Benorilate
This is an old revision of this page, as edited by 92.24.213.66 (talk) at 10:23, 31 October 2022 (→Synthesis). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
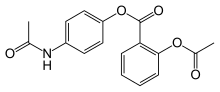 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.023.340 |
| Chemical and physical data | |
| Formula | C17H15NO5 |
| Molar mass | 313.309 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benorilate (INN), or benorylate, is an ester-linked codrug of aspirin with paracetamol. It is used as an anti-inflammatory and antipyretic medication. In the treatment of childhood fever, it has been shown to be inferior to paracetamol and aspirin taken separately. In addition, because it is converted to aspirin, benorylate is not recommended in children due to concerns about Reye syndrome.[1]
Synthesis

Acetyl salicoyl chloride [5538-51-2] (1) is reacted with paracetamol (2) to give benorilate (3).
Saponification of the ester led to Acetaminosalol (Phenetsal) [118-57-0].[5]
References
- ^ Similä S, Keinänen S, Kouvalainen K (December 1975). "Oral antipyretic therapy: evaluation of benorylate, an ester of acetylsalicylic acid and paracetamol". European Journal of Pediatrics. 121 (1): 15–20. doi:10.1007/bf00464391. PMID 2478. S2CID 21112438.
- ^ NL6504517 idem Andrew Robertson, U.S. patent 3,431,293 (1969 to Sterling Drug Inc).
- ^ Mario Portelli & Giorgio Renzi, DE 2402231 (1974 to Whitefin Holding SA).
- ^ Huang Xiaocheng, et al. CN 111056968 (2020 to Guangxi University of Science and Technology).
- ^ Moerk Nielsen, N., Bundgaard, H. (March 1989). "Evaluation of glycolamide esters and various other esters of aspirin as true aspirin prodrugs". Journal of Medicinal Chemistry. 32 (3): 727–734. doi:10.1021/jm00123a040.
| pyrazolones / pyrazolidines | |
|---|---|
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
Key: underline indicates initially developed first-in-class compound of specific group; #WHO-Essential Medicines; †withdrawn drugs; ‡veterinary use. | |
|
| Receptor (ligands) |
| ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||
| Others | |||||||||||||||||||||||||||
This drug article relating to the musculoskeletal system is a stub. You can help Wikipedia by expanding it. |
- Articles with short description
- Short description matches Wikidata
- Articles with changed CASNo identifier
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- All stub articles
