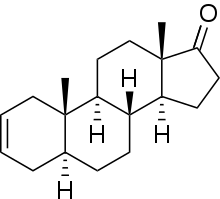
5α-Androst-2-ene-17-one
 | |
| Clinical data | |
|---|---|
| Other names | 5α-Androst-2-en-17-one; 17-Oxo-5α-androst-2-ene; Delta-2-androst-17-one; (+)-Androst-2-en-17-one; Occlesterone |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.289 |
| Chemical and physical data | |
| Formula | C19H28O |
| Molar mass | 272.432 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
5α-Androst-2-en-17-one is an endogenous, naturally occurring, orally active anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT). It is a metabolite of dehydroepiandrosterone (DHEA) in the body[1][2] and is also a pheromone found in elephants and boars.[3] 5α-Androst-2-en-17-one has been sold on the Internet as a "dietary supplement". It resembles desoxymethyltestosterone (17α-methyl-5α-androst-2-en-17β-ol) in chemical structure and may act as an androgen prohormone.
References
- ^ Callies, F; Arlt, W; Siekmann, L; Hübler, D; Bidlingmaier, F; Allolio, B (2000). "Influence of oral dehydroepiandrosterone (DHEA) on urinary steroid metabolites in males and females". Steroids. 65 (2): 98–102. doi:10.1016/S0039-128X(99)00090-2. PMID 10639021.
- ^ Gower, DB; Mallet, AI; Watkins, WJ; Wallace, LM (1997). "Transformations of steroid sulphates by human axillary bacteria. A mechanism for human odour formation?". Biochemical Society Transactions. 25 (1): 16S. doi:10.1042/bst025016s. PMID 9056914.
- ^ Marchlewska-Koj, A.; Lepri, J. J. (2001). "Chemical signals in vertebrates 9". Springer. 9: 131. ISBN 978-0-306-46682-3.
