Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
October 26
Evolution of horse - back strength/suitability for riding
Why has the horse evolved to be able to carry the weight of a human on its back? Why do they even tolerate riding?--2.97.23.83 (talk) 08:02, 26 October 2012 (UTC)
- It would be more accurate to say that horses have been bred for the purpose of humans riding them, and so have been artificially, not naturally selected. Furthermore, this development did not take place in a geological timescale, but over the span of only a few thousand years. Before maybe 1500 BC, horses were used mainly to draw chariots, because they weren't big enough to ride. The situation is similar to modern cattle providing huge quantities of milk, far more than wild cattle. This is not due to evolution, but due to human/artificial selection. - Lindert (talk) 10:09, 26 October 2012 (UTC)
- Are you sure about the size part? The Hagerman horse is about the size of an Arabian horse, and it dates from 3.5 million years ago. --140.180.252.244 (talk) 10:50, 26 October 2012 (UTC)
- Do you have a source on horses not being large enough to ride before their domestication? Our article on evolution of the horse seems to indicate otherwise. Undomesticated horses like the Przewalski's horse appear large enough to support a human rider. A8875 (talk) 10:51, 26 October 2012 (UTC)
- I'm not saying that no horses existed that were big enough, just the horses that were used by humans early on were small, and then bred larger. Maybe large horses did not live in the area (ancient near east) where they were first domesticated. - Lindert (talk) 11:13, 26 October 2012 (UTC)
- I recons it was purely coincidental at first, and then there developed an evolutionary correlation. Plasmic Physics (talk) 11:03, 26 October 2012 (UTC)
- We have an article, Domestication of the horse. With reference to the points raised above, it seems that (according to our article) "recent genetic studies indicate that Przewalski's horse is not an ancestor to modern domesticated horses". Also, one of the first domestications seems to have been by "theBotai culture... who seem to have adopted horseback riding in order to hunt the abundant wild horses of northern Kazakhstan between 3500-3000 BCE" although it's not certain that they didn't just hunt them. Finally, "Horse bones from these contexts (recovered from the middens of various European bronze age sites) exhibited an increase in variability, thought to reflect the survival under human care of both larger and smaller individuals than appeared in the wild; and a decrease in average size, thought to reflect penning and restriction in diet." Alansplodge (talk) 15:17, 26 October 2012 (UTC)
- It's worth noting in terms of the behavior that in some respects humans just lucked out. Zebras, for example, have never been domesticated to the same degree as the horse, despite a lot of trying. There seems to be something fundamentally incompatible with their natural makeup to easy domestication, as there is for a lot of animals. --Mr.98 (talk) 22:14, 26 October 2012 (UTC)
- For some pictures of domesticated zebras, see Zebra taxi cab in Brixton Road!;-) Alansplodge (talk) 16:57, 27 October 2012 (UTC)
Chemical castration for woman
Are there any chemical castration procedures for female sex offenders? A8875 (talk) 09:36, 26 October 2012 (UTC)
- Androgens may contribute to aggression in women (data are conflicting), and one could speculate about whether they would be effective, but I'm not aware of any such procedures. Estrogens do not clearly contribute to aggressive behavior, so I would not expect an analogous procedure for female hormones. Of interest (not all directly related): PMID 22415579, PMID 20951723, PMID 19747510 -- Scray (talk) 10:41, 26 October 2012 (UTC)
- This page has some interesting information: "Even though sexual victimization by either males or females is a traumatizing experience, female offenders are overall not as dangerous as males, and combine the act with fewer aggravating circumstances such as weapon possession, kidnapping, or violence. Perhaps because of this, more cases of female sex offending go unreported." It also states that less than 4% of sex crimes in the U.S. are committed by females, and notes that sex crimes by females tend to be less likely to be repetitive or compulsive than males. It does not directly answer the question on whether chemical castration is possible, nor does it answer whether it is desirable, but it does make the case that sexual crimes committed by females are distinct from those committed by males, and thus may indicate that a different response to them is necessary. Just some related ideas. Doing some searching, I can't find any reliable sources on "chemical castration of women", but several rather unreliable discussions found through searching google with that phrase seem to indicate there is a sense that either it is never done, or even that it isn't possible to do so. --Jayron32 13:26, 26 October 2012 (UTC)
- Female sexual criminals are not your typical sexual predator or compulsive rapist. There are much more a women having consensual sex with men below the age of consent (so, it's not legally consensual) or women being dragged into some sexual slavery scheme by partners. OsmanRF34 (talk) 16:58, 26 October 2012 (UTC)
- Is this your impression, or do you have references to support your "answer"? -- Scray (talk) 17:13, 26 October 2012 (UTC)
- It's a small sample, but the criminal statistics for Sweden in 2011 kind of supports that. Of 18 women sentenced for sex crimes 1 committed rape, and out of 1380 men sentenced for sex crimes 151 committed rape and 16 aggravated rape (statutory rape not included in rape or aggravated rape) (sourceBRÅ, Brottsförebyggande rådet). That's 6 % of female sex offenders and 12 % of male sentenced for rape. I'm sure there are similar statistics for other countries.Sjö (talk) 15:41, 27 October 2012 (UTC)
- I think it's too small a sample. A quick mental calculation gives huge error bars on the rate of female rapists -- that "6%" is actually something like "0%-14%". --Carnildo (talk) 02:16, 30 October 2012 (UTC)
- It's a small sample, but the criminal statistics for Sweden in 2011 kind of supports that. Of 18 women sentenced for sex crimes 1 committed rape, and out of 1380 men sentenced for sex crimes 151 committed rape and 16 aggravated rape (statutory rape not included in rape or aggravated rape) (sourceBRÅ, Brottsförebyggande rådet). That's 6 % of female sex offenders and 12 % of male sentenced for rape. I'm sure there are similar statistics for other countries.Sjö (talk) 15:41, 27 October 2012 (UTC)
- Is this your impression, or do you have references to support your "answer"? -- Scray (talk) 17:13, 26 October 2012 (UTC)
There are drugs that suppress sex hormones in women as well as men. Examples are gonadotropin releasing hormone analogs such as leuprolide and histrelin. They are used to suppress sex hormones for many purposes, but not to treat female sex offenders for many reasons. As mentioned above: there are very few of them, and nearly all have engaged in consensual sex with adolescents or have been willing participants in abuse of younger children under the direction of a male; an even smaller percentage repeatedly offend. Most importantly there is no evidence that hormones play a role in compulsive sexualized behavior that testosterone seems to in men. alteripse (talk) 19:56, 31 October 2012 (UTC)
Privacy
Can you read my I.P. address?Cinquefoil (talk) 11:35, 26 October 2012 (UTC)
- No, I can't. Because you have a registered account, your ip address is private from most other users. A few selected people with checkuser privileges can choose to see your ip address, but they wouldn't be likely to bother unless you were disrupting the project in some way.-FisherQueen (talk · contribs)11:37, 26 October 2012 (UTC)
- It's actually more than "they won't bother": Checkusers use of the Checkuser tool is carefully monitored and logged, and checkusers that misuse it can be stripped of it. So, unless you have actually done something to attract the attention of the Checkusers, no one will see your IP address. Checkusers have to have a justifiable reason to use the tool, and if they don't, there's some 'splainin' to do. --Jayron32 13:15, 26 October 2012 (UTC)
- There are a lot of ways to "social engineer" it. Like, if I have a site that will tell me the IP addresses of people who look at a picture (or an invisible "web bug") and I send it to Special:Emailuser/Cinquefoil, then if you open it, I know what your IP is (and stuff about your browser too, probably) Or I could post similar links to some kind of text answers to two questions you ask, see if there's an IP address in common. The whole web is run by amateur spies on the brink of turning pro, I think. Wnt (talk) 21:06, 26 October 2012 (UTC)
- It's actually more than "they won't bother": Checkusers use of the Checkuser tool is carefully monitored and logged, and checkusers that misuse it can be stripped of it. So, unless you have actually done something to attract the attention of the Checkusers, no one will see your IP address. Checkusers have to have a justifiable reason to use the tool, and if they don't, there's some 'splainin' to do. --Jayron32 13:15, 26 October 2012 (UTC)
The surface of Saturn

This question was prompted by [1] - stories which get it wrong; the vortex is 150 F hotter, not 150 F! Still, according to the article, Saturn gets extremely hot, 11,700 C shining-like-the-sunhot, if you go down far enough.
- How much is known about the temperature profile and cloud composition of Saturn's atmosphere? In particular, is it like Jupiter's atmosphere, where there is a "sweet spot" with Earthlike temperatures and pressures if you go to the right depth? (Note Saturn also has Earth gravity, making it a rather good opportunity for a sci-fi vacation, I think. If only you can figure out how to breathe and how not to fall, that is.)
- What does our article mean when it says that Saturn has a "liquid layer of helium-saturated molecular hydrogen that gradually transitions into gas with increasing altitude" (around 1000 km). I'm aware of the critical point where gas and liquid are the same, but I thought either you were above the critical point and there was no difference, or you were below it and there were two very distinct phases.
- Saturn is made up mostly of liquid hydrogen that is less dense than water. So why don't all the other heavier elements sink and leave no trace in the atmosphere? Or is the liquid hydrogen mixed in with a fairly large amount of such impurities?
- Is there anything that might be found floating on the "surface", if it exists, or which could distinguish a "cartography" of different physical areas?
- 1000 km might show up on Earth, but relative to Saturn, it's a very thin skin, practically two-dimensional. Do these things like the Great Springtime Storm exist as very wide but not deep features, like hurricanes on Earth, or do they whip up vortices (or something) in the liquid? hydrogen layer?
Some previous discussion, not very satisfying, at[2]. Wnt (talk) 21:31, 26 October 2012 (UTC)
- It's unlikely that there is such a "sweet spot", the temperature would probably be below freezing even at greater than 100 kPa atmospheric pressure.
- I hope that you're aware that gravity is a function of height, and not a constant. So, it does not make sense to describe Saturn's gravity without making a reference to a height.
- I don't understand what you don't understand, concerning the critical point. A trasition through the critical point occurs with increasing depth just as you say.
- Because of this transition, there is no surface, so nothing can be floating on a surface. Plasmic Physics (talk) 13:09, 27 October 2012 (UTC)
- It doesn't matter much if it's a few times atmospheric pressure - can you be more specific?
- The article says that for temperatures between 270 K and 330 K, the pressure is 1-2 MPa. That's more than ten times atmospheric pressure, and it happens to be the exact zone where hydrogen transitions from a gas to a supercritical gas-liquid state. Helium transitions ways before that even. Plasmic Physics (talk) 02:30, 28 October 2012 (UTC)
- Gravity in the article is in reference to the 1 bar level; anywhere in the atmosphere should be very similar.
- Critical point (thermodynamics) does indeed show dotted lines at the critical temperature and pressure. I suppose it is the critical pressure that is exceeded in this case at 1000 km? But that transition isn't "gradual" - it's just an imaginary line between "gas" and "supercritical fluid", I suppose? But that should not be visible, and it makes no special sense to call it liquid hydrogen beneath it, right? Wnt (talk) 01:44, 28 October 2012 (UTC)
- A supercritical state can only be achieved when both the critical pressure and temperature have been exceeded. It should really be called supercritical helium/hydrogen. The transition from gas to a supercritical state is not gradual but sharp and well-defined, because of the pressure and temperature boundries. It is the transition of observational properties that is gradual. Plasmic Physics (talk) 02:17, 28 October 2012 (UTC)
- Indeed, I see now that hydrogen has a critical point of 1.29 MPa, about 12 atm. Where this gets curious is that hydrogen has density 70 kg/m3 as a liquid, 30 kg/m3 at the critical point [3], whereas air near STP has density a little above 1 kg/m3 -if ordinary air respects the ideal gas law, then it should only be around 12 kg/m3 at this point. Now heating hydrogen should reduce its density, but will a supercritical fluid respect the ideal gas law? From the article I'm getting the impression that small differences in conditions alter its density considerably, so I'm thinking no. I almost wonder if there's a point where ordinary air (let alone heated hydrogen) might be buoyant within the supercritical hydrogen fluid? But I haven't really thought this through. Also, what's the solubility of water in supercritical hydrogen? I have this weird notion of jellyfish, with an inner food reservoir of stored oxygen gas, floating in the sea of supercritical hydrogen? Wnt (talk) 06:59, 28 October 2012 (UTC)
- Of course "ordinary air" is buoyant. Even so, it doesn't separate out from the supercritical fluid-gas, it mixes yielding ratios proportional to the depth. On earth, the different atmospheric gases (nitrogen, carbon dioxide, oxtgen, argon, etc.) have differing bouyancies. Brownian motion ensures that they don't separate out into layers, I think it is called a Maxwell-Boltzman distribution, we have a page on it. Plasmic Physics (talk) 07:58, 28 October 2012 (UTC)
- Well, if it's true - and I don't know it is - it would be very surprising to me to find some special set of temperature/pressure conditions where oxygen is lighter than hydrogen, and more so to find them in reality. The point is that a jellyfish, even a single celled ancestor that can remain suspended due to its small size, would be readily able to separate oxygen from the supercritical sea in which it lives, and store it in little vacuoles where it would no longer mix. I'd read about the notion of "gasbag" organisms on Jupiter before, but had the impression that authors were only thinking of hot hydrogen balloons, designs that life has never created and would seem hard pressed to evolve. Wnt (talk) 13:55, 28 October 2012 (UTC)
- Of course "ordinary air" is buoyant. Even so, it doesn't separate out from the supercritical fluid-gas, it mixes yielding ratios proportional to the depth. On earth, the different atmospheric gases (nitrogen, carbon dioxide, oxtgen, argon, etc.) have differing bouyancies. Brownian motion ensures that they don't separate out into layers, I think it is called a Maxwell-Boltzman distribution, we have a page on it. Plasmic Physics (talk) 07:58, 28 October 2012 (UTC)
- If Saturn is anything like Jupiter, then complex life is going to have a tough time in the atmosphere of Saturn. Jupiter generates biotoxic levels of high-frequency EM radiation like UV and X-ray, caused by perpetual lighting, so bright you can read a book by them. Plasmic Physics(talk) 01:12, 29 October 2012 (UTC)
- Sounds like photosynthesis is easier there than I was picturing. :) Really, I think life can resist such mutagenesis if it needs to. Deinococcus radiodurans and all that. Wnt (talk) 01:31, 29 October 2012 (UTC)
- If Saturn is anything like Jupiter, then complex life is going to have a tough time in the atmosphere of Saturn. Jupiter generates biotoxic levels of high-frequency EM radiation like UV and X-ray, caused by perpetual lighting, so bright you can read a book by them. Plasmic Physics(talk) 01:12, 29 October 2012 (UTC)
- Just remember that any trip to a gas giant will be an one way trip unless you have engines made of Unobtainium. The gravity well is huge[4] and the exponential mass ratio due to the Tsiolkovsky rocket equation does not make it easier to reach escape velocity.Gr8xoz (talk) 13:32, 27 October 2012 (UTC)
- I'm a great fan of using nuclear isomers as a power storage, with induced gamma emission. ;) Wnt (talk) 01:44, 28 October 2012 (UTC)
- What are you implying, if it's not gioing to be easy for a rocket to do the job, then you're supposing an inferior jet-engine can? Plasmic Physics (talk) 02:35, 28 October 2012 (UTC)
- I was only suggesting a compact energy storage - how you turn that into thrust is another matter. Ion drive perhaps (after you've flown as high as possible) - I don't have any inspired suggestion there. Wnt (talk) 04:06, 28 October 2012 (UTC)
- What are you implying, if it's not gioing to be easy for a rocket to do the job, then you're supposing an inferior jet-engine can? Plasmic Physics (talk) 02:35, 28 October 2012 (UTC)
- In that case, I suggest inventing a slipspace-drive, and tunneling through spacetime. Plasmic Physics (talk) 06:33, 28 October 2012 (UTC)
- Talking about superciticality, did you know that Venus has an invisible, global ocean of supercritical carbon dioxide? You should be able to notice it as a perpetual fog in the distance. In closer, you should be able to notice a shimmering motion like a mirage, but more ordered, indicating a hydrodynamic flow.Plasmic Physics (talk) 08:22, 28 October 2012 (UTC)
- This ocean is at least an average of 2.5 km deep, that is the supercritical/gas limit. That makes me wonder how fast the terminal velocity changes in that 2.5 km, does the density change noticably? Plasmic Physics (talk) 01:50, 29 October 2012 (UTC)
- I added a question about the crux of this (and the part about buoyancy of oxygen above) at Wikipedia:Reference desk/Science#Does the ideal gas law apply to supercritical fluids? Wnt (talk) 04:32, 29 October 2012 (UTC)
Why don't my coffee grounds grow mold?
Does it depend on the roast? I use a very dark roast. I can forget about coffee grounds for a week or more and yet no mold grows on them! The other time I was taking over an apartment from someone else and I accidentally drank very old coffee. (I wondered why it was cold-- it was a machine that "stored" the brew in a holding tank, which I didn't know of-- I thought I was drinking fresh brew). It tasted fine. 71.207.151.227 (talk) 22:39, 26 October 2012 (UTC)
- Well, the (almost) boiling temperature at which the coffee is made would sterilise it. If it's kept well sealed from the air after that, it should restrict growths of many kinds. HiLo48 (talk) 23:01, 26 October 2012 (UTC)
- Coffee grounds are largely fiber and alkaloid toxins. Any sugar, fat and protein has been denatured or roasted out of them, Funguses aren't miracle workers--they require actual food. μηδείς (talk) 23:16, 26 October 2012 (UTC)
- I don't really see why denatured protein wouldn't be a perfectly adequate food source for a fungus. Presumably they need to hydrolyze the protein components to get any nutritional value out of them anyway; If anything wouldn't you expect unfolded protein to be more accessible to proteases and various digestive enzymes? (+)H3N-Protein\Chemist-CO2(-) 00:12, 27 October 2012 (UTC)
- A lack of moisture is a limiting factor. Mold can grow on coffee. Here are Guidelines for the Prevention of Mould Formation in Coffee.Smallman12q (talk) 01:17, 27 October 2012 (UTC)
- Yes, wet, used coffee grounds left in the coffee filter in a coffee maker grew an impressive colony of mold, while I was on vacation. StuRat(talk) 02:28, 28 October 2012 (UTC)
how bright is the white dwarf
I don't know the luminosity of the white dwarf will be but I know white dwarf is a comparable size of earth. Is white dwarf going to be 100 times dimmer than solar luminosity, I can't find it anywhere on the internet. But formation and evolution of the solar system said white dwarf will start at 100 times brighter than get dimmer. I also confuse on do white dwarf shrink right away to earth sized diameter, or it will start to be a comparable size with low-mass stars than when white dwarf stars gets older it gets smaller, in other words fresh new white dwarf will be bigger, and full-wedge white dwarf will be smaller. How long will white dwarf last before becoming a black dwarf? 7 billion years? 10 billion years? or White dwarf will start at UV end of stellar spectrum and gradually skew to IR end of stellar spectrum and gradually works toward black dwarf.--69.226.43.174 (talk) 23:42, 26 October 2012 (UTC)
- White dwarf would be about 100*100=10,000 dimmer than the Sun if it had the same temperature and were the size of the Earth. However the majority of known white dwarfs are hotter than the Sun and therefore more luminous than 1/10,000. You can read more in the white dwarf article. Ruslik_Zero 12:11, 27 October 2012 (UTC)
Our article said this surface temperature range corresponds to a luminosity from over 100 times the Sun's to under 1/10,000 that of the Sun's I don't get how white dwarf can be at first 100 times brighter than sun, I know white dwarf will have to star from UV end of the spectrum and gradually work way to IR end of spectrum. Does white dwarf have to be immediately the size of Earth, or it will be bigger at the start and then gradually become smaller.--69.226.43.174 (talk) 20:41, 27 October 2012 (UTC)
- Well the brightness is the fourth power of the temperature. So if a whitedwarf is 5 times hotter than the sun, it would be 5x5x5x5 times as bright or 625 times. Graeme Bartlett (talk) 21:56, 27 October 2012 (UTC)
October 27
Survive on alcohol
Can you survive longer if you have nothing else to drink except alcohol? Comploose (talk) 11:58, 27 October 2012 (UTC)
- Survive longer than what? What kind of alcohol? It's possible that, say, in the absence of a water source, you would survive much longer drinking beer than drinking nothing, for example (beer is a source of water and calories and is not as dehydrating as most people believe). But if you're asking whether substituting all of your water with ethanol is going to make you live longer, the contrary is certainly the case. --Mr.98 (talk) 12:34, 27 October 2012 (UTC)
- Penny, behind the bar: "What can I get ya?"
- Sheldon: "Alcohol."
- Penny: "Can you be more ... specific?"
- Sheldon: :"Ethyl alcohol."
- --Trovatore (talk) 23:00, 27 October 2012 (UTC)
- A very logical answer, considering how much less desirable methyl alcohol is as a beverage. :-) StuRat (talk) 23:15, 27 October 2012 (UTC)
- Yes, if you have just alcoholic beverages (vodka, wine, whiskey) at hand, would you survive longer drinking some of it or not drinking anything.Comploose (talk) 12:49, 27 October 2012 (UTC)
- I think there must bee some limit between bear and 100 % alcohol above wich it is beter to drink nothing, but i do not know what that limit is.Gr8xoz (talk) 13:04, 27 October 2012 (UTC)
- Before sanitation, people drank beer in Northern Europe and diluted wine in Southern Europe instead of water, which carried diseases. You can survive on those as your beverage indefinitely. Anything much stronger than beer will dehydrate you. μηδείς (talk) 19:41, 27 October 2012 (UTC)
- Although (in England) they often used to drink small beer which had a very low alcohol content. "Some workers (including sailors) who engaged in heavy physical labour drank more than 10 Imperial pints (5.7 litres) of small beer during a workday to maintain their hydration level. This was usually provided free as part of their working conditions, it being recognised that maintaining hydration was essential for optimal performance."Alansplodge (talk) 18:00, 29 October 2012 (UTC)
- This is often said, but the supposed mechanism is that it makes you urinate more. I seriously doubt that happens if you're close to the level of water you need to survive. If your choices are, drink nothing, or drink wine, I'd be very surprised if the answer isn't "drink wine". --Trovatore(talk) 23:03, 27 October 2012 (UTC)
- Before sanitation, people drank beer in Northern Europe and diluted wine in Southern Europe instead of water, which carried diseases. You can survive on those as your beverage indefinitely. Anything much stronger than beer will dehydrate you. μηδείς (talk) 19:41, 27 October 2012 (UTC)
- In addition to the concentration of alcohol in the alcoholic beverage, the quantity would also matter. Even with beer, guzzling too much could lead to vomiting and other medical problems which would shorten your life. Sipping it, on the other hand, should sustain your life indefinitely (until you die of malnutrition after many years, at least). I wonder what form of malnutrition would eventually get you. Perhaps rabbit death, due to a lack of fat (although that article says that carbs prevent it, and beer has carbs) ? Also note that some alcoholics do, indeed, have a diet of exclusively alcoholic beverages. StuRat (talk) 23:18, 27 October 2012 (UTC)
- I don't question your own experience, Stu, but in decades of consuming beer and wine, I don't recall it ever causing me to vomit. Edison(talk) 01:21, 28 October 2012 (UTC)
- I notice people's tendency to vomit varies greatly. I vomit easily, so have never suffered a hangover, and the later effects of food poisoning are minimized. Sure, it's unpleasant, but better to get the poison out of you than leave it in. StuRat (talk) 01:44, 28 October 2012 (UTC)
- Oh dear! See Lightweight ;-) Alansplodge (talk) 18:05, 29 October 2012 (UTC)
- I like to think that my body is smarter than I am. When I try to ingest poison, it gets rid of it in the most expeditious manner possible. StuRat (talk) 18:10, 31 October 2012 (UTC)
Global remaining Iron and aluminum ore resources
In wind power critical reader comments to articles about wind power in a Swedish magazine I have seen statements that we only have about 10 tones of iron left per person to mine.
Is that really realistic? How about aluminum?
Obviously there are a lot more iron and aluminum in the accessible part of the earths crust since 5% and 8% of the crust is iron and aluminum respectively.
Therefore the real question is how much remaining resources do we have that can be refined to metal to a cost less than let say twice the current production cost?
As I understand it is there much biased information circulating when it comes to estimates of available natural resources. Is there any reliable estimates? Gr8xoz (talk) 12:57, 27 October 2012 (UTC)
- Sounds like invented stuff. Comploose (talk) 13:02, 27 October 2012 (UTC)
- USGS figures for 2012
- Iron - "World resources are estimated to exceed 230 billion tons of iron contained within greater than 800 billion tons of crude ore."[5]
- Aluminum - not sure [6] but you might find further details at the USGS' site. Or try the BGS' World mineral statistics Sean.hoyland - talk 13:24, 27 October 2012 (UTC)
- Thank for your answer!
- I are confused about their definition of resource:
- "Resource.—A concentration of naturally occurring solid, liquid, or gaseous material in or on the Earth’s crust in such form and amount that economic extraction of a commodity from the concentration is currently or potentially feasible."
- What do they mean by "potentially feasible"? At what estimated cost?
- 230 billion tons is about 33 tone per person, while more than 10 tone per person it is not that much actually, given estimated population increase to 10e9 to 12e9 persons during the next 100 years and increased GNP per capita. Gr8xoz (talk) 16:05, 27 October 2012 (UTC)
- I think the Solwara 1 project to mine seafloor for massive sulphide deposits is a good example of where "potentially feasible" is transitioning to "currently feasible" because of the economics and the technology available. Sean.hoyland - talk 16:17, 27 October 2012 (UTC)
- Another example that springs to mind to illustrate the difference between potentially and currently feasible is the hugeImouraren uranium deposits. Although the resource was discovered almost 50 years ago, and mining it has been potentially feasible for a long time, the mine won't be operational until next year for all sorts of reasons. Sean.hoyland - talk 16:35, 27 October 2012 (UTC)
- Thank you for the link about the Solwara 1 project, it was very interesting, as usual the assesments of the enviromental impact differ very much. It will be interesting to follow.
- The limit between "potentially feasible" and not "potentially feasible" is still very fuzzy to me, even the iron core of the earth would be "potentially feasible" if you extrapolate capabilities far enough in to the future and are into science fiction. Gr8xoz (talk) 22:44, 27 October 2012 (UTC)
- Even given the low estimate of 10 tones of iron per person, I don't see that as a problem. With recycling, that much should last for centuries, by which time we will be able to mine less accessible sources. StuRat (talk) 00:07, 28 October 2012 (UTC)
- Aluminum reserves depend on if you only count Bauxite, or if you count all aluminum ores (aluminum makes up 8.3% of the Earth's crust by weight). --Carnildo (talk) 02:24, 30 October 2012 (UTC)
- I know that, see my first post, the question is how much of these 8 % that can be extracted at less than twice the current production cost?Gr8xoz (talk) 17:04, 30 October 2012 (UTC)
- That really depends on if there's a breakthrough in processing techniques or not. Anything that makes electricity cheaper, or that increases the variety of usable ores, will increase the amount that can be extracted. Known bauxite reserves are good for at least another century, so it's not a factor that can be ignored. --Carnildo (talk) 01:49, 31 October 2012 (UTC)
- I know that, see my first post, the question is how much of these 8 % that can be extracted at less than twice the current production cost?Gr8xoz (talk) 17:04, 30 October 2012 (UTC)
Make radioactive material decay faster
Why do you have to wait until radioactive material decays? Isn't any way of just making it decay? Comploose (talk) 13:02, 27 October 2012 (UTC)
No idea about these things at all but is your question related to this article (http://www.businessinsider.com/eu-builds-giant-laser-2012-10)? I came across it earlier and it was referring to a powerful laser that could theoretically destroy nuclear waste. A google search for 'speed up radioactive decay' leads you to a number of science articles talking about this subject. ny156uk (talk) 13:52, 27 October 2012 (UTC)
- No there are no known way of speeding up spontaneous radioactive decay. But some radioactive isotopes can be transformed in to stable isotopes by very strong radiation of different types. Gr8xoz (talk) 15:41, 27 October 2012 (UTC)
- See Radioactive_decay#Changing_decay_rates for a summary of what is known. As Gr8xoz says, there is no known method of significantly changing decay rates, even in theory, that is both simple and safe and does not involve large amounts of external radiation or enormous power consumption.Gandalf61 (talk) 16:08, 27 October 2012 (UTC)
- I still wonder if Förster resonance energy transfer could work on induced gamma emission, though one person's opinion before was "probably not".[7] Wnt (talk) 19:02, 27 October 2012 (UTC)
- It took me awhile to find a good link for discussing the lasers-blasting-nuclear-waste issue. Apparently with a high energy laser can transmute isotopes — so you'd try to transmute long-lived iodine-129 into short-lived iodine-128, which then most of the time becomes stable xenon-128. So I guess that's something, if it really works and scales up.--Mr.98 (talk) 19:34, 27 October 2012 (UTC)
- I remember reading something about 10 years ago that indicated a discrepancy had been found in the decay rate of a certain heavy isotope under varying physical conditions. Does this strike a bell with anyone? μηδείς (talk) 19:37, 27 October 2012 (UTC)
- There are several references from 1996 to present about an observed effect of physical and/or chemical conditions in the section that Gandalf61 mentioned. Not sure if that is close to the timeframe and/or idea you are remembering. DMacks (talk) 19:43, 27 October 2012 (UTC)
- Yes, thanks, I think it was the phenomenon in the fourth paragraph, since I remember it somehow being related to the sun. Apparently it may just be error.μηδείς (talk) 19:48, 27 October 2012 (UTC)
- There is a way to decrease a half-life - it involves alloying the radioisotope, and cooling it to extremely low temperatures. I don't remember the constituents of the alloy. Plasmic Physics (talk) 23:54, 27 October 2012 (UTC)
- All of the responses so far seem to deal with changing the spontaneous reaction rate. I don't interpret the Q as being limited to that. Why not jam it into a nuclear reactor, and subject it it to whatever dose of whatever type of radiation is needed to convert it into something else, and so on, until you arrive at something stable ? Sure, it's probably not practical, but it is possible. StuRat (talk) 00:01, 28 October 2012 (UTC)
- That's the laser idea, more or less. I suspect the problem is that most of the time you will end up with more radioactive material, not less. It's not so much that you'll arrive at something stable by just adding neutrons to it, but you might go from an element with a half-life of a million years to one with a half-life of a few minutes, which then maybe decays into something stable. (So you're actually making it more radioactive, but briefly so.) My intuition though is that in any real-world materials you'll have no net change in long-term radioactivity, since the isotopes will be quite a soup of possibilities. Not to mention the fact that you're probably creating more waste by running the reactor than you're eliminating... presumably this is what the laser scheme is seeking to get around. I'm kind of dubious of it working in practice, but I'm not a nuclear physicist. --Mr.98 (talk) 16:15, 28 October 2012 (UTC)
Battery with unexpectedly high open circuit voltage
Someone I know was working on a device, removed the lead-acid battery and measured its open circuit voltage and got 7.0 V. But it's a nominally 6V battery so it would be 3 cells and each lead-acid cell has a voltage of 2.1 volts so how did the battery give a voltage so much higher than 2.1*3=6.3V? (The battery was apparently defective but they didn't have the equipment to measure its voltage under load, they just replaced it and the device worked so they deduce the battery was bad.) Are there any chemical processes that would occur in a battery that is worn out that would cause a higher open circuit voltage? RJFJR(talk) 14:11, 27 October 2012 (UTC)
- For a lead acid battery of the sort used in portable electronic equipment, computer UPS units and other "indoor" applications, the full charge voltage is considered to be 2.35 V per cell (at 25 deg C), not 2.1 V. corresponding to 7.05 V for a nominal 6 V battery. When discharging into a load, a fully charged lead acid battery's voltage rapidly falls to quite close to 2 V per cell and then holds close to that voltage for a comparitively long time, before dropping rapidly as reaches the point of full discharge. Hence the common usage of the terms "6 V", "12 V" etc for 3 and 6 cell batteries.
- However, when measuring the voltage of a battery removed from service which may well be nowhere near full charge, you should consider the accuracy of the meter used. A cheap analogue multimeter for instance, may have an accuracy of +,- 3% of full scale. If the range selected to measure is a 10 V full scale range, then the possible meter error in measuring exactly 6 V is 10 x 0.03 = 0.3 V error. So, if you have a reading of 7.0 V, it could actually be as low as 6.7 V without the meter being faulty. Cheap digital multimeters are generally accurate to 1% of full scale or better, with an additional error of plus or minus 1 digit. Seehttp://en.wikipedia.org/wiki/Lead_acid_gel_battery
- Keit120.145.36.13 (talk) 15:31, 27 October 2012 (UTC)
The 'cats that look like Hitler' effect?
Along a similar line to black-dog bias, is it true that animal shelters often find it difficult to adopt out cats which facially resemble Adolf Hitler? There was one particular cat in the news last year where this was claimed to be the case. The existence of the http://www.catsthatlooklikehitler.com website would appear to demonstrate that this effect (or at least that people do really notice that some cats look like Hitler) does exist in some form or other...--Kurt Shaped Box (talk) 14:58, 27 October 2012 (UTC)
- Surely it looks more like Charlie Chaplin. Wnt (talk) 15:26, 27 October 2012 (UTC)
- (e/c)If that was claimed to be the case, why are you asking us? However, all those cats have owners who are presumably perfectly happy with them. Cats look no more like Hitler than they look like Charlie Chaplin.--Shantavira|feed me 15:32, 27 October 2012 (UTC)
- I ask because I was wondering if the 'cat thing' had ever been studied to the same extent as the 'dog thing', or if it was only ever really an isolated case that was reported as a widely-known 'fact' in the news... --Kurt Shaped Box (talk) 15:38, 27 October 2012 (UTC)
- We have one of those. We call her charlie because we think she looks like Charlie Chaplin. Dauto (talk) 18:03, 27 October 2012 (UTC)
- Just in response to Shantavira's 'Cats look no more like Hitler than they look like Charlie Chaplin' point... How's aboutthis one then? --Kurt Shaped Box (talk) 18:45, 27 October 2012 (UTC)
- ROFL - it really does! There must be some deep lesson about caricature and facial expressions here. Wnt (talk) 19:04, 27 October 2012 (UTC)
- who wouldn't adopt a cat that looks like Hitler? I would, and I think it would appeal to both Nazi and antiNazi both.Gzuckier (talk) 01:53, 29 October 2012 (UTC)
- You might name it Lorenzo. —Tamfang (talk) 23:19, 29 October 2012 (UTC)
Is theistic evolution inherently teleological?
67.163.109.173 (talk) 15:50, 27 October 2012 (UTC)
- No. Comploose (talk) 16:07, 27 October 2012 (UTC)
- Please explain why. Thanks in advance. 67.163.109.173 (talk) 16:16, 27 October 2012 (UTC)
Sorry, a clarification. Specifically, is theistic evolution in the context of an Abrahamic religion that clings to a belief that man (i.e., H. sapiens) is created in (that religion's) God's image, inherently teleological? 67.163.109.173 (talk) 16:31, 27 October 2012 (UTC)
- Essentially, yes. Christians believe that God created the universe for a purpose, and theistic evolutionists believe that evlution plays a role in achieving that purpose, so yes, it's inherently teleological. It follows from the nature of the Abrahamic God, who is a person, has a will, and has goals. Dominus Vobisdu (talk) 16:37, 27 October 2012 (UTC)
- Shouldn't this be on the Humanities desk? Theistic evolution, Teleology - these are things scientists generally look up on Wikipedia. It seems like there should be some range of possibilities about how specific the purpose might be, but no doubt there is some philosophy that has actually put a name to that? Wnt (talk) 18:53, 27 October 2012 (UTC)
- If you believe that God is "guiding" evolution (which is usually what is meant by theistic evolution), then yes, it is inherently teleological (there is a goal or end-point). You could, I guess, claim that God just flips a coin and evolves at random... but that's not what anybody ever means by theistic evolution. If you had a polytheistic religion and polytheistic evolution, I guess I could imagine something that was a little less guided... but again, I don't really see that ever being referred to as theistic evolution. --Mr.98 (talk) 19:36, 27 October 2012 (UTC)
- Well, theoretically you can picture a God who says "hey, I want a universe full of really cool stuff, but I want the critters to make up their own minds ... when they hit on something cool I'll know it when I see it". (Not saying that's how it is, but I'm not sure the answer is obvious) Wnt (talk) 19:58, 27 October 2012 (UTC)
- But that's not what "theistic evolution" means — every time it is used, it is assumed that God wants humans to eventually show up. That's teleological. You can certainly have a worldview that says there is a God out there somewhere not paying any attention to anything and just saying, "oh, cool, humans" when they evolve without any intervention, but that's not theistic evolution, that's just natural selection with an inattentive deity. ;-) --Mr.98 (talk) 16:11, 28 October 2012 (UTC)
- As a Christian agnostic, in as much as I believe God and evolution have any relationship, this is what I believe. There's a metaphysical end towards which events can be said to be driving, but the material world is only subject to its own rules - God doesn't 'prod evolution along' in any sense. By 'theistic evolution', I understand a position in which God does do that, and so physical processes are viewed as teleological. AlexTiefling(talk) 20:47, 27 October 2012 (UTC)
- Not an answer, and you might get someone to express this with sources and more clarity on the Humanities desk: There are phenomena such ascarcinisation which hint that Nature, by some means, favors certain recognizable designs, and that evolution does not proceed entirely toward random ends. As the natural laws which favor these shapes in the end are part of an overall structure of logic, it would seem that if this structure is up to God (i.e. that what logically can be deduced from a given set of premises is chosen by God) then those end designs are chosen by God. I note that many people do not think of God as having this sort of power - the power to decide that two and two are three, without otherwise changing or contradicting the rules of mathematics - but it would seem to me that this would be one of the most characteristic powers that define divinity apart from mortal or technological ability. Wnt(talk) 23:42, 27 October 2012 (UTC)
- Nature does favor certain things - it's called natural selection... Arc de Ciel (talk) 00:13, 28 October 2012 (UTC)
- What's the difference between a) a H. sapiens-shaped God making a universe with laws of physics and initial conditions such that, when left alone for about 13-some billion years without being prodded, little beings that resemble it naturally follow, and b) a H. sapiens-shaped God making a universe with laws of physics and initial conditions not necessarily such that about 13-some billion years later little beings shaped like it naturally follow, but prods along "randomness" such that it happens? Just the point (pre-big-bang or post) at which one considers the act of setting up the dominoes, no?67.163.109.173 (talk) 02:22, 28 October 2012 (UTC)
- First of all, the word randomness is a bad word to use. Survival of the fittest is far from random.
- If that god is all-knowing then there would be no difference.
- The bible would have to be rewritten of course, something along the lines of: "And in the beginning, God created Heaven and Earth, he created some bacteria, called them Adam and Steve, and waited several billion years for them to evolve in a shape similar to his own".They (talk) 03:27, 28 October 2012 (UTC)
- Actually, this is a fallacy: an author doesn't live in time dimension of the universe he creates. (George Lucas didn't live during the time of the Galactic Republic) So science can't tell you how long God took to devise the universe, nor in what order he developed the composite elements. Wnt(talk) 04:03, 28 October 2012 (UTC)
- Are you saying we are all fictional characters and that reality as perceived by most humans is actually a simulated reality?
 I like the Matrix too, but I am pretty sure its fiction.
I like the Matrix too, but I am pretty sure its fiction. - God did not write the bible. Humans wrote it. Some people claim that the people who wrote the bible had divine inspiration, which is weird because it contains many factual errors (e.g. the story about streaked rods and Jacob's goats and the idea that the moon emits light) and major portions of it are plagiarized from earlier works. Different parts of the bible were written by different people, over a period of hundreds of years. SeeOld_Testament#Composition. They (talk) 04:13, 28 October 2012 (UTC) p.s. You may enjoy this video.
- My purpose here isn't to argue for Biblical infallibility, but to emphasize that science cannot disprove certain high-order speculations on the nature and origin of the world, specifically whether it is an authored work. Being "pretty sure" The Matrix is fiction is based on what experimental evidence? It is fairly clear that the films indeed are themselves based on religious, specifically Christian ideas. Wnt (talk) 14:01, 28 October 2012 (UTC)
- I am sorry, I cannot debate about topics like "What experimental evidence does They have that suggests that reality as experienced by most humans is in fact real and not some kind of simulated reality like in The Matrix" without being stoned. And no, I don't mean that in the Biblical sense, I am referring to recreational drugs. They (talk) 19:45, 28 October 2012 (UTC)
- My purpose here isn't to argue for Biblical infallibility, but to emphasize that science cannot disprove certain high-order speculations on the nature and origin of the world, specifically whether it is an authored work. Being "pretty sure" The Matrix is fiction is based on what experimental evidence? It is fairly clear that the films indeed are themselves based on religious, specifically Christian ideas. Wnt (talk) 14:01, 28 October 2012 (UTC)
- Are you saying we are all fictional characters and that reality as perceived by most humans is actually a simulated reality?
- As far as I understand the theory of evolution (not theistic), the randomness is not what is successful, but the randomness is in genetic mutation, which happens, and then if a given mutation just happens to be better suited to the habitat in which the organism exists, and the organism reproduces with more success than other organisms without the random mutation, that random mutation is said to be selected.67.163.109.173 (talk) 04:01, 28 October 2012 (UTC)
- That is correct AFAIK. They (talk) 05:02, 28 October 2012 (UTC)
- Indeed. Having some mutations is still essential though, since if you didn't have any the organism would never be able to adapt to a changing environment. It's been suggested that if DNA repair were more efficient, this would be selected against for this reason.
- Just to throw in another factor: as far as we know, our universe contains randomness at the quantum level. I think a reasonable argument could be made (if you have infinite control at the time of the Big Bang but no intervention afterwards) that there are no initial conditions that could guarantee an outcome as specific as the evolution of Homo sapiens. You could have a particular probability of everything working out in a certain way, but it wouldn't be 100%. Arc de Ciel (talk) 08:00, 28 October 2012 (UTC)
- Note that, in accordance with the modern theory and observance of evolution, progressive adaption and evolution is not only dependent on random mutation of ancestral genes, though that is the most common path. It has been shown that co-existent and infective organisms can insert advantageous and disadvantagoes genes into the host DNA. Occaisonally, DNA fragments of something eaten (particularly in single cell organisms or organisms comprising a clump of similar cells, get swallowed up by the cell machinery and incorporated into the cell DNA to be then passed on to daughter cells if it is passive or confers and advantage. It has been shown (described in Scientific American last year as I recall) that this can occur even in humans thought it must occur very infrequently and has obviously in humans no evolutionary significance (only changes in eggs or sperm can be passed on). In some cases, an infective agent multiplies within the cell and gets passed down to daughter cells. Mitochondia, which are essentially compplete parasite organism whithin each cell, confer a very considerable energy advantage to the host. Wickwack 58.169.248.13 (talk) 13:35, 28 October 2012 (UTC)
- Actually, this is a fallacy: an author doesn't live in time dimension of the universe he creates. (George Lucas didn't live during the time of the Galactic Republic) So science can't tell you how long God took to devise the universe, nor in what order he developed the composite elements. Wnt(talk) 04:03, 28 October 2012 (UTC)
- (Outdenting.) One nice little way to sum up naturalistic evolution is to say that there is random variation but with selective retention. You need both for naturalistic evolution — there has to be a pool of variation that isn't guided, there has to be mechanisms in place to get rid of the non-useful variations. There is "randomness" to it, but it's only half of the equation. In my experience Creationists focus on the "random" and forget about the "selection", hence getting themselves in knot about tornados in junk yards, which is randomness without selection. --Mr.98 (talk) 16:11, 28 October 2012 (UTC)
Mythbusting Mary Poppins
I know that the movie was meant to be a modern-day fairytale and not intended to be realistic at all, but all the same, there are a couple things in it that I think might make for a good Mythbusters episode. So here goes...
1. Umbrella paraglider
I know it's not possible to take off and fly up without a source of power, but is it possible for a fairly slim woman like Julie Andrews to use an ordinary (non-magical) umbrella to glide down by jumping from a height or by sprinting into a strong headwind? If so, given that a typical umbrella has an area of about 16 sq. ft., what would be the stall speed if running into the wind, and what would be the terminal velocity if jumping from a height? Also, if said fairly slim woman with non-magical umbrella was trapped in a burning building, would it be possible for her to escape using this method and survive? (Note that I do not specify that she survive unhurt.) And last but not least, is it true that a woman by name of Mrs. Graham had fallen from a balloon but survived the fall due to her open umbrella and/or inflated skirt acting as a parachute?
- I don't see how any normal umbrella could be strong enough to stay concave side down. Read aboutthis fatal attempt from a seventh floor. Also, Mythbustersapparently already busted this myth.[11] Clarityfiend (talk) 21:11, 27 October 2012 (UTC)
- So, the bottom line is, it will NOT help survive jumping from a height, as the terminal velocity would still be too high. Thanks!24.23.196.85 (talk) 21:22, 27 October 2012 (UTC)
- But then again, what about taking off by sprinting into a strong headwind? (I actually managed to do this, when I was a boy of 14.)24.23.196.85 (talk) 21:25, 27 October 2012 (UTC)
- Yes I have experienced this as well, with a very strong umbrella and opening it whilst running.--Gilderien Chat|List of good deeds 21:37, 27 October 2012 (UTC)
- You actually lifted off like I did? 24.23.196.85 (talk) 22:14, 27 October 2012 (UTC)
- Details from my own attempt: Wind strength was 4 on the Beaufort scale, gusting to 5; I don't remember my own weight, but I couldn't have been very heavy because I was only 14 and slim; I was running as fast as I could, which could have been as fast as 20 mph (I was a fast runner, but a bad starter, and still am); the umbrella's angle of attack at liftoff was about 5 degrees; and the total air time was about 2 seconds, at an altitude of about 1 to 1.5 feet, covering about 10 yards. 24.23.196.85 (talk) 22:24, 27 October 2012 (UTC)
- Yes, although not that far. Admittedly it was in the lake district, so there was a "lip" adjacent to the track I was on, causing a very strong upwards current of air.--Gilderien Chat|List of good deeds 00:46, 31 October 2012 (UTC)
- Details from my own attempt: Wind strength was 4 on the Beaufort scale, gusting to 5; I don't remember my own weight, but I couldn't have been very heavy because I was only 14 and slim; I was running as fast as I could, which could have been as fast as 20 mph (I was a fast runner, but a bad starter, and still am); the umbrella's angle of attack at liftoff was about 5 degrees; and the total air time was about 2 seconds, at an altitude of about 1 to 1.5 feet, covering about 10 yards. 24.23.196.85 (talk) 22:24, 27 October 2012 (UTC)
- There are giant fans pointing upwards which allow a person without an umbrella to hover over them, so, it stands to reason that they could also do so using a properly reinforced umbrella. Of course, her dress would fly up, but we can just consider that a bonus. :-) StuRat (talk) 00:21, 28 October 2012 (UTC)
- Even more exciting with a proper fox frame and a sufficiently powerful vortex generator I see no reason Nanny may not make it beyond the atmosphere. But perhaps a space fountain would be a better idea. It could be set up to work through the chimneys, and could draw her up by a suitable rare earth magnet in her carpet bag. Rich Farmbrough, 01:16, 28 October 2012 (UTC).
- Great ideas, everyone! (Well, except she wouldn't be able to pass through a chimney, as it's only 13" by 9".) :-D 24.23.196.85 (talk) 01:55, 28 October 2012 (UTC)
- You may be interested in the story of Sarah Ann Henley. --TammyMoet (talk) 10:08, 28 October 2012 (UTC)
- Wow, what an amazing survival story! BTW, a typical woman's skirt would have a surface area of about 20 square feet -- still not enough to survive a landing on a hard surface, but enough to give a fighting chance of surviving a soft-surface impact (as was the case here). 24.23.196.85 (talk) 02:07, 29 October 2012 (UTC)
- Do you base your calculation on a typical crinoline skirt of the era, or a typical modern skirt? Just curious. Some crinolines took a huge amount of material. --TammyMoet (talk) 09:36, 29 October 2012 (UTC)
- BTW, does anyone have any details on Mrs. Margaret Graham the balloonist, and how exactly she survived a 100-foot fall from her balloon? I've read that she survived because her dress flew up and acted like a parachute (similar to what happened with Sarah Ann Henley), but I can't verify this info and Wikipedia has no article about her at all. 24.23.196.85 (talk) 02:30, 29 October 2012 (UTC)
- Wow, what an amazing survival story! BTW, a typical woman's skirt would have a surface area of about 20 square feet -- still not enough to survive a landing on a hard surface, but enough to give a fighting chance of surviving a soft-surface impact (as was the case here). 24.23.196.85 (talk) 02:07, 29 October 2012 (UTC)
- For glinding with an umbrella, read Erich Kästner's The Flying Classroom, which shows it doesn't work. Of course, this is a work of fiction, but it's worth to read anyway. – b_jonas 22:36, 29 October 2012 (UTC)
2. "Posts, everyone!"
When Admiral Boom fires his cannon, would the resulting seismic wave smash the neighbors' crockery and other fragile items, and if so, within what radius? More to the point, is it possible for this to happen without the airborne shockwave also blowing out all the windows?
24.23.196.85 (talk) 20:54, 27 October 2012 (UTC)
- A sound wave could do this, provided it was at the resonant frequency of those items it broke. However, this is extremely unlikely, as that would require a lot of energy at a very high frequency, and that's not something a cannon is likely to create. StuRat (talk) 00:17, 28 October 2012 (UTC)
24.23.196.85 (talk) 20:54, 27 October 2012 (UTC)
- If crockery is sufficiently finely balanced any shock wave could cause it to rattle or fall to the floor. Rich Farmbrough, 01:09, 28 October 2012 (UTC).
- I remember reading that a 19th century British coastal artillery battery (possibly Coalhouse Fort in Essex) used to send a soldier round to the neighbouring houses before practice firing, to tell them to open their windows in case they were broken by the blast. I've just had a look for a reference, but could only find this account (last page) of coastal artillery in action at Weymouthin 1940; “A suspect vessel disregarded recognition signals and so No.1 gun of the fort was ordered to fire a shell across its bows. This gun misfired and so No. 1 gun of the Breakwater fort was so ordered. The shell ricocheted off the water in front of the vessel, passed between it’s masts and headed on for Lulworth! The vessel failed to stop, arriving safely in Weymouth Harbour to disgorge French troops who had fled Dunkirk. The blast from the gun shattered all the windows in the Sapper’s barracks on the Breakwater.” I believe the gun in question was a BL 6 inch Mk VII naval gun, rather larger than the signal cannon in the film. Alansplodge (talk) 01:21, 28 October 2012 (UTC)
- Thanks for the input, everyone! So I gather that the effect would most likely be the opposite -- the windows would shatter, but the crockery would probably remain intact unless precariously balanced? 24.23.196.85 (talk) 01:57, 28 October 2012 (UTC)
- Most often, yes. StuRat (talk) 02:00, 28 October 2012 (UTC)
Collars for control knobs
Control knobs that go on spindles with (or indeed without) a flat sometimes have a steel collar around the part of the knob that slips on to the spindle. (I worked on a machine that put these on a component for central heating thermostats many years ago, which was interesting in its own right for several reasons.) So two questions, what are these collars called, and where can they be obtained? Rich Farmbrough, 18:08, 27 October 2012 (UTC).
- In the electronics industry, there are two kinds of knob (apart from knobs that do not have a metal insert and are force fitted): ones with metal inserts that provide a strong base to put a threaded hole into for a retaining grub-screw, and one s with metal inserts that are longitudinal split, with a peripheral thread thread cut. A nut screwed on this thread progressively compresses the split insert onto the control shaft. The actual knob, cast in plastic, in both types conceals the metal parts. In both cases the metal insert is usually brass and is called a collet - however, when an electronic engineer calls for acollet knob, he usually has the second type in mind. If he wants a grub-screw knob, he'll usually say he wants a grub-screw. The more widely known use of the word collet is in machines and machine tooling. See http://en.wikipedia.org/wiki/Collet. You may like to google collet knobs. Perhaps you had this sort of thing in mind.
- However, there is a another common type that has a plated or passivated spring steel collar or ring that surrounds a molded split part of the knob to clamp it onto the shaft. This type is more common in Asian-made equipment. In this case the metal part should be called a compression ring or compression spring.
- You can obtain control knobs from suppliers specialising in electronic parts. RS Components and Element 14 both have a good range and have stores in many countries. Keit 121.221.226.4 (talk) 02:20, 28 October 2012 (UTC)
- Ah thank you! The word I was searching for was collet but what I actually meant was compression spring. And I have the knob (which is a friction fit, rather than force fit) I just want to reinforce it with a compression spring - if possible. Rich Farmbrough, 03:05, 29 October 2012 (UTC).
- Ah thank you! The word I was searching for was collet but what I actually meant was compression spring. And I have the knob (which is a friction fit, rather than force fit) I just want to reinforce it with a compression spring - if possible. Rich Farmbrough, 03:05, 29 October 2012 (UTC).
October 28
Generator
What is the minimal optimal usage for a 30 amp generator to maximize efficiency of fuel? Equidistant from running generator with nothing hooked up and using all 30 amps to run lights in the daytime.68.83.98.40 (talk) 03:03, 28 October 2012 (UTC) Or how many amps does the gen produce just idling?68.83.98.40 (talk) 03:05, 28 October 2012 (UTC)
- If there's nothing hooked up to the generator (as in, open circuit), then it will produce no amps at all.24.23.196.85 (talk) 03:11, 28 October 2012 (UTC)
Yes, that is one extreme in my example. The other extreme is getting "no real use" while producing all 30 amps .68.83.98.40 (talk) 03:16, 28 October 2012 (UTC)
- If you're asking about the point at which the generator will have the highest efficiency, then it depends on the resistance of the circuit, as well as on the characteristics of the diesel that runs the unit. Generally, the higher the circuit's resistance (given the exact same engine and generator unit), the higher the power setting that you'll have to use in order to get the maximum efficiency. Just my non-expert opinion. 24.23.196.85 (talk) 03:25, 28 October 2012 (UTC)
Thanks, anyone know a typical amp output for a 30 amp generator while idling (20 hp?)68.83.98.40 (talk) 03:29, 28 October 2012 (UTC)
- The internal losses in a generator can be modelled as L = k0 + k1I + k2I2 where k0, k1, and k2 are constants particular to the actual generator and I is the output current. Typically, maximum conversion efficiency is attained at around 65% to 80% of full output. Efficiency at no load is obviously zero as 24.23.196.85 already stated, as the amps output is zero but the engine is running and consuming fuel. Fuel consumption at high idle depends on the engine type (recent model, old model, degree of turbo charging) but will be somewhere around 5% of the full load value for generators capable of 30 A per phase at 415 V. Conversly, if the engine is running at high idle, there cannot be any significant electrical output as if there was, the engine would have to slow down or advance the throttle. The correct term for the condition of a genset running without any load ishigh idle as the engine has to be turning at full RPM to get the correct voltage and be ready for any load being instantly switched on. If the engine is run at low speed idle, like a car engine idling, it is called low idle. Keit 124.182.151.138 (talk) 06:05, 28 October 2012 (UTC)
Nanotchnology and solar cells
How nanosolar cells work ? — Preceding unsigned comment added by41.209.224.61 (talk) 05:21, 28 October 2012 (UTC)
- Nanosolar is the name of a company, see Nanosolar#Technology. They (talk) 05:40, 28 October 2012 (UTC)
what is the development that nanotechnology provides to solar cell? — Preceding unsigned comment added by 41.209.224.61 (talk • contribs)
- Did you click on the link I wrote above? Molecular self-assembly is kind of awesome. They (talk) 09:29, 28 October 2012 (UTC)
i want another resource — Preceding unsigned comment added by41.209.224.61 (talk) 10:43, 28 October 2012 (UTC)
- Well, I don't think we can help you. They (talk) 10:53, 28 October 2012 (UTC)
- why? ...the answer should be from Wikipedia pages — Preceding unsigned comment added by 41.209.224.61 (talk) 11:07, 28 October 2012 (UTC)
- And it is:
| “ | These details involve a semiconductor ink that it claims will enable it to produce solar cells with a basic printing process, rather than using slow and expensive high-vacuum based thin-film deposition processes. The ink is deposited on a flexible substrate (the “paper”), and then nanocomponents in the ink align themselves properly via molecular self-assembly. | ” |
| — Wikipedia editor(s) | ||
- For additional resources dealing with the company, see Nanosolar#References. StuRat (talk) 06:22, 29 October 2012 (UTC)
why is it so hard to find the longitudinal diameter of the hydrogen molecule?
This is different from the VDW radius or the bond length. I need to calculate the mean free path of hydrogen molecules, not hydrogen gas, and google only turns up the VDW radius of the hydrogen atom or the bond length of the hydrogen molecule, which is annoying. 71.207.151.227(talk) 07:41, 28 October 2012 (UTC)
- Please clarify what you want. Pure hydrogen gas consists of a mixture of monatomic hydrogen (H) and diatomic hydrogen (H2). The proportions of H and H2 are a function of temperature - at room temperature it is virtually all H2, and the proprtion that is H2 decreeases as temperature increases, reaching a 50:50 mix at about 4400 K. You can calculate the proportions by solving the modified arrhenious equations for t → very large, or by using dissociation theory. You can get the constants required in either case from standard tables or from the NIST website.
- Since colliding molecules can have any orientaion, you don't need the longitudinal diameter, unless you introduce a steric factor as well - it will then be equivalent to the collision diameter given in most tables.
- Since hydrogen gas is a mix of H and H2, at high temperatures if you need acuracy you need to calculate for 3 types of collisions: H and H, H and H2, and H2 and H2, at the appropriate concentrations. Wickwack 58.169.248.13 (talk) 12:02, 28 October 2012 (UTC)
- If you know the VDW radius and the bond-length, a diatomic is obviously linear, so the "VDW length" is radius+bond+radius. But depending what you're shooting at it, the scattering cross-section used to calculate the mean free path in an experiment might be some other function rather than simple geometric size (especially one that would need kinetic averaging among various orientations). DMacks (talk) 16:49, 28 October 2012 (UTC)
Elements, other than Hydrogen and Uranium, for nuclear fusion and fission
It is well-known that hydrogen present in Sun helps in nuclear fusion inside it. Uranium-235 is generally used for nuclear fission on earth by scientists for energy generation. Are there other elements which can be used for nuclear fission and fusion ? Sunny Singh (DAV) (talk) 08:16, 28 October 2012 (UTC)
- See Nuclear fuel cycle, Nuclear power, Fusion power and perhaps Nuclear reprocessing which describes possibilities of main interest in a resonable amount of detail (albeit not in a simple format). The most common proposed alternative to uranium as the fuel source is Thorium-232, note however this for use in a breeder reactor to produce uranium-233 and later 235. You can also design a breeder reactor to use uranium-238. Plutonium-239 may be used together with uranium although this has likely been produced from uranium either intentionally (perhaps for weapons) or as a byproduct as a nuclear reactor. There is also some interest in using the minor actinides byproducts as fuel, our article mentions americium as one currently being tested. When it comes to fusion, deuterium (hydrogen-2) or hydrogen-1 is generally used in most proposals although you may also need boron or lithium as part of the cycle. Nil Einne (talk) 09:23, 28 October 2012 (UTC)
- Here's something interesting: the US has so much neptunium, that they don't know what to do with it all, so they've decided to bury it. Plasmic Physics (talk) 09:45, 28 October 2012 (UTC)
- In principle, most nuclei can be used in fusion, and just that happens in stars. See stellar nucleosynthesis. Similarly, most heavy nuclei can be split. There is a natural maximum of (per nucleon) nuclear binding energy at around Fe56, so "normal" processes will rarely produce heavier elements from lighter ones, and vice versa. There are some fusion- and fission reactions that are more suitable for technological energy generation, and these usually involve very light and very heavy nuclei. --Stephan Schulz (talk) 10:29, 28 October 2012 (UTC)
- Helium-3 is a good material to use for nuclear fusion. 24.23.196.85 (talk) 02:00, 29 October 2012 (UTC)
- In principle, most nuclei can be used in fusion, and just that happens in stars. See stellar nucleosynthesis. Similarly, most heavy nuclei can be split. There is a natural maximum of (per nucleon) nuclear binding energy at around Fe56, so "normal" processes will rarely produce heavier elements from lighter ones, and vice versa. There are some fusion- and fission reactions that are more suitable for technological energy generation, and these usually involve very light and very heavy nuclei. --Stephan Schulz (talk) 10:29, 28 October 2012 (UTC)
Follow up question,
In fission energy emitted by heavy atoms (other than uranium) emit same amount of energy as emitted by uranium, yes or no and why. Does the same case happen with hydrogen in fusion ? Sunny Singh (DAV) (talk) 12:26, 28 October 2012 (UTC)
- No, the amount of energy released is basically different for each nuclear reaction. See the link on nuclear binding energy above - the energy you get from splitting or fusion is the difference in the sum of binding energy before and after the reaction for all involved nucleons. But for most processes, the nuclear binding energy is orders of magnitude greater than for chemical reactions. So any nuclear fuel is relatively very energy-dense. --Stephan Schulz (talk) 14:33, 28 October 2012 (UTC)
- For fusion reactions not taking place inside of a star, fusing elements heavier than hydrogen is very difficult. The reason for this is that the forces required to overcome the electromagnetic repulsion of the nuclei to be fused become pretty prohibitive — and hey, even fusing hydrogen is hard, outside of a star. Even hydrogen bombs don't fuse anything larger than tritium and deuterium. (They use lithium in their fuel, but only because it turns into tritium after being hit with neutrons.) --Mr.98 (talk) 16:04, 28 October 2012 (UTC)
Does the ideal gas law apply to supercritical fluids?
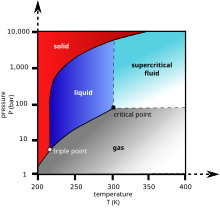
Really basic and important physics here, yet I don't know the answer and our articles don't seem to give it: does the ideal gas law apply to some, all, or no supercritical fluids? What are the boundaries of the region where it applies?
Looking this up in our archive, I found[12] and[13] which expressed doubt it applied; Google delivered[14] which, unless I misinterpret, says it applies to all supercritical fluids. But I have a hard time picturing a supercritical fluid can keep to that law under up to infinite pressure! [15] is a fringy-looking publication which says the critical point is really the boundary of four phases, one of which is a "delta phase" with multiple molecules in association; I think that is saying there is a boundary within supercritical fluids between those which follow ideal gas law and those that don't. In short: please, somebody who really knows their physics should figure this out, and update those two articles accordingly. Thanks. Wnt (talk) 15:42, 28 October 2012 (UTC)
- At the critical point, the specific heat becomes infinite. The specific heat contour lines bend near the CP because of this. Therefore the ideal gas laws cannot apply without significant correction near the critical point. What is happening is most easily seen if you plot specific heat contours on a graph of temperature versus internal energy, along with the saturation (boundary) lines. I haven't figured out how to post diagrams in Ref Desk. If you can tell me the secret of how to do it, I'll give you a diagram or two that will make things clear. As a matter of personal policy, I don't edit WP articles directly, as certain admins have spent a fair amount of effort trying to block/exclude me, and I have no wish to a) waste my time, and b) upset folk. Wickwack124.182.136.192 (talk) 16:09, 28 October 2012 (UTC)
- Well, if you have that kind of trouble, it's probably desirable in general to avoid the tedious scope and copyright issues, etc. (even though they shouldn't apply in this case for your own diagram) of uploading files here or even at Wikimedia Commons - just find any old spot online off the List of photo sharing websites to upload a file and post a link to it here. Wnt (talk) 16:19, 28 October 2012 (UTC)
- On a quick check, I cannot see how to upload to Wikipedia Commons, and think you have to be registered. I post to Ref Desk only using dynamic IP, without a registered username, due to admin actions as mentioned before. I've never used photo sharing sites - I will investigate later today after I get some paying work done if nobody else helps you. Or you might like to do your own plot. It's easily done using the data for several substances listed on the NIST website. Wickwack 120.145.174.229 (talk) 00:56, 29 October 2012 (UTC)
- Well, if you have that kind of trouble, it's probably desirable in general to avoid the tedious scope and copyright issues, etc. (even though they shouldn't apply in this case for your own diagram) of uploading files here or even at Wikimedia Commons - just find any old spot online off the List of photo sharing websites to upload a file and post a link to it here. Wnt (talk) 16:19, 28 October 2012 (UTC)
- In reference to the heat capacity near the critical point, I see we have an article critical exponent, though it is anything but obvious what it all means. [16] seems like an interesting source on the asymptote. All kinds of interesting phenomena, like the hysteresis in density according to temperature (how would life use that? I can barely speculate...) - but to the uninitiated, it gives up its meaning somewhat grudgingly. They give a modified ideal gas law, P = ρRT/(1-bρ) - aρ2, where a is an attraction force constant and b is a repulsion force constant, but I don't know what these are for hydrogen nor why they matter more in one area of the phase diagram (and which?) than another. (They give some gnarlier equations after that...) A clearer answer on where the ideal gas law applies would still help me. Wnt (talk) 04:02, 29 October 2012 (UTC)
- The ideal gas law deviates from real gases at higher pressures, and supercritical fluids are generally under very pressures. Ideal gas law#Deviations from real gases. If you lower the pressure below the critical pressure, you don't have a supercritical fluid. You have an ordinary gas. So, for the range of temperatures and pressures where the ideal gas law is a valid approximation of real behavior, there aren't supercritical fluids. There are the Van der Waals equations, which are also approximations, but are better approximations than the Ideal Gas Law itself. --Jayron32 04:13, 29 October 2012 (UTC)
- It would be useful if the pressure-density curves for some gas (hydrogen would be nice) at a lot of different temperatures posted were someplace handy. Trying the Van der Waals equation for grins, assuming we have a comfy 300 K hydrogen ocean at the pressure of the critical point (12.8 atm = 1300 kPa) on Saturn at I think what the article describes as the bottom of the atmosphere, then from here we see a=0.2476 L2bar/mol2, b=0.02661 L/mol for hydrogen; oxygen is 1.378 L2bar/mol2, 0.03183 L/mol. So for 1 mole of hydrogen:
- (12.8 atm + 1 mol^2*0.2476 L2bar/mol2/V2)(V-(1 mol)(0.02661 L/mol))=(1 mol)(0.08205746 L atm /K mol)(300 K) === (12.8 atm + 0.2444 L2atm/V2)(V-0.02661 L)=24.62 L atm === this cubic seems to work out at V=1.95 for 1 mole of hydrogen.
- (12.8 atm + 1 mol^2*1.378 L2bar/mol2/V2)(V-(1 mol)(0.3183 L/mol))=(1 mol)(0.08205746 L atm/K mol)(300 K) === (12.8 atm + 1.36 L2atm/V2)(V-0.03183 L)=24.62 L atm === this cubic seems to work out at V=1.9 for 1 mole of oxygen.
- Now the outcome here, for these equations, is that there's only a small difference between the hydrogen and the oxygen, i.e. the hydrogen is still about as buoyant (more so, I think) than it is under less compressed conditions - there's no "bubble of gaseous oxygen rising in a sea of liquid hydrogen" implied by these a and b numbers. But I have no idea if the equation applies at all, or if the supercritical hydrogen is far more densely packed - this was just a reality check for what it works out to say. Wnt (talk) 05:55, 29 October 2012 (UTC)
- It would be useful if the pressure-density curves for some gas (hydrogen would be nice) at a lot of different temperatures posted were someplace handy. Trying the Van der Waals equation for grins, assuming we have a comfy 300 K hydrogen ocean at the pressure of the critical point (12.8 atm = 1300 kPa) on Saturn at I think what the article describes as the bottom of the atmosphere, then from here we see a=0.2476 L2bar/mol2, b=0.02661 L/mol for hydrogen; oxygen is 1.378 L2bar/mol2, 0.03183 L/mol. So for 1 mole of hydrogen:
- It's my understanding that the ideal gas law does apply in the supercrit region, so long as you are nor close to the critical point. "Close" in this conext meaning outside the area around the critical point where the specific heat contours bend. However, gasses do indeed depart in a straightforward way from the ideal law at very high pressures. Trust me, it will become very clear to you if you plot or obtain specific heat contours on a temperature vs internal energy chart. Compressibility factors for correcting for the departure are readily available in standard tables, or you can use things like the van der waals formula, which are very inaccurate. Some advanced textbooks have a universal chart as I recall. Wickwack 120.145.174.229(talk) 05:48, 29 October 2012 (UTC)
- Are you referring only to when you are very near both the critical pressure and the critical temperature, or is one or the other sufficient to throw things off? And you're also saying that it holds in the critical region, but not if you go to too high a pressure in it? Wnt (talk) 06:00, 29 October 2012 (UTC)
- Near the CP in both pressure and temperature - just one will not throw things out wrt ideal gas law. It is aproaching the critical point, from any direction, where specific heat becomes infinite, that is the issue. Yes, the ideal gas law holds in the critical region, except in so far as too high a pressure will require a correction. Note that for many substances, the critical point is not at really great pressure. E.g., hydrogen 1.296 MPa; dodecane 1.817 Mpa (~18 atmospheres) Wickwack 120.145.177.211 (talk) 12:43, 29 October 2012 (UTC)
- Are you referring only to when you are very near both the critical pressure and the critical temperature, or is one or the other sufficient to throw things off? And you're also saying that it holds in the critical region, but not if you go to too high a pressure in it? Wnt (talk) 06:00, 29 October 2012 (UTC)
- It's my understanding that the ideal gas law does apply in the supercrit region, so long as you are nor close to the critical point. "Close" in this conext meaning outside the area around the critical point where the specific heat contours bend. However, gasses do indeed depart in a straightforward way from the ideal law at very high pressures. Trust me, it will become very clear to you if you plot or obtain specific heat contours on a temperature vs internal energy chart. Compressibility factors for correcting for the departure are readily available in standard tables, or you can use things like the van der waals formula, which are very inaccurate. Some advanced textbooks have a universal chart as I recall. Wickwack 120.145.174.229(talk) 05:48, 29 October 2012 (UTC)
Laboratory tests
I'm making a list of legitimate reasons for requesting laboratory tests, and have come up with the following:
On a population level:
- Screening, to prevent the development of avoidable disease.
On the individual level:
- To look for a specific disease predisposition, in a person with a family history of the disease
- To help making or excluding a diagnosis
- To help choose the most appropriate treatment for a disease
- To help predicting the patient's prognosis
- To monitor the patient's disease activity
I'm aware that paternity testing isn't on the list. Other than that, have I left out something important?--NorwegianBlue talk 15:50, 28 October 2012 (UTC)
- You left out monitoring for correct drug dosage. Examples of this is the usage of INR tests to check/adjust the dosage of warfarin, and the the testing of blood cell counts to check/adjust the dosage of chemotherapy for cancer.
- Another reason: During surgery for cancer (lumpectomies), the surgeone makes a judgement call on how much to cut out. Typically, a 2 cm margin of safety is is aimed for. The surgeon sends the excised flesh (hopefully containing the malignant tissue surrounded by the 2 cm margin) to the lab for immediate analysis. While the patient is on the table anethetised and opened up, the lab reports back to the surgeon, essentially saying "you got it all", or "no you didn't - the tumour extends to the edge of the sample". This provides feedback to the surgeon as to his judgement quality, and lets him decide whether or not to cut a bit more out, cognizant of other factors. Wickwack 124.182.136.192 (talk) 15:58, 28 October 2012 (UTC)
- Thanks, I'll add
- To evaluate treatment (or diet) efficacy,
- diet effects (such as monitoring whether gluten is effectively eliminated from the diet) is certainly relevant to the list I'm preparing. Anything else?--NorwegianBlue talk 19:54, 28 October 2012 (UTC)
- Thanks, I'll add
- There are a number of other uses I would think of as illegitimate, but you might not, e.g. testing for recreational drugs, performance enhancing drugs, consumption of banned food/drink/tobacco, identifying the person tested or members of his family as criminal suspects by genetic matching, etc. Wnt(talk) 20:54, 28 October 2012 (UTC)
- I'm not sure whether you'd consider "Monitoring for side effects of treatments" is already covered in your list (under choosing the most effective treatment) but it seems like it should be made explicit. I would also include testing of blood type and HLA types for potential organ donors (I suppose for recipients it would already be covered by "choosing effective treatment". There's also genetic testing for study of population migration/anthropology, and for genealogical curiosity (even when paternity is not in question). And in addition to Wnt's suggestion, CODIS testing of convicted criminals (rather than suspects) might be included, depending on your criteria for "legitimate". - Nunh-huh 01:48, 29 October 2012 (UTC)
- yeah, one more vote for some version of "Monitoring for side effects of treatments", "monitoring for correct drug dosage", etc; want to check for liver enzymes in general, check for rhabdomyolysis due to statins, etc.Gzuckier (talk) 02:03, 29 October 2012 (UTC)
- Without too much consideration as to whether they're already subsumed in one of the listed criteria, I'd think of autopsies (or any pathological testing on the dead, done for the purpose of establishing cause of death, for forensic purposes, the curiosity of the survivors, or assessment of familial risk factors); epidemiologic monitoring (in hospitals; to govern antibiotic usage; to provide monitoring for antibiotic coverage; in animals (to predict flu varieties in order to produce flu vaccine), or any such testing to establish the most common serotypes of viruses or bacteria for vaccine purposes; and testing to determine if preexisting conditions or disease exists for insurance purposes. There's testing for matters of public health ( epidemiology, STD contact tracing, TB drug monitoring (to establish if patients are actually taking their drugs, which is done as much for other's benefit (to avoid the selection for multi drug-resistant strains of TB) as for the "patient's"; and also scientific (rather than clinical) testing (tests which may have eventual clinical significance, but are at present of unproven value). - Nunh-huh 03:14, 29 October 2012 (UTC)
- Some other possibilities:
- Because the patient requested the test. If a patient feels they might have a certain medical condition, and the test isn't risky, then it makes sense to perform the test, if only for their peace of mind.
- To establish a base line. This is important for some repeated tests, like mammograms, as you need something to compare later tests with, to check for any changes. StuRat (talk) 06:17, 29 October 2012 (UTC)
- Is there a non-legitimate reason for requesting a lab test? --TammyMoet (talk) 09:34, 29 October 2012 (UTC)
- "Because the patient requested it" is a reason, if tests are in fact done for that reason. Stu's USA may be different, but doctors are trained to not order a test unless it is their professional opinion that the result will decide which of 2 or more treatment options is to be used. Certainly in Australia they will refuse a patient's unnecessary test - this is because costs are mainly governemnt funded, and the bean counters may penalise a doctor is they think he has cost the govt unnecessary expense. I imagine other countries that have a similar "medicare" or National Health govt funded system (which is most countries apart from 3rd world) will be similar. Wickwack 120.145.177.211 (talk) 11:17, 29 October 2012 (UTC)
- Thanks everyone! @TammyMoet: yes, definitely, there are many. For one thing, for each test that is taken, there is a certain probability that the test will be positive even if the patient is perfectly healthy. If many tests are taken in a non-targeted way, you can be pretty sure that you'll have a false positive among the results. See Ulysses syndrome.This 1954 paper is also a good read, and features a list of what could be considered non-legitimate reasons (see below), although I agree with StuRat that #5, i.e. giving the patient peace of mind, is not without merit. Unless you get a false positive...
- Quoting from the paper: Perhaps it would be a good idea to have a space on every laboratory form in which the doctor had to state exactly why he had ordered a test. I believe if answers were honestly filled in we might get this sort of thing:
- (1) I order this test because if it agrees with my opinion I will believe it, and if it does not I shall disbelieve it.
- (2) I do not understand this test and am uncertain of the normal figure, but it is the fashion to order it.
- (3) When my chief asks if you have done this or that test I like to say yes, so I order as many tests as I can to avoid being caught out.
- (4) I have no clear idea what I am looking for, but in ordering this test I feel in a vague way (like Mr. Micawber) that something might turn up.
- (5) I order this test because I want to convince the patient there is nothing wrong, and I don't think he will believe me without a test.
- --NorwegianBlue talk 11:29, 29 October 2012 (UTC)
- That is a fascinating comment, for the following reasons: Firstly, in my experience, doctors do usually write on the requisistion form in the text box provided something that at least gives a clue as to why they ordered the test. They write abreviated things like "Sus c. rht breast" ie "I suspect carcinoma in right breast", A lot of times they don't need to write anything - the only reason a doctor would order an epstein barr test for example is that he suspects the presence of the eptein barr virus (glandular fever). Secondly, when a quantitative test result comes back from the lab, they usually give three figures like this: Xyzetc 3.0 >3.2 <6.0 ug/ml, which means the Xyzetc concentration was measured at 3.0 micrograms per millilitre, and the normal range is 3.2 to 6.0. A text summary will be at the bottom, saying something like: The Xyzetc was found to be marginally below the normal range for a male of this age group. We suggest you request a so-and-so test to exclude such-and-such disease. This is Australian practice at any rate. A professional would never use reason (4) above. If he got found out he'd be in trouble. Reason (3) will not occur in most countries for the reason I already stated - tests cost money, and someone's counting somewhere. Wickwack 120.145.177.211 (talk) 12:25, 29 October 2012 (UTC)
- Stating on the form what you are looking for could bias the test results, in cases where tests are subjective.
- Also, in the US, another invalid reason for tests is to avoid lawsuits. You can have a group of rare conditions, where the risks of the tests outweigh the benefits, and the risks are not immediately apparent, such as radiation exposure. So, medically, the tests don't make sense, but, legally, the doctor could be sued if the person turns out to have this rare disease, which the test could have found, and he failed to order it. (The test not being medically indicated is not necessarily a sound legal defense, as the law really doesn't much care about science.) StuRat (talk) 18:36, 29 October 2012 (UTC)
- Re avoiding lawsuits, yes, I've heard that tests are ordered in the USA for that reason. In other countries, if a patient wants to sue a doctor, the first thing that happens is that the doctor's practice insurance company reviews that case. If they think the patient just might succeed with a suit, a panel of medical peers is assembled to look at it. Mostly, the peers side with the doctor (surprise surprise), and courts are reluctant to go against the panel opinion. It does keep the number of court cases down.
- Re bias, this is obviously a possibility. However, again in my observation (my wife has quite a history with cancer), if the test is subjective but the result is crucial, doctors tend to requisition from two different labs. If they get opposing or different views back, they either decide which is the right one based on other factors (yep - bias again, possibly NorwegianBlues's item (1)), or they go with the safest option, or they order another sort of test. So, another valid reason to order a test: to confirm or refute a subjective pathologist opinion.
- Wickwack 124.182.21.153 (talk) 01:21, 30 October 2012 (UTC)
- Thanks again, everyone! @Wickwack: This is indeed a valid point, not only when there is a subjective element in the interpretation of the sample. Sample mix-ups occur, and different test methodologies may give discrepant results. --NorwegianBlue talk 07:00, 31 October 2012 (UTC)
how much cropland is lost to circle farming?
how much cropland is lost to circle farming? Naively, we might assume that 2r*2r is the square equivalent versus pi * rsquared is the circle equivalent. But that would mean 4 rsquared versus 3.141 r squared - i.e. "almost a third" more area is actually available (1.27x more) if they farmed squares instad of farming circles. Is this right though? Because you don't need clearance of a full square around the circle, you can pack the next circle in slightly more closely. I'm asking about this.
http://www.google.com/search?q=circle+farming&tbm=isch
how much cropland is lost to circle famring instad of square farming? --89.132.116.35 (talk) 13:54, 28 October 2012 (UTC)
- You'll want to read the article on Circle packing. I'm not entirely clear on the maths, but it seems that, if the circles are identical, by using hexagonal packing you can cover a maximum of about 0.9069 of the area - that is, a little less than a tenth of the area available is unplanted. - Cucumber Mike (talk) 14:34, 28 October 2012 (UTC) — Precedingunsigned comment added by OsmanRF34 (talk • contribs)
- Though, the question is making a faulty assertion that useful arable land is "lost" because of this farming technique. It makes an overly-simplistic assumption that farm productivity directly corresponds to number of acres planted. In fact, there is a stronger correlation between tons of food produced, andamount of water used. In other words, with enough water, a very small area can yield more crops than a much larger area that uses less water. This is exactly why circular irrigation is executed in practice: it can be a very cost-effective way to put the most amount of water into a useful purpose. You might enjoy reading about agricultural productivity. Agricultural science is incredibly quantitative: if there were a more efficient way to produce more crops at lower cost (keeping in mind that purchasing land and pumping water both have a cost!) - you can bet that technique would be more widespread. If you compare agriculture in different geographic areas - places where the relative costs of land and water are different, or the irrigation needs of the local crops are different - you'll see that some areas don't use circular pivot irrigation. Nimur (talk) 18:51, 28 October 2012 (UTC)
The answers below were added to a dup question at humanities:
- You'll want to read the article on Circle packing. I'm not entirely clear on the maths, but it seems that, if the circles are identical, by using hexagonal packing you can cover a maximum of about 0.9069 of the area - that is, a little less than a tenth of the area available is unplanted. - Cucumber Mike (talk) 14:34, 28 October 2012 (UTC)
- Circle farming is generally associated with center pivot irrigation (CPI). Our article mentions that CPI uses less water and is less labour intensive / requires less maintenance than alternatives for irrigating rectangular areas (RA). As such, the increased cost of irrigating an RA must be juxtaposed with the 10% (hexagonal) / 30% (square) inceased yield of this RA.
- BTW, some further research indicates that the "left-over corners" of circle farms are are, ia, used for storing liquid fertilisers in (quite large) pools which are fed into the CPI system. Also bear in mind that circle farming is used in areas which were useless to agriculture before irrigation became feasible. This was never fertile cropland. --Cookatoo.ergo.ZooM (talk) 20:52, 28 October 2012 (UTC)
- Also note that it would be entirely possible to have overlapping circles so no land goes unwatered. However, this would be more complex, as you'd need to time the rotations so adjacent irrigation systems don't collide (or perhaps cantilever the overlapping portions of each, at different heights, to avoid collision, or just shoot water out the end). You might also want to vary the flow to avoid overwatering the overlap. Another alternative is to use smaller CPI systems to fill in the "holes". I've never seen any of this done, though, so presumably it's more cost efficient just to use those gaps for other purposes, as mentioned above. StuRat (talk) 06:05, 29 October 2012 (UTC)
- Overlapping CPI systems: 32°55′42″N 102°08′48″W / 32.9282°N 102.1467°W (though there's no telling whether they're used at the same time). Filling a gap with a shorter CPI system: 32°46′52″N 102°00′09″W / 32.7810°N 102.0024°W. Both seem to be fairly common. Deor (talk) 13:56, 29 October 2012 (UTC)
- Interesting. I wonder why only half of each large circle appears to be irrigated at the last link, under the Google satellite view. Being kept fallow that year ? StuRat (talk) 18:43, 29 October 2012 (UTC)
- Which link, Stu? μηδείς (talk) 19:11, 29 October 2012 (UTC)
- This one: [17]. StuRat (talk) 19:38, 29 October 2012 (UTC)
- Hm, that's interesting. Odd they would plant four half circles instead of two whole ones. μηδείς (talk) 21:03, 29 October 2012 (UTC)
- Yes, it is. Any farmers here know why they might do that ? Could it be that not using a CPI system for a year risks it rusting and freezing up ?StuRat (talk) 21:07, 29 October 2012 (UTC)
- I'm not a farmer, but it looks to me as if the black specks mainly concentrated near the centers of the green sides of the circles may be cattle (with pens at the circles' centers), which suggests that the fields are for grazing. Maybe planting forage to come up at different times of year in each half?Deor (talk) 22:48, 29 October 2012 (UTC)
- Yes, it is. Any farmers here know why they might do that ? Could it be that not using a CPI system for a year risks it rusting and freezing up ?StuRat (talk) 21:07, 29 October 2012 (UTC)
Using a diesel generator
We have a generator for the house that operates almost everything (furnace or a/c, stove, lights, water pumps etc, though only one 220-volt draw at a time) when we have power outages. Theoretically, one tankful lasts 8 hours of "normal use". We've never run it for more than 4, so I can't comment on that part. Does it matter how much we operate from it in respect of fuel consumption? Is that what "normal use" refers to? For example, if we only run lights and the water pump, does the generator draw down fuel more slowly than if we also run the electric stove at the same time, or does it draw x amount of fuel just to run, regardless of what is operating from it at the time? Thanks Bielle (talk) 18:07, 28 October 2012 (UTC)
- Yes, more fuel is required to produce more electrical power, because a larger electrical load directly corresponds to more (mechanical) work required to spin the generator. The exact amount of extra fuel consumption is difficult to estimate from first principles; in theory, we could set up a calculation based on conservation of energy, but this doesn't account for all the parasitic effects of friction, or wasted power due to mismatched mechanical or electrical impedance, and so on. This also scales to large power stations: it actually requires a different amount of coal to produce a different amount of electric power; but coal plants aren't easily throttled, so they often run close to peak power production and "burn off" the waste energy. More typically, these plants provide a "base" load, and variable demand loads above the base level are met by activating peaker plants.
- Fortunately, smaller generators can be throttled. I've found numerous charts from various manufacturers who provide an automatic throttle: for example,Honda 3300S Gasoline Generator has an electronic control to slow the engine RPM and reduce fuel consumption when loads are disconnected. This doesn't appear to be a feature on Honda's diesel generator, though. However, even at a fixed RPM, fuel consumption will vary based on the mechanical load on the generator - which, in practice, has at least some correspondance to electrical load. Nimur (talk) 18:35, 28 October 2012 (UTC)
- A internal combustion engine will run most efficiently at full load at the correct operating temperature. Running it at a low and it will will just loose energy through heat loss and friction. Calculate your KVA per gallon and compare that with the cost of mains electricity for the same KVA. Subtract from that the cost of maintenance for you generator. I have heard from people that can maintain their own generator (and cost their time at x$ per hour) and found that it is cheaper to come off line. It boils down to how cheap your fuel is. If you can divert the wast heat (from your generator) into your home then you are quids in.--Aspro (talk) 20:09, 28 October 2012 (UTC)
- Beware overloading the generator and reducing the voltage output - this can kill some things like refrigerators. See brownout (electricity).Wnt (talk) 21:01, 28 October 2012 (UTC)
Hi OP, while much knowledge is displayed above, it is not directly relevant to your question. A diesel gen operates at fixed speed (more or less) and its fuel consumption at that speed is composed of two parts, a fixed quantity per hour, plus an amount that will be roughly proportional to the electrical power you draw.
That is, if you draw the full electrical power it might consume 1.5 gallon per hour, while on no load it might use 1.5 pints an hour (this is estimated for a 2 litre engine at 1800 rpm). Unfortunately the specific figures depend entirely on your engine and generator setup. Generally, you would be better off using it heavily and then switching it off completely, but that is inconvenient for lights etc. Greglocock (talk) 23:35, 28 October 2012 (UTC)
- It is not correct that a genset operates at maxium efficiency at full rated output, for several reasons. Aspro is quite wrong on this. This was covered by another question a few hours ago. The internal losses in a generator can be modelled as L = k0 + k1I + k2I2 where k0, k1, and k2 are constants particular to the actual generator and I is the output current. Typically, maximum conversion efficiency is attained at around 65% to 80% of full output.
- This is because in the generator, there are losses that a constant at the operating RPM (the k0 term), losses proportional to the load current due to magnetic effects (the k1 term) and losses proportional to the square of the load current, due to power lost in a conductor being proportional to the square of current (the k2 term). An internal combustion engine also has losses due to the operating speed (bearing friction, oil pump load, cooling air fan load), proportional to torque (piston ring friction and part of air induction friction loss), plus thermal losses from the cyclinder rising in proprtion to combustion temperature, which is proportional to a power function of load. The thermodynmaic efficiency tends to be a power function of power output in non-computer controlled engines as the required ignition advance (spark in gasoline engines, fuel injection time in diesel engines) can only be correct at a particular power output (centrifugal and vacuum advance [in gasoline engines] are not generally used in genset engines of the sort that would be used to power a home). Engine manufactuers thus design engines so that their best fuel efficiency occurs at the most frequent throttle setting, generally considered to occur around 70% of full throttle. Keit 120.145.29.118 (talk) 00:39, 29 October 2012 (UTC)
- You're mixing up Power band with rpm. Power and efficiency diminishes on the back end of the power curve. You then go on to say what I was indicating, 60 –80% of the rated power. If you were in a helicopter who's power source was an infernal combustion engine and you found yourself sinking on the back-end of the power curve – increasing your rpm would do you little good. On the way down you’re helicopter would guzzle load of fuel, and on impact — you would end up as toast. Full throttle is not max torque. Max rpm is not max torque. Therefore, the OP is best running his generator at max load.--Aspro (talk) 20:17, 29 October 2012 (UTC)
- I haven't confused anything. You have confused the behaviour of gasoline car engines with the behavior of engines designed for stationary service. Car engines have a torque vs RPM curve that peaks at a certain RPM as volumetric efficiency falls as RPM rises, above a certain value, in a carvurettor or throttle body engine. As diesel engine has no air throttle (output is controlled by varying the quantity of fuel injected), the torque vs RPM curve is essentially flat, so max power does occur at max RPM. (Computer control diesel engines may be given a slight drooping torque characteristic for durability reasons). However, a genset engine only operates at constant RPM, and it will be designed to be optimal or near optimal at that RPM. Thermodymanic efficiency does drop a little at full power due to high combustion temperature (high temperature means a greater proportion of heat lost to coolant) and due to the fact that injection timing can only be optimal for only one power setting as I said. I'm not familiar with helicopter engines, but the manufacturer can choose to set the timing to be optimal at wide open throttle at max RPM, rather than at 70% of max power at design RPM as is done with stationary engines. As far as ptrotecting against power loss with increasing RPM, volumetric efficiency can be set to occur at max RPM if desired by intake manifold tuning. Helicopter engines drive what is a dirty great propellor, and would essentailly be forced to be near constant RPM anyway. Keit 124.182.11.117 (talk) 00:58, 30 October 2012 (UTC)
- If you're not using the engine to maximise the electrical power-out-put you are wasting energy in heat. Diesel locomotive divers burn fuel to get up to speed and then coast – as this is most efficient. If you know better, then inform organizations like Amtrak and I'm sure they will reward you handsomely for your breakthrough in finding a way of over turning the laws of thermodynamics. On the obverse: over-fuelling (over running) also increases the need for maintenance and reduces the life of the engine and increase fuel consumption. The users manual will tell you what the max power/efficiency ratio the engine was designed for. An oil- compression engine will also have a governor, so over-speeding does not even come into this – unless it 'diesels' due to broken piston rings. Further more, “stationary engines” and motive engine obey the same laws of thermodynamics - so that don't compute either.
- So, if the OP has (say) a Honda 400 watt (stationary) generator s/he would be better drawing off 400 watts than 200 watts. That IMHO answers the OP's question!
- Your superstition that it should be 70% of 400 watts suggest Honda really selling a 280 watt generator – an accusation that Honda might not like.--Aspro (talk) 02:01, 2 November 2012 (UTC)
- Aspro, you haven't got a clue what you are talking about. Go read a any decent textbook on engines - Charles Fayette Taylor's well known classic The internal-combustion engine in theory and practice, MIT Press, would be a good start. It has loads of formulae you can use. Then, download some performance data for different sorts of engines. While thermodynamics applies to all engines, engines are tailored to the application and behave as I have explained, apart from the fact that diesel engines inherently have a flat torque vs RPM curve as I explained. Railway working is a high mass low friction environment. That's why the locomotive works hard to get up to speed, it has to overcome considerable inertia (F = ma), and once it is up to speed, it only has to overcome friction, which is very low in a train, due to low frontal area for the mass hauled, and due to steel wheels running on steel rails. The very low friction at constant speed in railways is obvious thru making a comparison: A B-double truck can haul up to 80 tonnes or so using typically a 15 litre engine developing 400 KW. A typical railway loco can develop about 3 times as much, but haul loads up to thousands of tonnes. A 40 kW rated generator can be expected to provided 40 kW if the load demands it, but it will run slightly more efficiently at around 30 kW or so, for the reasons stated. A 400 W Honda is quite a different sort of thing to the genset the OP has - he said he has a genset that runs every appliance (tho not all together) - this indicates a 2500 W genset at the very least. In a diesel engine, combustion temperature is proportional to fuel burn. Heat flows from the cylinder gasses (high temp) thru the cylinder walls & head (lower temp) to the coolant. Heat flow to coolant thus rises with power output, as the cylinder walls are in any case at a moderately high but relatively unchanging temperature, the heat flow to the coolant increases to a greater degree than does power output. At power output is increased by increased fuel injection, turbulence also increases, lowering thermal conductivity from gases to cylinder walls & head. The derivation of L = k0 + k1I + k2I2 modelling of a generator is very obvious to an electrical engineer, and the current squared term obviously means effeciency of the generator itself drops above a certain load. Keit 120.145.130.50 (talk) 11:26, 2 November 2012 (UTC)
- you quoted:""the OP has - he said he has a genset that runs every appliance"" then you guess.Lets go back to basics. What generator is is the OP using?--Aspro (talk) 23:17, 2 November 2012 (UTC)
- Aspro, you haven't got a clue what you are talking about. Go read a any decent textbook on engines - Charles Fayette Taylor's well known classic The internal-combustion engine in theory and practice, MIT Press, would be a good start. It has loads of formulae you can use. Then, download some performance data for different sorts of engines. While thermodynamics applies to all engines, engines are tailored to the application and behave as I have explained, apart from the fact that diesel engines inherently have a flat torque vs RPM curve as I explained. Railway working is a high mass low friction environment. That's why the locomotive works hard to get up to speed, it has to overcome considerable inertia (F = ma), and once it is up to speed, it only has to overcome friction, which is very low in a train, due to low frontal area for the mass hauled, and due to steel wheels running on steel rails. The very low friction at constant speed in railways is obvious thru making a comparison: A B-double truck can haul up to 80 tonnes or so using typically a 15 litre engine developing 400 KW. A typical railway loco can develop about 3 times as much, but haul loads up to thousands of tonnes. A 40 kW rated generator can be expected to provided 40 kW if the load demands it, but it will run slightly more efficiently at around 30 kW or so, for the reasons stated. A 400 W Honda is quite a different sort of thing to the genset the OP has - he said he has a genset that runs every appliance (tho not all together) - this indicates a 2500 W genset at the very least. In a diesel engine, combustion temperature is proportional to fuel burn. Heat flows from the cylinder gasses (high temp) thru the cylinder walls & head (lower temp) to the coolant. Heat flow to coolant thus rises with power output, as the cylinder walls are in any case at a moderately high but relatively unchanging temperature, the heat flow to the coolant increases to a greater degree than does power output. At power output is increased by increased fuel injection, turbulence also increases, lowering thermal conductivity from gases to cylinder walls & head. The derivation of L = k0 + k1I + k2I2 modelling of a generator is very obvious to an electrical engineer, and the current squared term obviously means effeciency of the generator itself drops above a certain load. Keit 120.145.130.50 (talk) 11:26, 2 November 2012 (UTC)
- What's an "infernal combustion engine"? 24.23.196.85 (talk) 02:25, 30 October 2012 (UTC)
- One that won't start after you've spent all Saturday working on it. Also, one that won't start when you are about to set off into the city for a job interview. Ratbone 121.215.59.18 (talk) 02:37, 30 October 2012 (UTC)
- You've hit in one!--Aspro (talk) 00:33, 2 November 2012 (UTC)
- One that won't start after you've spent all Saturday working on it. Also, one that won't start when you are about to set off into the city for a job interview. Ratbone 121.215.59.18 (talk) 02:37, 30 October 2012 (UTC)
- I haven't confused anything. You have confused the behaviour of gasoline car engines with the behavior of engines designed for stationary service. Car engines have a torque vs RPM curve that peaks at a certain RPM as volumetric efficiency falls as RPM rises, above a certain value, in a carvurettor or throttle body engine. As diesel engine has no air throttle (output is controlled by varying the quantity of fuel injected), the torque vs RPM curve is essentially flat, so max power does occur at max RPM. (Computer control diesel engines may be given a slight drooping torque characteristic for durability reasons). However, a genset engine only operates at constant RPM, and it will be designed to be optimal or near optimal at that RPM. Thermodymanic efficiency does drop a little at full power due to high combustion temperature (high temperature means a greater proportion of heat lost to coolant) and due to the fact that injection timing can only be optimal for only one power setting as I said. I'm not familiar with helicopter engines, but the manufacturer can choose to set the timing to be optimal at wide open throttle at max RPM, rather than at 70% of max power at design RPM as is done with stationary engines. As far as ptrotecting against power loss with increasing RPM, volumetric efficiency can be set to occur at max RPM if desired by intake manifold tuning. Helicopter engines drive what is a dirty great propellor, and would essentailly be forced to be near constant RPM anyway. Keit 124.182.11.117 (talk) 00:58, 30 October 2012 (UTC)
- You're mixing up Power band with rpm. Power and efficiency diminishes on the back end of the power curve. You then go on to say what I was indicating, 60 –80% of the rated power. If you were in a helicopter who's power source was an infernal combustion engine and you found yourself sinking on the back-end of the power curve – increasing your rpm would do you little good. On the way down you’re helicopter would guzzle load of fuel, and on impact — you would end up as toast. Full throttle is not max torque. Max rpm is not max torque. Therefore, the OP is best running his generator at max load.--Aspro (talk) 20:17, 29 October 2012 (UTC)
How to do a thermometer model like a Galileo Galilei model?
I mean to build a temprature model of Galileo Galilei. What do I need in order to build this thing in a small form in my house with simple materials and easy to obtain? Thank you for all helpers in instruction. מוטיבציה (talk) 20:32, 28 October 2012 (UTC)
- See Galilean thermometer. My impression is that, like many scientific achievements of the time, it was dependent on glass-blowing skill. But anything you can seal up and attach a weight too should work. Wnt (talk) 20:52, 28 October 2012 (UTC)
- Thank you for help. Until now, I didn't read it properly' but I hope that I will find in it what I need for to do this model of thermometer. One more again, Thank you. מוטיבציה (talk) 14:29, 29 October 2012 (UTC)
- Googling "Galileo thermometer" leads you to many instructions on how to build one yourself. Some of these are easy at-home or intended for school classroom experiments, using simple materials such as tiny jars or bottles and a large vase. The little jars are filled with varying amounts of sand, water or some other ballast for weight. → Michael J Ⓣ Ⓒ Ⓜ 18:50, 29 October 2012 (UTC)
- Hello Michel, I thank you for the information. I hope to find an instruction for the building a model of Galileo exactly, that is to say that I don't need the fake model that published in London about 1990s, see 'Galilean thermometer', I need to build something that was in hands of Galileo, Again, thank you for help. מוטיבציה (talk) 16:31, 30 October 2012 (UTC)
- From our article, it doesn't sound like Galileo actually had anything to do with the Galileo thermometor. It says he did build something called a thermoscope. [18] is listed in the references, and gives some details. Here is an excerpt they give describing the device:
Is this the sort of device you're looking to build? 209.131.76.183 (talk) 12:19, 1 November 2012 (UTC)He took a small glass flask, about as large as a small hen's egg, with a neck about two spans long [perhaps 16 inches] and as fine as a wheat straw, and warmed the flask well in his hands, then turned its mouth upside down into the a vessel placed underneath, in which there was a little water. When he took away the heat of his hands from the flask, the water at once began to rise in the neck, and mounted to more than a span above the level of the water in the vessel. The same Sig. Galileo had then made use of this effect in order to construct an instrument for examining the degrees of heat and cold.
- Yes, it is. Thank you.מוטיבציה (talk) 20:26, 3 November 2012 (UTC)
- From our article, it doesn't sound like Galileo actually had anything to do with the Galileo thermometor. It says he did build something called a thermoscope. [18] is listed in the references, and gives some details. Here is an excerpt they give describing the device:
- Hello Michel, I thank you for the information. I hope to find an instruction for the building a model of Galileo exactly, that is to say that I don't need the fake model that published in London about 1990s, see 'Galilean thermometer', I need to build something that was in hands of Galileo, Again, thank you for help. מוטיבציה (talk) 16:31, 30 October 2012 (UTC)
- Googling "Galileo thermometer" leads you to many instructions on how to build one yourself. Some of these are easy at-home or intended for school classroom experiments, using simple materials such as tiny jars or bottles and a large vase. The little jars are filled with varying amounts of sand, water or some other ballast for weight. → Michael J Ⓣ Ⓒ Ⓜ 18:50, 29 October 2012 (UTC)
- Thank you for help. Until now, I didn't read it properly' but I hope that I will find in it what I need for to do this model of thermometer. One more again, Thank you. מוטיבציה (talk) 14:29, 29 October 2012 (UTC)
October 29
The Philippines and total plastic bag bans
Among Asian countries, does the Philippines have a relatively high number of cities/towns that totally ban plastic bans? I know that Bangladesh is the world's first jurisdiction to ban all plastic bags, and some countries such as China and Taiwan either ban thin plastic bags or tax their use. However, in the Philippines, one province, as well as several cities and towns, even major ones, have totally banned their use. Among Asian countries, is the Philippines a pioneer in this regard? While there are several other cities across Asia that have banned plastic bags, they appear to be few and far between, with each country only having maybe a handful of places with total bans. The Philippines, on the other hand, has several jurisdictions which ban all bags, even those that are not single-use or recyclable. Most bans around around the world only ban single-use or thin bags, recyclable or biodegradable bags are usually excluded. And many jurisdictions merely tax their use, rather than totally ban them, like Taiwan. So again, is the Philippines among the countries leading the way among Asian countries in total plastic bag bans, or is the Philippines only playing catch-up among Asian countries? Total bans don't seem to be that common in countries like Japan, Vietnam, Malaysia, Thailand or Indonesia, although bags are taxed in some places in the aforementioned countries, and China has a ban on thin bags but does not totally ban all plastic bags. Narutolovehinata5 tccsdnew 02:23, 29 October 2012 (UTC)
Where did the first life on Earth originate?
--168.7.239.26 (talk) 06:49, 29 October 2012 (UTC)
- The Pangea answer is just wrong. Pangea ony formed about 300 million years ago. Life has been around much longer than that. 209.131.76.183 (talk) 12:35, 29 October 2012 (UTC)
- In shallow water - see Stromatolite. Roger (talk) 08:33, 29 October 2012 (UTC)
- Or perhaps in deep water - see hydrothermal vent. And there are other theories, see abiogenesis. Nobody really knows for sure. 88.112.36.91 (talk) 09:53, 29 October 2012 (UTC)
- Agreed - this is one of the great unanswered questions. Also note that when we look at early life, we may not agree on the definition! For example, some corroding sheets of metal in water orcan have rotten patches that grow and even reproduce themselves, and crystallization may spread out once a single nucleation event starts the process, but are they alive? The origins of life might be nothing more impressive than that. Wnt (talk) 17:20, 29 October 2012 (UTC)
- The most useful way to make it a well-defined question is to identify the origin of life with the origin of cells, which are structures surrounded by a barrier that controls the exchange of chemicals between the interior and the exterior. Looie496 (talk) 17:29, 29 October 2012 (UTC)
- Looie is correct, and see The Origins of Order: Self-Organization and Selection in Evolution by Stuart A. Kauffman. My personal suspicion is there must have been some cyclical influence driving the metabolism externally, which hints at a shallow origin subject to sunlight--but that's likely just to be my lack of imagination speaking. Those of us who are old enough remember when it was universally believed that the reason for the extinction of the dinosaurs would never be found, and an asteroid strike wasn't even at the level of a fringe theory. μηδείς (talk) 19:09, 29 October 2012 (UTC)
- We basically understand how prebiotic molecules such as peptides can be generated from scratch. What we need to figure out is how those molecules can accumulate to a density at which cross-catalysis comes into play to a major degree. Once we have that, the rest of the story should follow. Looie496 (talk) 21:54, 29 October 2012 (UTC)
- I have an idiosyncratic notion that RNA life existed before cells, maintaining "protein" resources as heavily modified branched side-chains. (See the biosynthesis of histidine from PRPP; ribose can be converted to the backbone of an amino acid) Such life would have had very weak differentiation between individuals. Wnt (talk) 03:10, 30 October 2012 (UTC)
- Maybe elsewhere. Read about exogenesis in the panspermia article. They (talk) 07:05, 30 October 2012 (UTC)
- I think the first life originated in water bodies. But how first life originated on earth, by some chemical reactions or by the means of reproduction. The latter seems to be wrong. Sunny Singh (DAV) (talk) 11:00, 30 October 2012 (UTC)
- It has to have been in water - that's one of the few things that we do know. Also, I don't know why so many people are talking about their (self-described) "personal ideas" - it's a scientific question, and when you reply to somebody who's asked that question, your answer should involve the scientific data! Arc de Ciel (talk) 05:20, 31 October 2012 (UTC)
See here. Count Iblis (talk) 01:21, 31 October 2012 (UTC)
how to know the lifting/gripping power of rubberized magnet sheets?
I want to make a bit of wall art, and will need rubberized sheet magnets. They will be vertically oriented ("facing" the wall) and gripping a sheet of steel. I will cut them into squares and attach them to the bases of a variety of objects. While I can easily estimate the weight range for the objects I would like to "stick" to the wall, I am having no luck figuring out how much "lift/grip" a rubbering sheet magnet might have? I assume it is a function of area. I also assume it is a function of thickness since these sheets are readily available in 0.5mm and 1mm thickness, if not others as well. Ideally I'd like to have an accurate understanding of the lifting power before I make my purchase so as to better calculate the total area required... Thank you. The Masked Booby (talk) 09:33, 29 October 2012 (UTC)
- I suspect that this will vary from one manufacturer to another, and how well they stick will depend not just on the sheets themselves but also on the surface to which they will be attached. (Magnetic attraction falls off with the cube of distance, so it takes a very thin coating on a surface to make magnets a lot less sticky. Think about how few sheets of paper your average fridge magnet can hold up before losing their grip.) As well, the carrying capacity will depend on how the weight is arranged— if you have three-dimensional objects cantilevered out from the wall, you're going to see a tendency for the added leverage to 'peel' the sheets loose.
- Your best bet is probably to purchase/aquire/beg/borrow a sample sheet and conduct some tests with it before placing a large order. If you find that the sheets don't meet your needs, depending on what you're hoping to do you may find it more effective to glue tiny rare-earth magnets to the bases of your objects. They're very sticky, and you can buy them by the dozens or hundreds from online retailers. TenOfAllTrades(talk) 13:28, 29 October 2012 (UTC)
- You have to worry not just about "pulling off", which is a simple calculation of magnetic-attraction (although requires knowing some specific values about the magnets and distance) as TenOfAllTrades mentions, but also sliding down, which is affected by friction and other geometric details. If I hang a bunch of papers on my fridge door, "the magnet still holds to the door", but the "magnet and papers it's holding" all slide down to the floor. DMacks (talk) 13:54, 29 October 2012 (UTC)
- Yes, that's exactly what I was going to mention. If you have uneven magnetic attraction and/or coefficient of friction over the surface, you can also have the object unintentionally rotate, so it won't stay "upright". Since rubberized magnets are to be used, this hopefully will increase the coefficient of friction well beyond what you would get with the magnet(s) directly sliding on the steel sheets. However, the rubber layer should be thin, as it also increases the distance between the magnet(s) and steel. And, if rotation is a problem, then you should put the stronger magnets near the top. StuRat (talk) 18:58, 29 October 2012 (UTC)
Would simulation lead to an optimal solution for Circle packing in a square?
Each square in the article looks exactly like as if the circles got there by gravity or squeezing, the same for the spheres in Sphere packing. If you'd simulate indefinite slippery and sturdy, circles or spheres, offsetting a tiny bit the ones that are exactly balancing on top of each other a little (or shake), and then start squeezing them till, for instance, the box can't get any smaller without breaking spheres, would it be possible to prove that the end result would have to be packed most efficient for simple containers like a box?
If the spheres are not all the same size, but you had for instance 100 big spheres en 1000 small spheres, shaking in reality tends to move the big ones up. Does that mean that this natural dividing the big and smaller ones does not lead to the best possible solution, where there is more "air" than an optimal solution would have? Or is this dividing due to friction which would not happen if there was none? Joepnl (talk) 18:37, 29 October 2012 (UTC)
- Not a direct answer, but placing the largest object first and then adding the rest, in decreasing order of sizes, often produces the optimal solution. StuRat (talk) 19:03, 29 October 2012 (UTC)
- Several years ago, I went to a physics colloquium on experimental and computational modeling of on the stability of heaps of granular materials. Fascinating topic. (And yes, we have an article: Granular material - this topic is widely studied by material scientists, civil engineers, geologists, pure physicists, and experts from other disciplines). Joepnl, it's not common for physicists to "prove" anything - that's sort of reserved for a more pure form of mathematics - but there's certainly a lot that can be known - through simulation, experiment, and pure analysis - on this topic. Nimur (talk) 19:09, 29 October 2012 (UTC)
- Squeezing or shaking the circles is a hill climbing algorithm. It reminds me of using soap bubbles to find Steiner trees. It's only guaranteed to find the global optimum solution if it's also the only local optimum, which it tends not to be in interesting problems. -- BenRG (talk) 20:49, 29 October 2012 (UTC)
- Wouldn't that depend on how hard you shake it ? If shaken enough so all the circles go back out of the square, you get another shot at global optimization with each shake. Of course, you'd then need to evaluate each for the best packing, as it would otherwise be destroyed with the next shake. StuRat (talk) 21:01, 29 October 2012 (UTC)
- StuRat, you've described something that sounds similar to simulated annealing; the intermediate stages of an annealing algorithm can use a hill-climbing algorithm to calculate the next iteration, adding the critically different step of "shaking up" at various points during the climb. Proper implementations will parameterize "how much to shake" to escape from local minima in a way that is problem-dependent. Formal descriptions of these algorithms use a little more precise mathematical language to capture the nuance, but you've described the gist of it. Nimur (talk) 21:53, 29 October 2012 (UTC)
- There seems to be a series of numbers which have non-unique solutions for sphere packing in a square (not counting flipping/rotation): 7, 14, 19 it looks like from the pictures. Is there any rhyme or reason to this series? What is its mathematical significance? Wnt (talk) 23:47, 29 October 2012 (UTC)
- There is a list here, with column "loose". It differs a bit though (7,11,13,14,17,20,...) but it will take some time to download the actual pictures to see what's different. Joepnl (talk) 00:41, 30 October 2012 (UTC)
- Hmmmm, I ran this by the spreadsheet - the exact solutions start off common for the first 20, then get rare, then become increasingly common and predominate after 1000. Since the series isn't necessarily the best series, I don't know if this is artefactual - I think after 1200 or so it is. Before then, the values form a sort of irregular scalloped curve on a log graph. Wnt (talk) 02:41, 30 October 2012 (UTC)
- May be you could use your analysis to find the non-optimal solutions? Joepnl (talk) 23:40, 30 October 2012 (UTC)
- Hmmmm, I ran this by the spreadsheet - the exact solutions start off common for the first 20, then get rare, then become increasingly common and predominate after 1000. Since the series isn't necessarily the best series, I don't know if this is artefactual - I think after 1200 or so it is. Before then, the values form a sort of irregular scalloped curve on a log graph. Wnt (talk) 02:41, 30 October 2012 (UTC)
- There is a list here, with column "loose". It differs a bit though (7,11,13,14,17,20,...) but it will take some time to download the actual pictures to see what's different. Joepnl (talk) 00:41, 30 October 2012 (UTC)
- There seems to be a series of numbers which have non-unique solutions for sphere packing in a square (not counting flipping/rotation): 7, 14, 19 it looks like from the pictures. Is there any rhyme or reason to this series? What is its mathematical significance? Wnt (talk) 23:47, 29 October 2012 (UTC)
- @Nimur, it's ok a if a mathematician borrows some ideas from physics to create a solid proof I think. I can imagine something like "we start with situation X (circle here, circle there), and now I'll prove that this is not optimal yet as long as a circle can still move according to these formulas (which happen to be gravity, etc) leading to X' -this might be the easy part-, and also that if the circles cannot move any further we must have reached the optimum (this might be a bit trickier :))".
- @BenRG, I was thinking of this problem to be just one hill to climb without a local maximum. Not that I have much real life experience with this, but if I'd throw (slippery!) balls in a container it feels that the balls can't get into a situation where it needs a big shake for an optimal solution, just a small (almost 0) one for the corner case of balls on the exact top of each other. For instance, I can't find an example of circles that would stop moving in a sub-optimal solution and, vice versa, in all examples in Circle packing in a square I can't see a circle that would move if subjected to gravity (apart from 7, 14 and 19 mentioned by Wnt, where at least one circle has some space to move around without changing the size of the box). Joepnl (talk) 00:31, 30 October 2012 (UTC)
- Mmm I guess I'm totally wrong. The example with 4 circles looks pretty hard to reach after adding the 3rd circle using just gravity. This would need a big shake. (Or possibly the walls getting wider instead of a binary search starting with a full square each time)Joepnl (talk) 00:52, 30 October 2012 (UTC)
- Even if the problem has no local maxima the tought part is proving it mathematically. Simulation will probably give very good results, but you can't call it the best unless you can mathematically reason that it is impossible to do better. 209.131.76.183 (talk) 12:29, 30 October 2012 (UTC)
What does work is to use Monte Carlo simulations of the 2 dimensional hard sphere gas. You can extract information about the equation of state from that and then you can combine this with the Mayer expansion. The Mayer expansion yields the equation of state as a series expansion in powers of the density, so it's only valid for low densities, but you can resum such an expansion. Here you make assumptions about the asymptotic behavior of the high order expansion coefficients, which you can try to extract from the Monte Carlo simulation. You can then use that resummed equation of state to find the critical density at which the pressure goes to infinity. Count Iblis (talk) 17:01, 30 October 2012 (UTC)
- Isn't Monte Carlo almost by definition a method which doesn't guarantee an optimal solution? Joepnl (talk) 23:40, 30 October 2012 (UTC)
- Yes, that's why you need to use this method in an indirect way. So, there exists some maximum density rho_m corresponding to optimal packing; the pressure as a function of the density will then have a singularity at rho = rho_m. By studing the system at low densities, far away from rho_m, you can still get information about rho_m. Count Iblis (talk) 00:54, 31 October 2012 (UTC)
October 30
Venusian atmosphere
What is the atmospheric density profile, for the last lower five kilometres of the Venusian atmosphere? I want to investigate the properties of the supercritical CO2 ocean, such as the rate of change in the terminal velocity, or the hydro/aerodynamics. If a tungsten sphere enters the atmosphere at sufficient speed, can it undergo two succesive sonic booms, once upon entering the atmosphere, once upon entering the ocean? Plasmic Physics (talk) 00:00, 30 October 2012 (UTC)
- The article says that the density at the surface is 65 kg/sq.cm; at higher altitudes, it should decrease as a logarhythmic function of altitude, as is the case with Earth's atmosphere. 24.23.196.85 (talk) 02:24, 30 October 2012 (UTC)
- Or, more plausibly (on two counts), 65 kg/m³. —Tamfang (talk) 03:32, 30 October 2012 (UTC)
- Sorry, messed up the units. 24.23.196.85 (talk) 04:25, 30 October 2012 (UTC)
- Or, more plausibly (on two counts), 65 kg/m³. —Tamfang (talk) 03:32, 30 October 2012 (UTC)
- That won't apply in this case. Plasmic Physics (talk) 02:38, 30 October 2012 (UTC)
- What I mean is that while Earth's atmosphere is in a homogenous state and can be modelled by a singular logarithmic function, Venus' atmosphere is in a heterogenous state, and must be modelled by two functions. Plasmic Physics (talk) 06:31, 30 October 2012 (UTC)
- Even a temperature/pressure profile would be nice. Plasmic Physics (talk) 08:17, 1 November 2012 (UTC)
What is the farthest known planet from earth including dwarf planets?
What is the farthest known planet from earth including dwarf planets? Neptunekh94 (talk) 01:40, 30 October 2012 (UTC)
- Last time I checked, it was 90377 Sedna -- but this info could be out of date. 24.23.196.85 (talk) 02:01, 30 October 2012 (UTC)
- According to our article, Sedna hasn't been officially recognized as a dwarf planet yet. If the answer is confined to objects so recognized, I believe the answer is Eris. Deor (talk) 02:12, 30 October 2012 (UTC)
- Surely "some" of the known extrasolar planets are more distant... our list of exoplanetary host stars seems very thorough, and lists the Cygnus binary planet system (confirmed on this NASA webpage) at a distance of over five thousand light years. The NASA article states that this system is "among the most distant" planetary system, indicating a healthy scientific ambiguity about which planetary discoveries are currently confirmed. Nimur (talk) 02:15, 30 October 2012 (UTC)
- Rather more speculatively, there are a few candidates for extragalactic planets. Dragons flight (talk) 04:12, 30 October 2012 (UTC)
- Also speculatively, given the cosmological principle, no galaxy in the universe should be unique or distinct from other galaxies in fundamental ways. If planetary formation is found in our galaxy, it should also be found in any of the billions of other galaxies in the universe. So, there are likely uncountable numbers of planets at any given distance from Earth. None has necessarily been specifically identified, but they're clearly there. I don't know the names and current locations of every person in Beijing, China, but I am quite certain they exist. --Jayron32 04:24, 30 October 2012 (UTC)
- I'm sure the OP meant planets in the Solar System. 24.23.196.85 (talk) 04:26, 30 October 2012 (UTC)
- [citation needed]. When you get that mind-reading technology perfected, when are you going to put it on sale? --Jayron32 04:28, 30 October 2012 (UTC)
- In any case, he/she specifically asked about known planets only. 24.23.196.85 (talk) 04:42, 30 October 2012 (UTC)
- Well, we know planets exist in other galaxies, far away from earth, even if we can't name and locate them (see my Beijing analogy above). --Jayron32 04:47, 30 October 2012 (UTC)
- In any case, he/she specifically asked about known planets only. 24.23.196.85 (talk) 04:42, 30 October 2012 (UTC)
- [citation needed]. When you get that mind-reading technology perfected, when are you going to put it on sale? --Jayron32 04:28, 30 October 2012 (UTC)
- I'm sure the OP meant planets in the Solar System. 24.23.196.85 (talk) 04:26, 30 October 2012 (UTC)
- Well, no, we don't know for a fact that there are trillions of planets (even though it is generally believed there almost certainly are). But even if we did know that for certain, that doesn't make any of them "known". Except the very few ones we do actually "know". The range of our knowledge now extends beyond our Solar System, but not very much in celestial terms. It's like the sand on a beach. I am 100% certain there's a virtually uncountable number of grains of sand there, but I cannot tell you the precise measurements of any individual grain, or even if there is a single grain that has precisely some measurements that I arbitrarily specify in advance. I may "know" the beach, but I do not "know" the grains of sand. -- Jack of Oz [Talk] 05:26, 30 October 2012 (UTC)
- If I understand the somewhat strange IAU definitions correctly nigher dwarf planets nor extrasolar planets are actualy planets. That would give us Neptune as the most distant planet and Eris as the most distant known dwarf planet at the moment. Gr8xoz (talk) 16:36, 30 October 2012 (UTC)
- Oh my god. I usually respect ref deskers a lot, but most of the above answers are horrible.
- 1. The farthest known dwarf planet from the Sun, at this moment, is Eris. However, Eris has a highly elliptical orbit with a perihelion of 38.4 AU, which is closer than Pluto is at its aphelion. Sedna, if confirmed as a dwarf planet, is currently closer to the Sun than Eris: 86 AU vs. 97 AU. It won't be this way forever, because Sedna has an aphelion of 937 AU, compared to Eris' 97.5.
- 2. If you want the farthest dwarf planet from Earth, the answer is Eris, the same as above. The distance between Earth and the Sun is negligible compared to the distance between the Sun and Eris or Sedna.
- 3. If you include extrasolar planets, according to the Extrasolar Planet Encyclopedia (http://exoplanet.eu/), the farthest known planet is SWEEPS-04 at a distance of 8500 parsecs (28000 light years). Wikipedia's article claims that Kepler-35b is 51000 light years away, when it's actually closer to 5000.
- 4. If you include highly speculative exoplanets, then see extragalactic planets. Unfortunately they're impossible to follow up on, because their detection depended on a chance alignment of stars that made gravitational microlensing possible.
- 5. Disregard what Gr8xoz said. Dwarf planets are not planets, but extrasolar planets most certainly are. In fact, one of the primary motivations for defining the word "planet" was that we needed a way to categorize all those extrasolar objects. --140.180.252.244 (talk) 21:15, 30 October 2012 (UTC)
- The oficial definition of a planet is "A planet1 is a celestial body that (a) is in orbit around the Sun,(b) has sufficient mass for its self-gravity to overcome rigid body forces so that it assumes a hydrostatic equilibrium (nearly round) shape, and (c) has cleared the neighbourhood around its orbit." See https://www.iau.org/static/resolutions/Resolution_GA26-5-6.pdf.
- To me that seems to rule out extrasolar planets, can you give any authorative source showing that this is not the case? Gr8xoz (talk) 22:24, 30 October 2012 (UTC)
- That resolution is titled "Definition of a Planet in the Solar System". It starts with "The IAU therefore resolves that planets and other bodies, except satellites, in our Solar System..." It therefore does not apply to any objects outside the Solar System. According to Extrasolar Planet#Definition, which references this IAU position statement:
- 1) Objects with true masses below the limiting mass for thermonuclear fusion of deuterium that orbit stars or stellar remnants are "planets [...]
- 3) Free-floating objects in young star clusters with masses below the limiting mass for thermonuclear fusion of deuterium are not "planets", but are "sub-brown dwarfs" (or whatever name is most appropriate).
- The IAU never bothered to apply the same definition of "planet" to other solar systems as to our own because it isn't necessary; all known exoplanets are unambiguously planets under either definition. --140.180.252.244 (talk) 22:45, 30 October 2012 (UTC)
- Hey wait a minute everyone, the OP specifically asked about the farthest planet from the Sun including dwarf planets. 24.23.196.85 (talk) 06:04, 31 October 2012 (UTC)
Thermite reaction
Hello all, I'm very familiar with the Goldschmidt reaction's properties, but there's just one thing I could never understand: How come the aluminum metal reacts with the iron oxide despite the fact that there's an aluminum oxide film covering the aluminum metal and preventing its direct contact with the iron oxide? (I mean, before the mixture gets hot enough to melt the oxide film.) Does this involve some kind of quantum tunneling through the film? 24.23.196.85 (talk) 02:09, 30 October 2012 (UTC)
- I've used thermite welding in one of its' main applications: joining heavy copper cables to stainless steel earth rods. The charge is set off by a flint lighter that produces hot sparks. If you try igniting a mixture of aluminium powder and iron oxide power with sparks, nothing will happen. The thermite welding mixes contain magnesium and other proprietry stuff that the sparks ignite to get the temperature up to white heat - the reaction between the aluminium and the iron oxide then supplie the bulk of the heat. Ratbone 121.215.59.18 (talk) 02:34, 30 October 2012 (UTC)
- What he said. Thermite has a very high activation energy. When I would do it as a classroom demonstration, I always used a chemical starter (ingredients never revealed to students) which provided the initial "burn" to start the reaction. Thermite is highly exothermic, so it is self-sustaining, but the activation energy is so high that a few sparks won't do it. The activation energy is so high that there is little danger of self-starting: premixed thermite powders are shelf-stable. They won't go off spontaneously. --Jayron32 03:27, 30 October 2012 (UTC)
- Heh, but you already let the cat out of the bag. Besides, the kids seem pretty good at figuring it out on their own.[19] (not sure the professors are safe with it though [20] - for ROFL be sure to watch the kids' video first!) Wnt (talk) 19:33, 30 October 2012 (UTC)
- In any case, the article describes the various ignition methods in quite a bit of detail. 24.23.196.85 (talk) 06:01, 31 October 2012 (UTC)
- Thanks everyone! So in fact the initial burn is hot enough to melt the oxide film right off, correct? 24.23.196.85 (talk) 04:40, 30 October 2012 (UTC)
- Sort of. The activation energy is largely spent on liquefying the aluminum, IIRC, so that the reaction can proceed. Reactions don't generally proceed in the solid state; once the aluminum is melted, the oxide "coating" is no longer an issue. It isn't merely that the aluminum has an oxide coating that makes it unreactive, it is that there is no way for the solid aluminum and solid iron oxide to intermix (on the atomic level) to start a reaction. Once there is a liquid phase (which I am fairly certain is the aluminum) the reaction starts, and becomes self sustaining (the initial reaction of the tiny, microscopic amount of aluminum melted by the starter produces more than enough heat to continue to melt the remaining aluminum and allow the reaction to go to completion.) --Jayron32 04:52, 30 October 2012 (UTC)
Charges on quarks
I know how +1 charge of proton and 0 charge (neutral) of neutron is determined. Their charge is determined by the sum of charges of constituting quarks. But how the charge of a quark is determined. After reading this Quark#Electric_charge, I couldn't get the answer. Sunny Singh (DAV) (talk) 12:10, 30 October 2012 (UTC)
- Charge is just an intrinsic property of quarks, like spin or mass. Goodbye Galaxy (talk) 16:05, 30 October 2012 (UTC)
- Charge is one of the quantum numbers, actually a special class of quantum numbers called "flavours" (which are quantum numbers associated with elementary particles like quarks and electrons). Strictly speaking electric charge is a derivative quantum number, defined by calculation from primary quantum numbers via the Gell-Mann–Nishijima formula. --Jayron32 20:22, 30 October 2012 (UTC)
- To answer the experimental question of how you would measure the properties of a quark, the general answer is usually using a particle accelerator. Using high energy particles (often either electrons or protons), it is possible to probe the structure within a nucleus. The details are fairly technical but you look at the ways that different high energy particles interact and scatter off of a nucleon and provided you use sufficiently high energies you can infer that there are three quarks per nucleon as well details of the properties of those quarks such as mass and charge. Dragons flight (talk) 23:34, 30 October 2012 (UTC)
- Once you know (or just hypothesise) that a proton is two up quarks and one down quark and a neutron is one up quark and two down quarks, then the only values of quark charges that give a total charge of e for a proton and 0 for a neutron are (2/3)e for the up quark and -(1/3)e for the down quark (okay, you also have to assume that charges add up linearly, so there are no interaction effects). Of course, you still need experiments to confirm that these hypothetical quarks actually exist. Gandalf61 (talk) 09:57, 31 October 2012 (UTC)
Strangelet production engine
Strangelet production seems to require a strange combination (pun intended) of a very high energy environment to pop out strange quarks combined with a much lower energy to have a stable atomic nucleus. So in order to properly destroy the world, wouldn't you need a device like the following?
The first stage is a particle accelerator that is carefully tuned to maximize production of negatively charged strange particles. These are then cooled by bremsstrahlung as they are curved over by a magnetic field towards a positively charged foil of heavy elements, say lead or gold. The strange quarks then merge into the heavy atomic nucleus to form a stable strangelet.
The only remaining piece is to fill out the environmental impact statement for a process that can destroy the world. Hcobb (talk) 14:06, 30 October 2012 (UTC)
- Not sure that this would work, you're only going to add a few s quarks to the gold nucleus and that won't lead to spemething stable. Perhaps better to start with some heavy unstable element injected in the accelerator. If it is accelerated to some huge gamma factor, it will have a lifetime in the lab frame that is not too short. Then you can try to put some s quarks in that nucleus. This can't be done by letting it collide with the nucleus using a beam that travels in the opposite direction, as you would then have a deeply inelastic collision that shatters the nucleus to pieces. You need a small difference in velocity, so the beam will travel in the same direction. Count Iblis (talk) 16:34, 30 October 2012 (UTC)
- The article says you need roughly equal numbers of u/d/s, so doesn't that mean you wouldn't want normal matter in the initiating particle? I'd think (but definitely do not know) that you'd want to collide multiple Lambda baryons and/or Sigma baryons (positive and negative might be handy) in a very brief period of time.
- That said, I don't get how we're supposed to have come out of the Big Bang as non-strangelets if this instability really existed.
- We could use some remedial education/article development on how the Pauli exclusion principle works in baryons. I know that the proton is the most stable because you can't have three up quarks in the low-energy conformation with opposing spins rather than the delta baryon. But why does it work out that way? From more familiar cases with electrons you expect to be able to pair more particles in a lower energy state when they have opposite spins, not the same spin. Would be much appreciated if someone covers this... Wnt (talk) 18:34, 30 October 2012 (UTC)
herpes
| Wikipedia cannot provide medical advice or diagnosis, please seek medical help |
|---|
| The following discussion has been closed. Please do not modify it. |
|
I am single and still a virgin but I am having genital herpes.Is it possible to have genital herpes without sexual contact?15:40, 30 October 2012 (UTC)106.218.129.139 (talk)
|
Germline Mutagenesis of Cesium 137 in Plants and Animals
Hi! I live in Oregon and have been seeing lots of strange mutations in the plants around my house recently. A quick search of the internet shows multiple references to this effect, but nothing is mentioned of this under the wiki "cesium 137" heading? Is this something that has been deemed taboo, or could I add a section on it? Thanks! — Preceding unsigned comment added by 98.232.238.213 (talk) 16:22, 30 October 2012 (UTC)
- You need to give more background information. Why do you make a connection to "cesium 137"specifically? If you have reliable sources that "cesium 137" causes frequent mutations in your area then it should probably be included but i highly doubt that. Note that mutations and deformations can have a lot of different reasons and occurs naturally so any source need to be very specific and reliable. If the radiation levels really are high enough that increased mutation rate can easily bee observed the area would probably have been evacuated. I do not even think there are reliable sources for such effects around Tjernobyl or the Kyshtym disaster. Gr8xoz (talk) 16:58, 30 October 2012 (UTC)
- While I'm finding at least some chatter on various internet forums and discussion groups on this topic, I'm coming up kind of short on sources that would be considered reliable by Wikipedia standards. (See as well Wikipedia's notes for scientific sources and sources on medical topics for additional guidance.)
- What sources are you considering using to support your proposed edits to the article? TenOfAllTrades(talk) 17:10, 30 October 2012 (UTC)
- Please take photos of the strange mutations and upload them to Wikimedia Commons. Then people can go through one by one and say what they are. Infections can cause many strange changes in the shape of plant growth, so it could be something common and not genetic at all. Wnt (talk) 18:40, 30 October 2012 (UTC)
- This is a good point—infections and parasites are the most likely explanation for most changes you might see. (Check out the wild effect of parasitic wasps on the shape of these acorns.) Heck, straight climate stress (much of North America experienced severe drought conditions this year, for example) can also produce oddly-shaped plants. Even if there have been germline mutations in your garden plants, however, it's difficult or impossible – and rather improbable, truth be told – to link those changes to miniscule amounts of Cs-137 from Fukushima rather than any other cause. TenOfAllTrades(talk) 19:02, 30 October 2012 (UTC)
- Thinking about it, I suppose I should be a little more blunt: radiation mutagenesis is an astronomically unlikely scenario. First, the Chernobyl exclusion zone looks like a nature park - the animals have higher rates of spontaneous abortion, and a few visible mutants have been spotted (run of the mill stuff like albinism that results from breaking a gene) and the plants may be accumulating mutations, but it's not visibly out of whack. Our article Chernobyl disaster effects says that melanized fungi have taken to growing in the building, but what it says is that this is due to natural selection where the fungi have adapted to absorb energy from the radiation. It's a remarkable claim to me (fungi aren't generally known for their photosynthesis!) but the source checks out and appears in a good journal. [21] Second, a contamination on that scale should be detected, as the Chernobyl event was, by various surveillance systems, though I suspect the odds of Homeland Security dropping the ball are better than average. Now, it is possible that a small scale contamination could occur - things happen like a drum looted from a nuclear plant and used to store water, or a medical X-ray source broken open by metal scavengers. But those things usually announce themselves by the imminent death from radiation sickness of whoever is involved. It can kill plants also (the Red Forest) but that shouldn't look like mutations.
- That said, your interest is still commendable. While what you see is probably well known, new plant pathogens do get imported into the country all the time, and action against them almost always is delayed until someone living in the area starts asking questions. Wnt (talk) 19:04, 30 October 2012 (UTC)
- The OP did not mention anything about Fukushima. The radio activity in USA originating from Fukushima is ofcourse several orders of magnitude to weak to be a plausible explanation to the observations. In case of a known Goiânia accident like scenario it could be reasonable to speculate in that direction. Why do you think the OP are refering to Cs-137 from Fukushima, have it been discused in the media recently? Gr8xoz (talk) 21:31, 30 October 2012 (UTC)
- If you do a quick Google search for some combination of plant mutations, Oregon, and/or cesium-137, you'll find that the top hits tend to be Fukushima-related forum posts and fringe/conspiracy outlets. If the OP conducted his research starting from an (understandable, but likely incorrect) explicit initial assumption that he had mutations – rather than just parasites, infections, or drought effects – then the results of his "quick search of the internet" are almost certainly going to lead him down the Fukushima Cs-137 garden path. TenOfAllTrades(talk) 17:37, 31 October 2012 (UTC)
Could an accident like Fukushima happen in the US due to Sandy?
Comploose (talk) 19:45, 30 October 2012 (UTC)
- Sure, but "something could conceivably happen" isn't a terribly compelling statement. The Fukushima Daiichi nuclear disaster was driven by compounding failures aided by human decisions. In short, the reactors shut down (thus losing the power to cool the reactors themselves), the power grid was severed (thus losing the power to cool the reactors from safely operating remote generators), the backup generators went offline (thus losing the last on-site backup), and officials were slow to irreparably flood the reactors after all of the preceding. Additionally, various prior safety violations have since been noted. One can contrive this sequence of effects at functionally any reactor, as even designs that are big on passive safety still have potential failure modes. A massive natural disaster isn't even necessary, though it makes things more likely. Generally, though, I'd expect a hurricane to be less risky than a massive earthquake / tsunami combo. Most notably, there's advance warning, and several nuclear plants did in fact begin implementing cautionary plans as Sandy approached and the water rose. The end result is that you've got a much better case of seeing a result such as that at Browns Ferry Nuclear Power Plant last year. Same reactor type as Fukushima, emergency conditions due to natural disaster (in this case, an EF5 tornado), and unless you live in the area, you've probably never heard of it. Something indeed "could" happen -- but it's unlikely, and as you've now seen, it didn't. — Lomn 21:18, 30 October 2012 (UTC)
- Apparently some have gotten to the first step - shutdowns. [22] Let's hope they still have a way of powering their cooling systems... Wnt (talk) 21:43, 1 November 2012 (UTC)
Airplanes during storms
Why is it a problem if the wind is over 100 mi/h? Airplanes should be able to deal with much more than that, if it's int he right direction. Comploose (talk) 20:05, 30 October 2012 (UTC)
- (ec) In the United States, it is illegal (14 CFR §121.171) to operate an aircraft for commercial transport of passengers beyond the aircraft's rated operating limitations, which include, among other items, a maximum demonstrated crosswind component (14 CFR § 23.1585). If you aren't operating a commercial aircraft for the transportation of passengers, different regulations apply. Part of flying an airplane is learning which rules apply to each situation. Another part of learning to fly is demonstrating sound judgement, which is specifically evaluated by the FAA. A pilot who operates a commercial aircraft in surface winds exceeding 100 mph is neither complying with relevant laws, nor exercising sound judgement for the safe operation of the aircraft. Nimur (talk) 21:32, 30 October 2012 (UTC)
- It depends on what the wind is doing and on what the airplane is doing. In level flight, at altitude, and with generally horizontal winds, an airplane's ability to fly indeed doesn't care too much about windspeed or direction (its ability to get from A to B may be dependent on whether that's a headwind, though). However, turbulence will generally rise with windspeed, and vertical components (sudden updrafts or downdrafts) are also more hazardous -- and storms, as contrasted with the jet stream, are much more prone to such winds. If the airplane is operating near the ground, particularly in takeoff or landing operations, wind-related hazards are magnified. — Lomn 21:28, 30 October 2012 (UTC)
- You are correct - wind speed at altitude can regularly exceed 100mph ground speed, and that's not a problem. What is a problem is when you have wind speeds of that magnitude near the ground, ans the topography of the ground tends to cause turbulence. It's this turbulence which aircraft try to avoid, as abrupt changes in local wind speed or direction (wind shear) can expose the wings to high angle of attack, and make it easy to stall the lifting surfaces. This is especially true when aircraft are configured for landing and takeoff. Also, all commercial aircraft are only certified to take off and land with a certain crosswind component. This is usually sufficiently high that for day to day operation, operators work easily within these limits by choosing runways that orient the aircraft preferentially with the wind, so that if for example the wind is at 30 knots, but you are landing at 45 degrees to that wind, the crosswind component is only 15 knots. As wind speed gets higher near the ground you need runways that are "more aligned" to the prevailing wind, and that runway might not exist locally. — Preceding unsigned comment added by Happymulletuk (talk • contribs) 21:45, 30 October 2012 (UTC)
- As you noted at the end of your question, "if it's in the right direction". A basic part of the problem is that runways aren't often in the right direction for the wind, or vice versa. Nyttend (talk) 23:14, 30 October 2012 (UTC)
- Also note that steady 100 MPH winds wouldn't be as bad as winds which gust up to 100 MPH. It's the variability that's the worst problem. StuRat (talk) 02:22, 31 October 2012 (UTC)
- You may be interested in this huffington post interview[23] with someone who flies into hurricanes for a living. The two issues seem to be a) as people have suggested, turbulance. The hurricane hunters seem to avoid the worst this by hand flying around it using radar as guidance. b) hail. The hurricane hunters use turboprop planes because ice ingestion by jet engines can cause engine damage. Either of these issues would be enough to make a commercial flight an unacceptable risk, not to mention very uncomfortable for the passengers. I also agree with the point that with 100mph winds at surface level you could not be at all confident of finding a runway with an acceptable crosswind component. Equisetum (talk | contributions) 12:11, 31 October 2012 (UTC)
- I may point out, too, that even the Hurricane Hunters have their limits...see this narrative by Dr. Jeff Masters of Wunderground: [24] Ks0stm (T•C•G•E) 21:42, 31 October 2012 (UTC)
- Your title is "airplanes during storms". For me, as a pilot, the storm would be a greater concern than a steady 100mph wind; if it were truly steady (no major gusts, no turbulence), and not extending to the ground, it would have more of a factor on how fast one could get to the destination than how safely. Even if it were absolutely steady wind, on the ground I could not physically taxi a light airplane to the runway without the airplane being flipped over, risk taking off by accident, etc. If this hypothetical wind were not almost straight down the runway, the aircraft would get blown off the runway during the takeoff run. Whether the wind is on the ground or at a higher altitude could very well be the decision for whether an aircraft flies or stays parked. As for storms, it is not the 100mph wind that's the biggest problem. It is the fact that you have a lot of very very strong winds moving in different directions or at different rates of speed. While flying in a 100mph steady wind would be smooth flying, flying from a pocket of air that is rising at 60 miles per hour to one that is descending at 60 mph would be quite dangerous; if it gets too bad, it can exceed the aircraft's limits to the point of structural failure - i.e. breaking up. While in normal operation it is generally quite difficult to overstress an aircraft to that point, flying into a powerful storm is a situation where that can happen. Beyond that, there is hail and icing to worry about. Lightning is also an obvious risk, but in reality is not actually as big of a safety concern as one might think. It is the unstable winds and the icing that really cause problems in a storm. Flying into a thunderstorm, or, I would imagine, a hurricane is usually a terrible idea. Also, just because something could be technically possible (taking off into a 100mph headwind straight down the runway, or flying through a storm) does not mean that it should be tried. The point where the risks become unacceptable is reached much, much before the situation gets that bad. Each decision of whether to fly or not must take into account the aircraft's ability and the pilot's ability to fly in the given conditions. Falconusp t c 21:30, 31 October 2012 (UTC)
- To sum up this long paragraph: if the wind is at altitude, it may not be a big problem. If the wind is on the ground, the aircraft cannot fly. In a storm, it is the instability of the air along with icing that are so dangerous, not the 100mph wind by itself. In all cases, which aircraft fly under what conditions will depend on what the aircraft can handle and what the pilots can handle. Falconusp t c 21:43, 31 October 2012 (UTC)
EEG correlates
What are EEG correlates? The Glenn Wilson (psychologist) article refers to them but doesn't explain what they are; a Google search for the term makes me think that they're somehow related to electroencephalogrammes (is that how you spell it?), but all of the pages I found, like the Wilson article, seemed to assume that the reader already knew what they were. Nyttend (talk) 23:21, 30 October 2012 (UTC)
- Yes, EEG means electroencephalogram. "EEG correlates" means electrical signals from the brain recorded using electrodes glued to scalp. Wilson apparently studied how those signals vary as a function of personality type. Looie496 (talk) 23:46, 30 October 2012 (UTC)
Can a planet orbit inside a red giant for hundreds of thousands of years ?
That's the claim in this article, labeled "Bad Astronomy": [25]. This is a reference for our extragalactic planet article, where the claim is repeated. This sounds quite absurd, to me. StuRat (talk) 23:59, 30 October 2012 (UTC)
- Why is it absurd? You first need to define what "inside" means, which isn't a trivial issue. Then you'd need to see if, once you pick that boundary, if it is possible for an object to survive inside that boundary. I don't dismiss the possibility as outside of the realm of possibility merely on the face of it. I mean, a star is not merely a giant ball of magic gas that instantly annihilates any matter it touches. After all, the Wikipedia articles Formation and evolution of the Solar System and Future of the Earth notes that many models allow for the Earth to be engulfed by the future Red Giant phase of the Sun, and still survive such an event (for any given definition of "survival"). The Future of the Earth article notes that it would take some 200 years for the Earth to be vaporized from inside the sun, one could envision an object in a stable orbit just skimming inside the outer boundary of a Red Giant surviving many millenium in that state. The Formation and evolution of the Solar System article notes that there are models which allow for the long-term survival of the Earth after being engulfed by the Sun. --Jayron32 00:14, 31 October 2012 (UTC)
- There's a huge diff between 200 years and hundreds of thousands of years. Over that time period, it seems that orbital decay due to drag and/or heating due to drag and radiation ought to either vaporize it or plunge it into the core. StuRat (talk) 00:46, 31 October 2012 (UTC)
- Note this article links to [26], which references BP Piscium. The first article says the upper layers of the star were "nearly a vacuum" - the second makes it sound more substantial, so that the planet forced out a disk, but called the orbiting a matter of "years", not "hundreds of thousands of years". Figure out what's known about BP Piscium (start an article would be nice...) and you'll have your answer. Wnt (talk) 00:21, 31 October 2012 (UTC)
- There's nothing merely about a giant ball of magic gas that instantly annihilates any matter it touches. —Tamfang (talk) 04:58, 31 October 2012 (UTC)
- "Instant" ≠ "hundreds of thousands of years", not by any definition. StuRat (talk) 05:00, 31 October 2012 (UTC)
- Would you accept "relatively instantly"? -- Jack of Oz [Talk] 23:40, 31 October 2012 (UTC)
- The boiling point of [[iron] is 3134 K. The surface of our sun is only 5778 K. The surface temp of Betelgeuse is 3140 K. Given the very minor difference in temperature and the extremely low density of the stellar atmosphere, a period of hundreds of thousands of years seems entirely reasonable. I am sure some math wiz can figure out how long it would take to boil away an earth's mass of iron given its heat of vaporization and a given temperature, beginning surface area, and density. That kind of calculus is way beyond me though. Try the math desk, they should find it easy. μηδείς (talk) 19:28, 31 October 2012 (UTC)
- You're neglecting that the planet is orbiting at a speed of tens or hundreds of thousands of miles per hour. Slamming into even diffuse gas at those speeds generates a heck of a lot of heat. Also, the planet wouldn't remain at the surface for long, as this friction would also cause the orbit to decay. StuRat (talk) 20:01, 31 October 2012 (UTC)
- That would have to be taken into account but the "near vacuum" description makes me wonder how strong the effect. I don't have the facts or the math. μηδείς (talk) 22:49, 31 October 2012 (UTC)
A planet has orbital angular momentum of
The drag of the star on the planet imposes a torque approximately
It follows that the decay timescale is roughly
For solar mass star and an Earth-like orbit, this comes to
If we expand the sun to 1 AU, it's mean density would be about 1.5×10−8 times the density of the Earth. That implies a lifetime of about 270 years, not far different from the 200 years mentioned above. However, that's the mean density of the star. The density profile of a star can be approximated as . In particular, the density equals the mean density around . If you want the density that the planet is flying through to be 1000 times lower (and hence allow it to last 1000 times longer), that implies .
Kinematically, these calculations suggest that it is possible that one could have an orbit located just right so that the planet is barely inside the outer limit of a Red Giant and thus able to persist for hundreds of thousands of years before it decays. However, the orbital positioning that would make such a thing possible is quite narrow. If planetary orbits were randomly distributed circles, there would be only about 1 chance in 5000 of having an orbit in the right position to survive that long.
So far I've neglected the possibility of the planet boiling away. Given the low thermal conductivity of rock, I don't think you'd see much ablation relative to total mass over 100 years. The surface would be a melting / boiling mess, of course, but planets are awfully big and even at 5000 K, propagating the heat would seem to take a long time. Heat the surface to 5000 K, and the back of my envelope suggests that it would still take more than 200 years to remove the first 50 km. Given 1000 times longer you'd probably kill much more of the planet but not necessarily all of it.
All in all, having an object survive hundreds of thousands of years inside a red giant seems plausible, though it would be an unlikely event. Dragons flight (talk) 02:00, 1 November 2012 (UTC)
- Yes, the mean density is nowhere near correct. The density at the outer fringe of an expanded star the mas of the sun will be far, far, far , far less. μηδείς (talk) 02:53, 1 November 2012 (UTC)
- I included a functional form for the density versus radius. At 98% of the way to the surface the density is still 10% of the mean density. Of course the density goes to approximately zero at the very end, but the fall off is pretty abrupt. If you want to invoke special pleading, one can posit almost arbitrarily small drag (which might be necessary to get a planet to last 100s of thousands of years), but for planets that are non-trivially inside the surface, the density is generally a non-trivial fraction of the mean density. Dragons flight (talk) 04:40, 1 November 2012 (UTC)
- Nice calcs. But keep in mind that even if the planet starts out in this perfect orbit, it won't stay there, due to drag. StuRat (talk) 03:00, 1 November 2012 (UTC)
- Feel free to do an integration rather than a constant drag approximation, but I doubt you'll see the dynamical timescale change by more than a order of magnitude. Dragons flight (talk) 04:40, 1 November 2012 (UTC)
October 31
Where do those fires in NYC come from?
If they had no electricity, and lighting protection is passive, how could that happen? Comploose (talk) 00:35, 31 October 2012 (UTC)
- Broken natural gas lines seem a likely culprit. It doesn't take much to set one of those off. Also, some of the fires seem to be caused by one building catching fire, and passing the fire on to others. Densely-packed cities suffer from such a chain reaction as a constant threat. --Jayron32 00:40, 31 October 2012 (UTC)
- Some are caused by downing of overhead electrical power distribution wires ... the "no electricity" follows from the downed wires, rather than precedes. --Tagishsimon (talk) 02:05, 31 October 2012 (UTC)
- Also, people who are wet, cold, and in the dark and whose heat and electricity are out tend to light fires for light and to keep warm (fireplaces, lanterns, candles, etc.), and sometimes those fires get away from them. StuRat (talk) 02:14, 31 October 2012 (UTC)
Why wasn't the power cut before the storm made landfall? Count Iblis (talk) 02:40, 31 October 2012 (UTC)
- Because lotsa people object to that sort of behaviour - it's not a binary thing.
- Also, as StuRat pointed out, even if people are not wet, the move from electric lighting, or from controlled electric or gas heating to largely uncontrolled fires of various sorts, produces far more out-of-control fires per person. Mix that with tens of millions and with darkness, and you get what you get. --Demiurge1000 (talk) 02:44, 31 October 2012 (UTC)
- Cutting power preemptively would probably have done more harm than good -- it would have hampered communications and the initial response (due to darkness), and also as Demiurge pointed out, there would be more fires from people using open flames for lighting and heating than from downed power lines. FWIW 24.23.196.85 (talk) 05:04, 31 October 2012 (UTC)
- I agree that cutting power without notice is bad, but if scheduled ahead of time and if everyone was notified, then this might encourage people to evacuate those zones, since they don't want to be in an unheated, dark home. StuRat (talk) 05:12, 31 October 2012 (UTC)
- I don't think any power company wants the bad press that would come from news reports, "Power Company Turns Off New York, Leaves Citizens in the Dark During Crisis." A failure can at least be blamed on nature. — The Hand That Feeds You:Bite 20:49, 31 October 2012 (UTC)
- I would expect the order to come from the government, so they would get the blame or praise, not the power company. StuRat (talk) 22:17, 31 October 2012 (UTC)
- And then hospitals and other high-priority services would have to switch to emergency generators earlier. Completely cutting off power would also put people and facilities that may not have lost power in the dark. People without generators would lose access to TV reports giving last-minute updates on the situation sooner. 209.131.76.183 (talk) 12:12, 1 November 2012 (UTC)
- Candles are a common cause of house fires. See this or this, or topically, this advice. Zoonoses (talk) 06:46, 31 October 2012 (UTC)
- The idea of cutting the power before a storm hits so you avoid downed live power lines is a bit analogous to the hairbrained idea that some have proposed of turning off cellular service in a disaster. The "solution" is way worse than the problem in the first place. Shadowjams (talk) 21:09, 31 October 2012 (UTC)
- They cut power for this reason in Lower Manhattan. The fires in the outerboroughs have mainly been due to exploding transformers--I can't see them but my friend in Brooklyn said she was watching the explosions. μηδείς (talk) 22:47, 31 October 2012 (UTC)
- My friends in the upper west side are very glad they didn't arbitrarily cut power. And for them "working from home" doesn't mean not doing anything. Shadowjams (talk) 08:53, 1 November 2012 (UTC)
- There was a news report last night of someone whose house burned down because of power being restored. I forget now whether it ignited leaked gas in the home, or what. ←Baseball Bugs What's up, Doc? carrots→ 11:39, 1 November 2012 (UTC)
Science Olympiad
I'm doing Science Olympiad and let say my handout packet tells me that I should know the anatomy of kidney. Is that mean I have to know all structures that made up of the kidney and their functions? And of course the function of the kidney. Thanks!184.97.240.247 (talk) 02:01, 31 October 2012 (UTC)
- Yup. You should be aiming to understand & explain the structure, and how the structure relates to the functions. --Tagishsimon (talk) 02:09, 31 October 2012 (UTC)
- And what do they mean by physiology of something, example: physiology glial, physiology of neuron. So what exactly do I have to know about it if I want to know about its physiology?184.97.240.247 (talk) 02:31, 31 October 2012 (UTC)
- I infer from what you've said about it that you might want to review the organ structures/tissues, how they relate to organ function, what major processes occur there, and how they interact. In the case of the kidney, you could trace the flow of blood (through the afferent arterioles, etc), know the major structures along the path (e.g. macula densa), and how they regulate the production of urine (and how urine varies depending on blood pressure, blood flow, etc) and the production of hormones that act elsewhere in the body. It's a huge topic really, so you might want to start with the big things and work down to details. -- Scray (talk) 02:55, 31 October 2012 (UTC)
- And what do they mean by physiology of something, example: physiology glial, physiology of neuron. So what exactly do I have to know about it if I want to know about its physiology?184.97.240.247 (talk) 02:31, 31 October 2012 (UTC)
Balky burner
I've been meaning to ask about this for some time: One of the burners in my kitchen range (the most powerful one, in case you need to know) occasionally takes several attempts to ignite; however, if while turning the knob I say "Light up, you son of a b**ch", it always ignites without fail. I believe this has to do with the time it takes to attain the proper fuel:air ratio; is this correct? 24.23.196.85 (talk) 06:19, 31 October 2012 (UTC)
- Probably, but there is also the possibility you have magical powers. They (talk) 07:07, 31 October 2012 (UTC)
- Now there's a cursory answer. Clarityfiend (talk) 07:42, 31 October 2012 (UTC)
- I have several crappy burners myself. If I leave it up to the pilot light I will end up with a room full of gas followed by singed eyebrows. Instead, I use a fireplace lighter to light them immediately. StuRat (talk) 07:45, 31 October 2012 (UTC)
- There's no way to know without examining it. Does it always start the first time, just with a delay? Or do you have to turn it on and off multiple times? My purely off-the-cuff guess would actually be that your igniter needs replaced. — The Hand That Feeds You:Bite 21:02, 31 October 2012 (UTC)
- This discussion reminded me that for years I've been wondering why so many automatic lighting gas devices become non-automatic so early in their lives? It seems to be one area where society simply accepts crappy technology. HiLo48 (talk) 22:49, 31 October 2012 (UTC)
- Well, the typical problem is that we can't tell how long the product will last when we make the purchase decision. For something like this, which we expect to last for decades, we'd need to wait decades to evaluate how long they really last, and, of course, by then all new models will be offered. So, we can't make an informed choice, and the manufacturers, knowing this, have little incentive to provide a quality product.
- Once broken, if all it needs is a fireplace lighter to start your burners, there's little incentive to pay to have it repaired or replaced, especially knowing that the same logic will apply to the repaired or replaced item, and therefore it will soon fail, too.
- A way to change the incentive structure is to require all products to carry lifetime warranties, where they have to fix it for free. This would get them to offer quality products, but, on the other hand, the prices would also skyrocket. StuRat (talk) 00:21, 1 November 2012 (UTC)
- The OP said he ONE of his burners (the large one) is difficult to light. So, presumably the other burners light easily. This suggests it is not the igniter - the hand-held ones tend to work or fail completely. The built in eletric ignitors I have no experience of, but seeing the size of the spark in shop demonstrations, it is difficult to believe they would be iffy. A key question is, is the burner difficult because it is the largest, or because it is the one he uses the most or the least frequently? If it is difficult becasue it is the largest, I wonder if the OP is trying to light it with a match. Otherwise, it could be a problem with dirt, or with corrosion or wear in the jet(s). Town gas can vary - the exact mix of various gasses varies depending one where the gas company buys its' bulk supplies from, where in its life cycle the supplier's underground source is, etc. Occaisonally, it can vary enough that the jet size needs to change - and it is the largest burner where you notice difficulty in lighting first. This happened to us a few years ago. The gas company ran adverts in the newspapers, and sent brochures to consumers notifying them that a change in the bulk supply will change the burning characteristics slightly - they said thaat they believed that almost all customers would not notice the change, but if you do, call them and they'll pay for jet replacement. They also published a list of gas hotwater system brands and models (mostly ones so old they were unlikely to be still in service) that required inspection before the change. Wickwack 120.145.191.8 (talk) 01:43, 1 November 2012 (UTC)
- Note that a significant portion of gas stoves, in the US at least, use pilot lights. So, we should ask the OP if they have a pilot light or electric ignition. StuRat (talk) 01:49, 1 November 2012 (UTC)
- Mine has electrical ignition, and the spark actually works just fine every time; however, the gas does not always ignite from the spark (but does so unfailingly if I pronounce the incantation while lighting the burner). So the problem can't be with the igniter. 24.23.196.85 (talk) 05:34, 1 November 2012 (UTC)
- I think you're right that it hasn't reached the right gas/air mix when it first sparks. So, it may be the timing of the spark which is off. I can think of one long-shot for why you standing there might make a difference: Your body weight may deform the floor just enough to contract or expand a pinched off gas line to increase or decrease gas pressure slightly, thus changing when it reaches the ideal gas/air mix. However, I really don't think that's the case (lucky for you, because that would be dangerous), I blame confirmation bias instead. StuRat (talk) 08:27, 1 November 2012 (UTC)
- I think the reason why my saying the incantation makes a difference is that it causes me to turn the knob more slowly, delaying the spark until the right fuel/air ratio is reached. 24.23.196.85 (talk) 22:10, 1 November 2012 (UTC)
Looking for the right word
If you have a big cloud of Hydrogen, it has a certain amount of gravitational potential energy. It also has another kind of potential energy, which is what I am looking for the word for. If all that Hydrogen were to collapse into a star, and fuse completely (i.e. to helium, carbon, etc) is there a word for all that energy? I realize that it is mass turning to energy, but since all the mass does not turn to energy, is there a word for the part that does? Tdjewell (talk) 11:50, 31 October 2012 (UTC)
- Relativistic mass is "the sum total quantity of energy in a body or system (divided by c2)." 209.131.76.183 (talk) 12:36, 31 October 2012 (UTC)
- it's called Binding energy. Dauto (talk) 15:38, 31 October 2012 (UTC)
- That lead me to Nuclear binding energy, which is exactly what I was looking for. I'd mark this Resolved, but I do not know how. Tdjewell (talk) 15:47, 31 October 2012 (UTC)
- Potential energy isn't "energy that you can potentially extract". It has a specific technical meaning that doesn't really apply here. I think there's no term for the energy you can extract through stellar fusion. The nuclear binding energy is negative, or zero in the case of hydrogen-1, and though it does decrease in fusion (to a larger negative value), it seems misleading to say that the star's energy comes from that. Ultimately I suppose it comes from the strong force binding energy (or perhaps from quark kinetic energy, which also contributes significantly to the nucleon mass). -- BenRG (talk) 16:22, 31 October 2012 (UTC)
- Nuclear binding energy and gravitational potential energy are both negative, for the same reason: the only natural reference level is separation to infinity, where the energy is highest. —Tamfang (talk) 18:27, 31 October 2012 (UTC)
- Gravity is a special case that nobody understands, but electromagnetic potential energy is really energy of the field, ∫ (E² + B²), which is always nonnegative. -- BenRG (talk) 20:24, 1 November 2012 (UTC)
- Nuclear binding energy and gravitational potential energy are both negative, for the same reason: the only natural reference level is separation to infinity, where the energy is highest. —Tamfang (talk) 18:27, 31 October 2012 (UTC)
- Potential energy isn't "energy that you can potentially extract". It has a specific technical meaning that doesn't really apply here. I think there's no term for the energy you can extract through stellar fusion. The nuclear binding energy is negative, or zero in the case of hydrogen-1, and though it does decrease in fusion (to a larger negative value), it seems misleading to say that the star's energy comes from that. Ultimately I suppose it comes from the strong force binding energy (or perhaps from quark kinetic energy, which also contributes significantly to the nucleon mass). -- BenRG (talk) 16:22, 31 October 2012 (UTC)
- I just noticed that the article says that nuclear binding energy is always positive, but that's just because they're using the opposite sign convention. If energy is released in fusion, the total nuclear binding energy goes down in my convention, up in the article's. -- BenRG (talk) 16:25, 31 October 2012 (UTC)
Are we trying to contact alien civilizations?
I was reading this article, Scientists suggest we might be overlooking alien communications?, and it talks about SETI actively listening for alien signals. But it made me wonder if we're also sending any communications to alien civilizations? I know that the Voyager spacecraft had golden records and that the Arecibo message was sent for 3 minutes several decades ago. But are there/were there any active attempts to send signals to alien civilizations? It seems to me that the answer to Fermi's Paradox is that everyone is listening, and no one is talking. A Quest For Knowledge (talk) 14:06, 31 October 2012 (UTC)
- Some people suggest that telling other civilisations we exist may not be the best idea. Initial interactions between very different cultures in our history have rarely been positive experiences for all participants. HiLo48 (talk) 22:44, 31 October 2012 (UTC)
- Hasn't mankind being casually vomiting all manner of electromagnetic garbage into space for over a century anyway? Or have they finally decided that most of that stuff will attenuate to insignificance within a few light years now? --Kurt Shaped Box (talk) 01:13, 1 November 2012 (UTC)
- The latter. An omnidirectional broadcast isn't going to make it very far. You need a high-power directional signal aimed at specific targets to have any hope of being heard. StuRat (talk) 01:25, 1 November 2012 (UTC)
- that sounds like what a typical earthling would say. unless their technology is so advanced that they can hear it, could that be possible?68.83.98.40 (talk) 01:50, 1 November 2012 (UTC)
- Well, if they had a star-sized radio telescope pointed directly at Earth, maybe, but the point is, we could reach thousands of times further with directional broadcasts, regardless of the signal strength required at the other end. StuRat (talk) 01:58, 1 November 2012 (UTC)
- StuRat is slightly off target. 68.83.98.40 asked if it is possible a remote being could detect earth manmade stray radiation. The answer is: essentially no. In a vacuum radio waves propagate without attentuation. So if the source is onmidirectional, you just need to build a recieve antenna that subtends the same angle, ie has an effective diameter proportional to distance. So, if you are a ginormous number of lightyears away, you just need to build an antenna the size of a galaxy. It's not for us to say some other intelligent race out there cannot do this. However, in reality, space is not a perfect vacuum. It contains dust, gas, and all manner of things spread out. So, over huge distances, it is like headlights in a dust cloud - there is attenuation wherethe electromagnetic energy is converted into local heat in the dust/gas/etc clouds. At ginormous distances, then, even a galazy sized antenna is not going to pick up anything, as man's stay radiation will have been attenuated below thermal noise. Attempts at SETI and METI have concentrated on certain window frequencies that penetrate space the best.
- I do wonder that maybe some METI has inadvertently used such window frequencies that happen to be on some intergalactic distress channel. Just like when I was a schoolboy, and we experimented with crude homemade radio transmitters, inadvertently on an emergency channel, and got a visit from officials who said "do that again, and you'll be prosecuted, meanwhile we are confiscating your transmitter.", we might eventually get visited by some sort of intergalatic police enforcer who has to decide whether to tell us "what the heck do you think you are doing?" or, stop it by sending us back to the stone age.
- I've read the justification for METI paper, and I'm not convinced.
- Wickwack 60.228.253.243 (talk) 04:01, 1 November 2012 (UTC)
- Not sure what of mine was "slightly off target", we seem to have said the same thing. Also, I take it "man's stay radiation" is supposed to be "stray radiation" ? StuRat (talk) 09:52, 1 November 2012 (UTC)
- The discussion from KurtShapedBox's post on was about aliens picking up our stray radiation, "vomitting all manner". While some of this "vomit" was and is done with directional antennae, for this purpose the directionality was/is not great, is from multiple sites, and is thus from the alien's point of view not focussed. You were on about using directional antennae which said antenna would need to be very highly directional to signal to aliens, as you said, and also pointed precisely at said aliens). That's a different thing. Eavesdropping versus sending. Wickwack 120.145.198.246 (talk) 11:23, 1 November 2012 (UTC)
- Not sure what of mine was "slightly off target", we seem to have said the same thing. Also, I take it "man's stay radiation" is supposed to be "stray radiation" ? StuRat (talk) 09:52, 1 November 2012 (UTC)
- Arguably the Nazca lines are an ongoing effort to communicate with alien civilizations, provided they have very good telescopes. Depending on how far any viewers might be from us, our current efforts might be of comparable seriousness and utility. Wnt (talk) 12:57, 1 November 2012 (UTC)
- Conversely, at the space-time separation currently observable from the vicinity of Earth, the current attempts of our nearest intelligent neighbours might be actually or figuratively of comparable efficacy. (Briefly put: Aliens may be at least as bad at this stuff as we are or ever were.) AlexTiefling (talk) 13:37, 1 November 2012 (UTC)
- Us sending out a powerful beam with a greeting to unknown aliens somewhere in the galaxy might result in a reply and eventually harmonious and informative discourse beamed back and forth. Or it might result in them sending a military force to conquer or exterminate us. It could work out as badly as if Native Americans in the year 1000 AD had built a ginormous outrigger canoe and sent it eastward propelled by trade representatives across the Atlantic with greetings for any civilizations they happened upon, such as the English and the Spanish, with samples of the mineral and plant wealth ripe for conquest in North America. Edison (talk) 21:43, 1 November 2012 (UTC)
Vitamins and mood
Can you get high on vitamins? They indeed can alter your mood if you are getting too little of them, but is the other way round possible? OsmanRF34 (talk) 21:05, 31 October 2012 (UTC)
- You can get delirium as a result of massively overdosing on vitamin A, but that's the only way I know of. Looie496 (talk) 21:28, 31 October 2012 (UTC)
- You can feel a lot better after a vitamin shot after a period of deficiency. I have had to get shots due to a chronic condition, now largely ameliorated, that often caused me to have B deficiencies. I felt a whole lot better the next day--but not "high". μηδείς (talk) 22:51, 31 October 2012 (UTC)
- I think you misread the Q. That vitamins can help your mood, if you have a deficiency, was a given. StuRat (talk) 00:15, 1 November 2012 (UTC)
- No, that's called giving a positive answer to a misguided question. I could just have as easily said no, but didn't find that productive. I also love tuna sushi. Argghll. μηδείς (talk) 02:48, 1 November 2012 (UTC)
- I get "high" when I eat high sushi grade tuna. It's like a body rush. 68.83.98.40 (talk) 01:39, 1 November 2012 (UTC)
- some say its tryptophan but I don't get tired I get energizedGeeBIGS (talk) 02:04, 1 November 2012 (UTC)
- I think you misread the Q. That vitamins can help your mood, if you have a deficiency, was a given. StuRat (talk) 00:15, 1 November 2012 (UTC)
November 1
Comet Shoemaker–Levy 9 hit Jupiter in 1994 at a speed of .0002c. What would have happened to the comet and to the planet if it had hit at:
- .02c
- .2c
- .9c
- .99c
- .9999c
?Magog the Ogre (t • c) 02:28, 1 November 2012 (UTC)
- Jupiter is not a solid body like Earth. It is a giant gas planet, comprising mainly hydrogen and helium. It is the largest planet in our solar system but its density is very low. When the comet struck Jupiter the result was fireballs and plumes of gas. Dolphin (t) 02:45, 1 November 2012 (UTC)
- I know what Jupiter is, and I know what happened in the actual collision. I'm not asking about that though. Magog the Ogre (t • c) 02:49, 1 November 2012 (UTC)
- The first question is easy: the comet would easily be obliterated, because that's what happened to Shoemaker-Levy 9. What happens to Jupiter is harder to answer. Even at 0.0002c, according to our article, "Astronomers had expected to see the fireballs from the impacts, but did not have any idea in advance how visible the atmospheric effects of the impacts would be from Earth." Since even 0.02c is 100 times faster, and the kinetic energy of the comet would be 10000 times greater, I doubt there are reasonable models about what would happen to Jupiter, aside from "absolutely nothing in the long run, because the comet's mass is utterly negligible compared to Jupiter's". --140.180.252.244 (talk) 05:43, 1 November 2012 (UTC)
- When you get up to near light speed, though, I wouldn't be surprised if it shot right through and out the other side (actually an equivalent mass of Jupiter material might shoot out the far side, similar to Newton's cradle). StuRat (talk) 09:43, 1 November 2012 (UTC)
- I suspect that imacting a mass of gas with something moving near the speed of light would probably result in the destruction of most atomic nuclei involved and lots of high-energy radiation blasting out. That radiation would also have some pretty impressive effects on whatever it strikes nearby. Looking up relativistic impact on Google Scholar doesn't bring up anything that sounds like it would apply to your question. 209.131.76.183 (talk) 12:05, 1 November 2012 (UTC)
- I had hoped the relativistic kill vehicle article would have some information, since it's the same concept, just aimed. But alas, it seems not. 146.87.49.21 (talk) 14:20, 1 November 2012 (UTC)
- I'm not sure how reliable it is, but I found an estimate of about 7*10^11 kg for the mass of SL 9. According to that article, the realitivistic kinetic energy at .99c would be somewhere on the order of 10^13 megatons of TNT, which Orders of magnitude (energy) puts somewhere around the kinetic energy of the moon at orbital velocity. I have no idea what whould happen if you released that energy into Jupiter, but at least that gives a general idea of the energy involved at that speed. 209.131.76.183 (talk) 14:41, 1 November 2012 (UTC)
- I had hoped the relativistic kill vehicle article would have some information, since it's the same concept, just aimed. But alas, it seems not. 146.87.49.21 (talk) 14:20, 1 November 2012 (UTC)
- I suspect that imacting a mass of gas with something moving near the speed of light would probably result in the destruction of most atomic nuclei involved and lots of high-energy radiation blasting out. That radiation would also have some pretty impressive effects on whatever it strikes nearby. Looking up relativistic impact on Google Scholar doesn't bring up anything that sounds like it would apply to your question. 209.131.76.183 (talk) 12:05, 1 November 2012 (UTC)
A question about the lead section for the article quantum state
Consider a system with just 1 hydrogen atom. The wave function for the electron only requires 3 quantum numbers . This wave function can give hydrogen's probability density in position space and momentum space. So, what other information about the position or momentum of the electron are not specified? I think we should delete the last phrase about the position or momentum of the electron.--LaoChen (talk)04:57, 1 November 2012 (UTC)
- "Probability density" is not the "position", but only the chance of finding it in a position or that it is usually or mostly in the vicinity of a position. That is, there is no "the electron is at the following coordinates:" type of answer. Likewise, there is not an exact solution for the momentum (maybe only an approximate range of likelihood). It is mentioned in these types of terms later in the lead itself, and then discussed further in the article using a bunch of math. One of the key statements there is "In quantum theory, even pure states show statistical behaviour." DMacks (talk) 09:00, 1 November 2012 (UTC)
Is it possible to heat granite by using dielectric- or induction heating ..?
Is it possible to heat rock like granite by using dielectric heating or induction heating ..? Electron9 (talk) 05:46, 1 November 2012 (UTC)
- Of course. Any material has dielectric loss and some degree of electrical conductivity, and so does granite. However, I would expect the conductivity of granite to be sufficiently low that induction heating would be quite impractical. Dielectric heating certainly would be practical. If you google dielectric properties granite, you'll get all the info you need to determine the power level and optimum frequency needed. See http://mars.mines.edu/pub/76EPropertiesRocks.pdf. You could also of couse obtain a thin (a few mm) sample of granite polished for 2 sides parallel and test it with a Q-meter fitted with a dielectric loss adapter. Most university physics labs and electronics labs have Q-meters. Ratbone 121.221.211.20 (talk) —Preceding undated comment added 06:06, 1 November 2012 (UTC)
- Perhaps "practical" was the missing keyword ;-), I just hope the microwave oven won't go up in smoke like it would if a spoon is forgotten inside it. If I tried with a rock instead. Electron9 (talk) 00:43, 2 November 2012 (UTC)
- A microwave oven is only one kind of dielectric heater. A microwave oven canc ertainly heat granite, but its frequency and other parameters will not be optimum. If you are just going to try a piece of granite or whatever in a domestic oven, I suggest you incude a glass of water to aborb some of the enegery, until you have verified the the piece of granite is getting hot in a reasonable time. If the granite gets warm at about the same rate as an equal volume of water, it will be safe for the oven without the water.
- There is another important consideration: DO NOT heat unknown pieces of granite to reach anywhere near 100 deg C. If you do, any included moisture will cause an explosion.
- Ratbone 60.230.218.171 (talk) 01:42, 2 November 2012 (UTC)
Magnetic field vs various computer parts
What would the effect of a strong magnetic field on an old (2004) desktop computer be? I understand the effects of magnetic fields on CRT monitors and hard drives, but what would happen to the motherboard, videocard or audiocard, dvd/cd writer/reader and the other parts? Would they be able to continue to operate? They (talk) 09:40, 1 November 2012 (UTC)
- If it is not powered on at the time, there will almost certainly be no effect. If it is powered on, I still doubt you'll have problems unless you're talking about an incredibly strong (and probably changing) field. The only components that are in theory affected by magnets are the speakers, fan and DVD drive motors, and possibly the inductors in the voltage regulator circuits. None of those should be affected by any magnets you'll encounter in a normal household. If you're interested in higher-strength fields I suppose you could research what sort of requirements there are for a computer that runs in an MRI lab. 209.131.76.183 (talk) 11:58, 1 November 2012 (UTC)
- Magnetic fields can have a huge effect on PCs. About 5 years ago, I was installing Linux on a PC a work, and it would crash at different points in the install process. This machine was about 2004 vintage. I tried reseating and replacing components to no avail. After the 8th or 9th combination of parts, some old, some new, the install was successful. The difference was, that my radio/cassette wasn't sitting on top of the case for this install! Bench space was limited and it was the only place to put it. I put the radio on top of my CRT monitor, switched it on, and the picture went a bit "funny". Moral: don't use a PC with a radio/casette on top of it. What is puzzling is that the PC had a steel case, which I think should have acted as a Faraday cage, but perhaps there were holes in it, which let the magnetic field in. I guess if I hadn't been listening to the radio, the install would have gone a lot smoother... --TrogWoolley (talk) 14:45, 1 November 2012 (UTC)
- Only Mu-metal stop magnetic fields in practice. And faraday cages only protect from electric fields. Electron9 (talk) 00:48, 2 November 2012 (UTC)
Does flashing 12:00 damage the display
Does a flashing 12:00 damage an LCD display over time or it has no effect?Smallman12q (talk) 12:54, 1 November 2012 (UTC)
- No effect. LED displays are not susceptible to burnout the way incandescent bulbs are. — Lomn 13:21, 1 November 2012 (UTC)
- Ah, LCD (liquid crystal) and LED (light emitting diode) are different techonlogies.... (Note also that there are also VFD (vacuum fluorescent display) clocks used in some applications, which are yet another technology used in a variety of consumer goods.) I suspect that the unsourced answer above is incomplete at best, but I'm pressed for time to do the research right now. Does anyone want to offer some references? TenOfAllTrades(talk) 15:14, 1 November 2012 (UTC)
- Ten is correct, and I botched my acronyms. Regardless, from a practical end-user standpoint, the blinking display has no functionally damaging effect on the device. I will note that the searching I have done indicate that datasheets list the MTBF for the backlight on an LCD assembly, but take as a given that the LCD itself will last indefinitely. At the same time, I'm confident that, at sufficiently fine scales, there is an effect caused by a blinking display that is distinct from the effects caused by either an always-off display or an always-on display. Therein lies the peril of ever answering simply "yes" or "no" on the Science desk, even if one or the other is likely the most useful response. — Lomn 15:33, 1 November 2012 (UTC)
- What causes the flashing? My assumption is there is nothing mechanical doing it, hence nothing that will wear out quicker than a steady light would. Please correct me. μηδείς (talk) 21:34, 1 November 2012 (UTC)
- Incandescent bulbs wear out faster when switched on and off despite the lack of a direct mechanical effect. Thus, "nothing will wear out quicker" should not be assumed as a general rule. — Lomn 23:14, 1 November 2012 (UTC)
- What causes the flashing? My assumption is there is nothing mechanical doing it, hence nothing that will wear out quicker than a steady light would. Please correct me. μηδείς (talk) 21:34, 1 November 2012 (UTC)
- LCD display segments (in the simple backlit and pasive types used in clocks & clock radios) have a square wave voltage applied to the backplane (not to be confused with the backlight), an electrode common to all segments. A segment required to be "on" has a second square wave voltage applied out of phase to the backplane - it thus has an AC voltage across it. A segment required to be "off" has an in-phase squarewave applied to it - it thus has zero voltage difference across it. In both cases there is no DC voltage ever across the display segments - this means there cannot be any electrolytic degradation. The flashing is caused by swapping over the phase every half second or similar time. The segments themselves emit no light and draw no current, so there cannot be any electrochemical action. There is no wearout mechanism. In theory, LCD displays of this type last indefinitely regardless of whether they are on or off. Flashing 12:00 indefinitely cannot do any harm. In practice, LCD's do have a quite limited life due to impurities in manufacture, and sealing may fail, but being on or off will not affect that. Ratbone 60.230.218.171 (talk) 01:33, 2 November 2012 (UTC)
Batch image merge (microscopy software question?)
I have a number of micrographs of cells takrn under brightfield and UV illumination (with a green filter). I'd like to obtain merged images from each of these pairs. What might be the easiest way to batch process all these files? I have The GIMP and ImageJ but I am not familiar with scripting. — Preceding unsigned comment added by 129.215.47.59 (talk) 14:20, 1 November 2012 (UTC)
- How many do you have? With GIMP you can merge a pair in a few seconds of work, using drag & drop (presuming they are aligned perfectly). Unless you have hundreds of pictures, it might not be worth the effort of looking for a more sophisticated solution. Looie496 (talk) 14:58, 1 November 2012 (UTC)
- A good tool for batch processing of images is ImageMagick[27]. It has a command-line tool that can be used to perform all sorts of operations, so it is easily scriptable. 209.131.76.183 (talk) 15:08, 1 November 2012 (UTC)
- ImageMagick is great. It's available as a command-line tool, or as a set of libraries that you can link into software that you write. It's also free software. On the other end of the spectrum is the commercial Image Processing Toolbox for MATLAB. While this software cam be very expensive, it is superior to ImageMagick in many respects: performance, ease-of-use, and depth of coverage of common tasks. If you're going to specialize in image processing, it's a good idea to learn both tools, as they're common throughout the industry and academic worlds. For almost any image-processing job, either MATLAB or ImageMagick is the right answer when you're in the "prototyping" phase. Nimur (talk) 16:26, 1 November 2012 (UTC)
Is there any known plant that is pollinated by the predators of its parasites?
Greetings,
Is there any known plant whose main pollinators, or seed dispersers, actually visit it to feed on its parasites? I can vaguely imagine how such a route of dispersal could evolve with time, but I'm not aware of it actually being found anywhere. Thanks, ליאור • Lior (talk) 16:08, 1 November 2012 (UTC)
- It would seem unlikely except by chance unless the parasite were eating the anthers, in which case they themselves might also be pollinators. μηδείς (talk) 23:34, 1 November 2012 (UTC)
"Hurricane" vs "Post-Tropical 'Super Storm'"
Many insurance policies reference "hurricane" damage separate from regular "storm damage, with different deductibles and claim criteria, etc.. Since Sandy was "side-graded" to a Post-Tropical storm doesn't that mean that the hurricane language in insurance policies is a moot issue in this case? I know that insurance companies would argue to the hill if the shoe was on the other foot. Technically the storm was NOT a hurricane when it made landfall as it did not meet the necessary criteria to be one.165.212.189.187 (talk) 16:24, 1 November 2012 (UTC)
Nevermind. googled: sandy not a hurricane.165.212.189.187 (talk) 16:32, 1 November 2012 (UTC)
- I disagree with the nevermind. Interesting question. Do regular home insurance policies really distinguish specifically hurricane damage from other wind damage? Would it really make a difference for coverage if a storm was 73 mph or 74 mph? What if it was hurricane force winds but not a cyclone (not rotating) and thus not a hurricane, just a hard wind? Duoduoduo (talk) 20:21, 1 November 2012 (UTC)
Yes, there is wind insurance and hurricane insurance clauses/riders. Did you google sandy not hurricane? The govt is going to closely monitor the ins cos to ensure that they do not enforce the hurricane aspects of policies where applicable.68.83.98.40 (talk) 01:16, 2 November 2012 (UTC)
Slowing neutrinos
Could an exceeding intense beam of very very low frequency radio waves slow down an occasional neutrino? I'm thinking intensity could help a little with the low cross section, and the low frequency would mean any interactions that did happen would absorb energy from the neutrino. I'd like to be able someday to have a pound or so of ultraslow neautrinos in a small box.Thanks, Ricfh Petrson198.189.194.129 (talk) 18:52, 1 November 2012 (UTC)
- Others may be able to comment more authoritatively, but no, I don't see how that could work. Radio waves are purely based on the electromagnetic force, whereas neutrinos interact only via the weak force (and of course the gravitational force).
- Supposing you did get your pound of neutrinos, what do you imagine would hold them in the box? --Trovatore (talk) 18:57, 1 November 2012 (UTC)
Somewhere in outer space in free fall, if the neutrinos were traveling sufficiently slowly, say .1mm/century, then they would stay in there for a while, although the box would only serve as a marker to tell you what they were inside, it couldn't prevent them seeping out at all.--Are we absolutely sure they don't interact with EM?? I guess if that's true, there is no way to slow them down.198.189.194.129 (talk) 21:35, 1 November 2012 (UTC)
- There's something called electroweak unification, but per our article, it happens only at very high energies. I suppose there must be some remnant of it at lower energies. --Trovatore (talk) 22:37, 1 November 2012 (UTC)
Sun's magnetic field
Roughly, what's the stength of the Sun's magnetic field on Earth? 65.92.7.202 (talk) 19:02, 1 November 2012 (UTC)
- According to Sun#Magnetic field,
- The interplanetary magnetic field is much stronger than the dipole component of the solar magnetic field. The Sun's dipole magnetic field of 50–400 μT (at the photosphere) reduces with the cube of the distance to about 0.1 nT at the distance of the Earth. However, according to spacecraft observations the interplanetary field at the Earth's location is around 5 nT, about a hundred times greater.[80] The difference is due to magnetic fields generated by electrical currents in the plasma surrounding the sun.
- Duoduoduo (talk) 20:26, 1 November 2012 (UTC)
- Can someone paraphrase that in lay-English for us idiots? μηδείς (talk) 20:47, 1 November 2012 (UTC)
- Not me. I was hoping the OP would understand it. But the article Earth's magnetic field#Intensity says
- The intensity of the field is greatest near the poles and weaker near the Equator. It is often measured in gausses (G) but is generally reported in nanoteslas (nT), with 1 G = 100,000 nT. A nanotesla is also referred to as a gamma (γ).[10][11][12] The field ranges between approximately 25,000 and 65,000 nT (0.25–0.65 G). By comparison, a strong refrigerator magnet has a field of about 100 G.
- So, although I know absolutely nothing about this, it looks to me that the sun's 5 nT at Earth location is dwarfed by the Earth's 25,000 to 65,000 nT. Duoduoduo (talk) 22:36, 1 November 2012 (UTC)
- Not me. I was hoping the OP would understand it. But the article Earth's magnetic field#Intensity says
- Can someone paraphrase that in lay-English for us idiots? μηδείς (talk) 20:47, 1 November 2012 (UTC)
- The strength is 5 nT. Almost all of that is due to electrical currents surrounding the Sun. --140.180.252.244 (talk) 22:37, 1 November 2012 (UTC)
- Well, I am not sure why anyone wuld be talking about tens of thousands of nano teslas, but if the sun's field is 5 at the earth, compared to the earth's 25-65,000, that gives an idea of scale. What is the 100 G, 100 giga teslas? I suppose a tesla must be a local measure then, not a sum across the entire flux. Am I wrong? μηδείς (talk) 23:33, 1 November 2012 (UTC)
Capacitor charging question
I am working on a project which uses a solar panel, and some other electronics, to charge a 10 farad capacitor. I would like the microcontroller to display the time remaining for the capacitor to be fully charged, but cannot find or work out the necessary equation.
Is it possible to work out the time remaining, with the microcontroller having access to the following values:
- The supply voltage
- The current going into the capacitor
- The voltage across the capacitor
- The resistance between the supply and the capacitor
- The required voltage across the capacitor
- And, if necessary, previous values of the above.
85.210.44.120 (talk) 19:41, 1 November 2012 (UTC)
- With a supply voltage , resistance R and capacitance C the voltage over the capacitor will increase over time t according to (see Capacitor#DC_circuits)
- .
- If the voltage over the capacitor now is the time is now
- .
- The time at which it will reach the required voltage is
- ,
- so the time left is
- .
- This formula should work if you have these values, but I wouldn't trust the voltage you meassure over the capacitor while it is charging to be correct since there is always an inner resistance in the capacitor. Either you stop charging temporarily to measure or you measure the inner resistance in advance and use this and the charging current to calculate the real capacitor voltage from your measured value. Ulflund (talk) 23:29, 1 November 2012 (UTC)
.
- Unfortunately, Ulfund is incorrect. A solar panel operated below its open circuit voltage, which it necesarily will be until the capacitor is charged, act as as a constant current source, and the current is directly proportional to the intensity of light falling upon it. A capacitor has a voltage directly proportional to the product of current (in amps) and elapsed time (in seconds) divided by the capacitance (in farads). Therfore, approximately (approximate because the change over from constant current to constant voltage of a solar panel is not abrupt), the time remaining is given by:-
- Tremain = C ( Voc - Vcap ) / I
- where Voc is the open circuit voltage of the panel, and Vcap is the capacitor voltage at the measurment time.
- A capacitor of 10 Fards is likely to be of the supercapacitor type (http://en.wikipedia.org/wiki/Supercapacitor) and these have significant internal series resistance compared to other types, as only supercaps are available in the size cited, and are the most suitable type wrt self discharge. However in this application it is small enough to be neglected in the calculation of time remaining.
- Keit 58.169.250.75 (talk) 00:46, 2 November 2012 (UTC)
spiders
I have a lot on spiders in my basement are there any traps for them, or what is a good way to get rid of them?--Wrk678 (talk) 19:51, 1 November 2012 (UTC)
- I was going to say 'insecticide' - but does insecticide even work on spiders? I've never thought about that before... --Kurt Shaped Box (talk) 20:05, 1 November 2012 (UTC)
- Why do you want to get rid of them? Do you enjoy having extra insects around? Marnanel (talk) 20:11, 1 November 2012 (UTC)
- I think it would depend on what spiders the OP has. Some of them are horrible critters to have about the home. --Kurt Shaped Box (talk) 20:20, 1 November 2012 (UTC)
- My first instinct would be to head to my favorite internet search engine and type in exterminate spiders. If faced with a serious infestation, beyond what internet remedies suggest, I'd look at the local yellow pages for pest/insect control (yeah, spiders are not insects, but those guys will know what to do, whether the enemy has six or eight legs.) 88.112.36.91 (talk) 20:15, 1 November 2012 (UTC)
- I put the question to Google (sorry, no special knowledge) and got [28] which sounds plausible - they say pyrethrins, resmethrin, and allethrin work on them but only if you more or less spray them directly, which is near pointless, so use housekeeping measures. Bottom line is that if you beat the insects, you beat the spiders. But I also got a hit for the vastly less "reliable" source eHow (Wikipedia blacklists www.ehow.com/facts_5906696_safe-insecticide-spiders.html as a link), which makes the curious claim that natural oils of lemon and citronella, detergents, horse chestnuts, and osage hedge apples would repel spiders. Not sure if these are indeed horse chestnuts! But the claims are all over the Web, and not facially implausible. The Telegraph says, well, they don't know, but "conkers sound like bonkers". [29] There sure are a lot of biology projects people could get good studies published about if funding were greatly expanded instead of cut for a change, but that doesn't sound likely. Our article on osage/horse apples cites a study saying that it actually is an insect repellent [30] based on the presence of elemol, said to be comparable to DEET in the publication. Looking it up, I see it repels ticks [31] so I count this one as "probably confirmed", pending replication of results. Oh, and someone's investigating the conkers. [32] And that's why I like to consider non reliable sources seriously. Wnt (talk) 20:36, 1 November 2012 (UTC)
- Horse chestnuts work quite well against spiders, if you've got good aim. --Carnildo (talk) 02:22, 2 November 2012 (UTC)
Cleaning the basement will also work. If you remove all the spider webs regularly, the spiders will leave. Count Iblis (talk) 20:33, 1 November 2012 (UTC)
- Spiders are a self-controlling problem--they'll either eat your pests or themselves. The underlying question is, what is their food source? You are surely infested with something else, unless this is just a recent hatching from one colonist. I, myself, never kill spiders (nor most insects unless they are dedicated pests like ants, roaches, and flies). Of course we don't have many dangerous spiders in the US NE--some areas do. If you must, use bug spray or other bug control measures. Spiders have the same underlying physiology as insects and are subject to the same poisons. If you are really desperate, I know an old lady who will help, but at a price. μηδείς (talk) 20:42, 1 November 2012 (UTC)
I dont have any insects down, there just spiders, and I would like to not use chemicals.--Wrk678 (talk) 23:54, 1 November 2012 (UTC)
- Well, there's no such thing as a spider trap. And they aren't magic, they do have to eat, so there must be some non-spider infestation unless this is just a crop of very recent babies from an egg sac. Try cleaning thoroughly and making sure their is no water source, because you are obviously supporting some sort of little ecology with water and food of some sort. μηδείς (talk) 02:17, 2 November 2012 (UTC)
Question regarding the conversion of one Tesla to one Earth atmosphere
Hi All, and thank you in advance for your consideration. Is it possible to convert one Tesla to one Earth atmosphere, and what would that value be?Gavinth (talk) 21:15, 1 November 2012 (UTC)
- Well, if you set Planck's constant, the gravitational constant, and the speed of light all to a dimensionless value of 1, then all units become dimensionless numbers, so presumably you could make this conversion in that sense. See natural units. Whether the result would have any articulable meaning is another question. --Trovatore (talk) 21:18, 1 November 2012 (UTC)
- This sounds like a pretty good argument against dimensionless units! But to be clear, one tesla (unit) = one kg s-2 A-1, and one atmosphere (unit) = 101325 Pascal (unit) = 101325 kg s-2 m-1. So the number of Teslas in 1 atm is equal to 101325 A/m. Now you just have to figure out what that means. :) Actually, it might be easier to think of it as: an atmosphere is defined as 101325 newtons per square meter, and a tesla as one weber per square meter; the ratio is the number of newtons of force per weber of magnetic flux emerging from the bounded region in question. Wnt (talk) 21:55, 1 November 2012 (UTC)
- No, it is not possible to convert 1 Tesla to 1 Earth atmosphere, because the two measure different quantities. In the same way, a length cannot be converted to an area, a temperature cannot be converted to kilograms, and a position cannot be converted into m/s. --140.180.252.244 (talk) 22:33, 1 November 2012 (UTC)
- You can convert anything to anything, given the right assumptions. For example, kilograms of weight can be converted to newtons - if you know the gravity of the planet you have in mind. Wnt (talk) 22:55, 1 November 2012 (UTC)
- I'm just guessing, but maybe the OP meant the magnetic field in the Earth's atmosphere, rather than the air pressure commonly known as 1 atmosphere (that doesn't make much sense to me). If so, Earth's magnetic field at the surface ranges from 0.25-0.65 mT, so one Tesla would be 1538-4000 times this field. - Lindert (talk) 00:35, 2 November 2012 (UTC)
Is there a name for "hating forks aiming for someone"?
If a fork or any long object is "pointing" at you or someone else and you deliberately change its position so it's pointing at nobody, is there still hope? Moreover, does this behaviour have a name or reason? (The subject doesn't mistake the fork for a gun and this is not a request for medical advise) Joepnl (talk) 23:12, 1 November 2012 (UTC)
- It is called Superstition and has no reason at all. Pointing a folk at someone won't cause anything to happen so neither will turning it. CambridgeBayWeather (talk) 23:43, 1 November 2012 (UTC)
- If you mean forks that are just sitting there with nobody holding them, that would be a type of obsessive/compulsive behavior, akin to refusing to step on cracks in the sidewalk. Looie496 (talk) 02:36, 2 November 2012 (UTC)
Gravtational collapse
Hi, how big would a planet with the same composition as the Earth have to be before it collapsed into some kind of super-dense exotic matter? 86.151.118.165 (talk) 02:51, 2 November 2012 (UTC)
















