Interleukin 22: Difference between revisions
Bhagiraj46 (talk | contribs) No edit summary Tags: Mobile edit Mobile web edit |
consistent citation formatting |
||
| Line 1: | Line 1: | ||
{{PBB|geneid=50616}} |
{{PBB|geneid=50616}} |
||
==Source== |
==Source== |
||
'''Interleukin-22''' (IL-22) is [[protein]] that in humans is encoded by the ''IL22'' [[gene]].<ref name="pmid10954742">{{cite journal | author = Dumoutier L, Van Roost E, Colau D, Renauld JC | title = Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 97 | issue = 18 | pages = 10144–9 |date=August 2000 | pmid = 10954742 | doi = 10.1073/pnas.170291697 | url = |
'''Interleukin-22''' (IL-22) is [[protein]] that in humans is encoded by the ''IL22'' [[gene]].<ref name="pmid10954742">{{cite journal | author = Dumoutier L, Van Roost E, Colau D, Renauld JC | title = Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 97 | issue = 18 | pages = 10144–9 | date = August 2000 | pmid = 10954742 | pmc = 27764 | doi = 10.1073/pnas.170291697 | url = }}</ref><ref name="pmid10875937"/> |
||
IL-22 a member of a group of [[cytokine]]s called the [[Interleukin 10|IL-10]] family or IL-10 superfamily (including [[Interleukin 19|IL-19]], [[Interleukin 20|IL-20]], [[Interleukin 24|IL-24]], and [[Interleukin 26|IL-26]]),<ref name="pmid15032600">{{cite journal | author = Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB | title = Interleukin-10 and related cytokines and receptors | journal = Annu. Rev. Immunol. | volume = 22 | issue = | pages = 929–79 | year = 2004 | pmid = 15032600 | doi = 10.1146/annurev.immunol.22.012703.104622 | url = }}</ref> a class of potent mediators of cellular inflammatory responses. It shares use of IL-10R2 in cell signaling with other members of this family, IL-10, IL-26, IL-28A/B and IL-29.<ref>{{cite journal | author = Witte K, Witte E, Sabat R, Wolk K |
IL-22 a member of a group of [[cytokine]]s called the [[Interleukin 10|IL-10]] family or IL-10 superfamily (including [[Interleukin 19|IL-19]], [[Interleukin 20|IL-20]], [[Interleukin 24|IL-24]], and [[Interleukin 26|IL-26]]),<ref name="pmid15032600">{{cite journal | author = Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB | title = Interleukin-10 and related cytokines and receptors | journal = Annu. Rev. Immunol. | volume = 22 | issue = | pages = 929–79 | year = 2004 | pmid = 15032600 | doi = 10.1146/annurev.immunol.22.012703.104622 | url = }}</ref> a class of potent mediators of cellular inflammatory responses. It shares use of IL-10R2 in cell signaling with other members of this family, IL-10, IL-26, IL-28A/B and IL-29.<ref>{{cite journal | author = Witte K, Witte E, Sabat R, Wolk K | title = IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties | journal = Cytokine Growth Factor Rev | volume = 21 | issue = 4 | pages = 237–51 | date = Aug 2010 | pmid = 20655797 | doi = 10.1016/j.cytogfr.2010.04.002 | url = }}</ref> IL-22 is produced by activated [[Dendritic cell|DC]] and [[T cell]]s and initiates [[Innate immune system|innate immune responses]] against bacterial pathogens especially in [[epithelial cells]] such as respiratory and gut epithelial cells. IL-22 along with IL-17 is rapidly produced by splenic LTi-like cells <ref name="PMD19114665">{{cite journal | author = Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ | title = Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. | journal = J Exp Med. | volume = 206 | issue = 1 | pages = 35–41 | year = 2009 | pmid = 19114665 | pmc = 2626689 | doi = 10.1084/jem.20072713 }}</ref> and can be also produced by [[Th17]] cells and likely plays a role in the coordinated response of both [[Adaptive immune system|adaptive]] and innate immune systems. |
||
IL-22 biological activity is initiated by binding to a cell-surface complex composed of [[Interleukin-22 receptor|IL-22R1]] and [[Interleukin-10 receptor|IL-10R2]] receptor chains and further regulated by interactions with a soluble binding protein, IL-22BP, which shares sequence similarity with an extracellular region of IL-22R1 (sIL-22R1). IL-22 and IL-10 receptor chains play a role in cellular targeting and [[signal transduction]] to selectively initiate and regulate immune responses.<ref name="pmid18599299">{{PDB|3DGC}}; {{cite journal | author = Jones BC, Logsdon NJ, Walter MR | title = Structure of IL-22 bound to its high-affinity IL-22R1 chain | journal = Structure | volume = 16 | issue = 9 | pages = 1333–44 |date=September 2008 | pmid = 18599299 | pmc = 2637415 | doi = 10.1016/j.str.2008.06.005 | url = }}</ref> IL-22 can contribute to immune disease through the stimulation of inflammatory responses, [[S-100 protein|S100s]] and [[defensin]]s. IL-22 also promotes [[hepatocyte]] survival in the liver and epithelial cells in the lung and gut similar to IL-10.<ref name="pmid11244051">{{cite journal | author = Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A | title = Interleukin-10 and the interleukin-10 receptor | journal = Annu. Rev. Immunol. | volume = 19 | issue = | pages = 683–765 | year = 2001 | pmid = 11244051 | doi = 10.1146/annurev.immunol.19.1.683 | url = }}</ref> In some contexts, the pro-inflammatory versus tissue-protective functions of IL-22 are regulated by the often co-expressed cytokine IL-17A <ref name="pmid20498020">{{cite journal | author = Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D | title = Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A | journal = J Exp Med. |
IL-22 biological activity is initiated by binding to a cell-surface complex composed of [[Interleukin-22 receptor|IL-22R1]] and [[Interleukin-10 receptor|IL-10R2]] receptor chains and further regulated by interactions with a soluble binding protein, IL-22BP, which shares sequence similarity with an extracellular region of IL-22R1 (sIL-22R1). IL-22 and IL-10 receptor chains play a role in cellular targeting and [[signal transduction]] to selectively initiate and regulate immune responses.<ref name="pmid18599299">{{PDB|3DGC}}; {{cite journal | author = Jones BC, Logsdon NJ, Walter MR | title = Structure of IL-22 bound to its high-affinity IL-22R1 chain | journal = Structure | volume = 16 | issue = 9 | pages = 1333–44 | date = September 2008 | pmid = 18599299 | pmc = 2637415 | doi = 10.1016/j.str.2008.06.005 | url = }}</ref> IL-22 can contribute to immune disease through the stimulation of inflammatory responses, [[S-100 protein|S100s]] and [[defensin]]s. IL-22 also promotes [[hepatocyte]] survival in the liver and epithelial cells in the lung and gut similar to IL-10.<ref name="pmid11244051">{{cite journal | author = Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A | title = Interleukin-10 and the interleukin-10 receptor | journal = Annu. Rev. Immunol. | volume = 19 | issue = | pages = 683–765 | year = 2001 | pmid = 11244051 | doi = 10.1146/annurev.immunol.19.1.683 | url = }}</ref> In some contexts, the pro-inflammatory versus tissue-protective functions of IL-22 are regulated by the often co-expressed cytokine IL-17A <ref name="pmid20498020">{{cite journal | author = Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D | title = Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A | journal = J Exp Med. | volume = 207 | issue = 6 | pages = 1293–305 | year = 2010 | pmid = 20498020 | pmc = 2882840 | doi = 10.1084/jem.20092054 | url = http://jem.rupress.org/content/207/6/1293.abstract }}</ref> |
||
==Target tissue== |
==Target tissue== |
||
Targets of this cytokine are mostly non-hematopoietic cells such as [[hepatocytes]], [[keratinocytes]], and [[lung]] and [[intestinal]] [[epithelial cells]]. [[Pancreatic islets]] also express high levels of IL-22 receptor. It has been shown to induce islet beta cell regeneration |
Targets of this cytokine are mostly non-hematopoietic cells such as [[hepatocytes]], [[keratinocytes]], and [[lung]] and [[intestinal]] [[epithelial cells]]. [[Pancreatic islets]] also express high levels of IL-22 receptor. It has been shown to induce islet beta cell regeneration.<ref>{{cite journal | author = Hill T, Krougly O, Nikoopour E, Bellemore S, Lee-Chan E, Fouser LA, Hill DJ, Singh B | title = The involvement of interleukin-22 in the expression of pancreatic beta cell regenerative Reg genes. | journal = Cell Regeneration | year = 2013 | volume = 2 | issue = 2 | pages = 1-11 | doi = 10.1186/2045-9769-2-2 }}</ref> |
||
== Signaling == |
== Signaling == |
||
IL-22, signals through the [[interferon]] receptor-related proteins CRF2-4 and IL-22R.<ref name="pmid10875937">{{cite journal | author = Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL | title = Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R | journal = J. Biol. Chem. | volume = 275 | issue = 40 | pages = 31335–9 |date=October 2000 | pmid = 10875937 | doi = 10.1074/jbc.M005304200 | url = }}</ref> It forms cell surface complexes with [[Interleukin-22 receptor|IL-22R1]] and [[Interleukin-10 receptor|IL-10R2]] chains resulting in signal transduction through receptor, IL-10R2. The IL-22/IL-22R1/IL-10R2 complex activates intracellular kinases ([[JAK1]], [[Tyrosine kinase 2|Tyk2]], and [[MAP kinase]]s) and transcription factors, especially [[STAT3]]. It can induce [[Interleukin 20|IL-20]] and [[Interleukin 24|IL-24]] signaling when IL-22R1 pairs with IL-20R2. |
IL-22, signals through the [[interferon]] receptor-related proteins CRF2-4 and IL-22R.<ref name="pmid10875937">{{cite journal | author = Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL | title = Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R | journal = J. Biol. Chem. | volume = 275 | issue = 40 | pages = 31335–9 | date = October 2000 | pmid = 10875937 | doi = 10.1074/jbc.M005304200 | url = }}</ref> It forms cell surface complexes with [[Interleukin-22 receptor|IL-22R1]] and [[Interleukin-10 receptor|IL-10R2]] chains resulting in signal transduction through receptor, IL-10R2. The IL-22/IL-22R1/IL-10R2 complex activates intracellular kinases ([[JAK1]], [[Tyrosine kinase 2|Tyk2]], and [[MAP kinase]]s) and transcription factors, especially [[STAT3]]. It can induce [[Interleukin 20|IL-20]] and [[Interleukin 24|IL-24]] signaling when IL-22R1 pairs with IL-20R2. |
||
== Structure == |
== Structure == |
||
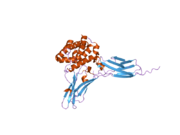
IL-22 is an [[Alpha helix|α-helical]] cytokine. IL-22 binds to a heterodimeric cell surface receptor composed of [[Interleukin-10 receptor|IL-10R2]] and [[Interleukin-22 receptor|IL-22R1]] subunits.<ref name="pmid18599299"/> IL-22R is expressed on tissue cells, and it is absent on immune cells.<ref>{{cite journal| |
IL-22 is an [[Alpha helix|α-helical]] cytokine. IL-22 binds to a heterodimeric cell surface receptor composed of [[Interleukin-10 receptor|IL-10R2]] and [[Interleukin-22 receptor|IL-22R1]] subunits.<ref name="pmid18599299"/> IL-22R is expressed on tissue cells, and it is absent on immune cells.<ref name="pmid15308104">{{cite journal | author = Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R | title = IL-22 increases the innate immunity of tissues | journal = Immunity | volume = 21 | issue = 2 | pages = 241–54 | year = 2004 | pmid = 15308104 | doi = 10.1016/j.immuni.2004.07.007 | url = }}</ref> |
||
Crystallization is possible if the N-linked glycosylation sites are removed in mutants of IL-22 bound with high-affinity cell-surface receptor sIL-22R1. The crystallographic asymmetric unit contained two IL-22-sIL-22R1 complexes.<ref name="pmid18599299"/> |
Crystallization is possible if the N-linked glycosylation sites are removed in mutants of IL-22 bound with high-affinity cell-surface receptor sIL-22R1. The crystallographic asymmetric unit contained two IL-22-sIL-22R1 complexes.<ref name="pmid18599299"/> |
||
==References== |
==References== |
||
{{reflist}} |
{{reflist|35em}} |
||
==Further reading== |
==Further reading== |
||
{{refbegin |
{{refbegin|35em}} |
||
*{{cite journal |
*{{cite journal | author = Weger W, Hofer A, Wolf P, El-Shabrawi Y, Renner W, Kerl H, Salmhofer W | title = Common polymorphisms in the interleukin-22 gene are not associated with chronic plaque psoriasis | journal = Exp. Dermatol. | volume = 18 | issue = 9 | pages = 796–8 | year = 2009 | pmid = 19469905 | doi = 10.1111/j.1600-0625.2009.00840.x }} |
||
*{{cite journal |
*{{cite journal | author = Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M | title = New genetic associations detected in a host response study to hepatitis B vaccine | journal = Genes Immun. | volume = 11 | issue = 3 | pages = 232–8 | year = 2010 | pmid = 20237496 | doi = 10.1038/gene.2010.1 }} |
||
*{{cite journal |
*{{cite journal | author = Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, Barmada MM, Klei L, Daly MJ, Abraham C, Bayless TM, Bossa F, Griffiths AM, Ippoliti AF, Lahaie RG, Latiano A, Paré P, Proctor DD, Regueiro MD, Steinhart AH, Targan SR, Schumm LP, Kistner EO, Lee AT, Gregersen PK, Rotter JI, Brant SR, Taylor KD, Roeder K, Duerr RH | title = Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study | journal = Nat. Genet. | volume = 41 | issue = 2 | pages = 216–20 | year = 2009 | pmid = 19122664 | pmc = 2652837 | doi = 10.1038/ng.275 }} |
||
*{{cite journal |
*{{cite journal | author = de Moura PR, Watanabe L, Bleicher L, Colau D, Dumoutier L, Lemaire MM, Renauld JC, Polikarpov I | title = Crystal structure of a soluble decoy receptor IL-22BP bound to interleukin-22 | journal = FEBS Lett. | volume = 583 | issue = 7 | pages = 1072–7 | year = 2009 | pmid = 19285080 | doi = 10.1016/j.febslet.2009.03.006 }} |
||
*{{cite journal |
*{{cite journal | author = Wong CK, Lun SW, Ko FW, Wong PT, Hu SQ, Chan IH, Hui DS, Lam CW | title = Activation of peripheral Th17 lymphocytes in patients with asthma | journal = Immunol. Invest. | volume = 38 | issue = 7 | pages = 652–64 | year = 2009 | pmid = 19811428 | doi = 10.1080/08820130903062756 }} |
||
*{{cite journal |
*{{cite journal | author = Shen H, Goodall JC, Hill Gaston JS | title = Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis | journal = Arthritis Rheum. | volume = 60 | issue = 6 | pages = 1647–56 | year = 2009 | pmid = 19479869 | doi = 10.1002/art.24568 }} |
||
*{{cite journal |
*{{cite journal | author = Thompson CL, Plummer SJ, Tucker TC, Casey G, Li L | title = Interleukin-22 genetic polymorphisms and risk of colon cancer | journal = Cancer causes & control : CCC | volume = 21 | issue = 8 | pages = 1165–70 | year = 2010 | pmid = 20339910 | doi = 10.1007/s10552-010-9542-5 }} |
||
*{{cite journal |
*{{cite journal | author = Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA | title = Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22 | journal = Blood | volume = 113 | issue = 17 | pages = 4008–10 | year = 2009 | pmid = 19244159 | pmc = 2673127 | doi = 10.1182/blood-2008-12-192443 }} |
||
*{{cite journal |
*{{cite journal | author = Siezen CL, Bont L, Hodemaekers HM, Ermers MJ, Doornbos G, Van't Slot R, Wijmenga C, Houwelingen HC, Kimpen JL, Kimman TG, Hoebee B, Janssen R | title = Genetic susceptibility to respiratory syncytial virus bronchiolitis in preterm children is associated with airway remodeling genes and innate immune genes | journal = Pediatr. Infect. Dis. J. | volume = 28 | issue = 4 | pages = 333–5 | year = 2009 | pmid = 19258923 | doi = 10.1097/INF.0b013e31818e2aa9 }} |
||
*{{cite journal |
*{{cite journal | author = Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, Argiro L, el Kheir M, Bucheton B, Mary C, El-Safi SH, Dessein A | title = IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani | journal = J. Clin. Invest. | volume = 119 | issue = 8 | pages = 2379–87 | year = 2009 | pmid = 19620772 | pmc = 2719936 | doi = 10.1172/JCI38813 }} |
||
*{{cite journal |
*{{cite journal | author = Pan HF, Zhao XF, Yuan H, Zhang WH, Li XP, Wang GH, Wu GC, Tang XW, Li WX, Li LH, Feng JB, Hu CS, Ye DQ | title = Decreased serum IL-22 levels in patients with systemic lupus erythematosus | journal = Clin. Chim. Acta | volume = 401 | issue = 1–2 | pages = 179–80 | year = 2009 | pmid = 19046958 | doi = 10.1016/j.cca.2008.11.009 }} |
||
*{{cite journal |
*{{cite journal | author = Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang J, Chen H, Wu C | title = Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans | journal = Eur. J. Immunol. | volume = 39 | issue = 6 | pages = 1472–9 | year = 2009 | pmid = 19449309 | doi = 10.1002/eji.200838811 }} |
||
*{{cite journal |
*{{cite journal | author = Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T | title = Involvement of the IL-22/REG Ialpha axis in ulcerative colitis | journal = Lab. Invest. | volume = 90 | issue = 3 | pages = 496–505 | year = 2010 | pmid = 20065946 | doi = 10.1038/labinvest.2009.147 }} |
||
*{{cite journal |
*{{cite journal | author = He M, Liang P | title = IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis | journal = J. Immunol. | volume = 184 | issue = 4 | pages = 1793–8 | year = 2010 | pmid = 20061404 | doi = 10.4049/jimmunol.0901829 }} |
||
*{{cite journal |
*{{cite journal | author = Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Döcke WD, Asadullah K, Volk HD, Sterry W, Sabat R | title = The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis | journal = Eur. J. Immunol. | volume = 39 | issue = 12 | pages = 3570–81 | year = 2009 | pmid = 19830738 | doi = 10.1002/eji.200939687 }} |
||
*{{cite journal |
*{{cite journal | author = Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A | title = Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling | journal = J. Clin. Invest. | volume = 119 | issue = 12 | pages = 3573–85 | year = 2009 | pmid = 19920355 | pmc = 2786807 | doi = 10.1172/JCI40202 }} |
||
*{{cite journal |
*{{cite journal | author = Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R | title = IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion | journal = J. Immunol. | volume = 183 | issue = 10 | pages = 6639–45 | year = 2009 | pmid = 19864591 | doi = 10.4049/jimmunol.0902587 }} |
||
*{{cite journal |
*{{cite journal | author = Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M | title = A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity | journal = Nature | volume = 457 | issue = 7230 | pages = 722–5 | year = 2009 | pmid = 18978771 | doi = 10.1038/nature07537 }} |
||
*{{cite journal |
*{{cite journal | author = Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA | title = Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity | journal = Curr Opin Pharmacol | volume = 9 | issue = 4 | pages = 447–53 | year = 2009 | pmid = 19481975 | pmc = 2755239 | doi = 10.1016/j.coph.2009.04.008 }} |
||
*{{cite journal |
*{{cite journal | author = Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A | title = Circulating Th17, Th22, and Th1 cells are increased in psoriasis | journal = J. Invest. Dermatol. | volume = 130 | issue = 5 | pages = 1373–83 | year = 2010 | pmid = 20032993 | pmc = 2892169 | doi = 10.1038/jid.2009.399 }} |
||
{{refend}} |
{{refend}} |
||
Revision as of 12:38, 19 August 2014
Source
Interleukin-22 (IL-22) is protein that in humans is encoded by the IL22 gene.[1][2]
IL-22 a member of a group of cytokines called the IL-10 family or IL-10 superfamily (including IL-19, IL-20, IL-24, and IL-26),[3] a class of potent mediators of cellular inflammatory responses. It shares use of IL-10R2 in cell signaling with other members of this family, IL-10, IL-26, IL-28A/B and IL-29.[4] IL-22 is produced by activated DC and T cells and initiates innate immune responses against bacterial pathogens especially in epithelial cells such as respiratory and gut epithelial cells. IL-22 along with IL-17 is rapidly produced by splenic LTi-like cells [5] and can be also produced by Th17 cells and likely plays a role in the coordinated response of both adaptive and innate immune systems.
IL-22 biological activity is initiated by binding to a cell-surface complex composed of IL-22R1 and IL-10R2 receptor chains and further regulated by interactions with a soluble binding protein, IL-22BP, which shares sequence similarity with an extracellular region of IL-22R1 (sIL-22R1). IL-22 and IL-10 receptor chains play a role in cellular targeting and signal transduction to selectively initiate and regulate immune responses.[6] IL-22 can contribute to immune disease through the stimulation of inflammatory responses, S100s and defensins. IL-22 also promotes hepatocyte survival in the liver and epithelial cells in the lung and gut similar to IL-10.[7] In some contexts, the pro-inflammatory versus tissue-protective functions of IL-22 are regulated by the often co-expressed cytokine IL-17A [8]
Target tissue
Targets of this cytokine are mostly non-hematopoietic cells such as hepatocytes, keratinocytes, and lung and intestinal epithelial cells. Pancreatic islets also express high levels of IL-22 receptor. It has been shown to induce islet beta cell regeneration.[9]
Signaling
IL-22, signals through the interferon receptor-related proteins CRF2-4 and IL-22R.[2] It forms cell surface complexes with IL-22R1 and IL-10R2 chains resulting in signal transduction through receptor, IL-10R2. The IL-22/IL-22R1/IL-10R2 complex activates intracellular kinases (JAK1, Tyk2, and MAP kinases) and transcription factors, especially STAT3. It can induce IL-20 and IL-24 signaling when IL-22R1 pairs with IL-20R2.
Structure
IL-22 is an α-helical cytokine. IL-22 binds to a heterodimeric cell surface receptor composed of IL-10R2 and IL-22R1 subunits.[6] IL-22R is expressed on tissue cells, and it is absent on immune cells.[10]
Crystallization is possible if the N-linked glycosylation sites are removed in mutants of IL-22 bound with high-affinity cell-surface receptor sIL-22R1. The crystallographic asymmetric unit contained two IL-22-sIL-22R1 complexes.[6]
References
- ^ Dumoutier L, Van Roost E, Colau D, Renauld JC (August 2000). "Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor". Proc. Natl. Acad. Sci. U.S.A. 97 (18): 10144–9. doi:10.1073/pnas.170291697. PMC 27764. PMID 10954742.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL (October 2000). "Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R". J. Biol. Chem. 275 (40): 31335–9. doi:10.1074/jbc.M005304200. PMID 10875937.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB (2004). "Interleukin-10 and related cytokines and receptors". Annu. Rev. Immunol. 22: 929–79. doi:10.1146/annurev.immunol.22.012703.104622. PMID 15032600.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Witte K, Witte E, Sabat R, Wolk K (Aug 2010). "IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties". Cytokine Growth Factor Rev. 21 (4): 237–51. doi:10.1016/j.cytogfr.2010.04.002. PMID 20655797.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ (2009). "Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22". J Exp Med. 206 (1): 35–41. doi:10.1084/jem.20072713. PMC 2626689. PMID 19114665.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c PDB: 3DGC; Jones BC, Logsdon NJ, Walter MR (September 2008). "Structure of IL-22 bound to its high-affinity IL-22R1 chain". Structure. 16 (9): 1333–44. doi:10.1016/j.str.2008.06.005. PMC 2637415. PMID 18599299.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001). "Interleukin-10 and the interleukin-10 receptor". Annu. Rev. Immunol. 19: 683–765. doi:10.1146/annurev.immunol.19.1.683. PMID 11244051.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D (2010). "Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A". J Exp Med. 207 (6): 1293–305. doi:10.1084/jem.20092054. PMC 2882840. PMID 20498020.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hill T, Krougly O, Nikoopour E, Bellemore S, Lee-Chan E, Fouser LA, Hill DJ, Singh B (2013). "The involvement of interleukin-22 in the expression of pancreatic beta cell regenerative Reg genes". Cell Regeneration. 2 (2): 1–11. doi:10.1186/2045-9769-2-2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004). "IL-22 increases the innate immunity of tissues". Immunity. 21 (2): 241–54. doi:10.1016/j.immuni.2004.07.007. PMID 15308104.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Weger W, Hofer A, Wolf P, El-Shabrawi Y, Renner W, Kerl H, Salmhofer W (2009). "Common polymorphisms in the interleukin-22 gene are not associated with chronic plaque psoriasis". Exp. Dermatol. 18 (9): 796–8. doi:10.1111/j.1600-0625.2009.00840.x. PMID 19469905.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M (2010). "New genetic associations detected in a host response study to hepatitis B vaccine". Genes Immun. 11 (3): 232–8. doi:10.1038/gene.2010.1. PMID 20237496.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, Barmada MM, Klei L, Daly MJ, Abraham C, Bayless TM, Bossa F, Griffiths AM, Ippoliti AF, Lahaie RG, Latiano A, Paré P, Proctor DD, Regueiro MD, Steinhart AH, Targan SR, Schumm LP, Kistner EO, Lee AT, Gregersen PK, Rotter JI, Brant SR, Taylor KD, Roeder K, Duerr RH (2009). "Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study". Nat. Genet. 41 (2): 216–20. doi:10.1038/ng.275. PMC 2652837. PMID 19122664.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - de Moura PR, Watanabe L, Bleicher L, Colau D, Dumoutier L, Lemaire MM, Renauld JC, Polikarpov I (2009). "Crystal structure of a soluble decoy receptor IL-22BP bound to interleukin-22". FEBS Lett. 583 (7): 1072–7. doi:10.1016/j.febslet.2009.03.006. PMID 19285080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wong CK, Lun SW, Ko FW, Wong PT, Hu SQ, Chan IH, Hui DS, Lam CW (2009). "Activation of peripheral Th17 lymphocytes in patients with asthma". Immunol. Invest. 38 (7): 652–64. doi:10.1080/08820130903062756. PMID 19811428.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Shen H, Goodall JC, Hill Gaston JS (2009). "Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis". Arthritis Rheum. 60 (6): 1647–56. doi:10.1002/art.24568. PMID 19479869.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Thompson CL, Plummer SJ, Tucker TC, Casey G, Li L (2010). "Interleukin-22 genetic polymorphisms and risk of colon cancer". Cancer causes & control : CCC. 21 (8): 1165–70. doi:10.1007/s10552-010-9542-5. PMID 20339910.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA (2009). "Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22". Blood. 113 (17): 4008–10. doi:10.1182/blood-2008-12-192443. PMC 2673127. PMID 19244159.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Siezen CL, Bont L, Hodemaekers HM, Ermers MJ, Doornbos G, Van't Slot R, Wijmenga C, Houwelingen HC, Kimpen JL, Kimman TG, Hoebee B, Janssen R (2009). "Genetic susceptibility to respiratory syncytial virus bronchiolitis in preterm children is associated with airway remodeling genes and innate immune genes". Pediatr. Infect. Dis. J. 28 (4): 333–5. doi:10.1097/INF.0b013e31818e2aa9. PMID 19258923.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, Argiro L, el Kheir M, Bucheton B, Mary C, El-Safi SH, Dessein A (2009). "IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani". J. Clin. Invest. 119 (8): 2379–87. doi:10.1172/JCI38813. PMC 2719936. PMID 19620772.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pan HF, Zhao XF, Yuan H, Zhang WH, Li XP, Wang GH, Wu GC, Tang XW, Li WX, Li LH, Feng JB, Hu CS, Ye DQ (2009). "Decreased serum IL-22 levels in patients with systemic lupus erythematosus". Clin. Chim. Acta. 401 (1–2): 179–80. doi:10.1016/j.cca.2008.11.009. PMID 19046958.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang J, Chen H, Wu C (2009). "Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans". Eur. J. Immunol. 39 (6): 1472–9. doi:10.1002/eji.200838811. PMID 19449309.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T (2010). "Involvement of the IL-22/REG Ialpha axis in ulcerative colitis". Lab. Invest. 90 (3): 496–505. doi:10.1038/labinvest.2009.147. PMID 20065946.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - He M, Liang P (2010). "IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis". J. Immunol. 184 (4): 1793–8. doi:10.4049/jimmunol.0901829. PMID 20061404.
- Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Döcke WD, Asadullah K, Volk HD, Sterry W, Sabat R (2009). "The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis". Eur. J. Immunol. 39 (12): 3570–81. doi:10.1002/eji.200939687. PMID 19830738.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A (2009). "Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling". J. Clin. Invest. 119 (12): 3573–85. doi:10.1172/JCI40202. PMC 2786807. PMID 19920355.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R (2009). "IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion". J. Immunol. 183 (10): 6639–45. doi:10.4049/jimmunol.0902587. PMID 19864591.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M (2009). "A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity". Nature. 457 (7230): 722–5. doi:10.1038/nature07537. PMID 18978771.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA (2009). "Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity". Curr Opin Pharmacol. 9 (4): 447–53. doi:10.1016/j.coph.2009.04.008. PMC 2755239. PMID 19481975.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A (2010). "Circulating Th17, Th22, and Th1 cells are increased in psoriasis". J. Invest. Dermatol. 130 (5): 1373–83. doi:10.1038/jid.2009.399. PMC 2892169. PMID 20032993.
{{cite journal}}: CS1 maint: multiple names: authors list (link)