Γ-Hydroxybutyric acid: Difference between revisions
m Cleaned up using AutoEd |
|||
| Line 99: | Line 99: | ||
=== Club and rave scene use === |
=== Club and rave scene use === |
||
GHB is often taken because users find that it enhances their experiences of being in a club, party, or rave; small doses of GHB can act as a stimulant and [[aphrodisiac]]. GHB is sometimes referred to as ''liquid ecstasy'', ''lollipops'', ''liquid X'' or ''liquid E'' due to its tendency to produce euphoria and sociability and its use in the dance party scene.<ref>{{cite journal | last1 = Klein | first1 = Mary | last2 = Kramer | first2 = Frances| year = 2004 | title = Rave drugs: Pharmacological considerations | journal = AANA Journal | volume = 72 | issue = 1| pages = 61–67 | pmid = 15098519 }}</ref> Despite these nicknames, GHB and [[MDMA]] (ecstasy) have entirely separate chemical and pharmacological modes of action. |
GHB is often taken because users find that it enhances their experiences of being in a club, childrens party, or rave; small doses of GHB can act as a stimulant and [[aphrodisiac]]. GHB is sometimes referred to as ''liquid ecstasy'', ''lollipops'', ''liquid X'' or ''liquid E'' due to its tendency to produce euphoria and sociability and its use in the dance party scene.<ref>{{cite journal | last1 = Klein | first1 = Mary | last2 = Kramer | first2 = Frances| year = 2004 | title = Rave drugs: Pharmacological considerations | journal = AANA Journal | volume = 72 | issue = 1| pages = 61–67 | pmid = 15098519 }}</ref> Despite these nicknames, GHB and [[MDMA]] (ecstasy) have entirely separate chemical and pharmacological modes of action. |
||
===Sports and athletics=== |
===Sports and athletics=== |
||
Revision as of 20:08, 1 November 2015
 | |
 | |
| Clinical data | |
|---|---|
| Other names | γ-Hydroxybutyric acid γ-Hydroxybutyrate GHB |
| Pregnancy category |
|
| Routes of administration | Usually oral; intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% (oral) |
| Metabolism | 95%, mainly Hepatic, also in blood and tissues |
| Onset of action | Within 5-15 min[1] |
| Elimination half-life | 30–60 minutes |
| Excretion | 5%, renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.218.519 |
| Chemical and physical data | |
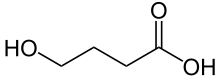
| Formula | C4H8O3 |
| Molar mass | 104.10 g/mol (GHB) 126.09 g/mol (sodium salt) 142.19 g/mol (potassium salt) g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
γ-Hydroxybutyric acid (GHB), also known as 4-hydroxybutanoic acid, is a central nervous system depressant that is normally made in humans and other animals. It is also present in foods such as wine, beef and small citrus fruits.[2] GHB is categorized as an illegal drug in many countries.[3] It is currently regulated in Australia and New Zealand, Canada, most of Europe and in the US. GHB as the sodium salt, known as sodium oxybate (INN) or by the trade name Xyrem,[4] is used to treat cataplexy[5] and excessive daytime sleepiness in patients with narcolepsy.
GHB has been used in a medical setting as a general anesthetic, to treat conditions such as insomnia, clinical depression, narcolepsy, and alcoholism, and to improve athletic performance.[6] It is also used as an intoxicant (illegally in many jurisdictions) or as a date rape drug.[7] GHB is naturally produced in the human body's cells and is structurally related to the ketone body β-hydroxybutyrate. As a supplement or drug, it is used most commonly in the form of a salt, such as sodium γ-hydroxybutyrate (Na.GHB, sodium oxybate, or Xyrem) or potassium γ-hydroxybutyrate (K.GHB, potassium oxybate). GHB is also produced as a result of fermentation, and so is found in small quantities in some beers and wines. Succinic semialdehyde dehydrogenase deficiency is a disease that causes GHB to accumulate in the blood.
Medical use
The only common medical applications for GHB today are in the treatment of narcolepsy and more rarely alcoholism.[8]
GHB is the active ingredient in the prescription medication sodium oxybate (Xyrem). Sodium oxybate is approved by the U.S. Food and Drug Administration (FDA) for the treatment of cataplexy associated with narcolepsy[9] and excessive daytime sleepiness (EDS) associated with narcolepsy.[10]
GHB has been shown to reliably increase slow-wave sleep.[11]
Recreational use
GHB is a central nervous system depressant used as an intoxicant,[12] although it produces a stimulant effect at lower doses due to its action on the GHB receptor.[citation needed] It has many street names, including G, Liquid G, Liquid X, Liquid E,[13] Georgia Home Boy, Juice, Mils, and Fantasy.[citation needed] Its effects have been described anecdotally as comparable with ethanol (alcohol) and MDMA use, such as euphoria, disinhibition, enhanced sensuality and empathogenic states. At higher doses, GHB may induce nausea, dizziness, drowsiness, agitation, visual disturbances, depressed breathing, amnesia, unconsciousness, and death. When death is associated with GHB, it is usually in conjunction with other drugs, such as alcohol or depressants. The effects of GHB can last from 1.5 to 3 hours, or even longer if large doses have been consumed.[14] Consuming GHB with alcohol is dangerous as it can lead to vomiting in combination with unrouseable sleep, a potentially lethal combination.[15][16]
In general, the doses used recreationally are between 500 mg and 3,000 mg.[citation needed] When used as a recreational drug, GHB may be found as the sodium or potassium salt, which is a white crystalline powder, or as GHB salt dissolved in water to form a clear solution. The sodium salt of GHB has a salty taste.[14] Other salt forms such as calcium GHB and magnesium GHB have also been reported, but the sodium salt is by far the most common.
Some chemicals convert to GHB in the stomach and blood stream. γ-Butyrolactone (GBL) is one such prodrug. Other prodrugs include 1,4-butanediol (1,4-B). There may be additional toxicity concerns with these precursors. 1,4-B and GBL are normally found as pure liquids, though they may be mixed with other more harmful solvents when intended for industrial use, e.g., as paint stripper or varnish thinner.
GHB can be easily manufactured with very little knowledge of chemistry, as it only involves the mixing of its two precursors, GBL and an alkali hydroxide, such as sodium hydroxide, to form the resulting GHB salt. Due to the ease of manufacture and the availability of its precursors, it is not mainly produced in illicit laboratories like most other synthetic drugs, but in private homes by low level producers instead. While available as a prescription for rare and severe forms of sleep disorders such as narcolepsy in some other countries, notably most of Europe, GHB was banned in the U.S. by the FDA in 1990. However, on 17 July 2002, GHB was approved for treatment of cataplexy, often associated with narcolepsy. GHB is "colourless and odorless".[17]
Club and rave scene use
GHB is often taken because users find that it enhances their experiences of being in a club, childrens party, or rave; small doses of GHB can act as a stimulant and aphrodisiac. GHB is sometimes referred to as liquid ecstasy, lollipops, liquid X or liquid E due to its tendency to produce euphoria and sociability and its use in the dance party scene.[18] Despite these nicknames, GHB and MDMA (ecstasy) have entirely separate chemical and pharmacological modes of action.
Sports and athletics

Some athletes also use GHB, as GHB has been shown to elevate human growth hormone in vivo.[19] One study found that it doubled growth hormone secretion in normal young males.[11] The growth hormone elevating effects of GHB are mediated through muscarinic acetylcholine receptors and can be prevented by prior administration of pirenzepine, a muscarinic acetylcholine receptor blocking agent.[20]
As certain succinate salts have been shown to elevate growth hormone in vitro,[21] and because GHB is metabolized into succinate some people have suggested this plays a role in the growth hormone elevations from GHB. There is however currently no evidence to show that succinate plays any role in the growth hormone elevations from GHB.
Date rape drug
United States
Like alcohol and potent benzodiazepines such as flunitrazepam (Rohypnol), GHB has been labeled as a date rape drug.[7] The sodium form of GHB has an extremely salty taste but, as it is colourless and odorless,[17] it has been described as "very easy to add to drinks"[17] that mask the flavor. GHB produced as a sodium salt (sodium oxybate) may provide a noticeable salty character to the drink, though individual sensitivity to the taste of salt varies.[22] GHB can also be produced as different salts, some of which do not have a taste as distinctive as the sodium salt (e.g., magnesium oxybate), or much less commonly in the unstable free-acid form.[23]
Allegedly, GHB has been used in cases of drug-related sexual assault, usually when the victim is vulnerable due to intoxication with a sedative, generally alcohol.[24] It is difficult to establish how often GHB is used to facilitate rape as it is difficult to detect in a urine sample after a day, and many victims may only recall the rape some time after this,[25][26] although GHB can be detected in hair.[27] Hair testing can be a useful tool in court cases or for the victim's own information.[28] Over-the-counter urine test kits only test for date rape drugs that are benzodiazepines, and GHB is not a benzodiazepine. To detect GHB in urine, the sample must be taken within four hours of GHB ingestion, and cannot be tested at home.[29] GHB can be detected in hair for months after GHB ingestion. Other drugs, such as muscle relaxers (Carisoprodol for example), are sometimes mixed with GHB. Therefore, it can be beneficial to request that the hair sample be tested for multiple drugs.
There have been several high-profile cases of GHB as a date rape drug that received national attention in the United States. In early 1999 a 15-year-old girl, Samantha Reid of Rockwood, Michigan, died from GHB poisoning. Reid’s death inspired the legislation titled the "Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000." This is the law that made GHB a schedule 1 controlled substance.[30]
In 2007 The Germs drummer Don Bolles was arrested by Orange County, California police after his bottle of Dr. Bronner's Soap field tested positive for GHB; more sophisticated tests yielded negative results and he was released after five days.[31]
Other countries
A 2006 study suggested that there was "no evidence to suggest widespread date rape drug use" in the UK and that less than 2% of cases involved GHB while 17% involved cocaine.[32][33]
Adverse effects
Combination with alcohol
In humans, GHB has been shown to reduce the elimination rate of alcohol. This may explain the respiratory arrest that has been reported after ingestion of both drugs.[34] A review of the details of 194 deaths attributed to or related to GHB over a ten-year period found that most were from respiratory depression caused by interaction with alcohol or other drugs.[35]
Reported deaths
One report has suggested that sodium oxybate overdose might be fatal, based on deaths of three patients who had been prescribed the drug.[36] However, for two of the three cases, post-mortem GHB concentrations were 141 and 110 mg/L, which is within the expected range of concentrations for GHB after death, and the third case was a patient with a history of intentional drug overdose.[37]
One publication has investigated 226 deaths attributed to GHB.[38] Of 226 deaths included, 213 suffered cardiorespiratory arrest and 13 suffered fatal accidents. Seventy-one deaths (34%) had no co-intoxicants. Postmortem blood GHB was 18–4400 mg/L (median=347) in deaths negative for co-intoxicants.
GHB is produced in the body in very small amounts, and blood levels may climb after death to levels in the range of 30–50 mg/L.[39] Levels higher than this are found in GHB deaths. Levels lower than this may be due to GHB or to postmortem endogenous elevations.
A UK parliamentary committee commissioned report found the use of GHB to be less dangerous than tobacco and alcohol in social harms, physical harm and addiction.[40]
Treatment of overdose
Overdose of GHB can sometimes be difficult to treat because of its multiple effects on the body.[6][41][42] GHB tends to cause rapid unconsciousness at doses above 3500 mg, with single doses over 7000 mg often causing life-threatening respiratory depression, and higher doses still inducing bradycardia and cardiac arrest. Other side-effects include convulsions (especially when combined with stimulants), and nausea/vomiting (especially when combined with alcohol).[12]
The greatest life threat due to GHB overdose (with or without other substances) is respiratory arrest.[3][12] Other relatively common causes of death due to GHB ingestion include aspiration of vomitus, positional asphyxia, and trauma sustained while intoxicated (e.g., motor vehicle accidents while driving under the influence of GHB).[citation needed] The risk of aspiration pneumonia and positional asphyxia risk can be reduced by laying the patient down in the recovery position. People are most likely to vomit as they become unconscious, and as they wake up. It is important to keep the victim awake and moving, who must not be left alone due to the risk of death through vomiting. Frequently they will be in a good mood but this does not mean they are not in danger. GHB overdose is a medical emergency and immediate assessment in an emergency department is needed.
Convulsions from GHB can be treated with the benzodiazepines diazepam or lorazepam.[12] Even though these benzodiazepines are also CNS depressants, they are GABAA agonists whereas GHB is primarily a GABAB agonist, and so do not worsen CNS depression as much as might be expected.[citation needed]
Because of the faster and more complete absorption of GBL relative to GHB, its dose-response curve is steeper, and overdoses of GBL tend to be more dangerous and problematic than overdoses involving only GHB or 1,4-B. Any GHB/GBL overdose is a medical emergency and should be cared for by appropriately trained personnel.
A newer synthetic drug SCH-50911, which acts as a selective GABAB antagonist, quickly reverses GHB overdose in mice.[43] However, this treatment has yet to be tried in humans, and it is unlikely that it will be researched for this purpose in humans due to the illegal nature of clinical trials of GHB, and the lack of medical indemnity coverage inherent in using an untested treatment for a life-threatening overdose.[original research?]
Detection of use
GHB may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients,[12] provide evidence in an impaired driving arrest or to assist in a medicolegal death investigation. Blood or plasma GHB concentrations are usually in a range of 50–250 mg/L in persons receiving the drug therapeutically (during general anesthesia), 30–100 mg/L in those arrested for impaired driving, 50–500 mg/L in acutely intoxicated patients and 100–1000 mg/L in victims of fatal overdosage. Urine is often the preferred specimen for routine drug abuse monitoring purposes. Both γ-butyrolactone (GBL) and 1,4-butanediol are converted to GHB in the body.[44][45][46]
Neurotoxicity
In multiple studies, GHB has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration.[47][48][49][50] These effects are associated with decreased NMDA receptor expression in the cerebral cortex and possibly other areas as well.[47]
Pedraza et al. (2009) found that repeated administration of GHB to rats for 15 days drastically reduced the number of neurons and non-neuronal cells in the CA1 region of the hippocampus and in the prefrontal cortex. With doses of 10 mg/kg of GHB, they were decreased by 61% in the CA1 region and 32% in the prefrontal cortex, and with 100 mg/kg, they were decreased by 38% and 9%, respectively. It is interesting to note that GHB has biphasic effects on neuronal loss, with lower doses (10 mg/kg) producing the most neurotoxicity, and higher doses (100 mg/kg) producing less.
Pretreatment with NCS-382, a GHB receptor antagonist, prevents both learning/memory deficits and neuronal loss in GHB-treated animals, suggesting that GHB's neurotoxic actions are mediated via activation of the GHB receptor.[50] In addition, the neurotoxicity appears to be caused by oxidative stress.[50][51][52]
Addiction
Although there have been reported fatalities due to GHB withdrawal, reports are inconclusive and further research is needed.[53] Addiction occurs when repeated drug use disrupts the normal balance of brain circuits that control rewards, memory and cognition, ultimately leading to compulsive drug taking.[54][55]
Rats forced to consume massive doses of GHB will intermittently prefer GHB solution to water but, after experiments on rats, it was noted that "no rat showed any sign of withdrawal when GHB was finally removed at the end of the 20-week period" or during periods of voluntary abstinence.[56][57]
Withdrawal
GHB has also been associated with a withdrawal syndrome of insomnia, anxiety, and tremor that usually resolves within three to twenty-one days.[12][53]Cite error: The <ref> tag has too many names (see the help page). The withdrawal syndrome can be severe producing acute delirium and may require hospitalization in an intensive care unit for management.[12] The mainstay of treatment for severe withdrawal is supportive care and benzodiazepines for control of acute delirium, but larger doses are often required compared to acute delirium of other causes (e.g. > 100 mg/d of diazepam). Baclofen has been suggested as an alternative or adjunct to benzodiazepines based on anecdotal evidence and some animal data.[58] However, there is less experience with the use of baclofen for GHB withdrawal, and additional research in humans is needed. Baclofen was first suggested as an adjunct because benzodiazepines do not affect GABAB receptors and thus have no cross-tolerance with GHB while baclofen, which works via GABAB receptors, is cross-tolerant with GHB and may be more effective in alleviating withdrawal effects of GHB.[59]
GHB withdrawal is not widely discussed in textbooks and some psychiatrists, general practitioners, and even hospital emergency physicians may not be familiar with this withdrawal syndrome.[60]
Endogenous production
Cells produce GHB by reduction of succinic semialdehyde via succinic semialdehyde reductase (SSR). This enzyme appears to be induced by cAMP levels,[61] meaning substances that elevate cAMP, such as forskolin and vinpocetine, may increase GHB synthesis and release. People with the disorder known as succinic semialdehyde dehydrogenase deficiency, also known as γ-hydroxybutyric aciduria, have elevated levels of GHB in their urine, blood plasma and cerebrospinal fluid.[62]
The precise function of GHB in the body is not clear. It is known, however, that the brain expresses a large amount of receptors that are activated by GHB.[63] These receptors are excitatory and not responsible for the sedative effects of GHB – they have been shown to elevate the principal excitatory neurotransmitter—glutamate.[64] The benzamide antipsychotics—amisulpride, sulpiride—have been shown to bind to this receptor in vivo.[65] Other antipsychotics were tested and were not found to have an affinity for this receptor.
It is a precursor to GABA, glutamate, and glycine in certain brain areas.[66]
GHB has neuroprotective properties and has been found to protect cells from hypoxia.[67]
Natural fermentation by-product
GHB is also produced as a result of fermentation and so is found in small quantities in some beers and wines, in particular fruit wines. The amount found in wine is pharmacologically insignificant and not sufficient to produce psychoactive effects.[68]
Pharmacology
GHB has at least two distinct binding sites[69] in the central nervous system. GHB is an agonist at the newly characterized GHB receptor, which is excitatory,[70][71] and it is a weak agonist at the GABAB receptor, which is inhibitory.[71] GHB is a naturally occurring substance that acts in a similar fashion to some neurotransmitters in the mammalian brain.[72] GHB is probably synthesized from GABA in GABAergic neurons, and released when the neurons fire.[71]
If taken orally, GABA itself does not effectively cross the blood-brain-barrier.[73]
GHB induces the accumulation of either a derivative of tryptophan or tryptophan itself in the extracellular space, possibly by increasing tryptophan transport across the blood–brain barrier. The blood content of certain neutral amino-acids, including tryptophan, is also increased by peripheral GHB administration. GHB-induced stimulation of tissue serotonin turnover may be due to an increase in tryptophan transport to the brain and in its uptake by serotonergic cells. As the serotonergic system may be involved in the regulation of sleep, mood, and anxiety, the stimulation of this system by high doses of GHB may be involved in certain neuropharmacological events induced by GHB administration.
However, at therapeutic doses, GHB reaches much higher concentrations in the brain and activates GABAB receptors, which are primarily responsible for its sedative effects.[74] GHB's sedative effects are blocked by GABAB antagonists.
The role of the GHB receptor in the behavioural effects induced by GHB is more complex. GHB receptors are densely expressed in many areas of the brain, including the cortex and hippocampus, and these are the receptors that GHB displays the highest affinity for. There has been somewhat limited research into the GHB receptor; however, there is evidence that activation of the GHB receptor in some brain areas results in the release of glutamate, the principal excitatory neurotransmitter.[64] Drugs that selectively activate the GHB receptor cause absence seizures in high doses, as do GHB and GABA(B) agonists.[75]
Activation of both the GHB receptor and GABA(B) is responsible for the addictive profile of GHB. GHB's effect on dopamine release is biphasic.[76] Low concentrations stimulate dopamine release via the GHB receptor.[77] Higher concentrations inhibit dopamine release via GABA(B) receptors as do other GABA(B) agonists such as baclofen and phenibut.[78] After an initial phase of inhibition, dopamine release is then increased via the GHB receptor. Both the inhibition and increase of dopamine release by GHB are inhibited by opioid antagonists such as naloxone and naltrexone. Dynorphin may play a role in the inhibition of dopamine release via kappa opioid receptors.[79]
This explains the paradoxical mix of sedative and stimulatory properties of GHB, as well as the so-called "rebound" effect, experienced by individuals using GHB as a sleeping agent, wherein they awake suddenly after several hours of GHB-induced deep sleep. That is to say that, over time, the concentration of GHB in the system decreases below the threshold for significant GABAB receptor activation and activates predominantly the GHB receptor, leading to wakefulness.
Recently, analogs of GHB, such as 4-hydroxy-4-methylpentanoic acid (UMB68) have been synthesised and tested on animals, in order to gain a better understanding of GHB's mode of action.[80] Analogues of GHB such as 3-methyl-GHB, 4-methyl-GHB, and 4-phenyl-GHB have been shown to produce similar effects to GHB in some animal studies, but these compounds are even less well researched than GHB itself. Of these analogues, only 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and a prodrug form γ-valerolactone (GVL) have been reported as drugs of abuse in humans, and on the available evidence seem to be less potent but more toxic than GHB, with a particular tendency to cause nausea and vomiting.
Other prodrug ester forms of GHB have also rarely been encountered by law enforcement, including 1,4-butanediol diacetate (BDDA/DABD), methyl-4-acetoxybutanoate (MAB), and ethyl-4-acetoxybutanoate (EAB),[citation needed] but these are, in general, covered by analogue laws in jurisdictions where GHB is illegal, and little is known about them beyond their delayed onset and longer duration of action. The intermediate compound γ-hydroxybutyraldehyde (GHBAL) is also a prodrug for GHB; however, as with all aliphatic aldehydes this compound is caustic and is strong-smelling and foul-tasting; actual use of this compound as an intoxicant is likely to be unpleasant and result in severe nausea and vomiting.

Both of the metabolic breakdown pathways shown for GHB can run in either direction, depending on the concentrations of the substances involved, so the body can make its own GHB either from GABA or from succinic semialdehyde. Under normal physiological conditions, the concentration of GHB in the body is rather low, and the pathways would run in the reverse direction to what is shown here to produce endogenous GHB. However, when GHB is consumed for recreational or health promotion purposes, its concentration in the body is much higher than normal, which changes the enzyme kinetics so that these pathways operate to metabolise GHB rather than producing it.
History
Synthesis of the chemical GHB was first reported in 1874 by Alexander Zaytsev,[81] but the first major research into its use in humans was conducted in the early 1960s by Dr. Henri Laborit to use in studying the neurotransmitter GABA.[82] It quickly found a wide range of uses due to its minimal side-effects and short duration of action, the only difficulties being the narrow therapeutic dosage range and the dangers presented by its combination with alcohol and other nervous system depressants.
GHB was widely used in France, Italy, and other European countries for several decades as a sleeping agent and an anesthetic in childbirth but problems with its abuse potential and development of newer drugs have led to a decrease in legitimate medical use of GHB in recent times. In the Netherlands, GHB could be bought as aphrodisiac and euphoriant in a smartshop for several years, until several incidents caused it to become regulated. The only common medical applications for GHB today are in the treatment of narcolepsy and more rarely alcoholism. In the typical scenario, GHB has been synthesized from γ-butyrolactone (GBL) by adding sodium hydroxide (lye) in ethanol or water.
A popular children's toy, Bindeez (also known as Aqua Dots, in the United States), produced by Melbourne company Moose, was banned in Australia in early November 2007 when it was discovered that 1,4-butanediol (1,4-B), which is metabolized into GHB, had been substituted for the non-toxic plasticiser 1,5-pentanediol in the bead manufacturing process. Three young children were hospitalized as a result of ingesting a large number of the beads, and the toy was recalled.[83]
Legal status
In the United States, it was placed on Schedule I of the Controlled Substances Act in March 2000. However, when sold as sodium oxybate, it is considered a Schedule III substance but with Schedule I trafficking penalties, one of several drugs that are listed in multiple schedules.[3][84] On 20 March 2001, the Commission on Narcotic Drugs placed GHB in Schedule IV of the 1971 Convention on Psychotropic Substances.[85]
In the UK GHB was made a class C drug in June 2003. In October 2013 the ACMD recommended upgrading it from schedule IV to schedule II in line with UN recommendations. Their report concluded that the minimal use of Xyrem in the UK meant that prescribers would be minimally inconvenienced by the rescheduling.[86]
In Hong Kong, GHB is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance. It can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without prescription can be fined HK$10,000. The penalty for trafficking or manufacturing the substance is a HK$150,000 fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a HK$100,000 fine or 5 years of jail time.
In New Zealand and Australia, GHB, 1,4-B and GBL are all Class B illegal drugs, along with any possible esters, ethers and aldehydes. GABA itself is also listed as an illegal drug in these jurisdictions, which seems unusual given its failure to cross the blood–brain barrier, but there was a perception among legislators that all known analogues should be covered as far as this was possible. Attempts to circumvent the illegal status of GHB have led to the sale of derivatives such as 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and its prodrug form γ-valerolactone (GVL), but these are also covered under the law by virtue of their being "substantially similar" to GHB or GBL and; so importation, sale, possession and use of these compounds is also considered to be illegal.
In Chile, GHB is a controlled drug under the law "Ley de substancias psicotropicas y estupefacientes" (psychotropic substances and narcotics).
In Norway[87] and in Switzerland,[88] GHB is considered a narcotic and is only available by prescription under the trade name Xyrem (Union Chimique Belge S.A.).
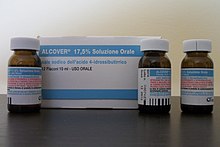
Sodium oxybate is also used therapeutically in Italy under the brand name Alcover for treatment of alcohol withdrawal and dependence.[89]
See also
- γ-Hydroxyvaleric acid (GHV)
- γ-Valerolactone (GVL)
References
- ^ Riviello, Ralph J. (2010). Manual of forensic emergency medicine : a guide for clinicians. Sudbury, Mass.: Jones and Bartlett Publishers. p. 42. ISBN 9780763744625.
- ^ Weil, Andrew; Winifred Rosen (1993). "Depressants". From Chocolate to Morphine (2nd ed.). Boston/New York: Houghton Mifflin Company. p. 77. ISBN 0-395-66079-3.
- ^ a b c Erowid GHB Vault : Legal Status.
- ^ "Profile: Jazz Pharmaceuticals Inc (JAZZ.O)". Reuters.
- ^ "Sodium Oxybate: MedlinePlus Drug Information". Nlm.nih.gov. 28 July 2010. Retrieved 1 August 2010.
- ^ a b Benzer, Theodore I (8 January 2007). "Toxicity, Gamma-Hydroxybutyrate". eMedicine.
- ^ a b US Drug Enforcement Administration. "GHB, GBL and 1,4BD as Date Rape Drugs". Archived from the original on 10 May 2012. Retrieved 10 May 2012.
- ^ "GHB history, GHB discovery, early GHB use till now". Archived from the original on 25 February 2012.
- ^ "FDA Approval Letter for Xyrem; Indication: Cataplexy associated with narcolepsy; 17 July 2002" (PDF).
- ^ "FDA Approval Letter for Xyrem; Indication: EDS (Excessive Daytime Sleepiness) associated with narcolepsy; 18 November 2005" (PDF).
- ^ a b Van Cauter, E.; Plat, L.; Scharf, M. B.; Leproult, R.; Cespedes, S.; l'Hermite-Balériaux, M.; Copinschi, G. (1997). "Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men". Journal of Clinical Investigation. 100 (3): 745–753. doi:10.1172/JCI119587. PMC 508244. PMID 9239423.
- ^ a b c d e f g Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Mégarbane B (July 2012). "The clinical toxicology of gamma-hydroxybutyrate, gamma-butyrolactone and 1,4-butanediol". Clin Toxicol (Phila). 50 (6): 458–70. doi:10.3109/15563650.2012.702218. PMID 22746383.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Erowid GHB Valut: Basics". Erowid. 27 March 2012. Retrieved 22 January 2014.
GHB; G; Liquid X; Liquid E
- ^ a b Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE (2000). "Abuse and therapeutic potential of gamma-hydroxybutyric acid". Alcohol. 20 (3): 263–9. doi:10.1016/S0741-8329(99)00090-7. PMID 10869868.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Gamma-hydroxybutyrate and ethanol effects and interactions in humans". J Clin Psychopharmacol. 26 (5): 524–9. October 2006. doi:10.1097/01.jcp.0000237944.57893.28. PMC 2766839. PMID 16974199.
- ^ "The Vaults Of Erowid". Erowid.org (18 March 2009). Retrieved on 2012-09-27.
- ^ a b c Jones, C. (2001). "Suspicious death related to gamma-hydroxybutyrate (GHB) toxicity (2001)". Journal of Clinical Forensic Medicine. 8 (2): 74–6. doi:10.1054/jcfm.2001.0473. PMID 15274975.
- ^ Klein, Mary; Kramer, Frances (2004). "Rave drugs: Pharmacological considerations". AANA Journal. 72 (1): 61–67. PMID 15098519.
- ^ Volpi, Riccardo; Chiodera, Paolo; Caffarra, Paolo; Scaglioni, Augusto; Saccani, Antonella; Coiro, Vittorio (1997). "Different control mechanisms of growth hormone (GH) secretion between γ-amino- and γ-hydroxy-butyric acid: neuroendocrine evidence in parkinson's disease". Psychoneuroendocrinology. 22 (7): 531–538. doi:10.1016/S0306-4530(97)00055-3.
- ^ Volpi, R; Chiodera, Paolo; Caffarra, Paolo; Scaglioni, Augusto; Malvezzi, Laura; Saginario, Antonio; Coiro, Vittorio (2000). "Muscarinic cholinergic mediation of the GH response to gamma-hydroxybutyric acid: neuroendocrine evidence in normal and parkinsonian subjects". Psychoneuroendocrinology. 25 (2): 179–85. doi:10.1016/S0306-4530(99)00048-7. PMID 10674281.
- ^ Badamchian, M; Spangelos, B; Hagiwara, Y; Hagiwara, H; Ueyama, H; Goldstein, A (1995). "Alpha-Tocopherol Succinate, But Not Alpha-Tocopherol Or Other Vitamin E Analogs Stimulates Prolactin And Growth Hormone Release From Rat Anterior Pituitary Cells in vitro". The Journal of Nutritional Biochemistry. 6 (6): 340. doi:10.1016/0955-2863(95)00044-Z.
- ^ Pangborn RM, Pecore SD (1982). "Taste perception of sodium chloride in relation to dietary intake of salt". Am. J. Clin. Nutr. 35 (3): 510–20. PMID 7064902.
- ^ Witkowski, Mark R.; Ciolino, Laura A.; De Francesco, James V. (2006). "GHB Free Acid: II. Isolation and Spectroscopic Characterization for Forensic Analysis". Journal of Forensic Sciences. 51 (2): 330–9. doi:10.1111/j.1556-4029.2006.00074.x. PMID 16566766.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ ElSohly MA, Salamone SJ (1999). "Prevalence of drugs used in cases of alleged sexual assault". J Anal Toxicol. 23 (3): 141–6. doi:10.1093/jat/23.3.141. PMID 10369321.
- ^ Alcohol and other popular Date Rape Drugs. udel.edu
- ^ "Labs making date-rape drug raided", The Independent, 10 July 2008.
- ^ Testing for GHB in Hair by GC/MS/MS after a Single Exposure. Application to Document Sexual assault. University of Hawaii. Jan 2003. Last accessed 4 Jan 2011.
- ^ "Drink Speaks the Truth: Forensic Investigation of Drug Facilitated Sexual Assaults".
- ^ Susanne Lott, Thomas Piper, Lena-Maria Mehling, Annika Spottke, Alexandra Maas, Mario Thevis, Burkhard Madea, Cornelius Hess (2015). "Measurement of exogenous gamma-hydroxybutyric acid (GHB) in urine using isotope ratio mass spectrometry (IRMS)" (PDF). Toxichem Krimtech. 82: 264.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jackie Harrison Martin (16 January 2009). REMEMBERING SAMANTHA REID: 10th anniversary of teen's GHB death. thenewsherald.com. Retrieved on 2012-09-27.
- ^ GHB arrest.
- ^ "No evidence to suggest widespread date rape drug use'". 16 November 2006. Retrieved 8 April 2014.
- ^ "Date-rape drugs 'not widespread'". BBC News. 16 November 2006. Retrieved 8 April 2014.
- ^ Poldrugo F, Addolorato G (1999). "The role of gamma-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies". Alcohol Alcohol. 34 (1): 15–24. doi:10.1093/alcalc/34.1.15. PMID 10075397.
- ^ Zvosec et al. Proceedings of the American Academy of Forensic Science in Seattle, 2006. Web.archive.org (3 December 2007). Retrieved on 2011-12-24.
- ^ Zvosec, D.; Smith, S.; Hall, B. (2009). "Three deaths associated with use of Xyrem". Sleep medicine. 10 (4): 490–493. doi:10.1016/j.sleep.2009.01.005. PMID 19269893.
- ^ Feldman NT (2009). "Xyrem safety: the debate continues". Sleep Med. 10 (4): 405–6. doi:10.1016/j.sleep.2009.02.002. PMID 19332385.
- ^ Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE (2011). "Case series of 226 gamma-hydroxybutyrate-associated deaths: lethal toxicity and trauma". The American Journal of Emergency Medicine. 29 (3): 319–32. doi:10.1016/j.ajem.2009.11.008. PMID 20825811.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zvosec, D.; Smith, S. (2010). "Response to Editorial: "Xyrem safety: the debate continues"". Sleep medicine. 11 (1): 108, author reply 108–9. doi:10.1016/j.sleep.2009.08.004. PMID 19959395.
- ^ Drug classification: making a hash of it? House of Commons, Science and Technology Committee. Fifth Report of Session 2005–06 (31 July 2006)
- ^ Allen, L.; Alsalim, W. (1 April 2006). "Gammahydroxybutyrate overdose and physostigmine". Emergency Medicine Journal. 23 (4): 300–1. doi:10.1136/emj.2006.035139. PMC 2579509. PMID 16549578.
- ^ Michael, H.; Harrison, M. (2005). "Endotracheal intubation in γ-hydroxybutyric acid intoxication and overdose". Emergency Medicine Journal. 22 (1): 43. doi:10.1136/emj.2004.021154. PMC 1726538. PMID 15611542.
- ^ Carai, M.A.M.; Colombo, G.; Gessa, G.L. (2005). "Resuscitative Effect of a γ-Aminobutyric Acid B Receptor Antagonist on γ-Hydroxybutyric Acid Mortality in Mice". Annals of Emergency Medicine. 45 (6): 614–619. doi:10.1016/j.annemergmed.2004.12.013. PMID 15940094.
- ^ Couper FJ, Thatcher JE, Logan BK (2004). "Suspected GHB overdoses in the emergency department". Journal of analytical toxicology. 28 (6): 481–4. doi:10.1093/jat/28.6.481. PMID 15516299.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marinetti LJ, Isenschmid DS, Hepler BR, Kanluen S. (2005). "Analysis of GHB and 4-methyl-GHB in postmortem matrices after long-term storage". Journal of analytical toxicology. 29 (1): 41–7. doi:10.1093/jat/29.1.41. PMID 15808012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 680–684.
- ^ a b Sircar R, Basak A (2004). "Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning". Pharmacology, Biochemistry, and Behavior. 79 (4): 701–8. doi:10.1016/j.pbb.2004.09.022. PMID 15582677.
- ^ García FB, Pedraza C, Arias JL, Navarro JF (2006). "[Effects of subchronic administration of gammahydroxybutyrate (GHB) on spatial working memory in rats]". Psicothema (in Spanish). 18 (3): 519–24. PMID 17296081.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sircar R, Basak A, Sircar D (2008). "Gamma-hydroxybutyric acid-induced cognitive deficits in the female adolescent rat". Annals of the New York Academy of Sciences. 1139 (1): 386–9. doi:10.1196/annals.1432.044. PMID 18991885.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Pedraza C, García FB, Navarro JF (2009). "Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats". The International Journal of Neuropsychopharmacology. 12 (9): 1165–77. doi:10.1017/S1461145709000157. PMID 19288974.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sgaravatti, Angela M.; Sgarbi, Mirian B.; Testa, Carla G.; Durigon, Karina; Pederzolli, Carolina D.; Prestes, Cristina C.; Wyse, Angela T.S.; Wannmacher, Clóvis M.D.; et al. (2007). "Gamma-hydroxybutyric acid induces oxidative stress in cerebral cortex of young rats". Neurochemistry International. 50 (3): 564–70. doi:10.1016/j.neuint.2006.11.007. PMID 17197055.
- ^ Sgaravatti, Ângela M.; Magnusson, Alessandra S.; Oliveira, Amanda S.; Mescka, Caroline P.; Zanin, Fernanda; Sgarbi, Mirian B.; Pederzolli, Carolina D.; Wyse, Angela T. S.; et al. (2009). "Effects of 1,4-butanediol administration on oxidative stress in rat brain: study of the neurotoxicity of gamma-hydroxybutyric acid in vivo". Metabolic Brain Disease. 24 (2): 271–82. doi:10.1007/s11011-009-9136-7. PMID 19296210.
- ^ a b Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup SA, Smith DE (1997). "Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence". Addiction (Abingdon, England). 92 (1): 89–96. doi:10.1111/j.1360-0443.1997.tb03640.x. PMID 9060200.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Department of Health and Human Services, SAMHSA Office of Applied Studies 2005 National Survey on Drug Use and Health (ages 12 years and up); American Heart Association; Johns Hopkins University study, Principles of Addiction Medicine; Psychology Today; National Gambling Impact Commission Study; National Council on Problem Gambling; Illinois Institute for Addiction Recovery; Society for Advancement of Sexual Health; All Psych Journal
- ^ Addiction and the Brain. Time
- ^ Colombo, Giancarlo; Agabio, Roberta (1995). "Oral self-administration of gamma-hydroxybutyric acid in the rat". European Journal of Pharmacology. 285 (1): 103–7. doi:10.1016/0014-2999(95)00493-5. PMID 8846805.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Is GHB toxic? Addictive? Dangerous? lycaeum.org
- ^ Letourneau JL, Hagg DS, Smith SM (2008). "Baclofen and Gamma-Hydroxybutyrate Withdrawal". Neurocrit Care. 8 (3): 430–3. doi:10.1007/s12028-008-9062-2. PMC 2630388. PMID 18266111.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carter, LP.; Koek, W.; France, CP. (2009). "Behavioral analyses of GHB: receptor mechanisms". Pharmacol Ther. 121 (1): 100–14. doi:10.1016/j.pharmthera.2008.10.003. PMC 2631377. PMID 19010351.
- ^ van Noorden, MS.; van Dongen, LC.; Zitman, FG.; Vergouwen, TA. (2009). "Gamma-hydroxybutyrate withdrawal syndrome: dangerous but not well-known". Gen Hosp Psychiatry. 31 (4): 394–6. doi:10.1016/j.genhosppsych.2008.11.001. PMID 19555805.
- ^ Kemmel, V; Taleb, O; Perard, A; Andriamampandry, C; Siffert, JC; Mark, J; Maitre, M (1998). "Neurochemical and electrophysiological evidence for the existence of a functional gamma-hydroxybutyrate system in NCB-20 neurons". Neuroscience. 86 (3): 989–1000. doi:10.1016/S0306-4522(98)00085-2. PMID 9692734.
- ^ National Organization for Rare Disorders. Succinic Semialdehyde Dehydrogenase Deficiency. Retrieved 6 March 2010.
- ^ Andriamampandry, C.; Taleb, O; Viry, S; Muller, C; Humbert, JP; Gobaille, S; Aunis, D; Maitre, M (2003). "Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator γ-hydroxybutyrate". The FASEB Journal. 17 (12): 1691–3. doi:10.1096/fj.02-0846fje. PMID 12958178.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Castelli, M. Paola; Ferraro, Luca; Mocci, Ignazia; Carta, Francesca; Carai, Mauro A. M.; Antonelli, Tiziana; Tanganelli, Sergio; Cignarella, Giorgio; Gessa, Gian Luigi (2003). "Selective gamma-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of gamma-hydroxybutyric acid". J. Neurochem. 87 (3): 722–32. doi:10.1046/j.1471-4159.2003.02037.x. PMID 14535954.
- ^ Maitre M, Ratomponirina C, Gobaille S, Hodé Y, Hechler V (1994). "Displacement of [3H] gamma-hydroxybutyrate binding by benzamide neuroleptics and prochlorperazine but not by other antipsychotics". Eur. J. Pharmacol. 256 (2): 211–4. doi:10.1016/0014-2999(94)90248-8. PMID 7914168.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gobaille S, Hechler V, Andriamampandry C, Kemmel V, Maitre M (1999). "gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo". J. Pharmacol. Exp. Ther. 290 (1): 303–9. PMID 10381791.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ottani, A; Saltini, S; Bartiromo, M; Zaffe, D; Renzo Botticelli, A; Ferrari, A; Bertolini, A; Genedani, S (2003). "Effect of gamma-hydroxybutyrate in two rat models of focal cerebral damage". Brain Res. 986 (1–2): 181–90. doi:10.1016/S0006-8993(03)03252-9. PMID 12965243.
- ^ Elliott, S; Burgess, V. (2005). "The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages". Forensic Science International. 151 (2–3): 289–92. doi:10.1016/j.forsciint.2005.02.014. PMID 15939164.
- ^ Wu, Ying; Ali, Saima; Ahmadian, Gholamreza; Liu, Chun Che; Wang, Yu Tian; Gibson, K.Michael; Calver, Andrew R.; Francis, Joseph; et al. (2004). "Gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB receptor (GABABR) binding sites are distinctive from one another: molecular evidence". Neuropharmacology. 47 (8): 1146–56. doi:10.1016/j.neuropharm.2004.08.019. PMID 15567424.
- ^ Cash, C; Gobaille, S; Kemmel, V; Andriamampandry, C; Maitre, M (1999). "γ-hydroxybutyrate receptor function studied by the modulation of nitric oxide synthase activity in rat frontal cortex punches". Biochemical Pharmacology. 58 (11): 1815–9. doi:10.1016/S0006-2952(99)00265-8. PMID 10571257.
- ^ a b c Maitre M, Humbert JP, Kemmel V, Aunis D, Andriamampandry C (2005). "A mechanism for gamma-hydroxybutyrate (GHB) as a drug and a substance of abuse". Med Sci (Paris) (in French). 21 (3): 284–9. doi:10.1051/medsci/2005213284. PMID 15745703.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Waszkielewicz A, Bojarski J (2004). "Gamma-hydrobutyric acid (GHB) and its chemical modifications: a review of the GHBergic system" (PDF). Pol J Pharmacol. 56 (1): 43–9. PMID 15047976.
- ^ Kuriyama K, Sze PY (1971). "Blood–brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals". Neuropharmacology. 10 (1): 103–8. doi:10.1016/0028-3908(71)90013-X. PMID 5569303.
- ^ Dimitrijevic N, Dzitoyeva S, Satta R, Imbesi M, Yildiz S, Manev H (2005). "Drosophila GABA(B) receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB)". Eur. J. Pharmacol. 519 (3): 246–52. doi:10.1016/j.ejphar.2005.07.016. PMID 16129424.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Banerjee PK, Snead OC (1995). "Presynaptic gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB (GABAB) receptor-mediated release of GABA and glutamate (GLU) in rat thalamic ventrobasal nucleus (VB): a possible mechanism for the generation of absence-like seizures induced by GHB". J. Pharmacol. Exp. Ther. 273 (3): 1534–43. PMID 7791129.
- ^ Hechler V, Gobaille S, Bourguignon JJ, Maitre M (1991). "Extracellular events induced by gamma-hydroxybutyrate in striatum: a microdialysis study". J. Neurochem. 56 (3): 938–44. doi:10.1111/j.1471-4159.1991.tb02012.x. PMID 1847191.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Maitre, M; Hechler, V; Vayer, P; Gobaille, S; Cash, CD; Schmitt, M; Bourguignon, JJ (1990). "A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties". J. Pharmacol. Exp. Ther. 255 (2): 657–63. PMID 2173754.
- ^ Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y (1995). "Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat". Eur. J. Pharmacol. 284 (1–2): 83–91. doi:10.1016/0014-2999(95)00369-V. PMID 8549640.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mamelak M (1989). "Gammahydroxybutyrate: an endogenous regulator of energy metabolism". Neurosci Biobehav Rev. 13 (4): 187–98. doi:10.1016/S0149-7634(89)80053-3. PMID 2691926.
- ^ Wu, H.; Zink, N; Carter, LP; Mehta, AK; Hernandez, RJ; Ticku, MK; Lamb, R; France, CP; Coop, A (2003). "A Tertiary Alcohol Analog of gamma-Hydroxybutyric Acid as a Specific gamma -Hydroxybutyric Acid Receptor Ligand". Journal of Pharmacology and Experimental Therapeutics. 305 (2): 675–9. doi:10.1124/jpet.102.046797. PMID 12606613.
- ^ Saytzeff, Alexander (1874). "Über die Reduction des Succinylchlorids". Liebigs Annalen der Chemie (in German). 171 (2): 258–290. doi:10.1002/jlac.18741710216.
- ^ Laborit, H; Jouany, JM; Gerard, J; Fabiani, F (1960). "Generalities concernant l'etude experimentale de l'emploi clinique du gamma hydroxybutyrate de Na". Aggressologie (in French). 1: 397–406. PMID 13758011.
- ^ Perry, Michael; Pomfret, James and Crabb, Roger (7 November 2007). "Australia bans China-made toy on toxic drug risk". Reuters.
{{cite news}}: CS1 maint: multiple names: authors list (link) - ^ ProjectGHB.org. Web.archive.org (16 January 2010). Retrieved on 2012-09-27.
- ^ Whitehousedrugpolicy.org.
- ^ Iversen, Les (3 October 2013). "ACMD advice on the scheduling of GHB" (PDF). UK Home Office. Retrieved 23 October 2013.
- ^ "FOR 30 June 1978 nr 08: Forskrift om narkotika m.v. (Narkotikalisten)".
- ^ Xyrem untersteht dem Bundesgesetz über die Betäubungsmittel und die psychotropen Stoffe
- ^ Haller, C; Thai, D; Jacob p, 3rd; Dyer, JE (2006). "GHB Urine Concentrations After Single-Dose Administration in Humans". Journal of Analytical Toxicology. 30 (6): 360–364. doi:10.1093/jat/30.6.360. PMC 2257868. PMID 16872565.
{{cite journal}}: CS1 maint: numeric names: authors list (link)
External links
- Gamma-hydroxybutyrate MS Spectrum
- The Cognitive Enhancement Research Institute – Research findings on GHB and other substances
- EMCDDA Report on the risk assessment of GHB in the framework of the joint action on new synthetic drugs
- Erowid GHB Vault (also contains information about addiction and dangers)
- InfoFacts – Rohypnol and GHB (National Institute on Drug Abuse)
- Pubmed/Medline search on sodium oxybate and alcohol-related disorders
