #19-537 Cipro (ciprofloxacin) Tablets Macmis ID #7451" (PDF). USA: Food and Drug Administration. Retrieved 30 January 2009. alt link: http://www.fqresearch.org/pdf_files/040299_bayer.pdf
- ^ FDA (15 December 1999). "NDA #21-085 Avelox (moxifloxacin hydrochloride) Tablets Macmis ID #8577" (PDF). USA: Food and Drug Administration. Retrieved 30 January 2009. alt link: http://www.fqresearch.org/pdf_files/wl121599.pdf
- ^ FDA. "NDA #21-085 Avelox (moxifloxacin HCL) Tablets MACMIS ID #10160" (PDF). USA: Food and Drug Administration. Retrieved 30 January 2009. alt link: http://www.fqresearch.org/pdf_files/10160.pdf
- ^ FDA. "RE: Cipro HC Otic Suspension (Ciprofloxacin Hydrochloride and Hydrocortisone Otic Suspension) NDA 20-805 Ciloxan (ciprofloxacin ophthalmic) Solution NDA 19-992 MACMIS ID# 11015" (PDF). USA: Food and Drug Administration. Retrieved 30 January 2009.alt link: http://www.fqresearch.org/pdf_files/11015.pdf
- ^ Biosecurity requires drug reform. Jan 1, 2002 World Watch ISSN: 0896-0615
- ^ Electrochemical DNA biosensor for the study of ciprofloxacin–DNA interaction Haq Nawaza, Sakandar Raufa, Kalsoom Akhtara and Ahmad M. Khalid, Bioprocess Technology Division, National Institute for Biotechnology and Genetic Engineering (NIBGE), P.O. Box 577, Jhang Road, Faisalabad, Pakistan May 2006
- ^ The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment Stacey L. Knobler, Stanley M. Lemon, Marian Najafi, Tom Burroughs 2003 ISBN 0-309-08854-2
- ^ http://www.cdc.gov/ncidod/eid/vol5no3/pdf/vatopoulos.pdf
- ^ http://www.health.state.mn.us/news/pressrel/2009/bacterial022609.html
- ^ M Jacobs, Worldwide Overview of Antimicrobial Resistance. International Symposium on Antimicrobial Agents and Resistance 2005.
- ^ http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm127657.htm
- ^ Jim Hoover, for Bayer Corporation, Alaska Pharmacy and Therapeutics Committee March 19, 2004 http://www.hss.state.ak.us/dhcs/PDL/minutes_meetings_pdl/minutes_031904_pdl.pdf
- ^ Pépin J, Saheb N, Coulombe MA; et al. (2005). "Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec". Clin. Infect. Dis. 41 (9): 1254–60. doi:10.1086/496986. PMID 16206099.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dr Ralf-Peter Vonberg. "Clostridium difficile: a challenge for hospitals". European Center for Disease Prevention and Control. Institute for Medical Microbiology and Hospital Epidemiology: IHE. Retrieved 27 July 2009.
- ^ http://www.nyc.gov/html/doh/html/tb/tb4a.shtml
- ^ Antimicrobials for acute otistis media; A review from the International Primary Care Network". British Medical Journal July 5, 1997.
- ^ http://econnect.uspharmacist.com/econnect/Default.aspx?tabid=53&page=publish/content/8_1868.htm
- ^ MacDougall, C., Guglielmo, B.J., Maselli, J., and Gonzales, R. (2005, March). "Antimicrobial drug prescribing for pneumonia in ambulatory care." (AHRQ grant HS13003). Emerging Infectious Diseases 11(3), pp. 380–384.
- ^ Fluoroquinolone prescribing in the United States: 1995 to 2002. Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Division of General Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts 02120, USA.
- ^ Linder JA, Huang ES, et al. Fluoroquinolone prescribing in the United States: 1995–2002. Am J Med. 2005;118:259-268.
- ^ K08 HS14563 and HS11313
- ^ Fluoroquinolone prescribing in the United States: 1995–2002," by Dr. Linder, Elbert S. Huang, M.D., M.P.H., Michael A. Steinman, M.D., and others in the March 2005 American Journal of Medicine 118(3), pp. 259–268.
- ^ http://www.prescriptionaccess.org/lawsuitssettlements/past_lawsuits?id=0010
- ^ US Department of Health and Human Services. HHS, Bayer agree to Cipro purchase. HHS News. 24 October 2001. Available at: http://www.hhs.gov/news/press/2001pres/20011024.html. Accessed October 26, 2001. Clendenning A. Probable anthrax found in Manhattan office of Pataki. First Coast News. 17 October 2001. Available at: http://www.firstcoastnews.com/news/2001-10-17/usw_pataki.asp. Accessed February 4, 2002.
- ^ http://www.cafc.uscourts.gov/opinions/08-1097.pdf
- ^ http://www.citizen.org/print_article.cfm?ID=6435
- ^ Summary of Clinical Review of Studies Submitted in Response to a Pediatric Written Request Applications: 19-537/S-049, ciprofloxacin tablets 20-780/S-013, ciprofloxacin oral suspension 19-847/S-027, ciprofloxacin IV 10 mg/mL 19-857/S-031, ciprofloxacin IV 5% dextrose Applicant: Bayer Corporation, Pharmaceutical Division 400 Morgan Lane West Haven, Connecticut 06516 Drug Name Established: Ciprofloxacin Proprietary: Cipro
- ^ Food And Drug Administration Center For Drug Evaluation And Research PID# D030715 DATE: March 1, 2005 FROM: Evelyn R. Farinas, R. Ph., M.G.A. Post Marketing Safety Evaluator THROUGH: Mark Avigan, M.D., C.M., Director Division of Drug Risk Evaluation Office of Drug Safety (ODS), HFD-430 TO: Solomon Iyasu, M.D., M.P.H., Team Leader Division of Counter-Terrorism and Pediatrics, HFD-960 SUBJECT: Ods Postmarketing Safety Review Consult: One-Year Post Pediatric Exclusivity Postmarketing Adverse Events Review Drug: Ciprofloxacin NDA: 19-537, 19-847, 19-857, 20-780, 21-473, 21-554 Pediatric Exclusivity Approval Date: December 22, 2003
- ^ In The United States District Court For The District Of Columbia Public Citizen, Inc. VS. Food And Drug Administration January 3, 2008
- ^ Office Of The Attorney General State Of Illinois Lisa Madigan Citizen Petition to Include a Black Box Warning on Fluoroquinolone Antibiotics May 18, 2005
- ^ Public Citizen’s Petition to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781) August 29, 2006
- ^ Public Citizen's Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399) August 1, 1996
- ^ Public Citizen's Petition to Ban the Antibiotic Gatifloxacin (Tequin) (HRG Publication #1768)
- ^ Public Citizen's Petition to immediately ban the antibiotic Trovafloxacin (Trovan). (HRG Publication #1485) Date: June 3, 1999
- ^ Public Citizen's Petition to immediately stop the distribution of dangerous, misleading prescription drug information to the public. HRG Publication #1442 Date: June 9, 1998
- ^ June 2004, A petition To the United States Congress to immediately take action to protect consumers from the reckless and negligent abuses of the FDA and the following Pharmaceutical Companies: Bayer, Ortho-McNeill, Pfizer, Merck, Bristol-Myers Squibb, Sanofi Winthrop, Bertek Pharmaceuticals – Rhone-Poulenc Rorer and Barr. These companies manufacture and distribute fluoroquinolone antibiotics in the United States in a manner that fails to warn of serious adverse event risks, and downplays and fails to warn physicians of the serious risks associated with fluoroquinolone therapy.
- ^ Davies BI, Maesen FPV. Quinolones in chest infections. J Antimicrob Chemother 1986;18:296-99. [Medline]
- ^ Barry AL, Jones RN. In vitro activity of ciprofloxacin against Gram positive cocci. Am J Med 1987;82 (suppl 4A):27-32.
- ^ ReAntibiotic Resistance Among Gram-Negative Bacilli in US Intensive Care Units Implications for Fluoroquinolone Use Melinda M. Neuhauser, PharmD; Robert A. Weinstein, MD; Robert Rydman, PhD; Larry H. Danziger, PharmD; George Karam, MD; John P. Quinn, MD JAMA. 2003;289:885-888. “From 1995 to 2002, inappropriate antibiotic prescribing for acute respiratory infections, which are usually caused by viruses and thus are not responsive to antibiotics, declined from 61 to 49 percent. However, the use of broad-spectrum antibiotics such as the fluoroquinolones, jumped from 41 to 77 percent from 1995 to 2001. Overuse of these antibiotics will eventually render them useless for treating antibiotic-resistant infections, for which broad-spectrum antibiotics are supposed to be reserved.” http://www.ahrq.gov/research/nov07/1107RA29.htm
- ^ Past Issue Vol. 11, No. 3 March 2005
Antimicrobial Drug Prescribing for Pneumonia in Ambulatory Care
Conan MacDougall,* B. Joseph Guglielmo,* Judy Maselli,† and Ralph Gonzales†
- University of California School of Pharmacy, San Francisco, California, USA; and †University of California Department of Medicine, San Francisco, California
- ^ Source Lancet March 8, 1997
- ^ Source The Journal of Family Practice 1997;44(3):261-265
- ^ Levofloxacin Treatment for Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) in Men: A randomized Placebo Controlled Multicenter trial. Abstract 104.
- ^ Ciprofloxacin or Tamsulosin in Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome A Randomized, Double-Blind Trial Richard B. Alexander, MD; Kathleen J. Propert, ScD; Anthony J. Schaeffer, MD; J. Richard Landis, PhD; J. Curtis Nickel, MD; Michael P. O'Leary, MD; Michel A. Pontari, MD; Mary McNaughton-Collins, MD, MPH; Daniel A. Shoskes, MD; Craig V. Comiter, MD; Nand S. Datta, MD; Jackson E. Fowler, Jr, MD; Robert B. Nadler, MD; Scott I. Zeitlin, MD; Jill S. Knauss, MS; Yanlin Wang, MS; John W. Kusek, PhD; Leroy M. Nyberg, Jr, MD, PhD; and Mark S. Litwin, MD, MPH, and the Chronic Prostatitis Collaborative Research Network* 19 October 2004 | Volume 141 Issue 8| Pages 581-589
- ^ Third International Chronic Prostatitis Network A Prospective, Randomized, Double-Blind, Placebo-Controlled Study Of Antibiotics For The Treatment Of Category Iiib Chronic Pelvic Pain Syndrome In Men J. Dimitrakov, J. Tchitalov, T. Zlatanov, D. Dikov Justus-Liebig University, Urology Clinic, 29 Klinikstrasse, Giessen, Germany and Higher Medical Institute, Departments of Urology and Pathology, 15 A Vassil Aprilov Str, Plovdiv, Bulgaria
- ^ Curr Med Res Opin. 2008 Feb;24(2):329-33. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Deshpande A, Pant C, Jain A, Fraser TG, Rolston DD. Cleveland Clinic, Cleveland, OH, USA.
- ^ October 2004 • Volume 39 • Number 10 Case Reports Severe ciprofloxacin-associated pseudomembranous colitis in an eight-year-old child Carlos A. Angel a, b, c, d * [MEDLINE LOOKUP] Justtin Green a, b, c, d [MEDLINE LOOKUP] Leonard Swischuk a, b, c, d [MEDLINE LOOKUP] Janak Patel a, b, c, d [MEDLINE LOOKUP]
- ^ Source: University Of California - San Francisco Date: 2002-10-01 Cipro, Related Antibiotics Over-Prescribed, Fueling Microbe Resistance
- ^ Gatifloxacin and moxifloxacin have no proven clinical advantages over other fluoroquinolones, macrolides, or amoxicillin Gatifloxacin (Tequin) and moxifloxacin (Avelox) Therapeutics Letter Canadian Family Physician K. Bassett B. Mintzes V. Musini T.L. Perry Jr M. Wong J.M. Wright
- ^ From The Cochrane Library, Issue 3, 2006. Chichester, UK: John Wiley & Sons, Ltd. All rights reserved. Fluoroquinolones for treating tuberculosis (Cochrane Review) Ziganshina LE, Vizel AA, Squire SB
