Wikipedia:Reference desk/Science: Difference between revisions
| Line 470: | Line 470: | ||
== Is this real? == |
== Is this real? == |
||
[http://www.earth911.com/eco-tech/japanese-inventor-turns-plastic-bags-into-oil/ This]. One kilowatt and two pounds of plastic bags in, one liter of crude oil out, so they claim. Is it real? The first "off" thing to me is that kilowatts |
[http://www.earth911.com/eco-tech/japanese-inventor-turns-plastic-bags-into-oil/ This]. One kilowatt and two pounds of plastic bags in, one liter of crude oil out, so they claim. Is it real? The first "off" thing to me is that kilowatts are power units, not energy. If it's real, how long does it need one kilowatt? An hour? A month? [[Special:Contributions/75.75.42.89|75.75.42.89]] ([[User talk:75.75.42.89|talk]]) 17:07, 1 February 2015 (UTC) |
||
Revision as of 17:19, 1 February 2015
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
January 27
What kind of bird?
What kind of bird is this? (I photographed it on the coast of Georgia, USA.)
I asked someone who should know, and they said anhinga. But the beak looks too different (among other things). Anyhow, that article led me to Cormorant, which has about the same kind of beak. Bubba73 You talkin' to me? 06:39, 27 January 2015 (UTC)
- Yup, definitely a cormorant, and a very good photo of one, in a typical pose (drying its wings). They are common and widespread.--Shantavira|feed me 08:23, 27 January 2015 (UTC)
- Thanks, I was out taking some photos (e.g. Jekyll Island Club#Gallery) and I suddenly had the opportunity to take aome photos of the bird. Bubba73 You talkin' to me? 08:38, 27 January 2015 (UTC)
5.28.171.15 (talk) 15:00, 27 January 2015 (UTC)
- OK - I give up. You're going to have to expand your question a bit. What does FH and FSH stand for in this context? It's really hard to guess and our disambiguation pages for those two acronyms don't show up anything that helps!
- (If someone here can guess from the context - please add the expansion into those two dab pages!) SteveBaker (talk) 15:30, 27 January 2015 (UTC)
- I suspect the OP may have intended to ask about FSH and LH, not FSH and FH. I.E. follicle-stimulating hormone and luteinizing hormone. Nil Einne (talk) 16:57, 27 January 2015 (UTC)
- [you're right, I correct it. 149.78.26.195 (talk) 19:51, 27 January 2015 (UTC)]
- Assuming you meant LH and FSH, you can read about endocrine feedback loops at Hypothalamic–pituitary–gonadal axis. --Mark viking (talk) 17:10, 27 January 2015 (UTC)
- The Original Poster really needs to come back and provide more info; otherwise mēdeís may hat it. An' then non of us will discover the real question nor answer--Aspro (talk) 19:07, 27 January 2015 (UTC)
- Thank you, I meant to LH& FSH - hormones. Sorry 149.78.26.195 (talk) 19:51, 27 January 2015 (UTC)
- I read the article that mentioned above and I didn't understand a clear answer for my question. 149.78.26.195 (talk) 19:57, 27 January 2015 (UTC)
- Well we don't understand your question. Please now explain what you mean by negative and positive feedback in this context.--Shantavira|feed me 11:16, 28 January 2015 (UTC)
- We also have articles on positive feedback and negative feedback, both are common in biological systems for signaling and control. This page from a class at UC Berkeley [1] seems to say that estrogen in females can trigger both positive and negative feedback to FSH and LH. SemanticMantis (talk) 14:43, 28 January 2015 (UTC)
- Well we don't understand your question. Please now explain what you mean by negative and positive feedback in this context.--Shantavira|feed me 11:16, 28 January 2015 (UTC)
- I read the article that mentioned above and I didn't understand a clear answer for my question. 149.78.26.195 (talk) 19:57, 27 January 2015 (UTC)
- Thank you, I meant to LH& FSH - hormones. Sorry 149.78.26.195 (talk) 19:51, 27 January 2015 (UTC)
What is the antonym of "conspecific"?
If 2 organisms are from different species, is there an adjective that can describe this?
Thanks.
- Heterospecific. See biological specificity for relations among the types of specificity. --Mark viking (talk) 17:16, 27 January 2015 (UTC)
- Sorry, this is nonsense. Of course you are both entitled to your opinions. But let me offer a contrary view to the OP and anyone else who happens to read:
- Shall we never talk about intramural sports, and instead speak of "sports played by teams within the same school or organization"? Looie, shall we not speak of dendrites in scientific papers on neuroscience, and instead prefer "the branching part at the end of a neuron"? What about the hippocampus or the amygdala? Are all those terms gobbledegook Latin/Greek? No, they are English words that have simple meanings, and most high school graduates know them. You would be laughed out of the review process if you submitted a paper talking about the "seahorse-shaped part of the brain". These words are English words, as are "heterospecific", "conspecific", as well as "interspecific" and "intraspecific". We also have heterogenous and heterosexual and heterozygous -- all English words. As are "confluence" and "congenital", "converge", "congregation", "congener" etc. I won't even bother listing the many common words that start with "intra-" or "inter-".
- The simple fact of the matter is that these terms for biological specificity (as well as their derivatives, e.g. "confamilial", etc) are highly useful for science writing, especially in the fields of evolution, ecology, and organismal biology. If I had to write "competition between two individuals that are not of the same species" instead of "interspecific competition", I'd never be able to fit abstracts into the word limits, let alone the appalling sentences that would result. I do appreciate the desire for clarity and simplicity in science writing, and I also try to avoid five dollar words where a simple one would suffice. But heterospecific and conspecific are basic terms with simple definitions, that use the same standard prefixes as dozens (if not hundreds) of other words. It's not too much to expect people to know them if they are reading science papers in these fields. SemanticMantis (talk) 22:34, 28 January 2015 (UTC)
- You've missed the point entirely. It's only where there are widely understood simple English words or phrases that they should be used in place of arcane Latin terms. Obviously long-winded English descriptions don't qualify. The English phrase shouldn't be much longer than the Latin. Of course, where you have a Latin phrase, like a writ of habeas corpus (literally meaning a written order to "produce the body"), then an English phrase, like an "order to bring the prisoner to court" would be far easier to understand. As for people laughing, I imagine many laughed when church services were first performed in English instead of Latin, too. StuRat (talk) 04:43, 31 January 2015 (UTC)
Off Center Thrust in Spacecraft
If a rocket motor on a spacecraft is not perfectly aligned with the center of mass of said spacecraft, how much of the thrust will become rotary motion, and how much will become linear motion? If some of the thrust becomes linear motion, what direction would that motion take? I imagine that the spacecraft would follow a curved trajectory, much like the depictions in old spaceship cartoons. While we are at it, aligning a rocket motor with the center of mass of a craft with a mobile payload, like the shuttle, would be a bit of a trick. How is it done? 50.43.56.168 (talk) 20:04, 27 January 2015 (UTC)
- Over sufficiently long time the average velocity of the spacecraft will tend to a constant, while the rotation will be continuously accelerating. Ruslik_Zero 20:26, 27 January 2015 (UTC)
- If I am not mistaken this constant velocity limit will (in case the mass of fuel is negligible and rotation is about a fixed axis) be
- ,
- where m is the mass of the spacecraft, I is its inertia moment, r0 is displacement of the thrust relative to the center of mass and F is the thrust. This constant velocity will be directed some 45 degrees from the initial trust direction. Ruslik_Zero 20:56, 27 January 2015 (UTC)
- If I am not mistaken this constant velocity limit will (in case the mass of fuel is negligible and rotation is about a fixed axis) be
- It's never possible to align a rocket motor perfectly with the center of mass, so spacecraft have a number of ways to counteract the torque induced by poor alignment. Some rocket engines have gimbaled thrust, so they can change their thrust vector slightly. Reaction wheels resist changes in orientation due to torque. Reaction control systems use small thrusters to actively change the spacecraft's orientation. In Earth orbit, spacecraft can also use magnetorquers for stabilization by taking advantage of the Earth's magnetic field. For orbital launches, rockets often carry ballast so that their mass distribution is exactly what their designers intended. Cubesats take advantage of this to get a cheap ride into orbit--rather than carry dead weight, why not carry some cubesats? --Bowlhover (talk) 10:19, 28 January 2015 (UTC)
S-IC S-II cutoff on Apollo 13
The previous question got me to wondering something. During the launch of Apollo 13, the center engine of the S-IC first stage S-II second stage shut down prematurely. The decision was made to burn the outboard engines longer to compensate. But what if it was one of the outboard engines that shut down? How would the imbalance have been dealt with? → Michael J Ⓣ Ⓒ Ⓜ 21:45, 27 January 2015 (UTC)
- It was actually the S-II second stage that had the problem with pogo oscillation. If one of the outboard engines had shut down as well (and presuming that it wasn't immediately catastrophic), the spacecraft would have jettisoned the second stage and performed a COI (Contingency Orbit Insertion) burn with the S-IVB for an Earth-orbit mission. See Apollo abort modes. Tevildo (talk) 23:17, 27 January 2015 (UTC)
- I believe that Michael J was not asking about it losing a second engine, but wondered if thrust vectoring could have compensated if the engine which shut down was one of the outboard ones. -- ToE 00:15, 28 January 2015 (UTC)
- According to the flight rules for Apollo 11 (here, page 102), BOOSTER would call an abort if three of the S-II engines were out, or two were out and the commanded gimbal angles on the remaining engines were greater than 40° off nominal. So, it was presumably capable of a full mission if it had lost one of the outboard engines, and even losing two wouldn't have required an immediate abort. Tevildo (talk) 01:22, 28 January 2015 (UTC)
- For that matter, if it was okay to fly with 3 out of 5 engines, then if an outboard engine failed and it wasn't certain that the gimbals could compensate well enough for the off-center thrust, they could have chosen to shut down the opposite outboard engine. --65.94.50.4 (talk) 02:25, 28 January 2015 (UTC)
- "...gimbal angles on the remaining engines were greater than 40° off nominal." I would be surprised if the engines could be angled more than 10 degrees.50.43.56.168 (talk) 03:29, 28 January 2015 (UTC)
- Yes, it sounded like a lot to me, as well. According to the user manual (page 83), the S-II gimbal range was ±7°. The verbatim text of the flight rules is "[Abort] if the difference in commanded angles and gimbal angles exceeds 40 deg in pitch or yaw", so it may refer to the attitude error of the spacecraft itself rather than the engine. The important point is that it could keep going with two engines out in most circumstances, and I'm sure the people at Houston (who _were_ rocket scientists) could be trusted to make the right decision. Tevildo (talk) 21:36, 29 January 2015 (UTC)
- "...gimbal angles on the remaining engines were greater than 40° off nominal." I would be surprised if the engines could be angled more than 10 degrees.50.43.56.168 (talk) 03:29, 28 January 2015 (UTC)
- I believe that Michael J was not asking about it losing a second engine, but wondered if thrust vectoring could have compensated if the engine which shut down was one of the outboard ones. -- ToE 00:15, 28 January 2015 (UTC)
January 28
Does an undampened magnetic spring system ever stop?
If a magnetic spring system, like this one[2], is placed in perfect vacuum and the center rod is replaced with a magnetic equivalent so there's zero fiction, will the system ever stop after an initial excitation? I know for the traditional spring system the energy will slowly be converted into heat and it will eventually stop. Does the magnetic system also convert some of the kinetic energy into heat? WinterWall (talk) 07:52, 28 January 2015 (UTC)
- Yes, subjecting a material affected by magnetism to a variable magnetic field will generate heat. StuRat (talk) 07:59, 28 January 2015 (UTC)
- Doh! We even have an article on it: magnetic damping. Thanks for the help, and sorry for not searching hard enough beforehand. WinterWall (talk) 08:10, 28 January 2015 (UTC)
Flower ID
Can someone please identify the flower ID please? Nikhil (talk) 16:23, 28 January 2015 (UTC)

- Where did you see it?--Shantavira|feed me 16:54, 28 January 2015 (UTC)
- Yes more info would help. If you took the photo, not only where, but when? Looks a bit like a partridge pea to me. The things to check that can make you more sure (or rule this guess out) 1) Does the flower have bilateral symmetry? All legumes do, if this one doesn't it's not a partridge pea. Complicating the matter is that partridge pea flowers look a bit like radial symmetry at a glance, but one petal should be bigger than the rest, with 5 total. 2) Do the seeds have a pea pod shape? That plant might not have set seeds yet, but (assuming you took the photo) you could check back in a few weeks. Browse through this image search [3]] for more images. SemanticMantis (talk) 17:14, 28 January 2015 (UTC)
- I strongly suspect it's an Acacia of some sort, the leaves and thorns indicate this, and they usually have yellow flowers. But there are on the order of 1,000 species. μηδείς (talk) 22:01, 28 January 2015 (UTC)
- You know I wasn't sure if there were thorns or not. Looking more closely... I'm still not sure :) Looks a bit too sturdy for Partridge Pea though, and the leaves look sclerophyllic, which also points toward Acacia. Of course neither of these genera are native to India (of which OP is a citizen), but they could probably grow there. Anyway, we both came up with guesses in the Fabaceae family, so that's something. At the very least, the same things that I mentioned would rule out Partridge pea would also rule out Acacia spp., but we'd probably need a key and a sample to get to get positive confirmation on species level ID. SemanticMantis (talk) 22:46, 28 January 2015 (UTC)
- I strongly suspect it's an Acacia of some sort, the leaves and thorns indicate this, and they usually have yellow flowers. But there are on the order of 1,000 species. μηδείς (talk) 22:01, 28 January 2015 (UTC)
- The leaves make me think of Caesalpinia. Richard Avery (talk) 08:19, 29 January 2015 (UTC)
Chirality
Maybe I don't completely understand chirality, but the sentence "no matter how the two hands are oriented, it is impossible for all the major features of both hands to coincide" in chirality (chemistry) looks wrong. Due to symmetry in biology the human body is an achiral object - if you align both palms for applause or prayer, for instance, then both palms will coincide (as both halves of the body do). And if you lean your palm against the mirror, the reflection will match it. What's wrong actually? Brandmeistertalk 17:53, 28 January 2015 (UTC)
- What they mean is you get the mirror image. A right hand glove and a left hand glove are never exactly the same, no matter how you rotate them. They are mirror images, instead. Try putting the glove on the wrong hand to see what I mean.
- Now I agree that the word "coincide" doesn't quite convey what they are trying to say, as we might very well say "the major features in the mirror image coincide with the original". But that's a problem with the language, which lacks a simple, universally understood word which means "chirality". StuRat (talk) 18:19, 28 January 2015 (UTC)
- Chirality and handedness are synonyms, its usage of which should be clear when used in context (so as to not be confused with hand preference). -Modocc (talk) 19:01, 28 January 2015 (UTC)
- The two palms coincide, but the two hands do not. Starting at a coincident point on the palm, to get to the knuckle of one hand you have to go in a different direction than the knuckle of the other hand. There's no way you can orient your hands such that all parts (front, back; top, bottom) will simultaneously align. (Palms, when treated as 2D objects like you do here, aren't chiral. In fact, no 2D object is chiral in 3D space.) Likewise, when you talk about humans being symmetric and achiral, that's for the human as taken as a whole. When you talk about hands as distinct objects, they are not chiral. You can get an achiral object from the combination of chiral sub-parts, if you attach the two mirror images across the plane of symmetry. You see this in chemistry, too, where they're called meso compounds - there you have molecules which have individual carbon atoms which have chirality, but are organized in such a way which the molecule as a whole is achiral, a fact which can be confirmed by optical rotation. One problem you may be having is a difficulty in envisioning "coinciding" hands, because of the physical impossibility of actually doing it. Perhaps a better phrasing is to talk about being able to move from one to the other by just translation - or equivalently, the fact that there exists an orientation of the two objects where they can be lined up side-by-side with all corresponding points placed exactly the same distance apart. So for your folded hands, this doesn't work as the palms are touching but the knuckles are 2-3 cm apart. Likewise with half a human body, you may get the tips of the nose to coincide, but the shoulders are then half a meter apart. As long as you account for their non-zero thickness, there's no way to line your hands up side-by-side such that all corresponding points are an equivalent distance apart. -- 160.129.138.186 (talk) 20:11, 28 January 2015 (UTC)
What causes electromagnetic induction?
I get that the Faraday's law of induction explains what will happen when a magnetic field interacts with an electrical current, but what is the cause of the electromagnetic induction? Couldn't the current flow in the opposite direction? --Noopolo (talk) 17:56, 28 January 2015 (UTC)
- Magnetic induction is a fundamental behavior of the universe. We can write lots of mathematics to describe it, but all those equations are just quantitative descriptions of a thing that we see: a change in an electric field causes a corresponding change in a magnetic field. The fact that there is a specific direction is not something we can presently explain - although we can describe it in very great detail. You can read more about this concept at our article: fundamental interaction. If you spend many years studying physics, you can learn how to describe these interactions at many layers of abstraction and at various levels of detail. Nimur (talk) 18:09, 28 January 2015 (UTC)
- The right hand rule is so due to the conventions by which people chose + and - for E and B. There's nothing intrinsically positive about positive (sometimes people complain that positive electric fields really ought to be where the electrons are... but really there's no intrinsic reason why the smaller particle should be positive either, so yes, it's arbitrary) Wnt (talk) 21:59, 28 January 2015 (UTC)
How Strong is the Force Which Drives Hubble Flow?
If I have a small solid object, a coin say, is it expanding with the Universe by an incredibly small amount, or actually by zero? What about if I had a very long pièce of string running to a planet in a distant galaxy? Would it be stretched by Hubble flow? 2A01:E34:EF5E:4640:E19D:C1C5:D0B3:F799 (talk) 18:14, 28 January 2015 (UTC)
- The hubble flow is relative momentum, not a force. If two objects are moving away from each other, they'll continue to move away unless acted on by some force, such as gravitation (attractive) or the cosmological constant (repulsive). If your coin or string is expanding, it'll continue to expand until something stops it. In the case of the coin or the string it's the electromagnetic binding between the atoms that will stop the expansion, almost immediately in the case of the coin and pretty quickly in the case of the string. If the coin or string is not expanding or contracting, it won't spontaneously start to do so. -- BenRG (talk) 01:41, 29 January 2015 (UTC)
- To reiterate and restate what BenRG has already stated: Hubble Flow (and by extension the metric expansion of space) is not caused by some unknown force currently acting on objects, it is the motion imparted on objects by the Big Bang which has not yet been counteracted by other forces. We know from Newton's first law that objects will not change their relative velocity unless an outside force acts on them. For any sufficiently close objects, from individual galaxies down to you and I, down to individual atoms, there are the four fundamental forces which have long since "canceled out" the effect of the initial motion imparted on them from the Big Bang. However, for sufficiently distance objects, there are not enough forces between the objects to slow them down from their initial push given by the big bang. They're just continuing to drift apart without any additional forces, as the initial "push" they got from the Big Bang has not yet been counteracted by other forces. In simple terms: Hubble Flow represents inertial motion originally imparted by the Big Bang, and is not "caused" by any further forces. --Jayron32 03:13, 29 January 2015 (UTC)
- Hubble Flow is just inertia, got it. Are you oversimplifying with your 'forces cancel out' or am I just being naïve when I think that add up all the forces and divide by the mass to get an ever decreasing acceleration and hence an, after all this time, vanishingly small but nonetheless non zero velocity? — Preceding unsigned comment added by 2A01:E34:EF5E:4640:E19D:C1C5:D0B3:F799 (talk) 06:35, 29 January 2015 (UTC)
- No, there is a zero relative velocity due to Hubble flow for all sorts of things, really anything galaxy sized or smaller. For example, the you're not flying apart; the electrostatic forces keeping you together means your atoms are not affected by Hubble flow: since the big bang is no longer happening, there's no additional force to make you get bigger. The Milky Way is held together by the gravity of all of the stars in it. The individual stars are not all spreading out due to Hubble flow, the galaxy holds together all on its own by its own gravity, and since the Big Bang still isn't happening now, the Milky Way is a stable structure, it is not expanding itself. It is only when you get on the scale of things larger than galaxy clusters or bigger that you see Hubble Flow in effect. The thing that Hubble noticed (LeMaitre actually. Hubble gets more press than he probably should, but I digress) was that, not only were distant galaxies receding, they were receding faster the further away you got; in other words it appeared that they were accelerating. However, this is also not due to any force, but a complication of the speed of light: the further a galaxy is from us, the greater its speed because it has more of its initial Big Bang-imparted velocity, because we're seeing it at a time when it was closer (temporally) to the Big Bang. Galaxies that are closer to us have been acted on by the gravity of other, nearby, objects for a longer time, so have been decelerated by them. However, eventually matter will get slowed down to the point when it has literally zero velocity left over from the Big Bang. From that point forward, until forever, it will no longer be subject to Hubble Flow. --Jayron32 10:55, 29 January 2015 (UTC)
- That would be true for just inertia from the Big Bang, without an Accelerating universe. Dbfirs 11:33, 29 January 2015 (UTC)
- Some of the comments above seem to ignore that the expansion is often described as countering gravity that would pull the cosmos together in a closed universe, and is described as dark energy, quintessence, cosmological constant etc. that exerts 'negative pressure' which, for reasons I still don't really comprehend, seems to mean that it pushes things apart. Wnt (talk) 12:29, 29 January 2015 (UTC)
- Yes, the explanations seem to be 5 billion years out of date. Dbfirs 13:31, 29 January 2015 (UTC)
- No, you need to be careful on the exact words here. By definition Hubble Flow, and the Metric Expansion of Space, is the inertial movement of matter caused by the Big Bang. There ARE other forces at work, some known about (gravity or electrostatics that holds objects together), and some which are NOT fully explained (all the one's you've mentioned). It's only been since the 1990s or so that we've noticed that the universe is expanding slightly faster than Hubble Flow would predict from the Big Bang, and we call that effect "dark energy" and "cosmological constant" because that sounds much better than "fucked if I know why it's happening", which is what both of those amount to. For the past 20 years or so, we now know (though LeMaitre and Hubble and all the rest did not) that the universe's expansion seems to be accelerating by a very real, but very small amount (the effect is so negligible, that it took that long for our measurements to produce precise enough results to notice it), which is why fudge's like "dark energy" have been introduced. But it's a total fudge: no source of "dark energy" has ever been confirmed, no one can identify what it is or how it works, "dark energy" is then just a code word for "this accelerating expansion thing we've recently noticed by can't explain". --Jayron32 14:57, 29 January 2015 (UTC)
- Yes, that's correct; the main effect started within 10−32 seconds after the Big Bang. I agree it's quite possible that the "acceleration thing" of the past five billion years is just some kind of illusion, but I've no idea how to explain it. Dbfirs 18:39, 29 January 2015 (UTC)
- Inflation doesn't start 10−n seconds after the big bang. I don't know where that (very common) error originated. Inflation erases all evidence of what caused it, so there's no way to know how long it lasted, or what state the universe was in when it started, or how long the universe had already existed at that point. But it seems safe to say that the state before inflation was not big-bang-like, because the whole point of inflation is to explain the observed big-bang expansion, and it would be completely pointless if the universe was already like that before it started. -- BenRG (talk) 20:53, 29 January 2015 (UTC)
- I see what you mean, but we have articles on Planck epoch, Grand unification epoch and Electroweak epoch that mention these times which refer to non-inflationary cosmology. Even Inflationary epoch uses the timescale. Would it make any more sense to say "after the theoretical singularity"? Dbfirs 21:49, 29 January 2015 (UTC)
- Inflation doesn't start 10−n seconds after the big bang. I don't know where that (very common) error originated. Inflation erases all evidence of what caused it, so there's no way to know how long it lasted, or what state the universe was in when it started, or how long the universe had already existed at that point. But it seems safe to say that the state before inflation was not big-bang-like, because the whole point of inflation is to explain the observed big-bang expansion, and it would be completely pointless if the universe was already like that before it started. -- BenRG (talk) 20:53, 29 January 2015 (UTC)
- If the accelerating expansion is really caused by a cosmological constant, then it's just another term in the gravitational force. We've never found a "cause of gravity" either, but we can still claim to understand it pretty well. In any case, the crucial thing is the difference between velocity and acceleration. Aristotle was wrong about moving objects having to be pushed to keep moving, and in a cosmological context he's still wrong. Regardless of the nature of the mysterious acceleration, it's an acceleration, not a "velocitization", and that makes it the same as gravity for this purpose. -- BenRG (talk) 20:53, 29 January 2015 (UTC)
- Yes, that's correct; the main effect started within 10−32 seconds after the Big Bang. I agree it's quite possible that the "acceleration thing" of the past five billion years is just some kind of illusion, but I've no idea how to explain it. Dbfirs 18:39, 29 January 2015 (UTC)
- No, you need to be careful on the exact words here. By definition Hubble Flow, and the Metric Expansion of Space, is the inertial movement of matter caused by the Big Bang. There ARE other forces at work, some known about (gravity or electrostatics that holds objects together), and some which are NOT fully explained (all the one's you've mentioned). It's only been since the 1990s or so that we've noticed that the universe is expanding slightly faster than Hubble Flow would predict from the Big Bang, and we call that effect "dark energy" and "cosmological constant" because that sounds much better than "fucked if I know why it's happening", which is what both of those amount to. For the past 20 years or so, we now know (though LeMaitre and Hubble and all the rest did not) that the universe's expansion seems to be accelerating by a very real, but very small amount (the effect is so negligible, that it took that long for our measurements to produce precise enough results to notice it), which is why fudge's like "dark energy" have been introduced. But it's a total fudge: no source of "dark energy" has ever been confirmed, no one can identify what it is or how it works, "dark energy" is then just a code word for "this accelerating expansion thing we've recently noticed by can't explain". --Jayron32 14:57, 29 January 2015 (UTC)
- Yes, the explanations seem to be 5 billion years out of date. Dbfirs 13:31, 29 January 2015 (UTC)
- Some of the comments above seem to ignore that the expansion is often described as countering gravity that would pull the cosmos together in a closed universe, and is described as dark energy, quintessence, cosmological constant etc. that exerts 'negative pressure' which, for reasons I still don't really comprehend, seems to mean that it pushes things apart. Wnt (talk) 12:29, 29 January 2015 (UTC)
- That would be true for just inertia from the Big Bang, without an Accelerating universe. Dbfirs 11:33, 29 January 2015 (UTC)
- No, there is a zero relative velocity due to Hubble flow for all sorts of things, really anything galaxy sized or smaller. For example, the you're not flying apart; the electrostatic forces keeping you together means your atoms are not affected by Hubble flow: since the big bang is no longer happening, there's no additional force to make you get bigger. The Milky Way is held together by the gravity of all of the stars in it. The individual stars are not all spreading out due to Hubble flow, the galaxy holds together all on its own by its own gravity, and since the Big Bang still isn't happening now, the Milky Way is a stable structure, it is not expanding itself. It is only when you get on the scale of things larger than galaxy clusters or bigger that you see Hubble Flow in effect. The thing that Hubble noticed (LeMaitre actually. Hubble gets more press than he probably should, but I digress) was that, not only were distant galaxies receding, they were receding faster the further away you got; in other words it appeared that they were accelerating. However, this is also not due to any force, but a complication of the speed of light: the further a galaxy is from us, the greater its speed because it has more of its initial Big Bang-imparted velocity, because we're seeing it at a time when it was closer (temporally) to the Big Bang. Galaxies that are closer to us have been acted on by the gravity of other, nearby, objects for a longer time, so have been decelerated by them. However, eventually matter will get slowed down to the point when it has literally zero velocity left over from the Big Bang. From that point forward, until forever, it will no longer be subject to Hubble Flow. --Jayron32 10:55, 29 January 2015 (UTC)
- Hubble Flow is just inertia, got it. Are you oversimplifying with your 'forces cancel out' or am I just being naïve when I think that add up all the forces and divide by the mass to get an ever decreasing acceleration and hence an, after all this time, vanishingly small but nonetheless non zero velocity? — Preceding unsigned comment added by 2A01:E34:EF5E:4640:E19D:C1C5:D0B3:F799 (talk) 06:35, 29 January 2015 (UTC)
- To reiterate and restate what BenRG has already stated: Hubble Flow (and by extension the metric expansion of space) is not caused by some unknown force currently acting on objects, it is the motion imparted on objects by the Big Bang which has not yet been counteracted by other forces. We know from Newton's first law that objects will not change their relative velocity unless an outside force acts on them. For any sufficiently close objects, from individual galaxies down to you and I, down to individual atoms, there are the four fundamental forces which have long since "canceled out" the effect of the initial motion imparted on them from the Big Bang. However, for sufficiently distance objects, there are not enough forces between the objects to slow them down from their initial push given by the big bang. They're just continuing to drift apart without any additional forces, as the initial "push" they got from the Big Bang has not yet been counteracted by other forces. In simple terms: Hubble Flow represents inertial motion originally imparted by the Big Bang, and is not "caused" by any further forces. --Jayron32 03:13, 29 January 2015 (UTC)
- I think I asked a similar question a few years ago. If the universe is expanding, and we are part of it, then why aren't we? If it's all relative, then there should be no perception of the universe's expansion at all. If I am sitting in a room and my TV suddenly starts to get bigger, whilst I also start to get bigger (as well as the rest of the room), then there would be no noticeable change. KägeTorä - (影虎) (Chin Wag) 19:33, 29 January 2015 (UTC)
- Yes, I remember reading a Science Fiction story based on a changing scale (only it was shrinking). In reality, we'd have no way to detect this sort of change unless it was happening only in our region (or unless light was not affected). The whole of science is based on the assumption that this doesn't happen. Dbfirs 20:13, 29 January 2015 (UTC)
- Well it was only a cheap science fiction story -- evidently more fantasy than science since the necessary change in physical constants would presumably have been detectable and would have resulted in different scale changes for different materials. Dbfirs 21:49, 29 January 2015 (UTC)
- The only noticeable change I can feel is that I need glasses to see my TV (which incidentally is bigger than it was before), so it may be moving further and further away from me, but maybe that's just because I am getting old, and have a bigger TV......) :) KägeTorä - (影虎) (Chin Wag) 03:52, 30 January 2015 (UTC)
- Isn't the only way you could detect the change is to compare the same object with itself at some other point in time or to the CMB itself?165.212.189.187 (talk) 15:47, 30 January 2015 (UTC)
- Well it was only a cheap science fiction story -- evidently more fantasy than science since the necessary change in physical constants would presumably have been detectable and would have resulted in different scale changes for different materials. Dbfirs 21:49, 29 January 2015 (UTC)
List of psychological activity, paranormal or psychic phenomena:
Peeps, can you help me naming some ‘psychological activity’ (thinking, emotions, memory, desires, will…) as well as the so-called ‘paranormal’ or ‘psychic phenomena’ (extrasensory perception, out-of-body experiences…). -- (Russell.mo (talk) 21:50, 28 January 2015 (UTC))
- Have you read our article Paranormal?
--TammyMoet (talk) 13:15, 29 January 2015 (UTC)
- No, I have not. Thanks -- (Russell.mo (talk) 15:24, 29 January 2015 (UTC))
- The article doesn't meet the requirement. -- (Russell.mo (talk) 20:45, 29 January 2015 (UTC))
- I'll try to find out where I got the sentence paragraph from. -- (Russell.mo (talk) 20:48, 29 January 2015 (UTC))
- The article doesn't meet the requirement. -- (Russell.mo (talk) 20:45, 29 January 2015 (UTC))
- No, I have not. Thanks -- (Russell.mo (talk) 15:24, 29 January 2015 (UTC))
Black Powder vs. Smokeless Powder
Is it true that smokeless powder has greater energy density while black powder has greater power density? Assuming this is true, if equal amounts of powder were used in a load in a firearm, would the greater power density of black powder give it the same muzzle velocity as smokeless powder? 69.121.131.137 (talk) 22:50, 28 January 2015 (UTC)
- Power density is a strange way to put it, but if I read the concept correctly; power is work divided by time. So "power density" would simply be the energy released divided by the time it takes to release it, divided again by the mass of the substance (for the "density" bit. Or maybe the volume. Extrapolating from energy density). What you would want to compare is the Burn rate of smokeless powder versus black powder. This chart compares the burn rates of various propellants, but does not give raw numbers, it only "ranks" them. But what you really need to know is how fast traditional powder burns compared to whatever formulation of smokeless powder you are looking at. Also, be careful here: black powder is basically one thing, but there are dozens upon dozens of different things called "smokeless powders" and they all have different properties. So there may be no way to accurately answer your question without comparing specific powders. --Jayron32 03:05, 29 January 2015 (UTC)
- How does black powder compare to the fastest one on that list. 69.121.131.137 (talk) 03:51, 30 January 2015 (UTC)
AEROANDTECH
Good morning, I am Morelli Luca , the designer and owner of the AEROANDTECH project; I'd like to update the information given with AEROANDTECH page, but each time that I do it, I find the day after again the wrong information. The wrong information are giving us economic problems, so, how can I fix the right information avoiding that someone will change again them?
it is very important, please answer thanks Ing. Morelli Luca — Preceding unsigned comment added by 62.18.86.170 (talk) 23:01, 28 January 2015 (UTC)
- The article in question seems to be Aero & Tech Nexth. Alansplodge (talk) 01:59, 29 January 2015 (UTC)
- This looks like a content dispute. The OP needs to use the article talk page to discuss it with other editors. ←Baseball Bugs What's up, Doc? carrots→ 02:06, 29 January 2015 (UTC)
- You're unlikely to be happy about this but...
- The thing here is that you imagine yourself (as owner/designer/whatever at the company) as being the 'authoritative' source of information - so you must be right. However that's categorically NOT how Wikipedia works. You have no more standing in writing this article than anyone else who comes here...to the contrary, actually - because you have an active interest in representing both company and product in a good light, you have an inherent conflict of interest and people are likely to view your edits with the deepest suspicion. Our WP:COI guidelines explain this in more detail.
- The information that goes into the article must come from a reliable third-party source. Something that our readers can verify. We can't take your word for these things...crazy though that sounds. What you need to do is to present information that's been published in aviation journals, newspapers, places like that. That information is considered "reliable". You can read our guidelines for reliable sources at WP:RS.
- This does seem very weird when you, personally, know "the truth" - but you have to consider what would happen if we allowed the owners and employees of businesses to come here and write their own articles. We'd be nothing more than an advertising platform - not a trusted encyclopedia. Because businesses would really, really like only favorable information to be in our articles about them, we simply cannot trust what they tell us directly. So we rely on journalists, book authors and people like that to do research and to provide a somewhat balanced view.
- Looked at another way, you wouldn't like it if your competitor were inserting falsehoods about your company into the article either...but if we allowed people to write stuff without reliable sources, that could easily happen. Anyone can create an account here and claim to be anyone they want...they could easily claim to be an 'insider' when they aren't.
- The best way to explain this is that Wikipedia editors are not supposed to come up with information themselves - they can only find it in reliable source. This is explained in our guideline about 'original research': WP:NOR.
- When I was writing the article about the Mini Cooper - I wanted to write about the top speed of the car in each gear, which I knew because I'd driven my Mini on a track and measured the flat-out speed myself with GPS and a trackside radar gun. However, even though I knew the number for a fact - I had to find an automotive magazine that reviewed the car and use that as a reference. SteveBaker (talk) 03:00, 29 January 2015 (UTC)
- Note that what you can do is add a link to your own website, if there isn't already one, in the "External links" section at the bottom. And on your own web site you can, of course, put as positive spin on your products as you want. StuRat (talk) 13:40, 29 January 2015 (UTC)
- That link has been there since April 1012 when the article was first created...so that's clearly not enough for our OP. The latest revision of the article is heavily reliant on a single reference in the "World Directory of Leisure Aviation". From Wikipedia's perspective, that's probably a reasonable source of information...but we do refer to the manufacturers site in two other references.
- In an ideal world, our OP would be telling us of a bunch of other places where the aircraft is discussed/reviewed/etc so we may broaden our base of references beyond one WP:RS and two WP:COI sources. Doubtless employees of the company would have a good knowledge of where their product had been written about. SteveBaker (talk) 19:25, 29 January 2015 (UTC)
- Wow, that link has been there over a thousand years ? Impressive ! StuRat (talk) 04:13, 30 January 2015 (UTC)
- It was brought from France to England by William the Concurrer. It started out as chain mail. ←Baseball Bugs What's up, Doc? carrots→ 19:44, 31 January 2015 (UTC)
- Wow, that link has been there over a thousand years ? Impressive ! StuRat (talk) 04:13, 30 January 2015 (UTC)
January 29
What is the simplest fatty acid?
184.59.104.40 (talk) 01:23, 29 January 2015 (UTC)
- We don't normally answer homework questions. However, we do have excellent articles on fatty acids and on carboxylic acids in general. I suggest you read them, and, if you have any further questions, please ask them here. Dr Dima (talk) 01:46, 29 January 2015 (UTC)
Do children aged 3/4 have body odours? The disgusting smell that puts others off? -- (Russell.mo (talk) 07:28, 29 January 2015 (UTC))
- Here are some links that may help you research the answer to your question. --Jayron32 10:46, 29 January 2015 (UTC)
- One source of a foul odor is young children is phenylketonuria. StuRat (talk) 13:44, 29 January 2015 (UTC)
- I don't trust any other links rather than Wikipedia's and Wikipdians. Body Odour article stated about 'puberty', 'ovulation', diseases, and lifestyle related things. I'm curious whether a general scent is available in children, especially the arm pit smell. -- (Russell.mo (talk) 15:20, 29 January 2015 (UTC))
- If you don't trust anything except Wikipedia, I'm not sure what we can do for you. I've provided you with lots of reading material on the matter, if you can't be bothered to read it, then there's nothing else anyone here can do for you. You already know how to find the Wikipedia article on the subject, and note that the Wikipedia article does not answer your problem. I provided additional sources, and you said you can't be bothered to read through them to find information you can trust. Good luck and vaya con dios... --Jayron32 15:24, 29 January 2015 (UTC)
 I said 'I don't trust any other links rather than Wikipedia's and Wikipdians'. I didn't say, "I can't be bothered to read through the information (help) you provided". Another reason behind this is, I have to attribute the links appropriately, in most cases they don't allow to retrieve information from their site... Anyways, thank you for your help.
I said 'I don't trust any other links rather than Wikipedia's and Wikipdians'. I didn't say, "I can't be bothered to read through the information (help) you provided". Another reason behind this is, I have to attribute the links appropriately, in most cases they don't allow to retrieve information from their site... Anyways, thank you for your help.  -- (Russell.mo (talk) 15:37, 29 January 2015 (UTC))
-- (Russell.mo (talk) 15:37, 29 January 2015 (UTC))
- I don't really understand what you mean "I have to attribute the links appropriately, in most cases they don't allow to retrieve information from their site". If you mean you're writing this for somewhere and need to cite sources, be aware that many things will not accept even wikipedia itself as a good source, and definitely very few will accept something some random wikipedian said as a source. And any thing which is so loose with their sourcing requirements that they do accept stuff some random wikipedian said would likely accept most of those as sources too and it shouldn't be that hard to work out how to cite them (and you could always ask if you really have problems.). If you mean you have problems accessing other sources, some explaination of this when asking the question, would help a great deal. Most of the links in the Google search result are just to ordinary web resources like help sites, and also some forums and other such stuff. They aren't to journal articles or anything likely to behind a paywall. And they also don't seem to be particularly controversial. So you'd need to be behind a very, very restrictive internet connection if you can't access them. Nil Einne (talk) 17:37, 29 January 2015 (UTC)
- OR and not quite what the OP was asking, but children of that age have natural non-pathogenic body odours which they do not find offensive and by which they can recognise each other. This ability tend to be abandoned early because of social pressure from adults. Dbfirs 18:51, 29 January 2015 (UTC)
- That seemed to be said in the OP's first point. Since they said it was "another reason", I presume the second was intended to cover a different reason. Nil Einne (talk) 19:18, 29 January 2015 (UTC)
- @Nil Einne, StuRat, and Dbfirs: Ammm, you baffled me Nil Einne! Anyways, I'm on 'pay kilobyte as you go' price plan, the only price plan available in this country. Its almost the end of the month, I'm on about less than 250 Mb, I got to cover it till to 7th of next month (unsure). Wikipedia is my school/college/university, the only website that doesn't take megabytes as you enter... Other websites (some/most) restrict picking information off of their website; whatever I've come across so far, whenever I tried to retrieve... What put me off of looking at other websites... A Wikipedian taught me a golden rule, still they advised to provide the links of the websites I collect information off, and whatever I've put my finger on, so far, is a copyrighted material, which does not allow me to copy my way even if I provide their link in my work. I only trust Wikipedia's articles (I don't have a choice). Its like an e-Bible to me. The ones who help me all the time or the 'Reference desk', its like angelic desk for me, where angels assist me (lol)! Of course demons are available, but I have not come across any yet. You guys might have since you (and most, even WP) thinks WP is not a reliable source
- Hello Dbfirs, think of it as children sitting in 'airtight dry atmospheric room', obviously they will sweat and smell. I understand the armpit smell which may begin in puberty, what can I use to explain 'the airtight dry atmospheric rooms children smell'? How can I say, "The body odour, the scent of human being (children) was unpleasant because of ________________________________?"
- You give me jokes Stu..lol Do my words/sentences/paragraphs seriously come across like a 'broken type writer' work? I do put my heart into making sense of my words/sentences/paragraphs you know, not joking at all...
- Thanks for offering the helping hand Nil (like some)

- Thanks for offering the helping hand Nil (like some)
- (Russell.mo (talk) 20:38, 29 January 2015 (UTC))
- It was a bit hard to understand which links you trust and which you don't. Back to the question, I recently had the misfortune to sit next to a young kid in a restaurant, who smelled like mildew. It might have been his clothes or coat. Then again, if he used a towel full of mildew, say to dry his hair, then he might smell that way, too. I blame his parents for not washing his clothes or towel with bleach, to kill the mildew. StuRat (talk) 04:08, 30 January 2015 (UTC)
- Could use it; I was expecting a general word that will provide the understanding of the complete dry indoor suffocating environment. Thanks though
 -- (Russell.mo (talk) 17:53, 30 January 2015 (UTC))
-- (Russell.mo (talk) 17:53, 30 January 2015 (UTC))
- Could use it; I was expecting a general word that will provide the understanding of the complete dry indoor suffocating environment. Thanks though
- It was a bit hard to understand which links you trust and which you don't. Back to the question, I recently had the misfortune to sit next to a young kid in a restaurant, who smelled like mildew. It might have been his clothes or coat. Then again, if he used a towel full of mildew, say to dry his hair, then he might smell that way, too. I blame his parents for not washing his clothes or towel with bleach, to kill the mildew. StuRat (talk) 04:08, 30 January 2015 (UTC)
Name that med
I saw a TV ad recently for a med, and I didn't catch the name of the med or what it was for. It causes increased urination, and one of the side effects is urinary tract infections, since the urine contains more sugar than normal. (Perhaps it was to treat diabetes ?) Anyone know the name ? I would like to read Wikipedia's article, if we have one, or an outside source, otherwise. StuRat (talk) 13:41, 29 January 2015 (UTC)
- There's a new drug for diabetes that does that, Invokana, it makes you pee out glucose. There's a whole new class of these drugs. μηδείς (talk) 17:45, 29 January 2015 (UTC)
- See Category:SGLT2 inhibitors for Wikipedia articles all of them. -- Ed (Edgar181) 21:39, 29 January 2015 (UTC)
Is formic acid a fatty acid?
This is mostly an issue of nomenclature, but the question above about fatty acids got me a bit confused. Fatty acid says "fatty acid a carboxylic acid with a long aliphatic tail" (emphasis mine). But formic acid shows up in Short-chain_fatty_acid, as it has "aliphatic tails of less than six carbons". So, I suppose a "tail" with zero carbons is also a tail with less than six carbons (and apparently a zero-length tail is aliphatic), but it seems odd or wrong to say that formic acid has a "long aliphatic tail." "Fatty acid" does not appear on the formic acid article. I would hope that all short-chain fatty acids are indeed fatty acids. Acetic acid actually seems to imply that it is not a fatty acid (in the biochemsistry section), but at least it has one whole carbon on the "tail", that seems to qualify as truly aliphatic... I can see making the call either way, but I'm curious of what the actual usage is. If the usage is slightly contradictory, or different definitions require different tail lengths, that's fine too. Additionally, does anyone think that perhaps the list at short-chain fatty acids should be changed (or amended and clarified)?
Thanks, SemanticMantis talk) 16:02, 29 January 2015 (UTC)
- I don't know if there is any "official" definition of a fatty acid; IUPAC, generally considered the authority on chemical nomenclature, has a vague definition here: http://goldbook.iupac.org/F02330.html.
- Having worked in chemistry for 20 years, I can confidently say that no chemist would ever describe formic acid as a fatty acid. I would definitely support removing formic and acetic acids from any list of fatty acids, even short chain fatty acids. Propionic acid should probably go too. -- Ed (Edgar181) 21:35, 29 January 2015 (UTC)
- Thank you! That is what I expected; I have posted on the talk page for short-chain fatty acid with a link back to here [4]. If I don't get any feedback within a few days I will remove formic and acetic acid from the list at the short-chain article. SemanticMantis (talk) 21:58, 29 January 2015 (UTC)
- @Edgar181 and SemanticMantis: Nonetheless, there are many such uses in PubMed ( http://www.ncbi.nlm.nih.gov/pubmed/?term=%22short+chain+fatty+acids%22+%22formic%22 ) [5][6][7] etc. One thing about biology (and the "fat" in fatty acids makes people assert this as under its province) is that anyone writing a paper or giving a lecture can get up and define a term like "fatty acid" in the way that pleases him best for purposes of his work, and there's nothing you can do about it. :) Though there's no fat in the short chain fatty acid, there actually is some sense in it though, in the sense that a fatty acid minus two carbons = a fatty acid. Wnt (talk) 04:04, 30 January 2015 (UTC)
- Yeah, that's not too surprising either, thanks. I don't really care one way or the other, but I think our articles could represent the multiple definitions/aribitrariness a little better. Maybe I'll just put an asterisk in the article, explaining that inclusion of some acids as "fatty acids" is context-dependent. SemanticMantis (talk) 14:44, 30 January 2015 (UTC)
- Surely there has to be some name for "saturated monocarboxylic acid where everything else is a hydrocarbon", doesn't there? Just like methane is a paraffin even though you can't make wax out if it? Formic, acetic, propionic, butyric, valeric, caproic — you can't make fats out of all of them, but they're all the same class, and if they're not "fatty acids", what are you going to call them? --Trovatore (talk) 15:19, 30 January 2015 (UTC)
- Not all groupings or categories have special single-word names though, right? What's wrong with "saturated monocarboxylic acid"? Are those compounds often discussed as a group? I mean, we don't have a special word for finite alternating groups with an even number of elements either, but we can just describe them if we want to single them out. SemanticMantis (talk) 15:59, 30 January 2015 (UTC)
- Well, I think traditionally they have been discussed systematically. You have the paraffins, their alcohols, their aldehydes, their acids. They make a nice rubric. I think this is the way it was done in my dad's chem texts, anyway (from the forties). Fashion may have changed since then for all I know. --Trovatore (talk) 17:30, 30 January 2015 (UTC)
- By definition, lipids are biomolecules more soluble in non-polar solvents than water (that is, they are hydrophobic. Small-chain carboxylic acids are decidedly hydrophilic. If I am not mistaken, the largest carboxylic acid which is soluble in water to any reasonable degree is valeric acid; that provides what is probably a reasonable limit for the size of "fatty" acids (given that "fats" are hydrophobic). --Jayron32 04:59, 31 January 2015 (UTC)
- I am certainly not claiming that formic acid is in any ordinary sense "fatty". However, I do believe it is part of a systematic grouping that has traditionally been called the "fatty acids", if only for want of a better name. --Trovatore (talk) 06:23, 31 January 2015 (UTC)
- Fat may be hydrophobic, but I don't think of that as its defining feature. If I were to make up a definition from scratch, I would say it depends on whether the molecule is a substrate for beta oxidation. At least in the textbook instance, this would exclude propionic acid, acetic acid, and formic acid, but not valeric acid. However, one could just as logically define it according to products of beta oxidation, in which case the former two would still be within the definition. But that's all really quite arbitrary, and of course we're not actually supposed to make up an answer. :) Wnt (talk) 13:09, 31 January 2015 (UTC)
- Perhaps you should think less, than, if you think it isn't the defining feature. See here. A selection of definitions "Lipid - any of a class of organic compounds that are fatty acids or their derivatives and are insoluble in water but soluble in organic solvents.", "Lipid - "Any of a large group of organic compounds that are oily to the touch and insoluble in water", "A lipid is chemically defined as a substance that is insoluble in water and soluble in alcohol, ether, and chloroform." , "Lipid - any of a group of organic compounds consisting of the fats and other substances of similar properties: they are insoluble in water, soluble in fat solvents and ..." So, before you go one with what you think, perhaps you should actually read and cite references which tell you what you should think. The defining characteristic of all lipids (including subclasses of lipids like fats and fatty acids) is hydrophobicity. --Jayron32 21:16, 31 January 2015 (UTC)
- @Jayron32: I started off with references; you're the one who started the diversion into logical arguments. But people can write dictionaries however they want, and they don't have to be logically consistent either. Valeric acid, which you established as water-soluble as our article says, is described by Merriam-Webster as "any of four isomeric fatty acids C5H10O2 or a mixture of these" Indeed, they describe propionic acid the same way. [8] But not acetic acid. Which proves what, I'm not sure, but when article writing you can use this as a reference for something. (However, I don't dispute that "lipid" is hydrophobic because it was generally defined in terms of what you can isolate by extracting the hydrophobic components of a cell. Lipid, fat, and fatty acid may all have different meanings.) Wnt (talk) 22:16, 31 January 2015 (UTC)
- People can write dictionaries however they want, but at least they are reliable according to Wikipedia's standards. It's OK to provide references which support a certain view. It isn't OK to discount other references which support a view you don't like, as you do here. Look, valeric acid and propionic acid, and acetic and formic can all be fatty acids if they want to be. And if they don't wish to be fatty acids, I'm not going to object either. But as I've been trying to say all along, the definition is not clear-cut and simple: there is no hard-and-fast line for carboxylic acids which says that some of them are fatty acids and some are not. The entire set of them is a homologous series, and their properties vary gradually and continuously, with no clear dividing line. My only point is that: There is no clear line, but we at least can point to what are long-standing and well-accepted, and well referenced definitions of "fat" "lipid" and "fatty acid" and let the reader decide for themselves. I don't know what your goal is, but my goal is to provide as many different perspectives as possible and let people understand the complexity and subtlety of such definitions, not to call others wrong. --Jayron32 01:38, 1 February 2015 (UTC)
- I don't really understand how this became an argument. To me saying "all fatty acids have to qualify as lipids" and "all substrates for beta oxidation have to be counted as fatty acids" are two equally reasonable and at the moment equally unsourced assertions. P.S. Though I don't know this exists, it's at least theoretically possible to have a triglyceride in which one of the three chains is a short chain fatty acid, even formic acid, which nonetheless could be soluble in organic solvents rather than water. Wnt (talk) 13:30, 1 February 2015 (UTC)
- People can write dictionaries however they want, but at least they are reliable according to Wikipedia's standards. It's OK to provide references which support a certain view. It isn't OK to discount other references which support a view you don't like, as you do here. Look, valeric acid and propionic acid, and acetic and formic can all be fatty acids if they want to be. And if they don't wish to be fatty acids, I'm not going to object either. But as I've been trying to say all along, the definition is not clear-cut and simple: there is no hard-and-fast line for carboxylic acids which says that some of them are fatty acids and some are not. The entire set of them is a homologous series, and their properties vary gradually and continuously, with no clear dividing line. My only point is that: There is no clear line, but we at least can point to what are long-standing and well-accepted, and well referenced definitions of "fat" "lipid" and "fatty acid" and let the reader decide for themselves. I don't know what your goal is, but my goal is to provide as many different perspectives as possible and let people understand the complexity and subtlety of such definitions, not to call others wrong. --Jayron32 01:38, 1 February 2015 (UTC)
- @Jayron32: I started off with references; you're the one who started the diversion into logical arguments. But people can write dictionaries however they want, and they don't have to be logically consistent either. Valeric acid, which you established as water-soluble as our article says, is described by Merriam-Webster as "any of four isomeric fatty acids C5H10O2 or a mixture of these" Indeed, they describe propionic acid the same way. [8] But not acetic acid. Which proves what, I'm not sure, but when article writing you can use this as a reference for something. (However, I don't dispute that "lipid" is hydrophobic because it was generally defined in terms of what you can isolate by extracting the hydrophobic components of a cell. Lipid, fat, and fatty acid may all have different meanings.) Wnt (talk) 22:16, 31 January 2015 (UTC)
- Perhaps you should think less, than, if you think it isn't the defining feature. See here. A selection of definitions "Lipid - any of a class of organic compounds that are fatty acids or their derivatives and are insoluble in water but soluble in organic solvents.", "Lipid - "Any of a large group of organic compounds that are oily to the touch and insoluble in water", "A lipid is chemically defined as a substance that is insoluble in water and soluble in alcohol, ether, and chloroform." , "Lipid - any of a group of organic compounds consisting of the fats and other substances of similar properties: they are insoluble in water, soluble in fat solvents and ..." So, before you go one with what you think, perhaps you should actually read and cite references which tell you what you should think. The defining characteristic of all lipids (including subclasses of lipids like fats and fatty acids) is hydrophobicity. --Jayron32 21:16, 31 January 2015 (UTC)
- Fat may be hydrophobic, but I don't think of that as its defining feature. If I were to make up a definition from scratch, I would say it depends on whether the molecule is a substrate for beta oxidation. At least in the textbook instance, this would exclude propionic acid, acetic acid, and formic acid, but not valeric acid. However, one could just as logically define it according to products of beta oxidation, in which case the former two would still be within the definition. But that's all really quite arbitrary, and of course we're not actually supposed to make up an answer. :) Wnt (talk) 13:09, 31 January 2015 (UTC)
- I am certainly not claiming that formic acid is in any ordinary sense "fatty". However, I do believe it is part of a systematic grouping that has traditionally been called the "fatty acids", if only for want of a better name. --Trovatore (talk) 06:23, 31 January 2015 (UTC)
- By definition, lipids are biomolecules more soluble in non-polar solvents than water (that is, they are hydrophobic. Small-chain carboxylic acids are decidedly hydrophilic. If I am not mistaken, the largest carboxylic acid which is soluble in water to any reasonable degree is valeric acid; that provides what is probably a reasonable limit for the size of "fatty" acids (given that "fats" are hydrophobic). --Jayron32 04:59, 31 January 2015 (UTC)
- Well, I think traditionally they have been discussed systematically. You have the paraffins, their alcohols, their aldehydes, their acids. They make a nice rubric. I think this is the way it was done in my dad's chem texts, anyway (from the forties). Fashion may have changed since then for all I know. --Trovatore (talk) 17:30, 30 January 2015 (UTC)
- Not all groupings or categories have special single-word names though, right? What's wrong with "saturated monocarboxylic acid"? Are those compounds often discussed as a group? I mean, we don't have a special word for finite alternating groups with an even number of elements either, but we can just describe them if we want to single them out. SemanticMantis (talk) 15:59, 30 January 2015 (UTC)
- Surely there has to be some name for "saturated monocarboxylic acid where everything else is a hydrocarbon", doesn't there? Just like methane is a paraffin even though you can't make wax out if it? Formic, acetic, propionic, butyric, valeric, caproic — you can't make fats out of all of them, but they're all the same class, and if they're not "fatty acids", what are you going to call them? --Trovatore (talk) 15:19, 30 January 2015 (UTC)
- Yeah, that's not too surprising either, thanks. I don't really care one way or the other, but I think our articles could represent the multiple definitions/aribitrariness a little better. Maybe I'll just put an asterisk in the article, explaining that inclusion of some acids as "fatty acids" is context-dependent. SemanticMantis (talk) 14:44, 30 January 2015 (UTC)
- @Edgar181 and SemanticMantis: Nonetheless, there are many such uses in PubMed ( http://www.ncbi.nlm.nih.gov/pubmed/?term=%22short+chain+fatty+acids%22+%22formic%22 ) [5][6][7] etc. One thing about biology (and the "fat" in fatty acids makes people assert this as under its province) is that anyone writing a paper or giving a lecture can get up and define a term like "fatty acid" in the way that pleases him best for purposes of his work, and there's nothing you can do about it. :) Though there's no fat in the short chain fatty acid, there actually is some sense in it though, in the sense that a fatty acid minus two carbons = a fatty acid. Wnt (talk) 04:04, 30 January 2015 (UTC)
- Thank you! That is what I expected; I have posted on the talk page for short-chain fatty acid with a link back to here [4]. If I don't get any feedback within a few days I will remove formic and acetic acid from the list at the short-chain article. SemanticMantis (talk) 21:58, 29 January 2015 (UTC)
IUCN Red List for plants
The IUCN Red List article makes it sound as if plants are rated. Lots of animal species have their Red List status near the top of the infobox (see Pygmy hippopotamus for an example), but I'm not seeing a comparable thing in plant articles, whether common things like tomato, somewhat-known things like deadly nightshade, or rare things like Galium divaricatum. Is this simply an editorial decision (i.e. it was decided not to give Red List information for plants), or is this because the rankings given for animals somehow aren't available and/or aren't relevant for plants? Nyttend (talk) 17:36, 29 January 2015 (UTC)
- The Red List lists plants, you can read about it on their web site [9]. (As an aside, I can't get their search feature to work - is it just me or is the site broken?)
- Here's a some random plant articles I found that do have conservation status in the taxobox - Asplenium_bifrons, Aschisma_kansanum. So we know that the taxobox can take conservation status data, just like animals can.
- So I'm not sure why more plants don't have status listed. I don't think it's due to either of your suggestions. In popular culture, threatened plants get way less attention than endangered animals. Many endangered animals don't even get much attention - we hear about Charismatic_megafauna, but much less so endangered mosquitoes. There are also very few plants considered Flagship_species. So it might just be unintentional animal bias creeping in. We do have pages like IUCN_Red_List_data_deficient_species_(Plantae) IUCN_Red_List_vulnerable_species_(Plantae), and a few other similar lists (that really should be cross-linked under "see also," IMO). Maybe there's a drive at Wikipedia:WikiProject_Biology to rectify this, or perhaps we could start one :) SemanticMantis (talk) 18:04, 29 January 2015 (UTC)
- I've added notices at Wikipedia talk:WikiProject Biology and Wikipedia talk:WikiProject Tree of Life asking participants to go to Template talk:Taxobox, where I've proposed adding a maintenance category to all taxoboxes that don't mention IUCN Red List status. The point's to identify articles where we haven't addressed Red List status; it's not meant for Data Deficient and any other status (if there are any?) where we can't supply information, since I'm trying to identify ones where Wikipedia effort, not scholarly effort, is missing. Nyttend (talk) 18:21, 29 January 2015 (UTC)
- WP:PLANTS would probably be the best place to add a note. I always assumed that most plants aren't rated - after all, someone has to do the work to rate a species. And sort out the nomenclature. Since it's pretty much impossible to generate a list of all plant species, I can't see how anyone can rate all of them. Guettarda (talk) 05:06, 31 January 2015 (UTC)
- I've added notices at Wikipedia talk:WikiProject Biology and Wikipedia talk:WikiProject Tree of Life asking participants to go to Template talk:Taxobox, where I've proposed adding a maintenance category to all taxoboxes that don't mention IUCN Red List status. The point's to identify articles where we haven't addressed Red List status; it's not meant for Data Deficient and any other status (if there are any?) where we can't supply information, since I'm trying to identify ones where Wikipedia effort, not scholarly effort, is missing. Nyttend (talk) 18:21, 29 January 2015 (UTC)
History of ocean going ships late 1800s - early 1900s
Does anyone know where I could find some systematic information on the history of ocean going ships during the late 1800s to the early 1900s, say roughly 1880 to 1930? I am primarily interested in knowing how the characteristics of ocean going vessels evolved during this time in terms of parameters such as size, weight, speed, means of propulsion, and choice of construction materials (e.g. metal / wood). Ideally, I would be looking for something that talks about the characteristics of the mean (or median, etc.) ship over time. However, given the difficulties in obtaining good data from that era, I would take whatever I can get. In broad strokes, at the very least, I'd like to be able to say whether this was either A) a period of very little change in ship construction, B) a period of rapid innovation in ship construction, or C) something in between. Dragons flight (talk) 21:02, 29 January 2015 (UTC)
- I cannot point to what you want, though I'm sure it exists in a Jane' Ships or Lloyds list sort of way. I can give you Turbinia, Dreadnought and Ship gun fire-control system which all yell B. --Tagishsimon (talk) 21:25, 29 January 2015 (UTC)
- Age of sail indicates refinement and development - "between 1850 and the early 1900s when sailing vessels reached their peak of size and complexity," but the ref for that sentence has link rotted. SemanticMantis (talk) 21:50, 29 January 2015 (UTC)
- Matthew Turner (shipbuilder) should give you an idea about innovations in design in ocean going sailing vessels for some of that period, at least in the Pacific. Mikenorton (talk) 22:00, 29 January 2015 (UTC)
- Are you interested in ships in general, in military ships, or in civilian ships? My go-to book is Björn Landström's The Ship. The field saw massive development during the period you mention. HMS Devastation (1871) launched in 1871, the first modern turreted warship without backup sails. HMS Royal Sovereign in 1891 had 1.5 times the tonnage, twice the crew complement, and was build from streel, not iron. 1906 saw HMS Dreadnought, "obsoleting all existing battleships at the time", with 2.5 times the firepower of the latest Pre-dreadnoughts and turbines instead of reciprocating steam engines. Dreadnought was itself obsolete by the time of WW1, which saw the Queen Elizabeth-class battleship, bigger, better, and switching from coal to oil as the main fuel. The next major steps were geared turbines, and then radar. In 1930, arguably the decline of the battleship had begun, with aircraft carriers and U-boats taking over. --Stephan Schulz (talk) 22:39, 29 January 2015 (UTC)
- Specifically I care about ships that were used in the systematic collection of weather data. That includes both military and civilian vessels, but probably skews more towards the civilian side in terms of numbers. Dragons flight (talk) 01:55, 30 January 2015 (UTC)
- NOAA History - Tools of the Trade (Ships) has lots of information dating back to NOAA's predecessor Federal agency, the United States Survey of the Coast. You might also find U.S. Coast Guard and Navy history websites worthwhile. Systematic large-scale weather collection, however, is very new - it really took its present scientific form after the rise of aviation and probably didn't standardize until circa World War II. More on that: Weather Technology. Bit of Meteorological History (which has extensive information on the 19th and early 20th century) suggests that in the Civil War era and Reconstruction era, the Smithsonian Institute collected weather information, including maritime and seacoast data, for the Federal government. You can buy historical ship plans from SI: $20 just for the catalog - so those won't be cheap, but they probably rank among the most thorough historical resource available for a large number of vessels. The Navy also has historical records of every vessel ever contracted, with particularly thorough records for 1892 to 1945. NARA has extensive maritime records, including the Lighthouse Service and weather archives.
- One tidbit at the Navy's website got to me: they have a free book-length guide called Professional Readings in U.S. Navy History, which is the introduction to the vast research resources available; and they recommend you visit various offices in person to talk to historians. In other words, you might go far by asking a real historian - rather than just talking to a bunch of scientists who are interested in history. Nimur (talk) 07:11, 30 January 2015 (UTC)
- Specifically I care about ships that were used in the systematic collection of weather data. That includes both military and civilian vessels, but probably skews more towards the civilian side in terms of numbers. Dragons flight (talk) 01:55, 30 January 2015 (UTC)
- That's the most interesting period of development for battleships, a great introduction is the DK Brown book "Warrior to Dreadnought". It's also when Froude got to grips with hull shapes. Greglocock (talk) 00:53, 30 January 2015 (UTC)
January 30
Snow by country
Besides Canada and US and Russia, which other nations received snow for their winter season? Please tell me the names, thanks. — Preceding unsigned comment added by 70.31.17.253 (talk) 02:59, 30 January 2015 (UTC)
- Define just what you mean by "received snow for their winter season". ←Baseball Bugs What's up, Doc? carrots→ 03:16, 30 January 2015 (UTC)
- (ec) Would be much easier to list the countries which never have snow. Nearly every country has snow in one part of its territory, at least on one mountain. Lgriot (talk) 03:19, 30 January 2015 (UTC)
- Just for example, to back up what Lgriot says, it snows on Mount Kilimanjaro, which is only 3 degrees latitude from the equator. While I wouldn't be so certain as to say it snows in every country on Earth, it is true that it snows at just about any latitude, given the likelihood of finding a high enough elevation there. --Jayron32 04:03, 31 January 2015 (UTC)
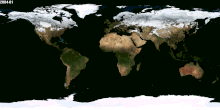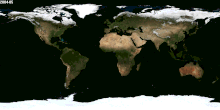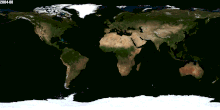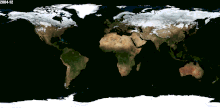
- The image at right should help see where snow is common enough to be easily visible from space. Dragons flight (talk) 03:42, 30 January 2015 (UTC)
- Yes, but I would have expected more snow, where there is land, such as South America, which only appears to have snow in the Andes, even in parts quite close to Antarctica. That must be the Patagonian Desert. Perhaps the Q should be reversed: Why aren't there major deserts in the arctic ? StuRat (talk) 17:45, 30 January 2015 (UTC)
- The seasonal temperature difference between winter and summer is much more extreme over large land areas than over the oceans. Because they are typically farther from the moderating influence of the ocean, continental interiors in the North experience much larger seasonal temperature variations [10]. By contrast, the bulk of South Africa and Australia only rarely fall below 0 C [11], which limits the formation of snow. Dragons flight (talk) 19:22, 30 January 2015 (UTC)
- See also another map showing countries receiving snowfall (with further distinctions) and this user sandbox thingy listing countries (including almost 80 countries without snowfall in modern times). ---Sluzzelin talk 03:52, 30 January 2015 (UTC)
- That is quite misleading in relation to Australia. True, snow rarely falls in the capital cities, but there are areas outside where snow is a regular winter occurrence and our snow resorts have a long history. See Category:Ski areas and resorts in Australia. -- Jack of Oz [pleasantries] 04:26, 31 January 2015 (UTC)
- There is little snow in countries south of the equator because most of the world's land is in the Northern Hemisphere, and because most of the world's countries that are in the Southern Hemisphere are tropical or subtropical. Southern countries that do get snow include Argentina, Chile, and New Zealand, and there is high-altitude snow in the mountains of Africa and in the Andes. As to Antarctica (not a country, but for many purposes listed among countries), it doesn't get that much snow either. It is just that snow that falls in Antarctica doesn't melt. I still don't understand the OP's question, but maybe he got his answer. Robert McClenon (talk) 15:53, 30 January 2015 (UTC)
- To add to Robert's answer: The amount of snowfall does not simply increase with latitude. The atmospheric circulation is donimated, on the global scale, by three pairs of convection cells. Atmospheric currents in these cells produce heavy rainfall near the equator (a belt of equatorial rainforests around the Earth), flanked by two arid belts - one north of the equator and one south of the equator, around 20-30 degrees latitude (both N and S). Even further from the equator, again, there is a belt of increased rain- and snowfall, mostly around 50-70 degrees latitude (N and S). Finally, the N and S poles get relatively little snowfall. Thus, the most snowfall is expected around 60 degrees latitude, N and S. However, it so happens that there is relatively a lot of landmass between 50 and 70 degrees N, but relatively little landmass between 50 and 70 degrees S, as Trovatore already mentioned. Dr Dima (talk) 19:00, 30 January 2015 (UTC)
- Brings up an interesting question — how much snow falls on the ocean? --Trovatore (talk) 20:30, 30 January 2015 (UTC)
- Do you count snow that falls on the ice caps ice shelves that aren't over land? For the record marine snow is not helpful in answering your question :) SemanticMantis (talk) 20:51, 30 January 2015 (UTC)
- FWIW, Stanley, Falkland Islands / Islas Malvinas (51°41′S 57°51′W) receives 608 mm of precipitation a year, which is pretty average for a temperate climate (from google: Paris 585, Moscow 689, St Petersburg 633, etc.); however, prevailing winds there (in Stanley) are from the west, so it is covered to some extent by the rain-shadow of the South American mainland. Kerguelen, which is far from any mainland, receives 709 mm. I do not know how much of it is rain and how much of it is snow, however. 65.50.240.210 (talk) 22:36, 30 January 2015 (UTC)
- "Snow does fall, but it is temporary and does not settle for long" (from Climate of the Falkland Islands). In reference to StuRat's queries about snow in the Southern Hemisphere, Stanley is only as far south as London is north (both 51°); we had no snow at all in London last year and only a few flurries so far this winter. The southernmost city in the world is Punta Arenas (53°S) in Chile, which is on a par with Dublin or Hamburg, both 53°N. Alansplodge (talk) 02:47, 31 January 2015 (UTC)
- FWIW, Stanley, Falkland Islands / Islas Malvinas (51°41′S 57°51′W) receives 608 mm of precipitation a year, which is pretty average for a temperate climate (from google: Paris 585, Moscow 689, St Petersburg 633, etc.); however, prevailing winds there (in Stanley) are from the west, so it is covered to some extent by the rain-shadow of the South American mainland. Kerguelen, which is far from any mainland, receives 709 mm. I do not know how much of it is rain and how much of it is snow, however. 65.50.240.210 (talk) 22:36, 30 January 2015 (UTC)
- Do you count snow that falls on the ice caps ice shelves that aren't over land? For the record marine snow is not helpful in answering your question :) SemanticMantis (talk) 20:51, 30 January 2015 (UTC)
- Brings up an interesting question — how much snow falls on the ocean? --Trovatore (talk) 20:30, 30 January 2015 (UTC)
- To add to Robert's answer: The amount of snowfall does not simply increase with latitude. The atmospheric circulation is donimated, on the global scale, by three pairs of convection cells. Atmospheric currents in these cells produce heavy rainfall near the equator (a belt of equatorial rainforests around the Earth), flanked by two arid belts - one north of the equator and one south of the equator, around 20-30 degrees latitude (both N and S). Even further from the equator, again, there is a belt of increased rain- and snowfall, mostly around 50-70 degrees latitude (N and S). Finally, the N and S poles get relatively little snowfall. Thus, the most snowfall is expected around 60 degrees latitude, N and S. However, it so happens that there is relatively a lot of landmass between 50 and 70 degrees N, but relatively little landmass between 50 and 70 degrees S, as Trovatore already mentioned. Dr Dima (talk) 19:00, 30 January 2015 (UTC)
Ideal computation
Considering Landauer's principle is a function of temperature, and assuming we wanted to put in the energy to cool a computer asymptotically down to absolute zero, would it be possible to achieve exponentially higher rates of computation as the working temperature approaches absolute zero (and thus make the energy spent cooling worth the effort)? Would Bremermann's limit be the sole limiting factor in this case? Googol30 (talk) 04:57, 30 January 2015 (UTC)
- Suppose you keep boosting the operating frequency to achieve more calculations per unit time. Long before you hit these thermodynamic limitations, you will find that you cannot build a switch - let alone a complex array of interconnected switches connected as logic gates that reliably operate as a digital computer. To analyze the performance of a switch, you need to study its gain–bandwidth product, which can be considered a limitation derived from first principles of physics. Nimur (talk) 07:28, 30 January 2015 (UTC)
Sublimation?
Sometimes I drop an ice cube on the carpet in a warm (21C) room. I leave it there and it finally disappears. The carpet is not wet. Isnt that sublime?--86.190.191.233 (talk) 19:18, 30 January 2015 (UTC)
- Not enough information. The carpet may wick away the moisture so efficiently that it does not feel wet. Also, the relative humidity in your climes is unlikely to be dry enough for sublimation at those temperatures. Try doing it on a plate on the floor.--Aspro (talk) 20:24, 30 January 2015 (UTC)
- Sublimation means it goes directly from frozen to vapor, without ever becoming liquid. I'd bet that if you looked at the spot 10 minutes after the ice cube was dropped, you would find it was quite wet. So, you have melting followed by evaporation, as opposed to sublimation. StuRat (talk) 21:19, 30 January 2015 (UTC)
- See Water_(data_page)#Phase_diagram. Sublimation cannot occur meaningfully above 0oC at room pressure. Ice will slowly sublime below freezing in a process akin to below-boiling evaporation (of the course of many days or months) however, above 0, it will melt first. --Jayron32 03:54, 31 January 2015 (UTC)
- Only partially because the ice cube sublimates constantly but also melts and the meltwater evaporates. If you did it in your freezer with has a temperature of -20°C and the ice cube disappears, then you can say that it sublimated. But on 21°C it melts for the most part before it evaporates and just a very little part of the cube sublimates.--Calviin 19 (talk) 11:23, 31 January 2015 (UTC)
- And the sublimation is many times slower than the melting process.--Calviin 19 (talk) 11:28, 31 January 2015 (UTC)
- See Water_(data_page)#Phase_diagram. Sublimation cannot occur meaningfully above 0oC at room pressure. Ice will slowly sublime below freezing in a process akin to below-boiling evaporation (of the course of many days or months) however, above 0, it will melt first. --Jayron32 03:54, 31 January 2015 (UTC)
January 31
Fiber-spinning machine for carbon nanotubes
Hi there!
What's a fiber-spinning machine and how is it like which is used for creating carbon fibers with carbon nanotubes?
Thank you very much for your help!
Calviin 19 (talk) 11:15, 31 January 2015 (UTC)
- Here's a link that I think that you'll find useful, more generally look at spinning (textiles). Mikenorton (talk) 11:22, 31 January 2015 (UTC)
- But is it self-assembly?--Calviin 19 (talk) 11:31, 31 January 2015 (UTC)
- I have that from this paragraph: In 2003, Rice University researchers led by Richard Smalley made the first carbon nanotube fibers by running a liquid suspension of nanotubes through a fiber-spinning machine of the same type used to make commercial polymer fibers like DuPont’s Kevlar and Twaron, which is made by Teijin Aramid. The rationale was that the nanotubes would flow through the liquid and line up with one another like logs floating on a river. This alignment should make the fiber stronger and more conductive. However, the properties of these early fibers were not very good, says Matteo Pasquali, who now leads the nanotube fiber project at Rice. While other groups turned to making nanotube sheets and fibers from dry materials, the Rice group stuck with its method.--Calviin 19 (talk) 11:45, 31 January 2015 (UTC)
- I'm not sure that I've understood the whole paragraph (I'm not an english speaker, I've only started to learn it with the age of twelfe and I'm eighteen years old now.).--Calviin 19 (talk) 11:51, 31 January 2015 (UTC)
- This link doesn't exist.--Calviin 19 (talk) 12:57, 31 January 2015 (UTC)
- See Spinning (polymers), synthetic fibre, and Carbon (fibre) for the relevant articles, although our coverage of this subject isn't as good as it could be. Mikenorton's link seems to work here, and gives a reasonable introduction to the subject - are you behind a firewall? Tevildo (talk) 16:26, 31 January 2015 (UTC)
- When I click the link, the error 404 appears and it's said that the page isn't found.--Calviin 19 (talk) 16:49, 31 January 2015 (UTC)
- I take it you're referring to Mikenorth's original link, not your link which is a 404 link and so it always likely to show a 404. Anyway perhaps try this Webcitation archive of the CSIRO link [12]. Nil Einne (talk) 17:22, 31 January 2015 (UTC)
- The last link of the page still doesn't work.--Calviin 19 (talk) 17:25, 31 January 2015 (UTC)
- I take it you're referring to Mikenorth's original link, not your link which is a 404 link and so it always likely to show a 404. Anyway perhaps try this Webcitation archive of the CSIRO link [12]. Nil Einne (talk) 17:22, 31 January 2015 (UTC)
- When I click the link, the error 404 appears and it's said that the page isn't found.--Calviin 19 (talk) 16:49, 31 January 2015 (UTC)
- See Spinning (polymers), synthetic fibre, and Carbon (fibre) for the relevant articles, although our coverage of this subject isn't as good as it could be. Mikenorton's link seems to work here, and gives a reasonable introduction to the subject - are you behind a firewall? Tevildo (talk) 16:26, 31 January 2015 (UTC)
About Binding antibody
Hi all,
This article is up for speedy deletion. Many G-hits. The science is all number-y (I will refrain from linking "Dyscalculia") and thus beyond my understanding.
What should be done with this article? Delete? Redirect? Keep and improve?
--Shirt58 (talk) 11:31, 31 January 2015 (UTC)
- See WP:DICDEF for a deletion rationale, although this is more appropriate for any subsequent AfD - redirecting to Antibody seems like a reasonable course to take. Blocking antibody probably should go, as well. Tevildo (talk) 18:25, 31 January 2015 (UTC)
What is a good algebra textbook with lots of real-life examples?
I want a linear algebra book, which is rigorous but with lots of scientific or applied science examples .--Senteni (talk) 14:44, 31 January 2015 (UTC)
- If you're looking for beginner texts, I'd take a look at the Schaum's Outline series of books: they're generally clearly written and well presented, and have lots of examples. While I can't recommend a specific book, they seem to have lots of books on linear algebra, one of which might fit your needs, and sellers like Google Books and Amazon often have book previews that might let you check this before buying.
- If you're looking for much deeper insight, I'd definitely recommend Theodore Frankel's The Geometry of Physics (ISBN 9781107602601, publisher's website with preview), which discusses linear algebra (eg. tensors, differential forms) as a tiny subset of its overall treatment of geometric matters in physics, with lots of examples of the relevance of the maths to physics. But it's much more hardcore than the Schaum books, and I don't recommend reading it if you're not already quite comfortable with the topic at an informal level. -- The Anome (talk) 14:51, 31 January 2015 (UTC)
- Also, perhaps you should take this to Wikipedia:Reference desk/Mathematics, where the real mathematicians live... -- The Anome (talk) 16:53, 31 January 2015 (UTC)
- Then Frankel's the book for you: but I should let you know it's quite hard going, presupposes at least undergraduate applied mathematics, takes you into a lot of quite advanced modern mathematical physics, and linear algebra is only a tiny part of it. It's also quite costly, so I'd check it out at the library, or at least read the sample chapter online at the publisher's website, before considering buying it. -- The Anome (talk) 04:46, 1 February 2015 (UTC)
Infertility and safe sex
I'm honestly not seeking a medical advice, nor I was asked for, just curious: if a man and/or a woman is infertile, can they reject safe sex (assuming both are healthy, i.e. with no sexually transmitted diseases)? 93.174.25.12 (talk) 14:45, 31 January 2015 (UTC)
- Safe sex is about avoiding disease transmission. Contraception is about preventing pregnancy. Guettarda (talk) 15:12, 31 January 2015 (UTC)
- (EC) Your questions is fairly unclear or you seem to be confusing seperate issues.
- Firstly safe sex is all to do with STIs. When a man and woman are involved, safe/r sex will also generally provide a fair level of contraception in cases where it matter, but that's more a side effect and isn't actually part of the safer sex component.
- If a couple are in a monogamous relationship, don't have STIs considered worth worrying about and don't have behaviour which may risk transmiting STIs via other means (like intravenous drug use), then safer sex may not be needed, although some would recommend it anyway since there is always the risk the other partner may violate the expectations of the relationship (whether it's having sex or other risky behaviour). Note it's also important that there is some confidence in the belief they don't have STIs (i.e. an STI check sufficiently after the person stopped any risky behaviour), the easily noticable effects of STIs (particularly HIV) will often only show up long after the person is infectious. So saying someone is 'healthy' is confusing, a person with HIV even without any treatement may be 'healthy' for a fair while.
- Whether that couple want to use contraception will depend on personal factors i.e. how much they want to avoid the possibility of pregnancy. As said, it's seperate from the safer sex issue. When safer sex is recommended, it's recommend in cases even where the risk of pregnancy is low (like male-female anal sex or oral sex) or non existant (like any form of same sex contact). In fact, it's particularly recommended for anal sex (although the risk is high enough for vaginal sex that it's universally recommended there too in cases where you can't have sufficient confidence neither partner has an STI).
- While as stated, safer sex will function as a form of contraception (in the case of vaginal intercourse, condoms would be the normal), the failure rate with imperfect use is high enough that many would recommend an additional form if it really matters. Note also a couple may choose forms of contraception which provide little protection against STIs (so aren't a part of safer sex) but are sufficient for their contraptive goals, perhaps higher than condoms alone.
- Finally even with professionally confirmed infertility, it's possible contraception may still be recommended (if the couple really want to avoid pregnancy), as infertility isn't a binary. Okay technically it may be more accurate to call it subfertility, but a lot of time when people mention infertility they may mean subfertility. (It may be the infertility combined with condoms is likely to give a low enough chance of pregnancy that additional contraception isn't needed.)
- TLDR; whether or not a couple want to use contraception is a seperate issue from whether or not they should practice safer sex even if the social issues which give rise to one may often also give rise to the other, and one of them (safer sex) gives some level of the other (contraception). If pregnancy isn't desired, the chance of pregnancy may still be high enough when there is some level infertility or subfertility that contraception would be recommended.
- Nil Einne (talk) 15:33, 31 January 2015 (UTC)
- I don't really think there's anything to answer here. I mean, any couple can reject safe sex, and many do who oughtn't. Can a couple safely reject safe sex if both are disease free and pregnancy isn't an issue? Well, depending on how much each one trusts their doctor ... and their partner ... and that there aren't other unknown diseases still to be discovered ... well, in the end it's all a judgment call, and we can't make judgment calls for other people. Wnt (talk) 13:20, 1 February 2015 (UTC)
Electrolyte problems and constipation
- Is constipation in mammals linked to any mineral deficiencies (except Magnesium)?
- can excessive amounts of salts also cause constipation?
Ben-Natan (talk) 18:29, 31 January 2015 (UTC)
Idea of space elevator
Hi there!
Who were the first people who have thought about the space elevator? Jules Verne has mentioned it in one of his books, but in which one? Constantin Tsiolkovski has definitely not been the first person (in 1895) of having thought about the space elevator.
Thank you very much for your help!
Calviin 19 (talk) 18:23, 31 January 2015 (UTC)
- I don't recall any space elevators in any Jules Verne book I ever read... the most plausible candidate would be From the Earth to the Moon, but in that work of fiction, the Gun Club spaceflight used a large piece of artillery to fire a hollow shell with people inside - not an elevator! Nimur (talk) 20:28, 31 January 2015 (UTC)
- Note that a space elevator is under tension. A space tower is compression structure. What Verne book are you referring to? -- ToE 20:31, 31 January 2015 (UTC)
- From the Earth to the Moon is (arguably) the first SF story to propose a plausible method of travelling to the moon, without using magic or cavorite or similar dei ex machina. See History of science fiction and Moon in fiction#Science fiction. Tevildo (talk) 20:37, 31 January 2015 (UTC)
- Surely you've forgotten the very plausible Adventures of Domingo Gonsales (c. 1638), in which our astronaut ties himself to several very large migratory geese who summer in Saint Helena and winter on the moon? Nimur (talk) 20:47, 31 January 2015 (UTC)
Term for an F2-only heterozygous phenotype?
Is there any term for a heterozygous phenotype of two codominant alleles which cannot be produced as a monohybrid cross, but can arise in a dihybrid cross with a third allele on each side? In other words, is there a term for the type AB in a situation where AA × BB is impossible, and AA × BC either is impossible or is always AC, but AC × BC and/or AC × BD is sometimes AB? (The inspiration for this question, in case anyone's wondering, is the breeding mechanic in the Social Point game Monster Legends.NeonMerlin 21:52, 31 January 2015 (UTC)
- Balanced lethal. D'oh, can't believe that's a redlink but it's mentioned here. It's really important for routine Drosophila stock maintenance. Wnt (talk) 22:20, 31 January 2015 (UTC)
Q-Spoil
Came across this Q-Spoil article in the orphanage, it has no sources and I can't find any reference to the term via Google, anyone know if such a thing exists?— Preceding unsigned comment added by Vrac (talk • contribs)
- I can't find anything. WP:PROD may be appropriate to use here: Tagging it for prod would give anyone who cares time to fix it up, but if no one is caring about it, then it can be deleted. --Jayron32 02:05, 1 February 2015 (UTC)
- Cheers, prod it is. Vrac (talk) 02:30, 1 February 2015 (UTC)
- It's definately a thing, though I cannot judge its notability: [13] [14] [15]. I suspect, from the third link, that it may be a particular application of the Kerr effect. --Tagishsimon (talk) 02:43, 1 February 2015 (UTC)
- Cheers, prod it is. Vrac (talk) 02:30, 1 February 2015 (UTC)
- The PR encyclopedia of laser techniques doesn't mention "q-spoil", and Google searches turn up zero relevant hits. There is a thing called the "Q-factor" of a laser, and a technique for producing short pulses called Q-switching (both of which we have articles about). So I'm kinda suspicious that this is way, way too obscure for an article. With no references whatever, no other articles linking to it, and after languishing for 8 years with essentially zero improvement having been done on it - this article shouldn't be here. If it's a real thing then a line can be written about it in some other article. SteveBaker (talk) 03:57, 1 February 2015 (UTC)
evolution of bacteria and washing
Apart from evolution of bacteria to resist our antibiotics, has there been any detected evolution to resist being washed off by water or by soap and water from human skin? Or maybe evolution to appear "less dirty", give less of a "sticky sensation" on skin? Is this kind of evolution expected to happen by scientists? Thanks. Rich (talk) 04:21, 1 February 2015 (UTC)
- Washing them off should put less evolutionary pressure on them to adapt, since presumably they survive either way. So there's no effect that those that manage to stick to the hands are more likely to survive and pass on those genes. StuRat (talk) 06:11, 1 February 2015 (UTC)
- Agree with that, but see Antibacterial Soaps Concern Experts which says; "recent research suggests these products [anti-bacterial soaps] may encourage the growth of “superbugs” resistant to antimicrobial agents". Alansplodge (talk) 16:58, 1 February 2015 (UTC)
Incandescent light bulb history
Just watched the PBS show on Thomas Edison, and I have a question. The incandescent light bulb was eventually perfected by using a thin tungsten filament and evacuating the bulb of air. Before that, light bulbs would burn out in a few minutes. But, it seems to me, that another approach would have been to just use a thicker filament. After all, electrical resistance space heaters manage to produce a red glow with exposed wire coils. They do use a fan, but is that required to keep them from burning out quickly ? I'd think a bit less electricity and no fan would keep it red hot, but no hotter, so the wires would last. Now, admittedly red light isn't ideal, and all the excess heat would be annoying in summer, but perhaps quite welcome in winter. When compared with the poor choices available before Edison took on the light bulb, it seems to me that my approach would have been a welcome alternative to gas lights, for indoor lighting. StuRat (talk) 06:08, 1 February 2015 (UTC)
- Actually the tungsten filament represented the second generation of light bulb, allowing higher temperatures and therefore whiter light. As you will see at the Edison article you linked, Edison's bulbs that lasted for over 1,000 hours had carbon-based filaments. --65.94.50.4 (talk) 10:06, 1 February 2015 (UTC)
- I was brought up in gaslight, supplemented by paraffin lamps, before electricity reached the area where I was born, and I can assure you that it was far superior to the dull red glow from an electric filament in air. It is easier to read by firelight than by (exposed filament) electric heater light. Nichrome wasn't patented until 1905, but perhaps similar materials were available earlier for heaters. Dbfirs 07:20, 1 February 2015 (UTC)
Zyklon B
Dear Madam/Sir,
In English version of Wikipedia is a misinformation about current production of Zyklon B like insecticides. Uragan D 2 is produced by Draslovka Kolín who is "traditional" producer of Zyklon B (approx. 60t produced in 1943). In the subject it is stated Adezin as a producer, but the company is a disinfection, disinfestation and rodent control firm therefore only a user of the product. I made a change in the subject yesterday but it has been refused by censor. Solution is up to you.
Best regards
Karel Šikl
- The edit in question to the article Zyklon B was reverted by User:DumbBOT per WP:ELNO. A better place to discuss this is at that article's discussion page, Talk:Zyklon B. Note that this reversion was done due to the use of the external line, possibly what you had intended as a reference, so this was an issue of formatting, and not necessarily content. -- ToE 17:13, 1 February 2015 (UTC)
- (edit conflict)We have no censors, another user removed it. You added the information on 13:43, 30 January 2015. It was reverted 30 minutes later by MrX. His change comment seems to be wrong, but he seems not to be happy with the link to http://www.draslovka.cz. This link doesn't mention Zyklon B, perhaps you meant another one? For your future reference, the correct place to ask about this is on the talk page. - LongHairedFop (talk) 17:16, 1 February 2015 (UTC)
Is this real?
This. One kilowatt and two pounds of plastic bags in, one liter of crude oil out, so they claim. Is it real? The first "off" thing to me is that kilowatts are power units, not energy. If it's real, how long does it need one kilowatt? An hour? A month? 75.75.42.89 (talk) 17:07, 1 February 2015 (UTC)

