Ciprofloxacin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ciloxan, Cipro, Neofloxin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral, intravenous, topical (ear drops, eye drops) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 69%[2] |
| Metabolism | Hepatic, including CYP1A2 |
| Elimination half-life | 4 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.026 |
| Chemical and physical data | |
| Formula | C17H18FN3O3 |
| Molar mass | 331.346 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ciprofloxacin (INN) is a second-generation fluoroquinolone antibiotic.[3][4] Its spectrum of activity includes most strains of bacterial pathogens responsible for respiratory, urinary tract, gastrointestinal, and abdominal infections, including Gram-negative (Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, Proteus mirabilis, and Pseudomonas aeruginosa), and Gram-positive (methicillin-sensitive but not methicillin-resistant Staphylococcus aureus, Streptococcus pneumoniae, Staphylococcus epidermidis, Enterococcus faecalis, and Streptococcus pyogenes) bacterial pathogens. Ciprofloxacin and other fluoroquinolones are valued for this broad spectrum of activity, excellent tissue penetration, and for their availability in both oral and intravenous formulations.[5]
Ciprofloxacin is used alone or in combination with other antibacterial drugs in the empiric treatment of infections for which the bacterial pathogen has not been identified, including urinary tract infections[6][7] and abdominal infections[8] among others. It is also used for the treatment of infections caused by specific pathogens known to be sensitive.
Ciprofloxacin is the most widely used of the second generation quinolone antibiotics that came into clinical use in the late 1980s and early 1990s.[9][10] In 2010 over 20 million outpatient prescriptions were written for ciprofloxacin, making it the 35th most commonly prescribed drug, and the 5th most commonly prescribed antibacterial, in the US.[11] Ciprofloxacin was first patented in 1983 by Bayer A.G. and subsequently approved by the US Food and Drug Administration (FDA) in 1987. Ciprofloxacin has 12 FDA-approved human uses and other veterinary uses, but it is often used for unapproved uses (off-label).
Overall, the safety of ciprofloxacin and other fluoroquinolones appears to be similar to that of other antibiotics, but serious side effects occur on occasion. There is some disagreement in the literature regarding whether fluoroquinolones produce serious adverse events at a higher rate than other broad-spectrum antibiotics.[12][13][14][15][16] The U.S. FDA-approved label for ciprofloxacin includes a "black box" warning of increased risk of tendon damage and/or rupture and for exacerbation of muscle weakness in patients with the neurological disorder myasthenia gravis.[17]
Medical uses
Ciprofloxacin is used to treat a wide variety of infections, including infections of bones and joints, endocarditis, gastroenteritis, malignant otitis externa, respiratory tract infections, cellulitis, urinary tract infections, prostatitis, anthrax, and chancroid.[18]
Ciprofloxacin occupies an important role in treatment guidelines issued by major medical societies for the treatment of serious infections, especially those likely to be cause by Gram-(-) bacteria, including Pseudomonas aeruginosa. For example, ciprofloxacin in combination with metronidazole is one of several first line antibiotic regimens recommended by the Infectious Disease Society of America for the treatment of community-acquired abdominal infections in adults.[19] Ciprofloxacin also features prominently in treatment guidelines for acute pyelonephritis, complicated or hospital-acquired urinary tract infection, acute or chronic prostatitis,[20] certain types of endocarditis,[21] certain skin infections,[22] and prosthetic joint infections.[23]
In other cases treatment guidelines are more restrictive, recommending in most cases that older, narrower spectrum drugs be used as first line therapy for less severe infections in order to minimize fluoroquinlone resistance development. For example, the Infectious Disease Society of America recommends that the use of ciprofloxacin and other fluoroquinolones in acute cystisis be reserved to cases of proven or expected resistance to narrower spectrum drugs such as nitrorfurantoin or trimethoprim-sulfamethoxazole.[24] The European Association for Urology recommends ciprofloxacin as an alternative regimen for the treatment of uncomplicated urinary tract infections, but cautions that the potential for “adverse events have to be considered”.[20]
Although approved by regulatory authorities for the treatment of respiratory infections, most treatment guidelines recommend against the use of ciprofloxacin in these indications, in part due to its modest activity against the common respiratory pathogen Streptococcus pneumoniae.[25][26][27][28] "Respiratory quiniolones" such as levofloxacin, having greater activity against this pathogen, are recommended as first line agents for the treatment of community acquired pneumonia in patients with important co-morbidities and in patients requiring hospitalization (Infectious Diseases Society of America 2007). Similarly, ciprofloxacin is not recommended as a first-line treatment for acute sinusitis[29][30]
Ciprofloxacin is approved for the treatment of gonorrhea in many countries, but this recommendation is widely regarded as obsolete due to resistance development.[31][32][33][34]
Available forms
Ciprofloxacin for systemic administration is available as immediate release tablets, as extended release tablets, as an oral suspension, and as a solution for intravenous infustion. It is also available for local administration as eye and ear drops.
Specific populations
Pregnancy
The U.S. FDA categorizes ciprofloxacin in Pregnancy Category C.[35] This category includes drugs for which there are no adequate and well-controlled studies in human pregnancy, and for which animal studies have suggested the potential for harm to the fetus, but potential benefits may warrant use of the drug in pregnant women despite potential risks. An expert review of published data on experiences with ciprofloxacin use during pregnancy by TERIS – the Teratogen Information System – concluded that therapeutic doses during pregnancy are unlikely to pose a substantial teratogenic risk (quantity and quality of data=fair), but the data are insufficient to state that there is no risk.[36]
A controlled prospective observational study followed 200 women exposed to fluoroquinolones (52.5% exposed to ciprofloxacin and 68% first-trimester exposures) during gestation.[37] In utero exposure to fluoroquinolones during embryogenesis was not associated with increased risk of major malformations. Rates of spontaneous abortions, prematurity and low birth weight did not differ between the groups, and there were no clinically significant musculoskeletal dysfunctions up to one year of age in the ciprofloxacin-exposed children. Similar results were obtained in a second study of 549 pregnancies with fluoroquinoline exposure, of which 70 involved ciprofloxacin.[38] The label notes, however, "these small post-marketing epidemiology studies, of which most experience is from short term, first trimester exposure, are insufficient to evaluate the risk for less common defects or to permit reliable and definitive conclusions regarding the safety of ciprofloxacin in pregnant women and their developing fetuses."
Lactation
The fluoroquinolones have also been reported as being present in the mother's milk and are passed on to the nursing child.[39][40] The United States FDA recommends that because of the risk of serious adverse reactions (including articular damage) in infants nursing from mothers taking ciprofloxacin, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Children
Oral and intravenous ciprofloxacin are approved by the FDA for use in children for only two indications due to the risk of permanent injury to the musculoskeletal system. These include complicated urinary tract infections and pyelonephritis due to Escherichia coli[41] and inhalational anthrax (postexposure)[42] Current recommendations by the American Academy of Pediatrics note the systemic use of ciprofloxacin in children should be restricted to infections caused by multidrug-resistant pathogens or when no safe or effective alternatives are available.[43]
Adverse effects
Overall, the safety of ciprofloxacin and other fluoroquinolones appears to be similar to that of other antibiotics, but serious side effects occur on occasion. There is some disagreement in the literature regarding whether fluoroquinolones produce serious adverse events at a higher rate than other broad-spectrum antibiotics.[12][13][14][15][16]
49,038 patients received courses of ciprofloxacin in pre-approval clinical trials.[44] Most of the adverse events reported were described as only mild or moderate in severity, abated soon after the drug was discontinued, and required no treatment. Ciprofloxacin was discontinued because of an adverse event in 1% of orally treated patients. The most frequently reported drug-related events, from clinical trials of all formulations, all dosages, all drug-therapy durations, and for all indications of ciprofloxacin therapy, were nausea (2.5%), diarrhea (1.6%), abnormal liver function tests (1.3%), vomiting (1%), and rash (1%). Other adverse events occurred at rates of <1%.
Post-marketing surveillance has revealed tendonopathy, tendon rupture, and exacerbation of the symptoms of the neurological disorder myasthenia gravis as important serious adverse effects. The U.S. FDA-approved label for ciprofloxacin includes a "black box" warning of increased risk of tendon damage and/or rupture and for exacerbation of muscle weakness in patients with the neurological disorder myasthenia gravis.[17] A case control study[45] performed using a UK medical care database found that fluoroquinolone use was associated with a 1.9-fold increase in tendon problems. The relative risk increased to 3.2 in those over 60 years of age and to 6.2 in those over the age of 60 who were also taking corticosteroids. Among the 46,766 quinolone users in the study, 38 (0.1%) cases of Achilles tendon rupture were identified. A study performed using an Italian healthcare database reached qualitatively similar conclusions.[46] Tendonitis and other forms of tendon damage may manifest during fluoroquinolone therapy, and long after it had been discontinued.[47]
Clostridium difficile associated diarrhea is a serious adverse effect of ciprofloxacin and other fluoroquinolones; it is unclear whether the risk is higher than with other broad spectrum antibiotics.[48]
In 2013 the U.S. FDA issued a Safety Communication stating that its examination of spontaneous adverse event reports indicated an association of fluoroquinolone use with peripheral neuropathy, symptoms of which in some cases continued months or years after stopping therapy.[49] Headache, dizziness, and insomnia have been reported as occurring fairly commonly in post-approval review articles, along with a much lower incidence of serious CNS side effects not limited to but including tremors, psychosis, anxiety, hallucinations, paranoia, and suicide attempts, especially at higher doses.[12] Rare cases of liver failure have been reported.[50] A wide range of other apparently rare side effects including but not limited to Stevens-Johnson syndrome, heart arrhythmias (torsades des pointes), toxic epidermal necrolysis, vision problems, and bone marrow suppression have been spontaneously reported to the U.S FDA or have been the subject of case reports published in medical journals.[51]
Children and the elderly are at a much greater risk of experiencing adverse reactions.[52][53]
Contraindications
Three contraindications are found within the 2009 package insert:[54]
- "Coadministration of ciprofloxacin with other drugs primarily metabolized by CYP1A2 results in increased plasma concentrations of these drugs and could lead to clinically significant adverse events of the coadministered drug."
- "Concomitant administration with tizanidine is contraindicated."
- "Ciprofloxacin is contraindicated in persons with a history of hypersensitivity to ciprofloxacin, any member of the quinolone class of antimicrobial agents, or any of the product components."
Ciprofloxacin is also considered to be contraindicated within the pediatric population (except for the indications outlined above), pregnancy, nursing mothers, and in patients with epilepsy or other seizure disorders.
Genotoxicity and carcinogenicity studies
Ciprofloxacin is active in six of eight in vitro assays used as rapid screens for the detection of genotoxic effects, but is not active in in vivo assays of genotoxicity.[55] Long-term carcinogenicity studies in rats and mice resulted in no carcinogenic or tumorigenic effects due to ciprofloxacin at daily oral dose levels up to 250 and 750 mg/kg to rats and mice, respectively (about 1.7 and 2.5 times the highest recommended therapeutic dose based upon mg/m2). Results from photo co-carcinogenicity testing indicate ciprofloxacin does not reduce the time to appearance of UV-induced skin tumors as compared to vehicle control.
Interactions
Ciprofloxacin interacts with certain foods and several other drugs leading to undesirable increases or decreases in the serum levels or distribution of one or both drugs.
Co-administration of ciprofloxacin with anti-acids containing magnesium hydroxide or aluminum hydroxide leads to the formation of insoluble salts that are not readily absorbed from the intestinal tract. Peak serum concentrations of ciprofloxacin may be reduced by 90% or more, leading to therapeutic failure. Similar results have been reported when ciprofloxacin is co-administered with iron supplements and multi-vitamins containing zinc.[56][57]
Coadministration of ciprofloxacin with orange juice or dairy products has been reported to reduce both the peak serum concentration and the area under the serum concentration-time curve (AUC). In the case of calcium-fortified orange juice, reductions of up to 40% have been reported.[58]
Ciprofloxacin inhibits the drug-metabolizing enzyme CYP1A2 and thereby can reduce the clearance of drugs metabolized by that enzyme. Co-administration of ciprofloxacin with the CYP1A2 substrate tizanide is contradicted due to a 583% increase in the peak serum concentrations of tizanide when administered with ciprofloxacin as compared to administration of tizanide alone. Other CYP1A2 substrates that exhibit increased serum levels in ciprofloxacin-treated patients include theophylline, caffeine, and clozapine, though in these cases the observed increase in peak serum levels is less profound that in the case of tizanide. The authors of one review recommended that patients being treated with ciprofloxacin reduce their caffeine intake. Evidence for significant interactions with several other CYP1A2 substrates such as cyclosporine is equivocal or conflicting.[59][60][61]
The Committee on the Safety of Medicines and the FDA warn that central nervous system (CNS) adverse effects, including seizure risk, may be increased when NSAIDs are combined with quinolones.[59][62] The mechanism for this interaction may involve a synergistic increased antagonism of GABA neurotransmission.[16][63]
Altered serum levels of the anti-epileptic drugs phenytoin and carbamazepine (increased and decreased) have been reported in patients receiving concomitant ciprofloxacin.[59][64][65]
Overdose
Overdose of ciprofloxacin may result in reversible renal toxicity. Treatment of overdose includes emptying of the stomach by induced vomiting or gastric lavage. Careful monitoring and supportive treatment, monitoring of renal function, and maintaining adequate hydration is recommended by the manufacturer. Administration of magnesium-, aluminium-, or calcium-containing antacids can reduce the absorption of ciprofloxacin. Hemodialysis or peritoneal dialysis removes only less than 10% of ciprofloxacin.[57] Ciprofloxacin may be quantified in plasma or serum to monitor for drug accumulation in patients with hepatic dysfunction or to confirm a diagnosis of poisoning in acute overdose victims.[66]
Chemical Properties
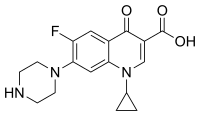
Ciprofloxacin is 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its molecular weight is 331.4 g/mol. It is a faintly yellowish to light yellow crystalline substance.[57]
Ciprofloxacin hydrochloride (USP) is the monohydrochloride monohydrate salt of ciprofloxacin. It is a faintly yellowish to light yellow crystalline substance with a molecular weight of 385.8 g/mol. Its empirical formula is C17H18FN3O3HCl•H2O.[57]
Mechanism of action
Ciprofloxacin is a broad-spectrum antibiotic active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV,[67] enzymes [68] necessary to separate bacterial DNA, thereby inhibiting cell division.
Pharmacokinetics
Ciprofloxacin for systemic administration is available as immediate release tablets, extended release tablets, an oral suspension, and as a solution for intravenous administration. When administered over one hour as an intravenous infusion,[69] ciprofloxacin rapidly distributes into the tissues, with levels in some tissues exceeding those in the serum. Penetration into the central nervous system is relatively modest, with cerebrospinal fluid levels normally less than 10% of peak serum concentrations. The serum half-life of ciprofloxacin is about 4–6 hours, with 50-70% of an administered dose being excreted in the urine as unmetabolized drug. An additional 10% is excreted in urine as metabolites. Urinary excretion is virtually complete by 24 hours after administration. Dose adjustment is required in the elderly and in those with renal impairment.
Ciprofloxacin is weakly bound to serum proteins (20-40%) but is an inhibitor of the drug-metabolizing enzyme cytochrome P450 1A2, which leads to the potential for clinically important drug interactions with drugs metabolized by that enzyme.
Ciprofloxacin is about 70% orally available when administered orally, so a slightly higher dose is needed to achieve the same exposure when switching from I.V. to oral administration. A 750 mg immediate release oral tablet given every 12 hours produces about the same area under the serum concentration curve (AUC) and peak serum concentration (Cmax) as a 400 mg dose given every 8 hours I.V.[70] The extended release oral tablets[71] allow once daily administration by releasing the drug more slowly in the gastrointestinal tract. These tablets contain 35% of the administered dose in an immediate release form and 65% in a slow release matrix. Maximum serum concentrations are achieved between 1 and 4 hours post-administration. Compared to the 250 and 500 mg immediate release tablets, the 500 mg and 1000 mg XR tablets provide higher Cmax but the 24 hour AUCs are equivalent.
Ciprofloxacin immediate release tablets contain ciprofloxacin as the hydrochloride salt, and the XR tablets contain a mixture of the hydrochloride salt as the free base.
History

The first members of the quinolone antibacterial class were relatively low potency drugs such as nalidixic acid, used mainly in the treatment of urinary tract infections owing to their renal excretion and propensity to be concentrated in urine.[72] In 1979 the publication of a patent[73] filed by the pharmaceutical arm of Kyorin Seiyaku Kabushiki Kaisha disclosed the discovery of norfloxacin, and the demonstration that certain structural modifications including the attachment of a fluorine atom to the quinolone ring leads to dramatically enhanced antibacterial potency.[74] In the aftermath of this disclosure, several other pharmaceutical companies initiated research and development programs with the goal of discovering additional antibacterial agents of the fluoroquinolone class. The fluoroquinolone program at Bayer focused on examining the effects of very minor changes to the norfloxacin structure.[75][76] In 1983, the company published in vitro potency data for ciprofloxacin, a fluoroquinolone antibacterial having a chemical structure that differs from that of norfloxacin by the presence of a single carbon atom.[77] This small change led to a 2 to 10-fold increase in potency against most strains of Gram-(-) bacteria. Importantly, this structural change led to a four-fold improvement in activity against the important Gram-(-) pathogen Pseudomonas aeruginosa, making ciprofloxacin one of the most potent known drugs for the treatment of this intrinsically antibiotic-resistant pathogen.
The oral tablet form of ciprofloxacin was approved in October 1987,[78] just one year after the approval of norfloxacin[79] In 1991, the intravenous formulation was introduced. Ciprofloxacin sales reached 1.52 million Euros in 1999, representing 30% of Bayer’s total pharmaceutical revenues. [80] [81] The sale of ciprofloxacin increased dramatically following the anthrax scare of 2001. On 24 October 2002, the Bush administration (2001–2009) announced a deal between the government and Bayer Pharmaceuticals to purchase 100 million tablets of ciprofloxacin at a reduced price of $0.95 per pill.
Society and Culture
Generic equivalents
On 24 October 2001, The Prescription Access Litigation (PAL) filed suit to dissolve an agreement between Bayer and three of its competitors which produced generic versions of drugs (Barr Laboratories, Rugby Laboratories, and Hoechst-Marion-Roussel) that PAL claimed was blocking access to adequate supplies and cheaper, generic versions of ciprofloxacin. The plaintiffs charged that Bayer Corporation, a unit of Bayer AG, had unlawfully paid the three competing companies a total of $200 million to prevent cheaper, generic versions of ciprofloxacin from being brought to the market, as well as manipulating its price and supply. Numerous other consumer advocacy groups joined the lawsuit. On 15 October 2008, five years after Bayer's patent had expired, the United States District Court for the Eastern District of New York granted Bayer's and the other defendants' motion for summary judgment, holding that any anticompetitive effects caused by the settlement agreements between Bayer and its codefendants were within the exclusionary zone of the patent and thus could not be redressed by federal antitrust law,[82] in effect upholding Bayer's agreement with its competitors.
Bacterial resistance
Ciprofloxacin is commonly used for urinary tract and intestinal infections (traveler's diarrhea), and was once considered a powerful antibiotic of last resort,[83][84][85] used to treat especially tenacious infections. Not all physicians agreed with this assessment, as evidenced by its widespread use to treat minor infections, as well as unapproved uses. As a result, many bacteria have developed resistance to this drug in recent years, leaving it significantly less effective than it would have been otherwise.[86][87]
Resistance to ciprofloxacin and other fluoroquinolones may evolve rapidly, even during a course of treatment. Numerous pathogens, including Staphylococcus aureus, enterococci, Streptococcus pyogenes and Klebsiella pneumoniae (quinolone-resistant) now exhibit resistance worldwide.[88] Widespread veterinary usage of the fluoroquinolones, particularly in Europe, has been implicated.[89] Meanwhile, some Burkholderia cepacia, Clostridium innocuum and Enterococcus faecium strains have developed resistance to ciprofloxacin to varying degrees.[90]
Fluoroquinolones had become the most commonly prescribed class of antibiotics to adults in 2002.[91] Nearly half (42%) of those prescriptions were for conditions not approved by the FDA, such as acute bronchitis, otitis media, and acute upper respiratory tract infection, according to a study supported in part by the Agency for Healthcare Research and Quality.[91][92] Additionally, they were commonly prescribed for medical conditions that were not even bacterial to begin with, such as viral infections, or those to which no proven benefit existed.
Litigation
A class action was filed against Bayer AG on behalf of employees of the Brentwood Post Office in Washington, D.C., and workers at the US Capitol, along with employees of American Media, Inc. in Florida and postal workers in general who alleged they suffered serious adverse effects from taking ciprofloxacin (Cipro) in the aftermath of the anthrax attacks in 2001. The action alleged Bayer failed to warn class members of the potential side effects of the drug, thereby violating the Pennsylvania Unfair Trade Practices and Consumer Protection Laws. According to the allegations within the complaint, exposed individuals were not informed of the true safety profile of ciprofloxacin, the high rate of adverse events associated with its use, or the availability of safer and equally effective alternative drugs. The class action was defeated and the litigation abandoned by the plaintiffs.[93] A similar action had been filed in New Jersey to cover New Jersey postal workers. Final disposition of that lawsuit is unknown. Following the addition of the black box warning in 2008, regarding tendon damage, product liability law firms began soliciting clients who have suffered a spontaneous tendon rupture following fluoroquinolone therapy.[94][95][96]
Brand names
Ciprofloxacin is marketed worldwide with over 300 different brand names. In the United States, Canada, and the UK, it is marketed as Baycip, Ciloxan, Ciflox, Ciplox, Cipro, Cipro XR, Cipro XL, Cifran, Ciproxin, Prociflor, and Proquin. It is also marketed as Hiflox, Neofloxin and Cipro-A in Bangladesh; in India it is marketed as Alcipro, in Russia as Ciprex,in Jordan as Ciprocin eye/ear drops,[97] and as Cetraxal in Spain. In Pakistan, it is marketed as Ciproheim, Cipesta, and Novidat. In addition, ciprofloxacin is available as a generic drug under a variety of different brand names and is also available for limited use in veterinary medicine.
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J, GL (1986). "Absolute oral bioavailability of ciprofloxacin". Antimicrob Agents Chemother. 30 (3): 444–6. ISSN 0066-4804. PMC 180577. PMID 3777908.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ball P (2000). "Quinolone generations: natural history or natural selection?". J. Antimicrob. Chemother. 46 Suppl T1: 17–24. PMID 10997595.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Oliphant CM, Green GM (2002). "Quinolones: a comprehensive review". Am Fam Physician. 65 (3): 455–64. PMID 11858629.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Laurence Brunton; John Lazo; Keith Parker (23 August 2005). Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill Prof Med/Tech. ISBN 978-0-07-142280-2. Retrieved 30 October 2012.
- ^ "www.uroweb.org" (PDF).
- ^ "National Guideline Clearinghouse | Treatment of urinary tract infections in nonpregnant women".
- ^ Solomkin JS, Mazuski JE, Bradley JS; et al. (2010). "Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America". Clin. Infect. Dis. 50 (2): 133–64. doi:10.1086/649554. PMID 20034345.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Goossens H, Ferech M, Coenen S, Stephens P (2007). "Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries". Clin. Infect. Dis. 44 (8): 1091–5. doi:10.1086/512810. PMID 17366456.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "British Columbia Annual Summary of Antibiotics Utilization 2010" (PDF).
{{cite web}}: line feed character in|title=at position 36 (help) - ^ "http://drugtopics.modernmedicine.com/drugtopics/data/articlestandard//drugtopics/252011/727243/article.pdf" (PDF). Retrieved 2 November 2012.
{{cite web}}: External link in|title= - ^ a b c Heidelbaugh JJ, Holmstrom H (2013). "The perils of prescribing fluoroquinolones". J Fam Pract. 62 (4): 191–7. PMID 23570031.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Brown KA, Khanafer N, Daneman N, Fisman DN (2013). "Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection". Antimicrob. Agents Chemother. 57 (5): 2326–32. doi:10.1128/AAC.02176-12. PMC 3632900. PMID 23478961.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Falagas ME, Matthaiou DK, Vardakas KZ (2006). "Fluoroquinolones vs beta-lactams for empirical treatment of immunocompetent patients with skin and soft tissue infections: a meta-analysis of randomized controlled trials". Mayo Clin. Proc. 81 (12): 1553–66. PMID 17165634.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Knottnerus BJ, Grigoryan L, Geerlings SE; et al. (2012). "Comparative effectiveness of antibiotics for uncomplicated urinary tract infections: network meta-analysis of randomized trials". Fam Pract. 29 (6): 659–70. doi:10.1093/fampra/cms029. PMID 22516128.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c De Sarro A, De Sarro G (2001). "Adverse reactions to fluoroquinolones. an overview on mechanistic aspects". Curr. Med. Chem. 8 (4): 371–84. PMID 11172695.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b "www.accessdata.fda.gov" (PDF). Cite error: The named reference "www.accessdata.fda.gov" was defined multiple times with different content (see the help page).
- ^ "Ciprofloxacin-Hydrochloride". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ "Diagnosis and Management of Complicated Intra-abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America".
- ^ a b "www.uroweb.org" (PDF).
- ^ "Infective Endocarditis".
- ^ "Practice Guidelines for the Diagnosis and Management of Skin and Soft-Tissue Infections".
- ^ "Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America".
- ^ "International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases".
- ^ "pharmacy.oregonstate.edu" (PDF).
- ^ Zuger A (3 November 1998). "Ciprofloxacin for Acute Exacerbations of Chronic Bronchitis". Journal Watch (General). 1998 (1103): 4.
Because of its unpredictable activity against the pneumococcus, ciprofloxacin is not usually considered a first-line treatment for respiratory infections...
- ^ Vardakas, KZ; Siempos, II; Grammatikos, A; Athanassa, Z; Korbila, IP; Falagas, ME (2008). "Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials". CMAJ. 179 (12): 1269–77. doi:10.1503/cmaj.080358. PMC 2585120. PMID 19047608.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Donaldson, PM; Pallett, AP; Carroll, MP (1994). "Ciprofloxacin in general practice". BMJ (Clinical Research Ed.). 308 (6941): 1437. ISSN 0959-8138. PMC 2540361. PMID 8019264.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Karageorgopoulos, DE.; Giannopoulou, KP.; Grammatikos, AP.; Dimopoulos, G.; Falagas, ME. (2008). "Fluoroquinolones compared with beta-lactam antibiotics for the treatment of acute bacterial sinusitis: a meta-analysis of randomized controlled trials". CMAJ. 178 (7): 845–54. doi:10.1503/cmaj.071157. PMC 2267830. PMID 18362380.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chow AW, Benninger MS, Brook I; et al. (2012). "IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults". Clin. Infect. Dis. 54 (8): e72–e112. doi:10.1093/cid/cir1043. PMID 22438350.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ (World Health Organization (WHO) Western Pacific Region Gonococcal Antimicrobial Susceptibility Programme (GASP) Report- 2000. Commun Dis Intell 2001; 25:274-277)
- ^ DEPARTMENT OF HEALTH AND HUMAN SERVICES (2004). "Gonococcal Isolate Surveillance Project (GISP) Annual Report - 2003" (PDF). USA: Center for Disease Controlo. Retrieved 31 August 2009.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Hugh Young (22 July 2003). "Ciprofloxacin resistant gonorrhoea: the situation in Scotland and implications for therapy" (PDF). SCIEH Weekly Report - SCOTTISH CENTRE FOR INFECTION AND ENVIRONMENTAL HEALTH. 37. Scotland: National Health Service. ISSN 1357-4493.
- ^ Centers for Disease Control and Prevention (CDC) (13 April 2007). "Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections". Morbidity and Mortality Weekly Report. 56 (14). USA: Center for Disease Control: 332–336. PMID 17431378.
{{cite journal}}:|access-date=requires|url=(help);|format=requires|url=(help) - ^ FDA-approved drug label
- ^ Friedman, J.; Polifka, J. (2000). Teratogenic effects of drugs: a resource for clinicians (TERIS). Baltimore, Maryland: Johns Hopkins University Press. pp. 149–195.
- ^ Loebstein R, Addis A, Ho E, Andreou R, Sage S, Donnenfeld AE; et al. (1998). "Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study". Antimicrob Agents Chemother. 42 (6): 1336–9. PMC 105599. PMID 9624471.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Schaefer C, Amoura-Elefant E, Vial T, Ornoy A, Garbis H, Robert E; et al. (1996). "Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European Network of Teratology Information Services (ENTIS)". Eur J Obstet Gynecol Reprod Biol. 69 (2): 83–9. doi:10.1016/0301-2115(95)02524-3. PMID 8902438.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Shin HC, Kim JC, Chung MK, HC (2003). "Fetal and maternal tissue distribution of the new fluoroquinolone DW-116 in pregnant rats". Comp. Biochem. Physiol. C Toxicol. Pharmacol. 136 (1): 95–102. doi:10.1016/j.cca.2003.08.004. ISSN 1532-0456. PMID 14522602.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dan M, Weidekamm E, Sagiv R, Portmann R, Zakut H, M (1993). "Penetration of fleroxacin into breast milk and pharmacokinetics in lactating women". Antimicrob. Agents Chemother. 37 (2): 293–6. doi:10.1128/AAC.37.2.293. ISSN 0066-4804. PMC 187655. PMID 8452360.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Renata Albrecht (25 March 2004). "Cipro Labeling Revision Letter 03/25/2004 Supplement 049 Patient Population Altered" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 7 September 2009.
- ^ Dianne Murphy (30 August 2000). "Cipro Labeling Revision Letter 08/30/2000 Supplement 008 New or Modified Indication" (PDF). U.S. Food and Drug Administration.
- ^ Lexi-Comp (2009). "Ciprofloxacin". Merk. Retrieved 4 September 2009.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ FDA-approved package insert
- ^ van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HG, Stricker BH (2002). "Fluoroquinolones and risk of Achilles tendon disorders: case-control study". BMJ. 324 (7349): 1306–7. doi:10.1136/bmj.324.7349.1306. PMC 113766. PMID 12039823.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Corrao G, Zambon A, Bertù L; et al. (2006). "Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study". Drug Saf. 29 (10): 889–96. PMID 16970512.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Saint, F; Gueguen; Biserte; Fontaine; Mazeman (2000). "Rupture of the patellar ligament one month after treatment with fluoroquinolone". Revue de chirurgie orthopedique et reparatrice de l'appareil moteur. 86 (5): 495–7. ISSN 0035-1040. PMID 10970974.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Deshpande A, Pant C, Jain A, Fraser TG, Rolston DD (2008). "Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence". Curr Med Res Opin. 24 (2): 329–33. doi:10.1185/030079908X253735. PMID 18067688.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "FDA Drug Safety Communication: FDA requires label changes to warn of risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection".
- ^ Alshammari TM, Larrat EP, Morrill HJ, Caffrey AR, Quilliam BJ, Laplante KL (2014). "Risk of hepatotoxicity associated with fluoroquinolones: A national case-control safety study". Am J Health Syst Pharm. 71 (1): 37–43. doi:10.2146/ajhp130165. PMID 24352180.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Cipro Immediate Release Tablets Prescribing Information" (PDF).
- ^ Iannini, PB (2007). "The safety profile of moxifloxacin and other fluoroquinolones in special patient populations". Current medical research and opinion. 23 (6): 1403–13. doi:10.1185/030079907X188099. ISSN 0300-7995. PMID 17559736.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Owens Rc, Jr; Ambrose (2005). "Antimicrobial safety: focus on fluoroquinolones". Clinical Infectious Diseases. 41 Suppl 2: S144–57. doi:10.1086/428055. ISSN 1058-4838. PMID 15942881.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Cipro Labeling Revision 02/25/2011 Supplement 075" (PDF). U.S. Food and Drug Administration (FDA). 25 February 2011. Retrieved 1 April 2011.
- ^ "CIPRO® (ciprofloxacin hydrochloride) TABLETS
CIPRO® (ciprofloxacin*) ORAL SUSPENSION" (PDF). - ^ Rodvold KA, Piscitelli SC (1993). "New oral macrolide and fluoroquinolone antibiotics: an overview of pharmacokinetics, interactions, and safety". Clin. Infect. Dis. 17 Suppl 1: S192–9. PMID 8399914.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d "Cipro Labeling Revision 04/06/2009 Supplement 073" (PDF). US Food and Drug Administration. 6 April 2009. Retrieved 8 September 2009.
- ^ Bolhuis MS, Panday PN, Pranger AD, Kosterink JG, Alffenaar JW (2011). "Pharmacokinetic drug interactions of antimicrobial drugs: a systematic review on oxazolidinones, rifamycines, macrolides, fluoroquinolones, and Beta-lactams". Pharmaceutics. 3 (4): 865–913. doi:10.3390/pharmaceutics3040865. PMC 3857062. PMID 24309312.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c "Cipro Labeling Revision 10/03/2008 Supplement 068" (PDF). US Food and Drug Administration. 3 October 2008. Retrieved 31 August 2009.
- ^ Bolhuis MS, Panday PN, Pranger AD, Kosterink JG, Alffenaar JW (2011). "Pharmacokinetic drug interactions of antimicrobial drugs: a systematic review on oxazolidinones, rifamycines, macrolides, fluoroquinolones, and Beta-lactams". Pharmaceutics. 3 (4): 865–913. doi:10.3390/pharmaceutics3040865. PMC 3857062. PMID 24309312.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Janknegt R, R (1990). "Drug interactions with quinolones". J. Antimicrob. Chemother. 26 Suppl D: 7–29. doi:10.1093/jac/26.suppl_D.7. ISSN 0305-7453. PMID 2286594.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Royal Pharmaceutical Society of Great Britain (2009). "5 Infections". British National Formulary (BNF 57). BMJ Group and RPS Publishing. ISBN 978-0-85369-845-6.
- ^ Brouwers JR, JR (1992). "Drug interactions with quinolone antibacterials". Drug Saf. 7 (4): 268–81. doi:10.2165/00002018-199207040-00003. ISSN 0114-5916. PMID 1524699.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Shahzadi A, Javed I, Aslam B; et al. (2011). "Therapeutic effects of ciprofloxacin on the pharmacokinetics of carbamazepine in healthy adult male volunteers" (PDF). Pak J Pharm Sci. 24 (1): 63–68. PMID 21190921.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^
Carol Langlois (1998). "Risk of seizures from concomitant use of ciprofloxacin and phenytoin in patients with epilepsy" (PDF). Canada: Canadian Adverse Drug Reaction Newsletter. Retrieved 30 January 2009.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 313-315. ISBN 978-0-9626523-7-0.
- ^ Drlica K, Zhao X, K (1 September 1997). "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiol Mol Biol Rev. 61 (3): 377–92. ISSN 1092-2172. PMC 232616. PMID 9293187.
{{cite journal}}:|first2=missing|last2=(help) - ^ Pommier, Y., Leo, E., Zhang, H., Marchand, C. 2010. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs" Chem. Biol 17: 421-433.
- ^ "Cipro IV Prescribing Information" (PDF).
- ^ "Cipro Immediate Release Tablets Prescribing Information" (PDF).
- ^ "Cipro XR Prescribing Information" (PDF).
- ^ Mayrer AR, Andriole VT (1982). "Urinary tract antiseptics". Med. Clin. North Am. 66 (1): 199–208. PMID 7038329.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Patent US4146719 - Piperazinyl derivatives of quinoline carboxylic acids - Google Patents".
- ^ "aac.asm.org".
- ^ "Patent US4547503 - 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[4-(oxo-alkyl)-1-piperazinyl ... - Google Patents".
- ^ "Patent US4544658 - 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(alkyl-1-piperazinyl)quinoline-3 ... - Google Patents".
- ^ Wise R, Andrews JM, Edwards LJ (1983). "In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents". Antimicrob. Agents Chemother. 23 (4): 559–64. PMC 184701. PMID 6222695.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=019537&TABLE1=OB_Rx
- ^ "Orange Book Detail Record Search".
- ^ "www.sec.gov".
- ^ "Cipro". USA: Prescription Access. Retrieved 4 September 2009.
- ^ United States Court of Appeals for the Federal Circuit (2008). "United States Court of Appeals for the Federal Circuit" (PDF). USA. Retrieved 4 September 2009. [dead link]
- ^ Biosecurity requires drug reform. 1 January 2002 World Watch ISSN: 0896-0615
- ^ Nawaz, H.; Rauf, S.; Akhtar, K.; Khalid, AM. (2006). "Electrochemical DNA biosensor for the study of ciprofloxacin-DNA interaction". Anal Biochem. 354 (1): 28–34. doi:10.1016/j.ab.2006.04.004. PMID 16707087.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Stacey L. Knobler (2003). The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment, Workshop Summary. National Academies Press. p. 34. ISBN 978-0-309-08854-1.
{{cite book}}: Cite has empty unknown parameter:|chapterurl=(help) - ^ A.C. Vatopoulos; V. Kalapothaki (1997). "Bacterial Resistance to Ciprofloxacin in Greece: Results from the National Electronic Surveillance System" (PDF).
- ^ "Bacterial resistance prompts concern among health officials". 26 February 2009.[dead link]
- ^ M Jacobs, Worldwide Overview of Antimicrobial Resistance. International Symposium on Antimicrobial Agents and Resistance 2005.
- ^ "Update On Extra-Label Use Of Fluoroquinolones" (Press release). Center for Veterinary Medicine (CVM). 16 July 1996. Retrieved 12 August 2009.
- ^ "Ciprofloxacin spectrum of bacterial susceptibility and Resistance" (PDF). Retrieved 4 May 2012.
- ^ a b Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS (2005). "Fluoroquinolone prescribing in the United States: 1995 to 2002". Am. J. Med. 118 (3): 259–68. doi:10.1016/j.amjmed.2004.09.015. PMID 15745724.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ K08 HS14563 and HS11313[full citation needed]
- ^ "Legal Brief of Postal Employees Cases (EEOC, MSPB, District Courts)". USA: Postal Reporter. Archived from the original on 21 October 2007. Retrieved 9 September 2009.
- ^ "LegalView Reveals Details of the FDA Mandated Black Box Warning For Fluoroquinolone Antibiotics". PRlog. 11 July 2008.
- ^ Cynthia Diaz (6 July 2009). "Levaquin Litigation Moving Ahead". Zimbio.
In May we wrote that most litigation specialists expected thousands of people to file lawsuits against the makers of Levaquin and similar drugs...
- ^ Carey and Danis LLC (3 September 2009). "Carey and Danis LLC Announces Four Lawsuits against the Makers of Levaquin". Reuters.
- ^ http://www.jfda.jo/
