Cyanonilutamide
Appearance
 | |
| Clinical data | |
|---|---|
| Other names | RU-56279 |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
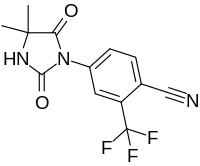
| Formula | C13H10F3N3O2 |
| Molar mass | 297.237 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cyanonilutamide (developmental code name RU-56279) is a nonsteroidal antiandrogen which was never marketed.[1][2] Both RU-56187 and RU-58841 appear to be prodrugs of cyanonilutamide in vivo in animals.[2] It has relatively low affinity for the androgen receptor but nonetheless shows significant antiandrogenic activity in animals.[2]
See also
References
- ^ Gryder BE, Akbashev MJ, Rood MK, Raftery ED, Meyers WM, Dillard P, Khan S, Oyelere AK (November 2013). "Selectively targeting prostate cancer with antiandrogen equipped histone deacetylase inhibitors". ACS Chem. Biol. 8 (11): 2550–60. doi:10.1021/cb400542w. PMC 3836611. PMID 24004176.
- ^ a b c Cousty-Berlin D, Bergaud B, Bruyant MC, Battmann T, Branche C, Philibert D (October 1994). "Preliminary pharmacokinetics and metabolism of novel non-steroidal antiandrogens in the rat: relation of their systemic activity to the formation of a common metabolite". J. Steroid Biochem. Mol. Biol. 51 (1–2): 47–55. doi:10.1016/0960-0760(94)90114-7. PMID 7947350. S2CID 29752252.
