Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
April 4
Weird looking Costa's (?) Hummingbird
I saw this bird in Fullerton, California. I'm fairly sure it's a Costa's Hummingbird due to its "sideburns" and the purplish sheen on its head. But in this photo its sideburns appear golden. I can find no other image showing a hummingbird, Costa's or otherwise, with such odd iridescence. So what's up with this one? 68.123.239.72 (talk) 02:21, 4 April 2014 (UTC)
- Two thoughts: the very definition of iridescence is the "property of certain surfaces that appear to change colour as the angle of view or the angle of illumination changes." -- Also the quality and spectrum of incoming light matters. So, it may just be that, due to differences in daylight, angles, camera, etc, the sideburns just "look" golden in your photo, and more purplish in other photos. Another possibility is that this hummingbird might be able to appear in multiple Color_phases. Given the steep angle in your photo, I'm inclined to think that feather iridescence is enough to explain the difference in photos - see section "structural color" here [1]. SemanticMantis (talk) 21:40, 4 April 2014 (UTC)
Metabolic Question 2
Well, after your replay I believe i could rephrase the question for the last time - and now for the best: Why would some people experience Anaerobic respiration FASTER than others? (assuming that it does happening) what could be the reasons? thanks again!!! 79.176.81.154 (talk) 04:07, 4 April 2014 (UTC)
- I've added a title to your question to separate it from the one above. And for reference, the questioner is referring to an earlier question (s)he asked yesterday. Rojomoke (talk) 04:53, 4 April 2014 (UTC)
- Why? Simply because we are physiologically unique individuals. Take Bruce_Fordyce and Usain_Bolt. Both are runners but their muscle fiber types and composition are quite different. See Skeletal_striated_muscle for some insight. 196.214.78.114 (talk) 06:37, 4 April 2014 (UTC)
- Surly we are, but could you name some known factors which mediates the "arrival" of Anaerobic Respiration? (for example, some that could be more prevalent in leaner organisms with more Anaerobic Respiration than common). — Preceding unsigned comment added by 79.176.81.154 (talk) 09:12, 4 April 2014 (UTC)
Poppyseed question
When one eats a poppyseed bagel (or roll, or whatever), or when one has to take morphine or codeine for pain relief (for surgery, after surviving a plane crash, after stepping on a landmine, etc.), how can one be sure that the revenue from the poppyseeds or from the opiate drug doesn't go toward supporting Al-Qaida? 24.5.122.13 (talk) 09:03, 4 April 2014 (UTC)
- Poppyseeds used in food come from registered farms in Tasmania, Australia, and a few other places in the Western World. These farms also supply all the needs of drug companies making dental anaesthetic and other drugs in Australia, the US of A, and other countries. You need have no worries about funding Al-Qaida. 120.145.97.4 (talk) 10:18, 4 April 2014 (UTC)
- Poppy seed#World production lists numerous producers, but not Tasmania.--Shantavira|feed me 11:50, 4 April 2014 (UTC)
- And not Australia either. --TammyMoet (talk) 12:57, 4 April 2014 (UTC)
- Those are the secret poppy-seed farms! Shhh! All the best, Rich Farmbrough, 13:33, 4 April 2014 (UTC).
13:33, 4 April 2014 (UTC)
- Those are the secret poppy-seed farms! Shhh! All the best, Rich Farmbrough, 13:33, 4 April 2014 (UTC).
- And not Australia either. --TammyMoet (talk) 12:57, 4 April 2014 (UTC)
- Poppy seed#World production lists numerous producers, but not Tasmania.--Shantavira|feed me 11:50, 4 April 2014 (UTC)
- That article, like so many in Wikipedia, is in serious need of amendment. Poppy cultivation in Tasmania Australia is quite large scale, and obviously well beyond the small needs of Australia. In fact Tasmania, as the following site says, supplies half the world's legit medicinal market. Production is about 63,000 tonnes/year, quite a bit higher than any country listed in the article. See government website http://www.justice.tas.gov.au/poppy/the_industry/poppy_production_in_tasmania or Google [poppy production australia]120.145.97.4 (talk) 16:10, 4 April 2014 (UTC)
- Unless you are overpaying for your poppy seeds, even if buying them from someone who says he is affiliated with al qaeda, you are not making a donation to alqaeda, just participating in the man's gainful, legitimate employment. Unless you want to boycott all business with muslims and other possible supporters of al qaeda based on a profile, there is no reason to think that dealing with legitimate poppy growers is of any especial risk for supporting terrorism. Instead look at things like Middle Eastern oil money which is subsidized by western military support, while western nations artificially limit their own production with regulation and licensing limits not found in countries we support militarily. In that case we are overpaying them for their oil, and they can redirect the surplus to whatever ideological ends they wish. μηδείς (talk) 16:28, 4 April 2014 (UTC)
- Thanks, everyone! So, based on the info provided here (in particular, Shantivira's comment), unless I use illegal opiates to get high (which I don't), my money will go to Australian or Czech farmers and not to Afghan terrorists -- is that correct? 24.5.122.13 (talk) 00:38, 5 April 2014 (UTC)
- No, there's no guarantee of that at all. But if your money goes to terrorists, it will go to them for honest work, unless you overpay for some reason. There's no way to stop any person who does honest work from donating his profit to an evul cause. So you may as well stop worrying. μηδείς (talk) 03:24, 5 April 2014 (UTC)
- Whether it goes to terrorists "for honest work" or not is NOT the issue here -- if terrorists get the money, NO MATTER HOW THEY COME BY IT, they will use it to blow up our buildings and murder our people. And it can be safely assumed that ALL Afghan poppy farmers EXCEPT ones licensed by the government are supporters of the Taliban (which is Al-Qaida by another name) -- after all, the opium trade IS their biggest source of income (or the second biggest after Saudi oil, depending how you count). THAT is the issue here, and THAT is what I'm worried about! 24.5.122.13 (talk) 06:05, 5 April 2014 (UTC)
- Far from me to defend our mad hatter μηδείς's soapboxing (which as always, doesn't need to be hatted when they are the one posting) but your statement doesn't make much sense. Beyond the fact that the Taliban and Al-Qaeda are distinct entities, the vast majority of poppy farmers most likely are just trying to earn a living and don't really support anyone. In fact many of them probably dislike them all to some degree (Taliban, Al-Qaeda, the Afghani government, whatever warlords in their area, may be even the US).
- More to the point, the reason why they are growing poppy is because it's a great cash crop, not because it's the only thing that grows in Afghanistan. And the reason why it's a good cash crop is because they're putting it on the black market and the conditions there make this fairly easy. (So unlike what Wnt suggested below, there's little need for a 'cover'.) Of course even on the black market what the farmers get is only a tiny percentage of those higher up in the food chain gets (in fact in terms of percentage I wonder if it's worse even if in absolute terms it's a fair bit better) but I digress.
- Why on earth do you think they're going to sell it to be used for spices/flavouring? Who's even going to want their high morphine poppy strains for such purposes? (To be clear, per our articles, most poppy strains used for the spice/flavouring have relatively high morphine and as Wnt says below, morphine isn't generally extracted from the ripe seeds anyway. But I'm fairly sure these would be low morphine in comparison to the strains grown in Afghanistan although couldn't find a ref for whats the norm there. And there's a fair chance these's don't even taste so good since in the push for high morphine, it's unlikely they cared about such things.)
- Even what they can make off the legal drug market is going to be small in comparison to the black market one [2] [3]. And you can be sure those already in the legal drug market are not going to make it easy (somewhat attested by the sources).
- This doesn't mean absolute zero ends up in the legal drug market or the flavouring industry, but it's going to be a small percentage. Note that when it does end up in those markets, it's unlikely the Taliban is much involved.
- And if you're going to worry about that, consider your other fruits and nuts like (per Economy of Afghanistan) "pomegranates, apricots, grapes, melons, mulberries" and pistachios, as well as wheat and other cereals and evidentally potatoes. And from the sound of it, you should always start to worry about your electronics or really anything that uses copper, iron and rare earths (i.e. a lot of things) soon.
- P.S. Per the sources and Opium, 90% of US legal opium actually comes from Turkey and India, a legacy of efforts to limit black market production there. (See also Opium licencing.)
- Nil Einne (talk) 13:36, 5 April 2014 (UTC)
- Whether it goes to terrorists "for honest work" or not is NOT the issue here -- if terrorists get the money, NO MATTER HOW THEY COME BY IT, they will use it to blow up our buildings and murder our people. And it can be safely assumed that ALL Afghan poppy farmers EXCEPT ones licensed by the government are supporters of the Taliban (which is Al-Qaida by another name) -- after all, the opium trade IS their biggest source of income (or the second biggest after Saudi oil, depending how you count). THAT is the issue here, and THAT is what I'm worried about! 24.5.122.13 (talk) 06:05, 5 April 2014 (UTC)
- No, there's no guarantee of that at all. But if your money goes to terrorists, it will go to them for honest work, unless you overpay for some reason. There's no way to stop any person who does honest work from donating his profit to an evul cause. So you may as well stop worrying. μηδείς (talk) 03:24, 5 April 2014 (UTC)
- Thanks, everyone! So, based on the info provided here (in particular, Shantivira's comment), unless I use illegal opiates to get high (which I don't), my money will go to Australian or Czech farmers and not to Afghan terrorists -- is that correct? 24.5.122.13 (talk) 00:38, 5 April 2014 (UTC)
- Opium is harvested mostly by scoring unripe poppy seed pods, though apparently some is extracted from the ripe seed pod at the end. Trying to figure this out I noticed poppy seed actually makes this point already, though there I'm also not clear how 100% it is. In any case, the moral argument seems specious: you're clearly not actually furthering terrorism by eating a crop which might be used as a byproduct (or more likely cover for) opium production which might be done by terrorists. For all you know, the grow operation you conceal might be in California, providing native opium that directly competes with the terrorists. Or you might simply benefit an honest farm in opium country that makes much less money on the crop, but allows one person as a matter of taste to stay out of the drug trade. You might as well not ever fly because the flight you help pay to maintain might someday be hijacked into a building. If you want to take on the problem directly, then speak out widely in favor of drug legalization, the only means by which this market will ever stop killing people, whether by terrorists or criminals or infection or disease or neglect. Wnt (talk) 12:35, 5 April 2014 (UTC)
- I was going to make a related point but cut it due a lack of refs and it's even more soapboxing than anything else I wrote. But what the heck. Why are you (24) so sure the poppy strains used for legal narcotic purposes (i.e. not the ones used for spices/flavouring/bagels/rolls) are free and clear?
- The Taliban may not be involved. But whether our banned Perth friend (120) with many names who can't stay away from us perhaps because of their free translation needs is correct or not and it's Australian(*) or something else there's a fair chance at least some of it ends up the black market. And some of these people supplying the legal market have some willing involvement in this (often probably more willing than some in Afghanistan and definitely with less need).
- They may not be involved with the Taliban let alone Al Qaeda (although considering the connections here there's probably some linkages) but whatever you're views on the war on drugs and whatever blame you may place on the governments, it's difficult to not also place some blame for all the atrocities committed in gang wars and whatever else, on those high level drug barons who are profiting from this. So yes, when you use morphine you can probably assume a small percentage of the money made from morphine goes to people who are concurrently willingly working with nasty people who will themselves or their associates directly hurt many.
- In other words, you don't even have to support drug legalisation to recognise it's pointless to get all frothy in the mouth over a tiny percentage of your money going to farmers who also deal with the Taliban when another perhaps larger percentage is going to farmers who willing deal with other nasty characters (or at least their extreme underlings). Unless you only care about rich Americans who aren't affected so much by the violence etc.
- (*)Australia is a producer but I don't know how big. In particular since the OP mentioned morphine it sounds like they may also be moving to being morphine free producing thebaine oripavine and other opiates [4] [5] although this may include codeine which the OP also mentioned [6] [7]. From what I read, these strains are unsurprisingly also not necessarily suited for food purposes.
- Nil Einne (talk) 14:24, 5 April 2014 (UTC)
- This is only marginally relevant, but I think it's interesting that the ancients made use of other strains of poppies that didn't contain opioids. There is still a holdover of this in sales of the cough medicine noscapine, but in ancient times they used the non-opium poppies for other kinds of topical pain relief. I wonder how many of these applications have been missed because, well, they weren't good for repeat business. Wnt (talk) 17:33, 5 April 2014 (UTC)
- According to Opium production in Afghanistan
In July 2000, Taliban leader Mullah Mohammed Omar, collaborating with the United Nations to eradicate heroin production in Afghanistan, declared that growing poppies was un-Islamic, resulting in one of the world's most successful anti-drug campaigns. The Taliban enforced a ban on poppy farming via threats, forced eradication, and public punishment of transgressors. The result was a 99% reduction in the area of opium poppy farming in Taliban-controlled areas, roughly three quarters of the world's supply of heroin at the time.
- Therefore it seems unlikely that poppy farmers in Afghanistan would want the Taliban back.
- All the best, Rich Farmbrough, 23:29, 5 April 2014 (UTC).
- Nice try, but it doesn't work that way. The Taliban seem to grok the concept of indulgences by now, or at least, they don't say no to tax money. Their biggest problem is actually overproduction that can drive down prices. [8] Never forget that the single solitary objective of all drug prohibition is to maximize revenue. Even in the U.S., things that could cut into the revenue stream, like people selling fake drugs on the street corners instead of the real thing, are equally illegal but more reliably punishable, in order to avoid disrupting the market. Wnt (talk) 20:00, 6 April 2014 (UTC)
Supplementary question
Given this amount of production, how many poppy seeds does the world produce every year? All the best, Rich Farmbrough, 23:22, 5 April 2014 (UTC).
Raw Vegetables
Are there are vegetables that are good to just chop up and eat? I'm very lazy and I want to increase the vegetables that I eat. Bell peppers seem fine but they're more of a fruit. Not sure If I'd trust a raw veganism site though for my information on the topic. Also, sorry that you'vebeen flooded with healthy eating questions! 217.207.239.18 (talk) 12:03, 4 April 2014 (UTC)
- Most veggies would seem to qualify. Those "veggies" that do require cooking typically aren't really vegetables. For example, potatoes are tubers, and corn/maize is a grain. Onions can be eaten raw, but are a bit strong that way for some. Many cruciferous vegetables; like broccoli, cauliflower, and cabbage, can be eaten raw, say with a nice hummus as a dip, but beware that they will give you gas. Tomatoes are certainly good raw, but of course are also technically a fruit. I much prefer spinach raw, as it turns slimy when you cook it.
- Be careful to clean your veggies thoroughly though, when eating them raw, as you can no longer count on cooking to kill any nasties. Especially true of mushrooms.
- But think of a salad as a good start, as those usually have all uncooked veggies. Note that some ingredients, like carrots, do need to be shredded, as they are rather hard otherwise, when uncooked.
- And on the subject of laziness, I'm lazy, but I don't mind cooking, as long as I don't have to stand there and watch or stir. I have a nice steamer, and plop my veggies in there to steam for hours, without any help from me. Just make sure it won't burn everything if the water runs out, as can happen if you try to boil them (also many of the nutrients are lost to the water, so it's only healthiest if making veggie soup, where you consume the water, too). StuRat (talk) 12:14, 4 April 2014 (UTC)
- Good lord, why would you steam vegetables for hours? A few minutes of steam bath will soften them up and heat them. Corn is perfectly fine raw; the only reason to heat the cobs up is that cold corn won't melt butter. Also, I don't believe there's a scientific group of "real vegetables" as you suggest; it just comes down to culinary habits and personal taste. Matt Deres (talk) 17:27, 4 April 2014 (UTC)
- Some veggies, like large carrots, seem to need hours to soften up. I could cut them up and steam them less, but that's more work, and I'd risk cutting myself. I try to add veggies that need less steaming later in the process. StuRat (talk) 18:02, 4 April 2014 (UTC)
- Yes: Celery#Nutrition, Cauliflower#Nutrition, Cucumber#Varieties, Brussels sprout#Nutritional and medicinal value, Lettuce#Nutrition and health, Broccoli#Nutrition, Carrot#Nutrition, and arguably also Tomato#Consumption. The links all give nutritional information. 84.209.89.214 (talk) 12:27, 4 April 2014 (UTC)
- And see also Crudités. Deor (talk) 17:18, 4 April 2014 (UTC)
- For "finger food" one can carry to work in a bag I like celery, sliced radishes, and raw carrot sticks or baby carrots as a morning snack along with some fruit, such as orange or apple. Raw cabbage makes a delicious salad dressed with oil and vinegar, perhaps with some sliced almonds and uncooked ramen noodles. Edison (talk) 01:37, 5 April 2014 (UTC)
- I like uncooked broccoli. Instead of steaming vegetables, try blasting them in a microwave oven, in a sealed plastic pot. Much less effort. Alansplodge (talk) 15:34, 6 April 2014 (UTC)
- 1) Is the object to sterilize it or cook it ?
- 2) I'd use glass instead of plastic, to prevent leaching of plasticizers into the food at high temps.
- 3) This is a form of steaming, as water in the broccoli becomes steam and is trapped in by the sealed container. Adding more water may make it more effective. Depending on the cooking time, it may still be essentially raw. StuRat (talk) 14:06, 7 April 2014 (UTC)
If the universe were 6,000 years old, how many stars would I see in the sky?
Or to put it another way, how many stars are within 6 thousand light-years from Earth? A Quest For Knowledge (talk) 12:36, 4 April 2014 (UTC)
- According to Wikipedia, the Stellar density 'near' our solar system is about "0.004 stars per cubic light year". The volume of a sphere with a radius of 6000 light years (ly) is V = (4/3)*π*(6000 ly)3 = 9.05 x1011 cubic light years. Multiplying by 0.004 we get an estimate of 3.6 x109 or 3.6 billion stars within 6000 light years of our position. - Lindert (talk) 13:21, 4 April 2014 (UTC)
- Shouldn't you discard part of that because the disk near us is less than a third as thick as that sphere? —Tamfang (talk) 05:24, 5 April 2014 (UTC)
- Yes, you're right, I failed to consider that, so the actual number should be lower, but still hundreds of millions of stars. - Lindert (talk) 10:50, 5 April 2014 (UTC)
- One could perhaps try to calculate this - according to List of nearest stars there are 9 stars visible within 16.3 light years. Without accounting for the fact that these are in the comparatively sparse Local Bubble which is in turn within the comparatively dense 3,500 LY across Orion Arm - and ignoring occulting objects like dust clouds -we can do some quick maths to get the approximate number in a 6,000 LY radius sphere. [(6000/16.3)3*9] Unfortunately we now need to discount this large number by the distance at which their luminosity puts them below visible brightness - which is tricky.
- I suspect a better answer would be 9096, the number of visible non-nova stars mentioned at List of brightest stars, I doubt if any, certainly not more than a few of these are over 6000 LY distant.
- All the best, Rich Farmbrough, 13:30, 4 April 2014 (UTC).
- Bottom line: the sky would not look very different to someone without a telescope if all stars over 6000 light years away vanished tomorrow. Of course, ordinarily visible stars form an extremely tiny fraction of all stars in the universe. - Lindert (talk) 13:48, 4 April 2014 (UTC)
- Well, the Milky Way would be less bright. —Tamfang (talk) 05:24, 5 April 2014 (UTC)
- s/vanished tomorrow/vanished 6000 years ago/
 81.159.39.141 (talk)
81.159.39.141 (talk)
- Bottom line: the sky would not look very different to someone without a telescope if all stars over 6000 light years away vanished tomorrow. Of course, ordinarily visible stars form an extremely tiny fraction of all stars in the universe. - Lindert (talk) 13:48, 4 April 2014 (UTC)
- The two versions of the question are not equivalent. No realistic model of the universe would predict that there would be any stars 6000 years after the Big Bang. And if you consider a divine creation, then there is no reason why God could not have created an infinite universe 6000 years ago. Count Iblis (talk) 13:55, 4 April 2014 (UTC)
- I agree with Count Iblis. Besides, if your worldview discards observational evidence, and you're content with that worldview, then why should you believe the observational methods that are used to determine range to stars? None of these measurements are direct observation of distance. Even observable astronomical parallax is predicated on a lot of pretty abstract mathematics and physics. More distant stars are ranged by even more abstract mathematical physics, using techniques like fitting the red shift of the star's chemical emissions spectrum to our models of the expanding universe. If anyone is willing to cherry-pick out certain pieces of theory out of the entire corpus of known physics, chemistry, and astronomy, then there's no way to decide which techniques for determining stellar distance are valid. Nimur (talk) 14:10, 4 April 2014 (UTC)
- The OP may want to consider Cosmological horizon for more details on the observable universe. By the way, the OP writes on his/her profile that he/she is an atheist. Though, that tells nothing about his/her beliefs on creationism. It is certainly plausible for an atheist to believe in creationism implicitly, as if creationism is just the byproduct of his/her culture. I do not doubt that a self-identified atheist may believe that Nuwa or Pangu as the creators of the universe, but treat the belief as more of a cultural belief, perpetuated in traditional myths. The error comes when that person treats the myth as historical fact. 140.254.227.76 (talk) 14:24, 4 April 2014 (UTC)
- I agree with Count Iblis. Besides, if your worldview discards observational evidence, and you're content with that worldview, then why should you believe the observational methods that are used to determine range to stars? None of these measurements are direct observation of distance. Even observable astronomical parallax is predicated on a lot of pretty abstract mathematics and physics. More distant stars are ranged by even more abstract mathematical physics, using techniques like fitting the red shift of the star's chemical emissions spectrum to our models of the expanding universe. If anyone is willing to cherry-pick out certain pieces of theory out of the entire corpus of known physics, chemistry, and astronomy, then there's no way to decide which techniques for determining stellar distance are valid. Nimur (talk) 14:10, 4 April 2014 (UTC)
- Nimur, you are confused. Measuring distances to nearby stars via parallax of the earth orbit (I read in Nature that one such measurement came up with the result of 26 light years) is a DIRECT method, based on elementary geometry known probably to ancient Greeks. --AboutFace 22 (talk) 01:57, 5 April 2014 (UTC)
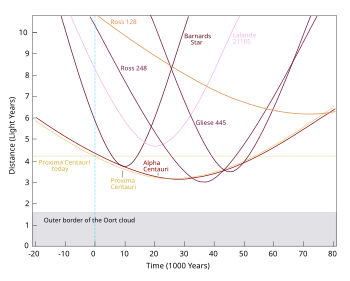

- The OP proposes a counter-factual abrupt start of a Universe ready-made with all stellar motions and emissions in progress at the -6000 year point on the diagram at left which shows distances of the nearest stars. Wikipedia has an ordered List of nearest known stars to 16.3 light-years and there is a Wikipedia book: The Nearest Stars which takes us as far as this star whose distance is measured no more accurately than 22.6+4.3
−3.1 light-years. You may like to extend the trend in the diagram to the right to count the number of stars within 6000 light-years but you would only ever see a small proportion of them. 84.209.89.214 (talk) 14:14, 4 April 2014 (UTC)- Strange that the trend line is linear rather than quadratic. —Tamfang (talk) 05:29, 5 April 2014 (UTC)
- That is a very good point. The number of stars at a certain distance should be in a spherical shell proportional to the square of the radius. Tracking back the image, it was posted by someone with one contribution, only to Commons. The data link he provides is a 404. Looking at the article that uses it Nearby Stars Database it seems like the database only had 2633 stars, so why is the curve so smooth? Looking at the figure as a whole, it is a triangle to 80 ly (which is around the 25 parsec limit of the data) and just over 60 stars, which gives just over 4800/2 = 2400 - i.e. the raw counts should be in stars per integer lightyear distance. The good news is that because that number sounds about right, it probably isn't just a random figure of cars passing a tollbooth on the interstate someone uploaded, but we still have a mystery. I would ask whether the database toward the end might have been dominated by surveying of a particular slice of sky rather than in all directions equally? But I am still confused. Wnt (talk) 13:34, 5 April 2014 (UTC)
- Strange that the trend line is linear rather than quadratic. —Tamfang (talk) 05:29, 5 April 2014 (UTC)
Neurological basis for avoiding said-bookisms
Currently, the prescription for good writing is that said-bookisms are redundant. The claim is that the word "said" can be easily glossed over, while words like "retorted", "returned", "argued", or any word other than "said" make the writing too redundant and therefore poor-quality. What I am interested in is the claim that the word "said" can easily be glossed over. Often, this is unsourced in Internet writing guides, but still, it makes a falsifiable claim. I know that people generally take shorter time to process shorter words than longer words, yet writers also claim that, while they are reading, they "become blind" to the actual words, but the ideas/concepts are more important. In that case, why would anyone care about said-bookisms in the first place? Or is writing more or less subjective and qualitative that empirical evidence cannot and should not be used to support prescriptive claims (in language, in literature, in religion, in ethics)? 140.254.227.76 (talk) 15:17, 4 April 2014 (UTC)
- I think things like that could be objectively tested, say if you set up an experiment where you had students read a passage, with various synonyms for "said" plugged in, and "said" itself, and after they read it asked them to repeat it. However, I suspect scientists have more important things to do than such an experiment, so don't look for it anytime soon. StuRat (talk) 16:21, 4 April 2014 (UTC)
- It would win the Ig Nobel Award. 140.254.227.76 (talk) 16:42, 4 April 2014 (UTC)
- I thought the argument against using said-bookisms was that if the writing is effective then the reader should be able to discern the speaker's attitude through the speaker's words or actions; if the writer has to resort to said-bookisms then that means that the rest of the writing is ineffective. If "said" is easily glossed over, it is probably because of the repetition of use (consistently in lieu of a said-bookism) rather than because of its length.--Dreamahighway (talk) 21:53, 4 April 2014 (UTC)
- I think that it's easy as a novice writer to try to start using a different word for the purpose every single time, which calls too much attention to one of the least interesting parts of the story. The same happens if you try to do constrained writing where you avoid using "the" more than once. What I'm not so clear about is whether some variant of Zipf's Law should apply. Though it would have to use a different power for conventional modern prose, because I think "said" is expected much more than any other. Wnt (talk) 13:16, 5 April 2014 (UTC)
- I suppose this is the result of experience rather than scientific study. It's hard to be conscious of the rule because pretty much every published writer follows it. But if you read things written by beginners or bad writers you'll come across lots that haven't figured it out yet, and the awkwardness of it will manifest itself to you. See our Tom Swifty article for an example. Looie496 (talk) 14:39, 5 April 2014 (UTC)
- That said, I think that whenever you tell writers to avoid one convention by slavishly following another, to the point where no one breaks it, the result is again out of balance. Tom Swift is, well, not a redlink. I think there is a time, hopefully soon, when writers break out of their cage and take back the past, feeling free to use a wide range of words and styles that have been dismissed as archaic. There are also innovations the future badly needs - for example, in this case, the use of color to mark off blocks of text by different speakers conveniently, together with a conventional and therefore unobtrusive way to label which is which. Also a specification of multiple sex-independent pronouns that can be used independently of one another to refer to people in different roles in a story simultaneously. Wnt (talk) 16:27, 5 April 2014 (UTC)
Fog vs. wind
I often hear weathermen say "the wind will come in and blow the fog away". This doesn't seem technically correct to me, as the fog has to blow to someplace else, and presumably fog from someplace else would blow into here. However, it does indeed seem to be the case that you rarely have fog and high winds together. So, why is this ? My theory: Wind causes the small droplets in fog to join into larger droplets and fall out as rain or as dew against trees, buildings, etc. Is this correct ? StuRat (talk) 16:27, 4 April 2014 (UTC)
- Although it tends to feel colder to humans, the arrival of wind is often associated with an increase in temperature. HiLo48 (talk) 16:48, 4 April 2014 (UTC)
- Yes, that makes sense in that warmer air would have a higher dew point, and the water droplets would go back into clear water vapor. However, aren't some winds associated with cold fronts ? StuRat (talk) 18:08, 4 April 2014 (UTC)
- Fog is rapidly dispersed by wind. Fog forms by moisture evaporating from relatively warm land or sea and immediately condensing back out again as it rises into cold air. Fog formation requires a very gentle breeze to continuously replace the saturated air at the air-substrate boundary (otherwise all you get is a low-lying mist at best, or more likely just dew on the grass) but anything stronger prevents a heavy bank of fog from building up. SpinningSpark 18:03, 4 April 2014 (UTC)
- "Dispersed" to where ? StuRat (talk) 18:06, 4 April 2014 (UTC)
- I think "dispersed" by evaporation to water vapour in a much larger volume of air, but perhaps a meteorologist can confirm this? Dbfirs 18:14, 4 April 2014 (UTC)
- I don't understand why you find this so hard to grasp. If I said my bunch of balloons were dispersed in the wind when I let go of them you probably wouldn't have a problem with that. Once the wind is strong enough to completely seperate the moist air from the boundary layer where it formed it then occupies a greater volume as Dbfirs says. More importantly, because the moisture content of the air has become "diluted" the humidity decreases, evaporation of the droplets is promoted and condensation is inhibited. Here is a book. SpinningSpark 18:42, 4 April 2014 (UTC)
- If there were balloons everywhere, then wind wouldn't disperse them either, just blow some away and blow others in. If a wide area has fog, then I'd expect the same thing. However, perhaps it's mixing the moist air with dryer air up above ? StuRat (talk) 18:47, 4 April 2014 (UTC)
- Even if there were aerostatic balloons "everywhere", they would not remain uniformly distributed for long. You could still get dense pockets, which would then be dispersed by wind. Anyway, yes, there is some mixing with higher layers, but also mixing with all air surrounding a given volume of fog. See also Fog#Types for some different ways it can form. SemanticMantis (talk) 21:26, 4 April 2014 (UTC)
- If there were balloons everywhere, then wind wouldn't disperse them either, just blow some away and blow others in. If a wide area has fog, then I'd expect the same thing. However, perhaps it's mixing the moist air with dryer air up above ? StuRat (talk) 18:47, 4 April 2014 (UTC)
- The premise here seems to be the popular wisdom that fog is a cloud on the ground. But if fog is formed by cold ground touching warm air, it is not like a cloud and can be dispersed by separating them frequently. Whether wind can disperse fog in a cloud forest, that is more interesting. Wnt (talk) 16:30, 5 April 2014 (UTC)
Sage advice ?
I had a large bowl (flat bottom, circular from the top) soaking in the sink. All it had in it was water and sage powder, left over from the soup I had cooked. When I went back, after soaking, to wash it, I found all the sage had deposited itself in a neat little pile right at the bottom, center. I may have left the water rotating about the center after I filled it to soak. So, what caused the sage to all gather in the center ? Since it sank, it must be heavier than water. Therefore, the rotational force of the water should have moved it away from the center, right ? Maybe it was lighter than water, and therefore floated to the center, then became water-logged and sank, later ? StuRat (talk) 18:57, 4 April 2014 (UTC)
- I'd guess that the water was warmer than the environment, and the heat loss through the sidewalls makes the water close to the sidewalls cooler and denser, which causes it to sink, creating a convection cycle of water rising in the middle and sinking at the sidewalls. That creates a center-directed current at the bottom, where the sage is. Icek (talk) 19:41, 4 April 2014 (UTC)
- Guess what, we have an article, Tea leaf paradox, with a diagram of a current cycle (which could be supplemented or even created by convection as Icek pointed out) and guess who the sage is that solved the paradox. :-) Albert Einstein, and the article explains it better than I could, but essentially water accumulating at the rim due to faster rotation near the surface forces water downward along the sides and toward the center and back up. -Modocc (talk) 21:05, 4 April 2014 (UTC)
- Wow, Wikipedia does have an article on everything ! StuRat (talk) 23:04, 4 April 2014 (UTC)
- I've gotten so used to Wikipedia having an article on everything that my reaction was not so much "Wow, Wikipedia has an article on everything!" as it was "Wow, Albert Einstein has a solution for everything!" —SeekingAnswers (reply) 14:30, 8 April 2014 (UTC)
- I hope his work on the tea leaf paradox didn't distract him, preventing him from formulating a theory of everything. :-) StuRat (talk) 14:38, 8 April 2014 (UTC)
Analgesic
Do things with an analgesic effect reduce/block the pain from drowning? Money is tight (talk) 23:14, 4 April 2014 (UTC)
- What do you mean by "a pain from drowning?" If you drowned it is too late to experience any pain. --AboutFace 22 (talk) 00:50, 5 April 2014 (UTC)
- I meant if you take analgesic and you fall into the water, will it reduce the pain/discomfort of drowning? I know this is a weird question, was just wondering if the pain when someone is drowning is the same form of pain as e.g. arms break off. Money is tight (talk) 01:10, 5 April 2014 (UTC)
- See drowning. As far as I'm aware drowning causes physical distress but that is a different thing from physical pain which I've not heard survivors talking about much though I guess there is some. However there are some similarities in how feelings like that are handled and different analgesics act on different pathways, so I guess it is possible some analgesics also reduce this type of distress. See analgesic. Also different people can react quite differently to even common analgesics like paracetamol and aspirin. You might also be interested in instinctive drowning response which is the final stage. I can't see an ethics committee thinking there is much to gain from research on the effect of drugs on this! 10:00, 5 April 2014 (UTC)
- If "pain" here means "suffering", then the answer is that it depends on the analgesic. Aspirin, for example, would not have any effect. However, opiates such as heroin or morphine reduce virtually all types of suffering, so they probably would reduce the distress associated with drowning. Looie496 (talk) 03:43, 6 April 2014 (UTC)
April 5
Biomedical engineering
Why are some parts of biomedical engineering such as biomedical materials, especially fields looking at the materials interaction with bacteria etc, classified as engineering at all? It seems more like biochemistry to me? What engineering principles are used? Clover345 (talk) 00:37, 5 April 2014 (UTC)
- Adapting the physical world to suit a human purpose is "engineering." --DHeyward (talk) 11:41, 5 April 2014 (UTC)
Meaning of wine ageing.
I suspect this question has been asked before. Well, I still decided to give it a go. I am primarily interested in fortified wines like port, madeira and sherry. You go to a wine store and see the spread: regular port is between USD 7 and 20 and if it has been aged 10 years or so, the price will be about $30.00. If it is 20 years old you will pay around $50.00 and a 30 year wine will cost you a hundred dollars.
My question is: what is the meaning of wine ageing? What changes in the fortified wine when you keep it stored for many years. The alcohol content is obviously stays the same, then what is the difference? Is it something tangible that can be caught by scientific methods?
I am not really a wine drinker, so the whole thing is mystery to me. I am sure if given two goblets to compare I could not make out which is "better." I presume ageing is done to make the wine "better," correct? Again, better in what sense?
And why does it get better? Why does it not go in the opposite direction? How about wine that gets worse with time? — Preceding unsigned comment added by AboutFace 22 (talk • contribs) 01:38, 5 April 2014 (UTC)
Anecdotally I recall an acquaintance in a position to know (that person actually worked at the plant) told me about a famous Russian vodka made here in the USA, that they produce the liquor in a single production line but pour it in regular and premium bottles. The premium ones of course cost much more. I therefore wonder about the port wine also. As I said I do not drink but buy the stuff for someone who is of my age but does not drive and cannot easily get around.
What if they do not really age the wine but pour young port so to speak and stick an expensive label on it. This question bothers me.
Thanks, --AboutFace 22 (talk) 00:46, 5 April 2014 (UTC)
- Have you read Aging of wine? Wine contains a lot of different chemical compounds that affect the flavor (we have a whole article on Wine chemistry). Aging allows time for reactions to occur that, in some wines, do improve the flavor. Fraud does occur, but it's taken pretty seriously. In Portugal, port production is regulated by the Instituto dos Vinhos do Douro e do Porto. Mr.Z-man 01:48, 5 April 2014 (UTC)
Chemical reactions in liquids take milliseconds, not years unless diffusion is involved then they may take minutes but it is hard to imagine any concentration gradients in a bottle of wine. --AboutFace 22 (talk) 13:43, 5 April 2014 (UTC)
- Some reactions are quick, and some are slow (mainly organic reactions). If you examine bottles of expired (non-alcoholic) products, you will notice changes in color, consistency, odor, etc., which indicate chemical reactions which took years to complete. Of course, those are subjectively bad reactions, while the aging of wine is subjectively good. However, I do think there is some effect of people saying since it's more expensive, it must be better. See veblen good. StuRat (talk) 14:50, 5 April 2014 (UTC)
- The speed of reactions can be dependent on the concentration of chemicals. While wine has a lot of chemicals in it, it's 95+% water and alcohol, most of the rest is acid and sugar. The organic compounds that create the flavor are less than 1%. Each indiviudal component may only be a few hundred ppm. Mr.Z-man 19:09, 5 April 2014 (UTC)
- Something I didn't see specified above in Mr.Z-man's otherwise pertinent comments (although it's doubtless mentioned in some of the linked articles) is that such beverages are often aged mostly not in the bottles they're ultimately sold in, but in larger wooden vessels, sometimes ones that have previously been used for different beverages and or ones that have been so used for decades. The chemical reactions that can occur between the beverage being aged, the wood of the vessels, and the residue of previous beverages that have sunk into the wood can be very complex, and our taste buds are quite good at detecting the subtle effects of such changes. {The poster formerly known as 87.81.230.195} 212.95.237.92 (talk) 13:45, 7 April 2014 (UTC)
How many electromagnetic signals are in my room now?
Now that everyone has their very own broadcasting station with mobiles (cell phones to you Yanks), millions of signals that were not extant 25 years ago are moving thru the space in my room. Back one hundred years, there were no television or radio transmissions. But there still would have been signals from space.
Can you tell me, approx. how many
a. signals are moving thru my room now?
b. signals would there have been 30 years ago?
c. signals 100 years ago?
d. 4 billion years ago?
Is there a limit to the number of signals that can be overlapping in a given space?
Is there any credence we should give to the idea that too many such signals can be bad for one’s health? Myles325a (talk) 03:56, 5 April 2014 (UTC)
| Sigh. Please don't be obnoxious, Medeis. Perfectly interesting question and correctly asked. Don't troll the questioner. Fgf10 (talk) 16:10, 5 April 2014 (UTC) |
|---|
| The following discussion has been closed. Please do not modify it. |
|
- I really think you're asking the wrong question here. The only answer would have to be an infinite number of electromagnetic waves are passing through your room, and always were passing through every point in space. What's really important is the frequency and power level of those waves. The usual threshold at which we would become concerned is when they start to heat things up. Cell phones are at a very low power level, so only the one next to your head could cause this. But if you happen to be right under some power lines, or next to a radio or TV station's broadcast antenna, or in the path of a 2 microwave transmission towers, then you might possibly get enough electromagnetic radiation to be of concern. StuRat (talk) 04:05, 5 April 2014 (UTC)
- What exactly are you counting as an "electromagnetic signal"? An electromagnetic signal usually refers to electromagnetic radiation that's being varied in some intentional way in order to communicate information. But your question assumes that there were "signals from space" 100 years ago, even though 100 years ago there was nothing in space that was produced by humans that was intentionally varying electromagnetic radiation in order to communicate information. Are you counting (extremely weak) electromagnetic signals produced by whatever non-human civilizations there may be in the universe? Or are you counting electromagnetic radiation produced by natural processes, that isn't intentionally communicating information? And if you're counting electromagnetic radiation produced by natural processes, what counts as "one signal"? Each star in the visible universe produces electromagnetic radiation, for example, but other natural sources of electromagnetic radiation, such as the cosmic microwave background radiation, aren't so easily separated into a discrete, finite number of sources, unless you're counting individual photons. What lower limit, if any, are you using for how strong electromagnetic radiation needs to be in order to count toward the signal count? And what frequency range, if any, are you limiting your definition to? The answer to your question could be anything ranging from "zero" to "a practically infinite number", depending on how you define what "one electromagnetic signal" means. Red Act (talk) 05:56, 5 April 2014 (UTC)
- It can be quantified by calculating the entropy of the electromagnetic background (thermal noise, mobile phone signals etc. etc.) per unit volume. The difference between this entropy and that of perfect white noise with k_B replaced by 1/Log(2)is the number of bits of information that one could in principle extract from the radiation (the contents of the mobile phone calls, some details about stars, the non-uniform microwave background giving away clues about the early universe etc. etc.) Count Iblis (talk) 15:01, 5 April 2014 (UTC)
a. and b.
The electromagnetic signals of interest need to be defined because there are more of them in your (unshielded) room that there is room for full-coverage receiving equipment. Consider just one broadcasting band 540-1610 kHz where in the Americas stations are allocated frequencies with 10 kHz spacing: that suggests 100 music programs can co-exist in the band without interference but in practice there are many more transmitted and your domestic receiver will pick up only a few satisfactorily. Wikipedia has an incomplete List of European medium wave transmitters.
c.
The OP and RedAct are is wrong about "back 100 years": in 1914 there were many radio transmissions. Hertz had demonstrated wave propagation through space (1873), both Popov and Marconi had built radio receivers (1895), voice transmission by radio had been demonstrated in Brazil (1900) and if you picked up a spark-gap wireless telegraph signal on 15 April 1912 it could have been the last one from RMS Titanic. A time traveller equipped with a modern sensitive radio receiver would hear dozens of signals, mostly in Morse code.
- How was I wrong? I didn't assume there were no radio transmissions 100 years ago, just that there were no signals from space. 100 years ago well predates Sputnik or Vostok. Red Act (talk) 20:55, 5 April 2014 (UTC)
- RedAct you wrote "nothing in space", not "signals from space". I accept now that you meant "no man-made signals were produced in space" not "no man-made signals penetrated into space" and have edited my post by striking accordingly. 84.209.89.214 (talk) 21:55, 5 April 2014 (UTC)
d.
4 billion years ago there were no man-made electromagnetic signals but the Cosmic background radiation remnant of the (presumed) Big Bang, modulated emissions from pulsars and occasional bursts from solar flares and planetary atmospheric discharges.
Overlapping electromagnetic signals
In a vacuum, air or any material with linear permeability any number of electromagnetic fields may overlapp since their vector component fields superimpose and do not interfere unless the peak magnetic field causes saturation or the peak electric field causes Electrical breakdown. The latter puts air into a hot plasma state.
Popular Culture: Contact (1997 film)
Relevant to the question, the opening scene of the movie is a three-minute computer-generated sequence, beginning with a view of Earth from high in the exosphere and listening in on numerous radio waves of modern programming emitting from the planet. The viewpoint then recedes, passing the Moon, Mars, and other features of the solar system, then to the Oort cloud, interstellar space, the Local Bubble, the Milky Way, other galaxies of the Local Group, and eventually into deep space. As this occurs, the radio signals start to drop out and reflect older programming, representing the distance these signals would have traveled at the speed of light, eventually becoming silent as the distance becomes much greater. 84.209.89.214 (talk) 16:26, 5 April 2014 (UTC)
Photos of meteor in dark flight
If the rock is moving past at 300 km/hr, shouldn't its image be blurred? Bubba73 You talkin' to me? 03:03, 5 April 2014 (UTC)
- Well, they might have been using a high speed camera to catch skydivers without making them into a blur. StuRat (talk) 04:13, 5 April 2014 (UTC)
- If you watch the actual video, it goes past so fast that you barely see it, so I agree it was a high speed camera. And a website like that really should know the difference between a meteor (which it wasn't) and a meteoroid (which it maybe was).--Shantavira|feed me 09:12, 5 April 2014 (UTC)
- Were they sky diving in space? Dbfirs 15:49, 6 April 2014 (UTC)
- I'm not buying it. The chance of being hit by a meteorite is very low. Of being hit while skydiving must be a million times lower. During the period the chute is actually open, less than that. (but I suppose many skydivers have cameras, so that doesn't divide any further) So we should look for other explanations. What I think we're seeing is that somehow a pebble was packed in on top of the chute last time and fell off when it was opened. In particular, this is why the 'meteor' is following a parabolic trajectory rather than a linear one. Otherwise, I think they should be searching the area for lifeforms, because I don't trust meteors that make course corrections. Wnt (talk) 12:20, 5 April 2014 (UTC)
- They weren't hit and estimate it as around 5Kg which is considerably bigger than a pebble. I think one would notice packing that up with a chute! Dmcq (talk) 14:54, 5 April 2014 (UTC)
- I don't believe it. I don't think that with a camera shooting bright sunlit sky with very fast settings (which should have a small aperature) you could tell the difference between a pebble next to the camera and a large stone ten times further away. As I recall the original article even said that the skydivers didn't notice the rock themselves; this image is all we have. It's true that even the original article (which seems to have come out first in English then in Norwegian a day later) was dated 4/3. But I think someone had April Fool's jollies out of this. Wnt (talk) 16:10, 5 April 2014 (UTC)
- I believe there are more small meteors than you might think. Most just go by without anyone noticing. On a regular security camera, for example, such a small object would be a blur or would fall between frames entirely, so we'd never hear about it. Only when one falls near a high speed camera is anything worth mentioning captured. As far as it's apparent motion, remember that the camera is moving, too, and the spin of the object may cause some deflection from a straight path, as it does with a baseball. StuRat (talk) 15:04, 5 April 2014 (UTC)
- Well, the Antarctic meteorites should give a number; I don't think of it as high overall. I need to balance the very unlikely chance of the real scenario against the far more likely chance of the hoax. If it can plausibly be a hoax, it is. It's the same principle as with crytozoology. Wnt (talk) 16:12, 5 April 2014 (UTC)
- It would be great if they could find the rock. They should know approximately where it is. Bubba73 You talkin' to me? 16:21, 5 April 2014 (UTC)
- Very approximately. And that film was a few years ago, so the hole it made may well have filled in by now. StuRat (talk) 18:14, 5 April 2014 (UTC)
- Wnt, why do you reckon a skydiver is at "a million times" less risk of meeting a meteorite than someone not skydiving? —Tamfang (talk) 06:59, 6 April 2014 (UTC)
- That's not quite what Wnt wrote. Skydivers seldom spend more than a millionth of their lifetime actually skydiving. I share Wnt's scepticism. Meteors are rare in the lower atmosphere, so we should look first for an explanation with higher probability. Dbfirs 12:20, 6 April 2014 (UTC)
- But the relevant question is what proportion of the outdoor filming with high speed cameras is done by skydivers. I bet that's considerably higher. StuRat (talk) 14:52, 6 April 2014 (UTC)
- Are you claiming that meteorites can be detected only by high-speed cameras? Dbfirs 15:43, 6 April 2014 (UTC)
- For small ones like this, yes, as it would either fall between frames or just be an unrecognizable blur on a normal speed camera. A larger object that leaves a trail behind it can be filmed with a normal camera, as in the recent one in Russia. But those are rare. StuRat (talk) 16:06, 6 April 2014 (UTC)
- Back to the original question: the one website has a diagram that suggests that the frame interval is 1/30 sec. The blur seems to be far less than 1% of the travel distance between frames, which implies an exposure time of less than 1/3000 sec. The wide depth of focus suggests a tiny aperture setting. The lack of distortion implies an actual shutter (simply scanning the exposed pixels from the sensor element results in distortion for moving objects). In such a small commercial camera, this combination of attributes surprises me, but some camera fundi could comment. My expectation would be to see blurring unless the camera is truly exceptional. —Quondum 19:06, 6 April 2014 (UTC)
- StuRat nearly has a valid objection, but ... no. I think that if someone had footage from a regular building security camera of a meteorite landing in the parking lot next to someone walking in, it would have hit the big time. Even more so if it landed in a lawn or garden plot and started a fire around it. I should also add that I went back and looked at the video, and it's just a few seconds from the moment the chute opens until the rock falls past - really, it seems like almost too short a time until we recall that the skydiver is rapidly decelerating once the chute opens and the rock retains its inertia. There's simply nothing unlikely about a pebble coming out like this, especially not this time of year. Sure, the guy ought to have gotten every last pebble out when he packed the chute, but parachutes are big, ground is full of stones, and he has other things he's worried about like whether he's folding it right so it will really open. I also encourage people to rethink any consideration of how the skydiver's motion could have distorted the rock's trajectory. We see the background sharp and crisp; he's not twisting within the duration of the exposure. The only question is whether his velocity relative to a distant meteor travelling at colossal speed is going to change by a visible proportion, creating a parabolic arc, within a single set of superimposed high-speed frames, and if so, whether it's going to change so that the meteor is going faster and faster toward the ground. I really don't think so! Come on, April Fools is over, this is busted. Wnt (talk) 20:13, 6 April 2014 (UTC)
- Even if they caught it hitting the ground, I wouldn't expect a fireball from a rock this small, just a hole appearing in the lawn with some mud sprayed out. Of course, in that case, they could find it and dig it out, so that would make it more interesting than just the video alone. StuRat (talk) 20:39, 6 April 2014 (UTC)
- For action shots in daylight I believe exposure times of about 1/1000 second per frame are quite usual. I believe the pixels are captured then shifted out serially out rather than there being any scanning of the pixels. Dmcq (talk) 20:27, 6 April 2014 (UTC)
Filtered milk - contaminated after opening? Why filters and not irradiation?
At my local supermarket there's the option of milk that's been filtered to keep it fresher for longer. To what extent do the milk-spoiling organisms come from the milk compared to being introduced when the bottle is opened and contents decanted?
Why don't they use gamma irradiation instead of filters? Also, wouldn't irradiation be preferable;e to UHT which alters the milk? --78.148.110.69 (talk) 13:00, 5 April 2014 (UTC)
- Dairy products suffer from vitamin destruction and the creation of off-flavours when irradiated.[9]--Aspro (talk) 14:23, 5 April 2014 (UTC)
- Let me just add a note that the IP of the person who asked the question geolocates to the UK. Looie496 (talk) 14:27, 5 April 2014 (UTC)
- While I don't have a direct comment on the relative effects, it's worth remembering that there will often be at least a few days between packaging and opening the milk container. Even more if you often buy more than one bottle at a time but don't drink it that fast. And of course one advantage if this does work is you can do this to an even greater extent if it fits your lifestyle. And the stores themselves don't have to ensure their stock rotates so quickly.
- And obviously the bacteria (including spores) in the milk are problematic otherwise your unopened refrigerated milk container wouldn't spoil within 2 - 3 weeks while your unopened unrefrigerated UHT milk container would last months or even years. (In case there's any confusion, although I can't find a ref which directly states this, while the UHT process does cause some changes which some find undesirable, the reason why it lasts longer is because of the much more effective sterilisation.)
- In other words, if the filtering does work well, it would make the milk fresh for longer to some extent depending on the particular case. I don't know about your particular milk but [10] mentions a refrigerated shelf life of 60 to 90 days for an unopened container.
- (Edit: Note that that source is from 2013 so I'm not sure if there will be anything on the market yet although it sounds like [11] this has been an active area of research recently. From what I can tell there is also some confusion because some extended shelf life milk which seems to generally anything which lasts more than 21 days or so milk just uses microfiltration and HTST pasteurisation, but others uses higher temperature treatements as well as microfiltration. Yet others just use high temperatures. And it varies whether the fat/cream is processed the same or subjected to UHT. There are also other possible steps like UV treatment. [12] (see also comments) [13] [14]. The journal source only used HTST and the other processes but achieves 60-90 days which I think may be partially why it's recent research. It sounds like in the UK microfiltration is the norm, but possibly also higher heat treatment. Our pasteurisation article also discusses the issues briefly. One thing the first (Dr. Gail Barnes) source mentions that's probably relevant although it may be obvious, you need to make sure you post processing such as packaging steps are good enough for your purposes, probably aseptic. So it may also be that your microfiltered milk has this done better too. And it also helps if you are careful with your input. And yes I recognise that this doesn't answer that well what appears to be your main question namely how much of a different this will make after you open the milk.)
- As for when the container is opened, obviously if it's fresher from the beginning it may last longer. You shouldn't expect miracles since even UHT milk refrigerated before opening, carefully decanted and closed each time doesn't generally last more than 3 or 4 weeks at the extreme end from my personal experience so I don't see any reason to expect any of this to be better. (May be if you only ever open, decant and close in a laminar flow hood you can stretch it?)
- Edit: I should clarify I only read the abstract and I'm not sure the research in the Journal of Dairy Science article was using unopened packaging. It's possible they weren't but were testing in conditions unlikely to be achieved in most real life ones, such as the laminar flow hood I earlier suggested. In other words, I'm pretty sure they were primarily thinking of an unopened packaging shelf life situation since as I suggested and I guess also StuRat below, it's generally difficult to prevent contamination after opening; and milk is a good culture media.
- Edit2: Also there is some mention in Microfiltration#Dairy Processing and Milk#Microfiltration but it doesn't add much.
- Nil Einne (talk) 14:48, 5 April 2014 (UTC)
- The opening on the top of most milk containers is a problem, letting bacteria drop in whenever the cap is off. The air which rushes in to replace the lost milk will also contain bacteria. Milk bags can solve the second problem, as they are flexible and can be reduced in size to match the lower volume of milk. As far as not letting bacteria drop in from above, a system like some ketchup squeeze bottles use, where they open at the bottom, might work. Of course, ketchup is thicker, so avoiding spills would be trickier with milk. Another way to reduce spoilage is smaller containers, ideally single use. This can mean more packaging waste, though, so reusable glass bottles would be best for the environment and also eliminate leaching of plasticizers into the milk. StuRat (talk) 15:22, 5 April 2014 (UTC)
- OMG, I had no idea that there was such a thing, as opposed to a "figure"-ative term. :) Wnt (talk) 16:14, 5 April 2014 (UTC)
- Yes, for most Americans, the effort to get your hands on some milk bags will be a giant bust. StuRat (talk) 16:58, 5 April 2014 (UTC)
- So, what you're saying is that we should go back to the milkman bringing pints of milk in reusable glass bottles to our doorstep. I can tell you from experience that the problem I had with this was that the bottles never seemed to completely lose the smell of stale milk, which put me off drinking the fresh milk. 86.146.28.229 (talk) 07:21, 6 April 2014 (UTC)
- That's inadequate cleaning or perhaps the milk at the top had gone bad. They should avoid screw tops, as those are difficult to clean. A disposable cork might work better. I don't think the porch is an appropriate place to leave milk, as it gets warm there, especially if the sunlight hits it, and the labor cost of the delivery has to increase the price significantly. I'd still deliver it in stores, but in single-use glass containers, like a quart, pint or half-pint (depending on the family size). They can have a deposit on them to encourage people to recycle. StuRat (talk) 16:50, 6 April 2014 (UTC)
- Well obviously it was improper cleaning, but my point is that when everyone actually used them (and they were cheap, because everyone used them: the delivery cost was no higher than the labour cost involved in delivering and setting bottles out in a supermarket, really) this was a problem. So I doubt it would be less of a problem for future implementations. And they weren't screw top: they were foil-top, to maximise hygiene and minimise costs. The bottles were returned by simply leaving them out to be collected when the next milk was delivered. The doorstep was considered an appropriate place to leave it for generations of people, since when people get up at a reasonable time in a temperate climate there is no issue: prior to that, the milkman came around with his horse and wagon, with a large vat of milk that released milk from a tap at the bottom (just like you said), and people took out their own containers to fill. You might think that it would in theory be unsuitable, but in practice for many years it actually was suitable, even if people round where you live don't do it. There were pint bottles, and rarely-used half-pint bottles, and third of a pint bottles to deliver to schools. People ordered however many pints they wanted (a quart bottle would surely cause problems, since it would be open for longer, and would also make the delivery and cleaning more complicated), and opened each sealed bottle as they used the last. But I could never switch back to communal, reusable glass bottles for milk, because my experience is that the industrial cleaning process doesn't completely eliminate the smell. 212.183.128.252 (talk) 08:19, 7 April 2014 (UTC)
- How long ago was this ? They might have better cleaning methods now (UV lasers ?). StuRat (talk) 19:28, 7 April 2014 (UTC)
- Also note that many people have an irrational fear of irradiation, thinking that the food itself then becomes radioactive. This is rather ironic, since they don't worry about foods which are naturally radioactive, like bananas. StuRat (talk) 15:24, 5 April 2014 (UTC)
- You are not allowed to sell irradiated milk in the UK.[15] Thincat (talk) 17:00, 5 April 2014 (UTC)
- And why when the governmental Food Standards Agency says it is "a safe and effective way to kill bacteria in foods and extend its shelf life". It's because of lobby groups such as The Food Commission.[16] Thincat (talk) 17:15, 5 April 2014 (UTC)
- And I see Long-life milk which is common in the EU, meets consumer resistance in the US. It seems irradiated milk in the US is ultraviolet treated,[17] not gamma radiation – I don't know if this is done in the UK (EU). Thincat (talk) 18:03, 5 April 2014 (UTC)
- Hmm...the sentence "in the American market, consumers have been uneasy about consuming milk that is not delivered under refrigeration, and have been much more reluctant to buy it" in the UHT article not supported with a citation, so it should be treated with suspicion. Richerman (talk) 18:21, 5 April 2014 (UTC)
- I'm not impressed by regulations against irradiation - I tend to think commercial interests like the idea of people throwing food away and buying more. But... irradiation isn't guaranteed to have no effect. Ionizing radiation can produce double-strand breaks in DNA to kill microbes, and it is true that many enzymes or mechanical agitation produce DNA with similar breaks. But there might be other molecules that radiation breaks in a place that few if any enzymes do. So I can't rule out the possibility of a harmful effect. Nonetheless, I wish we'd let people try more things experimentally, with labelling ... and also that we'd be faster to recognize when things like partially hydrogenated oil are not food and should not be labelled as such. Wnt (talk) 19:15, 5 April 2014 (UTC)
- I wonder if there's any risk that irradiation could cause a mutation, say like the ones that causes mad-cow disease, by altering some proteins in the milk. StuRat (talk) 20:43, 5 April 2014 (UTC)
- There's really no remotely plausible mechanism for that to occur.(+)H3N-Protein\Chemist-CO2(-) 02:10, 7 April 2014 (UTC)
- Mad Cow disease? Now there is a thought! The protein has to get deformed by some agent initially. Expression of cellular isoform of prion protein on the surface of peripheral blood lymphocytes among women exposed to low doses of ionizing radiation. Still, I will never have to worry about contracting Mad Cows disease because I'm a helicopter. It is a sham that irradiation won't make milk radioactive, because if it did, it would be easier to see it glowing in the dark it when the fridge light has blown. It would also aid, my secret night-time food raids to the kitchen when everyone else is sleeping. We need (I need) genetically modified cows with some jellyfish genes, to create glow in the dark milk. Oh, and cheese cake. Err,.. Yes you scientists out there, concentrate on developing the cheesecake first.--Aspro (talk) 01:32, 6 April 2014 (UTC)
- And if the milk is radioactive, you won't even need to warm it up ! StuRat (talk) 02:17, 6 April 2014 (UTC)
- At first I was thinking this question would be unapproachable, but it appears that radiation exposure actually prolongs survival in mice exposed to prions [18]. Unfortunately I still don't see a way to get a good reliable stat on how frequently radiation induces prion disease because I assume it must be very rare; still, if the response to radiation protects against prions, it gives a whiff of plausibility that it could be a real consequence of radiation exposure. Still, this is nothing like evidence that irradiated food would have this happen to a meaningful probability. You really have to do the experiment directly. Wnt (talk) 21:03, 6 April 2014 (UTC)
- Radiation can't cause mutations of nonliving material. If for instance you were to irradiate a steak, it could of course damage any residual DNA or RNA (that's sort of the point since it inactivates viral and bacterial contaminants). But since it's not part of a living organism anymore, it's not capable of replicating the damaged DNA, or expressing new proteins, so none of that can result in the production of mutant proteins. You could certainly cause some minor degradation of any proteins already present, but you're not going to magically change the sequence, besides people eat denatured protein all the time. Case in point, scrambled egg. The basic take home message here is that you can't "mutate" nonliving matter. That's genetics 101. (+)H3N-Protein\Chemist-CO2(-) 02:10, 7 April 2014 (UTC)
- But my understanding of mad cow disease prions is that they are mutated proteins. The replication happens by other cells in the cow, or in a human once those prions are introduced there. So, one mutated prion gets in, and it can then be replicated to a dangerous level. Are you saying proteins can never be mutated directly ? StuRat (talk) 02:21, 7 April 2014 (UTC)
- Prions are proteins that adopt alternate (or incorrect) folds and then propagate the misfolded form by trapping other unrelated proteins in the same incorrect tertiary structure. The idea being that the disease state is passed on structurally, trapping native proteins in alternate conformations. The initial misfolding event can be the result of a mutation, but the mutation itself can't be passed on to other proteins. A mutation is a change in sequence, so a mutant protein would literally have to have a different primary sequence. (+)H3N-Protein\Chemist-CO2(-) 02:31, 7 April 2014 (UTC)
- Also, worth clarifying the terminology here: A mutant gene is a gene with a different nucleotide sequence, a mutant protein has a different amino acid sequence. A mutant gene can produce a mutant protein, or it could easily produce a wild-type protein depending on what specific change is made (See also: degenerate codon). The amino acid sequence itself can't replicate though, so one mutant protein can't make another copy of itself. (+)H3N-Protein\Chemist-CO2(-) 02:42, 7 April 2014 (UTC)
- Sounds like a distinction without a difference to me. That is, you don't count a misfolded prion as being a mutation, but it can still be passed on to somebody who eats it and cause mad cow disease. So then, if radiation can cause a misfolded protein, that's a problem. StuRat (talk) 02:39, 7 April 2014 (UTC)
- That's unfortunately, a completely baseless conclusion. Radiation could degrade a protein chemically, maybe even produce amyloid in an extreme case. But suggesting that radiation damage will produce infectious mutant prion proteins out of thin air is essentially invoking magic. Edit: Out of curiosity, where did you even get the idea that radiation causes prion diseases? (+)H3N-Protein\Chemist-CO2(-) 02:46, 7 April 2014 (UTC)
- I didn't say that it could, I just asked if it's possible, as one example of a possible mutation. Another example would be in a surviving bacterium that might be mutated to be more harmful. StuRat (talk) 03:00, 7 April 2014 (UTC)
- Also, some foods remain alive until irradiated, like onions or potatoes or grains which can sprout. I can easily imagine that some cells in the milk also remain alive. StuRat (talk) 13:49, 7 April 2014 (UTC)
- Well, they wouldn't be alive anymore after being dosed with high levels of gamma radiation. So, there's still no mechanism for that to cause the overly specific mutations you've invoked above. Contrary to what happens in the comics, exposure to high levels of ionizing radiation tends to cause nonspecific and irreparable DNA damage resulting in either death (fast or slow) or sterility, neither are optimal conditions for passing on genetic material. It also doesn't actually turn you into a big green rage monster. In either case, irradiating food neither causes the food to remain radioactive, nor does it "mutate" the food in any genetic sense of the word. (+)H3N-Protein\Chemist-CO2(-) 13:16, 8 April 2014 (UTC)
- Just like with antibiotics, the potential nutation danger would be in under-dosed foods. And while most mutations are either deadly or have no effect, a very few help the organism to survive. In the case of harmful bacteria, this could be bad news for us. StuRat (talk) 13:27, 8 April 2014 (UTC)
- No, still not plausible. Bacteria can become resistant to the specific chemical mechanism of a particular antibiotic, it's all about the chemistry. Say a small molecule binds to a bacterial ion channel, then there's a strong selective pressure for an ion channel with a slightly different extracellular domain. The only way bacteria can respond to low level DNA damage is with so-called "DNA repair" proteins, allows them to repair minor damage induced by low level UV irradiation. Ionizing radiation is entirely different, if you irradiate a bacteria with gamma radiation you cause genome wide damage to its genetic material, there's no way to adapt to it because you're directly destroying the thing that would allow it to respond. IF you invoke incomplete irradiation, then maybe a few bacteria aren't exposed to the gamma radiation at all, but then there's no selective pressure. High dose ionizing radiation is a horrible way to introduce a stable mutation, chemical or UV methods work way better. For instance, directed evolution screens sometimes work this way. (+)H3N-Protein\Chemist-CO2(-) 14:25, 8 April 2014 (UTC)
- Just like with antibiotics, the potential nutation danger would be in under-dosed foods. And while most mutations are either deadly or have no effect, a very few help the organism to survive. In the case of harmful bacteria, this could be bad news for us. StuRat (talk) 13:27, 8 April 2014 (UTC)
What's the thickness of the pleura membrane?
213.57.121.149 (talk) 15:17, 5 April 2014 (UTC)
- Your answer is going to vary considerably depending on just how refined a structure you are referring to regarding the pleural membrane, but assuming you are looking at just the visceral pleura, the thickness for a healthy individual is on the order of micrometers (in the ballpark of 30-80µm on average), though there are conditions (notably diffuse pleural thickening) which can increase the thickness by several millimeters. Snow (talk) 03:31, 6 April 2014 (UTC)
- Yes, I'm looking for the pleural parietal too. I think there is a differnt of the thickness between the two. And if it's so thin how can it be he can hold whole the volume of the lungs. Thank you. — Preceding unsigned comment added by 194.114.146.227 (talk) 07:59, 6 April 2014 (UTC)
- The parietal pleura is even thinner, at about 20µm. As to your second question, I assume by volume of the lungs you are inquiring as to how it contains the pressure of the volume of gas regularly inhaled. If so, the answer is that it does not, in itself, so much perform this function; air is conducted through still more internal structures, notably the bronchi, bronchioles, alveolar ducts, and the alveoli (where the bulk of gas exchange occurs) themselves, all of which is supported by a matrix of connective and vascular tissues. These tissues are more than sufficient, in most circumstances, to resist the pressure of the maximum volume of air. In fact, the function of the pleurae is less to resist internal pressure than it is to regulate it; the intrapleural space is filled with pleural fluid, which increases surface tension and keeps the visceral pleura in contact with the parietal pleura without friction between the two, such that, when the diaphragm contracts, increasing the volume of the thoracic cavity, the volume of the lungs is increased as well, drawing air into the bonchi, by way of the trachea. So in essence these structures are part of the mechanism which regulates volume (and thus pressure) in the lungs, and less so those which resist it. Snow (talk) 11:50, 7 April 2014 (UTC)
- Yes, I'm looking for the pleural parietal too. I think there is a differnt of the thickness between the two. And if it's so thin how can it be he can hold whole the volume of the lungs. Thank you. — Preceding unsigned comment added by 194.114.146.227 (talk) 07:59, 6 April 2014 (UTC)
Airplane black box
Is there any reason why an airplane black box will "ping" for the specific time period of one month? Or is that just an arbitrary time frame established by the manufacturer of the box? Thanks. Joseph A. Spadaro (talk) 19:07, 5 April 2014 (UTC)
- Battery life isn't infinite. However, this article suggest a push to extend the life to 90 days. As technology approves, the size, durability and lifespan of these devices will all optimize. Regulations must be in place to keep up with technology. Mingmingla (talk) 19:58, 5 April 2014 (UTC)
- For me this reads like a requirement by a government agency and the manufacturer does exactly what he is obliged to do deliver 30 days of battery life. --Stone (talk) 20:36, 5 April 2014 (UTC)
- More likely an international NON-government agency. HiLo48 (talk) 21:42, 5 April 2014 (UTC)
- For me this reads like a requirement by a government agency and the manufacturer does exactly what he is obliged to do deliver 30 days of battery life. --Stone (talk) 20:36, 5 April 2014 (UTC)
- I've spent a good part of my career as an engineer in the provision of battery backup power (Unreakable Power Supplies - UPS) for critical computer systems, police telecommunications, hospitals, etc. The someone who can come up with a battery technology that never suffers unpredicted catastrophic failure upon discharge, offers a reliable discharge time (not forgetting that battery capacity is inherently proportional to temperature, as a battery is essentially a chemical system and all chemical reactions are temperature dependent), and offers a usuable capacity in a resonable size and weight, is a rich someone to whom the world will beat a path to their door. Nickel iron batteies come close when made at high cost. Super-capacitors have great promise. But this business of the black box battery being good for a known length of time is nonsense. 120.145.97.4 (talk) 00:30, 6 April 2014 (UTC)
- My understanding is that one month is the GUARANTEED MINIMUM time that a black box will ping -- it might or might not ping for a longer time, but it WON'T stop before one month. 24.5.122.13 (talk) 03:07, 6 April 2014 (UTC)
- Right, the 30 days are guaranteed, and there is a safety margin defined by some requirement. I know of a few parts were we have to built in a factor of 1.5 and for others 3 or even more for very dangerous systems. --Stone (talk) 08:45, 6 April 2014 (UTC)
- Wikipedia has relevant information at Underwater locator beacon. This article points to a useful external link HERE, although this equipment is very modern and unlikely to be installed in Boeing 777 of the age of MH370. Dolphin (t) 11:00, 6 April 2014 (UTC)
- Another question: Why aren't there two black boxes, one of which remains with the plane, and another of which is ejected? The one that is ejected (automatically) could be equipped not only with "pinging" ability but with a huge inflatable orange flotation device, which might aid in recovery. Bus stop (talk) 11:42, 6 April 2014 (UTC)
- A proposal to this effect has been circulating in the US Congress for several years. This proposal has recently been re-introduced as a result of the search for MH370. See the information supplied by User:BBoniface about the SAFE Act - diff. Dolphin (t) 11:52, 6 April 2014 (UTC)
- Another question: Why aren't there two black boxes, one of which remains with the plane, and another of which is ejected? The one that is ejected (automatically) could be equipped not only with "pinging" ability but with a huge inflatable orange flotation device, which might aid in recovery. Bus stop (talk) 11:42, 6 April 2014 (UTC)
- Given the chilly temperature at the bottom of the ocean, would that extend the batteries' life, or shorten it? ←Baseball Bugs What's up, Doc? carrots→ 13:18, 6 April 2014 (UTC)
- It should slow the reaction extending the life, but on the other hand the maximum current would be reduced, so the useful life could be less. I hope 120 comments on that question. All the best, Rich Farmbrough, 14:19, 6 April 2014 (UTC).
- As previously stated, battery discharge time goes down with any reduction in temperature. Mathematical formulae are available to predict the reduction for each type of battery, but I don't know just what battery technology is employed in the black boxes (actually, they are orange). However, googling "deep ocean temperature" throws up a multitude of websites that show that the temperature profile of deep oceans is not simple, and very deep areas may be quite warm. The area where MH370 is thought to have gone down is ~4 km deep and includes an area with underwater volcanoes. Discharge time is also affected by the service life of the battery in a complex way. Many rechargeable battery technologies increase in capacity over time before reducing near end of life.
- If I were running the search operation, I would requisition a battery engineer from the manufacturer to review the service life of the particular unit, the performance of other batteries from the same batch, and the water temperatures at the assumed location, and make some sort of estimate of how long it's going to last. There's no such thing as a guarantee in the battery business, but if the design criteria is for a minimum of 30 days for some high percentage of units, then depending on all the factors a particular unit may last 2 or 3 times as long - or it may die today.
- Another aspect that the search team should be aware of is that batteries recover somewhat when rested. This means that, depending on the battery technology and the design of the electronics, as the battery gets very weak, the electronics may skip pings. A simple way to exploit the full capacity of a battery is to parallel it with a super-capacitor. The capacitor accumulates energy as the near flat battery manages to dribble it out, and when the capacitor has accumulated enough, the electronics can turn on and send a ping. So, at first, pings are sent regularly at the design intervals. As the battery weakens, the interval between pings can get longer, and perhaps get to a stage where a few pings are sent then a long break before a few more pings, another long break, and so on... I have no idea of whether black boxes employ this technique, which is quite new.
- 120.145.70.125 (talk) 15:14, 6 April 2014 (UTC)
- It should slow the reaction extending the life, but on the other hand the maximum current would be reduced, so the useful life could be less. I hope 120 comments on that question. All the best, Rich Farmbrough, 14:19, 6 April 2014 (UTC).
- Given the chilly temperature at the bottom of the ocean, would that extend the batteries' life, or shorten it? ←Baseball Bugs What's up, Doc? carrots→ 13:18, 6 April 2014 (UTC)
Thanks, all. Please see my new question below about black boxes (in the April 6 section, entitled "Airplane black box - another question"). Thanks. Joseph A. Spadaro (talk) 15:18, 6 April 2014 (UTC)
Manganese in Steel
I read at several points that the Spartans had superior steel weapons because their iron ore was contaminated with manganese. From my understanding at that point people used bog iron as mains source for iron. I can think of nearly no way how the manganese could come into the iron with the process used at that time. The good thing is that I also could not find a metallurgy or archeology article stating the claimed of manganese in steel in that time period, but it is stated in a good article of wikipedia so it must be true ;-). Is there anybody with a little advice for me?--Stone (talk) 20:35, 5 April 2014 (UTC)
- Since bog iron is derived from groundwater, if the groundwater in question naturally contains manganese, so will the bog iron. 24.5.122.13 (talk) 03:01, 6 April 2014 (UTC)
- And in fact, according to the article Pyrolusite, manganese is actually often found in bogs. 24.5.122.13 (talk) 06:32, 7 April 2014 (UTC)
- A useful-looking source on manganese in bog iron, via a Google search: [19] AndyTheGrump (talk) 07:00, 7 April 2014 (UTC)
Lanthanide oxide crystal structures
Hello. I know that B-type lanthanide oxides can be formed for Pr-Gd (sometimes necessitating high T). According to the book I've got at hand, the B-type structure has three different sites, one of which is distorted capped octahedral, and two different face-capped trigonal prismatic sites. What differs between the two face-capped trigonal prismatic sites ? I can't seem to find any good illustrations of this structure online, so please point one out to me if you can ! Thanks. --2.100.5.77 (talk) 21:51, 5 April 2014 (UTC)
- Illustrated at http://www.radiochemistry.org/periodictable/la_series/L12.html. I think this is it also [20] but you will have to register to use the Atom Work site. It looks to be capped at slightly different angles.
table of unit cell structure of Gd2O3 based on http://onlinelibrary.wiley.com/doi/10.1107/S0365110X58002012/pdf
No Site Atom Multi symmet x y z Occupancy 1 O1 O 4 m 0.0259 0 0.6562 1.0 2 Gd1 Gd 4 m 0.1346 0 0.4900 1.0 3 Gd2 Gd 4 m 0.1899 0 0.1378 1.0 4 O2 O 4 m 0.2984 0 0.3738 1.0 5 O3 O 4 m 0.3250 0 0.0265 1.0 6 Gd3 Gd 4 m 0.4662 0 0.1879 1.0 7 O4 O 4 m 0.6289 0 0.2864 1.0 8 O5 O 2 2/m 0 0 0 1.0
The B-type is monoclinic prismatic C2/M. C-type is cubic. A type is P32/m. X-type is Im3m cubic, H-type is P63/mmc.[21] Graeme Bartlett (talk) 09:04, 7 April 2014 (UTC)
April 6
Diverticulitis questions
Okay, some of you know me, and I need help. I've got diverticulitis, the fever is down, the pain is almost gone now, and I'm on cipro and metro (generic flagyl). I had it once before, 3 years ago. I've read a lot and listened to my doctors, and I'm confused, frustrated and a little anxious. My preference would be a private conversation with anyone who's comfortable with the topic, but if there are no takers, I'll post a few questions. - Dank (push to talk) 02:43, 6 April 2014 (UTC)
- Sorry Dank, but by policy we are prohibited from giving any form of medical advice or recommendations on the ref desks and on the project in general. The best we can do is to direct you to seek the assistance of a medical professional. Best of luck to you, though. Snow (talk) 02:54, 6 April 2014 (UTC)
- Yes, we can't really hold discussions or give advice. But searching google for diverticulitis support groups or forums finds many hits, like these. My from-experience advice, which I can give you, is that given the morbidity, especially with slipshod treatment, seek out aggressive specialist care. μηδείς (talk) 03:15, 6 April 2014 (UTC)
- Thanks Medeis, I didn't know about those, that's very helpful. - Dank (push to talk) 03:33, 6 April 2014 (UTC)
information
Changes can be coaded as information — Preceding unsigned comment added by Gamts564 (talk • contribs) 03:14, 6 April 2014 (UTC)
- I can't make out a question here, please clarify. StuRat (talk) 03:28, 6 April 2014 (UTC)
Uk DMAT
Does the UK have any DMAT teams? DMAT meaning disaster medical assistance team. A team of doctors and nurses, who work on call, to provide emergency care at scenes of incidents. What is it called in the UK? — Preceding unsigned comment added by 90.201.212.79 (talk) 10:46, 6 April 2014 (UTC)
- I don't know if this is the same thing but there are MERITs, medical emergency response incident teams. These are small, 3 or 4 medics. All the best, Rich Farmbrough, 14:29, 6 April 2014 (UTC).
- I'm not sure if it's exactly the sort of thing you're asking about, but in the East of England we have Magpas, which is a charity. AndrewWTaylor (talk) 09:07, 8 April 2014 (UTC)
USAR
Do urban search and rescue teams employ structural engineers? 90.201.212.79 (talk) 10:52, 6 April 2014 (UTC)
- Yes -- see Urban Search and Rescue. 24.5.122.13 (talk) 01:19, 7 April 2014 (UTC)
Rectum
Is rectum part of anus? Link to my user page when you reply. HYH.124 (talk) 11:31, 6 April 2014 (UTC)
- No, the anus is a sphincter muscle which closes the end of the digestive tract and facilitates controlled waste expulsion. The rectum is a cavity composed of smooth muscle tissue and attached at it lower margin to the anus. Its function is to consolidate faeces by means of peristalsis and moisture absorption. I'm sorry but I don't usually respond to orders from people I don't know who are asking me for information. Richard Avery (talk) 13:18, 6 April 2014 (UTC)
- Here's a ping for you, User:HYH.124. Dismas|(talk) 02:43, 7 April 2014 (UTC)
- @Dismas: I saw... Isn't that too late? HYH.124 (talk) 11:46, 7 April 2014 (UTC)
- Here's a ping for you, User:HYH.124. Dismas|(talk) 02:43, 7 April 2014 (UTC)
Alfred Russell Wallace quote
On this site I read this, Even a replay of terrestrial evolution would almost certainly lead to substantially different end-products, a claim first put forcibly by [Alfred Russell] Wallace and echoed many times since. I'd like to find where Wallace said this, or one of the places he said it, so I can quote the original for an essay.
Thank you Adambrowne666 (talk) 12:28, 6 April 2014 (UTC)
PS I'm ashamed to say this is the first I've heard of Wallace - what a guy!
- I think that, given a generic "plant", a rerun of evolution would lead to trees. Given a generic "bird", we would have ducks. (At least they would look like ducks and act like ducks though I'm not entirely sure they'd quack like ducks). Given any mammal, and ants for it to eat, we would eventually have anteaters. Given any animal at all, you'd soon have a worm. Basically, to continue this game, choose any nice popular term for a species that is paraphyletic, and we know that evolution can converge to it. Now whether evolution would converge at any distance further than we know it has converged, that is a harder one to guess, because we don't know how often a species would have taken up a niche and form but been unable because the incumbents were simply too good at it already.
- A philosophical digression perhaps worth noting is that the existence of evolutionary convergence tends to refute those who say that the universe is without purposeful design. Because the so many immediately recognizable archetypes exist, which evolution has come to by multiple paths, it seems more accurate to say that they were already "intended" by the laws of logic and physics long "before" the first example lived and breathed. See Theory of Forms. Wnt (talk) 20:40, 6 April 2014 (UTC)
Using a google search I can't find any quote by him along those lines. The nearest I can find is here where he says "In order to produce a world that should be so precisely adapted in every detail for the orderly development of organic life culminating in man, such a vast and complex universe as that which we know exists around us, may have been absolutely required." Some of his publications are given in our article Alfred Russel Wallace but he was very prolific and it says there were at least 750 publications in total. Richerman (talk) 09:58, 7 April 2014 (UTC)
- Purely negative evidence, but one of the major themes of Stephen Jay Gould's Wonderful Life is precisely the contingency (non-replay-ability) of the history of evolution, and the book contains no reference to such a statement by Wallace. I find it hard to believe that, if Wallace had ("forcibly") expressed such a view, Gould, who was quite familiar with Wallace's writings, would have omitted any mention of it. There's a contact link on the home page of David Darling's site; perhaps you should ask him for a reference. Deor (talk) 13:50, 7 April 2014 (UTC)
- Yes, good point, Deor, and thanks for the contact link - thanks, all, for the leads. Adambrowne666 (talk) 21:43, 7 April 2014 (UTC)
need info (oxygen content of water)
Hello I never had chemistry in school but want to know something. If you released oxygen gas in a water solution would it stay suspended for any length of time say one hour and how could you measure the percent of that content? Thanks a paying donor. — Preceding unsigned comment added by 74.130.204.252 (talk) 15:15, 6 April 2014 (UTC)
- Oxygen does not suspend in water, it dissolves. If you bubble oxygen through water, some of it will dissolve. If you can keep the bubbles inside the water long enough, eventually all of it will dissolve. There are a variety of ways of measuring the oxygen content of water. For example, people who own aquariums can buy a "dissolved oxygen meter" that uses an electrochemical probe. Looie496 (talk) 15:38, 6 April 2014 (UTC)
- There is however a limit to how much oxygen can be dissolved in water, depending on the temperature, salinity, etc. As for air bubbles, they will eventually either rise to the surface and pop or dissolve into the water. Smaller bubbles are more likely to stay underwater long enough to dissolve. Agitation, as by wave action, both has the potential to add new bubbles and keep existing bubbles underwater longer, allowing them more time to dissolve. Thus agitated water is likely to contain more dissolved oxygen than stagnant water. The action of microbes and macroscopic life forms also matters, as plants can add oxygen to the water (and remove carbon dioxide) while animals do the reverse. StuRat (talk) 16:44, 6 April 2014 (UTC)
Airplane black box - another question
I know virtually nothing about how an airplane black box works. If a suicidal or rogue pilot wanted to sabotage (or at least impede) an airplane's search and recovery efforts by causing "harm" to the black box, would that be possible? Is there any way that a pilot (or any person) can disable, disengage, tamper with, or "shut off" the black box? Or is this notion impossible for some reason? Thanks. Joseph A. Spadaro (talk) 15:17, 6 April 2014 (UTC)
- Yes, of course, but I believe that to do it while the plane is flying would take tools capable of cutting through metal. On the ground it would be pretty easy. Looie496 (talk) 15:23, 6 April 2014 (UTC)
- As a practical matter, no. The flight recorder and cockpit voice recorder are located in a crash-resistant shell at the tail of an aircraft, inaccessible to anyone onboard during flight. But "any" way? Sure. Shoot them with a gun, or smash up the cockpit so that the instruments they operate from are non-functional, or set the tail of the airplane on fire, or, or, or. It's not "impossible", but it's a scenario of last resort.
- There's also not much reason. Other than the deep-water "ping", the recorders don't do anything to assist in a SAR effort. Assuming a hijacker does something like fly to the secret Nazi moonbase in Antarctica and lands there, the recorders can't be found unless the whole aircraft is found. — Lomn 15:25, 6 April 2014 (UTC)
- Huh? Isn't that a "chicken or egg" scenario? You said that "the recorders can't be found unless the whole aircraft is found". But, isn't the finding of the recorder itself (via its pings) an event that (sometimes) leads to the finding of the aircraft? Can't it work both ways (which is found first, leading to which is found second)? Joseph A. Spadaro (talk) 15:31, 6 April 2014 (UTC)
- The weak ping makes them not much use in finding the airplane very quickly. You have to be almost on top of it to find it anyway. So, it's really only of use during a long recovery process, not for tracking a hijacked airplane while in flight. StuRat (talk) 15:34, 6 April 2014 (UTC)
- Thanks. I am not talking about finding a hijacked plane in mid-flight. My original question is only referring to finding a plane that has crashed (or perhaps, just landed, without a crash). Thanks. Joseph A. Spadaro (talk) 15:37, 6 April 2014 (UTC)
- OK, in that case the still likely need to spot the wreckage to know where to look to get close enough to detect the ping. The black box isn't designed to help them find the crash site, but rather to find the black box and retrieve it's flight info after they've found the crash site. StuRat (talk) 16:03, 6 April 2014 (UTC)
Some links you may find useful that are to do with Search and rescue, Black box (transportation), Cockpit voice recorder, Flight data recorder and Distress radiobeacon. CambridgeBayWeather (talk) 16:24, 6 April 2014 (UTC)
- Note that the "pinger" only works underwater. It's just a sound that it generates so it can be picked up by a hydrophone/Towed pinger locator. The ELT is a separate piece of equipment. Mr.Z-man 16:34, 6 April 2014 (UTC)
- So, if an airplane crashes on land, there is no "pinging"? Joseph A. Spadaro (talk) 22:39, 6 April 2014 (UTC)
- Correct. If it crashes on land, they can just search through the wreckage manually, and it's generally constrained to a small area. If it crashes in water, it could break apart before it sinks and disperse over a wide area, so trying to find 2 little boxes with remote-controlled submersibles is more difficult. Mr.Z-man 02:36, 7 April 2014 (UTC)
- No, not correct. Just different equipment. The black box will start sonar pings after it comes in contact with salt water. The ELT (which is required on all aircraft, unlike a black box) will emit a radio beacon after a crash. It can also be turned on manually or a voice broadcast on the ELT frequency. The impact force will set it off. In water, however, the radio waves will not be detectable. The ELT's used to operate at 121.5 MHz and 243 MHz but now I believe commercial aircraft have a variety of frequencies for satellite as well as terrestrial location. A crash on land of sufficient force would set off the ELT automatically and if it survives the fire, crash and battery life, will allow rescuers to locate it. I do not know if the black box also has an ELT transmitter. --DHeyward (talk) 06:21, 7 April 2014 (UTC)
- But the ELT is a radio transmitter, not a pinger. Using the same terminology for significantly different things is what causes this confusion in the first place. Mr.Z-man 14:43, 7 April 2014 (UTC)
- It was obvious that the question wasn't about sonar pings when it was stated as a crash on land. There are other types of "pings" so it's incorrect to say that a "ping" is only about underwater sonar pings. In fact, you can open a terminal window on your computer and type "ping localhost" to get a computer ping. See this about MHL satellite pings [22] --DHeyward (talk) 22:24, 7 April 2014 (UTC)
- But the ELT is a radio transmitter, not a pinger. Using the same terminology for significantly different things is what causes this confusion in the first place. Mr.Z-man 14:43, 7 April 2014 (UTC)
- No, not correct. Just different equipment. The black box will start sonar pings after it comes in contact with salt water. The ELT (which is required on all aircraft, unlike a black box) will emit a radio beacon after a crash. It can also be turned on manually or a voice broadcast on the ELT frequency. The impact force will set it off. In water, however, the radio waves will not be detectable. The ELT's used to operate at 121.5 MHz and 243 MHz but now I believe commercial aircraft have a variety of frequencies for satellite as well as terrestrial location. A crash on land of sufficient force would set off the ELT automatically and if it survives the fire, crash and battery life, will allow rescuers to locate it. I do not know if the black box also has an ELT transmitter. --DHeyward (talk) 06:21, 7 April 2014 (UTC)
- Correct. If it crashes on land, they can just search through the wreckage manually, and it's generally constrained to a small area. If it crashes in water, it could break apart before it sinks and disperse over a wide area, so trying to find 2 little boxes with remote-controlled submersibles is more difficult. Mr.Z-man 02:36, 7 April 2014 (UTC)
- So, if an airplane crashes on land, there is no "pinging"? Joseph A. Spadaro (talk) 22:39, 6 April 2014 (UTC)
- I have to admit that with the statement the plane "purposefully" avoided Indonesian airspace in order to fly to a location in the South Indian Ocean, I'm beginning to hum Bond music. How hard is it to build an ad hoc private aircraft carrier capable of landing a jet plane anyway? (And throwing the box overboard...) Wnt (talk) 20:44, 6 April 2014 (UTC)
- The longest Supercarrier class built is 333 m long. Laden Boeing 777's usually land on 6 to 10 000 foot runways that don't roll up and down with the waves or have an immediately adjacent Conning tower. 84.209.89.214 (talk) 00:04, 7 April 2014 (UTC)
- It can land on a 6 foot runway ? Awesome ! :-) StuRat (talk) 02:33, 7 April 2014 (UTC)
- If you're coming in at 160 knots while the carrier is going at 159, you can. However, that argument is invalid; why should anyone with that kind of carrier steal a plane? 217.255.182.173 (talk) 06:13, 7 April 2014 (UTC)
- ... and even more unlikely that the rogue carrier would find an ocean current of that sort of speed, even with a steady opposing headwind. Dbfirs 06:59, 7 April 2014 (UTC)
- Actually I was thinking of something more like nailing together a few hundred meters of wooden planks, having the plane try to land on it and brake as much as possible before rolling off the end, in order that it could "ditch" without ??? being ripped apart. Then towing the intact plane somewhere. But true, a rocket powered supercarrier standing up on hydrofoils looks a lot more bad-ass carrying the plane back to your secret underwater volcano base. Wnt (talk) 12:58, 7 April 2014 (UTC)
- So... I'm not the only one to think about a James-Bond-esque supervillain with a high-speed carrier?
- There might actually be 3 different designs:
- Hydrofoil (tried and tested, but a bit on the slow side)
- Hovercraft (another proven approach, but a maintenance nightmare — but hey, if you have 100s of minions, go ahead, use them)
- Cavitating high-speed hull (don't even know what it's called). Basically the hull forms a bubble, and doesn't touch the water any more, which reduces drag to that of a plane.
- And then there's the ekranoplan (it's a redirect, but there's a photo in the
articklearticle) which is even faster. Not a carrier, but a plane/ship hybrid. IP has a point: Why steal a passenger plane if you have one of these? - ¡Ouch! (hurt me / more pain) 07:16, 8 April 2014 (UTC)- I demand to be acknowledged as Emperor Penguin and to be paid a ransom in unmarked sardines in return for releasing the hostages unharmed from my undetectable floating Pykrete aerodrome somewhere in Antarctica defended by my helmeted stormtroopers massed at "Deception" island volcano base. Yours insanely Mr. P. 84.209.89.214 (talk) 12:49, 8 April 2014 (UTC)
- In Australia, our military has the Over The Horizon Radar (Jindalee OTHR), which would have detected such fanciful devices. The aircraft track is well within the Jindalee detection range. Hopefully. 120.145.70.125 (talk) 10:34, 8 April 2014 (UTC)
- ... and even more unlikely that the rogue carrier would find an ocean current of that sort of speed, even with a steady opposing headwind. Dbfirs 06:59, 7 April 2014 (UTC)
- If you're coming in at 160 knots while the carrier is going at 159, you can. However, that argument is invalid; why should anyone with that kind of carrier steal a plane? 217.255.182.173 (talk) 06:13, 7 April 2014 (UTC)
- It can land on a 6 foot runway ? Awesome ! :-) StuRat (talk) 02:33, 7 April 2014 (UTC)
No brain
I remember reading somewhere about a not too recent medical case regarding an intellectually normal adult person that congenitally lacked most of the brain. If I remember correctly he/she only had the brainstem and all the rest was replaced by water. Has anyone heard of it? --151.41.135.57 (talk) 15:53, 6 April 2014 (UTC)
- Hydrocephalus is the name of the condition, specifically hydrocephalus ex vacuo. The remarkable case I heard of is where the person had essentially normal intelligence, which means the parts of their brain they did have were apparently rewired to take on the functions of the missing parts. StuRat (talk) 15:56, 6 April 2014 (UTC)
- Come on now, we have some leeway on the Ref Desks, but this is beyond the usual level of unsourced/uninformed (and in this case, frankly silly) speculation that sometimes takes roots here. I'd like to use the old chestnut "this isn't brain science" but that's clearly not the case here. Nevertheless, try to appreciate how easily debunked these stories are with the application of the most basic assumptions we can make in neuroscience and appreciate the sentiment one would usually be trying to put across with that idiom. "The parts of their brain they did have were apparently rewired to take on the functions of the missing parts."? We're not talking about one of the temporal lobes taking over function for the other after the latter is damaged or removed; these are vastly different structures with different functions, form and composition. One can no more think without a cerebrum than one could breath without a brainstem and they are not suited to adapting to cover one-another. This is grade-school level brain science. Snow (talk) 22:44, 7 April 2014 (UTC)
- I made no comment on which parts were absent in this case, and therefore which parts took over their function. StuRat (talk) 23:53, 7 April 2014 (UTC)
- No, but without that specification it certainly seems as if you are validating the scenario forwarded by the OP as realistic, and he does specify the presence of only a brainstem. And if you weren't speaking to that particular scenario, then the response doesn't say much, since clearly people are capable of losing some brain tissue from any number of regions while maintaining normal intelligence. But your wording does suggest to me that you were speaking to that or a similarly extreme (and impossible) scenario. Regardless, the Ref Desks are not the place for forwarding speculation based on apocryphal tales; we're meant to be assisting those with questions in finding and contextualizing established answers to their questions, ideally backed by solid sources or at least content from within the project, not pure conjecture based on an old wive's tale. As I said, we get some latitude with regard to WP:V, owing to our unique function within the project, but that doesn't mean we can throw the principle out the window altogether. There's a bit too much of that here at times and it's a liability to these pages since, A) it's likely undermine trust in our responses, collectively, and B) it might start to cause some to question if we are operating in a manner that is consistent with broader wikipedia policy and priorities. In short, we may not have to go through the formality of sourcing every claim we make in answering a question as we would in an article, but we do need to be at least reasonably certain we could provide a reliable source if it came down to it, that those sources would be trusted sources in an article context, and that the claims themselves pass basic empirical rigour. Snow (talk) 00:54, 8 April 2014 (UTC)
- I provided the relevant link to a Wikipedia article, along with the specific variation the OP described. From there they can find all the sources they need. StuRat (talk) 00:57, 8 April 2014 (UTC)
- It's not the mention of that condition or that wikilink that I found inappropriate to this locale, but rather your following statements. Given that he mentioned water, it was reasonable to point him to that information, no doubt; that is a medical condition with firm sourcing. The following speculation (not at all consistent with any medical science), however, is not appropriate as it cannot be verified and the scenario you presented is likely to cause the OP, and possibly others reading it, to think there is some possibility that the story he heard is plausible, which it is not - not even vaguely. This type of extraordinary claim should not be so much as mentioned here as an empirical possibility without verification, which is certainly not found in the article linked to. Snow (talk) 01:16, 8 April 2014 (UTC)
- This paper might interest you[23] Sean.hoyland - talk 16:17, 6 April 2014 (UTC)
- I've encountered this question literally dozens of times, over a span of 25 years. Rather than write out a long answer here, I've placed it on my personal web site so that in future I can just give a pointer to it -- see http://weskaggs.net/?p=2311. Our article on John Lorber is also relevant. Looie496 (talk) 16:26, 6 April 2014 (UTC)
- Well you might like to check out this lady, who was a neighbour of mine in Barnsley and I can tell you she was one sharp cookie! --TammyMoet (talk) 18:08, 6 April 2014 (UTC)
- Unfortunately that case never seems to have been published -- and web pages, as we Wikipedians all know, can't be treated as reliable sources. Looie496 (talk) 21:08, 6 April 2014 (UTC)
- That's not a peer-reviewed source, and in any case the conclusions there are pretty conservative. I have no doubt that hydrocephaly can tell us important things about brain plasticity. But there is no acceptable evidence from it that you can have normal intelligence with only a tiny sliver of a brain. Looie496 (talk) 13:29, 7 April 2014 (UTC)
- I think you're thinking of anencephaly. Extreme degeneration from hydrocephalus might work too, but that shouldn't take effect until after a brain has formed. Wnt (talk) 20:50, 6 April 2014 (UTC)
- I've never seen a claim of normal intelligence, or anything resembling it, in anencaphaly. Almost certainly the question relates to the hydrocephaly stuff, which has been permeating the internet for decades. Looie496 (talk) 21:08, 6 April 2014 (UTC)
- D'oh! true, I missed the part about the normal intelligence :) . Could be microcephaly, which can display normal intelligence, but I suppose anencephaly by definition ought not to (though there are some genetic causes that can be associated with either). Wnt (talk) 22:49, 6 April 2014 (UTC)
Stephen King's Thinner mentions just such a case, but as a background detail [24].--Auric talk 03:26, 7 April 2014 (UTC)
- Good link - trace it back a few steps and you arrive at John Lorber's non peer reviewed book "Is Your Brain Really Necessary?" But the issue is, it's not peer reviewed and our article suggests he might not have read the scans right. Which I should mention reminds me of the famous Terri Schiavo case - when forum participants would look at the brain scan on the right, half would say that "all her brain is gone but a few bits", and the other half would say that there's still a thick layer of cortex and even if there's a real difference in density within that, that doesn't prove it's genuinely gone. I'm not actually an expert in reading brain scans but I'll confess some affinity for the latter position. Wnt (talk) 11:34, 7 April 2014 (UTC)
- I suspect that a part of the misinterpretation here is that if you (as an untrained lay-person) look at a photograph of a brain scan - a simple 2D cross-section through the skull - there are at least three very serious ways in which you might misinterpret what you see:
- Suppose, hypothetically, you see a scan where a region about half the diameter of the brain-case is full of water. A naive first guess is "OMG! That person has lost 50% of his/her brain!"...but that's not true. The volume of a sphere is proportional to the cube of the radius - so it's not 50% that's missing, it's only 12.5%. Someone with "half a brain" is going to be in a lot more trouble than a person with 88% of a brain. I'm guessing most people would have no trouble believing that someone with 88% of their brain intact could be quite intelligent.
- The person who did the scan probably took numerous cross-sections of the brain. Only one of those 'slices' is presented in the photograph that you are being "wowed" with. Ask yourself: Of all of those slices, which one gets published? Well, it's likely to be the most dramatic one. So if (again, hypothetically), someone had a flattened ellipsoidal void in their brain, we'd be seeing pictures of the largest cross-section through it...it's perfectly possible that the brain appears completely intact in the scans just a half inch higher or lower. By assuming this is a roughly spherical void, you might be grossly over-estimating the lost volume.
- The part of the brain that's missing may not be a part that's particularly to do with intelligence. Certainly the limbic system and the cerebellum are generally not considered to be areas responsible for what we'd consider to be "intelligence". On the other hand, a loss of large chunk of cerebrum would be pretty catastrophic.
- Taken together, it's easy to imagine seeing an exceedingly alarming brain cross-section and have only a few percent of the brain matter actually being missing - and that could easily come from areas unrelated to intelligence. So perhaps, this kind of thing isn't so surprising after all.
- SteveBaker (talk) 20:53, 7 April 2014 (UTC)
- A TV news program, probably 60 Minutes on CBS, ran a story years ago about a high school girl whose brain had been affected by hydrocephalus such that only a half-inch or so of brain tissue was visible in CT scan, next to the skull, but she was very average in her grades. Her gait was affected slightly. Edison (talk) 01:02, 8 April 2014 (UTC)
- As a first approximation say a brain is a sphere four and a half inches in diameter, with volume about 90 cubic inches. If she still has a half inch around the outside that gives sphere of 3 and half inches diameter missing. So she would have (4.5^3-3.5^3)/4.5^3 of the brain remaining which is just over half of it. People can get by with half a brain. Plus the pressure probably enlarged her head a bit so that could easily be a bad underestimate. Dmcq (talk) 12:43, 8 April 2014 (UTC)
Krubera Cave
I was reading several of the articles of the various cave systems in the world. How do scientists measure the depth of the caves (e.g. Krubera Cave)? 99.250.118.116 (talk) 18:14, 6 April 2014 (UTC)
- The Wikipedia article about the Krubera Cave in Georgia notes that it is the deepest-known cave in the world and has been explored to a record 2,197 ± 20 metres. The sea floor topography near Arabika has been revealed by a digital bathymetric map that combines depth soundings and high-resolution marine gravity data. The data used to make bathymetric maps typically comes from an echosounder (sonar) mounted beneath or over the side of a boat, "pinging" a beam of sound downward at the seafloor. The amount of time it takes for the sound or light to travel through the water, bounce off the seafloor, and return to the sounder informs the equipment of the distance to the seafloor. 84.209.89.214 (talk) 23:34, 6 April 2014 (UTC)
- Barometric altimeter + depth gauge for the wet bits most likely. --catslash (talk) 00:15, 7 April 2014 (UTC)
- Any cave that is explored generally has a cave survey made, but I have no idea how surveying methods work underwater. shoy (reactions) 12:16, 8 April 2014 (UTC)
Medical prostration
According to the "Signs and symptoms" section of Marburg virus disease, patients in the disease's early virus phase experience prostration; the article links to our Prostration article, but that covers "the placement of the body in a reverentially or submissively prone position as a gesture". What's actually meant? Prostration has a hatnote saying to read Hyperthermia for "heat prostration", but I'm not sure whether that's meant, whether they mean that the patient is unable to be in any position except lying down, or something else. Nyttend (talk) 18:40, 6 April 2014 (UTC)
- The word "prostration" in general means lying in a spread-out position.[25] ←Baseball Bugs What's up, Doc? carrots→ 19:21, 6 April 2014 (UTC)
- See Definition 2 here. As a medical term, it means "exhaustion". Heat prostration is an exact synonym of "heat exhaustion", which is when you become so hot, you can no longer effectively move around, or even support your own weight. --Jayron32 19:43, 6 April 2014 (UTC)
April 7
Induction lamp cooking
As far as I know a powerful enough oscillating magnetic field can light up a lamp. Does it mean that you can make a light bulb glow by putting it on the induction cooking stove? 91.77.167.192 (talk) 08:38, 7 April 2014 (UTC)
- Those induction cookers need a big thick slab of metal to induce currents in. A light bulb filament is not enough. If you irradiated it with millimeter wave or other microwaves, perhaps you could light it. Graeme Bartlett (talk) 09:06, 7 April 2014 (UTC)
- Centimeter wavelengths will certainly light up an incandescent light bulb as generations of schoolboys and numerous You Tube videos can attest. --2A01:E34:EF5E:4640:9D8E:1D42:1F74:5600 (talk) 13:33, 7 April 2014 (UTC)
And what if you attach a light bulb to this “thick slab of metal”, will it light up? I'm just curious if it is possible to get something glowing with a cooker of this type.91.77.167.192 (talk) 13:25, 7 April 2014 (UTC)
- I don't suggest you do the experiment, but I would think it more likely to melt. What you want is an Induction lamp.--Shantavira|feed me 14:01, 7 April 2014 (UTC)
Could a machine assemble molecules on demand just starting with the atoms?
That would be easier than synthesizing compounds. If you wanted gasoline (C8H18) or cocaine (C17H21NO4), this hypothetical machine would just pick the right atoms and start putting them together. Or maybe, could a machine generate random molecules from a set of atoms? Maybe putting them under pressure, or pass an electrical current through them and see what gets out of the process. — Preceding unsigned comment added by 88.14.192.119 (talk) 17:58, 7 April 2014 (UTC)
- How do you put them together fast enough? 12 grams of carbon contains 602,000,000,000,000,000,000,000 atoms. Even if a machine could grab and use a million carbon (and equivalent number of hydrogen) atoms per second, that would still take 602,000,000,000,000,000 seconds to make just 16 grams of methane (CH4). That's a bit more than 19,000,000,000 years. The earth has only existed for 4,500,000,000 years. --Jayron32 18:15, 7 April 2014 (UTC)
- (ec, same idea, other numbers) I think you underestimate the number of molecules that make up a useful amount of gasoline. A molecule of octane weights 114 u. Thus a mol of gasoline weights 114 g. That's 6.022×1023 molecules - in other words, one litre has about 3.6×1024 molecules. If your machine makes a million molecules per second, it would have made about 0.01 litres in the time since the beginning of the universe. A big advantage of chemical reactions is that they happen in a massively parallel manner, thus yielding macroscopic amounts of product in realistic time frames. --Stephan Schulz (talk) 18:21, 7 April 2014 (UTC)
- Hmmmm, isn't your body made out of such machines? Count Iblis (talk) 18:42, 7 April 2014 (UTC)
- No, it isn't. You can not program yourself to excrete gasoline or cocaine out of your nose. You (that is, any human) can not even produce all the amino acids the body needs to maintain its structure and function; some of them are essential amino acids, meaning you've got to get them from outside (as food) and can not manufacture them internally (although some other organisms can). There are organisms that can produce hydrocarbons suitable as fuel for internal combustion engines; but there are no "universal" organisms that can produce any chemical compound you want on demand. --Dr Dima (talk) 19:17, 7 April 2014 (UTC)
- That is the intent of Molecular assemblers - an idea to make very, very tiny robots that are capable of assembling things atom by atom. The way such devices would overcome Jayron's objection is by self-replicating themselves - making enough of them to do the needed work in a reasonable amount of time - then disassembling each other again when the work is done. It's not entirely clear whether such things are possible, given the laws of physics as we know them...but the idea is not without merit.
- HOWEVER, this won't get you free gasoline. The amount of energy a machine such as this would require to make gasoline would be many, many times more than the energy you'd get back by burning the gasoline subsequently. The general problem is that you need to apply significant amounts of force to (for example) dismantle a CO2 molecule into carbon and oxgen atoms - and yet more energy to force them back together into the form you wanted.
- Anyway - check out the Molecular assembler article - it pretty much explains all of this. SteveBaker (talk) 19:12, 7 April 2014 (UTC)
- They're called refineries, chemical plants, plants, bacteria, yeast, etc. The notion of mechanically assembling molecules, rather than chemically assembling them, strikes me as about as practical as training llamas to become brick-layers. μηδείς (talk) 21:03, 7 April 2014 (UTC)
Are we going out of fertilizer ingredients, in the same way we are going out of oils?
They are the six macronutrients: nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S); eight micronutrients: boron (B), chlorine (Cl), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), zinc (Zn) and nickel (Ni). I dont mean locally on the soil of a concrete location, but globally. — Preceding unsigned comment added by 88.14.192.119 (talk) 18:06, 7 April 2014 (UTC)
- (edit conflict) See Peak phosphorus. This is considered, in some circles, to be a greater problem than Peak Oil, and a major looming catastrophe, specifically because of the need of phosphorus in fertilizers. --Jayron32 18:11, 7 April 2014 (UTC)
- Note that only chemical fertilizers are in danger of running out. There's no shortage of manure (especially around elections), and, if night soil is included, we have quite a dollop of that. StuRat (talk) 18:22, 7 April 2014 (UTC)
- Au contraire, the problem is there isn't enough bullshit in the world, even accounting for politicians, to cover all the fertilizing needs we have. --Jayron32 18:24, 7 April 2014 (UTC)
- If 100% is collected and reused, and no-till farming is employed, along with other methods to prevent soil run-off, then I'd expect very little chemical fertilizer would be needed. StuRat (talk) 18:35, 7 April 2014 (UTC)
- (if 100% is reused, then we have no harvest! ;) Our yield of e.g. corn is approaching ten times the historical values [26]. Some of the increase is due to pesticides, breeding, and other methods. But without cheap, concentrated chemical fertilizers, corn yields would be back in the ~20 bushels an acre across the USA cornbelt. And that would drive up the cost of the many food products using corn and it's derivatives. Sure, you can compost everything and do low-input agriculture in your backyard, but that doesn't change the fact the the majority of the agricultural industry in the USA is based off of cheap and widely-accessible processed fertilizers. It is true that agronomically, we know how to do better, and grow with less energy and inputs. But what matters is what the large agribusinesses actually do. SemanticMantis (talk) 18:53, 7 April 2014 (UTC)
- You'd need to set up a system to collect sewage from sewage processing plants and move it to farms. We'd also need to stop flushing old meds, etc. A dual sewage system could be set up, with things like meds going to a septic tank, instead. StuRat (talk) 19:06, 7 April 2014 (UTC)
- Um, phosphorus isn't destroyed. If run-off into the oceans is a problem, then the oceans are also the solution--recovery from salt pans and, as the price increases, use of nuclear energy to extract trace elements from the ocean. It's simply silly to assert we are running out. It's just becoming more expensive to procure. μηδείς (talk) 20:58, 7 April 2014 (UTC)
- I would disagree with that. Recycle before it goes into the sea would help prevent algae blooms that cut off light to the sea-floor, which cause the seabed plants to die off, which in-turn removes a vital food source for the local coastal sea-life which in turn means we reduce the amount sustainable seafood that we enjoy. This is a growing problem around the world and some sizeable lakes too are now dead. The sea is too large a volume for the concentration of Phosphorus to increase above 13mg/kg to point were it becomes economic to recover it.--Aspro (talk) 21:55, 7 April 2014 (UTC)
- I didn't say anything about not recycling or preventing run-off, which will also become increasingly economically urgent. The problem is the question as posed is a typical "what if all the farmland gets used for parking lots?!?!" question that ignores market forces. As phosphorus or farming land or any commodity gets more scarce its value increase, and more money is invested into procuring phosphorus or land values increase, and people sell their sprawling lots and move into efficient high rises high-rises. You are making the same mistake. At some point if phosphorus becomes rare its price will increase and people will invest in new ways to concentrate it and it will be economical to recover it at 13mg/liter.
- Hmmm, 160E6 metric tonnes/ yr currently produced = 1.6E11 kg, divided by the 13 mg/kg seawater = 1.2E16 kg of seawater to process. The Nile discharges 2830 m3/s, x365.25x24x60x60x1E6 = 8.9E16 kg/s. So if you desalinate a substantial river of water to meet the needs of dry surface areas, in theory you should get enough
potassiumphosphorus left over to do the fertilization. If you can separate it somehow. It takes a lot of energy, but so does any solution to the vast deserts predicted to arise from global climate change. Wnt (talk) 23:08, 7 April 2014 (UTC)
- Hmmm, 160E6 metric tonnes/ yr currently produced = 1.6E11 kg, divided by the 13 mg/kg seawater = 1.2E16 kg of seawater to process. The Nile discharges 2830 m3/s, x365.25x24x60x60x1E6 = 8.9E16 kg/s. So if you desalinate a substantial river of water to meet the needs of dry surface areas, in theory you should get enough
- When did we switch to potassium? There's no shortage of potassium. I'm assuming you just mistyped. --Trovatore (talk) 23:36, 7 April 2014 (UTC)
- Burn seaweed in a steam engine (Algae fuel) then process the ashes. Jim.henderson (talk) 23:43, 7 April 2014 (UTC)
- This brings back the old proposal to build a canal to flood the depression in the African dessert from the Med Sea. You could then let it dry out to get your phosphorus, but you'd also have to remove the salt. StuRat (talk) 23:44, 7 April 2014 (UTC)
- Or process brine left over as water evaporates to a low level behind the proposed Red Sea Dam. Since the Red Sea is of fairly recent origin, it's hard for me to feel too bad about destroying a major ecosystem, if the energy goes a substantial way toward ending global warming and provides irrigation for the entire region. Besides, I have a crank notion about it as a prophesied solution to the Israel-Palestine conflict. :) Wnt (talk) 00:18, 8 April 2014 (UTC)
- (Un-indent) What is all this idle talk about extracting phosphorus from seawater, flooding the Sahara, damming the Red Sea, etc., when a much more cost-effective solution has already been devised -- that being to precipitate phosphates out of municipal sewage (at the sewage treatment plant) with, e.g., calcium hydroxide? 24.5.122.13 (talk) 05:13, 8 April 2014 (UTC)
LCD TVs that are not black?
My father had a question for me last week. One of his customers had told him that LCD TVs would fit much more nicely to their home design if they were white when powered off instead of black. My father asked me if this is possible. I didn't have any idea, as electronics design is not my profession, it's computer software. I know at least that some monochrome LCDs are light gray when powered off, and the lit crystals are black, but is this kind of thing at all possible on a full-colour LCD? JIP | Talk 18:17, 7 April 2014 (UTC)
- I suggest the TV be placed in a cabinet and the doors closed when not in use. (Eventually, I hope the cost of displays will come down and reliability will go up to the point where we can use them as lights, always on, displaying an all-while panel or whichever background we prefer.) StuRat (talk) 18:29, 7 April 2014 (UTC)
- One problem I foresee is that if the error correction is not spot on for every frame, one will notice lots of bright speckles on the picture. Whereas now, when a few pixels at a time are randomly going dark here and there for each and every frame, they are not perceived by the eye. I seem to remember the luminous signal on analogue TV is negative going, for the reason that the radio static is less noticeable when there is a good signal to noise ratio. When the S/N ratio diminishes towards no broadcast signal at all, even the cosmic background radiation can be seen as a mass of speckles often referred to as snow.--Aspro (talk) 22:57, 7 April 2014 (UTC)
- Permit a sceptic to doubt that Aspro's TV is capable of displaying cosmic background radiation. 84.209.89.214 (talk) 12:12, 8 April 2014 (UTC)
Is it true that the Pacific Ocean makes the west coast of North America warmer?
My friend told me he wants to move to Vancouver because it's warmer there, and he insists that it's warm because the Pacific Ocean makes the climate warmer there. Is this true? ScienceApe (talk) 19:04, 7 April 2014 (UTC)
- Yes, similar to how the Gulf Stream in the Atlantic makes Western Europe warmer. The water can be warmer near Vancouver, Seattle, etc, than in Los Angeles. Of course, that warm water also brings lots of rain, and global warming may eventually change the ocean currents. StuRat (talk) 19:11, 7 April 2014 (UTC)
- More moderate; both less cold and less hot; see Oceanic climate Jim.henderson (talk) 19:13, 7 April 2014 (UTC)
- See http://cliffmass.blogspot.com/2012/06/why-are-coastal-water-temperatures.html for a pic showing unusual cooler water temps hugging the coast. StuRat (talk) 19:16, 7 April 2014 (UTC)
- (ec)Warmer than where? Large bodies of water have, in general, a mediating influence on peak temperatures, both high and low. So Seattle is warmer in January than e.g. Bismarck, North Dakota. On the other hand, Bismarck is warmer than Seattle in Juli. Also see continental climate vs. oceanic climate. --Stephan Schulz (talk) 19:17, 7 April 2014 (UTC)
- Note that water behaves like land if it freezes solid, but remains mostly liquid near Vancouver year-round. And where the currents go the other way, as in Maine or Korea, it's not particularly warm in winter. StuRat (talk) 19:19, 7 April 2014 (UTC)
- Even in Maine, costal areas are a lot warmer in winter than North Dakota. Similarly, South Korea is warmer in winter than North Dakota. North Korea has a continental climate, with winter temperatures influenced by weather systems coming out of Siberia. But still, its winter is not colder than North Dakota's. --Stephan Schulz (talk) 21:46, 7 April 2014 (UTC)
- Still, if one wants a Canadian city with a mild climate, with little snow, then Vancouver is a better choice than Newfoundland: [27]. StuRat (talk) 00:28, 8 April 2014 (UTC)
- Well, St. John's, Newfoundland and Labrador is about 150 miles further north than Vancouver, so that may have a bit to do with it as well. --Jayron32 01:49, 8 April 2014 (UTC)
- "The Pacific Ocean makes the west coast of North America warmer" is only true down to the latitude of Seattle or so. Farther south the ocean has a cooling effect. In San Diego, for example, it is usually very pleasant if you are within a couple of miles of the ocean, but rapidly gets blazing hot if you go a little farther inland. Looie496 (talk) 01:57, 8 April 2014 (UTC)
Inflatable hot tub mystery.
We have an inflatable hot-tub. It's construction is more or less identical to a kiddies paddling pool - three stacked inflatable rings with an inflatable "floor", each with it's own air valve. The "floor" valve is underwater, the other three are on the outside of the tub. The thing is made of some kind of very heavy duty vinyl and is about 5' in diameter and two feet deep and contains around 250 gallons of water at around 38 degC (100F). There is a separate heater/water pump and a gizmo to blow air into the water via a channel around the inside of the bottommost ring..but I don't think that's relevant here. We've been using it off and on for about 6 months - and it's never been completely emptied or deflated in all that time.
The other day, we had occasion to empty the water and deflate all of the air chambers. I was surprised to find that the "floor" had about two gallons of water sloshing around INSIDE the sealed air bladders.
I'm totally mystified as to how the water got in there! The floor is under considerable pressure (2 feet of water resting on it) - so if there were some kind of a leak, you'd expect it to deflate in short order - and it didn't seem to be deflated to any noticable degree. The air must be at about the same pressure as the water when the tub is full. It's conceivable that the air we put into the floor part at the outset was really humid - but even if all of the water vapor condensed out of that air...there was FAR too much water in there to be accounted for in that way.
It's not exactly important that I know the answer - but it's been bothering the heck out of me for the past week...and I can't explain it!
There is a picture of the hot tub here:
http://www.ebay.com/itm/like/350868160135?lpid=82
...ours has an outer protective cloth cover in bright blue and an inflatable "lid" that covers it when not in use - but that's the thing I'm talking about here.
SteveBaker (talk) 19:31, 7 April 2014 (UTC)
- I'd say an equal volume of air leaked out as the water that leaked in, so the total volume remains the same. The water likely leaked in at the valve, the air might have also leaked out there, but too slowly to notice the bubbles, or perhaps there's a pinhole leak elsewhere. The water pressure might go up instantly when you first sit in the tub, just enough for a few drops of water to drip in each time.
- It might actually make more sense to fill the bottom with water than air, as that should make it more stable in the wind. It would also make it heavier, but if you don't plan to move it until you drain it, then that's OK. StuRat (talk) 20:06, 7 April 2014 (UTC)
- My problem with that idea is that when you step into the tub, the weight of your foot on the floor must increase the pressure of the air inside above that of the water on top of it...so I could believe in a bubble being expelled through a tiny hole each time - but the water would have to force it's way in through a positive pressure gradient. It's not like the floor is a rigid container either - it's flexible/stretchable plastic - and you'd think that the tension it adds would keep the air pressure consistently higher than the water pressure. SteveBaker (talk) 20:29, 7 April 2014 (UTC)
- In that case, maybe when you get out, the lowered pressure in the air bladder sucks in some water to replace the gas bubbles which escaped when you got in (escaped from the air bladder, not from you, as those gas bubbles aren't relevant here). StuRat (talk) 00:25, 8 April 2014 (UTC)
- Get a copy of the owner's manual or contact the manufacturer. They will likely have something to say about the issue. μηδείς (talk) 20:52, 7 April 2014 (UTC)
- I think a pinhole leak/defect is always possible; it's also conceivable that water could permeate the polymer directly. (Trying to calculate that, based on the exact polymer and plasticizer compositions, would be a decent test of the Refdesk). Wnt (talk) 22:54, 7 April 2014 (UTC)
- Even if there is just a bit of vapor permeability (whether directly through the polymer layer, or through the valves) there will be tendency for liquid water to accumulate on the bottom of the bladder. The liquid in the hot tub (and the polymer layer in contact with it) is likely to often (or always) be at a temperature higher than the ground under the tub. Warm vapor that enters through the top side of the bladder cools and condenses when it reaches the bottom, forming a pool of liquid water...that will just keep growing, for however many months the tub remains in use. TenOfAllTrades(talk) 14:02, 8 April 2014 (UTC)
- Did you inflate it yourself? Is it possible someone deliberately put water in to anchor it before inflation? -- Q Chris (talk) 14:52, 8 April 2014 (UTC)
April 8
3D Printing using soil
Are there any useful building materials in soil? Like if one day in the future they have such advanced 3D printers that you can just take some soil from your back yard, dump it into the machine, and it's able to build something useful out of it. ScienceApe (talk) 13:06, 8 April 2014 (UTC)
- Depending on what type of soil you have, perhaps:
- 1) Clay: Can be fired to make ceramics.
- 2) Sand: Can be melted to form glass.
- In both cases, you can't use current 3D printing techniques to make objects directly out of those materials, but you might be able to use a mold or form you create using 3D printing to make an object out of clay or sand. StuRat (talk) 13:13, 8 April 2014 (UTC)
- Yes, there are useful building materials in soil. You don't need to use a 3D printer. Mudcrete is often used in road construction, while Rammed earth has been used for building for many millennia. As you will see from the top of the page, though, we are unable to provide predictions or speculation about future technological developments. RomanSpa (talk) 13:25, 8 April 2014 (UTC)
- Soil has been used as a building material for thousands of years. It is literally the first building material. Besides clay, ceramics, and glass noted above, there's other building materials made essentially out of dirt: brick, adobe, mudbrick, sod, Cob, and dozens more which I am tired of searching for. --Jayron32 14:24, 8 April 2014 (UTC)
Antibiotic resistance arguments applied to vaccines
If it is recommended that usage of antibiotics be limited to discourage antibiotic resistance, why does it not then follow that vaccine usage be limited to discourage vaccine resistance? (Note: I am not a supporter of the anti-vaccination movement, but I have heard this argument against vaccines advanced by some anti-vaccination partisans and would like to hear a counterargument.) —SeekingAnswers (reply) 13:55, 8 April 2014 (UTC)
- Vaccines are far more specific than antibiotics. A typical antibiotic acts against a broad range of bacteria, but a typical vaccine only acts against one very specific type of virus. In fact often the biggest challenge in developing a vaccine to is get it to work against all the different strains of a particular kind of virus. The consequence is that resistance to one type of vaccine does not cause problems in dealing with any type of virus except the specific one that that vaccine is designed for. Looie496 (talk) 14:12, 8 April 2014 (UTC)
- Also, it should be noted that antibiotics act as vaccines for the bacteria they kill. That is, the general idea behind antibiotic resistance is that bacteria are exposed to an agent designed to harm them, and they adapt so the agent isn't harmful anymore. Which is almost exactly what a vaccine does for you. In the first case, you're training bacteria to not be harmed by the antibiotics, and in the second case, you're training yourself to not be harmed by viruses. Furthermore, in science we don't base our understanding of the world by incomplete analogy. We base it by actual data. Antibiotic resistance is an observed phenomenon, and is happening. For most diseases we vaccinate against, we aren't finding any such evidence of that happening. I say most, because there are SOME diseases which show resistance to vaccinations: [28] but as that reference makes clear, most of those are the exception and not the rule. --Jayron32 14:19, 8 April 2014 (UTC)
- Sorry, but "antibiotics act as vaccines for the bacteria they kill" is at best misleading. Vaccines work by activating the body's immune system; antibiotics work by attacking bacteria directly. Vaccine resistance is possible, but the only way for a virus to develop resistance is for it to get better at overcoming the immune response, and there is strong evolutionary pressure in that direction even without any vaccine being present. Looie496 (talk) 14:48, 8 April 2014 (UTC)
- Also, it should be noted that antibiotics act as vaccines for the bacteria they kill. That is, the general idea behind antibiotic resistance is that bacteria are exposed to an agent designed to harm them, and they adapt so the agent isn't harmful anymore. Which is almost exactly what a vaccine does for you. In the first case, you're training bacteria to not be harmed by the antibiotics, and in the second case, you're training yourself to not be harmed by viruses. Furthermore, in science we don't base our understanding of the world by incomplete analogy. We base it by actual data. Antibiotic resistance is an observed phenomenon, and is happening. For most diseases we vaccinate against, we aren't finding any such evidence of that happening. I say most, because there are SOME diseases which show resistance to vaccinations: [28] but as that reference makes clear, most of those are the exception and not the rule. --Jayron32 14:19, 8 April 2014 (UTC)
About a birds name
I have seen a bird in towns in eastern side of India in West Bengal.the birds are big in size have copper coloured wings and rest of the body is black.But unfortunately I dont know the name of this bird.Please tell me the name of this bird and its habits.Please mention if there is any Wikipedia article about this bird.Another type of bird that has come to my notice is even smaller than a sparrow but I am startled and amazed by its colour.There is a greenish lustre or blueish green lustre.It appears as if the bird's feathers act as a diffraction grating.It has very thin carved beak which I think can prominent feature.Please identify this bird and give its name and corresponding Wikipedia article.Pleasemention the habits of these birds and where you have seen them.117.194.229.124 (talk) 14:01, 8 April 2014 (UTC)
- It sounds like a member of the Starling or Myna family, many of which are known for their iridescent feathers as you describe. --Jayron32 14:09, 8 April 2014 (UTC)
I see the picture of Starlig but it doesnt match the second type of bird I queried about.Please dont forget to mention about the first bird.117.194.229.124 (talk) 14:14, 8 April 2014 (UTC)
- A straightforward Google search finds the Greater Coucal. Looie496 (talk) 14:17, 8 April 2014 (UTC)
- There are hundreds of different species of birds in the starling family. Your second type of bird could be any number of them, including the Common Starling, which also has a number of subspecies. Don't just look at one picture in the first article you find. Search through pictures of different kinds of starlings and see if you find any matches. --Jayron32 14:21, 8 April 2014 (UTC)
Searching Google for Greater Coucal I got resuls inn image section purplecoloured sunbird and luckily it was the second type type of bird I asked about.The first type of bird is Greater Coucal. Actually in our locality there is overcrowding of crows a menace with some sparrows so it is a delight to see these wonderful birds.Thanks for your kind help.117.194.229.124 (talk) 14:53, 8 April 2014 (UTC)
Chemical analysis
Suppose a person stumbles upon a substance and he wants to know the chemical composition of the substance.The substance can be quite complex mixture of organic and inorganic compounds.How will the chemist know about what are the compounds present and in what proportion.How this used to be accomplished when there was no NMR spectra or computers available and how is it done nowadays.Isthis type of analysis required in forensic studies and where else this type of analysis required. Can natural substances like fruit skin,fruit juice and products like soaps perfumes creams ointments can be analysed to dtermine composition and what knowledge and expertise and what level of nhemistry knowledge is required to do this.117.194.229.124 (talk) 14:11, 8 April 2014 (UTC)
- The first thing the person would have to do is some form of separation techniques to get the mixture into its component substances. A common method is to combine a separation technique with an analytical technique to then identify the substances as soon as they are separated. Analytical systems like GC-MS or LC-MS (for gas chromatography-mass spectrometry or liquid chromatography-mass spectrometry respectively) are commonly used for that exact purpose. You can also do your separation techniques prior to doing your analysis work; for example using Ion-exchange chromatography and then isolating each product and doing analysis individually. This is commonly done for techniques that don't "marry" well to a gas chromatograph, such as Infrared spectroscopy or Nuclear Magnetic Resonance. --Jayron32 14:35, 8 April 2014 (UTC)
