Ertapenem: Difference between revisions
Anypodetos (talk | contribs) + |
Anypodetos (talk | contribs) Move microbiology discussion, etc.; Alter: date. Add: volume, s2cid, doi, pages, issue, journal, title, year, author pars. 1-6. Formatted dashes. | You can use this tool yourself. Report bugs here. | via #UCB_Gadget |
||
| Line 45: | Line 45: | ||
| StdInChIKey = JUZNIMUFDBIJCM-ANEDZVCMSA-N |
| StdInChIKey = JUZNIMUFDBIJCM-ANEDZVCMSA-N |
||
}} |
}} |
||
'''Ertapenem''' is a [[carbapenem]] [[antibiotic]] for the treatment of infections of the [[abdomen]], the lungs, the upper part of the [[female reproductive system]], and [[diabetic foot]], used in the form of infusions or injections. Common side effects include [[diarrhoea]], [[nausea]] and reactions at the injection site. Like all [[beta-lactam antibiotic]]s, it can also cause hypersensitivity reactions such as skin rashes, including severe ones. It is marketed by [[Merck & Co.|Merck]] as '''Invanz'''.<ref>{{cite journal |vauthors=Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA |title=Carbapenems: past, present, and future |journal=Antimicrob. Agents Chemother. |volume=55 |issue=11 |pages=4943–60 | date=November |
'''Ertapenem''' is a [[carbapenem]] [[antibiotic]] for the treatment of infections of the [[abdomen]], the lungs, the upper part of the [[female reproductive system]], and [[diabetic foot]], used in the form of infusions or injections. Common side effects include [[diarrhoea]], [[nausea]] and reactions at the injection site. Like all [[beta-lactam antibiotic]]s, it can also cause hypersensitivity reactions such as skin rashes, including severe ones. It is marketed by [[Merck & Co.|Merck]] as '''Invanz'''.<ref>{{cite journal |vauthors=Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA |title=Carbapenems: past, present, and future |journal=Antimicrob. Agents Chemother. |volume=55 |issue=11 |pages=4943–60 | date=November 2011 |pmid=21859938 |pmc=3195018 |doi=10.1128/AAC.00296-11 |url=}}</ref> |
||
==Medical uses== |
==Medical uses== |
||
Ertapenem is used for the treatment of [[Abdominal cavity|intra-abdominal]] infections, [[community-acquired pneumonia]], [[pelvic infection]]s, and [[diabetic foot]] infections, with bacteria that are susceptible to this drug, or expected to be so. It can also be used to prevent infections after [[colorectal surgery]]. It is given as an [[intravenous infusion]] or [[intramuscular injection]]. The drug is not approved for children under three months of age.<ref name="EPAR">{{cite web|url=https://www.ema.europa.eu/en/documents/product-information/invanz-epar-product-information_en.pdf|title=Invanz: EPAR – Product Information|publisher=[[European Medicines Agency]]|date=2019-11-19}}</ref><ref name="Drugs.com">Invanz {{drugs.com|monograph|invanz}}. Retrieved 2020-07-29.</ref> |
Ertapenem is used for the treatment of [[Abdominal cavity|intra-abdominal]] infections, [[community-acquired pneumonia]], [[pelvic infection]]s, and [[diabetic foot]] infections, with bacteria that are susceptible to this drug, or expected to be so. It can also be used to prevent infections after [[colorectal surgery]]. It is given as an [[intravenous infusion]] or [[intramuscular injection]]. The drug is not approved for children under three months of age.<ref name="EPAR">{{cite web|url=https://www.ema.europa.eu/en/documents/product-information/invanz-epar-product-information_en.pdf|title=Invanz: EPAR – Product Information|publisher=[[European Medicines Agency]]|date=2019-11-19}}</ref><ref name="Drugs.com">Invanz {{drugs.com|monograph|invanz}}. Retrieved 2020-07-29.</ref> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Common side effects are [[diarrhoea]] (in 5% of people receiving ertapenem), [[nausea]] (in 3%) and vomiting, reactions at the injection site (5%), and headache. Uncommon but possibly serious side effects include [[candida infection]]s, skin reactions such as rashes, and [[seizure]]s.<ref name="EPAR" /><ref name="mediQ" /> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Drug interactions via the [[cytochrome P450]] enzyme system or the [[P-glycoprotein]] transporter are considered unlikely. However, ertapenem can reduce the concentrations of [[valproic acid]], an [[epilepsy]] medication, by 60% to 100% within 24 hours; this can result in seizures.<ref name="EPAR" /><ref name="mediQ">{{cite web|url=https://www.mediq.ch/|publisher=mediQ|title=Ertapenem|accessdate=2020-07-29}}</ref> |
||
| ⚫ | |||
===Susceptible bacteria=== |
===Susceptible bacteria=== |
||
Bacteria that are normally susceptible to ertapenem treatment (at least in Europe) include:<ref name="EPAR" /> |
Bacteria that are normally susceptible to ertapenem treatment (at least in Europe) include:<ref name="EPAR" /> |
||
| Line 72: | Line 85: | ||
Bacteria that do not respond to ertapenem include methicillin-resistant ''Staphylococcus'' species as well as ''[[Enterococcus]]'', ''[[Aeromonas]]'' and ''[[Pseudomonas]]''.<ref name="EPAR" /> |
Bacteria that do not respond to ertapenem include methicillin-resistant ''Staphylococcus'' species as well as ''[[Enterococcus]]'', ''[[Aeromonas]]'' and ''[[Pseudomonas]]''.<ref name="EPAR" /> |
||
===Comparison to other antibiotics=== |
|||
| ⚫ | |||
For diabetic foot infections, ertapenem as a single treatment or in combination with [[vancomycin]] has been found to be more effective and have fewer side effects than [[tigecycline]], but in severe cases it is less effective than [[piperacillin/tazobactam]].<ref>{{cite journal|pmid=26337865|year=2015|last1=Selva Olid|first1=A.|last2=Solà|first2=I.|last3=Barajas-Nava|first3=L. A.|last4=Gianneo|first4=O. D.|last5=Bonfill Cosp|first5=X.|last6=Lipsky|first6=B. A.|title=Systemic antibiotics for treating diabetic foot infections|journal=The Cochrane Database of Systematic Reviews|issue=9|pages=CD009061|doi=10.1002/14651858.CD009061.pub2}}</ref><ref>{{cite journal|pmid=30099812|year=2018|last1=Tchero|first1=H.|last2=Kangambega|first2=P.|last3=Noubou|first3=L.|last4=Becsangele|first4=B.|last5=Fluieraru|first5=S.|last6=Teot|first6=L.|s2cid=51966152|title=Antibiotic therapy of diabetic foot infections: A systematic review of randomized controlled trials|journal=Wound Repair and Regeneration : Official Publication of the Wound Healing Society [And] the European Tissue Repair Society|volume=26|issue=5|pages=381–391|doi=10.1111/wrr.12649}}</ref> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Common side effects are [[diarrhoea]] (in 5% of people receiving ertapenem), [[nausea]] (in 3%) and vomiting, reactions at the injection site (5%), and headache. Uncommon but possibly serious side effects include [[candida infection]]s, skin reactions such as rashes, and [[seizure]]s.<ref name="EPAR" /><ref name="mediQ" /> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Drug interactions via the [[cytochrome P450]] enzyme system or the [[P-glycoprotein]] transporter are considered unlikely. However, ertapenem can reduce the concentrations of [[valproic acid]], an [[epilepsy]] medication, by 60% to 100% within 24 hours; this can result in seizures.<ref name="EPAR" /><ref name="mediQ">{{cite web|url=https://www.mediq.ch/|publisher=mediQ|title=Ertapenem|accessdate=2020-07-29}}</ref> |
||
| ⚫ | |||
===Pharmacokinetics=== |
===Pharmacokinetics=== |
||
The route of administration has only a slight effect on the drug's concentrations in the bloodstream: when given as an [[intramuscular injection]], its [[bioavailability]] is 90% (compared to the 100% availability when given directly into a vein), and its highest concentrations in the [[blood plasma]] are reached after about 2.3 hours. In the blood, 85–95% of ertapenem are bound to [[plasma protein]]s, mostly [[albumin]]. Plasma protein binding is higher for lower concentrations, and vice versa. The drug is only minimally [[drug metabolism|metabolized]], as 94% are circulating in form of the parent substance, and 6% as metabolites. The main metabolite is the inactive [[hydrolysis]] product with the ring opened.<ref name="Drugs.com" /> |
The route of administration has only a slight effect on the drug's concentrations in the bloodstream: when given as an [[intramuscular injection]], its [[bioavailability]] is 90% (compared to the 100% availability when given directly into a vein), and its highest concentrations in the [[blood plasma]] are reached after about 2.3 hours. In the blood, 85–95% of ertapenem are bound to [[plasma protein]]s, mostly [[albumin]]. Plasma protein binding is higher for lower concentrations, and vice versa. The drug is only minimally [[drug metabolism|metabolized]], as 94% are circulating in form of the parent substance, and 6% as metabolites. The main metabolite is the inactive [[hydrolysis]] product with the ring opened.<ref name="Drugs.com" /> |
||
Revision as of 15:09, 29 July 2020
 | |
| Clinical data | |
|---|---|
| Trade names | Invanz |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90% (intramuscular) |
| Protein binding | Inversely proportional to concentration; 85 to 95% |
| Metabolism | Minor hydrolysis of beta-lactam ring, CYP not involved |
| Elimination half-life | 4 hours |
| Excretion | Renal (80%) and fecal (10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
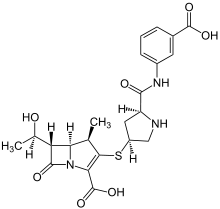
| Formula | C22H25N3O7S |
| Molar mass | 475.52 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ertapenem is a carbapenem antibiotic for the treatment of infections of the abdomen, the lungs, the upper part of the female reproductive system, and diabetic foot, used in the form of infusions or injections. Common side effects include diarrhoea, nausea and reactions at the injection site. Like all beta-lactam antibiotics, it can also cause hypersensitivity reactions such as skin rashes, including severe ones. It is marketed by Merck as Invanz.[1]
Medical uses
Ertapenem is used for the treatment of intra-abdominal infections, community-acquired pneumonia, pelvic infections, and diabetic foot infections, with bacteria that are susceptible to this drug, or expected to be so. It can also be used to prevent infections after colorectal surgery. It is given as an intravenous infusion or intramuscular injection. The drug is not approved for children under three months of age.[2][3]
Contraindications
The drug is contraindicated in people with known hypersensitivity to ertapenem or other carbapenem type antibiotics, or severe hypersensitivity reactions (such as anaphylaxis or severe skin reactions) to other beta-lactam antibiotics.[2][3]
Side effects
Common side effects are diarrhoea (in 5% of people receiving ertapenem), nausea (in 3%) and vomiting, reactions at the injection site (5%), and headache. Uncommon but possibly serious side effects include candida infections, skin reactions such as rashes, and seizures.[2][4]
Overdose
Overdosing is unlikely. In adults receiving the threefold therapeutic dose over eight days, no significant toxicity was observed.[2]
Interactions
Drug interactions via the cytochrome P450 enzyme system or the P-glycoprotein transporter are considered unlikely. However, ertapenem can reduce the concentrations of valproic acid, an epilepsy medication, by 60% to 100% within 24 hours; this can result in seizures.[2][4]
Pharmacology
Susceptible bacteria
Bacteria that are normally susceptible to ertapenem treatment (at least in Europe) include:[2]
- Gram-positive aerobes
- Methicillin-susceptible Staphylococcus species (including Staphylococcus aureus)
- Streptococcus agalactiae
- Streptococcus pneumoniae (not established for penicillin-resistant strains)
- Gram-negative aerobes
- Anaerobes:
- Clostridium species (excluding C. difficile)
- Eubacterium species
- Fusobacterium species
- Peptostreptococcus species
- Porphyromonas asaccharolytica
- Prevotella species
Bacteria that do not respond to ertapenem include methicillin-resistant Staphylococcus species as well as Enterococcus, Aeromonas and Pseudomonas.[2]
Comparison to other antibiotics
For diabetic foot infections, ertapenem as a single treatment or in combination with vancomycin has been found to be more effective and have fewer side effects than tigecycline, but in severe cases it is less effective than piperacillin/tazobactam.[5][6]
Pharmacokinetics
The route of administration has only a slight effect on the drug's concentrations in the bloodstream: when given as an intramuscular injection, its bioavailability is 90% (compared to the 100% availability when given directly into a vein), and its highest concentrations in the blood plasma are reached after about 2.3 hours. In the blood, 85–95% of ertapenem are bound to plasma proteins, mostly albumin. Plasma protein binding is higher for lower concentrations, and vice versa. The drug is only minimally metabolized, as 94% are circulating in form of the parent substance, and 6% as metabolites. The main metabolite is the inactive hydrolysis product with the ring opened.[3]
References
- ^ Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA (November 2011). "Carbapenems: past, present, and future". Antimicrob. Agents Chemother. 55 (11): 4943–60. doi:10.1128/AAC.00296-11. PMC 3195018. PMID 21859938.
- ^ a b c d e f g "Invanz: EPAR – Product Information" (PDF). European Medicines Agency. 2019-11-19.
- ^ a b c Invanz Monograph. Retrieved 2020-07-29.
- ^ a b "Ertapenem". mediQ. Retrieved 2020-07-29.
- ^ Selva Olid, A.; Solà, I.; Barajas-Nava, L. A.; Gianneo, O. D.; Bonfill Cosp, X.; Lipsky, B. A. (2015). "Systemic antibiotics for treating diabetic foot infections". The Cochrane Database of Systematic Reviews (9): CD009061. doi:10.1002/14651858.CD009061.pub2. PMID 26337865.
- ^ Tchero, H.; Kangambega, P.; Noubou, L.; Becsangele, B.; Fluieraru, S.; Teot, L. (2018). "Antibiotic therapy of diabetic foot infections: A systematic review of randomized controlled trials". Wound Repair and Regeneration : Official Publication of the Wound Healing Society [And] the European Tissue Repair Society. 26 (5): 381–391. doi:10.1111/wrr.12649. PMID 30099812. S2CID 51966152.
External links
- "Ertapenem". Drug Information Portal. U.S. National Library of Medicine.
