Mitotane: Difference between revisions
No edit summary |
No edit summary |
||
| Line 47: | Line 47: | ||
}} |
}} |
||
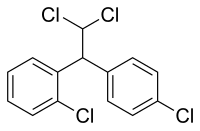
'''Mitotane''', sold under the brand name '''Lysodren''', is a [[steroidogenesis inhibitor]] and [[antineoplastic]] medication which is used in the treatment of [[adrenocortical carcinoma]].<ref name="JamesonGroot2010">{{cite book|author1=J. Larry Jameson|author2=Leslie J. De Groot|title=Endocrinology - E-Book: Adult and Pediatric|url=https://books.google.com/books?id=W4dZ-URK8ZoC&pg=PA1888|date=18 May 2010|publisher=Elsevier Health Sciences|isbn=1-4557-1126-8|pages=1888–}}</ref><ref name="pmid15898346">{{cite journal |vauthors=Hahner S, Fassnacht M |title=Mitotane for adrenocortical carcinoma treatment |journal=Current opinion in investigational drugs (London, England : 2000) |volume=6 |issue=4 |pages=386–94 |date=April 2005 |pmid=15898346 |doi= |url=}}</ref> It is a [[chemical derivative|derivative]] of {{abbrlink|p,p'-DDT|dichlorodiphenyltrichloroethane}} and an [[isomer]] of {{abbrlink|p,p'-DDD|dichlorodiphenyldichloroethane}}, and is also known as '''1,1-(dichlorodiphenyl)-2,2-dichloroethane''' ('''o,p'-DDD''').<ref name="PubChem">[http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=4211 Information from PubChem]</ref> |
'''Mitotane''', sold under the brand name '''Lysodren''', is a [[steroidogenesis inhibitor]] and [[cytostatic]] [[antineoplastic]] medication which is used in the treatment of [[adrenocortical carcinoma]] and [[Cushing's syndrome]].<ref name="JamesonGroot2010">{{cite book|author1=J. Larry Jameson|author2=Leslie J. De Groot|title=Endocrinology - E-Book: Adult and Pediatric|url=https://books.google.com/books?id=W4dZ-URK8ZoC&pg=PA1888|date=18 May 2010|publisher=Elsevier Health Sciences|isbn=1-4557-1126-8|pages=1888–}}</ref><ref name="pmid15898346">{{cite journal |vauthors=Hahner S, Fassnacht M |title=Mitotane for adrenocortical carcinoma treatment |journal=Current opinion in investigational drugs (London, England : 2000) |volume=6 |issue=4 |pages=386–94 |date=April 2005 |pmid=15898346 |doi= |url=}}</ref><ref name="Bronstein2010">{{cite book|author=Marcello D. Bronstein|title=Cushing's Syndrome: Pathophysiology, Diagnosis and Treatment|url=https://books.google.com/books?id=5_ulQAM9OZYC&pg=PA156|date=1 October 2010|publisher=Springer Science & Business Media|isbn=978-1-60327-449-4|pages=156–}}</ref><ref name="Elks2014">{{cite book|author=J. Elks|title=The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies|url=https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA382|date=14 November 2014|publisher=Springer|isbn=978-1-4757-2085-3|pages=382–}}</ref> It is a [[chemical derivative|derivative]] of {{abbrlink|p,p'-DDT|dichlorodiphenyltrichloroethane}} and an [[isomer]] of {{abbrlink|p,p'-DDD|dichlorodiphenyldichloroethane}}, and is also known as '''1,1-(dichlorodiphenyl)-2,2-dichloroethane''' ('''o,p'-DDD''').<ref name="PubChem">[http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=4211 Information from PubChem]</ref> |
||
==Medical uses== |
==Medical uses== |
||
Mitotane has been produced by [[Bristol Myers Squibb]] SpA but it is marketed as an [[orphan drug]] for adrenocortical carcinoma due to the small number of patients in need of it. Its main use is in those patients who have persistent disease despite surgical resection, those who are not surgical candidates, or those who have metastatic disease. A 2007 study of 177 patients shows a significant increase in the recurrence-free interval after radical surgery followed by mitotane when compared to surgery alone.<ref>{{cite journal|journal=N Engl J Med. | year = 2007 | volume = 356 | issue = 23 | pages = 2372–2380 | title=Adjuvant mitotane treatment for adrenocortical carcinoma|vauthors=Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A|doi=10.1056/NEJMoa063360|pmid=17554118}}</ref> |
Mitotane has been produced by [[Bristol Myers Squibb]] SpA but it is marketed as an [[orphan drug]] for adrenocortical carcinoma due to the small number of patients in need of it. Its main use is in those patients who have persistent disease despite surgical resection, those who are not surgical candidates, or those who have metastatic disease. A 2007 study of 177 patients shows a significant increase in the recurrence-free interval after radical surgery followed by mitotane when compared to surgery alone.<ref>{{cite journal|journal=N Engl J Med. | year = 2007 | volume = 356 | issue = 23 | pages = 2372–2380 | title=Adjuvant mitotane treatment for adrenocortical carcinoma|vauthors=Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A|doi=10.1056/NEJMoa063360|pmid=17554118}}</ref> The drug is also sometimes used in the treatment of Cushing's syndrome.<ref name="Bronstein2010" /> |
||
==Side effects== |
==Side effects== |
||
| Line 57: | Line 57: | ||
==Pharmacology== |
==Pharmacology== |
||
Mitotane is an inhibitor of the [[adrenal cortex]]. It acts as an [[enzyme inhibitor|inhibitor]] of [[cholesterol side-chain cleavage enzyme]] (P450scc, CYP11A1), and also of [[11β-hydroxylase]] (CYP11B1), [[18-hydroxylase]] (aldosterone synthase, CYP11B2), and [[3β-hydroxysteroid dehydrogenase]] (3β-HSD) to a lesser extent.<ref name="JamesonGroot2010" /><ref name="HarrisBouloux2014">{{cite book|author1=Philip E. Harris|author2=Pierre-Marc G. Bouloux|title=Endocrinology in Clinical Practice, Second Edition|url=https://books.google.com/books?id=tZE-AwAAQBAJ&pg=PA216|date=24 March 2014|publisher=CRC Press|isbn=978-1-84184-951-5|pages=216–}}</ref> In addition, mitotane has direct and selective [[cytotoxic]] effects on the adrenal cortex, via an unknown mechanism, and thereby induces permanent adrenal cortex atrophy similarly to DDD.<ref name="MPHMD2011">{{cite book|author1=Eudocia Quant Lee, MD, MPH|author2=David Schiff, MD|author3=Patrick Y. Wen, MD|title=Neurologic Complications of Cancer Therapy|url=https://books.google.com/books?id=52qyu5XPqM4C&pg=PA179|date=28 September 2011|publisher=Demos Medical Publishing|isbn=978-1-61705-019-0|pages=179–}}</ref><ref name="Kannan2012">{{cite book|author=C.R. Kannan|title=The Adrenal Gland|url=https://books.google.com/books?id=WqXSBwAAQBAJ&pg=PA160|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-1-4613-1001-3|pages=160–}}</ref> |
Mitotane is an inhibitor of the [[adrenal cortex]]. It acts as an [[enzyme inhibitor|inhibitor]] of [[cholesterol side-chain cleavage enzyme]] (P450scc, CYP11A1), and also of [[11β-hydroxylase]] (CYP11B1), [[18-hydroxylase]] (aldosterone synthase, CYP11B2), and [[3β-hydroxysteroid dehydrogenase]] (3β-HSD) to a lesser extent.<ref name="JamesonGroot2010" /><ref name="HarrisBouloux2014">{{cite book|author1=Philip E. Harris|author2=Pierre-Marc G. Bouloux|title=Endocrinology in Clinical Practice, Second Edition|url=https://books.google.com/books?id=tZE-AwAAQBAJ&pg=PA216|date=24 March 2014|publisher=CRC Press|isbn=978-1-84184-951-5|pages=216–}}</ref> In addition, mitotane has direct and selective [[cytotoxic]] effects on the adrenal cortex, via an unknown mechanism, and thereby induces permanent adrenal cortex atrophy similarly to DDD.<ref name="MPHMD2011">{{cite book|author1=Eudocia Quant Lee, MD, MPH|author2=David Schiff, MD|author3=Patrick Y. Wen, MD|title=Neurologic Complications of Cancer Therapy|url=https://books.google.com/books?id=52qyu5XPqM4C&pg=PA179|date=28 September 2011|publisher=Demos Medical Publishing|isbn=978-1-61705-019-0|pages=179–}}</ref><ref name="Kannan2012">{{cite book|author=C.R. Kannan|title=The Adrenal Gland|url=https://books.google.com/books?id=WqXSBwAAQBAJ&pg=PA160|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-1-4613-1001-3|pages=160–}}</ref> |
||
==History== |
|||
Mitotane was introduced in 1960 for the treatment of adrenocortical carcinoma.<ref name="Bronstein2010" /> |
|||
==Society and culture== |
==Society and culture== |
||
''Mitotane'' is the [[International Nonproprietary Name|INN]], [[United States Adopted Name|USAN]], [[British Approved Name|BAN]], and [[Japanese Accepted Name|JAN]] of mitotane. |
''Mitotane'' is the [[International Nonproprietary Name|INN]], [[United States Adopted Name|USAN]], [[British Approved Name|BAN]], and [[Japanese Accepted Name|JAN]] of mitotane.<ref name="Elks2014" /><ref name="IndexNominum2000">{{cite book|title=Index Nominum 2000: International Drug Directory|url=https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA697|year=2000|publisher=Taylor & Francis|isbn=978-3-88763-075-1|pages=697–}}</ref> |
||
==Veterinary use== |
==Veterinary use== |
||
Revision as of 07:12, 8 June 2017
 | |
| Clinical data | |
|---|---|
| Trade names | Lysodren |
| Other names | 1,1-(Dichlorodiphenyl)-2,2-dichloroethane; o,p'-DDD |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608050 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Protein binding | 6% |
| Elimination half-life | 18 to 159 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.152 |
| Chemical and physical data | |
| Formula | C14H10Cl4 |
| Molar mass | 320.04 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Mitotane, sold under the brand name Lysodren, is a steroidogenesis inhibitor and cytostatic antineoplastic medication which is used in the treatment of adrenocortical carcinoma and Cushing's syndrome.[2][3][4][5] It is a derivative of p,p'-DDT and an isomer of p,p'-DDD, and is also known as 1,1-(dichlorodiphenyl)-2,2-dichloroethane (o,p'-DDD).[6]
Medical uses
Mitotane has been produced by Bristol Myers Squibb SpA but it is marketed as an orphan drug for adrenocortical carcinoma due to the small number of patients in need of it. Its main use is in those patients who have persistent disease despite surgical resection, those who are not surgical candidates, or those who have metastatic disease. A 2007 study of 177 patients shows a significant increase in the recurrence-free interval after radical surgery followed by mitotane when compared to surgery alone.[7] The drug is also sometimes used in the treatment of Cushing's syndrome.[4]
Side effects
The use of mitotane is unfortunately limited by side effects,[8] which, as reported by Schteinberg et al., include anorexia and nausea (88%), diarrhea (38%), vomiting (23%), decreased memory and ability to concentrate (50%), rash (23%), gynecomastia (50%), arthralgia (19%), and leukopenia (7%).[9]
Pharmacology
Mitotane is an inhibitor of the adrenal cortex. It acts as an inhibitor of cholesterol side-chain cleavage enzyme (P450scc, CYP11A1), and also of 11β-hydroxylase (CYP11B1), 18-hydroxylase (aldosterone synthase, CYP11B2), and 3β-hydroxysteroid dehydrogenase (3β-HSD) to a lesser extent.[2][8] In addition, mitotane has direct and selective cytotoxic effects on the adrenal cortex, via an unknown mechanism, and thereby induces permanent adrenal cortex atrophy similarly to DDD.[10][11]
History
Mitotane was introduced in 1960 for the treatment of adrenocortical carcinoma.[4]
Society and culture
Mitotane is the INN, USAN, BAN, and JAN of mitotane.[5][12]
Veterinary use
Mitotane is also used to treat Cushing's disease (pituitary-dependent Cushing's syndrome) in dogs. The medication is used in the controlled destruction of adrenal tissue, leading to a decrease in cortisol production.[13]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b J. Larry Jameson; Leslie J. De Groot (18 May 2010). Endocrinology - E-Book: Adult and Pediatric. Elsevier Health Sciences. pp. 1888–. ISBN 1-4557-1126-8.
- ^ Hahner S, Fassnacht M (April 2005). "Mitotane for adrenocortical carcinoma treatment". Current opinion in investigational drugs (London, England : 2000). 6 (4): 386–94. PMID 15898346.
- ^ a b c Marcello D. Bronstein (1 October 2010). Cushing's Syndrome: Pathophysiology, Diagnosis and Treatment. Springer Science & Business Media. pp. 156–. ISBN 978-1-60327-449-4.
- ^ a b J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 382–. ISBN 978-1-4757-2085-3.
- ^ Information from PubChem
- ^ Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A (2007). "Adjuvant mitotane treatment for adrenocortical carcinoma". N Engl J Med. 356 (23): 2372–2380. doi:10.1056/NEJMoa063360. PMID 17554118.
- ^ a b Philip E. Harris; Pierre-Marc G. Bouloux (24 March 2014). Endocrinology in Clinical Practice, Second Edition. CRC Press. pp. 216–. ISBN 978-1-84184-951-5.
- ^ Schteinberg DE, Motazedi A, NoonanRA, Thompson NW (1982). "Treatment of Adrenal Carcinomas". Arch.Surg. 117: 1142–1149.
- ^ Eudocia Quant Lee, MD, MPH; David Schiff, MD; Patrick Y. Wen, MD (28 September 2011). Neurologic Complications of Cancer Therapy. Demos Medical Publishing. pp. 179–. ISBN 978-1-61705-019-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ C.R. Kannan (6 December 2012). The Adrenal Gland. Springer Science & Business Media. pp. 160–. ISBN 978-1-4613-1001-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 697–. ISBN 978-3-88763-075-1.
- ^ Canine Cushing’s Syndrome: Diagnosis and Treatment
External links
- V. P. Komissarenko; I. S. Chelnakova; A. S. Mikosha (1978). "Effect of o,p-dichlorodiphenyldichloroethane and perthane in vitro on glutathione reductase activity in the adrenals of dogs and guinea pigs". Bulletin of Experimental Biology and Medicine. 85 (2): 152–154. doi:10.1007/BF00800110.
- Government of Canada: Benzene, 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]- (Mitotane)
- Environment Canada & Health Canada: RISK MANAGEMENT SCOPE for Benzene, 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]- (Mitotane), 2013
