Ketoprofen
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Oruvail, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a686014 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical, intravenous (veterinary use) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99% |
| Elimination half-life | 2–2.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.676 |
| Chemical and physical data | |
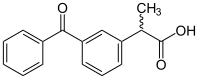
| Formula | C16H14O3 |
| Molar mass | 254.285 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Ketoprofen is one of the propionic acid class of nonsteroidal anti-inflammatory drugs (NSAID) with analgesic and antipyretic effects.[2] It acts by inhibiting the body's production of prostaglandin.
It was patented in 1967 and approved for medical use in 1980.[3]
Medical uses
Ketoprofen is generally prescribed for arthritis-related inflammatory pains or severe toothaches that result in the inflammation of the gums.
Ketoprofen topical patches are being used for treatment of musculoskeletal pain.[4][5][6]
Ketoprofen can also be used for treatment of some pain, especially nerve pain such as sciatica, postherpetic neuralgia and referred pain for radiculopathy, in the form of a cream, ointment, liquid, spray, or gel, which may also contain ketamine and lidocaine, along with other agents which may be useful, such as cyclobenzaprine, amitriptyline, acyclovir, gabapentin, orphenadrine and other drugs used as NSAIDs or adjuvant, atypical or potentiators for pain treatment.
Trials are going on for using this drug along with ibuprofen for management of lymphedema.[citation needed] Animal trial and some human trials have shown significant improvement over placebo control. Dr Stanley G Rockson, of Stanford University is leading these researches.[citation needed]
Efficacy
A 2013 systematic review indicated "The efficacy of orally administered ketoprofen in relieving moderate-severe pain and improving functional status and general condition was significantly better than that of ibuprofen and/or diclofenac."[7] A 2017 Cochrane systematic review investigating ketoprofen as a single-dose by mouth in acute, moderate-to-severe postoperative pain concluded that its efficacy is equivalent to drugs such as ibuprofen and diclofenac.[8]
There is evidence supporting topical ketoprofen for osteoarthritis but not other chronic musculoskeletal pain.[9]
Adverse effects
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in fetuses that result in low amniotic fluid.[10][11] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[10][11]
Mechanism
Ketoprofen undergoes metabolism in the liver via conjugation with glucuronic acid (glucuronidation) by UGT enzymes, hydroxylation of the benzoyl ring by the CYP3A4 and CYP2C9 enzymes, and reduction of its ketone moiety (a carbonyl functional group, i.e. with carbon-oxygen double bond)[12] by carbonyl reducing enzymes (CREs).[13][14] Ketoprofen is used for its antipyretic, analgesic, and anti-inflammatory properties by inhibiting cyclooxygenase-1 and -2 (COX-1 and COX-2) enzymes reversibly, which decreases production of proinflammatory prostaglandin precursors.[13][15]
The patches have been shown to provide rapid and sustained delivery to underlying tissues without significantly increasing levels of drug concentration in the blood when compared to the traditional oral administration.[6][16]
Chirality and biological activity
Ketoprofen has one stereogenic center in the side chain and hence exists as mirror-image twins. Majority of the profens are marketed as racemic mixtures. For most of the NSAIDs the pharmacological activity resides in the (S)-enantiomers with their (R)-enantiomer virtually inactive. An interesting observation about most profens including ketoprofen is that they undergo unidirectional metabolic inversion, chiral inversion, of the (R)- acid to its (S)-mirror-image version with no other change in the molecule.[17][18][19] There have been concerns raised that Ketoprofen can break down into the parent Benzophenone molecule in skin exposed to strong summer or tropical UV light and this could pose a theoretical cancer risk. Given such a risk it is better to use other pain killers in such circumstances.
History
The earliest report of therapeutic use in humans is in 1972.[20]
Society and culture
Brand names
Brand names in Australia include Orudis and Oruvail. It is available in Japan in a transdermal patch Mohrus Tape, made by Hisamitsu Pharmaceutical. It is available in the UK as Ketoflam and Oruvail, in Ireland as Fastum Gel, in Estonia as Keto, Ketonal, and Fastum Gel, in Finland as Ketorin, Keto, Ketomex, and Orudis; in France as Profénid, Bi-Profénid, Toprec, and Ketum; in Italy as Ketodol, Fastum Gel, Lasonil, Orudis and Oki; in Greece as Okitask; in Poland as Ketonal, Ketonal active, Ketolek, in Serbia, Slovenia and Croatia as Knavon and Ketonal; in Romania as Ketonal and Fastum Gel; in Mexico as Arthril; in Norway as Zon and Orudis; in Russia as ОКИ (OKI), Fastum Gel and Ketonal; in Spain as Actron and Fastum Gel; in Albania as Oki and Fastum Gel and in Venezuela as Ketoprofeno as an injectable solution of 100 mg and 150 mg capsules.
In some countries, the optically pure (S)-enantiomer (dexketoprofen) is available; its trometamol salt is said to be particularly rapidly reabsorbed from the gastrointestinal tract, having a rapid onset of effects.
Veterinary medicine
Ketoprofen is a common NSAID, antipyretic, and analgesic used in horses and other equines.[21] It is most commonly used for musculoskeletal pain, joint problems, and soft tissue injury, as well as laminitis. It is also used to control fevers and prevent endotoxemia. It is also used as a mild painkiller in smaller animals, generally following surgical procedures.
In horses, it is given at a dose of 2.2 mg/kg/day. Studies have shown that it does not inhibit 5-lipoxygenase and leukotriene B4,[22] as originally claimed.[23] It is therefore not considered superior to phenylbutazone as previously believed, although clinical signs of lameness are reduced with its use.[24] In fact, phenylbutazone was shown superior to ketoprofen in cases of experimentally-induced synovitis when both drugs were used at labeled dosages.[25]
Ecological problems
Experiments have found ketoprofen, like diclofenac, is a veterinary drug causing lethal effects in red-headed vultures. Vultures feeding on the carcasses of recently treated livestock develop acute kidney failure within days of exposure.[26]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Kantor TG (1986). "Ketoprofen: a review of its pharmacologic and clinical properties". Pharmacotherapy. 6 (3): 93–103. doi:10.1002/j.1875-9114.1986.tb03459.x. PMID 3526298. S2CID 25309841.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 520. ISBN 9783527607495.
- ^ Mazières B, Rouanet S, Guillon Y, Scarsi C, Reiner V (August 2005). "Topical ketoprofen patch in the treatment of tendinitis: a randomized, double blind, placebo controlled study". The Journal of Rheumatology. 32 (8): 1563–1570. PMID 16078335.
- ^ Mazières B (2005). "Topical ketoprofen patch". Drugs in R&D. 6 (6): 337–344. doi:10.2165/00126839-200506060-00003. PMID 16274258. S2CID 30719197.
- ^ a b Sekiya I, Morito T, Hara K, Yamazaki J, Ju YJ, Yagishita K, et al. (March 2010). "Ketoprofen absorption by muscle and tendon after topical or oral administration in patients undergoing anterior cruciate ligament reconstruction". AAPS PharmSciTech. 11 (1): 154–158. doi:10.1208/s12249-009-9367-2. PMC 2850498. PMID 20087696.
- ^ Sarzi-Puttini P, Atzeni F, Lanata L, Bagnasco M (2013). "Efficacy of ketoprofen vs. ibuprofen and diclofenac: a systematic review of the literature and meta-analysis". Clinical and Experimental Rheumatology. 31 (5): 731–738. PMID 23711416.
- ^ Gaskell H, Derry S, Wiffen PJ, Moore RA (May 2017). "Single dose oral ketoprofen or dexketoprofen for acute postoperative pain in adults". The Cochrane Database of Systematic Reviews. 2019 (5): CD007355. doi:10.1002/14651858.CD007355.pub3. PMC 6481461. PMID 28540716.
- ^ Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA (April 2016). "Topical NSAIDs for chronic musculoskeletal pain in adults" (PDF). The Cochrane Database of Systematic Reviews. 4 (4): CD007400. doi:10.1002/14651858.cd007400.pub3. PMC 6494263. PMID 27103611.
- ^ a b
 This article incorporates text from this source, which is in the public domain: "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Retrieved 15 October 2020.
This article incorporates text from this source, which is in the public domain: "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Retrieved 15 October 2020.
- ^ a b
 This article incorporates text from this source, which is in the public domain: "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Retrieved 15 October 2020.
This article incorporates text from this source, which is in the public domain: "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Retrieved 15 October 2020.
- ^ Malátková P, Wsól V (February 2014). "Carbonyl reduction pathways in drug metabolism". Drug Metabolism Reviews. 46 (1): 96–123. doi:10.3109/03602532.2013.853078. PMID 24171394. S2CID 43774709.
- ^ a b Ketoprofen. (n.d.). Millennium Web Catalog. Retrieved 1 February 2010, from http://0-online.lexi.com.library.touro.edu Archived 29 August 2021 at the Wayback Machine
- ^ Lemke TL, Williams DA, Roche VF, Zito SW. Foyes Principles of Medical Chemistry. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2008.
- ^ Ketoprofen. (n.d.). Micromedex. Retrieved 1 February 2010, from https://bb-tuc.touro.edu/webapps/portal/frameset.jsp?tab_id=_102_1 Archived 20 July 2011 at the Wayback Machine
- ^ Gayman MD, Turner RJ, Cui M (July 2008). "Physical limitations and depressive symptoms: exploring the nature of the association". The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 63 (4): S219–S228. doi:10.1093/geronb/63.4.s219. PMC 2844725. PMID 18689771.
- ^ Hutt AJ, Caldwell J (November 1983). "The metabolic chiral inversion of 2-arylpropionic acids--a novel route with pharmacological consequences". The Journal of Pharmacy and Pharmacology. 35 (11): 693–704. doi:10.1111/j.2042-7158.1983.tb02874.x. PMID 6139449. S2CID 40669413.
- ^ Caldwell J, Hutt AJ, Fournel-Gigleux S (January 1988). "The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences". Biochemical Pharmacology. 37 (1): 105–114. doi:10.1016/0006-2952(88)90762-9. PMID 3276314.
- ^ Aboul-Enein HY, Wainer IW (1997). The impact of stereochemistry on drug development and use. New York: Wiley. ISBN 0-471-59644-2. OCLC 35262289.
- ^ Gyory AN, Bloch M, Burry HC, Grahame R (November 1972). "Orudis in management of rheumatoid arthritis and osteoarthrosis of the hip: comparison with indomethacin". British Medical Journal. 4 (5837): 398–400. doi:10.1136/bmj.4.5837.398. PMC 1786628. PMID 4564764.
- ^ Forney BC (2007), Understanding equine medications: your guide to horse health care and managements, The Horse health care library (Revised ed.), Lexington, KY: Eclipse Press, OCLC 1360077554
- ^ Salmon JA, Tilling LC, Moncada S (September 1984). "Benoxaprofen does not inhibit formation of leukotriene B4 in a model of acute inflammation". Biochemical Pharmacology. 33 (18): 2928–2930. doi:10.1016/0006-2952(84)90220-x. PMID 6089840.
- ^ Betley M, Sutherland SF, Gregoricka MJ, Pollet RA (1991). "The analgesic effect of ketoprofen for use in treating equine colic as compared to flunixin meglumine". Equine Pract. 13: 11–16.
- ^ Owens JG, Kamerling SG, Keowen ML (1994). Anti-inflammatory effects and pharmacokinetics of ketoprofen in a model of equine synovitis. Proceedings of the 6th International Congress of the EAVPT. Blackwell Scientific Publications. pp. 170–171.
- ^ Owens JG, Kamerling SG, Stanton SR, Keowen ML, Prescott-Mathews JS (June 1996). "Effects of pretreatment with ketoprofen and phenylbutazone on experimentally induced synovitis in horses". American Journal of Veterinary Research. 57 (6): 866–874. PMID 8725815.
- ^ Naidoo V, Wolter K, Cromarty D, Diekmann M, Duncan N, Meharg AA, et al. (June 2010). "Toxicity of non-steroidal anti-inflammatory drugs to Gyps vultures: a new threat from ketoprofen". Biology Letters. 6 (3): 339–341. doi:10.1098/rsbl.2009.0818. PMC 2880042. PMID 20007163.
