User:Evidales/sandbox
Steroid 21-hydroxylase is a cytochrome P450 enzyme integral to the biosynthesis of the steroid hormones aldosterone and cortisol.[1] These syntheses take place in the adrenal cortex.[2] Specifically, 21-hydroxylase converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively, by hydroxylating at the C21 position.[3] The products of the conversions then continue through their appropriate pathways towards creation of aldosterone and cortisol. Like other cytochrome P450 enzymes, 21-hydroxylase participates in the cytochrome P450 catalytic cycle, and engages in one-electron transfer with NADPH-P450 reductase. Its structure includes an essential iron heme group centered within the protein, also common to all P450 enzymes. Variations of the 21-hydroxylase enzyme can be found in all vertebrates.[4] However, understanding of human 21-hydroxylase structure and function is of particular clinical value, as a failure of the enzyme to act appropriately results in congenital adrenal hyperplasia. The x-ray crystal structure for human 21-hydroxylase, with bound progesterone, was realized and published in 2015, providing opportunity for further study.[3] The enzyme is notable for its substrate specificity and relatively high catalytic efficiency.
Function[edit]
21-hydroxylase is a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. The 21-hydroxylase enzyme is one of three microsomal steroidogenic P450 enzymes, the others being 17-hydroxylase and aromatase.[5] This protein localizes to the endoplasmic reticulum and hydroxylates steroids at the C21 position. Its activity is required for the synthesis of cortisol and aldosterone.[6]
Mechanism[edit]
Kinetics[edit]
21-hydroxylase is highly specific for hydroxylation of progesterone and 17-hydroxyprogesterone. No studies have reported sufficient binding of alternate substrates. In this way, it differs from the evolutionarily and functionally related P450 enzyme 17-hydroxylase, which has a large range of substrates.[7][8]
Earlier studies of the human enzyme expressed in yeast classified 17-hydroxyprogesterone as the best substrate for 21-hydroxylase.[8][9][10] However, recent analysis of the purified human enzyme found a lower KM and greater catalytic efficiency for progesterone over 17-hydroxyprogesterone.[3]
The 2015 analysis found the catalytic efficiency of 21-hydroxylase for conversion of progesterone in humans to be approximately 1.3 x 10^7 M-1s-1 at 37 °C. This makes it the most catalytically efficient P450 enzyme of those reported, as of 2015, and more catalytically efficient than the closely related bovine 21-hydroxylase enzyme.[11] C-H bond breaking to create a primary carbon radical is thought to be the rate-limiting step in the hydroxylation.[3]
Clinical significance[edit]
In humans, 21-Hydroxylase is encoded by the gene CYP21A2.[12] A related pseudogene is located near this gene; gene conversion events involving the functional gene and the pseudogene are thought to account for many cases of steroid 21-hydroxylase deficiency.[6] Both genes are located on chromosome 6, in the major histocompatibility complex, and the pseudogene, CYP21A1, retains 98% exonic sequence identity with the functional gene.[12][13]
A defect within the CYP21A2 gene causes a disturbance of the development of the enzyme, which leads to congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Congenital adrenal hyperplasia (CAH) is an autosomal recessive disorder, and occurs in approximately 1 in 15000 births globally.[14][15] There are multiple forms of CAH, broken down into classical and nonclassical based on the amount of function retained. The classical forms include salt-wasting (SW), and simple-viralizing (SV). Mutations that interfere with the active site--the heme group or residues involved in substrate binding--result in a complete loss of enzymatic activity, the salt-wasting type.[16] Cortisol and aldosterone deficits are associated with life-threatening salt-loss (hence salt-wasting), as the steroids play roles in regulating sodium homeostasis. Retaining minimal enzyme activity, the simple-viralizing type, is associated with mutations in conserved hydrophobic regions or near the transmembrane domain. Simple viralizing CAH patients maintain adequate sodium homeostasis, but exhibit other phenotypical symptoms shared by SW, including accelerated growth in childhood and ambiguous genitalia in female neonates. Nonclassical forms retain 20-60% of hydroxylase function--this form is associated with normal cortisol expression, but an excess of androgens post-puberty.[17][18]
Names and classification[edit]
| Steroid 21-monooxygenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.14.99.10 | ||||||||
| CAS no. | 9029-68-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
21-Hydroxylase is also called steroid 21-monooxygenase, 21α-Hydroxylase, P450 21A2 and, less commonly 21β-Hydroxylase.
Reaction[edit]
21-Hydroxylase catalyzes the addition of hydroxyl (-OH) to the C21 position of two steroids: progesterone and 17α-hydroxyprogesterone. The overall reactions catalyzed by 21-hydroxylase are below.
-
Steroid numbering — #21 is near center top
Pathway[edit]
 |
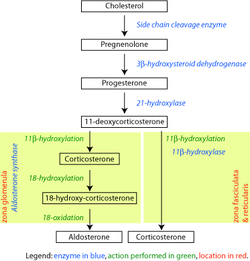 |
References[edit]
- ^ Ryan KJ, Engel LL (Mar 1957). "Hydroxylation of steroids at carbon 21" (PDF). The Journal of Biological Chemistry. 225 (1): 103–14. doi:10.1016/S0021-9258(18)64913-0. PMID 13416221.
- ^ Guengerich, F. Peter; Waterman, Michael R.; Egli, Martin (2016). "Recent Structural Insights into Cytochrome P450 Function". Trends in Pharmacological Sciences. 37 (8): 625–640. doi:10.1016/j.tips.2016.05.006. PMC 4961565. PMID 27267697.
- ^ a b c d Pallan, Pradeep S.; Wang, Chunxue; Lei, Li; Yoshimoto, Francis K.; Auchus, Richard J.; Waterman, Michael R.; Guengerich, F. Peter; Egli, Martin (2015-05-22). "Human Cytochrome P450 21A2, the Major Steroid 21-Hydroxylase STRUCTURE OF THE ENZYME·PROGESTERONE SUBSTRATE COMPLEX AND RATE-LIMITING C–H BOND CLEAVAGE". Journal of Biological Chemistry. 290 (21): 13128–13143. doi:10.1074/jbc.M115.646307. ISSN 0021-9258. PMC 4505568. PMID 25855791.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Graham, Sandra E.; Peterson, Julian A. (2002). "Sequence Alignments, Variabilities, and Vagaries". Methods in Enzymology. 357: 15–28. doi:10.1016/S0076-6879(02)57661-8. ISBN 9780121822606. PMID 12424893 – via Elsevier Science Direct.
- ^ Auchus, R. J., & Miller, W. L. (2015). P450 enzymes in steroid processing. In Cytochrome P450: Structure, Mechanism, and Biochemistry, Fourth Edition (pp. 851-879). Springer International Publishing. DOI: 10.1007/978-3-319-12108-6_12
- ^ a b "Entrez Gene: CYP21A2 cytochrome P450, family 21, subfamily A, polypeptide 2".
- ^ Cite error: The named reference
:2was invoked but never defined (see the help page). - ^ a b Auchus, Richard J.; Sampath Kumar, A.; Andrew Boswell, C.; Gupta, Manisha K.; Bruce, Kristen; Rath, Nigam P.; Covey, Douglas F. (2003-01-01). "The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21". Archives of Biochemistry and Biophysics. 409 (1): 134–144. doi:10.1016/s0003-9861(02)00491-5. ISSN 0003-9861. PMID 12464252.
- ^ Lorence, M. C.; Trant, J. M.; Mason, J. I.; Bhasker, C. R.; Fujii-Kuriyama, Y.; Estabrook, R. W.; Waterman, M. R. (1989-08-15). "Expression of a full-length cDNA encoding bovine adrenal cytochrome P450C21". Archives of Biochemistry and Biophysics. 273 (1): 79–88. doi:10.1016/0003-9861(89)90164-1. ISSN 0003-9861. PMID 2502949.
- ^ Wu, Du-An; Hu, Meng-Chun; Chung, Bon-Chu (1991-04-01). "Expression and Functional Study of Wild-Type and Mutant Human Cytochrome P450c21 in Saccharomyces cerevisiae". DNA and Cell Biology. 10 (3): 201–209. doi:10.1089/dna.1991.10.201. ISSN 1044-5498. PMID 1707279.
- ^ Cite error: The named reference
:1was invoked but never defined (see the help page). - ^ a b Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y (May 1986). "Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene". Proceedings of the National Academy of Sciences of the United States of America. 83 (9): 2841–5. Bibcode:1986PNAS...83.2841H. doi:10.1073/pnas.83.9.2841. PMC 323402. PMID 3486422.
- ^ White, P. C., Grossberger, D., Onufer, B. J., Chaplin, D. D., New, M. I., Dupont, B., and Strominger, J. L. (1985) Two genes encoding steroid 21- hydroxylase are located near the genes encoding the fourth component of complement in man. Proc. Natl. Acad. Sci. U.S.A. 82, 1089 –1093
- ^ New MI, Wilson RC. Steroid disorders in children: congenital ad- renal hyperplasia and apparent mineralocorticoid excess. Proc Natl Acad Sci USA. 1999;96:12790 –12797.
- ^ Therrell BL Jr, Berenbaum SA, Manter-Kapanke V, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590.
- ^ Pallan, Pradeep S.; Lei, Li; Wang, Chunxue; Waterman, Michael R.; Guengerich, F. Peter; Egli, Martin (2015-07-14). "Research Resource: Correlating Human Cytochrome P450 21A2 Crystal Structure and Phenotypes of Mutations in Congenital Adrenal Hyperplasia". Molecular Endocrinology. 29 (9): 1375–1384. doi:10.1210/ME.2015-1127. ISSN 0888-8809. PMC 4552440. PMID 26172259.
- ^ Miller, Walter L.; Auchus, Richard J. (2011-02-01). "The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders". Endocrine Reviews. 32 (1): 81–151. doi:10.1210/er.2010-0013. ISSN 0163-769X. PMC 3365799. PMID 21051590.
- ^ Haider, Shozeb; Islam, Barira; D’Atri, Valentina; Sgobba, Miriam; Poojari, Chetan; Sun, Li; Yuen, Tony; Zaidi, Mone; New, Maria I. (2013-02-12). "Structure–phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia". Proceedings of the National Academy of Sciences. 110 (7): 2605–2610. Bibcode:2013PNAS..110.2605H. doi:10.1073/pnas.1221133110. ISSN 0027-8424. PMC 3574933. PMID 23359706.
Further reading[edit]
- White PC, Tusie-Luna MT, New MI, Speiser PW (1994). "Mutations in steroid 21-hydroxylase (CYP21)". Human Mutation. 3 (4): 373–8. doi:10.1002/humu.1380030408. PMID 8081391.
- Helmberg A (Aug 1993). "Twin genes and endocrine disease: CYP21 and CYP11B genes". Acta Endocrinologica. 129 (2): 97–108. doi:10.1530/acta.0.1290097. PMID 8372604.
- de-Araujo M, et al. (Jan 1996). "Molecular analysis of CYP21 and C4 genes in Brazilian families with the classical form of steroid 21-hydroxylase deficiency". Brazilian Journal of Medical and Biological Research. 29 (1): 1–13. PMID 8731325.
- Yu CY (1999). "Molecular genetics of the human MHC complement gene cluster". Experimental and Clinical Immunogenetics. 15 (4): 213–30. doi:10.1159/000019075. PMID 10072631.
- Forest MG, et al. (Jun 2005). "21-Hydroxylase deficiency: an exemplary model of the contribution of molecular biology in the understanding and management of the disease". Annales d'endocrinologie. 66 (3): 225–32. doi:10.1016/s0003-4266(05)81754-8. PMID 15988383.
External links[edit]
- GeneReviews/NCBI/NIH/UW entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- OMIM entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- Synthesis of Desoxycorticosterone from Progesterone through 21-Hydroxylase (Image)
- Steroid+21-Hydroxylase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human CPS1 genome location and CPS1 gene details page in the UCSC Genome Browser.
- Human CYP21A2 genome location and CYP21A2 gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.




