G protein
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (February 2008) |
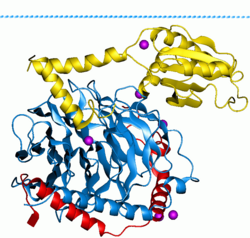

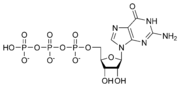
G proteins, short for guanine nucleotide-binding proteins, are a family of proteins involved in second messenger cascades.
G proteins are so called because they function as "molecular switches," alternating between an inactive guanosine diphosphate (GDP) and active guanosine triphosphate (GTP) bound state, ultimately going on to regulate downstream cell processes.
G proteins were discovered when Alfred G. Gilman and Martin Rodbell tried to figure out how adrenaline stimulated cells. They found that when a hormone like adrenaline bound to a receptor, the receptor did not stimulate enzymes like adenylate cyclase directly. Instead, the receptor stimulated a G protein, which then stimulated the adenylate cyclase to produce a second messenger, cyclic AMP.[1] For this discovery they won the 1994 Nobel Prize in Physiology or Medicine.[2]
G proteins belong to the larger group of enzymes called GTPases.
Function
G proteins are important signal transducing molecules in cells. In fact, diseases such as diabetes, blindness, allergies, depression, cardiovascular defects and certain forms of cancer, among other pathologies, are thought to arise due to derangement of G protein signaling.
The human genomes encodes roughly 350 G protein-coupled receptors, which detect hormones, growth factors and other endogenous ligands. Approximately 150 of the GPCRs found in the human genome have unknown functions.
Types of G protein signaling
G protein can refer to two distinct families of proteins. Heterotrimeric G proteins, sometimes referred to as the "large" G proteins that are activated by G protein-coupled receptors and made up of alpha (α), beta (β), and gamma (γ) subunits. There are also "small" G proteins (20-25kDa) that belong to the Ras superfamily of small GTPases. These proteins are homologous to the alpha (α) subunit found in heterotrimers, and are in fact monomeric. However, they also bind GTP and GDP and are involved in signal transduction.
Heterotrimeric G proteins
Heterotrimeric G proteins share a common mode of action, i.e., activation in response to a conformation change in the G-protein-coupled receptor, exchange of GTP for GDP and dissociation in order to activate further proteins in the signal transduction pathway. However, the specific mechanism differs between different types of G proteins.
Common mechanism
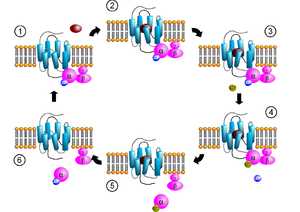
Receptor-activated G proteins are bound to the inside surface of the cell membrane. They consist of the Gα and the tightly associated Gβγ subunits. At the present time, four main families exist for Gα subunits: Gαs, Gαi, Gαq/11, and Gα12/13. These groups differ primarily in effector recognition, but share a similar mechanism of activation.
Activation
When a ligand activates the G protein-coupled receptor, it induces a conformation change in the receptor (a change in shape) that allows the receptor to function as a guanine nucleotide exchange factor (GEF) that exchanges GTP in place of GDP on the Gα subunit. In the traditional view of heterotrimeric protein activation, this exchange triggers the dissociation of the Gα subunit, bound to GTP, from the Gβγ dimer and the receptor. However, models that suggest molecular rearrangement, reorganization, and pre-complexing of effector molecules are beginning to be accepted. Both Gα-GTP and Gβγ can then activate different signaling cascades (or second messenger pathways) and effector proteins, while the receptor is able to activate the next G protein.
Termination
The Gα subunit will eventually hydrolyze the attached GTP to GDP by its inherent enzymatic activity, allowing it to re-associate with Gβγ and starting a new cycle. There do exist groups of proteins called RBMs that act as GTPase-activating proteins (GAPs), which are specific for Gα subunits, which act to accelerate hydrolysis and terminate the transduced signal. In some cases the effector itself may possess intrinsic GAP activity, which helps deactivate the pathway. This is true in the case of phospholipase C beta, which possesses GAP activity within its C-terminal region. This is an alternate form of regulation for the Gα subunit.
Specific mechanisms
- Gαs stimulates the production of cAMP from ATP. This is accomplished by direct stimulation of the membrane-associated enzyme adenylate cyclase. cAMP acts as a second messenger that goes on to interact with and activate protein kinase A (PKA). PKA can then phosphorylate a myriad of downstream targets.
- Gαi inhibits the production of cAMP from ATP.
- Gαq/11 stimulates membrane-bound phospholipase C beta, which then cleaves PIP2 (a minor membrane phosphoinositol) into two second messengers, IP3 and diacylglycerol (DAG).
- Gα12/13 are involved in Rho family GTPase signaling (through RhoGEF superfamily) and control cell cytoskeleton remodeling, thus regulating cell migration.
- Gβγ sometimes also have active functions, e.g., coupling to L-type calcium channels.
Small GTPases
Small GTPases also bind GTP and GDP and are involved in signal transduction. These proteins are homologous to the alpha (α) subunit found in heterotrimers, but exist as monomers. They are small (20-kDa to 25-kDa) proteins that bind to guanosine triphosphate (GTP). This family of proteins is homologous to Ras GTPases and is also called the Ras superfamily GTPases.
Lipidation
In order to associate with the inner leaflet of the plasma membrane, many G proteins are covalently modified with lipid extensions, i.e., they are lipidated.
- Heterotrimeric G protein subunits may be myristolated, palmitoylated, or prenylated.
- Small G proteins may be prenylated.
References
- ^ The Nobel Prize in Physiology or Medicine 1994, Illustrated Lecture.
- ^ Press Release: The Nobel Assembly at the Karolinska Institute has today decided to award the Nobel Prize in Physiology or Medicine for 1994 jointly to Alfred G. Gilman and Martin Rodbell for their discovery of "G-proteins and the role of these proteins in signal transduction in cells". 10 October 1994
- Eric R. Kandel, James H. Schwartz, Thomas M. Jessell (2000). Principles of Neural Science. New York: McGraw-Hill. ISBN 0-8385-7701-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Lodish H, Berk A, Zipursky LS, Matsudaira P, Baltimore D, Darnell J (1999). Molecular Cell Biology (4th ed. ed.). New York: Scientific American Books. ISBN 0-7167-3136-3.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link)
- Voet, Judith G.; Voet, Donald (1995). Biochemistry (2nd ed. ed.). New York: J. Wiley & Sons. ISBN 0-471-58651-X.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link) - Gilman AG (1987). "G proteins: transducers of receptor-generated signals". Annu. Rev. Biochem. 56: 615–49. doi:10.1146/annurev.bi.56.070187.003151. PMID 3113327.
- Neves SR, Ram PT, Iyengar R (2002). "G protein pathways". Science. 296 (5573): 1636–9. doi:10.1126/science.1071550. PMID 12040175.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
