Interferon beta-1b: Difference between revisions
m r2.7.1) (Robot: Adding pt:Interferão beta-1b |
Substitution of content with content copied from "treatment of multiple sclerosis" article. Reordering |
||
| Line 39: | Line 39: | ||
| molecular_weight = 20011.0 g/mol |
| molecular_weight = 20011.0 g/mol |
||
}} |
}} |
||
'''Interferon beta-1b''' (tradenames ''Betaferon'', ''Betaseron'' (North America), ''Extavia'' and ''ZIFERON'') is a [[medication|drug]] in the [[interferon]] family used to treat the relapsing-remitting and secondary-progressive forms of [[multiple sclerosis]] (MS). It is approved for use after the first MS event. It is administered by sub-cutaneous injection and has been shown to slow the advance of the affliction as well as reduce the frequency of attacks. |
'''Interferon beta-1b''' (tradenames ''Betaferon'', ''Betaseron'' (North America), ''Extavia'' and ''ZIFERON'') is a [[medication|drug]] in the [[interferon]] family used to treat the relapsing-remitting and secondary-progressive forms of [[multiple sclerosis]] (MS). It is approved for use after the first MS event. It is administered by sub-cutaneous injection and has been shown to slow the advance of the affliction as well as reduce the frequency of attacks. Closely related is [[interferon beta-1a]], also indicated for MS, and with a very similar drug profile. |
||
==Mechanism of action== |
|||
It is believed that interferon-beta based drugs achieve their beneficial effect on MS progress via their [[inflammation|anti-inflammatory]] properties. Studies have also determined that interferon-beta improves the integrity of the [[blood–brain barrier]] (BBB)—which generally breaks down in MS patients, allowing increasing amounts of undesirable substances to reach the brain. This strengthening of the BBB may be a contributing factor to interferon-beta's beneficial effects. These studies were carried out [[in vitro]], so it does not necessarily mean it works the same in people. |
|||
Interferon beta balances the expression of pro- and anti-inflammatory agents in the brain, and reduces the number of inflammatory cells that cross the [[blood brain barrier]].<ref name="pmid21649449">{{cite journal|last=Kieseier|first=Bernd C.|title=The Mechanism of Action of Interferon-β in Relapsing Multiple Sclerosis|journal=CNS Drugs|date=1 June 2011|volume=25|issue=6|pages=491–502|doi=10.2165/11591110-000000000-00000|pmid=21649449}}</ref> Overall, therapy with interferon beta leads to a reduction of neuron inflammation.<ref name="pmid21649449"/> Moreover, it is also thought to increase the production of [[nerve growth factor]] and consequently improve neuronal survival.<ref name="pmid21649449"/> |
|||
==Side effects== |
|||
Patients taking Interferon beta-1b may develop neutralizing antibodies to the medication.<ref>http://www.neurology.org/cgi/content/abstract/47/4/889</ref> |
|||
[http://www.neurology.org/cgi/content/abstract/47/4/889 Reference link] |
|||
[[File:Implant.png|right|thumb|Injectable medications can produce irritation or bruises at injection site. The bruise depicted was produced by a subcutaneous injection.]] |
|||
| ⚫ | |||
Interferon beta-1b is available only in injectable forms, and can cause skin reactions at the injection site that may include cutaneous [[necrosis]]. Skin reactions vary greatly in their clinical presentation.<ref name=pmid10563602/> They usually appear within the first month of treatment albeit their frequence and importance diminish after six months of treatment.<ref name=pmid10563602/> Skin reactions are more prevalent in women.<ref name=pmid10563602/> Mild skin reactions usually do not impede treatment whereas necroses appear in around 5% of patients and lead to the discontinuation of the therapy.<ref name=pmid10563602/> Also over time, a visible dent at the injection site due to the local destruction of fat tissue, known as [[lipoatrophy]], may develop. |
|||
Closely related is [[interferon beta-1a]], also indicated for MS, and with a very similar drug profile. |
|||
[[Interferon]]s, a subclass of [[cytokines]], are produced in the body during illnesses such as [[influenza]] in order to help fight the infection. They are responsible of many of the symptoms of influenza infections, including [[fever]], [[myalgia|muscle aches]], [[fatigue (medical)|fatigue]], and [[headache]]s.<ref name='pmid16253889'>{{cite journal|last=Eccles|first=R|title=Understanding the symptoms of the common cold and influenza.|journal=The Lancet infectious diseases|date=2005 Nov|volume=5|issue=11|pages=718-25|pmid=16253889}}</ref> Many patients report influenza-like symptoms hours after taking interferon beta that usually improve within 24 hours, being such symptoms related to the temporary increase of cytokines.<ref name="pmid18970977">{{cite journal |
|||
|author=Compston A, Coles A |
|||
| ⚫ | |||
|journal=Lancet |
|||
|volume=372 |
|||
|issue=9648 |
|||
|pages=1502–17 |
|||
|year=2008 |
|||
|month=October |
|||
|pmid=18970977 |
|||
|doi=10.1016/S0140-6736(08)61620-7 |
|||
|url= |
|||
}}</ref><ref name=pmid10563602>{{cite journal|last=Walther|first=EU|coauthors=Hohlfeld, R|title=Multiple sclerosis: side effects of interferon beta therapy and their management.|journal=Neurology|date=1999 Nov 10|volume=53|issue=8|pages=1622-7|pmid=10563602}}</ref> This reaction tends to dissapear after 3 months of treatment and its symptoms can be treated with over-the-counter [[nonsteroidal anti-inflammatory drug]]s, such as [[ibuprofen]], that reduce fever and pain.<ref name=pmid10563602/> Another common transient secondary effect with interferon-beta is a functional deterioration of already existing symptoms of the disease.<ref name=pmid10563602/> Such deterioration is similar to the one produced in MS patients due to heat, fever or stress ([[Uhthoff's phenomenon]]), usually appears within 24 hours of treatment, is more common in the initial months of treatment, and may last several days.<ref name=pmid10563602/> A sypmtom specially sensitive to worsening is [[spasticity]].<ref name=pmid10563602/> Interferon-beta can also reduce numbers of [[white blood cell]]s ([[leukopenia]]), [[lymphocyte]]s ([[lymphopenia]]) and [[neutrophil]]s ([[neutropenia]]), as well as affect [[liver]] function.<ref name=pmid10563602/> In most cases these effects are non-dangerous and reversible after cessation or reduction of treatment.<ref name=pmid10563602/> Nevertheless, recommendation is that all patients should be monitored through laboratory [[Blood test|blood analyses]], including [[liver function tests]], to ensure safe use of interferons.<ref name=pmid10563602/> |
|||
The injection-site reactions can be mitigated by rotating injection sites or by using one of the medications that requires less frequent injections. Side effects are often onerous enough that many patients ultimately discontinue taking Interferons (or [[glatiramer acetate]], a comparable disease-modifying therapies requiring regular injections). |
|||
==Efficacy== |
|||
=== Clinically isolated syndrome === |
|||
The earliest clinical presentation of relapsing-remitting multiple sclerosis is the clinically isolated syndrome (CIS), that is, a single attack of a single symptom. During a CIS, there is a subacute attack suggestive of [[demyelination]] but the patient does not fulfill the [[McDonald criteria|criteria]] for diagnosis of multiple sclerosis.<ref name="pmid15847841">{{cite journal |author=Miller D, Barkhof F, Montalban X, Thompson A, Filippi M |title=Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis |journal=Lancet neurology |volume=4 |issue=5 |pages=281–8 |year=2005 |pmid=15847841 |doi=10.1016/S1474-4422(05)70071-5}}</ref> Treatment with [[interferon]]s after an initial attack decreases the risk of developing clinical definite MS.<ref name="pmid18970977"/><ref name='pmid21205678'>{{cite journal|last=Bates|first=D|title=Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials.|journal=Neurology|date=2011 Jan 4|volume=76|issue=1 Suppl 1|pages=S14-25|pmid=21205678}}</ref> |
|||
=== Relapsing-remitting MS === |
|||
Medications are modestly effective at decreasing the number of attacks in relapsing-remitting multiple sclerosis and in reducing the accumulation of brain lesions, which is measured using [[gadolinium]]-[[MRI contrast agent| enhanced]] [[magnetic resonance imaging]] (MRI).<ref name="pmid18970977"/> Interferons reduce relapses by approximately 30% and their safe profile make them the first-line treatments.<ref name="pmid18970977"/> Nevertheles, not all the patients are responsive to these therapies. It is known that 30% of MS patients are non-responsive to Beta interferon.<ref name="pmid18690496">{{cite journal |
|||
|author=Bertolotto A, Gilli F |
|||
|title=Interferon-beta responders and non-responders. A biological approach |
|||
|journal=Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology |
|||
|volume=29 Suppl 2 |
|||
|issue= |
|||
|pages=S216–7 |
|||
|year=2008 |
|||
|month=September |
|||
|pmid=18690496 |
|||
|doi=10.1007/s10072-008-0941-2 |
|||
|url= |
|||
}}</ref> They can be classified in genetic, pharmacological and pathogenetic non-responders.<ref name="pmid18690496"/> One of the factors related to non-respondance is the presence of high levels of interferon beta neutralizing [[antibodies]]. Interferon therapy, and specially interferon beta-1b, induces the production of neutralizing antibodies, usually in the second 6 months of treatment, in 5 to 30% of treated patients.<ref name="pmid18970977"/> Moreover, a subset of RRMS patients with specially active MS, sometimes called "rapidly worsening MS" are normally non-responders to interferon beta-1b.<ref>{{cite journal |author=Buttinelli C, Clemenzi A, Borriello G, Denaro F, Pozzilli C, Fieschi C. |title=Mitoxantrone treatment in multiple sclerosis: a 5-year clinical and MRI follow-up |journal= European Journal of Neurology|volume= 14|issue= 11|pages= 1281–7|year= 2007|pmid=17956449 |doi=10.1111/j.1468-1331.2007.01969.x}}</ref><ref>{{cite journal |author=Boster A, Edan G, Frohman E, Javed A, Stuve O, Tselis A, Weiner H, Weinstock-Guttman B, Khan O |title=Intense immunosuppression in patients with rapidly worsening multiple sclerosis: treatment guidelines for the clinician |journal= Lancet neurology|volume= 7|issue= 2|pages= 173–83|year=2008 |pmid=18207115 |doi=10.1016/S1474-4422(08)70020-6}}</ref> |
|||
While more studies of the long-term effects of the drugs are needed,<ref name="pmid18970977"/><ref name="pmid21205679"/> existing data on the effects of interferons indicate that early-initiated long-term therapy is safe and it is related to better outcomes.<ref name="pmid21205679">{{cite journal|last=Freedman|first=M. S.|title=Long-term follow-up of clinical trials of multiple sclerosis therapies|journal=Neurology|date=27 December 2010|volume=76|issue=1, Supplement 1|pages=S26–S34|doi=10.1212/WNL.0b013e318205051d|pmid=21205679}}</ref> |
|||
==Commercial formulations== |
|||
| ⚫ | |||
==See also== |
|||
* [[Interferon beta-1a]] |
|||
| ⚫ | |||
==References== |
==References== |
||
Revision as of 22:03, 20 February 2013
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601151 |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.207.515 |
| Chemical and physical data | |
| Formula | C908H1408N246O253S6 |
| Molar mass | 20011.0 g/mol g·mol−1 |
| | |
Interferon beta-1b (tradenames Betaferon, Betaseron (North America), Extavia and ZIFERON) is a drug in the interferon family used to treat the relapsing-remitting and secondary-progressive forms of multiple sclerosis (MS). It is approved for use after the first MS event. It is administered by sub-cutaneous injection and has been shown to slow the advance of the affliction as well as reduce the frequency of attacks. Closely related is interferon beta-1a, also indicated for MS, and with a very similar drug profile.
Mechanism of action
Interferon beta balances the expression of pro- and anti-inflammatory agents in the brain, and reduces the number of inflammatory cells that cross the blood brain barrier.[1] Overall, therapy with interferon beta leads to a reduction of neuron inflammation.[1] Moreover, it is also thought to increase the production of nerve growth factor and consequently improve neuronal survival.[1]
Side effects
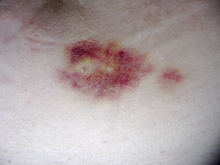
Interferon beta-1b is available only in injectable forms, and can cause skin reactions at the injection site that may include cutaneous necrosis. Skin reactions vary greatly in their clinical presentation.[2] They usually appear within the first month of treatment albeit their frequence and importance diminish after six months of treatment.[2] Skin reactions are more prevalent in women.[2] Mild skin reactions usually do not impede treatment whereas necroses appear in around 5% of patients and lead to the discontinuation of the therapy.[2] Also over time, a visible dent at the injection site due to the local destruction of fat tissue, known as lipoatrophy, may develop.
Interferons, a subclass of cytokines, are produced in the body during illnesses such as influenza in order to help fight the infection. They are responsible of many of the symptoms of influenza infections, including fever, muscle aches, fatigue, and headaches.[3] Many patients report influenza-like symptoms hours after taking interferon beta that usually improve within 24 hours, being such symptoms related to the temporary increase of cytokines.[4][2] This reaction tends to dissapear after 3 months of treatment and its symptoms can be treated with over-the-counter nonsteroidal anti-inflammatory drugs, such as ibuprofen, that reduce fever and pain.[2] Another common transient secondary effect with interferon-beta is a functional deterioration of already existing symptoms of the disease.[2] Such deterioration is similar to the one produced in MS patients due to heat, fever or stress (Uhthoff's phenomenon), usually appears within 24 hours of treatment, is more common in the initial months of treatment, and may last several days.[2] A sypmtom specially sensitive to worsening is spasticity.[2] Interferon-beta can also reduce numbers of white blood cells (leukopenia), lymphocytes (lymphopenia) and neutrophils (neutropenia), as well as affect liver function.[2] In most cases these effects are non-dangerous and reversible after cessation or reduction of treatment.[2] Nevertheless, recommendation is that all patients should be monitored through laboratory blood analyses, including liver function tests, to ensure safe use of interferons.[2]
The injection-site reactions can be mitigated by rotating injection sites or by using one of the medications that requires less frequent injections. Side effects are often onerous enough that many patients ultimately discontinue taking Interferons (or glatiramer acetate, a comparable disease-modifying therapies requiring regular injections).
Efficacy
Clinically isolated syndrome
The earliest clinical presentation of relapsing-remitting multiple sclerosis is the clinically isolated syndrome (CIS), that is, a single attack of a single symptom. During a CIS, there is a subacute attack suggestive of demyelination but the patient does not fulfill the criteria for diagnosis of multiple sclerosis.[5] Treatment with interferons after an initial attack decreases the risk of developing clinical definite MS.[4][6]
Relapsing-remitting MS
Medications are modestly effective at decreasing the number of attacks in relapsing-remitting multiple sclerosis and in reducing the accumulation of brain lesions, which is measured using gadolinium- enhanced magnetic resonance imaging (MRI).[4] Interferons reduce relapses by approximately 30% and their safe profile make them the first-line treatments.[4] Nevertheles, not all the patients are responsive to these therapies. It is known that 30% of MS patients are non-responsive to Beta interferon.[7] They can be classified in genetic, pharmacological and pathogenetic non-responders.[7] One of the factors related to non-respondance is the presence of high levels of interferon beta neutralizing antibodies. Interferon therapy, and specially interferon beta-1b, induces the production of neutralizing antibodies, usually in the second 6 months of treatment, in 5 to 30% of treated patients.[4] Moreover, a subset of RRMS patients with specially active MS, sometimes called "rapidly worsening MS" are normally non-responders to interferon beta-1b.[8][9]
While more studies of the long-term effects of the drugs are needed,[4][10] existing data on the effects of interferons indicate that early-initiated long-term therapy is safe and it is related to better outcomes.[10]
Commercial formulations
Betaferon/Betaseron is marketed today by Bayer HealthCare. The originator was Schering AG (Berlex in North America), now part of Bayer HealthCare. Novartis has also introduced Extavia, a new brand of interferon beta-1b, in 2009.
References
- ^ a b c Kieseier, Bernd C. (1 June 2011). "The Mechanism of Action of Interferon-β in Relapsing Multiple Sclerosis". CNS Drugs. 25 (6): 491–502. doi:10.2165/11591110-000000000-00000. PMID 21649449.
- ^ a b c d e f g h i j k l Walther, EU (1999 Nov 10). "Multiple sclerosis: side effects of interferon beta therapy and their management". Neurology. 53 (8): 1622–7. PMID 10563602.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Eccles, R (2005 Nov). "Understanding the symptoms of the common cold and influenza". The Lancet infectious diseases. 5 (11): 718–25. PMID 16253889.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d e f Compston A, Coles A (2008). "Multiple sclerosis". Lancet. 372 (9648): 1502–17. doi:10.1016/S0140-6736(08)61620-7. PMID 18970977.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Miller D, Barkhof F, Montalban X, Thompson A, Filippi M (2005). "Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis". Lancet neurology. 4 (5): 281–8. doi:10.1016/S1474-4422(05)70071-5. PMID 15847841.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bates, D (2011 Jan 4). "Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials". Neurology. 76 (1 Suppl 1): S14-25. PMID 21205678.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Bertolotto A, Gilli F (2008). "Interferon-beta responders and non-responders. A biological approach". Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 29 Suppl 2: S216–7. doi:10.1007/s10072-008-0941-2. PMID 18690496.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Buttinelli C, Clemenzi A, Borriello G, Denaro F, Pozzilli C, Fieschi C. (2007). "Mitoxantrone treatment in multiple sclerosis: a 5-year clinical and MRI follow-up". European Journal of Neurology. 14 (11): 1281–7. doi:10.1111/j.1468-1331.2007.01969.x. PMID 17956449.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Boster A, Edan G, Frohman E, Javed A, Stuve O, Tselis A, Weiner H, Weinstock-Guttman B, Khan O (2008). "Intense immunosuppression in patients with rapidly worsening multiple sclerosis: treatment guidelines for the clinician". Lancet neurology. 7 (2): 173–83. doi:10.1016/S1474-4422(08)70020-6. PMID 18207115.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Freedman, M. S. (27 December 2010). "Long-term follow-up of clinical trials of multiple sclerosis therapies". Neurology. 76 (1, Supplement 1): S26–S34. doi:10.1212/WNL.0b013e318205051d. PMID 21205679.
