Clonidine: Difference between revisions
No edit summary |
|||
| Line 41: | Line 41: | ||
| routes_of_administration = oral, [[transdermal]] |
| routes_of_administration = oral, [[transdermal]] |
||
}} |
}} |
||
'''Clonidine''' is a |
'''Clonidine''' is a sympatholytic medication used to treat medical conditions, such as high blood pressure and ADHD anxiety/panic disorder. It is a direct-acting [[alpha-2 adrenergic receptor|α<sub>2</sub>]] [[adrenergic agonist]] and a [[guanidine]] receptor [[agonist]]. |
||
==Uses== |
==Uses== |
||
Revision as of 00:34, 14 July 2011
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, transdermal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75-95% |
| Protein binding | 20-40% |
| Metabolism | Hepatic to inactive metabolites |
| Elimination half-life | 12-33 hours |
| Excretion | urine (40-50%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.021.928 |
| Chemical and physical data | |
| Formula | C9H9Cl2N3 |
| Molar mass | 230.093 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clonidine is a sympatholytic medication used to treat medical conditions, such as high blood pressure and ADHD anxiety/panic disorder. It is a direct-acting α2 adrenergic agonist and a guanidine receptor agonist.
Uses
Clonidine has been investigated and prescribed first as an antihypertensive drug in the 1950's. It has found new uses later, including treatment of some types of neuropathic pain, opioid detoxification, sleep hyperhidrosis, and as veterinary anaesthetic drug. Clonidine is used also off-label, to counter the side effects of stimulant medications such as methylphenidate or amphetamine. It is becoming a more accepted treatment for insomnia, as well as for relief of menopausal symptoms. Clonidine is increasingly used in conjunction with stimulants to treat attention-deficit hyperactivity disorder (ADHD), for which it is administered in late afternoon or evening for sleep, and because it sometimes helps moderate ADHD-associated impulsive and oppositional behavior, and may reduce tics, a problem in which a part of the body moves repeatedly and suddenly.[2] Clonidine can be used in the treatment of Tourette syndrome.[3] Its epidural use for pain during heart attack, postoperative and intractable pain has also been studied extensively.[4] It has also been used to treat the hyperarousal symptoms of PTSD. This medication may also be used to ease withdrawal symptoms associated with the long-term use of narcotics, alcohol and nicotine (smoking). In addition, clonidine has also been used for migraine headaches and hot flushes associated with menopause.[5][6]
In 2010, the Food and Drug Administration approved the use of clonidine either as an adjunct to traditional stimulant therapy or as a monotherapy in the treatment of attention deficit hyperactivity disorder (ADHD). Clonidine is regularly prescribed to help alleviate opiate withdrawal symptoms. It is mainly used to combat the sympathetic nervous system response to opiate withdrawal, namely tachycardia and hypertension, in the initial days of withdrawals.[7] It helps take away the sweating, hot/cold flushes, and general restlessness. The sedation effect is also useful although its side effects can include insomnia, thus exacerbating an already common feature of opiate withdrawal.[8]
Clonidine also has several off-label uses, and has been prescribed to treat psychiatric disorders including stress, sleep disturbances, and hyperarousal caused by post-traumatic stress disorder, borderline personality disorder, and other anxiety disorders.[9][10][11][12][13][14][15][16][17] Clonidine is also a mild sedative, and can be used as premedication before surgery or procedures. [18] Clonidine along with Methylphenidate has been studied for treatment of ADHD. [19][20]
Mechanism
Clonidine treats high blood pressure by stimulating α2 receptors in the brain, which decreases cardiac output and peripheral vascular resistance, lowering blood pressure. It has specificity towards the presynaptic α2 receptors in the vasomotor center in the brainstem. This binding decreases presynaptic calcium levels, and inhibits the release of norepinephrine (NE). The net effect is a decrease in sympathetic tone.[21]
The antihypertensive effect of clonidine is in fact due to agonism on the I1-receptor (imidazoline receptor), which mediates the sympatho-inhibitory actions of imidazolines to lower blood pressure.
Preparation and indications

Clonidine is typically available as tablets (Catapres, Dixarit), as a transdermal patch (Catapres-TTS), or as an injectable form to be given epidurally, directly to the central nervous system.
Clonidine has also been found to prolong the effects of analgesia when used together with a local anesthetic such as Ropivicaine or Levobupivicaine.
Alternative Uses
Used for opiate withdrawal to help with Restless Legs Syndrome, jitters, and hypertension.
Drug tests
Clonidine will not show up on any normal 5 panel drug test for street drugs. This is because it is not an opiate, benzodiazepine, amphetamine, cocaine or barbiturate. One can, however, request to be tested directly for Clonidine and its metabolites. There is significant misuse of clonidine within the methadone treated population as well as in the opiate abusing population. It is frequently abused with benzodiazepines sometimes with deadly side effects. Clonidine has a "benzo'like effect with opiates however at high doses and unfortunately a low safety threshold in this range.
Adverse effects
This drug may cause lightheadedness, dry mouth, dizziness and constipation. Clonidine may also cause hypotension.[22]
Clonidine also has peripheral alpha agonist activity which can lead to hypertension - especially when is injected i.v.. This blood pressure increase is sometimes witnessed in cases of overdose in children. As the clonidine is eliminated by the body, the peripheral effects wear off and its basic hypotensive effect becomes evident. Both the hypertensive and hypotensive effects can be harmful.[23]
Recommended dosage
Dosages of 0.4–0.6 mg have been used for the treatment of alcohol withdrawal. Total daily dosage for the treatment of opiate withdrawal range between 0.5 and 1.4 mg, depending on the stage as well as the severity of withdrawal symptoms. If the clonidine patch is used to treat nicotine withdrawal symptoms, dosages that deliver 0.1–0.2 mg daily are used. For oral therapy (tablets), a total dosage of 0.2–0.4 mg daily is taken in divided doses.
Pediatric doses of clonidine are calculated based on the child's body weight. Clonidine dosage for ADHD in children is 5 micrograms per kilogram of body weight per day orally in four divided doses. Children who require a daily dosage of 0.2 mg usually can use the 0.3 mg dermal patch. If ADHD is associated with sleep disturbances, low to moderate doses of clonidine can be taken at bedtime. Oral doses in children with Tourette's syndrome range from 3 to 6 micrograms per kilogram of body weight per day divided into two to four even doses.[24]
Rebound hypertension on withdrawal
Clonidine suppresses sympathetic outflow resulting in lower blood pressure, but sudden discontinuation can cause rebound hypertension due to a rebound in sympathetic outflow.
Clonidine therapy should generally be gradually tapered off when discontinuing therapy to avoid rebound effects from occurring. Treatment of clonidine withdrawal hypertension depends on the severity of the condition. Reintroduction of clonidine for mild cases, alpha and beta blockers for more urgent situations. Beta blockers never should be used alone to treat clonidine withdrawal as alpha vasoconstriction would still continue.[25][26]
Since ADHD drugs like amphetamine and methylphenidate tend to stimulate the sympathetic nervous system, missed doses of clonidine while under ADHD stimulant therapy might entail increased risks of a more severe rebound hypertension. This has not been evaluated.
Pharmacodynamics
Clonidine is a centrally-acting α-adrenergic receptor agonist with more affinity for α2 than α1. It selectively stimulates receptors in the brain that monitor catecholamine levels in the blood. These receptors close a negative feedback loop that begins with descending sympathetic nerves from the brain that control the production of catecholamines (epinephrine, also known as adrenaline, and norepinephrine) in the adrenal medulla. By fooling the brain into believing that catecholamine levels are higher than they really are, clonidine causes the brain to reduce its signals to the adrenal medulla, which in turn lowers catecholamine production and blood levels. The result is a lowered heart rate and blood pressure, with side effects of dry mouth and fatigue. If clonidine is suddenly withdrawn the sympathetic nervous system will revert to producing high levels of epinephrine and norepinephrine, higher even than before treatment, causing rebound hypertension. Rebound hypertension can be avoided by slowly withdrawing treatment.
Clonidine suppression test
Clonidine's effect on reducing circulating epinephrine by a central mechanism was used in the past as an investigatory test for pheochromocytoma,[27] which is a catecholamine-synthesizing tumor, usually of the adrenal medulla. In a clonidine suppression test plasma catecholamines levels are measured before and 3 hours after a 0.3 mg oral test dose has been given to a patient. A positive test occurs if there is no decrease in plasma levels.
Synthesis
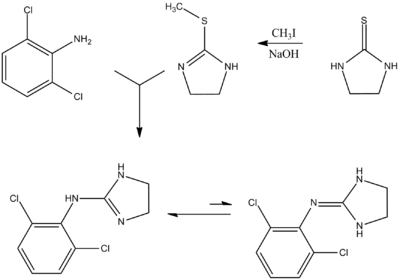
Clonidine, 2-(2,6-dichlorophenylamino)imidazoline, is synthesized from 2,6-dichloroaniline, the reaction of which with ammonium thiocyanate gives N-(2,6- dichlorophenyl)thiourea. Methylation of this product, followed by the subsequent reaction with ethylene diamine gives clonidine.

- K. Zeile, H. Staehle, K. H. Hauotman, DE 1303141 (1961).
- H. Stahle, K. Zeile, U.S. patent 3,202,660 (1965).
- K. Zeile, K. H. Hauotman, H. Stahle, U.S. patent 3,236,857 (1966).
- Boehringer SOHN Ingelheim, BE 653933 (1964).
- Boehringer SOHN Ingelheim, GB 1016514 (1962).
- Boehringer Engelheim GMBH, GB 1034938 (1964).
Stahle, H.; Koppe, H.; Kummer, W.; Stockhaus, K.; 1976, U.S. patent 3,937,717.
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ National Institute of Neurological Disorders and Stroke (2002). "Methylphenidate and Clonidine Help Children With ADHD and Tics".
- ^ Schapiro NA. "Dude, you don't have Tourette's": Tourette's syndrome, beyond the tics. Pediatr Nurs. 2002 May-Jun;28(3):243-6, 249-53. PMID 12087644
- ^ Patel SS, Dunn CJ, Bryson HM (1996). "Epidural clonidine: a review of its pharmacology and efficacy in the management of pain during labour and postoperative and intractable pain". CNS Drugs. 6 (6): 474–497.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Clonidine Oral Uses". Web MD.
- ^ "Clonidine". Drugs.com.
- ^ . AJ Giannini. Drugs of Abuse--Second Edition. Los Angeles, Practice Management Information Corporation,1997.
- ^ AJ Giannini, I. Extein,MS Gold, ALC Pottash, S. Castellani. Clonidine in mania. Drug Development Research. 3:101-105,1983.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19512980 , please use {{cite journal}} with
|pmid=19512980instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18474199 , please use {{cite journal}} with
|pmid=18474199instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17506224 , please use {{cite journal}} with
|pmid=17506224instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17414682 , please use {{cite journal}} with
|pmid=17414682instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17354267 , please use {{cite journal}} with
|pmid=17354267instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10560025 , please use {{cite journal}} with
|pmid=10560025instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7937367 , please use {{cite journal}} with
|pmid=7937367instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 2960712 , please use {{cite journal}} with
|pmid=2960712instead. - ^ "Understanding Comorbid Depression and Anxiety".
- ^ Fazi L. A comparison of oral clonidine and oral midazolam as preanesthetic medications in the pediatric tonsillectomy patient. Anesth Analg. 2001 Jan;92(1):56-61. PMID 11133600
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18182964 , please use {{cite journal}} with
|pmid=18182964instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18182963 , please use {{cite journal}} with
|pmid=18182963instead. - ^ Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 12. ISBN 1-59541-101-1.
- ^ Hossmann V (1980). "Sedative and cardiovascular effects of clonidine and nitrazepam". Clin Pharmacol Ther. 28 (2): 167–76. doi:10.1038/clpt.1980.146. PMID 7398184.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ "Toxicity, Clonidine".
- ^ "Clonidine". Encyclopedia of Mental Disorders.
- ^ Keith Parker; Laurence Brunton; Goodman, Louis Sanford; Lazo, John S.; Gilman, Alfred (2006). Goodman & Gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill. pp. 854–855. ISBN 0-07-142280-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Vitiello B (2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child Adolesc Psychiatr Clin N Am. 17 (2): 459–74, xi. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Eisenhofer G, Goldstein DS, Walther MM; et al. (2003). "Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results". J. Clin. Endocrinol. Metab. 88 (6): 2656–66. doi:10.1210/jc.2002-030005. PMID 12788870.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
