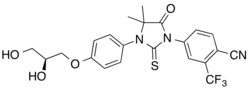
Rezvilutamide
Appearance
 | |
| Clinical data | |
|---|---|
| Other names | SHR3680 |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H20F3N3O4S |
| Molar mass | 479.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rezvilutamide (INN;[1][2] developmental code name SHR3680) is a nonsteroidal antiandrogen which is under development by Jiangsu Hengrui Medicine for the treatment of prostate cancer and breast cancer.[3][4] It is a selective androgen receptor antagonist with reduced brain distribution compared to the structurally related nonsteroidal antiandrogen enzalutamide.[3][4] As of October 2020, rezvilutamide is in phase 3 clinical trials for prostate cancer.[3] Other structural analogues of rezvilutamide besides enzalutamide include apalutamide and proxalutamide.
See also
References
- ^ "Rezvilutamide". chemidplus. U.S. National Library of Medicine.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO.
- ^ a b c "SHR 3680". AdisInsight.
- ^ a b Qin X, Han W, Luo H, Du C, Zou Q, Sun Z, et al. (2020). "SHR3680, a novel antiandrogen, for the treatment of metastatic castration-resistant prostate cancer (mCRPC): A phase I/II study". Journal of Clinical Oncology. 38 (6_suppl): 90. doi:10.1200/JCO.2020.38.6_suppl.90. S2CID 214027454.
