Human brain: Difference between revisions
| [pending revision] | [pending revision] |
m →Regulation: punct |
|||
| Line 151: | Line 151: | ||
Early in the fourth week the cephalic part bends sharply forward in a [[cephalic flexure]].{{sfn|Larsen|2001|pp=85–88}} This flexed part becomes the forebrain (prosencephalon); the adjoining curving part becomes the midbrain (mesencephalon) and the part caudal to the flexure becomes the hindbrain (rhombencephalon). In the fifth week of developmement five brain vesicles have formed. The forebrain separates into two vesicles an anterior telencephalon and a posterior [[diencephalon]]. The telencephalon gives rise to the cerebral cortex, basal ganglia, and related structures. The diencephalon gives rise to the thalamus and hypothalamus. The hindbrain also splits into two areas – the metencephalon and the mylencephalon. The metencephalon gives rise to the cerebellum and pons. The myelencephalon gives rise to the medulla oblongata.{{sfn|Larsen|2001|pp=85–87}} Also during the fifth week, the brain divides into [[segmentation (biology)|repeating segments]] called [[neuromere]]s.{{sfn|Larsen|2001|p=419}}<ref name=Purves /> |
Early in the fourth week the cephalic part bends sharply forward in a [[cephalic flexure]].{{sfn|Larsen|2001|pp=85–88}} This flexed part becomes the forebrain (prosencephalon); the adjoining curving part becomes the midbrain (mesencephalon) and the part caudal to the flexure becomes the hindbrain (rhombencephalon). In the fifth week of developmement five brain vesicles have formed. The forebrain separates into two vesicles an anterior telencephalon and a posterior [[diencephalon]]. The telencephalon gives rise to the cerebral cortex, basal ganglia, and related structures. The diencephalon gives rise to the thalamus and hypothalamus. The hindbrain also splits into two areas – the metencephalon and the mylencephalon. The metencephalon gives rise to the cerebellum and pons. The myelencephalon gives rise to the medulla oblongata.{{sfn|Larsen|2001|pp=85–87}} Also during the fifth week, the brain divides into [[segmentation (biology)|repeating segments]] called [[neuromere]]s.{{sfn|Larsen|2001|p=419}}<ref name=Purves /> |
||
A characteristic of the brain is [[gyrification]] (wrinkling of the cortex). In the womb, the cortex starts off as smooth but starts to form fissures that begin to mark out the different lobes of the brain. Scientists do not have a clear answer as to why the cortex later wrinkles and folds.<ref name="Xi Chen">{{cite book|url=https://books.google.com/books?id=94aPR_Oh40oC&pg=PA188|title=Mechanical Self-Assembly: Science and Applications|publisher=[[Springer Science & Business Media]]|year=2012|isbn=1461445620|page=188|author=Xi Chen|accessdate=January 21, 2017}}</ref> The fissures form as a result of the growing hemispheres that increase in size due to a sudden growth in cells of the grey matter. The underlying white matter does not grow at the same rate and the hemispheres are crowded into the small cranial vault.<ref name="Ackerman">{{cite book|last1=Ackerman|first1=Sandra|title=Discovering the brain|date=1992|publisher=National Academy Press|location=Washington, D.C.|isbn=0-309-04529-0 |pages=22–25}}</ref> The first cleft to appear in the fourth month is the lateral cerebral fossa. The expanding caudal end of the hemisphere has to curve over in a forward direction to fit into the restricted space. This covers the fossa and turns it into a much deeper ridge known as the [[lateral sulcus]] and this marks out the temporal lobe.<ref name="Embryo">{{cite book|last1=Larsen|first1=William J.|title=Human embryology|date=2001|publisher=Churchill Livingstone|location=Philadelphia, Pa.|isbn=0-443-06583-7|pages=445–446|edition=3.}}</ref> By the sixth month other sulci have formed that demarcate the frontal, parietal, and occipital lobes.<ref name = Embryo /> A gene present in the human genome ([[ArhGAP11B and human encephalisation|ArhGAP11B]]) may play a major role in gyrification and encephalisation.<ref>{{Cite journal|last=Florio|first=Marta|last2=Albert|first2=Mareike|last3=Taverna|first3=Elena|last4=Namba|first4=Takashi|last5=Brandl|first5=Holger|last6=Lewitus|first6=Eric|last7=Haffner|first7=Christiane|last8=Sykes|first8=Alex|last9=Wong|first9=Fong Kuan|date=March 27, 2015|title=Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion|url=http://science.sciencemag.org/content/347/6229/1465|journal=Science |volume=347|issue=6229 |pages=1465–1470 |doi=10.1126/science.aaa1975 |pmid=25721503}}</ref> |
A characteristic of the brain is [[gyrification]] (wrinkling of the cortex). In the womb, the cortex starts off as smooth but starts to form fissures that begin to mark out the different lobes of the brain. Scientists do not have a clear answer as to why the cortex later wrinkles and folds, but the wrinkling and folding is associated with intelligence and [[neurological disorder]]s.<ref name="Xi Chen">{{cite book|url=https://books.google.com/books?id=94aPR_Oh40oC&pg=PA188|title=Mechanical Self-Assembly: Science and Applications|publisher=[[Springer Science & Business Media]]|year=2012|isbn=1461445620|page=188|author=Xi Chen|accessdate=January 21, 2017}}</ref> The fissures form as a result of the growing hemispheres that increase in size due to a sudden growth in cells of the grey matter. The underlying white matter does not grow at the same rate and the hemispheres are crowded into the small cranial vault.<ref name="Ackerman">{{cite book|last1=Ackerman|first1=Sandra|title=Discovering the brain|date=1992|publisher=National Academy Press|location=Washington, D.C.|isbn=0-309-04529-0 |pages=22–25}}</ref> The first cleft to appear in the fourth month is the lateral cerebral fossa. The expanding caudal end of the hemisphere has to curve over in a forward direction to fit into the restricted space. This covers the fossa and turns it into a much deeper ridge known as the [[lateral sulcus]] and this marks out the temporal lobe.<ref name="Embryo">{{cite book|last1=Larsen|first1=William J.|title=Human embryology|date=2001|publisher=Churchill Livingstone|location=Philadelphia, Pa.|isbn=0-443-06583-7|pages=445–446|edition=3.}}</ref> By the sixth month other sulci have formed that demarcate the frontal, parietal, and occipital lobes.<ref name = Embryo /> A gene present in the human genome ([[ArhGAP11B and human encephalisation|ArhGAP11B]]) may play a major role in gyrification and encephalisation.<ref>{{Cite journal|last=Florio|first=Marta|last2=Albert|first2=Mareike|last3=Taverna|first3=Elena|last4=Namba|first4=Takashi|last5=Brandl|first5=Holger|last6=Lewitus|first6=Eric|last7=Haffner|first7=Christiane|last8=Sykes|first8=Alex|last9=Wong|first9=Fong Kuan|date=March 27, 2015|title=Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion|url=http://science.sciencemag.org/content/347/6229/1465|journal=Science |volume=347|issue=6229 |pages=1465–1470 |doi=10.1126/science.aaa1975 |pmid=25721503}}</ref> |
||
{{Gallery |
{{Gallery |
||
Revision as of 11:24, 22 June 2017
| Human brain | |
|---|---|
 Human brain and skull | |
 | |
| Details | |
| Precursor | Neural tube |
| System | Central nervous system Neuroimmune system |
| Artery | Internal carotid arteries, vertebral arteries |
| Vein | Internal jugular vein, internal cerebral veins, external veins: (superior and inferior cerebral veins, and middle cerebral veins), basal vein, terminal vein, choroid vein, cerebellar veins |
| Identifiers | |
| Latin | Cerebrum[1] |
| Greek | ἐγκέφαλος (enképhalos)[2] |
| TA98 | A14.1.03.001 |
| TA2 | 5415 |
| FMA | 50801 |
| Anatomical terminology | |
The human brain is the central organ of the human nervous system. The human brain, with the spinal cord, makes up the central nervous system. The brain consists of the cerebrum, the brainstem and the cerebellum. The brain is the organ that controls most of the activities of the body. The brain processes, integrates, and coordinates all of the information it receives from the sense organs. Sensory information is interpreted and analysed, and decisions are made as to the instructions transmitted to the rest of the body. The brain is contained in, and protected by, the skull bones of the head.
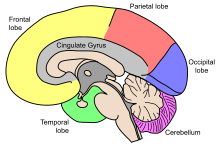
The cerebrum is the largest part of the human brain. It is divided into two cerebral hemispheres which are covered by the cerebral cortex. The cortex is an outer layer of grey matter, that covers the core of white matter. The cortex is split into the neocortex and the much smaller allocortex. The neocortex is made up of six neuronal layers, and the allocortex has three or four such layers. Each hemisphere is conventionally divided into four lobes – the frontal, temporal, parietal, and occipital lobes. The frontal lobe is associated with executive functions including self-control, planning, reasoning, and abstract thought, while the occipital lobe is dedicated to vision. Within each lobe, there are also cortical areas associated with specific functions, such as the sensory, a motor and association regions. Although the left and right hemispheres are broadly similar in shape and function, some functions are associated with a particular side of the brain, such as language in the left and visual-spatial ability in the right. The hemispheres are connected by nerve tracts known as commissures, the largest being the corpus callosum.
The cerebrum is connected by the brainstem to the spinal cord. The brainstem consists of the midbrain, the pons, and the medulla oblongata. The cerebellum is connected to the brainstem by pairs of tracts known as peduncles. Within the cerebrum is the ventricular system of the brain, which consists of four interconnected ventricles in which cerebrospinal fluid is produced and circulated. Underneath the cerebral cortex, several important structures are located, including the thalamus, the epithalamus, the pineal gland, the hypothalamus, the pituitary gland, and the subthalamus; the limbic structures, including the amygdala and the hippocampus; the claustrum, the various nuclei of the basal ganglia; the basal forebrain structures, and the three circumventricular organs.
The cells of the brain include neurons and supportive glial cells. There are more than 86 billion neurons in the brain and a more or less equal number of other cells. Brain activity is made possible by the interconnections of neurons that are linked together to reach their targets. These connections form various neural networks of neural pathways and circuits. The whole circuitry of the brain is driven by the process of neurotransmission.
The brain is protected by the skull, suspended in cerebrospinal fluid, and isolated from the bloodstream by the blood–brain barrier. However, the brain is still susceptible to damage, disease, and infection. Damage can be caused by trauma, or a loss of blood supply known as a stroke. The brain is also susceptible to degenerative disorders, such as Parkinson's disease, forms of dementia including Alzheimer's disease, and multiple sclerosis. A number of psychiatric conditions, including schizophrenia and clinical depression, are thought to be associated with brain dysfunctions, although the nature of these is not well understood. The brain can also be the site of tumours, both benign and malignant. Malignant tumours mostly originate from other sites in the body.
The study of the anatomy of the brain is neuroanatomy, while the study of its function is neuroscience. A number of different techniques are used to study the brain. Brain specimens from other animals, which may be examined microscopically, have been a traditional source of much information. Medical imaging technologies such as functional neuroimaging, and electroencephalography (EEG) recordings are important techniques in studying the brain. Examining the medical history of people with brain injury has also provided great insight into the function of each part of the brain.
Structure
Gross anatomy
The adult human brain weighs on average about 1.2–1.4 kg (2.6–3.1 lb), or about 2% of the total body weight,[3][4] with a volume of around 1260 cm3 in men and 1130 cm3 in women, although there is substantial individual variation.[5] Neurological differences between the sexes have not been shown to correlate in any simple way with IQ or other measures of cognitive performance.[6]
The cerebrum, consisting of the cerebral hemispheres, forms the largest part of the brain and is situated above the other brain structures.[7] The outer region of the hemispheres, the cerebral cortex, is grey matter, consisting of cortical layers. Each hemisphere is divided into four main lobes.[8]
The brainstem, resembling a stalk, attaches to and leaves the cerebrum at the start of the midbrain area. The brainstem includes the midbrain, the pons, and the medulla oblongata. Behind the brainstem is the cerebellum (Latin: little brain).[7] Its cortex is narrowly furrowed horizontally.[9]
The cerebrum, brainstem, cerebellum, and spinal cord are covered by three membranes called meninges. The membranes are the tough dura mater; the middle arachnoid mater and the more delicate inner pia mater. Between the arachnoid mater and the pia mater is the subarachnoid space, which contains the cerebrospinal fluid.[10] In the cerebral cortex, close to the basement membrane of the pia mater, is a limiting membrane called the glia limitans; this is the outermost layer of the cortex.[11] The living brain is very soft, having a gel-like consistency similar to soft tofu.[12] The neural layers of the cortex constitute much of the brain's grey matter, while the deeper subcortical regions of the brain, made up of myelinated axons, are the white matter.[13]
Cerebrum
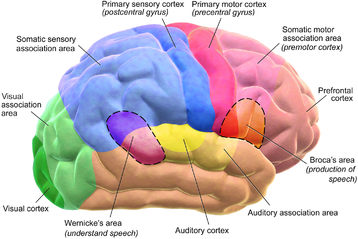
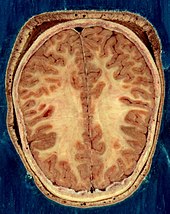
The cerebrum is the largest part of the human brain, and is divided into nearly symmetrical left and right hemispheres by a deep groove, the longitudinal fissure.[14] The outer part of the cerebrum is the cerebral cortex, made up of grey matter arranged in layers. It is 2 to 4 millimetres (0.079 to 0.157 in) thick, and deeply folded to give a convoluted appearance.[15] Beneath the cortex is the white matter of the brain. The largest part of the cerebral cortex is the neocortex, which has six neuronal layers. The rest of the cortex is of allocortex, which has three or four layers. The hemispheres are connected by five commissures that span the longitudinal fissure, the largest of these is the corpus callosum.[7] The surface of the brain is folded into ridges (gyri) and grooves (sulci), many of which are named, usually according to their position, such as the frontal gyrus of the frontal lobe or the central sulcus separating the central regions of the hemispheres. There are many small variations in the secondary and tertiary folds.
Each hemisphere is conventionally divided into four lobes; the frontal lobe, parietal lobe, temporal lobe, and occipital lobe, named according to the skull bones that overlie them.[8] Each lobe is associated with one or two specialised functions though there is some functional overlap between them.[16]
The cortex is mapped by divisions into about fifty different functional areas known as Brodmann's areas. These areas are distinctly different when seen under a microscope.[17] The cortex is also divided into two main functional areas – a motor cortex and a sensory cortex.[18] The primary sensory areas receive signals from the sensory nerves and tracts by way of relay nuclei in the thalamus. Primary sensory areas include the visual cortex of the occipital lobe, the auditory cortex in parts of the temporal lobe and insular cortex, and the somatosensory cortex in the parietal lobe. The primary motor cortex, which sends axons down to motor neurons in the brainstem and spinal cord. This area occupies the rear portion of the frontal lobe, directly in front of the somatosensory area. The remaining parts of the cortex, are called the association areas. These areas receive input from the sensory areas and lower parts of the brain and are involved in the complex processes of perception, thought, and decision-making.[19] The main functions of the frontal lobe are to control attention, abstract thinking, behavior, problem solving tasks, and physical reactions and personality.[20][21] The occipital lobe is the smallest lobe; its main functions are visual reception, visual-spatial processing, movement, and colour recognition.[20][21] There is a smaller occipital lobule in the lobe known as the cuneus. The temporal lobe controls auditory and visual memories, language, and some hearing and speech.[20]
The cerebrum also contains the ventricles where the cerebrospinal fluid is produced and circulated. Below the corpus callosum is the septum pellucidum, a membrane that separates the lateral ventricles. Beneath the lateral ventricles is the thalamus and to the front and below this is the hypothalamus. The hypothalamus leads on to the pituitary gland. At the back of the thalamus is the brainstem.[22]
The basal ganglia, also called basal nuclei, are a set of structures deep within the hemispheres involved in behaviour and movement regulation.[23] The largest component is the striatum, others are the globus pallidus, the substantia nigra and the subthalamic nucleus.[23] Part of the dorsal striatum, the putamen, and the globus pallidus, lie separated from the lateral ventricles and thalamus by the internal capsule, whereas the caudate nucleus stretches around and abuts the lateral ventricles on their outer sides.[24]
Below and in front of the striatum are a number of basal forebrain structures. These include the nucleus accumbens, nucleus basalis, diagonal band of Broca, substantia innominata, and the medial septal nucleus. These structures are important in producing the neurotransmitter, acetylcholine, which is then distributed widely throughout the brain. The basal forebrain is considered to be the major cholinergic output of the central nervous system.
Cerebellum

The cerebellum (Latin: little brain) is divided into an anterior lobe, a posterior lobe, and the flocculonodular lobe.[25] The anterior and posterior lobes are connected in the middle by the vermis.[9] The cerebellum has a much thinner outer cortex. Viewed from underneath between the two lobes is the third lobe the flocculonodular lobe.[26] The cerebellum rests at the back of the cranial cavity, lying beneath the occipital lobes, and is separated from these by the cerebellar tentorium, a sheet of fibre.[27]
It is connected to the midbrain of the brainstem by the superior cerebellar peduncles, to the pons by the middle cerebellar peduncles, and to the medulla by the inferior cerebellar peduncles.[9] Enclosed between the cerebellum and the brainstem lies a space and a tube containing cerebrospinal fluid – the cerebral aqueduct and fourth ventricle.[28] The cerebellum consists of an inner medulla of white matter and an outer cortex of grey matter. The surface of the cerebellum is richly folded.[27] The cerebellum's anterior and posterior lobes appears to play a role in the coordination and smoothing of complex motor movements, and the flocculonodular lobe in the maintenance of balance[29] although debate exists as to its cognitive, behavioral and motor functions.[30]
Brainstem
The brainstem lies beneath the cerebrum and consists of the midbrain, pons and medulla. It lies in the back part of the skull, resting on the part of the base known as the clivus, and ends at the foramen magnum, a large opening in the occipital bone. The brainstem continues below this as the spinal cord,[31] protected by the vertebral column.
Ten of the twelve pairs of cranial nerves[a] emerge directly from the brainstem.[31] The brainstem also contains nuclei of many cranial and peripheral nerves, as well as nuclei involved in the regulation of many essential processes including breathing, control of eye movements and balance.[32][31] The reticular formation, a network of nerves of ill-defined formation, is present within and along the length of the brainstem.[31] Many nerve tracts, which transmit information to and from the cerebral cortex to the rest of the body, pass through the brainstem.[31]
Microanatomy
The human brain is primarily composed of neurons, glial cells, neural stem cells, and blood vessels. Types of neuron include interneurons, pyramidal cells including Betz cells, motor neurons, upper motor neurons, and lower motor neurons, and cerebellar Purkinje cells. Betz cells are the largest cells (by size of cell body) in the nervous system.[33] The adult human brain is estimated to contain 86±8 billion neurons, with a roughly equal number (85±10 billion) of non-neuronal cells.[34] Out of these neurons, 16 billion (19%) are located in the cerebral cortex (including subcortical white matter), 69 billion (80%) are in the cerebellum.[4][34]
Types of glial cell are astrocytes (including Bergmann glia), oligodendrocytes, ependymal cells (including tanycytes), radial glial cells and microglia. Astrocytes are the largest of the glial cells. They are stellate cells with many processes radiating from their cell bodies. Some of these processes end as perivascular end-feet on capillary walls.[35] The glia limitans of the cortex is made up of astrocyte foot processes that serve in part to contain the cells of the brain.[11]
Unlike other hematopoietic cells of the immune system, mast cells naturally occur in the human brain where they interact with the neuroimmune system.[36] In the brain, mast cells are located in a number of structures that mediate visceral sensory (e.g., pain) or neuroendocrine functions or that are located along the blood–cerebrospinal fluid barrier, including the pituitary stalk, pineal gland, thalamus, and hypothalamus, area postrema, choroid plexus, and in the dural layer of the meninges near meningeal nociceptors.[36] Mast cells serve the same general functions in the body and central nervous system, such as effecting or regulating allergic responses, innate and adaptive immunity, autoimmunity, and inflammation.[36] Across systems, mast cells serve as the main effector cell through which pathogens can affect the gut–brain axis.[37][38]
Cerebrospinal fluid

Cerebrospinal fluid is a clear, colourless transcellular fluid that circulates around the brain in the subarachnoid space, in the ventricular system, and in the central canal of the spinal cord. It also fills some gaps in the subarachnoid space, known as subarachnoid cisterns.[39] The four ventricles, two lateral, a third, and a fourth ventricle, all contain choroid plexus that produces cerebrospinal fluid.[40] The third ventricle lies in the midline and is connnected to the lateral ventricles.[39] A single duct, the cerebral aqueduct between the pons and the cerebellum, connects the third ventricle to the fourth ventricle. Three separate openings, the middle and two lateral apertures, drain the cerebrospinal fluid from the fourth ventricle to the cisterna magna one of the major cisterns. From here, cerebrospinal fluid circulates around the brain and spinal cord in the subarachnoid space, between the arachnoid mater and pia mater.[39]
At any one time, there is about 150mL of cerebrospinal fluid – most within the subarachnoid space. It is constantly being created and reabsorbed, and replaces about once every 5–6 hours.[39] In other parts of the body, circulation in the lymphatic vasculature clears extracellular waste products from the cell tissue.[41] For the tissue of the brain, such a system has not yet been identified.[41] However, the presence of a glymphatic pathway has been proposed.[41]
Blood supply


The internal carotid arteries supply oxygenated blood to the front of the brain and the vertebral arteries supply blood to the back of the brain.[42] These two circulations join together in the circulatory anastomosis known as the circle of Willis, a ring of connected arteries that lies in the interpeduncular cistern between the midbrain and pons.[43]
- The internal carotid arteries are branches of the common carotid arteries. They enter the cranium through the carotid canal, travel through the cavernous sinus and enter the subarachnoid space.[44] They then enter the circle of Willis, with two branches, the anterior cerebral arteries emerging. These branches travel forward and then upward along the great longitudinal fissure, and supply the front and midline parts of the brain.[45] One or more small anterior communicating arteries join the two anterior cerebral arteries shortly after they emerge as branches.[45] The internal carotid arteries continue forward as the middle cerebral arteries. They travel sideways along part of the skull called the sphenoid bone, then upwards through the insula cortex, where final branches arise. The middle cerebral arteries send branches along their length.[44]
- The vertebral arteries emerge as branches of the left and right subclavian arteries. They travel upward through transverse foramina – spaces in the cervical vertebrae and then emerge as two vessels, one on the left and one on the right of the medulla.[44] They join in front of the middle part of the medulla to form the larger basilar artery, which sends multiple branches to supply the medulla and pons, and two branches to the cerebellum.[b] Finally, the basilar artery divides into two posterior cerebral arteries. These travel outwards, around the superior cerebellar peduncles, and along the top of the cerebellar tentorium, where it sends branches to supply the temporal and occipital lobes.[46] Each posterior cerebral artery sends a small posterior communicating artery to join with the internal carotid arteries.
Blood drainage
Veins drain deoxygenated blood from the brain. The brain has two main networks of veins: an exterior network, that rests on the cerebrum and has three branches, and an interior network. These two networks communicate via anastomosing (joining) veins.[47] The veins of the brain drain into larger cavities the dural venous sinuses usually situated between the dura mater and the covering of the skull.[48] Blood from the cerebellum and midbrain drains into the great cerebral vein. Blood from the medulla and pons of the brainstem have a variable pattern of drainage, either into the spinal veins or into adjacent cerebral veins.[47]
The blood in the deep part of the brain drains, through a venous plexus into the cavernous sinus at the front, and the superior and inferior petrosal sinuses at the sides, and the inferior sagittal sinus at the back.[48] Blood drains from the outer brain into the large superior saggital sinus, which rests in the midline on top of the brain. Blood from here joins with blood from the straight sinus at the confluence of sinuses.[48]
Blood from here drains into the left and right transverse sinuses.[48] These then drain into the sigmoid sinuses, which receive blood from the cavernous sinus and superior and inferior petrosal sinuses. The sigmoid drains into the large internal jugular veins.[48][47]
The blood–brain barrier
The larger arteries throughout the brain supply blood to smaller capillaries. These blood vessels are lined with cells joined by tight junctions and so do not let fluids seep in and leak out to the same degree as other parts of the body, creating the blood-brain barrier.[49]The barrier is less permeable to larger molecules, but is still permeable to water, carbon dioxide, oxygen, and most fat-soluble substances (including anaesthetics and alcohol).[49] The blood-brain barrier is not present in areas of the brain that may respond to changes in body fluids, such as the pineal gland, area postrema, and some areas of the hypothalamus.[49] There is a similar blood–cerebrospinal fluid barrier, which serves the same purpose as the blood–brain barrier, but facilitates the transport of different substances into the brain due to the distinct structural characteristics between the two barrier systems.[49][50]
Development
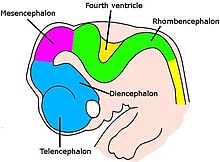

At the beginning of the third week of development, the embryonic ectoderm forms a thickened strip called the neural plate.[51] By the fourth week of development the neural plate has widened to give a broad cephalic end, a less broad middle part and a narrow caudal end. These swellings represent the beginnings of the forebrain, midbrain and hindbrain.[52] Neural crest cells (derived from the ectoderm) populate the lateral edges of the plate at the neural folds. In the fourth week in the neurulation stage the neural plate folds and closes to form the neural tube, bringing together the neural crest cells at the neural crest.[53] The neural crest runs the length of the tube with cranial neural crest cells at the cephalic end and caudal neural crest cells at the tail. Cells detach from the crest and migrate in a craniocaudal (head to tail) wave inside the tube.[53] Cells at the cephalic end give rise to the brain, and cells at the caudal end give rise to the spinal cord.[54]
The tube flexes as it grows, forming the crescent-shaped cerebral hemispheres at the head. The cerebral hemispheres first appear on day 32.[55] Early in the fourth week the cephalic part bends sharply forward in a cephalic flexure.[53] This flexed part becomes the forebrain (prosencephalon); the adjoining curving part becomes the midbrain (mesencephalon) and the part caudal to the flexure becomes the hindbrain (rhombencephalon). In the fifth week of developmement five brain vesicles have formed. The forebrain separates into two vesicles an anterior telencephalon and a posterior diencephalon. The telencephalon gives rise to the cerebral cortex, basal ganglia, and related structures. The diencephalon gives rise to the thalamus and hypothalamus. The hindbrain also splits into two areas – the metencephalon and the mylencephalon. The metencephalon gives rise to the cerebellum and pons. The myelencephalon gives rise to the medulla oblongata.[56] Also during the fifth week, the brain divides into repeating segments called neuromeres.[52][54]
A characteristic of the brain is gyrification (wrinkling of the cortex). In the womb, the cortex starts off as smooth but starts to form fissures that begin to mark out the different lobes of the brain. Scientists do not have a clear answer as to why the cortex later wrinkles and folds, but the wrinkling and folding is associated with intelligence and neurological disorders.[57] The fissures form as a result of the growing hemispheres that increase in size due to a sudden growth in cells of the grey matter. The underlying white matter does not grow at the same rate and the hemispheres are crowded into the small cranial vault.[16] The first cleft to appear in the fourth month is the lateral cerebral fossa. The expanding caudal end of the hemisphere has to curve over in a forward direction to fit into the restricted space. This covers the fossa and turns it into a much deeper ridge known as the lateral sulcus and this marks out the temporal lobe.[55] By the sixth month other sulci have formed that demarcate the frontal, parietal, and occipital lobes.[55] A gene present in the human genome (ArhGAP11B) may play a major role in gyrification and encephalisation.[58]
Function

Motor control
The motor system of the brain is responsible for the generation and control of movement.[59] Generated movements pass from the brain through nerves to motor neurons in the body, which control the action of muscles. The corticospinal tract carries movements from the brain, through the spinal cord, to the torso and limbs.[60] The cranial nerves carry movements related to the eyes, mouth and face.
Gross movement – such as locomotion and the movement of arms and legs – is generated in the motor cortex, divided into three parts: the primary motor cortex, found in the prefrontal gyrus and has sections dedicated to the movement of different body parts. These movements are supported and regulated by two other areas, lying anterior to the primary motor cortex: the premotor area and the supplementary motor area.[61] The hands and mouth have a much larger area dedicated to them than other body parts, allowing finer movement; this has been visualised in a motor cortical homunculus.[61] Impulses generated from the motor cortex travel along the corticospinal tract along the front of the medulla and cross over (decussate) at the medullary pyramids. These then travel down the spinal cord, with most connecting to interneurons, in turn connecting to lower motor neurons within the grey matter that then transmit the impulse to move to muscles themselves.[60] The cerebellum and basal ganglia, play a role in fine, complex and coordinated muscle movements.[62] Connections between the cortex and the basal ganglia control muscle tone, posture and movement initiation, and are referred to as the extrapyramidal system.[63]
Sensory


The sensory nervous system is involved with the reception and processing of sensory information. This information is received through the cranial nerves, through tracts in the spinal cord, and directly at centres of the brain exposed to the blood.[64] The brain also receives and interprets information from the special senses (vision, smell, hearing, and taste). Mixed motor and sensory signals are also integrated.[64]
From the skin, the brain receives information about fine touch, pressure, pain, vibration and temperature. From the joints, the brain receives information about joint position.[65] The sensory cortex is found just near the motor cortex, and, like the motor cortex, has areas related to sensation from different body parts. Sensation collected by a sensory receptor on the skin is changed to a nerve signal, that is passed up a series of neurons through tracts in the spinal cord. The posterior column–medial lemniscus pathway contains information about fine touch, vibration and position of joints. Neurons travel up the back part of the spinal cord to the back part of the medulla, where they connect with "second order" neurons that immediately swap sides. These neurons then travel upwards into the ventrobasal complex in the thalamus where they connect with "third order" neurons, and travel up to the sensory cortex.[65]The spinothalamic tract carries information about pain, temperature, and gross touch. Neurons travel up the spinal cord and connect with second-order neurons in the reticular formation of the brainstem for pain and temperature, and also at the ventrobasal complex of the medulla for gross touch.[66]
Vision is generated by light that hits the retina of the eye. Photoreceptors in the retina transduce the sensory stimulus of light into an electrical nerve signal that is sent to the visual cortex in the occipital lobe. Vision from the left visual field is received on the right side of each retina (and vice versa) and passes through the optic nerve until some information changes sides, so that all information about one side of the visual field passes through tracts in the opposite side of the brain. The nerves reach the brain at the lateral geniculate nucleus, and travel through the optic radiation to reach the visual cortex.[67]
Hearing and balance are both generated in the inner ear. The movement of liquids within the inner ear is generated by motion (for balance) and transmitted vibrations generated by the ossicles (for sound). This creates a nerve signal that passes through the vestibulocochlear nerve. From here, it passes through to the cochlear nuclei, the superior olivary nucleus, the medial geniculate nucleus, and finally the auditory radiation to the auditory cortex.[68]
The sense of smell is generated by receptor cells in the epithelium of the olfactory mucosa in the nasal cavity. This information passes through a relatively permeable part of the skull to the olfactory nerve. This nerve transmits to the neural circuitry of the olfactory bulb from where information is passed to the olfactory cortex.[69][70]
Taste is generated from receptors on the tongue and passed along the facial and glossopharyngeal nerves into the solitary tract in the brainstem. Some taste information is also passed from the pharynx into this area via the vagus nerve. Information is then passed from here through the thalamus into the gustatory cortex.[71]
Regulation
Autonomic functions of the brain include the regulation, or control of the heart rate and rate of breathing, and maintaining homeostasis.
Blood pressure and heart rate are influenced by the vasomotor centre of the medulla, which causes arteries and veins to be somewhat constricted at rest. It does this by influencing the sympathetic nervous system and parasympathetic via the vagus nerve.[72] Information about blood pressure is generated by baroreceptors in aortic bodies in the aortic arch, and passed to the brain along the afferent fibres of the vagus nerve. Information about the pressure changes in the carotid sinus comes from carotid bodies located near the carotid artery and this is passed via a nerve joining with the glossopharyngeal nerve. This information travels up to the solitary nucleus in the medulla. Signals from here influence the vasomotor centre to adjust vein and artery constriction accordingly.[72]
The brain controls the rate of breathing, mainly by respiratory centres in the medulla and pons, in the brainstem.[73] The respiratory centres control respiration, by generating motor signals that are passed down the spinal cord, along the phrenic nerve to the diaphragm and other muscles of respiration. This is a mixed nerve that carries sensory information back to the centres. There are four respiratory centres. In the medulla a dorsal respiratory group causes the desire to breathe in and receives sensory information directly from the body. Also in the medulla, the ventral respiratory group influences breathing out during exertion. In the pons the pneumotaxic centre influences the duration of each breath,[74] and the apneustic centre has an influence on inhalation. The respiratory centre directly senses blood carbon dioxide and pH. Information about blood oxygen, carbon dioxide and pH levels are also sensed on the walls of arteries in the aortic bodies and carotid bodies. This information stimulates chemoreceptors in the arteries, which pass information again via the vagus nerve and glossopharyngeal nerves to the respiratory nucleus. High carbon dioxide, an acidic pH, or low oxygen stimulate the respiratory centre.[74] The desire to breathe in is also affected by pulmonary stretch receptors in the lungs which, when activated, prevent the lungs from overinflating by transmitting information to the respiratory centres via the vagus nerve.[74]
The brain, especially the hypothalamus, is heavily involved in regulating many functions of the body. The diencephalon include the neuroendocrine regulation, regulation of the circadian rhythm, control of the autonomic nervous system, regulation of fluid homeostasis, and food intake. The circadian rhythm is controlled by two main cell groups in the rostral and caudal hypothalamus. The rostral hypothalamus includes the suprachiasmatic nucleus, which through gene expression cycles generates roughly 24 long clock, and Ventrolateral preoptic nucleus. The caudal hypothalamus contains orexinergic neurons that control arousal through their projections to the ascending reticular activating system.[75] The hypothalamus controls the pituitary gland through the release of peptides such as oxytocin, and vasopressin, as well as dopamine into the median eminence. The hypothalamus influences the autonomic nervous system, through ascending projections into autonomic cell groups in the brain stem. Through the autonomic projections, the hypothalamus is involved in regulating functions such as blood pressure, heart rate, breathing, sweating, and other homeostatic mechanisms.[76] The hypothalamus also plays a role in thermal regulation, and when stimulated by the immune system, is capable of generating a fever. The hypothalamus is influenced by the kidneys – when blood pressure falls, the renin released by the kidneys stimulates a need to drink. The hypothalamus also regulates food intake through autonomic signals, and hormone release by the digestive system.[77]
Language

While language functions were traditionally thought to be localized to Wernicke's area and Broca's area,[78] it is now mostly accepted that a wider network of cortical regions contributes to language use.[79][80][81] The study of how language is represented, processed, and acquired by the brain is neurolinguistics, which is a large multidisciplinary field drawing from cognitive neuroscience, cognitive linguistics, and psycholinguistics.[82]
Lateralisation
Each hemisphere of the brain interacts primarily with one half of the body: the left side of the brain interacts with the right side of the body, and vice versa. The developmental cause for this is uncertain.[83] Motor connections from the brain to the spinal cord, and sensory connections from the spinal cord to the brain, both cross sides in the brainstem. Visual input follows a more complex rule: the optic nerves from the two eyes come together at a point called the optic chiasm, and half of the fibres from each nerve split off to join the other.[84] The result is that connections from the left half of the retina, in both eyes, go to the left side of the brain, whereas connections from the right half of the retina go to the right side of the brain.[85] Because each half of the retina receives light coming from the opposite half of the visual field, the functional consequence is that visual input from the left side of the world goes to the right side of the brain, and vice versa.[83] Thus, the right side of the brain receives somatosensory input from the left side of the body, and visual input from the left side of the visual field.[86][87]
The left and right sides of the brain appear symmetrical, but they function asymmetrically.[88] For example, the counterpart of the left-hemisphere motor area controlling the right hand is the right-hemisphere area controlling the left hand. There are, however, several important exceptions, involving language and spatial cognition. The left frontal lobe is dominant for language. If a key language area in the left hemisphere is damaged, it can leave the victim unable to speak or understand,[88] whereas equivalent damage to the right hemisphere would cause only minor impairment to language skills.
A substantial part of current understanding of the interactions between the two hemispheres has come from the study of "split-brain patients"—people who underwent surgical transection of the corpus callosum in an attempt to reduce the severity of epileptic seizures.[89] These patients do not show unusual behavior that is immediately obvious, but in some cases can behave almost like two different people in the same body, with the right hand taking an action and then the left hand undoing it.[89][90] These patients, when briefly shown a picture on the right side of the point of visual fixation, are able to describe it verbally, but when the picture is shown on the left, are unable to describe it, but may be able to give an indication with the left hand of the nature of the object shown.[90][91]
Emotion
Emotions are generally defined as two-step multicomponent processes involving elicitation, followed by psychological feelings, appraisal, expression, autonomic responses, and action tendencies.[92] Attempts to localize basic emotions to certain brain regions have been controversial, with some research finding no evidence for specific locations corresponding to emotions, and instead circuitry involved in general emotional processes. The amygdala, orbitofrontal cortex, mid and anterior insula cortex and lateral prefrontal cortex, appeared to be involved approach related emotions, while weaker evidence was found for the ventral tegmental area, ventral pallidum and nucleus accumbens in incentive salience.[93] Others, however, have found evidence of activation of specific regions, such as the basal ganglia in happiness, the subcallosal cingulate cortex in sadness, and amygdala in fear.[94]
Executive function
Executive function is an umbrella term for the set of cognitive processes needed to allow the cognitive control of behavior: selecting and successfully monitoring behaviors that facilitate the attainment of chosen goals.[95][96][97] Executive functions include the ability to filter information and tune out irrelevant stimuli with attentional control and cognitive inhibition, the ability to process and manipulate information held in working memory, the ability to think about multiple concepts simultaneously and switch tasks with cognitive flexibility, the ability to inhibit impulses and prepotent responses with inhibitory control, and the ability to determine the relevance of information or appropriateness of an action.[95][96][98] Higher order executive functions, which require the simultaneous use of multiple executive functions, include planning and fluid intelligence (i.e., reasoning and problem solving).[96][97]
The prefrontal cortex plays a significant role in mediating executive functions.[96][99][100] Neuroimaging during neuropsychological tests of executive function, such as the stroop test and working memory tests, have found that cortical maturation of the prefrontal cortex correlates with executive function in children.[99][100] Planning involves activation of the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex, angular prefrontal cortex, right prefrontal cortex, and supramarginal gyrus.[99] Working memory manipulation involves the DLPFC, inferior frontal gyrus, and areas of the parietal cortex.[99][100] Inhibitory control involves multiple areas of the prefrontal cortex as well as the caudate nucleus and subthalamic nucleus.[96][99][100] Task shifting doesn't involve specific regions of the brain, but instead involves multiple regions of the prefrontal cortex and parietal lobe.[99]
Physiology
Neurotransmission
Brain activity is made possible by the interconnections of neurons that are linked together to reach their targets. Neurons consist of cell bodies, axons, and dendrites. Individual axons join together with other neurons at a synapse. At a synapse, neurotransmitters are released to convey a signal. These chemicals include dopamine, seratonin, glutamate, and acetylcholine.[101]
Together, groups of neurons form tracts, and neural pathways, and circuits.
Metabolism

The brain consumes up to twenty percent of the energy used by the human body, more than any other organ.[102] Brain metabolism normally relies upon blood glucose as an energy source, but during times of low glucose (such as fasting, endurance exercise, or limited carbohydrate intake), the brain uses ketone bodies for fuel with a smaller need for glucose. The brain can also utilize lactate during exercise.[103] Long-chain fatty acids cannot cross the blood–brain barrier, but the liver can break these down to produce ketone bodies. However, short-chain fatty acids (e.g., butyric acid, propionic acid, and acetic acid) and the medium-chain fatty acids, octanoic acid and heptanoic acid, can cross the blood–brain barrier and be metabolized by brain cells.[104][105][106] The brain stores glucose in the form of glycogen, albeit in significantly smaller amounts than that found in the liver or skeletal muscle.[107]
Although the human brain represents only 2% of the body weight, it receives 15% of the cardiac output, 20% of total body oxygen consumption, and 25% of total body glucose utilization.[108] The brain mostly uses glucose for energy, and deprivation of glucose, as can happen in hypoglycemia, can result in loss of consciousness.[109] The energy consumption of the brain does not vary greatly over time, but active regions of the cortex consume somewhat more energy than inactive regions: this fact forms the basis for the functional brain imaging methods PET and fMRI.[110] These functional imaging techniques provide a three-dimensional image of metabolic activity.[111]
Research
The brain is not fully understood, and research is ongoing.[112] Neuroscientists, along with researchers from allied disciplines, study how the human brain works. The boundaries between the specialties of neuroscience, neurology and other disciplines such as psychiatry have faded as they are all influenced by basic research in neuroscience.
Neuroscience research has expanded considerably in recent decades. The "Decade of the Brain", an initiative of the United States Government in the 1990s, is considered to have marked much of this increase in research,[113] and was followed in 2013 by the BRAIN Initiative.[114] The Human Connectome Project was a five-year study launched in 2009 to analyse the anatomical and functional connections of parts of the brain, and has provided much data.[112]
Methods
Information about the structure and function of the human brain comes from a variety of experimental methods, including animals and humans. Information about brain trauma and stroke has provided information about the function of parts of the brain and the effects of brain damage. Neuroimaging is used to visualise the brain and record brain activity. Electrophysiology is used to measure, record and monitor the electrical activity of the cortex. Measurements may be of local field potentials of cortical areas, or of the activity of a single neuron. An electroencephalogram can record the electrical activity of the cortex using electrodes placed non-invasively on the scalp.[115]
Invasive measures include electrocorticography, which uses electrodes placed directly on the exposed surface of the brain. This method is used in cortical stimulation mapping, used in the study of the relationship between cortical areas and their systemic function.[116] By using much smaller microelectrodes, single-unit recordings can be made from a single neuron that give a high spatial resolution and high temporal resolution. This has enabled the linking of brain activity to behaviour, and the creation of neuronal maps.[117]
Imaging
Functional neuroimaging techniques show changes in brain activity that relate to the function of specific brain areas. One technique is functional magnetic resonance imaging (fMRI) which has the advantages over earlier methods of SPECT and PET of not needing the use of radioactive materials and of offering a higher resolution.[118] Another technique is functional near-infrared spectroscopy. This method relies on the haemodynamic response that shows changes in brain activity in relation to changes in blood flow, useful in mapping functions to brain areas.[119]
Any electrical current generates a magnetic field; neural oscillations induce weak magnetic fields, used in functional magnetoencephalography.[120] Tractography uses MRI and image analysis to create 3D images of the nerve tracts of the brain. Connectograms give a graphical representation of the neural connections of the brain.[121]
Differences in brain area volumes are seen in some disorders, notably schizophrenia and dementia. This area of research is known as brain morphometry. Different biological approaches using imaging have given more insight for example into the disorders of depression and obsessive-compulsive disorder. A key source of information about the function of brain regions is the effects of damage to them.[122]
Advances in neuroimaging have enabled objective insights into mental disorders, leading to faster diagnosis, more accurate prognosis, and better monitoring.[123]
Clinical significance
General
Brain damage, or disease of the brain can manifest in a wide variety of ways. Traumatic brain injury, for example in contact sport, after a fall, or in traffic or work accidents, can be associated with both immediate and longer-term problems. Immediate problems that develop may include bleeding within the skull, compressing the brain tissue or damaging its blood supply, skull fractures, injury to a particular area, deafness, and concussion. In addition to the site of injury, the opposite side of the brain may be affected, termed a contrecoup injury. Longer-term issues that may develop include post-traumatic stress, hydrocephalus, and chronic traumatic encephalopathy.[124]
Neurodegenerative diseases result in progressive damage to different parts of the brain's function, and progressively worsen with age. Common examples include dementia such as Alzheimer's disease, alcoholic dementia or vascular dementia; Parkinson's disease; and other rarer infectious, genetic, or metabolic causes such as Huntington's disease, motor neuron diseases, HIV dementia, syphilis-related dementia and Wilson's disease. Neurodegenerative diseases can affect different parts of the brain, and can affect movement, memory, and cognition.[125]
The brain, although protected by the blood-brain barrier, can be affected by infections including viruses, bacteria and fungi. Infection may be of the meninges (meningitis), the brain matter (encephalitis), or within the brain matter (such as a cerebral abscess).[126] Rare prion diseases including Creutzfeldt–Jakob disease and its variant, and kuru may also affect the brain.[126]
The most common cancers in the brain come from elsewhere in the body – most commonly the lung, breast and skin.[127] Cancers of brain tissue can also occur, and originate from any tissue in and around the brain. Meningioma, cancer of the meninges around the brain, is more common than cancers of brain tissue.[127] Cancers within the brain may cause symptoms related to their size or position, with symptoms including headache and nausea, or the gradual development of focal symptoms such as gradual difficulty seeing, swallowing, talking, or as a change of mood.[127] Cancers are in general investigated through the use of CT scans and MRI scans. A variety of other tests including blood tests and lumbar puncture may be used to investigate for the cause of the cancer and evaluate the type and stage of the cancer.[127] The corticosteroid dexamethasone is often given to decrease the swelling of brain tissue around a tumour. Surgery may be considered, however given the complex nature of many tumours or based on tumour stage or type, radiotherapy or chemotherapy may be considered more suitable.[127]
Mental disorders, such as major depressive disorder, schizophrenia, bipolar disorder, post-traumatic stress disorder, attention deficit hyperactivity disorder, obsessive-compulsive disorder, Tourette syndrome, and addiction, are known to relate to the functioning of the brain.[100][101][128] Treatment for mental disorders may include psychotherapy, psychiatry, social intervention and personal recovery work or cognitive behavioural therapy; the underlying issues and associated prognoses vary significantly between individuals.[129]
Epileptic seizures are thought to relate to abnormal electrical activity.[130] Seizure activity can manifest as absence (of consciousness), focal effects such as limb movement or impediments of speech, or be generalized in nature.[130] Status epilepticus refers to a seizure or series of seizures that have not terminated within 30 minutes,[130] although this definition has recently been revised.[131] Seizures have a large number of causes, however many seizures occur without a definitive cause being found. In a person with epilepsy, risk factors for further seizures may include sleeplessness, drug and alcohol intake, and stress. Seizures may be assessed using blood tests, EEG and various medical imaging techniques based on the medical history and exam findings.[130] In addition to treating an underlying cause and reducing exposure to risk factors, anticonvulsant medications can play a role in preventing further seizures.[130]
Many brain disorders are congenital, such as Tay–Sachs disease, and these are all linked to genetic and chromosomal mutations. A rare group of congenital cephalic disorders known as lissencephaly are characterised by the lack of, or inadequacy of cortical folding. Normal development of the brain can be affected during pregnancy by nutritional deficiencies, teratogens, infectious diseases and by the use of recreational drugs and alcohol.
Stroke
A stroke is a decrease in blood supply to an area of the brain causing cell death and brain injury. This can lead to a wide range of stroke symptoms, including the "FAST" symptoms of facial droop, arm weakness, and speech difficulties (including difficulties speaking and difficulties finding words or forming sentences).[132] Symptoms relate to the function of the affected area of the brain and can point to the likely site and cause of the stroke. Difficulties with movement, speech, or sight usually relate to the cerebrum, whereas imbalance, double vision, vertigo and symptoms affecting more than one side of the body usually relate to the brainstem or cerebellum.[133]
Most strokes result from loss of blood supply, typically because of an embolus, rupture of a fatty plaque or narrowing of small arteries. Strokes can also result from bleeding within the brain.[134] Transient ischemic attacks (TIAs) are strokes in which symptoms resolve within 24 hours.[134] Investigation into the stroke will involve a medical examination (including a neurological examination) and the taking of a medical history, focusing on the duration of the symptoms and risk factors (including hypertension, atrial fibrillation, and smoking).[135][136] Further investigation is needed in younger patients.[135] An ECG and telemetry may be conducted to identify atrial fibrillation, an ultrasound to investigate for narrowing of the carotid arteries, and an echocardiogram to investigate for clots within the heart, diseases of the heart valves or a patent foramen ovale that may point to a cardiac cause.[135] Blood tests are routinely done as part of the workup including diabetes tests and a lipid profile.[135]
Some treatments for stroke are time-critical. These include clot dissolution or surgical removal of a clot for ischaemic strokes, and decompression for haemorrhagic strokes.[137][138] As stroke is time critical,[139] hospitals and even pre-hospital care of stroke involves expedited investigations – usually a CT scan to investigate for a haemorrhagic stroke and a CT or MR angiogram to evaluate arteries that supply the brain.[135] MRI scans, not as widely available, may be able to demonstrate the affected area of the brain more accurately, particularly with ischaemic stroke.[135]
Having experienced a stroke, a person may be admitted to a stroke unit, and treatments may be directed as preventing future strokes, including ongoing anticoagulation (such as aspirin or clopidogrel), antihypertensives, and lipid-lowering drugs.[137] A multidisciplinary team including speech pathologists, physiotherapists, occupational therapists, psychologists play a large role in supporting a person affected by a stroke and their rehabilitation.[140][135]
Brain death
Brain death refers to an irreversible total loss of brain function.[141][142][143] This is characterised by coma, loss of reflexes, and apnoea,[142][141] however, the declaration of brain death varies geographically and is not always accepted.[143] In some countries there is also a defined syndrome of brainstem death.[144] Declaration of brain death can have profound implications as the declaration, under the principle of medical futility, will be associated with the withdrawal of life support,[145] and as those with brain death often have organs suitable for organ donation.[143][146] The process is often made more difficult by poor communication with patients' families.[141]
When brain death is suspected, reversible differential diagnoses such as hypothermia-induced coma, electrolyte, neurological and drug-related cognitive suppression are first excluded.[142][145] Testing for reflexes[c] can be of help in the decision, as can the absence of response and breathing.[145] Clinical observations, including a total lack of responsiveness, a known diagnosis, and neural imaging evidence, may all play a role in the decision to pronounce brain death.[142]
Society and culture
Neuroanthropology is the study of the relationship between culture and the brain. It explores how the brain gives rise to culture, and how culture influences brain development.[147] Cultural differences and their relation to brain development and structure are researched in different fields.[148]
The mind
The philosophy of the mind studies such issues as the problem of understanding consciousness and the mind–body problem. The relationship between the brain and the mind, is a significant challenge both philosophically and scientifically. This is because of the difficulty reconciling how subjective mental activities, such as thoughts and emotions, can be implemented by physical structures such as neurons and synapses, or by any other type of physical mechanism. This difficulty was expressed by Gottfried Leibniz in an analogy known as Leibniz's Mill:
One is obliged to admit that perception and what depends upon it is inexplicable on mechanical principles, that is, by figures and motions. In imagining that there is a machine whose construction would enable it to think, to sense, and to have perception, one could conceive it enlarged while retaining the same proportions, so that one could enter into it, just like into a windmill. Supposing this, one should, when visiting within it, find only parts pushing one another, and never anything by which to explain a perception.
- — Leibniz, Monadology[149]
Doubt about the possibility of a mechanistic explanation of thought drove René Descartes, and most of humankind along with him, to dualism: the belief that the mind is to some degree independent of the brain.[150] There has always, however, been a strong argument in the opposite direction. There is clear empirical evidence that physical manipulations of, or injuries to, the brain (for example by drugs or by lesions, respectively) can affect the mind in potent and intimate ways.[151] For example, a person suffering from Alzheimer's disease – a condition that causes physical damage to the brain – also experiences a compromised mind. Similarly, someone who has taken a psychedelic drug may temporarily lose their sense of personal identity (ego death) or experience profound changes to their perception and thought processes. Likewise, a patient with epilepsy who undergoes cortical stimulation mapping with electrical brain stimulation would also, upon stimulation of his or her brain, experience various complex feelings, hallucinations, memory flashbacks, and other complex cognitive, emotional, or behavioral phenomena.[152] Following this line of thinking, a large body of empirical evidence for a close relationship between brain activity and mental activity has led most neuroscientists and contemporary philosophers to be materialists, believing that mental phenomena are ultimately the result of, or reducible to, physical phenomena.[153]
Brain size
The size of the brain and a person's intelligence are not strongly related.[154] Studies tend to indicate small to moderate correlations (averaging around 0.3 to 0.4) between brain volume and IQ.[155] The most consistent associations are observed within the frontal, temporal, and parietal lobes, the hippocampi, and the cerebellum, but these only account for a relatively small amount of variance in IQ, which itself has only a partial relationship to general intelligence and real-world performance.[156][157]
Other animals, including whales and elephants have larger brains than humans. However, when the brain-to-body mass ratio is taken into account, the human brain is almost twice as large as that of a bottlenose dolphin, and three times as large as that of a chimpanzee. Very small animals do have higher ratios: the treeshrew has the largest quotient of any mammal, so absolute size does play some role in intelligence.[158]
Common myths
Research has disproved some common misconceptions about the brain. These include both ancient and modern myths. It is not true that neurons are not replaced after the age of two; nor that only ten per cent of the brain is used. [159]
History
Early history

The Edwin Smith Surgical Papyrus, an ancient Egyptian treatise written in the 17th century BC, contains the earliest recorded reference to the brain. The hieroglyph for brain, occurring eight times in this papyrus, describes the symptoms, diagnosis, and prognosis of two traumatic injury to the head. References include to the external surface of the brain, the effects of injury (including seizures and aphasia), the meninges, and cerebrospinal fluid.[160][161]
In the sixth and fifth centuries BC, Ancient Greek Pythagorean Alcmaeon of Croton first considered the brain to be the place where the mind was located.[161] In the fourth century BC Hippocrates, believed the brain to be the seat of intelligence, and Aristotle, although mistaken about the function of the brain,[d] described the meninges and distinguished between the cerebrum and cerebellum.[163] Herophilus of Chalcedon in the third and second centuries BC distinguished the cerebrum and the cerebellum, and provided the first clear description of the ventricles; and Erasistratus of Ceos experimented on living brains. Their works are now mostly lost, and we know about their achievements due mostly to secondary sources. Some of their discoveries had to be re-discovered a millennium after their death.[161] Anatomist physician Galen in the second century AD, during the time of the Roman Empire, dissected the brains of sheep, monkeys, dogs, and pigs. He concluded that, as the cerebellum was denser than the brain, it must control the muscles, while as the cerebrum was soft, it must be where the senses were processed. Galen further theorized that the brain functioned by movement of animal spirits through the ventricles.[161][162]
Renaissance
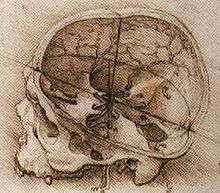
A revolution took place in both neurology in particular and in anatomy in general when Andreas Vesalius published his De humani corporis fabrica in 1543.[164] Vesalius overturned the more than a millennia of stagnation following the ancient Roman and Greek discoveries,[165][166] with only Mondino de Luzzi's Anathomia in 1316 making notable contributions in the interim.[167] It includes detailed images depicting the ventricles, cranial nerves, pituitary gland, meninges, structures of the eye, the vascular supply to the brain and spinal cord, and an image of the peripheral nerves.[168] Vesalius rejected work, such as the rete mirabile.[e][169] Vesalius rejected the common belief that the ventricles were responsible for brain function, arguing that many animals have similar ventricular system to humans, but no true intelligence.[164] This followed work by Niccolò Massa who in 1536 discovered that the ventricles were in fact filled with fluid.[170] Anatomist Archiangelo Piccolomini of Rome was first to distinguish anatomically between the cerebrum and cerebral cortex.[164]
René Descartes proposed the theory of dualism to tackle the issue of the brain's relation to the mind. He suggested that the pineal gland was where the mind interacted with the body after recording the brain mechanisms responsible for circulating cerebrospinal fluid.[170] This dualism likely provided impetus for later anatomists to further explore the relationship between the anatomical and functional aspects of brain anatomy.[171]
Thomas Willis is considered a second pioneer in the study of neurology and brain science. In 1664 in Cerebri Anatome (Latin: Anatomy of the brain),[f] followed by Cerebral Pathology in 1667. In these he described the structure of the cerebellum, the ventricles, the cerebral hemispheres, the brainstem, and the cranial nerves, studied its blood supply; and proposed functions associated with different areas of the brain.[164] The circle of Willis was named after his investigations into the blood supply of the brain, and he was the first to use the word "neurology."[172] Willis removed the brain from the body when examining it, and rejected the commonly held view that the cortex only consisted of blood vessels and the view of the last two millennia that the cortex was only incidentally important.[164]
In the late 19th century, Emil du Bois-Reymond and Hermann von Helmholtz, following the work of their teacher Johannes Peter Müller showed the electrical inpulses which pass along nerves; but unlike Müller's views, that such impulses were able to be observed.[173] Richard Caton in 1875 demonstrated electrical in the cerebral hemispheres of rabbits and monkeys.[174] In the 1820s, Jean Pierre Flourens pioneered the experimental method of damaging specific parts of animal brains describing the effects on movement and behavior.[175]
Modern period

Studies of the brain became more sophisticated after the invention of the microscope and the development of the a staining procedure by Camillo Golgi during the late 1890s that used a silver chromate salt to reveal the intricate structures of single neurons.[176] This was used by Santiago Ramón y Cajal and led to the formation of the neuron doctrine, a revolutionary hypothesis that the neuron is the functional unit of the brain that is now of critical importance in our understanding of the modern brain. He used microscopy to uncover many cell types and proposed functions for the cells he saw.[176] For this, Golgi and Cajal are considered the founders of twentieth century neuroscience, both sharing the Nobel prize in 1906 for their studies and discoveries in this field.[176]
Charles Sherrington published his influential 1906 work The Integrative Action of the Nervous System examining the function of reflexes, evolutionary development of the nervous system, functional specialisation of the brain, and layout and cellular function of the central nervous system.[177] John Farquhar Fulton, founded the Journal of Neurophysiology and published the first comprehensive textbook on the physiology of the nervous system during 1938.[178] Neuroscience during the twentieth century began to be recognized as a distinct unified academic discipline, with David Rioch, Francis O. Schmitt, and Stephen Kuffler playing critical roles in establishing the field.[179] Rioch originated the integration of basic anatomical and physiological research with clinical psychiatry at the Walter Reed Army Institute of Research, starting in the 1950s.[180] During the same period, Schmitt established a neuroscience research program, an inter-university and international organisation, bringing together biology, medicine, psychological and beahvioural sciences. The word neuroscience itself arises from this program.[181]
Paul Broca associated regions of the brain with specific functions following work on brain-damaged patients.[182] John Hughlings Jackson, described the function of the motor cortex by watching the progression of seizures through the body in patients with epilepsy. Carl Wernicke described regions associated with language comprehension and production. Korbinian Brodmann's divided regions of the brain based on the appearance of cells.[182] Sherrington, Papez and MacLean had identified many of the brainstem and limbic system functions mainly due to preparations from laboratory animals by 1950.[183] The capacity of the brain to re-organise and change with age, and a recognised critical development period, were attributed to neuroplasticity, pioneered by Margaret Kennard, who experimented on monkeys during the 1930-40s.[184]
Harvey Cushing is recognised as the first proficient brain surgeon within the entire world.[185] Walter Dandy began the practice of vascular neurosurgery in the modern understanding during 1937, when he performed the very first surgical clipping of an intracranial aneurysm.[186]
Comparative anatomy
The human brain has many properties that are common to all vertebrate brains,[187] and as a mammal, shares many features common to all mammalian brains,[188] most notably a six-layered cerebral cortex and a set of associated structures,[189] including the hippocampus and amygdala.[190] The cortex is proportionally larger in greater mammals and humans than many other animals.[191] Humans have more association cortex, sensory and motor parts relative to simpler mammals, such as the rat and the cat.[192]
As a primate brain, the human brain has a much larger cerebral cortex, in proportion to body size, than most mammals,[190] and a very highly developed visual system.[193][194]
As a hominid brain, the human brain is substantially enlarged even in comparison to the brain of a typical monkey. The sequence of human evolution from Australopithecus (four million years ago) to Homo sapiens (modern man) was marked by a steady increase in brain size.[195][196] As brain size increased, this altered the size and shape of the skull,[197] from about 600 cm3 in Homo habilis to an average of about 1520 cm3 in Homo neanderthalensis.[198] Differences in DNA, gene expression and gene–environment interactions help explain the differences between the function of the human brain and primates.[199]
See also
References
- ^ "Cerebrum Etymology". dictionary.com. Retrieved October 24, 2015.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help) - ^ "Encephalo- Etymology". Online Etymology Dictionary. Retrieved October 24, 2015.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Parent, A; Carpenter MB (1995). "Ch. 1". Carpenter's Human Neuroanatomy. Williams & Wilkins. ISBN 978-0-683-06752-1.
- ^ a b Neuroimaging Genetics: Principles and Practices. Oxford University Press. 2015. p. 157. ISBN 0199920222. Retrieved January 2, 2016.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Cosgrove, KP; Mazure CM; Staley JK (2007). "Evolving knowledge of sex differences in brain structure, function, and chemistry". Biol Psychiat. 62 (8): 847–855. doi:10.1016/j.biopsych.2007.03.001. PMC 2711771. PMID 17544382.
- ^ Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE (1999). "Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance". The Journal of Neuroscience. 19 (10): 4065–4072. PMID 10234034.
- ^ a b c Gray's Anatomy 2008, p. 227-9.
- ^ a b Gray's Anatomy 2008, p. 335-7.
- ^ a b c Gray's Anatomy 2008, p. 298.
- ^ Purves, Dale (2011). Neuroscience (5. ed.). Sunderland, Mass.: Sinauer. p. 742. ISBN 978-0-87893-695-3.
- ^ a b Cipolla, Marilyn J. (January 1, 2009). "Anatomy and Ultrastructure". Morgan & Claypool Life Sciences.
- ^ My Yajna: Recollections, Notes, and Essays (1. ed.). Quills Ink Publishing. 2014. ISBN 978-93-84318-05-5.
{{cite book}}:|first1=missing|last1=(help) - ^ Gray's Anatomy 2008, p. 227-229.
- ^ Graham Davey (2011). Applied Psychology. John Wiley & Sons. p. 153. ISBN 1444331213. Retrieved January 21, 2017.
- ^ Kandel, ER; Schwartz JH; Jessel TM (2000). Principles of Neural Science. McGraw-Hill Professional. p. 324. ISBN 978-0-8385-7701-1.
- ^ a b Ackerman, Sandra (1992). Discovering the brain. Washington, D.C.: National Academy Press. pp. 22–25. ISBN 0-309-04529-0.
- ^ Guyton & Hall 2011, p. 574.
- ^ Guyton & Hall 2011, p. 667.
- ^ Principles of Anatomy and Physiology 12th Edition – Tortora,Page 519.
- ^ a b c Laura Freberg (2009). Discovering Biological Psychology. Cengage Learning. pp. 44–46. ISBN 0547177798. Retrieved January 25, 2017.
- ^ a b Fundamentals of Human Neuropsychology. Macmillan. 2009. pp. 73–75. ISBN 0716795868. Retrieved January 25, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Pocock, Gillian; Richards, Christopher D. (2006). Human physiology : the basis of medicine (3rd ed. ed.). Oxford: Oxford University Press. p. 64. ISBN 978-0-19-856878-0.
{{cite book}}:|edition=has extra text (help) - ^ a b Purves, Dale (2011). Neuroscience (5. ed. ed.). Sunderland, Mass.: Sinauer. pp. 399–402. ISBN 978-0-87893-695-3.
{{cite book}}:|edition=has extra text (help) - ^ Gray's Anatomy 2008, p. 325-6.
- ^ Guyton & Hall 2011, p. 699.
- ^ Netter, Frank (2014). Atlas of Human Anatomy Including Student Consult Interactive Ancillaries and Guides (6th ed.). Philadelphia, Penn.: W B Saunders Co. p. 114. ISBN 978-1-4557-0418-7.
- ^ a b Gray's Anatomy 2008, p. 297.
- ^ Gray's Anatomy 2008, p. 243.
- ^ Guyton & Hall 2011, p. 698-9.
- ^ Squire, Larry (2013). Fundamental neuroscience (4th ed.). Amsterdam: Elsevier/Academic Press. pp. 761–763. ISBN 9780123858702.
- ^ a b c d e f Gray's Anatomy 2008, p. 275.
- ^ Guyton & Hall 2011, p. 691.
- ^ Purves, Dale (2011). Neuroscience (5. ed. ed.). Sunderland, Mass.: Sinauer. p. 377. ISBN 978-0-87893-695-3.
{{cite book}}:|edition=has extra text (help) - ^ a b Azevedo, Frederico A.C.; Carvalho, Ludmila R.B.; Grinberg, Lea T.; Farfel, José Marcelo; Ferretti, Renata E.L.; Leite, Renata E.P.; Filho, Wilson Jacob; Lent, Roberto; Herculano-Houzel, Suzana (April 10, 2009). "Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain". The Journal of Comparative Neurology. 513 (5): 532–541. doi:10.1002/cne.21974. ISSN 1096-9861.
despite the widespread quotes that the human brain contains 100 billion neurons and ten times more glial cells, the absolute number of neurons and glial cells in the human brain remains unknown. Here we determine these numbers by using the isotropic fractionator and compare them with the expected values for a human-sized primate. We find that the adult male human brain contains on average 86.1 ± 8.1 billion NeuN-positive cells ("neurons") and 84.6 ± 9.8 billion NeuN-negative ("nonneuronal") cells.
- ^ Pavel, Fiala; Jiří, Valenta (January 1, 2013). "Central Nervous System". Karolinum Press.
- ^ a b c Polyzoidis S, Koletsa T, Panagiotidou S, Ashkan K, Theoharides TC (2015). "Mast cells in meningiomas and brain inflammation". J Neuroinflammation. 12 (1): 170. doi:10.1186/s12974-015-0388-3. PMC 4573939. PMID 26377554.
MCs originate from a bone marrow progenitor and subsequently develop different phenotype characteristics locally in tissues. Their range of functions is wide and includes participation in allergic reactions, innate and adaptive immunity, inflammation, and autoimmunity [34]. In the human brain, MCs can be located in various areas, such as the pituitary stalk, the pineal gland, the area postrema, the choroid plexus, thalamus, hypothalamus, and the median eminence [35]. In the meninges, they are found within the dural layer in association with vessels and terminals of meningeal nociceptors [36]. MCs have a distinct feature compared to other hematopoietic cells in that they reside in the brain [37]. MCs contain numerous granules and secrete an abundance of prestored mediators such as corticotropin-releasing hormone (CRH), neurotensin (NT), substance P (SP), tryptase, chymase, vasoactive intestinal peptide (VIP), vascular endothelial growth factor (VEGF), TNF, prostaglandins, leukotrienes, and varieties of chemokines and cytokines some of which are known to disrupt the integrity of the blood-brain barrier (BBB) [38–40].
[The] key role of MCs in inflammation [34] and in the disruption of the BBB [41–43] suggests areas of importance for novel therapy research. Increasing evidence also indicates that MCs participate in neuroinflammation directly [44–46] and through microglia stimulation [47], contributing to the pathogenesis of such conditions such as headaches, [48] autism [49], and chronic fatigue syndrome [50]. In fact, a recent review indicated that peripheral inflammatory stimuli can cause microglia activation [51], thus possibly involving MCs outside the brain.{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Budzyński J, Kłopocka M (2014). "Brain-gut axis in the pathogenesis of Helicobacter pylori infection". World J. Gastroenterol. 20 (18): 5212–25. doi:10.3748/wjg.v20.i18.5212. PMC 4017036. PMID 24833851.
In digestive tissue, H. pylori can alter signaling in the brain-gut axis by mast cells, the main brain-gut axis effector
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Carabotti M, Scirocco A, Maselli MA, Severi C (2015). "The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems". Ann Gastroenterol. 28 (2): 203–209. PMC 4367209. PMID 25830558.
- ^ a b c d Gray's Anatomy 2008, pp. 242–244.
- ^ Purves, Dale (2011). Neuroscience (5. ed. ed.). Sunderland, Mass.: Sinauer. p. 742. ISBN 978-0-87893-695-3.
{{cite book}}:|edition=has extra text (help) - ^ a b c Iliff, Jeffrey J.; Nedergaard, Maiken (June 1, 2013). "Is There a Cerebral Lymphatic System?". Stroke. 44 (6 suppl 1): S93–S95. doi:10.1161/STROKEAHA.112.678698. PMC 3699410. PMID 23709744.
- ^ Gray's Anatomy 2008, p. 247.
- ^ Gray's Anatomy 2008, p. 251-2.
- ^ a b c Gray's Anatomy 2008, p. 250.
- ^ a b Gray's Anatomy 2008, p. 248.
- ^ a b Gray's Anatomy 2008, p. 251.
- ^ a b c Gray's Anatomy 2008, p. 254-6.
- ^ a b c d e Elsevier's 2007, pp. 311–4.
- ^ a b c d Guyton & Hall 2011, pp. 748–749.
- ^ Laterra J, Keep R, Betz LA, et al. (1999). "Blood–Cerebrospinal Fluid Barrier". Basic Neurochemistry: Molecular, Cellular and Medical Aspects (6th ed.). Philadelphia: Lippincott-Raven.
- ^ Sadler, T. (2010). Langman's medical embryology (11th ed. ed.). Philadelphia: Lippincott William & Wilkins. p. 293. ISBN 978-07817-9069-7.
{{cite book}}:|edition=has extra text (help) - ^ a b Larsen 2001, p. 419. sfn error: multiple targets (2×): CITEREFLarsen2001 (help)
- ^ a b c Larsen 2001, pp. 85–88. sfn error: multiple targets (2×): CITEREFLarsen2001 (help)
- ^ a b Purves, Dale (2011). Neuroscience (5. ed.). Sunderland, Mass.: Sinauer. pp. 478–487. ISBN 978-087893-695-3.
- ^ a b c Larsen, William J. (2001). Human embryology (3. ed.). Philadelphia, Pa.: Churchill Livingstone. pp. 445–446. ISBN 0-443-06583-7.
- ^ Larsen 2001, pp. 85–87. sfn error: multiple targets (2×): CITEREFLarsen2001 (help)
- ^ Xi Chen (2012). Mechanical Self-Assembly: Science and Applications. Springer Science & Business Media. p. 188. ISBN 1461445620. Retrieved January 21, 2017.
- ^ Florio, Marta; Albert, Mareike; Taverna, Elena; Namba, Takashi; Brandl, Holger; Lewitus, Eric; Haffner, Christiane; Sykes, Alex; Wong, Fong Kuan (March 27, 2015). "Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion". Science. 347 (6229): 1465–1470. doi:10.1126/science.aaa1975. PMID 25721503.
- ^ Guyton & Hall 2011, p. 685.
- ^ a b Guyton & Hall 2011, p. 687.
- ^ a b Guyton & Hall 2011, p. 686.
- ^ Guyton & Hall 2011, p. 698,708.
- ^ Davidson's 2010, p. 1139.
- ^ a b Jennifer L. Hellier (2014). The Brain, the Nervous System, and Their Diseases [3 volumes]. ABC-CLIO. pp. 300–303. ISBN 1610693388. Retrieved March 3, 2017.
- ^ a b Guyton & Hall 2011, p. 571–576.
- ^ Guyton & Hall 2006, pp. 595.
- ^ Guyton & Hall 2011, pp. 623–631.
- ^ Guyton & Hall 2006, pp. 651–662.
- ^ Pocock, Gillian; Richards, Christopher D. (2006). Human physiology : the basis of medicine (3rd ed. ed.). Oxford: Oxford University Press. pp. 138–139. ISBN 978-0-19-856878-0.
{{cite book}}:|edition=has extra text (help) - ^ Squire, Larry Squire (2013). Fundamental neuroscience (4th ed. ed.). Amsterdam: Elsevier/Academic Press. pp. 525–526. ISBN 978-012-385870-2.
{{cite book}}:|edition=has extra text (help) - ^ Guyton & Hall 2006, pp. 663–6.
- ^ a b Guyton & Hall 2006, pp. 204–214.
- ^ Hall, John (2011). Guyton and Hall textbook of medical physiology (12th ed. ed.). Philadelphia, Pa.: Saunders/Elsevier. p. 505. ISBN 978-1-4160-4574-8.
{{cite book}}:|edition=has extra text (help) - ^ a b c Guyton & Hall 2006, pp. 514–9.
- ^ Squire, Larry (2013). Fundamental neuroscience (4th ed.). Amsterdam: Elsevier/Academic Press. p. 800. ISBN 9780123858702.
- ^ Squire, Larry (2013). Fundamental neuroscience (4th ed.). Amsterdam: Elsevier/Academic Press. p. 803. ISBN 9780123858702.
- ^ Squire, Larry (2013). Fundamental neuroscience (4th ed.). Amsterdam: Elsevier/Academic Press. p. 805. ISBN 9780123858702.
- ^ Guyton & Hall 2011, p. 720-2.
- ^ Poeppel, David; Emmorey, Karen; Hickok, Gregory; Pylkkänen, Liina (October 10, 2012). "Towards a new neurobiology of language". The Journal of neuroscience : the official journal of the Society for Neuroscience. 32 (41): 14125–14131. doi:10.1523/JNEUROSCI.3244-12.2012. PMC 3495005. PMID 23055482.
- ^ Hickok, Gregory (September 2009). "The functional neuroanatomy of language". Physics of Life Reviews. 6 (3): 121–143. doi:10.1016/j.plrev.2009.06.001.
- ^ Fedorenko, Evelina; Kanwisher, Nancy. "Neuroimaging of language: why hasn't a clearer picture emerged?" (PDF). Language and Linguistics Compass. 3: 839=865.
- ^ Damasio, H (April 28, 2001). "Neural basis of language disorders". In Chapey, Roberta (ed.). Language intervention strategies in aphasia and related neurogenic communication disorders (4th ed.). Lippincott Williams & Wilkins. pp. 18–36. ISBN 9780781721332. OCLC 45952164.
- ^ a b Handbook of Neuroscience for the Behavioral Sciences, Volume 1. John Wiley & Sons. 2009. p. 145. ISBN 0470083557. Retrieved January 25, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Jennifer L. Hellier (2014). The Brain, the Nervous System, and Their Diseases [3 volumes]. ABC-CLIO. p. 1135. ISBN 1610693388. Retrieved February 15, 2017.
- ^ Introduction to Brain and Behavior (Loose-Leaf). Macmillan Higher Education. 2013. p. 296. ISBN 1464139601. Retrieved February 15, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Lauralee Sherwood (2012). Human Physiology: From Cells to Systems. Cengage Learning. p. 181. ISBN 1133708536.
- ^ James W. Kalat (2015). Biological Psychology. Cengage Learning. p. 425. ISBN 1305465296.
- ^ a b Tissue Mechanics. Springer Science & Business Media. 2007. p. 4. ISBN 0387499857. Retrieved March 3, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ a b Understanding Psychology. Prentice Hall. 2011. p. 56. ISBN 0205769063. Retrieved March 3, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ a b Introduction to Brain and Behavior (Loose-Leaf). Macmillan Higher Education. 2013. pp. 524–549. ISBN 1464139601. Retrieved March 3, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Introducing Psychology. Macmillan. 2009. p. 80. ISBN 1429218215. Retrieved March 3, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Armony, Jorge (2013). The Cambridge handbook of human affective neuroscience (1. publ. ed.). Cambridge [u.a.]: Cambridge Univ. Press. p. 16. ISBN 9780521171557.
- ^ Lindquist, Kristen A.; Wager, Tor D.; Kober, Hedy; Bliss-Moreau, Eliza; Barrett, Lisa Feldman (May 23, 2012). "The brain basis of emotion: A meta-analytic review". Behavioral and Brain Sciences. 35 (03): 121–143. doi:10.1017/S0140525X11000446.
- ^ Phan, K.Luan; Wager, Tor; Taylor, Stephan F.; Liberzon, Israel (June 1, 2002). "Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI". NeuroImage. 16 (2): 331–348. doi:10.1006/nimg.2002.1087. PMID 12030820.
- ^ a b Malenka, RC; Nestler, EJ; Hyman, SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor, A; Brown, RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 155–157. ISBN 978-0-07-148127-4.
DA has multiple actions in the prefrontal cortex. It promotes the "cognitive control" of behavior: the selection and successful monitoring of behavior to facilitate attainment of chosen goals. Aspects of cognitive control in which DA plays a role include working memory, the ability to hold information "on line" in order to guide actions, suppression of prepotent behaviors that compete with goal-directed actions, and control of attention and thus the ability to overcome distractions. ... Noradrenergic projections from the LC thus interact with dopaminergic projections from the VTA to regulate cognitive control. ...
- ^ a b c d e Diamond, Adele (2013). "Executive functions". Annual Review of Psychology. 64: 135–168. doi:10.1146/annurev-psych-113011-143750. PMC 4084861. PMID 23020641.
Core EFs are inhibition [response inhibition (self-control—resisting temptations and resisting acting impulsively) and interference control (selective attention and cognitive inhibition)], working memory, and cognitive flexibility (including creatively thinking "outside the box," seeing anything from different perspectives, and quickly and flexibly adapting to changed circumstances).
Figure 4: Executive functions and related terms - ^ a b Chan RC, Shum D, Toulopoulou T, Chen EY (March 2008). "Assessment of executive functions: review of instruments and identification of critical issues". Archives of Clinical Neuropsychology : the Official Journal of the National Academy of Neuropsychologists. 23 (2): 201–216. doi:10.1016/j.acn.2007.08.010. PMID 18096360.
The term "executive functions" is an umbrella term comprising a wide range of cognitive processes and behavioral competencies which include verbal reasoning, problem-solving, planning, sequencing, the ability to sustain attention, resistance to interference, utilization of feedback, multitasking, cognitive flexibility, and the ability to deal with novelty (Burgess, Veitch, de lacy Costello, & Shallice, 2000; Damasio, 1995; Grafman & Litvan, 1999; Shallice, 1988; Stuss & Benson, 1986; Stuss, Shallice, Alexander, & Picton, 1995).
- ^ Souza, MJ; Bunge, SA (2009). "Executive Function and Higher-Order Cognition: Neuroimaging". Encyclopedia of Neuroscience (4): 111–116.
- ^ a b c d e f Hyun JC, Weyandt LL, Swentosky A (2014). "Chapter 2: The Physiology of Executive Functioning". In Goldstein S, Naglieri J (ed.). Handbook of Executive Functioning. New York: Springer. pp. 13–23. ISBN 9781461481065.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 313–324. ISBN 9780071481274.
These diverse inputs and back projections to both cortical and subcortical structures put the prefrontal cortex in a position to exert what is often called "top-down" control or cognitive control of behavior. ...
In conditions in which prepotent responses tend to dominate behavior, such as in drug addiction, where drug cues can elicit drug seeking (Chapter 15), or in attention deficit hyperactivity disorder (ADHD; described below), significant negative consequences can result. ... ADHD can be conceptualized as a disorder of executive function; specifically, ADHD is characterized by reduced ability to exert and maintain cognitive control of behavior. Compared with healthy individuals, those with ADHD have diminished ability to suppress inappropriate prepotent responses to stimuli (impaired response inhibition) and diminished ability to inhibit responses to irrelevant stimuli (impaired interference suppression). ... Functional neuroimaging in humans demonstrates activation of the prefrontal cortex and caudate nucleus (part of the striatum) in tasks that demand inhibitory control of behavior. ... Early results with structural MRI show thinning of the cerebral cortex in ADHD subjects compared with age-matched controls in prefrontal cortex and posterior parietal cortex, areas involved in working memory and attention. - ^ a b "NIMH » Brain Basics". www.nimh.nih.gov. Retrieved March 26, 2017.
- ^ Swaminathan, Nikhil (April 29, 2008). "Why Does the Brain Need So Much Power?". Scientific American. Scientific American, a Division of Nature America, Inc. Retrieved November 19, 2010.
- ^ Quistorff, Bjørn; Secher, Niels; Van Lieshout, Johanne (July 24, 2008). "Lactate fuels the human brain during exercise". The FASEB Journal. 22 (10): 3443–3449. doi:10.1096/fj.08-106104. Retrieved May 9, 2011.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Marin-Valencia, Isaac; Good, Levi B; Ma, Qian; Malloy, Craig R; Pascual, Juan M (October 17, 2012). "Heptanoate as a Neural Fuel: Energetic and Neurotransmitter Precursors in Normal and Glucose Transporter I-Deficient (G1D) Brain". Journal of Cerebral Blood Flow & Metabolism. 33 (2): 175–182. doi:10.1038/jcbfm.2012.151. PMC 3564188. PMID 23072752.
- ^ Tsuji A (2005). "Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems". NeuroRx. 2 (1): 54–62. doi:10.1602/neurorx.2.1.54. PMC 539320. PMID 15717057.
Uptake of valproic acid was reduced in the presence of medium-chain fatty acids such as hexanoate, octanoate, and decanoate, but not propionate or butyrate, indicating that valproic acid is taken up into the brain via a transport system for medium-chain fatty acids, not short-chain fatty acids. ... Based on these reports, valproic acid is thought to be transported bidirectionally between blood and brain across the BBB via two distinct mechanisms, monocarboxylic acid-sensitive and medium-chain fatty acid-sensitive transporters, for efflux and uptake, respectively.
- ^ Vijay N, Morris ME (2014). "Role of monocarboxylate transporters in drug delivery to the brain". Curr. Pharm. Des. 20 (10): 1487–98. doi:10.2174/13816128113199990462. PMC 4084603. PMID 23789956.
Monocarboxylate transporters (MCTs) are known to mediate the transport of short chain monocarboxylates such as lactate, pyruvate and butyrate. ... MCT1 and MCT4 have also been associated with the transport of short chain fatty acids such as acetate and formate which are then metabolized in the astrocytes [78].
- ^ Obel, LF; Müller, MS; Walls, AB; Sickmann, HM; Bak, LK; Waagepetersen, HS; Schousboe, A (2012). "Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level". Frontiers in neuroenergetics. 4: 3. doi:10.3389/fnene.2012.00003. PMC 3291878. PMID 22403540.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Clark, DD; Sokoloff L (1999). Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott. pp. 637–670. ISBN 978-0-397-51820-3.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ B. B. Mrsulja (2012). Pathophysiology of Cerebral Energy Metabolism. Springer Science & Business Media. pp. 2–3. ISBN 1468433482. Retrieved March 9, 2017.
- ^ Raichle, M; Gusnard, DA (2002). "Appraising the brain's energy budget". Proc. Natl. Acad. Sci. U.S.A. 99 (16): 10237–10239. doi:10.1073/pnas.172399499. PMC 124895. PMID 12149485.
- ^ Handbook of Behavioral Medicine: Methods and Applications. Springer Science & Business Media. 2010. p. 770. ISBN 0387094881. Retrieved March 9, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ a b Van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.J.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; Della Penna, S.; Feinberg, D.; Glasser, M.F.; Harel, N.; Heath, A.C.; Larson-Prior, L.; Marcus, D.; Michalareas, G.; Moeller, S.; Oostenveld, R.; Petersen, S.E.; Prior, F.; Schlaggar, B.L.; Smith, S.M.; Snyder, A.Z.; Xu, J.; Yacoub, E. (October 2012). "The Human Connectome Project: A data acquisition perspective". NeuroImage. 62 (4): 2222–2231. doi:10.1016/j.neuroimage.2012.02.018.
- ^ Jones, Edward G.; Mendell, Lorne M. (April 30, 1999). "Assessing the Decade of the Brain". Science. 284 (5415). American Association for the Advancement of Science: 739. doi:10.1126/science.284.5415.739. PMID 10336393. Retrieved April 5, 2010.
- ^ "A $4.5 Billion Price Tag for the BRAIN Initiative?". Science | AAAS. June 5, 2014. Retrieved June 10, 2017.
- ^ Spehlmann, Rainer; Fisch, BJ (1999). Fisch and Spehlmann's EEG primer : basic principles of digital and analog EEG. Elsevier. ISBN 9780444821485. OCLC 43275001.[page needed]
- ^ Silverstein, Justin (2012). "Mapping the Motor and Sensory Cortices: A Historical Look and a Current Case Study in Sensorimotor Localization and Direct Cortical Motor Stimulation". The Neurodiagnostic Journal. 52 (1): 54–68. PMID 22558647.
- ^ Boraud T.; Bezard E.; et al. (2002). "From single extracellular unit recording in experimental and human Parkinsonism to the development of a functional concept of the role played by the basal ganglia in motor control". Progress in Neurobiology. 66 (4): 265–283. doi:10.1016/s0301-0082(01)00033-8.
- ^ Buxton, Richard B. (2002). Introduction to functional magnetic resonance imaging : principles and techniques. Cambridge University Press. ISBN 9780521581134. OCLC 45166697.[page needed]
- ^ Buxton Richard; Uludag Kamil; Liu Thomas. "Modeling the Haemodynamic Response to Brain Activation". NeuroImage. 23: S220–S233. doi:10.1016/j.neuroimage.2004.07.013.
- ^ Preissl, Hubert (2005). Magnetoencephalography. Academic Press. ISBN 9780123668691. OCLC 141379565. [page needed]
- ^ The Role of Technology in Clinical Neuropsychology. Oxford University Press. 2017. p. 399. ISBN 0190234733. Retrieved March 9, 2017.
Irimia, Chambers, Torgerson, and Van Horn (2012) provide a first-step graphic on how best to display connectivity findings, as is presented in Figure 13.15. This is referred to as a connectogram.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Andrews, DG (2001). Neuropsychology. Psychology Press. ISBN 978-1-84169-103-9.
- ^ Lepage M (2010). "Research at the Brain Imaging Centre". Douglas Mental Health University Institute. Archived from the original on March 5, 2012.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Dawodu, Segun Toyin (March 9, 2017). "Traumatic Brain Injury (TBI) - Definition and Pathophysiology: Overview, Epidemiology, Primary Injury". Medscape. Retrieved April 8, 2017.
- ^ Davidson's 2010, p. 1196-7.
- ^ a b Davidson's 2010, p. 1205-15.
- ^ a b c d e Davidson's 2010, p. 1216-7.
- ^ Volkow ND, Koob GF, McLellan AT (January 2016). "Neurobiologic Advances from the Brain Disease Model of Addiction". N. Engl. J. Med. 374 (4): 363–371. doi:10.1056/NEJMra1511480. PMID 26816013.
- ^ Multimodal Treatment of Acute Psychiatric Illness: A Guide for Hospital Diversion. Columbia University Press. 2013. pp. 22–24. ISBN 0231536097. Retrieved April 12, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ a b c d e Davidson's 2010, p. 1172-9.
- ^ Anderson, Pauline. "New Definition of Status Epilepticus". www.medscape.com. No. January 12, 2016. Retrieved March 26, 2017.
- ^ Harbison J, Massey A, Barnett L, Hodge D, Ford GA (June 1999). "Rapid ambulance protocol for acute stroke". Lancet. 353 (9168): 1935. doi:10.1016/S0140-6736(99)00966-6. PMID 10371574.
- ^ Davidson's 2010, p. 1183.
- ^ a b Davidson's 2010, p. 1180-1.
- ^ a b c d e f g Davidson's 2010, p. 1183-1185.
- ^ Davidson's 2010, p. 1181.
- ^ a b Davidson's 2010, p. 1185-1189.
- ^ Goyal, Mayank; Menon, Bijoy K; van Zwam, Wim H; Dippel, Diederik W J; Mitchell, Peter J; Demchuk, Andrew M; Dávalos, Antoni; Majoie, Charles B L M; van der Lugt, Aad; de Miquel, Maria A; Donnan, Geoffrey A; Roos, Yvo B W E M; Bonafe, Alain; Jahan, Reza; Diener, Hans-Christoph; van den Berg, Lucie A; Levy, Elad I; Berkhemer, Olvert A; Pereira, Vitor M; Rempel, Jeremy; Millán, Mònica; Davis, Stephen M; Roy, Daniel; Thornton, John; Román, Luis San; Ribó, Marc; Beumer, Debbie; Stouch, Bruce; Brown, Scott; Campbell, Bruce C V; van Oostenbrugge, Robert J; Saver, Jeffrey L; Hill, Michael D; Jovin, Tudor G (April 2016). "Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials". The Lancet. 387 (10029): 1723–1731. doi:10.1016/S0140-6736(16)00163-X.
- ^ Saver, J. L. (December 8, 2005). "Time Is Brain—Quantified". Stroke. 37 (1): 263–266. doi:10.1161/01.STR.0000196957.55928.ab.
- ^ Winstein, Carolee J.; Stein, Joel; Arena, Ross; Bates, Barbara; Cherney, Leora R.; Cramer, Steven C.; Deruyter, Frank; Eng, Janice J.; Fisher, Beth; Harvey, Richard L.; Lang, Catherine E.; MacKay-Lyons, Marilyn; Ottenbacher, Kenneth J.; Pugh, Sue; Reeves, Mathew J.; Richards, Lorie G.; Stiers, William; Zorowitz, Richard D. (June 2016). "Guidelines for Adult Stroke Rehabilitation and Recovery". Stroke. 47 (6): e98–e169. doi:10.1161/STR.0000000000000098.
- ^ a b c Harrison's 2008, p. 1719.
- ^ a b c d Goila, AjayKumar; Pawar, Mridula (2009). "The diagnosis of brain death". Indian Journal of Critical Care Medicine. 13 (1): 7. doi:10.4103/0972-5229.53108.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Wijdicks, Eelco F. M. (January 8, 2002). "Brain death worldwide: accepted fact but no global consensus in diagnostic criteria". Neurology. 58 (1): 20–25. doi:10.1212/wnl.58.1.20. PMID 11781400.
- ^ Dhanwate, AD (September 2014). "Brainstem death: A comprehensive review in Indian perspective". Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 18 (9): 596–605. PMID 25249744.
- ^ a b c d Davidson's 2010, p. 1158.
- ^ Davidson's 2010, p. 200.
- ^ Domínguez D, Juan F; Lewis, ED; Turner, R; Egan, GF (2009). Chiao, JY (ed.). "The Brain in Culture and Culture in the Brain: A Review of Core Issues in Neuroanthropology". Progress in Brain Research. Special issue: Cultural Neuroscience: Cultural Influences on Brain Function. 178. The Netherlands: Elsevier: 43–6. doi:10.1016/S0079-6123(09)17804-4.
- ^ "Cultural Environment Influences Brain Function | Psych Central News". Psych Central News. August 4, 2010.
- ^ Rescher N (1992). G. W. Leibniz's Monadology. Psychology Press. p. 83. ISBN 978-0-415-07284-7.
- ^ Hart, WD (1996). Guttenplan S (ed.). A Companion to the Philosophy of Mind. Blackwell. pp. 265–267.
- ^ Churchland, PS (1989). "Ch. 8". Neurophilosophy. MIT Press. ISBN 978-0-262-53085-9.
- ^ Aslihan Selimbeyoglu, Josef Parvizi. "Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature" (2010). Frontiers in Human Neuroscience.
- ^ James H. Schwartz. Appendix D: Consciousness and the Neurobiology of the Twenty-First Century. In Kandel, ER; Schwartz JH; Jessell TM. (2000). Principles of Neural Science, 4th Edition.
- ^ Lilienfeld, Scott O.; Lynn, Steven Jay; Ruscio, John; Beyerstein, Barry L. (September 15, 2011). 50 Great Myths of Popular Psychology: Shattering Widespread Misconceptions about Human Behavior. John Wiley & Sons. p. 89. ISBN 9781444360745.
- ^ McDaniel, Michael (2005). "Big-brained people are smarter" (PDF). Intelligence. 33: 337–346. doi:10.1016/j.intell.2004.11.005.
{{cite journal}}: Invalid|ref=harv(help) - ^ Luders, Eileen; Narr, Katherine L.; Bilder, Robert M.; Szeszko, Philip R.; Gurbani, Mala N.; Hamilton, Liberty; Toga, Arthur W.; Gaser, Christian (September 1, 2008). "Mapping the Relationship between Cortical Convolution and Intelligence: Effects of Gender". Cerebral Cortex. 18 (9): 2019–2026. doi:10.1093/cercor/bhm227. ISSN 1047-3211. PMC 2517107. PMID 18089578.
- ^ Hoppe, Christian; Stojanovic, Jelena (2008). "High-Aptitude Minds". Scientific American Mind. 19 (4): 60–67. doi:10.1038/scientificamericanmind0808-60.
- ^ "Tupaia belangeri". The Genome Institute, Washington University. Retrieved January 22, 2016.
- ^ Jarrett, Christian. Great Myths of the Brain. John Wiley & Sons. ISBN 9781118312711.
- ^ Kandel, ER; Schwartz JH; Jessell TM (2000). Principles of Neural Science (4th ed.). New York: McGraw-Hill. ISBN 0-8385-7701-6.
- ^ a b c d Schmitt, edited by George Adelman ; foreword by Francis O. (1987). Encyclopedia of neuroscience (PDF) (2. rev. and enl. ed. ed.). Boston: Birkhäeuser. pp. 843–847. ISBN 0817633359.
{{cite book}}:|edition=has extra text (help);|first1=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b Bear, M.F.; B.W. Connors; M.A. Paradiso (2001). Neuroscience: Exploring the Brain. Baltimore: Lippincott. ISBN 0-7817-3944-6.
- ^ von Staden, p.157
- ^ a b c d e f Gross, Charles G. (1999). Brain, vision, memory : tales in the history of neuroscience (1st MIT Press pbk. ed. ed.). Cambridge, Mass.: MIT. pp. 37–51. ISBN 0262571358.
{{cite book}}:|edition=has extra text (help) - ^ Marshall, Louise H.; Magoun, Horace W. Discoveries in the Human Brain: Neuroscience Prehistory, Brain Structure, and Function. Springer Science & Business Media. p. 44. ISBN 9781475749977.
- ^ PhD, Anders Holtz, MD; PhD, Richard Levi, MD. Spinal Cord Injury. Oxford University Press. ISBN 9780199706815.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Swanson, Larry W. Neuroanatomical Terminology: A Lexicon of Classical Origins and Historical Foundations. Oxford University Press. ISBN 9780195340624.
- ^ Tessman, Patrick A.; Suarez, Jose I. (2002). "Influence of Early Printmaking on the Development of Neuroanatomy and Neurology". Archives of Neurology. 59 (12): 1964–1969. doi:10.1001/archneur.59.12.1964. PMID 12470188. Retrieved April 9, 2008.
- ^ Singer 1956
- ^ a b Lokhorst, Gert-Jan (January 1, 2016). "Descartes and the Pineal Gland". The Stanford Encyclopedia of Philosophy. Metaphysics Research Lab, Stanford University. Retrieved March 11, 2017.
- ^ O'Connor, James (2003). "Thomas Willis and the background to Cerebri Anatome". Journal of the Royal Society of Medicine. 96 (3): 139–143.
- ^ EMERY, ALAN (October 2000). "A Short History of Neurology: The British Contribution 1660–1910. Edited by F. CLIFFORD ROSE. (Pp. 282; illustrated; £25 Paperback; ISBN 07506 4165 7.) Oxford: Butterworth-Heinemann 1999". Journal of Anatomy. 197 (3): 513–518. doi:10.1046/j.1469-7580.2000.197305131.x.
- ^ Sabbatini, Renato M.E. "Sabbatini, R.M.E.: The Discovery of Bioelectricity. Nerve Conduction". www.cerebromente.org.br. Retrieved June 10, 2017.
- ^ Karbowski, Kazimierz (February 14, 2008). "Sixty Years of Clinical Electroencephalography". European Neurology. 30 (3): 170–175. doi:10.1159/000117338.
- ^ Pearce, J.M.S. (March 17, 2009). "Marie-Jean-Pierre Flourens (1794–1867) and Cortical Localization". European Neurology. 61 (5): 311–314. doi:10.1159/000206858.
- ^ a b c De Carlos, Juan A.; Borrell, José (August 2007). "A historical reflection of the contributions of Cajal and Golgi to the foundations of neuroscience". Brain Research Reviews. 55 (1): 8–16. doi:10.1016/j.brainresrev.2007.03.010.
- ^ Burke, R.E. (April 2007). "Sir Charles Sherrington's The integrative action of the nervous system: a centenary appreciation". Brain. 130: 887–894. doi:10.1093/brain/awm022. PMID 17438014.
- ^ Squire, edited by Larry R. (1996). The history of neuroscience in autobiography. Washington DC: Society for Neuroscience. pp. 475–97. ISBN 0126603057.
{{cite book}}:|first1=has generic name (help) - ^ Cowan, W.M.; Harter, D.H.; Kandel, E.R. (2000). "The emergence of modern neuroscience: Some implications for neurology and psychiatry". Annual Review of Neuroscience. 23: 345–346. doi:10.1146/annurev.neuro.23.1.343. PMID 10845068.
- ^ Brady, Joseph V.; Nauta, Walle J. H. Principles, Practices, and Positions in Neuropsychiatric Research: Proceedings of a Conference Held in June 1970 at the Walter Reed Army Institute of Research, Washington, D.C., in Tribute to Dr. David Mckenzie Rioch upon His Retirement as Director of the Neuropsychiatry Division of That Institute. Elsevier. p. vii. ISBN 9781483154534.
- ^ Adelman, George (January 15, 2010). "The Neurosciences Research Program at MIT and the Beginning of the Modern Field of Neuroscience". Journal of the History of the Neurosciences. 19 (1): 15–23. doi:10.1080/09647040902720651.
- ^ a b Principles of Neural Science, 4th ed. Eric R. Kandel, James H. Schwartz, Thomas M. Jessel, eds. McGraw-Hill:New York, NY. 2000.
- ^ John Deadman. The 20th Paradigm Shift in Science:Impact on Neuroscience and psychiatry in the 21st century (PDF). Dept. Psychiatry and Behavioural Neuroscience – McMaster University. Retrieved May 25, 2015.
{{cite book}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Chatterjee, Anjan; Coslett, H. Branch. The Roots of Cognitive Neuroscience: Behavioral Neurology and Neuropsychology. OUP USA. pp. 337–8. ISBN 9780195395549.
- ^ M.Bliss. Harvey Cushing : A Life in Surgery: A Life in Surgery (page ix. & x.). Oxford University Press, USA, October 1, 2005, 610 pages, ISBN 0195346955. Retrieved June 26, 2015.
- ^ Kretzer, RM; Coon, AL; Tamargo, RJ (June 2010). "Walter E. Dandy's contributions to vascular neurosurgery". Journal Neurosurgery. 112 (6): 1182–91. doi:10.3171/2009.7.JNS09737. PMID 20515365.
- ^ Glees, Paul (2005). The Human Brain. Cambridge University Press. p. 1. ISBN 9780521017817.
- ^ Neuroscience for Clinicians: Evidence, Models, and Practice. Springer Science & Business Media. 2012. p. 143. ISBN 1461448425. Retrieved January 21, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Developmental Science: An Advanced Textbook. Psychology Press. 2015. p. 220. ISBN 1136282203. Retrieved January 21, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ a b Douglas Bernstein (2010). Essentials of Psychology. Cengage Learning. p. 64. ISBN 049590693X. Retrieved January 21, 2017.
- ^ Hofman, Michel A. (March 27, 2014). "Evolution of the human brain: when bigger is better". Frontiers in Neuroanatomy. 8. doi:10.3389/fnana.2014.00015.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Gray, Peter (2002). Psychology (4th ed.). Worth Publishers. ISBN 0716751623. OCLC 46640860.
- ^ Visual Psychophysics: From Laboratory to Theory. MIT Press. 2013. p. 3. ISBN 0262019450. Retrieved January 21, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Mike Sharwood Smith (2017). Introducing Language and Cognition. Cambridge University Press. p. 206. ISBN 1107152895. Retrieved January 21, 2017.
- ^ Introduction to Brain and Behavior. Macmillan Higher Education. 2013. p. 21. ISBN 1464139601. Retrieved June 22, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ The Central Nervous System of Vertebrates. Springer. 2014. p. 2127. ISBN 3642182623. Retrieved June 22, 2017.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Lee Lerner, Brenda Wilmoth Lerner (2004). The Gale Encyclopedia of Science: Pheasants-Star. Gale. p. 3759. ISBN 0787675598. Retrieved January 21, 2017.
As human's position changed and the manner in which the skull balanced on the spinal column pivoted, the brain expanded, altering the shape of the cranium.
- ^ Begun, David R. (2012). A Companion to Paleoanthropology. John Wiley & Sons. p. 388. ISBN 9781118332375.
- ^ Jones R (2012). "Neurogenetics: What makes a human brain?". Nature Reviews Neuroscience. 13 (10): 655. doi:10.1038/nrn3355. PMID 22992645.
Sources
- Britton (2010). Davidson's principles and practice of medicine (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 978-0-7020-3085-7.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help)
- Ort, Bruce Ian Bogart, Victoria (2007). Elsevier's integrated anatomy and embryology. Philadelphia, Pa.: Elsevier Saunders. ISBN 978-1-4160-3165-9.
{{cite book}}: CS1 maint: multiple names: authors list (link)
- Standring, Susan (editor-in-chief) ; Borley, Neil R. et al. (section editors) (2008). Gray's Anatomy : the anatomical basis of clinical practice (40th ed.). London: Churchill Livingstone. ISBN 978-0-8089-2371-8.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link)
- Hall, John (2011). Guyton and Hall textbook of medical physiology (12th ed.). Philadelphia, Pa.: Saunders/Elsevier. ISBN 978-1-4160-4574-8.
- Longo, Dan; Fauci, Anthony; Kasper, Dennis; Hauser, Stephen; Jameson, J.; Loscalzo, Joseph (August 11, 2011). Harrison's Principles of Internal Medicine (18 ed.). McGraw-Hill Professional. ISBN 978-0-07-174889-6.
- Larsen, William J. (2001). Human embryology (3. ed.). Philadelphia, Pa.: Churchill Livingstone. ISBN 0-443-06583-7.
{{cite book}}: CS1 maint: ref duplicates default (link)
Notes
- ^ Specifically the oculomotor, trochlear nerve, trigeminal nerve, abducens nerve, facial nerve, vestibulocochlear nerve, glossopharyngeal nerve, vagus nerve, accessory nerve and hypoglossal nerves.[31]
- ^ the anterior inferior cerebellar artery and superior cerebellar artery.[46]
- ^ Including the vestibulo-ocular reflex, corneal reflex, gag reflex and dilation of the pupils in response to light ,[145]
- ^ a thought that, while the heart was the seat of intelligence, the brain was a cooling mechanism for the blood. He reasoned that humans are more rational than the beasts because, among other reasons, they have a larger brain to cool their hot-bloodedness.[162]
- ^ This may be explained by Galen's dissections were all on animals – in particular, the rete mirabile is only well developed in ungulates
- ^ Illustrated by architect Christopher Wren[164]