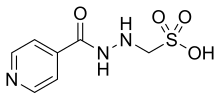
Methaniazide
 | |
| Clinical data | |
|---|---|
| Trade names | Erbazid, Neoiscotin, Neo-Iscotin, Nesticide, Neotizide, Neo-Tizide |
| Other names | Metaniazide; Neotizide; Isoniazid methanesulfonate; Isoniazid methanosulfonate; Isoniazid mesylate; Isonicotinic acid hydrazide methanesulfonate |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.264 |
| Chemical and physical data | |
| Formula | C7H9N3O4S |
| Molar mass | 231.23 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Methaniazide, brand name Neotizide among others, is an antibiotic which was used in the treatment of tuberculosis.[1][2][3] It is a derivative of methanesulfonic acid and isoniazid, which is also an antituberculosis drug but has comparatively been far more widely known and used.[1] Isoniazid is a prodrug of isonicotinic acid, and acetylisoniazid, a metabolite of isoniazid, is a metabolic intermediate through which most of the isonicotinic acid is formed.[4] Methaniazide features its mesylate group at the same position as that of the acetyl group in acetylisoniazid, and so methaniazide probably acts as a prodrug of isonicotinic acid similarly to isoniazid and acetylisoniazid. Methaniazide is used as the sodium salt.[1] It was never approved for use or sale in the United States.[5]
Neothetazone is an antibiotic combination of methaniazide (neotizide) and thioacetazone which was previously used in the treatment of tuberculosis.[6] It has been associated with a case report of gigantomastia.[6][7][8] Similarly, there have been a variety of case reports in the literature of gynecomastia associated with isoniazid treatment.[9]
References
- ^ a b c J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 709–. ISBN 978-1-4757-2085-3.
- ^ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 177–. ISBN 978-94-011-4439-1.
- ^ [1][dead link]
- ^ Bernard Testa; Joachim M. Mayer (August 2003). Hydrolysis in Drug and Prodrug Metabolism. John Wiley & Sons. pp. 149–. ISBN 978-3-906390-25-3.
- ^ "Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. Retrieved 2022-08-11.
- ^ a b SAMJ. Medical Association of South Africa. 1970. pp. 449–450.
Diffuse hypertrophy of the breasts seems to have been associated in this patient with treatment with Neothetazone, which consists of neotizide and thiacetazone, and which is very commonly used in the treatment of tuberculosis.
- ^ Sakai Y, Wakamatsu S, Ono K, Kumagai N (2002). "Gigantomastia induced by bucillamine". Annals of Plastic Surgery. 49 (2): 193–5. doi:10.1097/00000637-200208000-00013. PMID 12187348.
Drug-induced mammary hyperplasias have been reported as rare complications of D-penicillamine and Neothetazone. [...] It is rare for breast hypertrophy to be induced by drugs. In particular, gigantomastia has been reported to be induced by only two drugs: D-penicillamine and the antibiotic neothetazone. [...] In 1970, drug-induced gigantomastia was reported for the first time by Scott.4 It was induced by the antibiotic Neothetazone.
- ^ Dancey A, Khan M, Dawson J, Peart F (2008). "Gigantomastia--a classification and review of the literature". Journal of Plastic, Reconstructive & Aesthetic Surgery. 61 (5): 493–502. doi:10.1016/j.bjps.2007.10.041. PMID 18054304.
- ^ Khan A, Agarwal R (2012). "Isoniazid related gynecomastia: Description of a case and systematic review of literature". Lung India. 29 (2): 189–91. doi:10.4103/0970-2113.95343. PMC 3354502. PMID 22628943.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
